Abstract
Platelet membrane phosphatidylserine (PS) exposure that regulates the production of thrombin represents an important link between platelet activation and the coagulation cascade. Here, we have evaluated the involvement of the Na+/H+ exchanger (NHE) in this process in human platelets. PS exposure induced in human platelets by thrombin, TRAP, collagen or TRAP+ collagen was abolished in a Na+-free medium. Inhibition of the Na+/H+ exchanger (NHE) by 5-(N-Ethyl-N-Isopropyl) Amiloride (EIPA) reduced significantly PS exposure, whereas monensin or nigericin, which mimic or cause activation of NHE, respectively, reproduced the agonist effect. These data suggest a role for Na+ influx through NHE activation in the mechanism of PS exposure. This newly identified pathway does not discount a role for Ca2+, whose cytosolic concentration varies together with that of Na+ after agonist stimulation. Ca2+ deprivation from the incubation medium only attenuated PS exposure induced by thrombin, measured from the uptake of FM1-43 (a marker of phospholipid scrambling independent of external Ca2+). Surprisingly, removal of external Ca2+ partially reduced FM1-43 uptake induced by A23187, known as a Ca2+ ionophore. The residual effect can be attributed to an increase in [Na+]i mediated by the ionophore due to a lack of its specificity. Finally, phosphatidylinositol 4,5-bisphosphate (PIP2), previously reported as a target for Ca2+ in the induction of phospholipid scrambling, was involved in PS exposure through a regulation of NHE activity. All these results would indicate that the mechanism that results in PS exposure uses redundant pathways inextricably linked to the physio-pathological requirements of this process.
Keywords: Platelet; Thrombin; Phosphatidylserine; Na+/H+ exchanger (NHE) phosphatidylinositol 4,5-bisphosphate (PIP2)
1. Introduction
In resting platelets, the constitutive plasma membrane aminophospholipids phosphatidylserine (PS) and phosphatidylethanolamine (PE) are almost exclusively restricted to the inner (cytoplasmic) leaflet, whereas sphingomyelin (SM) and phosphatidylcholine (PC) are more concentrated in the outer (exoplasmic) leaflet [1]. Randomization of the lipids over both membrane leaflets, i.e., lipid scrambling, is most clearly manifested by PS translocation to the exoplasmic leaflet in which PS becomes accessible to circulating blood-clotting factors [2]. Phosphatidylserine molecules bind to regulatory sites on factors Xa and Va and allosterically alter their proteolytic and cofactor activities, resulting in the enhancement of prothrombinase activity relative to intrinsic factor Xa activity in solution. Due to its important role in regulating the activity of critical enzymes, the exposure of PS on the surface of activated platelets is a key regulatory event in blood coagulation [3–6] suggesting an important role of PS in normal hemostasis and thrombotic disease.
A Ca2+-dependent scramblase activation downstream of an increase in Ca2+ concentration was proposed initially as a necessary step for plasma membrane phospholipid scrambling; however, subsequent studies of pure recombinant scramblase activity reconstituted in phospholipid liposomes or large unilamellar vesicles (LUVs) show only slow and limited phospholipid redistribution after Ca2+ addition [7]. There is also evidence that scramblase activity is not the sole effector of phospholipid redistribution and that it may be regulated by factors other than Ca2+ [8,9]. Adult knockout phospholipid scramblase 1, PLSCR1 (−/−) mice, and mice overexpressing PLSCR1 show no obvious hematologic or hemostatic differences, and blood cells from PLSCR1(−/−) mice mobilize PS normally to the cell surface upon stimulation [10]. These data argue that the relationship between this protein and the scrambling activity observed in intact cells is uncertain. Other studies show that the increase in cytosolic Ca2+ is not sufficient to stimulate plasma membrane phospholipid scrambling [11] and the hypothesis that scrambling could result from the formation of microvesicles [12] induced by Ca2+ has been experimentally invalidated [13–15].
During platelet stimulation with agonists such as thrombin, a rapid rise in intracellular pH is observed. This alkalinization (found by some investigators to be preceded by brief acidification [16]) is mediated by an increase in the transport activity of the NHE1 isoform of the Na+/H+ exchanger. The regulation of NHE1 activity is complex, not completely understood, and includes several heterotrimeric G proteins, small G proteins (ras, cdc42, rhoA), mitogen activated protein kinase (MAP), protein kinase C (PKC) and the Ca2+/calmodulin system [17,18].
Recent observations suggest that Na+ influx through NHE is necessary for the creation of procoagulant activity of porcine platelets [19] and of human platelets treated by desmopressin [20], and an enhanced platelet NHE exchanger activity found in type 2 diabetic patients is associated with elevated phospholipid-dependent procoagulant activity and increased risk of vascular damage [21]. Additionally, Na+ influx resulting from P2X7 receptor activation by extracellular ATP was recently reported to induce rapid PS externalization in thymocytes [22].
The relationship between Ca2+ mobilization and NHE1 function during platelet activation is not well established. There are data suggesting that inositol triphosphate (IP3)-induced calcium mobilization is pH-sensitive [23] and inactivation of Na+/H+ exchange inhibits Ca2+ mobilization [17,24]. On the other hand, in human platelets stimulated with thrombin, Ca2+ mobilization was found to occur without a functional exchanger and in an acidified cytoplasm [25].
Phosphatidylinositol 4,5-bisphosphate (PIP2) was reported previously to play a role in PS exposure as a direct Ca2+ target [9,26,27] by forming specific PIP2 domains [9] and/or as a cofactor of an enzyme such as scramblase [26]. Since PIP2 interacts with a large spectrum of proteins as well as peptides, cations and polycations, finding a PIP2-regulated cofactor responsible for PS exposure is the long-term goal of our study. Based on a number of findings, we propose NHE activity, reported to be PIP2-dependent [28] as a novel candidate for such an intermediate.
2. Materials and methods
2.1. Materials
Thrombin (T-6884), calcium ionophore A23187 (C-7522), BSA1 (A6003), apyrase (A-6535), sepharose CL-2B (CL-2B-300), 5-(N-methyl-N-Isobutyl) Amiloride (EIPA) (A3085), N-methyl-d-glucamine (M-2004), FURA 2-AM (A-9210), quercetin (Q-0125), neomycin (N-6386), monensin (M-5273), gramicidin (G-5002), nigericin (N-7143), and lactate dehydrogenase (LDH) kit (500) were obtained from Sigma (St. Louis, USA). Thrombin receptor agonist fragment (SFLLRN) (TRAP); (H-2936) was from Bachem (King of Prussia, PA USA). Calf tendon collagen reagent (385) and luciferin-luciferase reagent (Chrono-Lume 395) were from Chrono-Log Corporation (Havertown, PA USA). Cell permeant sodium green Indicator (S-6901) and Pluronic F-127 (P-6867) were from Molecular Probes (Eugene, OR USA). Green synaptracer (FM1-43); (FPT2982A) was from Fluo Probes (Montluçon, France). IgG1 PE-conjugated mouse monoclonal antibody to human CD41 (platelet GP II b) (12-0419) was from Bioscience (Franklin Lake, NJ USA), Annexin V-FITC (BMS306FI) and PE (BMS306PE) were from Bender MedSystems (Burlingame, CA, USA).
2.2. Preparation of human platelets
Human blood from several healthy donors was collected by venipuncture in a tube containing 0.1 vol of ACD (111 mM dextrose, 85 mM trisodium citrate, 71 mM citric acid) as an anticoagulant. Platelet-rich plasma (PRP) was prepared by centrifugation of the blood for 15 min at 150×g at room temperature (RT). After apyrase (0.5 U/ml) addition, contaminating erythrocytes were removed by centrifugation at 300×g for 5 min. The platelets were sedimented by centrifugation at 1100×g for 12 min and carefully resuspended in buffer A (145 mM NaCl, 2.8 mM KCl, 0.8 mM MgCl2, 0.8 mM KH2PO4, 10 mM HEPES, 5.6 mM glucose, 0.3% albumin at pH 7.35) or in sodium-free buffer B in which Na+ was replaced by N-methyl-d-glucamine (NMDG+) [25,29] and filtered on a column of Sepharose 2B equilibrated with the respective buffers. Osmolarity of buffer A (309±10.5 mosM/kg) and B (315±5.7 mosM/kg) was determined using a vapor pressure osmometer (Vapor™, Vescor). Cell count was obtained using a Coulter Z2 particle count and size analyzer.
