Abstract
Glycine receptors are widely expressed in the mammalian central nervous system, and previous studies have demonstrated that glycine receptors are modulated by endogenous zinc. Zinc is concentrated in synaptic vesicles in several brain regions but is particularly abundant in the hippocampus and olfactory bulb. In the present study, we used patch-clamp electrophysiology of rat hippocampal and olfactory bulb neurons in primary culture to examine the effects of zinc on glycine receptors. Although glycine has been reported to reach millimolar concentrations during synaptic transmission, most previous studies on the effects of zinc on glycine receptors have used relatively low concentrations of glycine. High concentrations of glycine cause receptor desensitization. Our current results extend our previous demonstration that the modulatory actions of zinc are largely prevented when co-applied with desensitizing concentrations of glycine (300 μM), suggesting that the effects of zinc are dependent on the state of the receptor. In contrast, pre-application of 300 μM zinc, prior to glycine (300 μM) application, causes a slowly developing inhibition with a slow rate of recovery, suggesting that the timing of zinc and glycine release also influences the effects of zinc. Furthermore, previous evidence suggests that synaptically released zinc can gain intracellular access, and we provide the first demonstration that low concentrations of intracellular zinc can potentiate glycine receptors. These results support the notion that zinc has complex effects on glycine receptors and multiple factors may interact to influence the efficacy of glycinergic transmission.
Keywords: Olfactory bulb, hippocampus, electrophysiology
Zinc is an essential element with diverse roles in protein function including acting as a cofactor and structural component. While the majority of zinc in the central nervous system is protein-bound, a significant pool of zinc is contained within synaptic vesicles (Frederickson, 1989, Cole et al., 1999) in subpopulations of glycinergic, GABAergic, and primarily glutamatergic neurons (Birinyi et al., 2001, Wang et al., 2001, Wang et al., 2002). Experimental evidence supports the notion that vesicular zinc is released upon depolarization (Assaf and Chung, 1984, Howell et al., 1984, Li et al., 2001) and can influence neuronal excitability via effects on several classes of voltage- and ligand-gated ion channels (Harrison and Gibbons, 1994, Trombley and Shepherd, 1996, Horning and Trombley, 2001, Blakemore and Trombley, 2004). One such channel is the glycine receptor for which basal concentrations of zinc enhance the amplitude and duration of glycinergic inhibitory postsynaptic potentials (Suwa et al., 2001). Furthermore, zinc has concentration-dependent biphasic effects on glycine receptors. Low micromolar concentrations of zinc potentiate glycine-evoked currents, whereas higher concentrations of zinc inhibit glycine-evoked currents (Bloomenthal et al., 1994, Laube et al., 1995, Trombley and Shepherd, 1996, Lynch et al., 1998). The potentiation site(s) appear(s) to be located on the external face of the N-terminal domain of the alpha subunit, while the inhibitory site(s) is/are located some distance away on the opposite external face of the subunit (Laube et al., 1995, Laube et al., 2000, Nevin et al., 2003, Miller et al., 2005a). Potentiation by zinc involves an increase in both the channel's open probability and burst duration; inhibition has the opposite effect (Laube et al., 2000). These authors concluded that zinc-mediated potentiation is due to a slowing of agonist dissociation, thus increasing glycine binding; in contrast, inhibition is due to effects of zinc on channel gating (Laube et al., 2000).
Although the potentiating and inhibiting concentrations of zinc are frequently reported as < 10 μM and > 10 μM, respectively, the concentration-response effects of zinc on glycine receptors appear to vary significantly with glycine receptor subunit composition, e.g. (Miller et al., 2005a). In addition, these effects only occur with use of low concentrations of glycine (e.g., 30 μM), that is, concentrations that do not desensitize glycine receptors. In contrast, when desensitizing concentrations of glycine (e.g., 300 μM) are applied, coapplied zinc has no effect on glycine-evoked currents (Trombley and Shepherd, 1996). This may have important implications for zinc modulation of glycinergic transmission, since the peak concentration of glycine in the synaptic cleft has been estimated to exceed 2 mM (Beato, 2008). To explore this issue, we externally co-applied several concentrations of zinc and glycine under a variety of conditions and analyzed the effects of zinc on glycine-evoked currents. To extend our previous results obtained in cultured rat olfactory bulb neurons, these experiments were performed on neurons in primary culture from two brain regions that are highly enriched in vesicular zinc: the hippocampus and the olfactory bulb (OB).
Because synaptically released zinc can gain access to the interior of the postsynaptic neuron, e.g. (Li et al., 2001), we hypothesized that intracellular zinc also can modulate currents mediated by glycine receptors. First, we used Newport Green fluorescence to demonstrate an increase in intracellular zinc following external application of zinc. To determine whether such an increase in intracellular zinc could contribute to the effects of zinc, we tested the effects of externally applied zinc on glycine-mediated currents in the presence of intracellular zinc chelators. In a final set of experiments, we determined the effect of a range of intracellular zinc concentrations on glycine-evoked currents.
EXPERIMENTAL PROCEDURES
Animal Use
The animals used for these experiments were used according to the guidelines of our protocol approved by Florida State University's Animal Care and Use Committee and the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23).
Tissue Culture and Electrophysiology
Olfactory bulb (OB) and hippocampal neurons from P1 to P5 Sprague-Dawley (Charles River Laboratories International, Inc., Wilmington, MA) rat pups were dissociated and then plated on a confluent layer of astrocytes using standard tissue culture methods as described by Trombley and Blakemore (Trombley and Blakemore, 1999). Experiments were conducted after the neurons remained in culture for 2-18 days. The data from OB neurons and hippocampal neurons were combined in experiments in which there were no significant differences between neuronal populations. To examine membrane currents evoked by glycine, whole-cell recordings were made with an Axoclamp 2B amplifier (Axon Instruments) using either the discontinuous (switch frequency of 10-15 kHz or continuous single-electrode voltage-clamp mode. Membrane currents were filtered at 1-3 kHz, digitized at 5-10 kHz, and analyzed using AxoGraph (AxoGraph Scientific, Sydney, Australia). Zinc (in the form of ZnCl2) and glycine were diluted in the bath solution and applied using a gravity-fed, flow-pipe, perfusion system, assembled from an array of 600 μm-I.D. square glass barrels. The flow pipes were positioned near the neuron being examined using an electronic manipulator (Warner Instruments, Hamdem, CT), and flow was controlled with pinch clamps. The speed of solution delivery allowed complete solution exchange within 100-200 msec. The intracellular electrode solution contained: (in mM): CsCl, 145; MgCl2, 1; HEPES, 10; Mg-ATP, 5; Mg-GTP, 0.5; and EGTA, 1.1; pH 7.2, osmolarity 310 mOsM. The recording chamber was perfused at 0.5-2.0 ml/min with a solution containing (in mM): NaCl, 162.5; KCl, 2.5; CaCl2, 2; HEPES, 10; glucose, 10; and MgCl2, 1. The pH was adjusted to 7.3 with 1 M NaOH and the osmolarity was 325 mOsM.
