Abstract
A series of compounds structurally related to aripiprazole (1), an atypical antipsychotic and antidepressant used clinically for the treatment of schizophrenia, bipolar disorder, and depression, have been prepared and evaluated for affinity at D2-like dopamine receptors. These compounds also share structural elements with the classical D2-like dopamine receptor antagonists, haloperidol, N-methylspiperone, domperidone and benperidol. Two new compounds, 7-(4-(4-(2-methoxyphenyl)piperazin-1-yl)butoxy)-3,4-dihydroquinolin-2(1H)-one oxalate (6) and 7-(4-(4-(2-(2-fluoroethoxy)phenyl)piperazin-1-yl)butoxy)-3,4-dihydroquinolin-2(1H)-one oxalate (7) were found to (a) bind to the D2 receptor subtype with high affinity (Ki values <0.3 nM), (b) exhibit >50-fold D2 versus D3 receptor binding selectivity and (c) be partial agonists at both the D2 and D3 receptor subtype.
Keywords: Dopamine D2 receptor, Aripiprazole, Dopamine partial agonist
1. Introduction
Dopamine receptors belong to a large superfamily of neurotransmitter and hormone receptors which are coupled to their specific effectors function via guanine nucleotide regulatory (G) proteins. Based upon genomic and cDNA cloning studies, it is currently thought that there are five functionally active dopamine receptor subtypes expressed in mammals. These five receptor subtypes have been classified into two major types (D1-like and D2-like) based on their amino acid sequence and pharmacological properties. The D1-like receptor subtypes consist of the D1 (D1a) and the D5 (D1b) dopamine receptors. The D2-like receptor subtypes include the D2short (D2S), D2long (D2L), D3, and D4 receptors.1 Stimulation of D1-like receptors results in an activation of adenylyl cyclase.2 Stimulation of D2-like receptors results in an inhibition of adenylyl cyclase activity, mitogenesis, an increase in the release of arachidonic acid and an increase in phosphatidylinositol hydrolysis.3
The D2 and D3 dopamine receptor subtypes are structurally and pharmacologically similar.4 Despite these similarities, the D2 and D3 receptors differ in the neuroanatomical localization, the levels of receptor expression, efficacy in response to agonist stimulation, regulation, desensitization and the intracellular trafficking properties.5–8 Because of the high degree of homology between D2 and D3 receptor binding sites, it has been difficult to develop compounds that can selectively stimulate or block D2 or D3 receptors.3,6,7,9–11 This has also been true for the development of radiotracers for imaging dopamine D2 and D3 receptors with the functional imaging technique positron emission tomography or PET. That is, all radiotracers used in PET imaging studies bind with similar affinity to D2 and D3 receptors, and receptor density measurements using radiotracers such as the antagonists [11C]raclopride and [18F]fally-pride, and the radiolabeled full agonist, [11C](+)_PHNO, are typically reported as D2/3 receptor binding potentials.12–14 There has been a need to develop highly selective dopamine receptor ligands capable of labeling D2 versus D3 and D3 versus D2 in order to gain a better insight into the behavioral pharmacology and in vivo regulation of these structurally-similar dopamine receptors in disorders of the central nervous system.