2.3. Thrombin activity
Enzymatic activity of thrombin in sodium buffer A and sodium-free buffer B was determined from optical density changes at 405 nm following cleavage of the synthetic peptide derivative tosyl-gly-pro-arg-4-nitroanilide (Chromozym TH, Roche, Indianapolis, IN, USA) based on the sequence at the N terminus of the alpha chain of human fibrinogen. The assay was done according to the manufacturer’s instructions.
2.4. Platelet aggregation and secretion
Platelet suspensions (2 × 108/ml) in buffer A or B (supplemented with 2 mM CaCl2) were added to a pre-warmed cuvette (37 °C) of a Chronolog Lumi-Aggregometer (Chrono-Log Corp. Havertown, PA) and stirred at 1000 rpm before the addition of thrombin (0.1–1 U/ml) or TRAP (10–100 µM). The aggregometer was calibrated with a platelet suspension for zero light transmission and with a buffer for 100% transmission. Changes in light transmission were recorded for 7 min, using a PowerLab/200 instrument and MacLab Chart program version 3.2. ATP secretion was monitored in platelet suspensions (2 × 108/ml) supplemented with luciferin–luciferase reagent according to the manufacturer’s instructions.
2.5. PS exposure
Samples of platelet suspension in buffer A or B were activated with different agonists (0.1–1 U/ml thrombin, TRAP 100 µM) and ionophores (A23187 2–5 µM, monensin 1–50 µM, gramicidin 1–50 µM, nigericin 10 µM) for 20 min at 37 °C in the presence of 2 mM CaCl2. When indicated, platelets were treated for 1 min with 30 µM EIPA, one h with 100 µM quercetin or 5 mM neomycin before activation. Samples of activated platelet suspensions (0.5 µl) were mixed with 13 µl of filtered buffer A or B (supplemented with 2 mM CaCl2), 1 µl of IgG1 anti-CD41-PE, and 0.5 µl of annexin V-FITC or PE [14]. After 10-min incubation to ensure antibody and annexin binding, samples were diluted with 185 µl of the appropriate buffer and subjected to flow cytometry analysis. To determine the percentage of annexin V binding platelets, the population of platelets was first characterized by gating the platelets labeled by a PE-conjugated antibody to CD41 and this population was reanalyzed on the FITC fluorescence channel to determine the percentage of events CD41 positive (platelets) and FITC positive (platelets exposing PS). The gate containing FITC negative platelets was fixed with the non treated cells and in treated cells all the platelets with a higher fluorescence were considered as PS positive. In each sample run, at least 10,000 events were acquired. Platelet lysis occurring during this assay was determined by LDH activity in the supernatant of activated platelets. Depending on the experimental conditions, lysis amounted to 1–3% of total cells. To evaluate plasma membrane phospholipid redistribution in platelets suspended in calcium-free buffer, binding of Factor Va or uptake of styryl dye FM1-43 have been described. As activation of platelets induces secretion of endogenous Factor Va [30], it is not possible to use this technique (Data not shown). In contrast, FM1-43 uptake has been widely used to investigate PS externalization in various cells and the results have been justified by comparison with other methods [31–34]. After platelet activation for 20 min, 5-µl samples were mixed with 20 µl of 50 µM FM1-43 solution, followed by a 1-min incubation and a 100-fold dilution prior to flow cytometry analysis. In each sample run, at least 10,000 events were acquired.
In all experiments, cells were sorted by flow cytometry using a BD Biosciences FaxVantage cytometer. Fluorochrome excitation was performed by argon laser at 488 nm, and fluorescence emissions were analyzed with a 530 nm filter for FITC or FM1-43 fluorescences and with a 575 nm filter for R-phycoerythrin.
2.6. [Ca2+]i measurement
Platelet suspensions in buffer A (4 × 108/ml) were incubated for 45 min at RT, in the dark, with 2 µM Fura 2-AM, centrifuged (10 min, 1000×g), and resuspended in buffer A or B containing 0.1 mM EGTA, to a density of 1.5 × 108/ml. After addition of platelets to a cuvette of a SLM-Aminco MC 200 fluorimeter, the transient Fura-2 fluorescence was recorded (Iex=340 nm, Iem=510 nm) before and after addition of agonists. [Ca2+]i was calculated from the general formula [Ca2+]i=Kd(F−Fmin/Fmax−F) in which Kd is the dissociation constant of Fura-2 for Ca2+ binding (224 nM), and F the fluorescence intensity of the sample. Fmax was determined after lysing the cells with 50 µM digitonin and Fmin after adjusting the pH of the lysed cells to 8.5 with 20 mM TRIS base, followed by addition of 10 mM EGTA.
2.7. Cytosolic Na+ changes
Platelets (0.5–1 × 108/ml) suspended in buffer A (containing 0.1 mM EGTA) were incubated with 5 µM sodium green indicator (stock solution in 20% pluronic F-127/DMSO) for 40 min at RT. After this period, platelet suspensions were supplemented with 2 mM Ca2+ (when required), and 100 µl samples were treated for 1 min with 30 µM EIPA, one h with 100 µM quercetin or 5 mM neomycin and activated with various agonists for 10 min at 37 °C. 0.5 µl samples of activated platelet suspensions were mixed with 13.5 µl of filtered buffer A, 1 µl of IgG1 anti-CD41-PE, and after 10 min incubation (to ensure antibody binding), samples were diluted with 185 µl of buffer A for flow cytometry analysis. In each sample run, at least 10,000 events were acquired.
2.8. Data analysis
Data are means±S.D. of a minimum of three independent experiments. Differences between means were evaluated by paired t test and the P values are indicated in the tables and figures.
3. Results
3.1. Platelet activation through the PAR-1 pathway in Na+-containing and Na+-free buffer
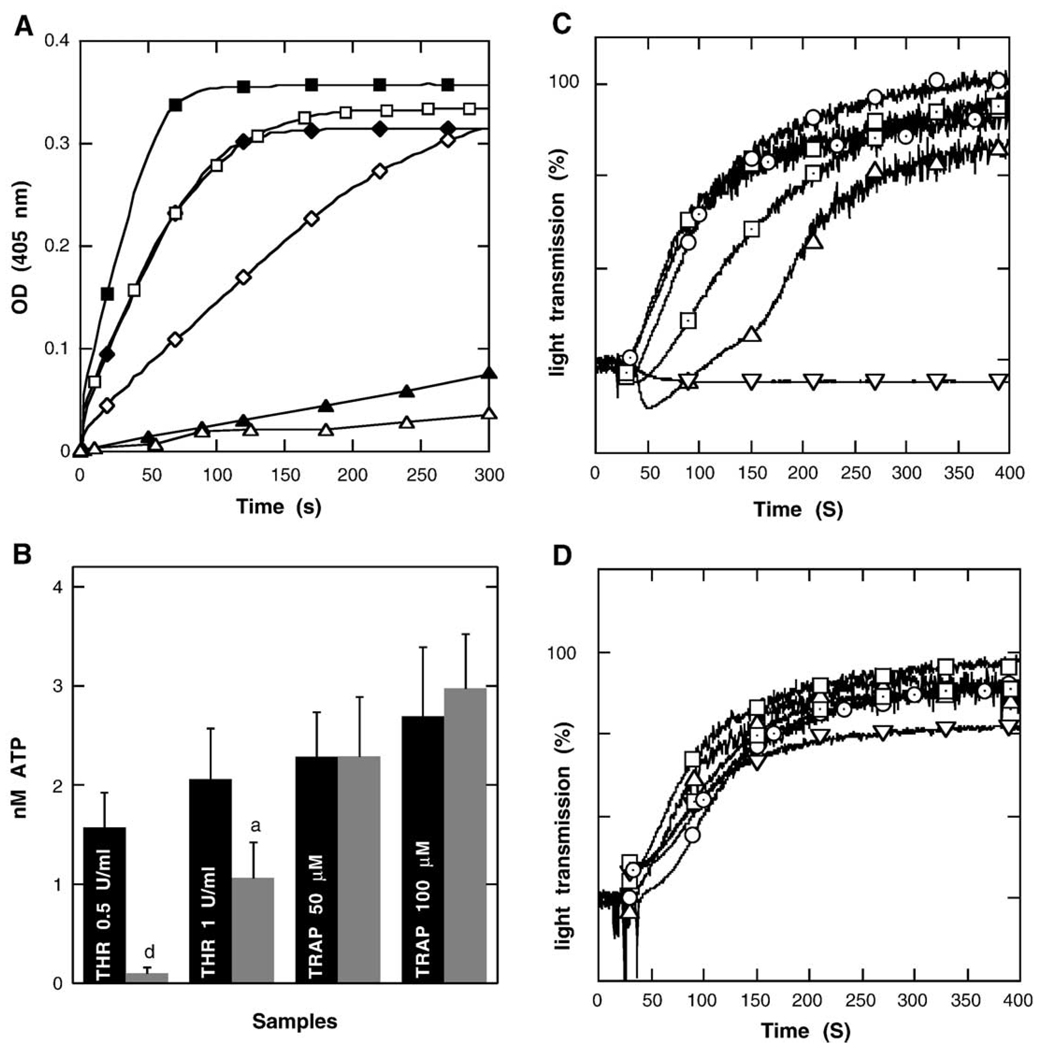
Thrombin is a Na+-activated protease [35,36]. Na+ binding near the primary specificity pocket of thrombin promotes the procoagulant and signaling functions of the enzyme. The effect is mediated allosterically by communication between the Na+ site and regions involved in substrate recognition [37,38]. Accordingly, the ability of thrombin to proteolyse the Chromozym substrate is drastically inhibited in a Na+-free buffer (Fig. 1A). Thrombin-induced platelet secretion and aggregation were also inhibited in a buffer lacking Na+ ions (Fig. 1 B and C), whereas the effect of TRAP peptide, a specific protease activated receptor 1 (PAR1) agonist, was not (Fig. 1B and D). These data confirm that in contrast to receptor activation through thrombin-mediated proteolysis, direct activation by TRAP is independent of external Na+. To investigate the role of external Na+ in the signaling cascades downstream of PAR1 activation, platelets were stimulated with TRAP.