Zinc Fluorescence
Cultured neurons were loaded with 5 μM Newport Green (Molecular Probes, Eugene, OR), a fluorescent indicator dye for Zn2+. Newport Green stock solutions were prepared by dissolving 1 mg of Newport Green into 800 μL of DMSO. The dye was applied to the cultures at a concentration of 5 μL/ml in buffer (2 mM CaCl2 and 1 mM MgCl2 in 10 mM HEPES, with 162.5 mM NaCl, 2.5 mM KCl, and 10 mM glucose). Cells were exposed to Newport Green for 30 min at room temperature and washed for another 30 min with buffer solution. For the experimental group, the cultures were then exposed to 300 μM ZnCl2 for 60 seconds. The Zinc solution was then removed by washing with buffer so that no extracellular zinc remained. The same procedure was used for the control group except that ZnCl2 was not added.
ZnCl2, glycine, GABA, and the zinc chelators (EDTA, dipicolinic acid, and 1,10-phenantroline) all were obtained from Sigma (St. Louis, MO).
Statistics
Statistical analyses were conducted using the t-test functions in KaleidaGraph (Synergy Software; Reading, PA). A P value of < 0.05 was considered statistically significant.
RESULTS
Zinc produces biphasic effects on glycine-evoked currents
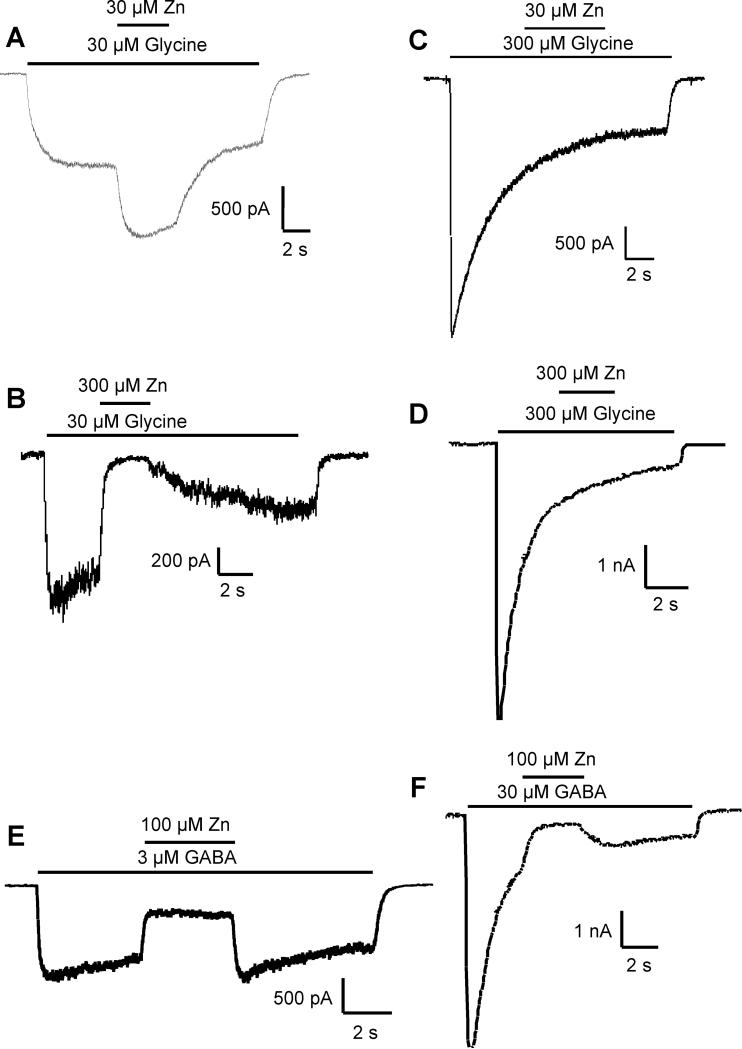
In both OB and hippocampal neurons, low concentrations of glycine (30 μM) produced membrane currents with little desensitization during 5-10 second applications. Co-application of zinc at 30 μM resulted in potentiation of the current (210 ± 45% of control, n=12) in both neuronal populations (Fig 1A). Higher concentrations of zinc (e.g., 300 μM) inhibited 30 μM glycine-evoked currents (5.3 ± 6.2% of control, n=10) in both OB and hippocampal neurons (Fig 1B).
Figure 1.
The concentration of glycine influences the effects of zinc. A) A low concentration of glycine (30 μM) evoked a nondesensitizing current, which is potentiated by co-application of 30 μM zinc. B) Co-application of a high concentration of zinc (300 μM) inhibited glycine-evoked currents. However, when a high concentration of glycine (300 μM) is applied, co-application of zinc neither potentiates (C) nor inhibits (D) the glycine-evoked current. This is in contrast to the effects of zinc on GABA-evoked currents, which are inhibited by zinc at either low (3 μM, E) or high (30 μM, F) concentrations of applied GABA.
Desensitizing concentrations of glycine block zinc's effects on glycine receptors
In contrast to the above effects of zinc, when high desensitizing concentrations of glycine (e.g., 300 μM) were applied, subsequent co-application of zinc had no effect; neither potentiation at low concentrations of zinc (Fig 1C, n=8) nor inhibition at high zinc concentrations (Fig 1D, n=8) was observed. This is in contrast to the effects of zinc on currents mediated by GABA receptors, which are ligand-gated chloride channels with properties very similar to glycine receptors. In both OB and hippocampal neurons, zinc produced only inhibitory effects and was effective at inhibiting GABA-evoked currents regardless of whether nondesensitizing concentrations (3 μM, 30 ± 9% of control, n=10, Fig 1E) or desensitizing concentrations (30 μM, 27 ± 8% of control, n=10, Fig 1F) of GABA were applied.