Previously, our group synthesized a series of ((1H-indol-3-yl)methyl)piperidin-4-ol analogs and evaluated their affinities and intrinsic activities for dopamine D2 and D3 receptors. These compounds share structural elements with the classical D2-like dopamine receptor antagonists, haloperidol, N-methylspiperone and benperidol. Several of the compounds structurally similar to haloperidol were found to have moderate to high affinity and selectivity at D2 versus D3 receptors.15,16 Functional assays revealed that these compounds were antagonists at D2 receptors.15,16
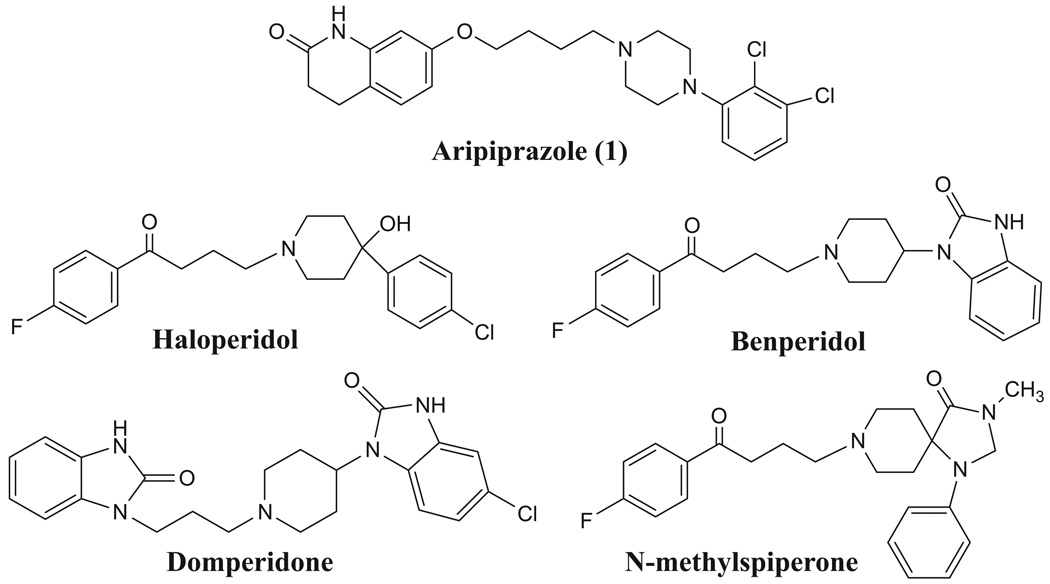
Aripiprazole, 7-[4-[4-(2,3-dichlorophenyl)-1-piperazinyl]butoxy]-3,4-dihydro-2(1H)-quinolinone (1, Fig. 1) has been used for the treatment of schizophrenia and various forms of depression. Aripiprazole has been reported to be an antagonist of postsynaptic dopamine receptor while acting as an agonist of dopamine autoreceptors.17 Using aripiprazole as a lead compound, we synthesized a series of new compounds and evaluated their binding affinities and intrinsic activities at D2-like receptors. These modifications include: (a) replacing the N-2,3-dichlorophenyl piperazine ring of aripiprazole with amine groups present in typical antipsychotics (haloperidol, benperidol, domperidone and N-methylspiperone), (b) replacing the N-2,3-dichlorophenyl group of aripiprazole with N-methoxyphenyl, or N-2-fluoroethoxy groups, (c) changing the length of the carbon atom spacer between the oxygen and nitrogen atoms, and, (d) introduction of a double bond into the 3,4-dihydro-2(1H)-quinolinone ring. The goal of this study is to modify the structure of aripiprazole in order to determine if it is possible to identify ligands having a higher affinity and selectivity for D2 versus D3 receptors for behavioral pharmacology and PET imaging studies.
Figure 1.
Structures of the lead compounds.