Fig. 1.
Na+-dependence of thrombin proteolytic activity (panel A). Filled and empty symbols represent thrombin activity in Na+-containing and Na+-free buffer, respectively, with equal concentration of chromozym substrate and after thrombin addition at a concentration 0.1 U/ml (triangles) 0.5 U/ml (diamonds) and 1 U/ml (squares). Data shown are representative of three independent experiments. Na+-dependence of platelet secretion after addition of thrombin (THR) or TRAP was evaluated by monitoring ATP release (panel B). Black and gray columns represent Na+-containing and Na+-free buffers, respectively. Data are means±S.D. of 3–4 experiments (aP<0.05; dP<0.001 vs. the respective value in Na+-containing buffer). Na+-dependence of platelet aggregation expressed as the percentage of light transmission in suspensions activated with thrombin (panel C) or TRAP (panel D). Platelet aggregation was induced with 0.1 U/ml thrombin or 10 µM TRAP (triangles up—with extracellular Na+, triangles down without extracellular Na+), 0.5 U/ml thrombin or 50 µM TRAP (squares—with extracellular Na+, pointed squares—without extracellular Na+), 1 U/ml thrombin or 100 µM TRAP (circles—with extracellular Na+, pointed circles—without extracellular Na+). Data shown are representative of three independent experiments.
3.2. Dependence of PS exposure on Na+ influx through NHE activation
As previously described [39] and shown in Table 1, platelet PS exposure can be induced by activation of several receptors, including the thrombin receptor PAR1 activated with thrombin/TRAP [40] and procoagulant αIIβ3 integrin and GpVI receptors activated with collagen/thrombin [41]. In a medium containing Ca2+ but no extracellular Na+, PS exposure induced by TRAP, collagen, and a combination of TRAP and collagen was drastically inhibited (80–90% inhibition) (Table 1). Determination of PS externalization by annexin V-FITC binding was not affected in the medium containing Ca2+ but deprived of Na+ as demonstrated by the increase of the percentage of annexin V-FITC positive cells when platelets were treated with A23187 (Table 1), and as in choline medium, PS externalization induced by ATP in thymocytes was always detectable [22]. Thrombin-induced PS exposure was also dependent on the presence of external Na+ (Table 1). The simultaneous determination of PS exposure and [Na+]i in platelets activated with thrombin provides evidence that [Na+]i was increased in a significant percentage of the cell population that bind annexin V-PE as measured by FACS (Fig. 2B). Together, these data lead to the conclusion that Na+ influx can be an effector of PS externalization in platelets. Na+ influx can be mediated by NHE activation consecutive to a rapid and transient intracellular acidification [42]. We have investigated the implication of NHE in PS exposure by using an amiloride-derived specific inhibitor (EIPA) of the exchanger. Although less strongly that Na+ deprivation, EIPA significantly inhibited (20–30% inhibition) PS exposure induced by TRAP, collagen and TRAP+ collagen. The effects of thrombin and thrombin + collagen were also reduced, suggesting that thrombin-induced PS exposure was also dependent on Na+ influx through NHE activation. The difference between the effect of Na+ deprivation and EIPA on PS externalization could be linked to the inability of EIPA to reduce totally the increase in [Na]i even at 30 µM (the highest concentration that did not affect the resting platelet state) (Table 2). Assuming that EIPA is a full inhibitor of NHE1 [19,20], this finding indicates that Na+ influx via other pathways than NHE could be involved in this process.
Table 1.
Annexin V-FITC positive platelets (% of CD41-PE positive), suspended in media with (buffer A) or without Na (buffer B) after treatment with different agonists for 20 min at 37 °C
| Agonist | Buffer A (Control) | Buffer B | Buffer A+EIPA 30 µM |
Buffer A+Quercetin 100 µM |
Buffer A+Neomycin 5 mM |
|---|---|---|---|---|---|
| Control | 1.2±0.4 | 1.7±0.3 | 1.7±1.3 | 1.7±1.0 | 1.2±0.3 |
| Thrombin 0.1 U/ml | 7.6±3.1 | 2.7±0.7b | ND | ND | ND |
| Thrombin 1U/ml | 30.1±7.9 | 2.8±0.8d | 11.7±6.1b | 11.0±2.7c | 3.0±1.5c |
| TRAP 100 µM | 11.6±4.6 | 2.9±0.8b | 5.5±3.4a | 5.7±3.6a | 4.4±3.5a |
| Collagen 20 µg/ml | 15.8±4.9 | 2.6±0.6d | 8.1±1.2 b | 9.2±2.3a | 7.1±2.9b |
| TRAP+Collagen | 36.0±4.9 | 6.7±2.1d | 13.4±4.9c | 16.1±1.7d | 12.5±2.0d |
| Thrombin 1U/ml+Collagen | 39.7±7.8 | ND | 29.9±4.3a | ND | ND |
| PMA 50 nM | 3.9±1.6 | 1.9±0.9a | ND | 3.1±2.3 | 3.3±1.1 |
| Thrombin 0.1 U/ml+PMA | 14.7±4.5 | 4.8±2.3a | ND | ND | ND |
| Thrombin 1U/ml+PMA | 35.4±3.5 | 6.4±3.7d | ND | 21.2±6.4b | 11.6±2.7d |
| Nigericin 10 µM | 28.3±9.1 | 5.8±3.0c | 8.4±4.2a | ND | ND |
| Monensin 50 µM | 50.2±11.4 | 45.2±5.1 | ND | 50.4±21.9 | 44.9±9.4 |
| Gramicidin 50 µM | 9.6±9.1 | 68.6±10.1d | ND | ND | ND |
| A 23187 2 µM | 70.2±11.3 | 91.2±7.8 | ND | ND | ND |
Data are means±S.D. of 4–8 experiments (aP<0.05; bP<0.02; cP<0.01; dP<0.001 vs. the respective value in control) ND: not determined.