Pre-application of zinc causes inhibition of currents evoked by desensitizing concentrations of glycine
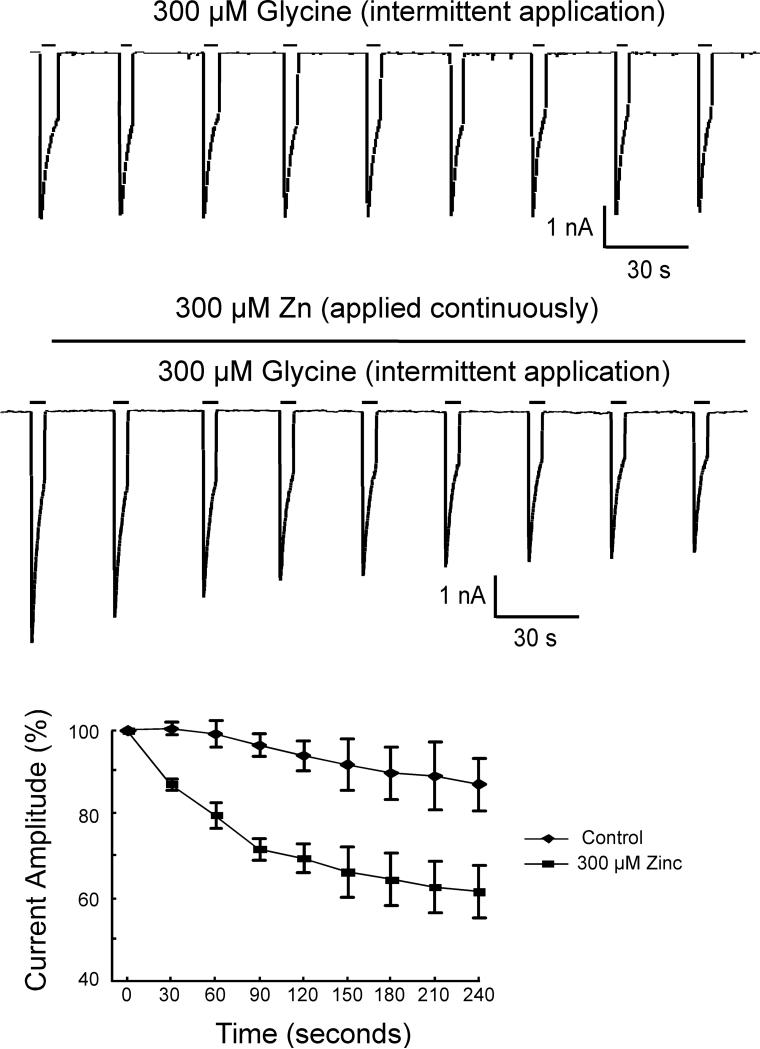
It has recently been proposed that the inhibitory actions of zinc on glycine receptors are mediated by stabilizing the closed state of the receptor (Lynch, 2004). Consequently, we hypothesized that, with high micromolar concentrations of glycine, all glycine binding sites are occupied and the resulting conformational change in the receptor prevents either zinc binding or the effects of bound zinc. To test this hypothesis, we continuously applied 300 μM zinc during intermittent pulse application of 300 μM glycine (n=12). Under these conditions, zinc inhibition of the peak glycine-mediated current developed slowly, producing about a 35% inhibition of the control amplitude in approximately 3 minutes (Fig 2).
Figure 2.
In contrast to the lack of an effect of zinc when co-applied with high concentrations of glycine, pre-application of zinc (300 μM) produces a slowly developing inhibition. Top) Pulse application of 300 μM glycine evokes reproducible inward currents. Middle) Continuous application of 300 μM zinc during pulse application of 300 μM glycine slowly inhibits the glycine-evoked currents. Bottom) Summary graph of the effects of zinc over time on glycine-evoked currents in both hippocampal and olfactory bulb neurons.
Zinc-mediated inhibition of glycine receptors increases with duration of zinc exposure
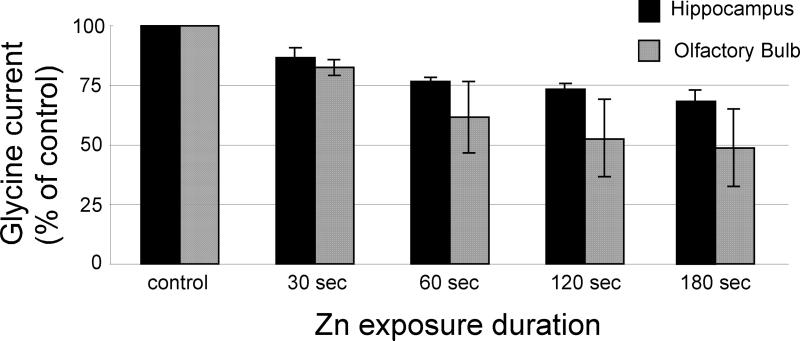
Using another approach to test this hypothesis, a control (300 μM) glycine-evoked current was recorded and then 300 μM zinc was applied for durations ranging from 30 to 180 seconds prior to a subsequent application of 300 μM glycine. Under these conditions, near-maximal inhibition of the current was observed after 60 seconds of zinc application (Fig 3). The glycine-evoked current was (as a percentage of control) 86.3 ± 4.3% (n=6) at 30 seconds; 76.8 ± 1.6% (n=5) at 60 seconds; 73.5 ± 2.35% (n=5) at 120 seconds; and 68 ± 4.6% (n=5) at 180 seconds for hippocampal neurons and 82.6 ± 3.5% (n=6) at 30 seconds; 61.6 ± 15.1% (n=10) at 60 seconds; 52.8 ± 16.0% (n=5) at 120 seconds; and 48 ± 16.3% (n =6) at 180 seconds for OB neurons (Fig 3). Each of these values is significantly different from control values (P <0.05).
Figure 3.
Zinc-mediated inhibition of glycine receptors increases with duration of zinc exposure. A control glycine-evoked current was initially measured. Zinc (300 μM) was then applied for durations between 30-180 seconds followed by a subsequent application of 300 μM glycine. Glycine receptor-mediated currents were inhibited as a function of zinc exposure duration with a near-maximal inhibition at approximately 60 seconds.