2. Chemistry
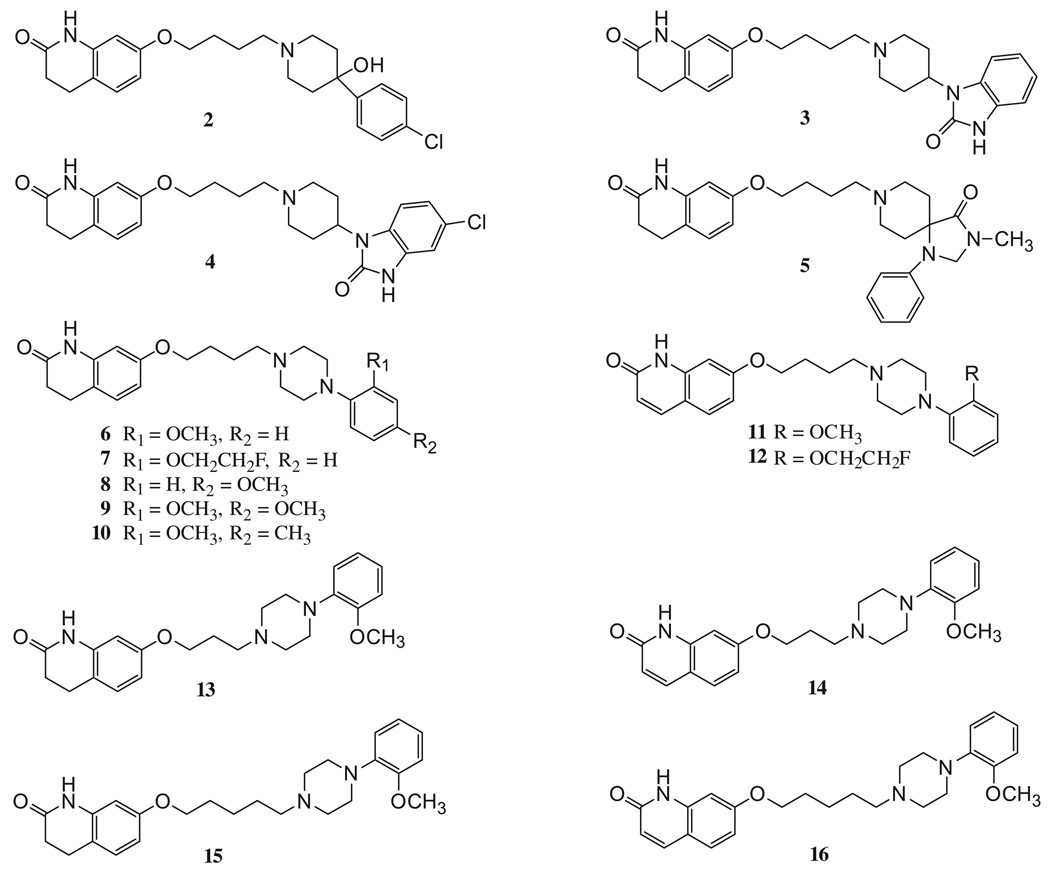
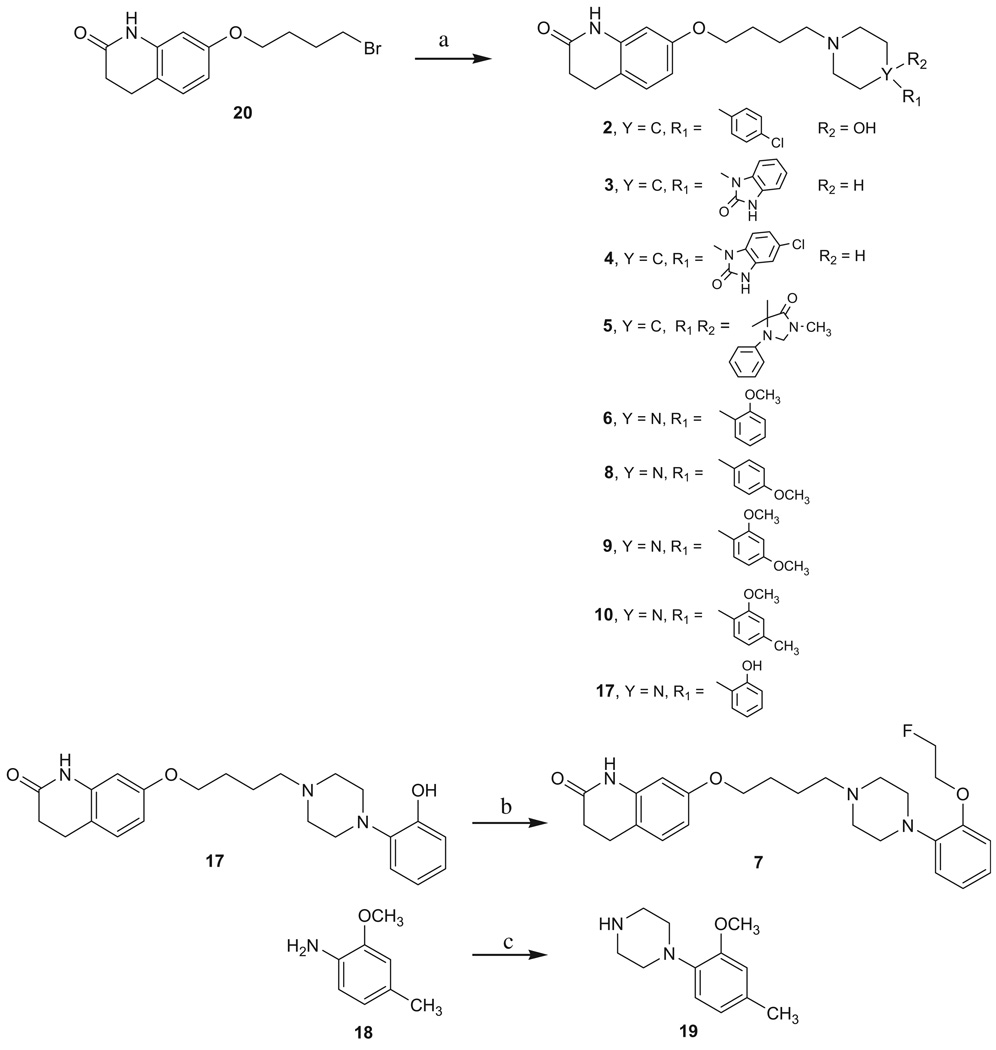
The syntheses of all target compounds (Fig. 2) are outlined in Schemes 1–4. Compounds 2–10 were prepared as outlined in Scheme 1. Starting from the commercially available 7-(4-bromobutoxy)-3,4-dihydroquinolin-2(1H)-one (20) and the corresponding piperidines or piperazines, the desired compounds 2–6, 8–10 and the intermediate 1718 were obtained in 67–96% yields. Subsequently, fluoroethylation of the aromatic hydroxyl group of 