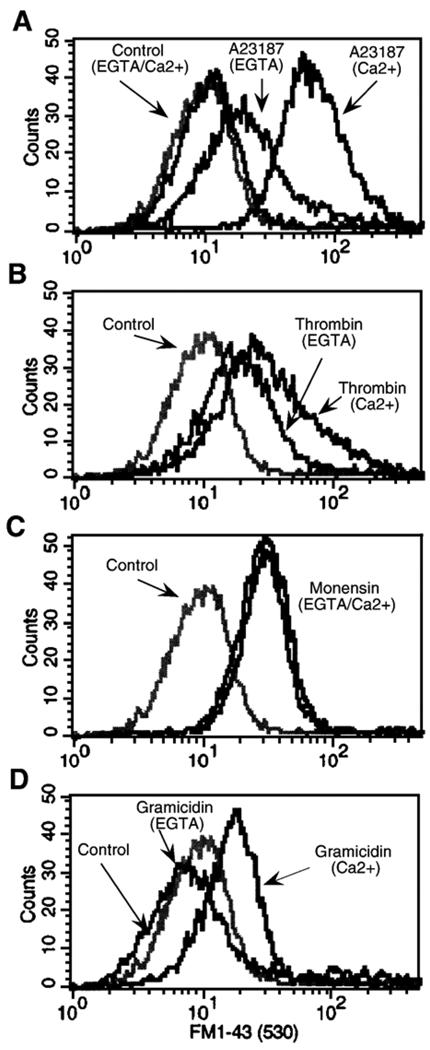
Fig. 2.
Simultaneous analysis of PS exposure and Na+ influx in sodium green (SG) loaded platelets (suspended in buffer A supplemented with 2 mM CaCl2) incubated for 10 min with annexin V-PE (AV-PE), after activation with thrombin (1 U/ml) (row B) or calcium ionophore (A23187; 5 µM) (row C). Data shown are representative of 4–6 independent experiments. Percentage of positive cells in each quadrant (row D). SG (+) Na+-positive, AV (+)-annexin V-PE positive (aP<0.05; bP<0.02; cP<0.01; dP<0.001 vs. the respective value in control).
Table 2.
Mean fluorescence intensities of sodium green indicator in platelets (CD41-PE positive) activated by different agonists for 20 min at 37 °C
| Agonist | Buffer A/Ca2+ | Buffer A/EGTA | Buffer A/Ca+30 µM EIPA |
Buffer A/Ca+100 µM Quercetin |
Buffer A/Ca+5 mM Neomycin |
|---|---|---|---|---|---|
| Control | 18.9±1.7 | 19.4±1.4 | 18.7±0.6 | 16.6±2.6 | 19.5±2.2 |
| Thrombin 1 U/ml | 34.9±2.5 | 32.8±1.8 | 28.3±4.4 | 23.3±1.4d | 23.4±3.1c |
| TRAP 100 µM | 32.8±3.1 | 27.9±1.3 | 22.8±1.6c | 20.6±2.3c | 24.5±4.0a |
| Collagen 20 µg/ml | 24.1±0.9 | 26.1±0.3 | 20.9±0.6c | 16.6±0.3d | (22.1)e |
| TRAP+Collagen | 36.7±5.4 | 29.6±4.5 | 24.3±1.1b | 20.7±1.3c | (27.8)e |
| Nigericin 10 µM | 34.9±2.7 | ND | 21.8±3.0c | ND | ND |
| Monensin 50 µM | 38.7±3.9 | 32.1±2.0a | 34.4±2.2 | 28.2±9.5 | 34.5±2.0 |
| Gramicidin 50 µM | 28.9±3.0 | 28.4±2.4 | 30.9±2.0 | 24.3±7.4 | 29.5±2.3 |
| A23187 2 µM | 38.1±9.9 | 51.8±1.5 | ND | ND | ND |
Data are means±S.D. of 4 experiments (aP<0.05; bP<0.02; cP<0.01; dP<0.001 vs. the respective value in bufferA/Ca2+). ND: not determined, ()e—means of two experiments.
Nigericin, a K+/H+ ionophore known to induce a decrease in intracellular pH, was as effective as the other agonists at inducing PS exposure (Table 1). Na+ deprivation and EIPA inhibited the effect of nigericin (Table 1). Nigericin also triggered an influx of Na+ attributable to NHE activation, as did the other agonists (thrombin, TRAP, and TRAP+ collagen), and it was partially inhibited by EIPA (Table 2). NHE activation could be a consequence of nigericin-induced acidification that precedes alkalinization [16,17]. These data confirm the possible role of Na+ influx through NHE activation but do not exclude the potential participation of pH variations in the PS exposure pathway.
PKC activation by PMA is known to activate NHE1 in platelets [17] by inducing its phosphorylation. Low concentrations of PMA (50–100 nM) were able to induce slow alkalinization in human platelets [16,17], but amplify recovery of pH after acidification induced by nigericin [16]. Higher concentrations (200 to 400 nM) were necessary to induce procoagulant activity [19] or serotonine secretion in porcine platelets [43]. Our data show that 50 nM PMA alone has no effect on PS externalization. However, the small increase of the effect of low or high concentrations of thrombin (Table 1) with PMA, argue in favor of a contribution of NHE in the regulation of PS exposure. Together, the data provide evidence that Na+ influx subsequent to NHE activation participates in PS exposure induced through PAR1 activation in platelets.
3.3. Dependence of PS externalization on Na+ influx, Ca2+ influx and intracellular pH
The involvement of Na+ in PS exposure does not discount a role for Ca2+. It is not easy to determine the respective contributions of these two cations as their concentrations vary concomitantly in response to the same agonists (Table 2 and Fig. 4A). Deprivation of either Na+ or Ca2+ in the external medium has either a deleterious effect on cell viability or prevents the binding of fluorescent annexin V to PS (which requires the presence of about 2 mM Ca2+). Moreover, the specificity of available cation ionophores is not absolute. The use of the Ca2+ ionophore A23187 has led to suggestions that Ca2+ is the major effector of phospholipid scrambling [44,45]. Here, A23187 induced PS externalization in 70% of platelets in a medium containing Na+ and Ca2+, while in the absence of Na+ externalization was observed in 91% of the cells (Table 1). The increased effect of A23187 on the percentage of platelets exposing PS would be due to an increase in the influx of Ca2+ resulting from the suppression of the competition with Na+ already described [46,47]. This hypothesis is supported by the fact that in a Na+/Ca2+ medium, A23187 induced a rise in [Na+]i similar to that of the other agonists, an effect enhanced in the absence of Ca2+ (Table 2) confirming the competition between Ca2+ and Na+ [46,47]. To investigate the contribution of external Ca2+ on PS redistribution, we measured the uptake of FM1–43, which reflects phospholipid scrambling and PS externalization and does not require external Ca2+ [31–34]. A23187 induced FM1–43 uptake in the majority of platelets in the presence of Ca2+, and a lesser uptake in its absence (Fig 3A and Table 3). The residual effect could be due to the release of Ca2+ from internal stores or to the Na+ influx induced by A23187 in the absence of Ca2+ (see Table 2). In the same way, thrombin induced FM1–43 uptake in a significant population of platelets in the presence of Ca2+, and this effect persisted but was attenuated without Ca2+ (Fig. 3B and Table 3). As for A23187, the residual effect of thrombin without external Ca2+ can be due either to Ca2+ released from internal stores or Na+ influx. However, since PS exposure mediated through PAR1 activation by TRAP was entirely abolished in solutions without Na+ (Table 1), an increase in [Ca2+]i, due to release from internal stores or to an influx similar to that induced by TRAP or collagen (Fig. 4A) is unlikely to be the sole mechanism regulating PS exposure. Indeed as previously described in T cells, an increase in [Ca2+]i was not sufficient to induce phospholipid redistribution [32], and the sole release from Ca2+ stores is likely insufficient as capacitive Ca2+ influx could be indispensable [48,49]. All together these data indicate that PS exposure induced not only by thrombin, TRAP, and collagen but also by A23187 was dependent, at least partially, on an influx of Na+. An effect of Na+ influx induced by A23187 was also suggested by the detection of an increase in [Na+]i in a population of platelets exposing PS (annexin V-PE positive) (Fig. 2C).
Fig. 4.
Evaluation of intracellular calcium concentration in platelets loaded with Fura-2 and suspended in buffer A supplemented with 2 mM [Ca2+] (right and left columns) or 0.1 mM EGTA (center columns). Changes in [Ca2+]i after activation with TRAP, collagen (panel A), 50 µM gramicidin or 50 µM monensin (panel B). Data are means±S.D. of 4–5 experiments (bP<0.02; dP<0.001 vs. the respective value in control).
Fig. 3.
Evaluation of PS exposure in calcium-containing (2 mM) and calcium-free buffers (0.1 mM EGTA), 20 min after platelet treatment with 2 µM A23187 (panel A),1 U/ml thrombin (panel B), 50 µM monensin (panel C) and 50 µM gramicidin (panel D). Remodeling of plasma membrane was assessed from FM1–43 staining. Data shown are representative of four independent experiments.