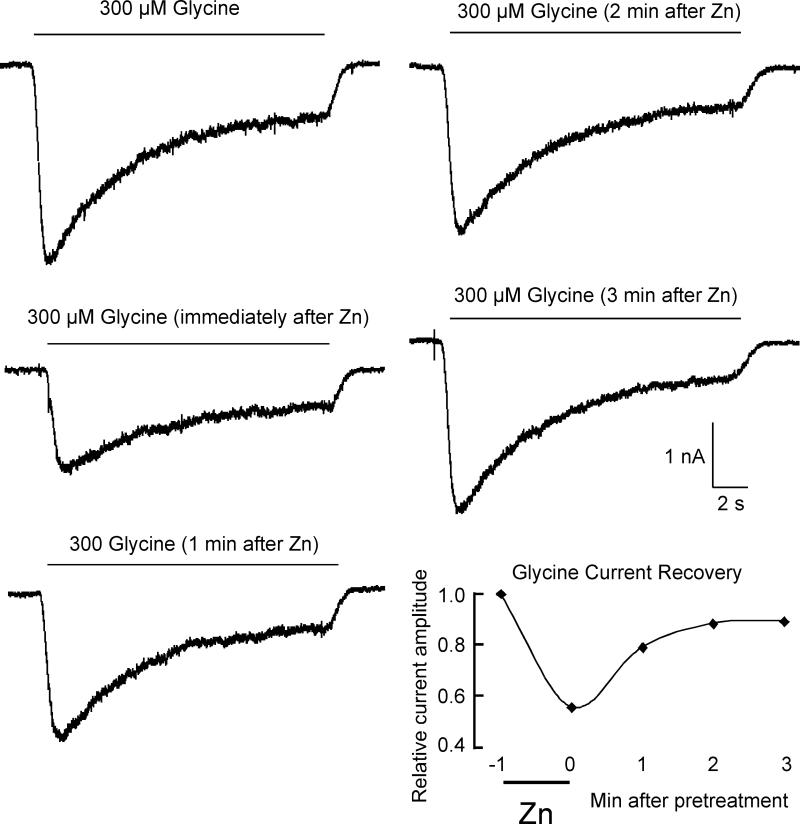
To explore this finding further, we preapplied 300 μM zinc and examined the time course of recovery from inhibition. A 60-second pre-application of 300 μM zinc resulted in a 33.3 ± 14.2% (n=15) reduction in the peak current evoked by 300 μM glycine (Fig 4). The recovery from this inhibition took several minutes with a time constant of 81 seconds, suggesting a slow dissociation rate constant from its inhibitory binding site(s).
Figure 4.
Inhibition of Glycine-evoked currents recovers slowly after pre-application of zinc. The series of currents evoked by 300 μM glycine shows an example of the time course of recovery from inhibition after a 60-second pre-application of 300 μM zinc. The final panel is a graphic representation of the recovery time course.
Extracellular application of zinc results in elevated intracellular zinc
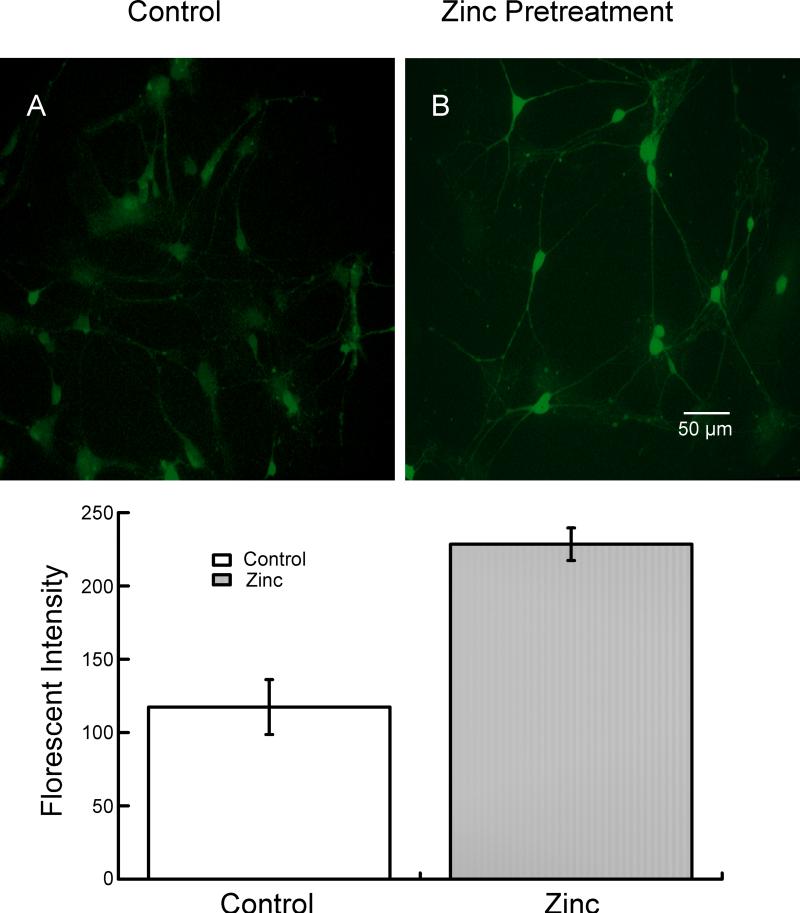
Because zinc inhibition of glycine currents is much slower to develop under these conditions (high glycine concentration, zinc pre-application) than is observed with low concentrations of glycine (compare Fig 1B with Fig 2), we wondered whether some of the effect of zinc was due to intracellular access and interaction with an intracellular domain of the glycine receptor. It has been previously reported that zinc can gain intracellular access via several types of ion channels (see (Nakashima and Dyck, 2009) for review). We examined whether extracellular application of zinc could result in an increase in intracellular zinc under our recording conditions. To examine this, we compared the fluorescence of Newport Green-loaded neurons under control conditions (no added zinc) to those in which 300 μM extracellular zinc was applied for 60 seconds. As shown in Fig 5, neurons exposed to 300 μM zinc were much more intensely labeled than those under control conditions, indicating a rise in the intracellular zinc concentration. The intensity of the fluorescence was quantified using ImageJ (National Institutes of Health) and a 0-255 scale. The intensity of the neurons under control conditions was 117.5 ± 18.6 and it increased to 228.8 ± 11.1 after treatment with zinc (mean ± S.D.; n=16; P < 0.001).
Figure 5.