17 with 1-bromo-2-fluoroethane gave 7 in 59% yield. The piperazine 19 was prepared from 2-methoxy-4-methylaniline (18) and bis-(2-chloroethyl)amine hydrochloride in the presence of potassium carbonate in butanol.19
Figure 2.
Structures of the target compounds.
Scheme 1.
Reagents: (a) substituted piperidines or piperazines, K2CO3, KI, acetonitrile, heat; (b) 1-bromo-2-fluoroethane, K2CO3, acetone, heat; (c) bis-(2-chloroethyl)amine hydrochloride, K2CO3, butanol, heat.
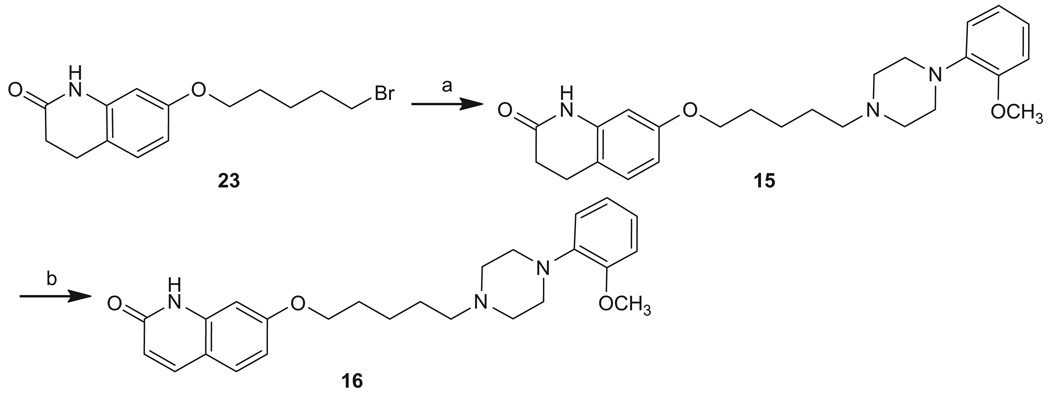
Scheme 4.