Table 3.
Mean fluorescence values (Geo mean) for data from Fig. 3
| Samples | EGTA | Ca2+ |
|---|---|---|
| Control | 2.70±0.24 | 2.78±0.42 |
| A23187 | 9.90±2.92c | 29.55±6.27d |
| Thrombin | 6.81±0.91d | 14.8±7.05a |
| Monensin | 12.04±3.78d | 10.38±5.14a |
| Gramicidin | 2.86±0.54 | 6.18±1.28c |
Data are means±S.D. of 4 experiments (a P<0.05; c P<0.01; d P<0.001 vs. the respective value in control).
Monensin, a Na+/H+ ionophore which mimics NHE function, has been shown previously to induce procoagulant activity in porcine platelets [19,20] and secretion in human platelets [43]. In human platelets, this ionophore raises the intracellular pH and Na+ concentration within seconds [50]. In a medium containing Na+ and Ca2+, 50 µM monensin induced PS externalization (Table 1), an increase in [Na+]i (Table 2), and no change in intracellular Ca2+ (Fig. 4B). Under these conditions, PS exposure appeared mostly dependent on Na+ influx. FM1–43 uptake was not affected by Ca2+ removal (Fig. 3C and Table 3) and even in presence of Ca2+, the [Ca2+]i remained unchanged (Fig. 4B) confirming that Ca2+ influx did not affect PS exposure in a Na+-containing medium. However, the effect of monensin on PS redistribution was not affected in a medium deprived of Na+ (Table 1), consistent with the fact that, under these conditions, monensin induced a weak increase in [Ca2+]i (Fig. 4B), which could favor PS exposure.
To investigate the effect of Na+ influx independently of pH variation, we have used gramicidin, an ionophore that induces Na+ influx without concomitant H+ efflux. In a Na+ medium with or without Ca2+, gramicidin induced a marked increase in [Na+]i (Table 2). Surprisingly, in a Ca2+ containing-medium, PS exposure occurred in a larger percentage of platelets (68.6%) in the absence of Na+ than in its presence (9.6%) (Table 1). This latter effect was likely due to an increase in [Ca2+]i. Indeed, gramicidin has only relative specificity for Na+ influx as it induced a drastic increase in [Ca2+]i in medium devoid of Na+ (Fig. 4B). The dependence of gramicidin-induced phospholipid redistribution on Ca2+ influx was confirmed by the abolition of FM1–43 uptake in a medium without Ca2+ (Fig. 3D and Table 3). In contrast, in medium containing Ca2+ and Na+, gramicidin induced an increase of [Na+]i comparable to that induced by agonists (thrombin, TRAP or collagen) (Table 2) but in contrast no increase in [Ca2+]i. (Fig 4B) and no PS externalization (Table 1). These data suggest that the sole increase in [Na+]i did not induce PS externalization and that an increase in [Ca2+]i. and/or variation in internal pH are necessary factors, which are induced in activated platelets.
These data indicate that the effect of monensin on PS exposure can be mediated through an increase in [Na+]i without any increase in [Ca2+]i. In contrast, the effect of gramicidin would require an increase in [Ca2+]i which is inhibited in the presence of external Na+, presumably as a result of a competition between Ca2+ and Na+ influx. In a normal medium (Na+ and Ca2+), both ionophores induced a significant increase in [Na+]i. Remarkably, only monensin induced PS externalization. In contrast to gramicidin, only monensin induced a change in cytosolic pH [50], thus mimicking NHE activation, leading to the hypothesis that variations in cytosolic pH linked to an increase in [Na+]i are necessary to induce PS externalization.
3.4. Implication of PIP2 in NHE-regulated PS exposure
The activity of NHE is optimized by PIP2 [28]. To investigate the role of PIP2 on PS exposure and Na+ influx through the NHE regulation, we have tested the effects of quercetin, a modulator of PIP2 concentration in platelets that inhibits phosphoinositide kinases and reduces phosphoinositide synthesis (32P-phosphate incorporation) by 50–60% or agonist-induced increase of PIP2 resynthesis by 70% [51]. We have also used neomycin, known for its high affinity interaction with PIP2 [52,53] preventing PIP2 interaction with proteins and resulting in NHE inhibition [28]. Preincubation of platelets with 100 µM quercetin or 5 mM neomycin significantly inhibited PS exposure induced by thrombin, TRAP, collagen or collagen + TRAP, (Table 1). The inhibitory effect of quercetin and neomycin on thrombin-induced PS exposure was partially reversed by a direct activation of NHE with PMA (Table 1). Quercetin and neomycin not only decreased PS externalization, but also abolished the increase in [Na+]i induced by thrombin and TRAP (Tables 1 and 2). This inhibition was larger than that promoted by EIPA, indicating that the blocking effect of quercetin and neomycin would affect NHE and other mediator(s) of Na+ influx. These data agree with the implication of NHE in PS externalization and suggest that PIP2 could play a regulatory role on Na+ influx and NHE activity. Quercetin and neomycin had no effect on PS exposure induced by monensin (Table 1), showing that this ionophore mimics NHE activation without involving PIP2.
4. Discussion
PS exposure on the surface of platelets is essential for their ability to activate the coagulation pathway. The intracellular signals responsible for triggering PS exposure are incompletely understood, with most studies focusing on the effects of a rise in intracellular Ca2+ on this process. Previous studies comparing the kinetics of Ca2+ influx and Na+ influx – Ca2+ influx being transient although Na+ influx is prolonged – have proposed a major role for Na+ through NHE activation, in procoagulant activity induced by collagen in porcine platelets [19] and by desmopressin in human platelets [20]. In the present study, when human platelets were activated by several physiologic agonists (thrombin, TRAP, collagen or combinations of collagen with thrombin or TRAP) or drugs that mimic agonist action, PS exposure was abolished in the absence of external Na+ and significantly diminished by EIPA. Accordingly, even for thrombin treatment, whose proteolytic action is dependent on external Na+ (see Results), Na+ influx through NHE activation plays a role in PS externalization.
Classically, Ca2+ influx has been implicated in PS exposure in activated platelets [14,54] and cell apoptosis [55,56]. In erythrocytes, Ca2+ channels have been shown to be regulated by PKC and phosphatases [57] and osmotic shock, oxidative stress or glucose depletion open Ca2+-permeable cation channels that induce apoptosis (cell-shrinkage and PS exposure) only partly dependent on Ca2+ entry [58]. Other effectors than Ca2+ have been implicated in phospholipids scrambling. Acidification induced phospholipid analog redistribution in inside-out vesicles from membrane erythrocytes [8,9]. Production of ceramides promotes PS externalization in erythrocytes exposed to osmotic shock [59], and oxidation of PS itself could play a role [60]. By measuring FM1–43 uptake, which reflects membrane remodeling and PS exposure independently of Ca2+ [31], we show that phospholipid redistribution generated by thrombin was not fully abolished by the suppression of external Ca2+, suggesting that Ca2+ influx is not the sole effector. Surprisingly, the effect of A23187 was also partially maintained without external Ca2+, an effect that can be attributed to an increase in [Na+]i mediated by the ionophore, due to a lack of competition with Ca2+. However, the deprivation of external Na+ increased the externalization of PS induced by A23187, an effect mediated this time by a larger increase in [Ca2+]i, in the absence of Na+, again resulting from the lack of competition between the two cations (Table 2) and previous reports [19,46,47]. In a similar way, in thymocytes, activation by external ATP of the purinergic P2X7 receptor, a non-specific cationic channel, induced a simultaneous Na+ and Ca2+ influx, and the very early PS externalization was predominantly generated through an effect of Na+ [22]. Our data provide various lines of evidence for the function of NHE in Na+-mediated PS exposure in activated platelets: (1) monensin, which mimics NHE activation by inducing an increase in Na+ influx and [pH]i promoted PS exposure as did the physiologic agonists; (2) nigericin, which activates NHE through primary acidification, induced Na+ influx and PS exposure; (3) EIPA, a specific inhibitor of NHE, significantly inhibited Na+ influx and PS exposure induced by the agonists and nigericin; (4) PMA, which activates NHE through a PKC-dependent phosphorylation [61], had no effect on PS exposure by itself, but enhanced the effect of thrombin.