Extracellular application of zinc results in elevated intracellular zinc. Neurons were loaded with Newport Green for 30 minutes and washed for 30 minutes with buffer to remove extracellular Newport Green. (A) Photograph showing Newport Green fluorescence in neurons not exposed to extracellular zinc. (B) Neurons exposed to 300 μM zinc for 60 seconds show a significant increase in Newport Green fluorescence, indicating that a portion of the extracellular zinc gained intracellular access. A quantitative analysis is presented in the lower panel.
Effects of intracellular zinc chelation on inhibitory effects of zinc
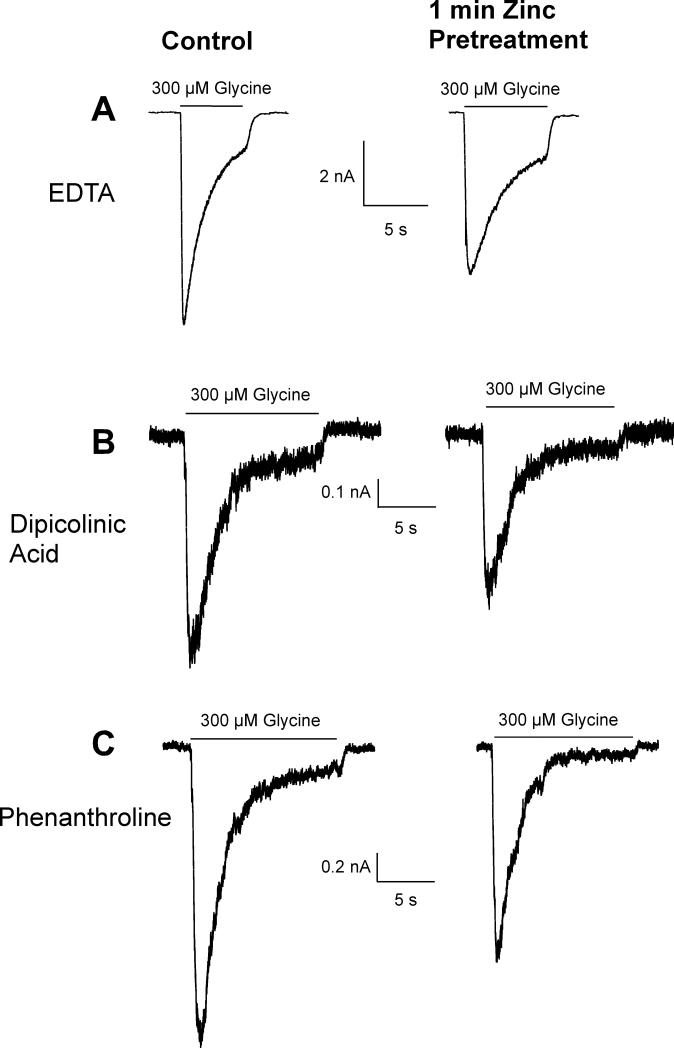
To determine whether intracellular access might contribute to the inhibitory effects of zinc, we tested the effects of intracellular zinc chelation by including several different types of chelators in our recording electrode. A control (300μM) glycine-mediated current was recorded and then 300 μM zinc was preapplied for 60 seconds and a subsequent glycine-evoked current was measured. Neither EDTA (10 mM), dipicolinic acid (100 μM), nor 1,10-phenanthroline (100 μM) was able to prevent the inhibitory effects of externally applied zinc (Fig 6). Without an intracellular chelator, a 60-second extracellular application of 300 μM zinc inhibited the glycine-evoked current by 33.3 ± 14.2% (n=15). A 60-second extracellular application of 300 μM zinc inhibited the glycine-evoked current by 24.0 ± 8% (n=6) with intracellular EDTA, by 22.6 ± 11% (n=6) with intracellular dipicolinic acid, and by 28.6 ± 9% (n=6) with intracellular 1,10-phenantroline. None of these values were significantly different from the degree of inhibition in the absence of an intracellular zinc chelator (P > 0.05). This suggests that either the rise in intracellular zinc had no effect on inhibition or, perhaps, the chelation was too slow to prevent an intracellular effect of zinc. However, the most probably explanation is that inhibition by extracellular application of zinc is mediated entirely by an extracellular binding site.
Figure 6.
Intracellular zinc chelation has little effect on the inhibitory actions of extracellular application of 300 μM zinc on glycine-evoked (300 μM) currents. After a 300 μM glycine-evoked control current was recorded, neurons were exposed to 300 μM zinc for 60 seconds and another glycine-evoked current was recorded. Neither 10 mM EDTA (A), nor 100 μM dipicolinic acid (B), nor 100 μM 1,10-phenantroline (C), when applied intracellularly via the recording electrode, were effective at reducing the inhibition of glycine-evoked currents by zinc.