Reagents: (a) 1-(2-methoxyphenyl)piperazine hydrochloride, N(C2H5)3, acetonitrile; (b) DDQ, CH2Cl2.
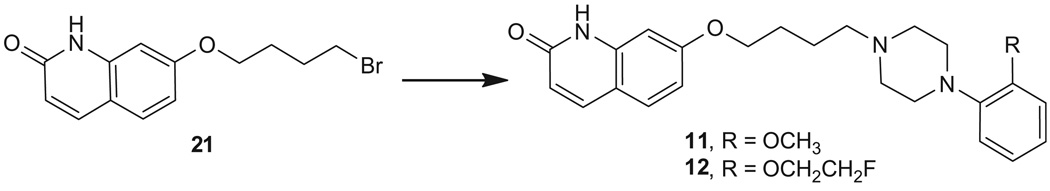
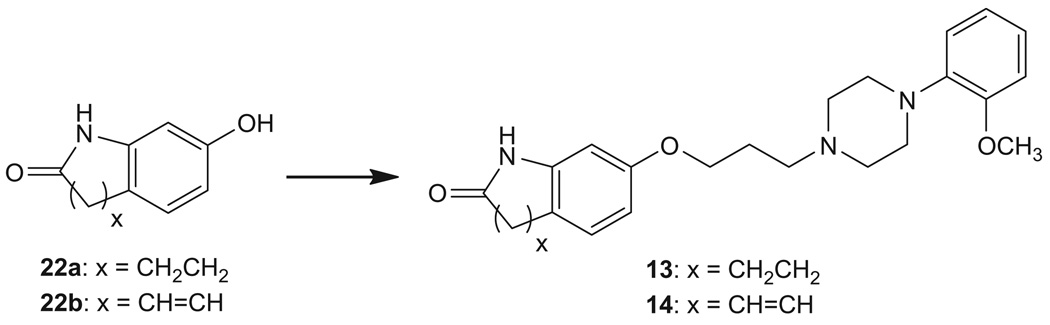
Compound 11 was synthesized from 7-(4-bromobutoxy)quinolin-2(1H)-one (21)17 and 1-(2-methoxyphenyl)piperazine, whereas 12 was synthesized from 21 and 1-(2-(2-fluoroethoxy)phenyl) piperazine20 (Scheme 2). Compounds 1321 and 1421 were prepared from O-alkylation of commercially available 7-hydroxy-3,4-dihydroquinolin-2(1H)-one (22a) or 7-hydroxyquinolin-2(1H)-one (22b) with 1-(3-chloropropyl)-4-(2-methoxyphenyl)piperazine22 (Scheme 3).
Scheme 2.
Reagents: 1-(2-methoxyphenyl)piperazine hydrochloride or 1-(2-(2-fluoroethoxy)phenyl)piperazine, N(C2H5)3, acetonitrile.
Scheme 3.
Reagents: 1-(3-chloropropyl)-4-(2-methoxyphenyl)piperazine, NaOCH3, ethanol.
Reaction of 7-(5-bromopentoxy)-3,4-dihydroquinolin-2(1H)-one (23)21 and 1-(2-methoxyphenyl)piperazine gave 15. Dehydrogenation of 15 with 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) gave 16 (Scheme 4).
3. Radioligand binding studies at dopamine receptors
Competitive radioligand binding studies were performed to determine the equilibrium dissociation constants of each compound at human D2, D3, and D4 dopamine receptors (Table 1). For these studies tissue homogenates from stably transfected HEK 293 cells were used in conjunction with the radioligand 125I-IABN. We have previously reported that the benzamide 125I-IABN binds with high affinity and selectively to D2-like dopamine receptors, but it binds non-selectively to the D2 and D3 dopamine receptor subtypes.23
Table 1.