Activation of NHE suggests that in addition to Na+ influx, a change in intracellular pH could be involved in the regulation of platelet PS exposure. In a previous report, the involvement of pH in procoagulant activation of porcine platelets was discarded [19]. However, only the effect of a cytosolic alkalinization was tested independently of agonist activation and consecutive variations of [Na]i and or [Ca2+]i. In our study, gramicidin, which triggered an increase of [Na+]i without changing the pH, had no effect on PS externalization. In contrast, the effect of A23187, which could be partly due to Na+ influx (see above), is potentially linked to intracellular alkalinization, as this carboxylic ionophore functions as a Ca2+/H+ exchanger [46]. The influence of pH on the effect of A23187 would deserve to be investigated. Changes in pH can modulate PS exposure by several mechanisms: (1) a direct effect of an increase in the concentration of protons as suggested from the induction of the redistribution of phospholipid analogs in IOVs by acidification [8,9]; (2) an inhibition of the aminophospholipid translocase mediated by acidic intracellular pH [62] could contribute to PS externalization, as previously shown in apoptotic cells [63]; (3) pH variations could modulate the affinity of intracellular messengers such as Ca2+ or Na+ for their targets, which include scramblase or phosphoinositides [15,26,64,65]. Since NHE activation leads to a prolonged Na+ influx coupled with alkalinization in response to platelet activation, only the latter hypothesis has to be considered in our conditions.
Polyphosphoinositide (PPI) turnover is a key signaling mechanism in platelets. During initiation of activation, PIP2 hydrolysis leads to Ca2+ signaling. Next, PIP2 resynthesis creates a new pool, which could be essential for the regulation of various processes by acting as a ligand or a cofactor in a variety of proteins. We have previously proposed that PIP2 could serve as a target for Ca2+ and other effectors, or as a cofactor of scramblase to induce phospholipid redistribution [9,15,26,64,65]. NHE1, the isoform of the Na+/H+ exchanger presumably implicated in activated platelets [19–21], possesses two potential PIP2-binding domains required for optimal activation [28]. Furthermore, intracellular regulation of pH has been shown to depend on PIP2 concentration or accessibility in plasma membranes [28,66]. Pretreatment of platelets with quercetin induces a decrease in PIP2 concentration [51], whereas neomycin, which interacts with PIP2, affects its accessibility [52,53]. A first consequence of quercetin and neomycin is an inhibition of the agonist-induced increase in [Ca2+]i [51,67] which can partly affect PS externalization. Here, quercetin and neomycin significantly inhibited Na+ influx and PS externalization when induced by agonists, suggesting that PS redistribution could depend on PIP2 metabolism through its regulatory role on Na+ influx mediated by NHE activation. Interestingly, PS externalization induced by monensin which mimics NHE activation was not prevented by quercetin or neomycin. In the same way, direct activation of NHE with PMA through PKC activation, overcame the inhibitory effect of the quercetin or neomycin.
In this work, we confirm that an increase of cytosolic Na+ concentration triggers PS exposure in activated platelets, similarly to PS exposure induced by P2X7 receptor in thymocytes [22]. Furthermore, the role of PIP2 in PS exposure may involve modulation of Na+ influx by regulating NHE activity. Together, these data suggest that NHE would be a checkpoint in the regulation of PS externalization. The reason for the existence of redundant pathways and/or effectors to induce PS exposure is inextricably linked to the physio-pathological requirements of this process. The identification of these pathways represents an opportunity for future investigations to provide new targets for anti-thrombotic therapies.
Acknowledgments
We gratefully acknowledge Dr. M. Hours from the Service de Cytometrie, Institut Federatif de Recherches 46, bat 440, Universite Paris XI for help with sample analysis using FACS. This study was supported by NIH grant AR38910.
Abbreviations
- BSA
bovine serum albumin
- EIPA
5-(N-Ethyl-N-Isopropyl) amiloride
- PC
phosphatidylcholine
- PE
phosphatidylethanolamine
- PS
phosphatidylserine
- LUVs
large unilamellar vesicles
- IP3
inositol triphosphate
- PIP2
phosphatidylinositol 4, 5-bisphosphate
- NHE
Na+/H+ exchanger
- TRAP
thrombin receptor agonist fragment
- PLSCR1
phospholipid scramblase 1
Footnotes
Recipient of a grant from the Centre National de la Recherche Scientifique (CNRS).
References
- 1.Devaux PF. Static and dynamic lipid asymmetry in cell membranes. Biochemistry. 1991;30:1163–1173. doi: 10.1021/bi00219a001. [DOI] [PubMed] [Google Scholar]
- 2.Bevers EM, Comfurius P, Dekkers DW, Zwaal RF. Lipid translocation across the plasma membrane of mammalian cells. Biochim. Biophys. Acta. 1999;1439:317–330. doi: 10.1016/s1388-1981(99)00110-9. [DOI] [PubMed] [Google Scholar]
- 3.Majumder R, Weinreb G, Zhai X, Lentz BR. Soluble phosphatidylserine triggers assembly in solution of a prothrombin-activating complex in the absence of a membrane surfac. J. Biol. Chem. 2002;277:29765–29773. doi: 10.1074/jbc.M200893200. [DOI] [PubMed] [Google Scholar]
- 4.Mann KG, Brummel K, Butenas S. What is all that thrombin for? J. Thromb. Haemost. 2003;1:1504–1514. doi: 10.1046/j.1538-7836.2003.00298.x. [DOI] [PubMed] [Google Scholar]
- 5.Lentz BR. Exposure of platelet membrane phosphatidylserine regulates blood coagulation. Prog. Lipid Res. 2003;42:423–438. doi: 10.1016/s0163-7827(03)00025-0. [DOI] [PubMed] [Google Scholar]
- 6.Weinreb GE, Mukhopadhyay K, Majumder R, Lentz BR. Cooperative roles of factor V(a) and phosphatidylserine-containing membranes as cofactors in prothrombin activation. J. Biol. Chem. 2003;278:5679–5684. doi: 10.1074/jbc.M208423200. [DOI] [PubMed] [Google Scholar]
- 7.Zhou Q, Zhao J, Stout JG, Luhm RA, Wiedmer T, Sims PJ. Molecular cloning of human plasma membrane phospholipid scramblase. A protein mediating transbilayer movement of plasma membrane phospholipids. J. Biol. Chem. 1997;272:18240–18244. doi: 10.1074/jbc.272.29.18240. [DOI] [PubMed] [Google Scholar]
- 8.Stout JG, Basse F, Luhm RA, Weiss HJ, Wiedmer T, Sims PJ. Scott syndrome erythrocytes contain a membrane protein capable of mediating Ca2+-dependent transbilayer migration of membrane phospholipids. J. Clin. Invest. 1997;99:2232–2238. doi: 10.1172/JCI119397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bucki R, Giraud F, Sulpice JC. Phosphatidylinositol 4,5-bisphosphate domain inducers promote phospholipid transverse redistribution in biological membranes. Biochemistry. 2000;39:5838–5844. doi: 10.1021/bi992403l. [DOI] [PubMed] [Google Scholar]
- 10.Zhou Q, Zhao J, Wiedmer T, Sims PJ. Normal hemostasis but defective hematopoietic response to growth factors in mice deficient in phospholipid scramblase 1. Blood. 2002;99:4030–4038. doi: 10.1182/blood-2001-12-0271. [DOI] [PubMed] [Google Scholar]