Intracellular zinc potentiates glycine-evoked currents
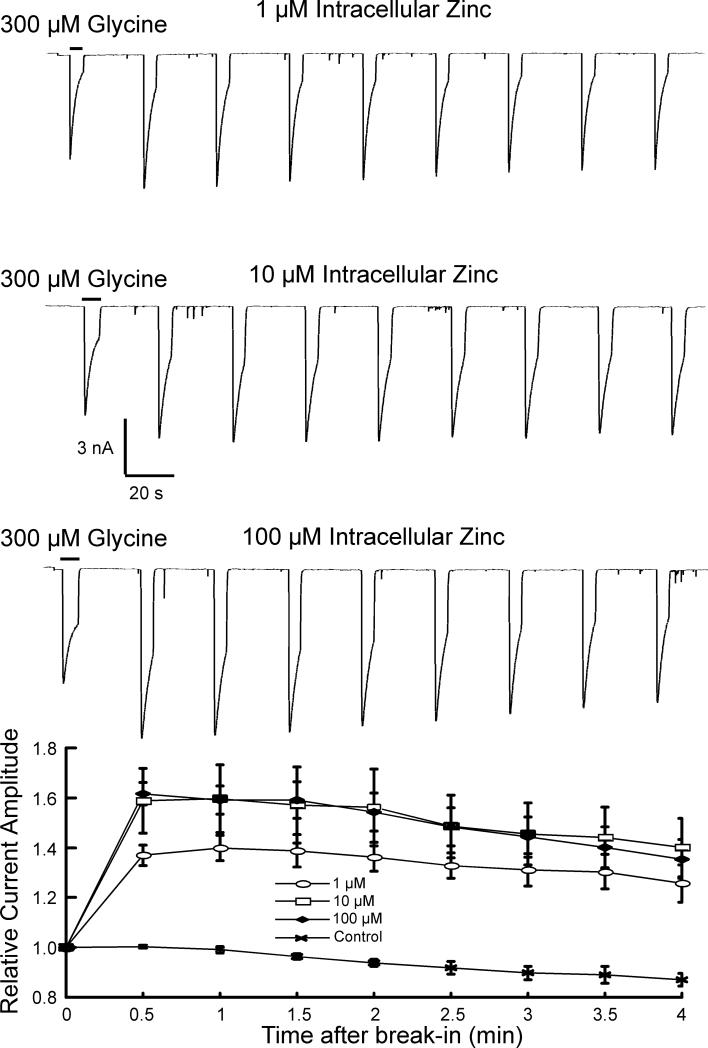
To examine potential intracellular effects of zinc further, we included a range of concentrations (1, 10,100 μM) of zinc in the recording electrode (Fig 7). The current evoked by 300 μM glycine was recorded immediately after break-in and compared to currents recorded every 30 seconds for approximately 4 minutes. There was a rapid increase in the current amplitude that peaked at the 30-second time point and declined slightly over the subsequent 3.5 minutes. This was observed with 1 μM zinc (137 ± 8% of control; n=4), with 10 μM zinc (158 ± 29% of control; n=6), and with 100 μM zinc (161 ± 10% of control; n=5). Current amplitudes in only the 1 μM and 100 μM groups were significantly different from each other (P = 0.006). Current amplitudes in each group were significantly different than control current amplitudes at the 30-second time point recorded without added zinc (P < 0.005). As expected, inclusion of a chelator with zinc in the electrode prevented the effects of intracellular zinc. Concentrations of zinc greater that 100 μM were not examined, as such concentrations likely exceed physiological concentrations. These results suggest an action of zinc on glycine receptors not previously reported, that is, that intracellular zinc can potentiate glycine receptor-mediated currents.
Figure 7.
Intracellular zinc potentiates glycine-evoked currents. In contrast to examining the effect of intracellular access of extracellularly applied zinc, a range of concentrations (1, 10, 100 μM) of zinc in the recording electrode was used to directly examine the effects of intracellular zinc. The current evoked by 300 μM glycine was recorded immediately after break-in and compared to currents recorded every 30 seconds for approximately 4 minutes. The peak glycine-evoked current increased and peaked at the 30-second time point with each zinc concentration and declined slightly over the subsequent 3.5 minutes.
DISCUSSION
The present study extends our previous findings (Trombley and Shepherd, 1996) and provides evidence supporting the notion that the modulatory effects of zinc on glycine receptors depend on the state of the receptor. That is, zinc has little to no effect on glycine-evoked currents when co-applied during receptor desensitization. Furthermore, this report is the first to provide evidence for the existence of an intracellular modulatory effect of zinc on glycine receptors.
The modulatory effects of zinc are dependent on agonist concentration
A number of studies (e.g. (Bloomenthal et al., 1994, Laube et al., 1995) have reported biphasic effects of zinc on glycine receptors. However, most studies used low concentrations of glycine to evoke membrane currents. In the present study, high concentrations of glycine were used since the concentration of glycine has been estimated to exceed 2 mM in the synaptic cleft (Beato, 2008). Such concentrations would rapidly saturate glycine binding sites on the receptor and generate changes that result in a rapid conformational switch to the desensitized state during glycinergic synaptic events. For example, it has been reported that glycine receptors progressively desensitize with short (1 ms) applications of saturating concentrations of glycine (3 mM) when application frequencies exceed 1 Hz (Rigo and Legendre, 2006). Furthermore, it has been demonstrated not only that pulse application of glycine, at physiologically relevant frequencies, results in glycine receptor desensitization, but also that much of this desensitization occurs even during interpulse intervals when no glycine is present (Papke et al., 2011). Collectively, these results support the notion that glycine receptor desensitization occurs under physiological conditions. In contrast to the effects of zinc on currents evoked by low concentrations of glycine, our results show that co-application of zinc had no effects, neither potentiation nor inhibition, on currents evoked by desensitizing concentrations of glycine. These results suggest that saturation of the agonist binding sites with glycine results in a conformational shift in the receptor that either prevents the binding of zinc (binding only to the unliganded, closed state) or the effects of bound zinc, which may be a consequence of the zinc binding site mediating inhibition being near the agonist binding site (Nevin et al., 2003).
Pre-application and zinc-mediated inhibition of glycine receptors
It has been previously reported that pre-application of zinc rapidly reduces the time to achieve maximal inhibition of glycine-evoked currents (Lynch et al., 1998). However, the conditions of these experiments were significantly different from those in the present study. The report by Lynch et al. is from a cell line (human embryonic kidney 293) used to express human glycine receptors. In contrast, the present study examined glycine receptors expressed by olfactory bulb and hippocampal neurons in primary culture. Also, the concentration of glycine used (20 μM) by Lynch et al. does not cause glycine receptor desensitization and, therefore, is insufficient to prevent the effects of zinc during co-application. In contrast, the data presented here demonstrate that zinc-mediated inhibition of currents evoked by desensitizing concentrations of glycine can still occur when a high concentration of zinc is pre-applied. This suggests that if zinc is already bound, high concentrations of glycine are not sufficient to prevent the inhibitory actions of zinc. These data support the notion that the receptor has to be in a desensitized conformation prior to zinc binding to prevent actions of zinc. Although zinc inhibition is more rapid in the absence of bound agonist (Lynch et al., 1998), it remains unclear why inhibition was slow to develop under these conditions in our study. One explanation may be that receptor desensitization during pulse application of glycine, used in the present study, results in a conformational shift in the receptor that reduces the access or affinity for zinc. However, since during pulse applications the receptors would be entering and exiting a desensitized conformation, a proportion of the receptors would still be accessible to zinc. Those receptors would still be under the influence of zinc during a subsequent application of glycine and lead to a steady, but slow, inhibition by zinc. Another viable interpretation is that is that zinc-mediated inhibition is due to zinc stabilizing glycine receptors in the unbound, closed state.
Recovery from zinc-mediated inhibition
Because the inhibitory effects of pre-applied zinc were slow to develop, we also examined the time course of recovery from zinc-mediated inhibition. Even though our perfusion system resulted in complete solution exchange within 200 ms, the recovery from inhibition had a time constant (66.67% recovery) of about 80 seconds. This suggests that bound zinc is slow to dissociate from the receptor under these conditions and implies that the effects of synaptically released zinc may influence subsequent glycinergic events for seconds after zinc release.