Binding affinities for dopamine D2/D3 and sigma σ1/σ2 receptors
| Compound | Ki (nM)a | |||||||
|---|---|---|---|---|---|---|---|---|
| D2b | D3c | D4d | D3:D2e | D4:D2f | σ1g | σ2h | log Pi | |
| 2 | 2604 ± 748 | 1536 ± 156 | 1311 ± 100 | 0.6 | 0.5 | 417 ± 105 | 8269 ± 1771 | 2.25 |
| 3 | 145 ± 20 | 879 ± 289 | 1029 ± 263 | 6.1 | 7.1 | 433 ± 33 | 36364 ± 5623 | 2.54 |
| 4 | 309 ± 97 | 574 ± 80 | 1352 ± 307 | 1.9 | 4.4 | 158 ± 55 | 16907 ± 403 | 3.58 |
| 5 | 611 ± 62 | 427 ± 36 | 865 ± 116 | 0.7 | 1.4 | 3087 ± 281 | 19930 ± 3524 | 1.17 |
| 6 | 0.22 ± 0.01 | 13.1 ± 2.3 | 212 ± 45 | 60 | 964 | 1176 ± 108 | 598 ± 160 | 3.63 |
| 7 | 0.26 ± 0.05 | 13.5 ± 3.5 | 185 ± 27 | 52 | 712 | 13574 ± 2463 | 4988 ± 837 | 3.89 |
| 8 | 102 ± 25 | 930 ± 239 | 359 ± 22 | 9.1 | 3.5 | 170 ± 49 | 665 ± 36 | 3.43 |
| 9 | 25.3 ± 5.9 | 785 ± 258 | 286 ± 27 | 31 | 11 | 620 ± 64 | 3340 ± 194 | 3.12 |
| 10 | 4.6 ± 1.0 | 251 ± 33 | 27.2 ± 1.7 | 54 | 5.9 | 377 ± 56 | 6393 ± 276 | 3.97 |
| 11 | 0.14 ± 0.03 | 5.4 ± 1.1 | 30.1 ± 7.3 | 39 | 215 | 2631 ± 235 | 1309 ± 133 | 3.67 |
| 12 | 0.07 ± 0.01 | 2.7 ± 0.5 | 31.9 ± 9.1 | 39 | 456 | 20904 ± 1594 | 4952 ± 412 | 3.93 |
| 13 | 4.8 ± 0.9 | 7.4 ± 0.7 | 38.5 ± 6.4 | 1.5 | 8.0 | 759 ± 43 | 1907 ± 213 | 3.29 |
| 14 | 2.6 ± 0.5 | 5.7 ± 1.2 | 52.7 ± 16.9 | 2.2 | 20 | 2600 ± 464 | 2838 ± 86 | 3.34 |
| 15 | 7.3 ± 1.3 | 7.0 ± 1.6 | 234 ± 15.4 | 1.0 | 32 | 1349 ± 213 | 231 ± 5.85 | 3.94 |
| 16 | 7.4 ± 0.5 | 3.8 ± 0.1 | 201 ± 7.6 | 0.5 | 27 | 1614 ± 426 | 608 ± 17 | 3.98 |
| Aripiprazole | 3.1 ± 0.5 | 6.8 ± 0.2 | 168 ± 16.6 | 2.2 | 54 | NDj | NDj | 4.50 |
Mean ± SEM, Ki values were determined by at least three experiments.
Ki values for D2 receptors were measured on human D2(long) expressed in HEK cells using [125I]ABN as the radioligand.
Ki values for D3 receptors were measured on human D3 expressed in HEK cells using [125I]ABN as the radioligand.
Ki values for D4 receptors were measured on human D4 expressed in HEK cells using [125I]ABN as the radioligand.