- 11.Wurth GA, Zweifach A. Evidence that cytosolic calcium increases are not sufficient to stimulate phospholipid scrambling in human T-lymphocytes. Biochem. J. 2002;362:701–708. doi: 10.1042/0264-6021:3620701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zwaal RF, Comfurius P, Bevers EM. Mechanism and function of changes in membrane–phospholipid asymmetry in platelets and erythrocytes. Biochem. Soc. Trans. 1993;21:248–253. doi: 10.1042/bst0210248. [DOI] [PubMed] [Google Scholar]
- 13.Dachary-Prigent J, Freyssinet JM, Pasquet JM, Carron JC, Nurden AT. Annexin Vas a probe of aminophospholipid exposure and platelet membrane vesiculation: a flow cytometry study showing a role for free sulfhydryl groups. Blood. 1993;81:2554–2565. [PubMed] [Google Scholar]
- 14.Dachary-Prigent J, Pasquet JM, Freyssinet JM, Nurden AT. Calcium involvement in aminophospholipid exposure and microparticle formation during platelet activation: a study using Ca2+-ATPase inhibitors. Biochemistry. 1995;34:11625–11634. doi: 10.1021/bi00036a039. [DOI] [PubMed] [Google Scholar]
- 15.Bucki R, Bachelot-Loza C, Zachowski A, Giraud F, Sulpice JC. Calcium induces phospholipid redistribution and microvesicle release in human erythrocyte membranes by independent pathways. Biochemistry. 1998;37:15383–15391. doi: 10.1021/bi9805238. [DOI] [PubMed] [Google Scholar]
- 16.Zavoico GB, Cragoe EJ, Jr, Feinstein MB. Regulation of intracellular pH in human platelets. Effects of thrombin, A23187, and ionomycin and evidence for activation of Na+/H+ exchange and its inhibition by amiloride analogs. J. Biol. Chem. 1986;261:13160–13167. [PubMed] [Google Scholar]
- 17.Siffert W, Siffert G, Scheid P. Activation of Na+/H+ exchange in human platelets stimulated by thrombin and a phorbol ester. Biochem. J. 1987;241:301–303. doi: 10.1042/bj2410301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosskopf D. Sodium–hydrogen exchange and platelet function. J. Thromb. Thrombolysis. 1999;8:15–24. doi: 10.1023/a:1008986329267. [DOI] [PubMed] [Google Scholar]
- 19.Samson J, Stelmach H, Tomasiak M. The importance of Na+/H+ exchanger for the generation of procoagulant activity by porcine blood platelets. Platelets. 2001;12:436–442. doi: 10.1080/09537100120078395. [DOI] [PubMed] [Google Scholar]
- 20.Tomasiak MM, Stelmach H, Bodzenta-Lukaszyk A, Tomasiak M. Involvement of Na+/H+ exchanger in desmopressin-induced platelet procoagulant response. Acta Biochem. Pol. 2004;51:773–788. [PubMed] [Google Scholar]
- 21.Telejko B, Tomasiak M, Stelmach H, Kinalska I. Platelet sodium–proton exchanger and phospholipid-dependent procoagulant activity in patients with type 2 diabetes. Metabolism. 2003;52:102–106. doi: 10.1053/meta.2003.50016. [DOI] [PubMed] [Google Scholar]
- 22.Courageot MP, Lepine S, Hours M, Giraud F, Sulpice JC. Involvement of sodium in early phosphatidylserine exposure and phospholipid scrambling induced by P2X7 purinoceptor activation in thymocytes. J. Biol. Chem. 2004;279:21815–21823. doi: 10.1074/jbc.M401426200. [DOI] [PubMed] [Google Scholar]
- 23.Brass LF, Joseph SK. A role for inositol triphosphate in intracellular Ca2+ mobilization and granule secretion in platelets. J. Biol. Chem. 1985;260:15172–15179. [PubMed] [Google Scholar]
- 24.Siffert W, Siffert G, Scheid P, Riemens T, Gorter G, Akkerman JW. Inhibition of Na+/H+ exchange reduces Ca2+ mobilization without affecting the initial cleavage of phosphatidylinositol 4,5-bisphosphate in thrombin-stimulated platelets. FEBS Lett. 1987;212:123–126. doi: 10.1016/0014-5793(87)81569-7. [DOI] [PubMed] [Google Scholar]
- 25.Zavoico GB, Cragoe EJ., Jr Ca2+ mobilization can occur independent of acceleration of Na+/H+ exchange in thrombin-stimulated human platelets. J. Biol. Chem. 1988;263:9635–9639. [PubMed] [Google Scholar]
- 26.Sulpice JC, Moreau C, Devaux PF, Zachowski A, Giraud F. Antagonist effects of Ca2+ and spermine on phosphatidylinositol 4,5-bisphosphate-mediated transmembrane redistribution of phospholipids in large unilamellar vesicles and in erythrocytes. Biochemistry. 1996;35:13345–13352. doi: 10.1021/bi960624a. [DOI] [PubMed] [Google Scholar]
- 27.Shiffer KA, Rood L, Emerson RK, Kuypers FA. Effects of phosphatidylinositol diphosphate on phospholipid asymmetry in the human erythrocyte membrane. Biochemistry. 1998;37:3449–3458. doi: 10.1021/bi972218c. [DOI] [PubMed] [Google Scholar]
- 28.Aharonovitz O, Zaun HC, Balla T, York JD, Orlowski J, Grinstein S. Intracellular pH regulation by Na(+)/H(+) exchange requires phosphatidylinositol 4,5-bisphosphate. J. Cell Biol. 2000;150:213–224. doi: 10.1083/jcb.150.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kimura M, Nakamura K, Fenton JW, Andersen TT, Reeves JP, Aviv A. Role of external Na+ and cytosolic pH in agonist-evoked cytosolic Ca2+ response in human platelets. Am. J. Physiol. 1994;267:C1543–C1552. doi: 10.1152/ajpcell.1994.267.6.C1543. [DOI] [PubMed] [Google Scholar]
- 30.Sims PJ, Faioni EM, Wiedmer T, Shattil SJ. Complement proteins C5b-9 cause release of membrane vesicles from the platelet surface that are enriched in the membrane receptor for coagulation factor Va and express prothrombinase activity. J. Biol. Chem. 1988;263:18205–18212. [PubMed] [Google Scholar]
- 31.Zweifach A. FM1-43 reports plasma membrane phospholipid scrambling in T-lymphocytes. Biochem. J. 2000;349:255–260. doi: 10.1042/0264-6021:3490255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wurth GA, Zweifach A. Evidence that cytosolic calcium increases are not sufficient to stimulate phospholipid scrambling in human T-lymphocytes. Biochem. J. 2002;362:701–708. doi: 10.1042/0264-6021:3620701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galitzine M, Capiod T, Le Deist F, Meyer D, Freyssinet JM, Kerbiriou-Nabias D. Ca2+ ionophores trigger membrane remodeling without a need for store-operated Ca2+ entry. Biochem. Biophys. Res. Commun. 2005;327:335–341. doi: 10.1016/j.bbrc.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 34.Frasch SC, Henson PM, Nagaosa K, Fessler MB, Borregaard N, Bratton DL. Phospholipid flip-flop and phospholipid scramblase 1 (PLSCR1) co-localize to uropod rafts in formulated Met-Leu-Phe-stimulated neutrophils. J. Biol. Chem. 2004;279:17625–17633. doi: 10.1074/jbc.M313414200. [DOI] [PubMed] [Google Scholar]
- 35.Workman EF, Jr, Lundblad RL. The effect of monovalent cations on the catalytic activity of thrombin. Arch. Biochem. Biophys. 1978;185:544–548. doi: 10.1016/0003-9861(78)90199-6. [DOI] [PubMed] [Google Scholar]
- 36.Di Cera E. Thrombin: a paradigm for enzymes allosterically activated by monovalent cations. C. R. Biol. 2004;327:1065–1076. doi: 10.1016/j.crvi.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 37.Wells CM, Di Cera E. Thrombin is a Na(+)-activated enzyme. Biochemistry. 1992;31:11721–11730. doi: 10.1021/bi00162a008. [DOI] [PubMed] [Google Scholar]
- 38.Di Cera E, Guinto ER, Vindigni A, Dang QD, Ayala YM, Wuyi M, Tulinsky A. The Na+ binding site of thrombin. J. Biol. Chem. 1995;270:22089–22092. doi: 10.1074/jbc.270.38.22089. [DOI] [PubMed] [Google Scholar]
- 39.Thiagarajan P, Tait JF. Binding of annexin V/placental anticoagulant protein I to platelets. Evidence for phosphatidylserine exposure in the procoagulant response of activated platelets. J. Biol. Chem. 1990;265:17420–17423. [PubMed] [Google Scholar]