Modulation of glycine receptors by intracellular zinc
The slowly developing inhibition of glycine receptors by pre-applied zinc led us to hypothesize that perhaps some of the effects of zinc were mediated by intracellular access to the externally applied zinc, since previous reports indicate that extracellular zinc can enter neurons, e.g. (Sensi et al., 1997, Li et al., 2001). Zinc can gain entry via a variety of routes. Mostly, this involves channels that are also permeable to Ca++, including NMDA receptors (Koh and Choi, 1994), voltage-gated calcium channels (Sensi et al., 1997, Kerchner et al., 2000), and AMPA or kainate receptors that can also flux calcium (Sensi et al., 1999). Other routes of entry include reverse operation of the Na+-Ca++ exchanger (Sensi et al., 1997), proton-zinc exchangers (Balaji and Colvin, 2005), and Zrt-Irt-like proteins (Gaither and Eide, 2001). That zinc can gain intracellular access during external application was confirmed in the present study by an increase in the fluorescent intensity of the zinc indicator probe, Newport Green. However, the intracellular inclusion of several types of zinc chelators was not sufficient to prevent the inhibitory effects of zinc. While this may be because the chelation rate was too slow to prevent zinc from binding to intracellular sites, the most likely interpretation is that zinc-mediated inhibition can be fully accounted for by binding to an extracellular site on the glycine receptor (Nevin et al., 2003). Furthermore, we suspect that the inclusion of intracellular chelators did not enhance inhibition mediated by extracellular application of zinc because extracellular zinc-mediated inhibition is already maximal and overrides any potentiation via an intracellular effect.
To examine the effects of intracellular zinc in more detail, and without the influence of extracellular zinc, we included a range of concentrations of zinc in the recording electrode. Under these conditions, we demonstrated, for the first time, that intracellular zinc can potentiate currents evoked by glycine, with a significant increase in current amplitude observed with a little as 1 μM zinc included in the electrode (the lowest concentration examined). Although not examined in detail, the glycine receptor contains several amino acids (His, Glu, Asp) on the intracellular loops that can bind zinc (Laube et al., 2002, Lynch, 2004, Miller et al., 2005b).
Collectively, these results demonstrate that zinc has complex effects on glycine receptor function and multiple factors may interact to influence the efficacy of glycinergic transmission. These include the synaptic concentrations of glycine, the extent of glycine spillover, receptor conformation, and mechanisms that regulate the concentration of zinc, both extracellularly (e.g., synaptic release) and intracellularly. The latter may include mechanisms that provide extracellular zinc intracellular access (e.g., NMDA receptors, calcium-permeable AMPA receptors, or voltage-gated calcium channels), and/or mechanisms involved in intracellular zinc homeostasis (e.g., metalloproteins).
Research Highlights.
>Modulation of glycine receptors by zinc is dependent on the state of the receptor
>The timing of zinc and glycine application influences the effects of zinc
>Low concentrations of intracellular zinc potentiates glycine receptors
ACKNOWLEDGMENTS
This work was supported, in part, by NIH/NIDCD and a grant from the Florida State University Council on Research and Creativity.
ABBREVIATIONS
- AMPA
α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate
- ATP
Adenosine-5'-triphosphate
- DMSO
Dimethyl sulfoxide
- EDTA
Ethylenediaminetetraacetic acid
- EGTA
ethylene glycol tetraacetic acid
- GABA
gamma-Aminobutyric acid
- GTP
Guanosine-5'-triphosphate
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- NMDA
N-Methyl-D-aspartic acid
- OB
olfactory bulb
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Assaf SY, Chung SH. Release of endogenous Zn2+ from brain tissue during activity. Nature. 1984;308:734–736. doi: 10.1038/308734a0. [DOI] [PubMed] [Google Scholar]
- Balaji RV, Colvin RA. A proton-dependent zinc uptake in PC12 cells. Neurochem Res. 2005;30:171–176. doi: 10.1007/s11064-004-2438-6. [DOI] [PubMed] [Google Scholar]
- Beato M. The time course of transmitter at glycinergic synapses onto motoneurons. J Neurosci. 2008;28:7412–7425. doi: 10.1523/JNEUROSCI.0581-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birinyi A, Parker D, Antal M, Shupliakov O. Zinc co-localizes with GABA and glycine in synapses in the lamprey spinal cord. J Comp Neurol. 2001;433:208–221. doi: 10.1002/cne.1136. [DOI] [PubMed] [Google Scholar]
- Blakemore LJ, Trombley PQ. Diverse modulation of olfactory bulb AMPA receptors by zinc. Neuroreport. 2004;15:919–923. doi: 10.1097/00001756-200404090-00037. [DOI] [PubMed] [Google Scholar]
- Bloomenthal AB, Goldwater E, Pritchett DB, Harrison NL. Biphasic modulation of the strychnine-sensitive glycine receptor by Zn2+. Mol Pharmacol. 1994;46:1156–1159. [PubMed] [Google Scholar]
- Cole TB, Wenzel HJ, Kafer KE, Schwartzkroin PA, Palmiter RD. Elimination of zinc from synaptic vesicles in the intact mouse brain by disruption of the ZnT3 gene. Proc Natl Acad Sci U S A. 1999;96:1716–1721. doi: 10.1073/pnas.96.4.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederickson CJ. Neurobiology of zinc and zinc-containing neurons. Int Rev Neurobiol. 1989;31:145–238. doi: 10.1016/s0074-7742(08)60279-2. [DOI] [PubMed] [Google Scholar]
- Gaither LA, Eide DJ. Eukaryotic zinc transporters and their regulation. Biometals. 2001;14:251–270. doi: 10.1023/a:1012988914300. [DOI] [PubMed] [Google Scholar]