Ki for D3 receptors/Ki for D2 receptors.
Ki for D4 receptors/Ki for D2 receptors.
Ki for inhibiting the binding of [3H](+)-pentazocine to guinea pig brain homogenates.
Ki for inhibiting the binding of [3H]DTG to rat liver homogenates.
Calculated value using the program C log P.
Not determined.
First, a comparison was made of the affinity at D2 and D3 dopamine receptors of the compounds which have structural elements similar to haloperidol, benperidol, domperidone, and N-methylspiperone. Surprisingly, these structural elements did not appear to have an effect on D2-like receptor affinity or selectivity, with compounds 2–5 displaying weak affinity for D2, D3, and D4 receptors. Next, a comparison of substituents at the piperazine moiety of aripiprazole was made in which the 2,3-dichloro substitution pattern of aripiprazole was replaced with either a 2-OCH3 (compound 6) or a 2-OCH2CH2F (compound 7) group. We found that both methoxy and 2-fluoroethoxy groups resulted in a 2-fold decrease in affinity at D3 receptors with a concomitant 14- and 12-fold increase in affinity at D2 receptors, with compounds 6 and 7 having a 60- and 52-fold selectivity at D2 receptors, respectively. This level of binding selectivity is 25 to 30 times greater than the lead compound aripiprazole (Table 1). Compounds 6, 7, 11, and 12, which contain a piperazine moiety and a saturated four carbon chain, exhibited the greatest affinity (Ki values 0.07–0.26 nM, Table 1) and selectivity (39- to 60-fold) at D2 receptors compared to the D3 receptor subtype. The positions of the substituents at the piperazine moiety were also compared. Changing the methoxy group from the 2-position on the benzene ring (compound 6) to the 4-position (compound 8) decreased both D2 and D3 receptors affinity. Substitution on both 2- and 4-positions (compounds 9 and 10) decreased D3 receptor affinity but had little effect on D2 receptor affinity. Decreasing (compounds 13 and 14) or increasing (compounds 15 and 16) the length of the four carbon spacer by one carbon atom decreased D2 receptor affinity, while having little to no effect on D3 receptor affinity, thereby reducing the receptor subtype binding selectivity (Table 1, Fig. 2). Introduction of a double bond into the 3,4-dihydro-2(1H)-quinolinone ring had little effect on both D2 and D3 receptors affinity (compounds 11, 12, 14 and 16).