- 40.Andersen H, Greenberg DL, Fujikawa K, Xu W, Chung DW, Davie EW. Protease-activated receptor 1 is the primary mediator of thrombin-stimulated platelet procoagulant activity. Proc. Natl. Acad. Sci. U. S. A. 1999;96:11189–11193. doi: 10.1073/pnas.96.20.11189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heemskerk JW, Vuist WM, Feijge MA, Reutelingsperger CP, Lindhout T. Collagen but not fibrinogen surfaces induce bleb formation, exposure of phosphatidylserine, and procoagulant activity of adherent platelets: evidence for regulation by protein tyrosine kinase-dependent Ca2+ responses. Blood. 1997;90:2615–2625. [PubMed] [Google Scholar]
- 42.Scholz W, Albus U, Counillon L, Gogelein H, Lang HJ, Linz W, Weichert A, Scholkens BA. Protective effects of HOE642, a selective sodium–hydrogen exchange subtype 1 inhibitor, on cardiac ischaemia and reperfusion. Cardiovasc. Res. 1995;29:260–268. [PubMed] [Google Scholar]
- 43.Tomasiak M, Ciborowski M, Stelmach H. The role of Na(+)/H(+) exchanger in serotonin secretion from porcine blood platelets. Acta Biochim. Pol. 2005;52:811–822. [PubMed] [Google Scholar]
- 44.Bevers EM, Tilly RH, Senden JM, Comfurius P, Zwaal RF. Exposure of endogenous phosphatidylserine at the outer surface of stimulated platelets is reversed by restoration of aminophospholipid translocase activity. Biochemistry. 1989;28:2382–2387. doi: 10.1021/bi00432a007. [DOI] [PubMed] [Google Scholar]
- 45.Chang CP, Zhao J, Wiedmer T, Sims PJ. Contribution of platelet microparticle formation and granule secretion to the transmembrane migration of phosphatidylserine. J. Biol. Chem. 1993;268:7171–7178. [PubMed] [Google Scholar]
- 46.Pressman BC. Biological applications of ionophores. Annu. Rev. Biochem. 1976;45:501–530. doi: 10.1146/annurev.bi.45.070176.002441. [DOI] [PubMed] [Google Scholar]
- 47.Flatman P, Lew VL. Does ionophore A23187 mediate Na transport in the absence of divalent cations? Nature. 1977;270:444–445. doi: 10.1038/270444a0. [DOI] [PubMed] [Google Scholar]
- 48.Martinez MC, Martin S, Toti F, Fressinaud E, Dachary-Prigent J, Meyer D, Freyssinet JM. Significance of capacitative Ca2+ entry in the regulation of phosphatidylserine expression at the surface of stimulated cells. Biochemistry. 1999;38:10092–10098. doi: 10.1021/bi990129p. [DOI] [PubMed] [Google Scholar]
- 49.Kunzelmann-Marche C, Freyssinet JM, Martinez MC. Regulation of phosphatidylserine transbilayer redistribution by store-operated Ca2+ entry: role of actin cytoskeleton. J. Biol. Chem. 2001;276:5134–5139. doi: 10.1074/jbc.M007924200. [DOI] [PubMed] [Google Scholar]
- 50.Borin M, Siffert W. Further characterization of the mechanisms mediating the rise in cytosolic free Na+ in thrombin-stimulated platelets. Evidence for inhibition of the Na+,K(+)-ATPase and for Na+ entry via a Ca2+ influx pathway. J. Biol. Chem. 1991;266:13153–13160. [PubMed] [Google Scholar]
- 51.Bucki R, Pastore JJ, Giraud F, Sulpice JC, Janmey PA. Flavonoid inhibition of platelet procoagulant activity and phosphoinositide synthesis. J. Thromb. Haemost. 2003;1:1820–1828. doi: 10.1046/j.1538-7836.2003.00294.x. [DOI] [PubMed] [Google Scholar]
- 52.Schacht J. Inhibition by neomycin of polyphosphoinositide turnover in subcellular fractions of guinea-pig cerebral cortex in vitro. J. Neurochem. 1976;27:1119–1124. doi: 10.1111/j.1471-4159.1976.tb00318.x. [DOI] [PubMed] [Google Scholar]
- 53.Gabev E, Kasianowicz J, Abbott T, McLaughlin S. Binding of neomycin to phosphatidylinositol 4,5-bisphosphate (PIP2) Biochim. Biophys. Acta. 1989;979:105–112. doi: 10.1016/0005-2736(89)90529-4. [DOI] [PubMed] [Google Scholar]
- 54.Zwaal RF, Schroit AJ. Pathophysiologic implications of membrane phospholipid asymmetry in blood cells. Blood. 1997;89:1121–1132. [PubMed] [Google Scholar]
- 55.Hampton MB, Vanags DM, Porn-Ares MI, Orrenius S. Involvement of extracellular calcium in phosphatidylserine exposure during apoptosis. FEBS Lett. 1996;399:277–282. doi: 10.1016/s0014-5793(96)01341-5. [DOI] [PubMed] [Google Scholar]
- 56.Bratton DL, Fadok VA, Richter DA, Kailey JM, Guthrie LA, Henson PM. Appearance of phosphatidylserine on apoptotic cells requires calcium-mediated nonspecific flip-flop and is enhanced by loss of the aminophospholipid translocase. J. Biol. Chem. 1997;272:26159–26165. doi: 10.1074/jbc.272.42.26159. [DOI] [PubMed] [Google Scholar]
- 57.Andrews DA, Yang L, Low PS. Phorbol ester stimulates a protein kinase C-mediated agatoxin-TK-sensitive calcium permeability pathway in human red blood cells. Blood. 2002;100:3392–3399. doi: 10.1182/blood.V100.9.3392. [DOI] [PubMed] [Google Scholar]
- 58.Lang KS, Duranton C, Poehlmann H, Myssina S, Bauer C, Lang F, Wieder T, Huber SM. Cation channels trigger apoptotic death of erythrocytes. Cell Death Differ. 2003;10:249–256. doi: 10.1038/sj.cdd.4401144. [DOI] [PubMed] [Google Scholar]
- 59.Lang KS, Myssina S, Brand V, Sandu C, Lang PA, Berchtold S, Huber SM, Lang F, Wieder T. Involvement of ceramide in hyperosmotic shock-induced death of erythrocytes. Cell Death Differ. 2004;11:231–243. doi: 10.1038/sj.cdd.4401311. [DOI] [PubMed] [Google Scholar]
- 60.Tyurina YY, Tyurin VA, Zhao Q, Djukic M, Quinn PJ, Pitt BR, Kagan VE. Oxidation of phosphatidylserine: a mechanism for plasma membrane phospholipid scrambling during apoptosis? Biochem. Biophys. Res. Commun. 2004;324:1059–1064. doi: 10.1016/j.bbrc.2004.09.102. [DOI] [PubMed] [Google Scholar]
- 61.Aharonovitz O, Granot Y. Stimulation of mitogen-activated protein kinase and Na+/H+ exchanger in human platelets. Differential effect of phorbol ester and vasopressin. J. Biol. Chem. 1996;271:16494–16499. doi: 10.1074/jbc.271.28.16494. [DOI] [PubMed] [Google Scholar]
- 62.Libera J, Pomorski T, Muller P, Herrmann A. Influence of pH on phospholipid redistribution in human erythrocyte membrane. Blood. 1997;90:1684–1693. [PubMed] [Google Scholar]
- 63.Williamson P, Schlegel RA. Transbilayer phospholipid movement and the clearance of apoptotic cells. Biochim. Biophys. Acta. 2002;1585:53–63. doi: 10.1016/s1388-1981(02)00324-4. [DOI] [PubMed] [Google Scholar]
- 64.Sulpice JC, Zachowski A, Devaux PF, Giraud F. Requirement for phosphatidylinositol 4,5-bisphosphate in the Ca(2+)-induced phospholipid redistribution in the human erythrocyte membrane. J. Biol. Chem. 1994;269:6347–6354. [PubMed] [Google Scholar]
- 65.Bucki R, Janmey PA, Vegners R, Giraud F, Sulpice JC. Involvement of phosphatidylinositol 4,5-bisphosphate in phosphatidylserine exposure in platelets: use of a permeant phosphoinositide-binding peptide. Biochemistry. 2001;40:15752–15761. doi: 10.1021/bi010899c. [DOI] [PubMed] [Google Scholar]
- 66.Hilgemann DW, Feng S, Nasuhoglu C. The complex and intriguing lives of PIP2 with ion channels and transporters. Sci. STKE. 2001:RE19. doi: 10.1126/stke.2001.111.re19. [DOI] [PubMed] [Google Scholar]
- 67.Jeng JH, Chan CP, Wu HL, Ho YS, Lee JJ, Liao CH, Chang YK, Chang HH, Chen YJ, Perng PJ, Chang MC. Protease-activated receptor-1-induced calcium signaling in gingival fibroblasts is mediated by sarcoplasmic reticulum calcium release and extracellular calcium influx. Cell Signal. 2004;16:731–740. doi: 10.1016/j.cellsig.2003.11.008. [DOI] [PubMed] [Google Scholar]