- Harrison NL, Gibbons SJ. Zn2+: an endogenous modulator of ligand- and voltage-gated ion channels. Neuropharmacology. 1994;33:935–952. doi: 10.1016/0028-3908(94)90152-x. [DOI] [PubMed] [Google Scholar]
- Horning MS, Trombley PQ. Zinc and copper influence excitability of rat olfactory bulb neurons by multiple mechanisms. J Neurophysiol. 2001;86:1652–1660. doi: 10.1152/jn.2001.86.4.1652. [DOI] [PubMed] [Google Scholar]
- Howell GA, Welch MG, Frederickson CJ. Stimulation-induced uptake and release of zinc in hippocampal slices. Nature. 1984;308:736–738. doi: 10.1038/308736a0. [DOI] [PubMed] [Google Scholar]
- Kerchner GA, Canzoniero LM, Yu SP, Ling C, Choi DW. Zn2+ current is mediated by voltage-gated Ca2+ channels and enhanced by extracellular acidity in mouse cortical neurones. J Physiol. 2000;528(Pt 1):39–52. doi: 10.1111/j.1469-7793.2000.00039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh JY, Choi DW. Zinc toxicity on cultured cortical neurons: involvement of N-methyl-D-aspartate receptors. Neuroscience. 1994;60:1049–1057. doi: 10.1016/0306-4522(94)90282-8. [DOI] [PubMed] [Google Scholar]
- Laube B, Kuhse J, Betz H. Kinetic and mutational analysis of Zn2+ modulation of recombinant human inhibitory glycine receptors. J Physiol. 2000;522(Pt 2):215–230. doi: 10.1111/j.1469-7793.2000.t01-1-00215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laube B, Kuhse J, Rundstrom N, Kirsch J, Schmieden V, Betz H. Modulation by zinc ions of native rat and recombinant human inhibitory glycine receptors. J Physiol. 1995;483(Pt 3):613–619. doi: 10.1113/jphysiol.1995.sp020610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laube B, Maksay G, Schemm R, Betz H. Modulation of glycine receptor function: a novel approach for therapeutic intervention at inhibitory synapses? Trends Pharmacol Sci. 2002;23:519–527. doi: 10.1016/s0165-6147(02)02138-7. [DOI] [PubMed] [Google Scholar]
- Li Y, Hough CJ, Suh SW, Sarvey JM, Frederickson CJ. Rapid translocation of Zn(2+) from presynaptic terminals into postsynaptic hippocampal neurons after physiological stimulation. J Neurophysiol. 2001;86:2597–2604. doi: 10.1152/jn.2001.86.5.2597. [DOI] [PubMed] [Google Scholar]
- Lynch JW. Molecular structure and function of the glycine receptor chloride channel. Physiol Rev. 2004;84:1051–1095. doi: 10.1152/physrev.00042.2003. [DOI] [PubMed] [Google Scholar]
- Lynch JW, Jacques P, Pierce KD, Schofield PR. Zinc potentiation of the glycine receptor chloride channel is mediated by allosteric pathways. J Neurochem. 1998;71:2159–2168. doi: 10.1046/j.1471-4159.1998.71052159.x. [DOI] [PubMed] [Google Scholar]
- Miller PS, Beato M, Harvey RJ, Smart TG. Molecular determinants of glycine receptor alphabeta subunit sensitivities to Zn2+-mediated inhibition. J Physiol. 2005a;566:657–670. doi: 10.1113/jphysiol.2005.088575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller PS, Da Silva HM, Smart TG. Molecular basis for zinc potentiation at strychnine-sensitive glycine receptors. J Biol Chem. 2005b;280:37877–37884. doi: 10.1074/jbc.M508303200. [DOI] [PubMed] [Google Scholar]
- Nakashima AS, Dyck RH. Zinc and cortical plasticity. Brain Res Rev. 2009;59:347–373. doi: 10.1016/j.brainresrev.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Nevin ST, Cromer BA, Haddrill JL, Morton CJ, Parker MW, Lynch JW. Insights into the structural basis for zinc inhibition of the glycine receptor. J Biol Chem. 2003;278:28985–28992. doi: 10.1074/jbc.M300097200. [DOI] [PubMed] [Google Scholar]
- Papke D, Gonzalez-Gutierrez G, Grosman C. Desensitization of neurotransmitter-gated ion channels during high-frequency stimulation: A comparative study of Cys-loop, AMPA and purinergic receptors. J Physiol. 2011 doi: 10.1113/jphysiol.2010.203315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigo JM, Legendre P. Frequency-dependent modulation of glycine receptor activation recorded from the zebrafish larvae hindbrain. Neuroscience. 2006;140:389–402. doi: 10.1016/j.neuroscience.2006.01.057. [DOI] [PubMed] [Google Scholar]
- Sensi SL, Canzoniero LM, Yu SP, Ying HS, Koh JY, Kerchner GA, Choi DW. Measurement of intracellular free zinc in living cortical neurons: routes of entry. J Neurosci. 1997;17:9554–9564. doi: 10.1523/JNEUROSCI.17-24-09554.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sensi SL, Yin HZ, Carriedo SG, Rao SS, Weiss JH. Preferential Zn2+ influx through Ca2+-permeable AMPA/kainate channels triggers prolonged mitochondrial superoxide production. Proc Natl Acad Sci U S A. 1999;96:2414–2419. doi: 10.1073/pnas.96.5.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwa H, Saint-Amant L, Triller A, Drapeau P, Legendre P. High-affinity zinc potentiation of inhibitory postsynaptic glycinergic currents in the zebrafish hindbrain. J Neurophysiol. 2001;85:912–925. doi: 10.1152/jn.2001.85.2.912. [DOI] [PubMed] [Google Scholar]
- Trombley PQ, Blakemore LJ. Mammalian Olfactory Bulb Neurons. In: Haynes LW, editor. The Neuron in Tissue Culture. John Wiley & Sons, Ltd.; Sussex: 1999. pp. 585–592. [Google Scholar]
- Trombley PQ, Shepherd GM. Differential modulation by zinc and copper of amino acid receptors from rat olfactory bulb neurons. J Neurophysiol. 1996;76:2536–2546. doi: 10.1152/jn.1996.76.4.2536. [DOI] [PubMed] [Google Scholar]
- Wang Z, Danscher G, Kim YK, Dahlstrom A, Mook Jo S. Inhibitory zinc-enriched terminals in the mouse cerebellum: double-immunohistochemistry for zinc transporter 3 and glutamate decarboxylase. Neurosci Lett. 2002;321:37–40. doi: 10.1016/s0304-3940(01)02560-5. [DOI] [PubMed] [Google Scholar]
- Wang Z, Li JY, Dahlstrom A, Danscher G. Zinc-enriched GABAergic terminals in mouse spinal cord. Brain Res. 2001;921:165–172. doi: 10.1016/s0006-8993(01)03114-6. [DOI] [PubMed] [Google Scholar]