4. Adenylyl cyclase studies with D2-like receptors
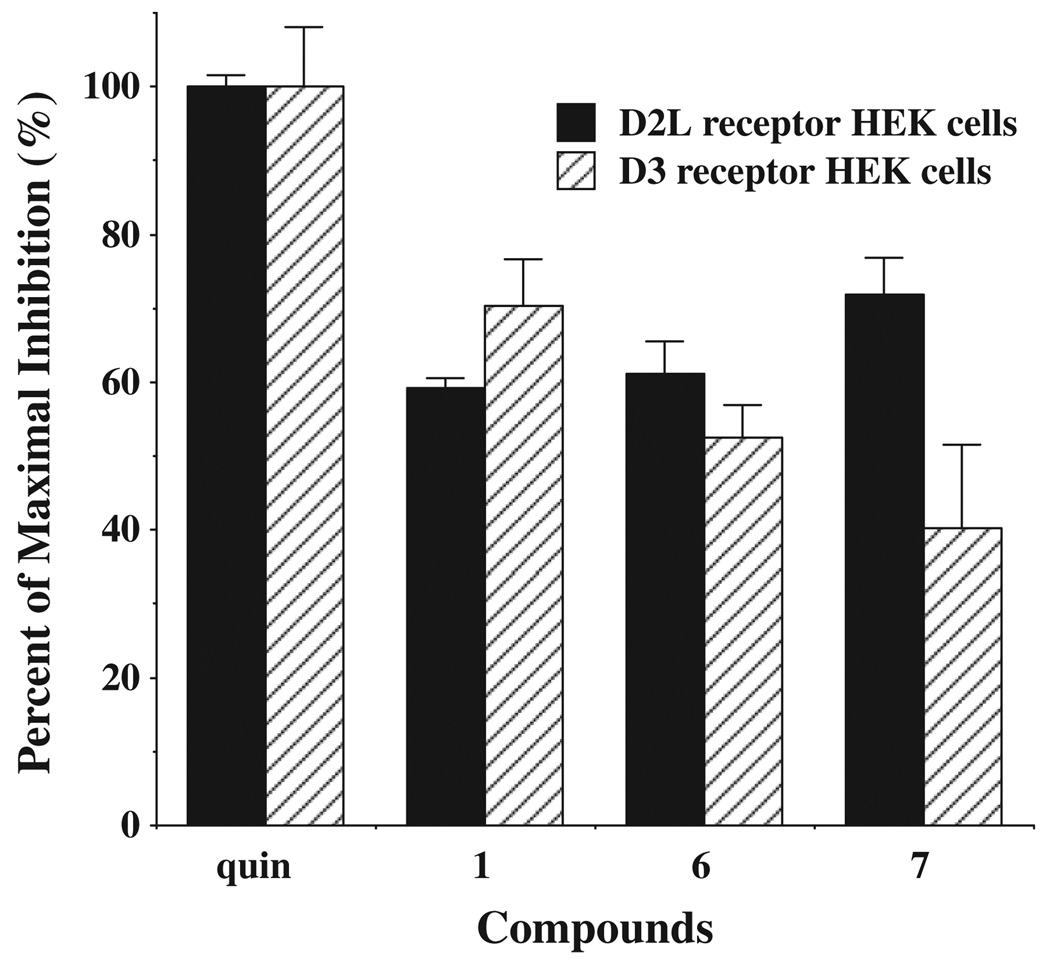
The pharmacological properties of the two compounds with the highest binding selectivity forD2 dopamine receptors (6 and 7) were further evaluated using a whole cell forskolin-dependent adenylyl cyclase inhibition assay. The intrinsic efficacy of these compounds were compared to the full agonist quinpirole, as previously described.15 Compounds 6 and 7 were found to be partial agonists at both D2 and D3 dopamine receptors. The intrinsic efficacy was found to be slightly greater (16–75%) at D2 receptors compared to D3 receptors. The intrinsic efficacy of these two compounds at human D2 receptors is similar to that found for the lead compound aripiprazole, while the efficacy at D3 receptors was somewhat lower (Fig. 3).
Figure 3.
Intrinsic efficacy at D2 and D3 dopamine receptor subtypes for adenylyl cyclase inhibition. Maximal inhibition for forskolin-dependent adenylyl cyclase inhibition was evaluated using the test ligands aripiprazole (1), compounds 6 and 7 at concentrations approximately equal to 10× the Ki values. The mean maximal percent inhibition ± SEM. (n ≥ 3) was obtained by normalizing the data to the mean inhibition achieved using the full agonist quinpirole (quin) at a final concentration of 1 µM for D2L HEK cells (solid bars) and 100 nM for D3-HEK cells (hatched bars).42
5. Radioligand binding studies at sigma receptors
In vitro binding studies were conducted to determine the affinity of the target compounds at sigma-1 (σ1) and sigma-2 (σ2) receptors. Since many structurally-diverse ligands are capable of binding to sigma receptors, we routinely screen our potential CNS PET radiotracers for binding affinity to σ1 and σ2 receptors. Any compound which binds with high affinity to σ1 and/or σ2 receptors would not be an appropriate candidate for the development of a dopaminergic PET imaging agent since sigma receptors are expressed ubiquitously in the CNS.
The σ1 binding studies were conducted using the σ1-selective radioligand, [3H](+)-pentazocine in guinea pig brain membranes; σ2 sites were assayed in rat liver membranes with [3H]DTG in the presence of 100 nM unlabeled (+)-pentazocine to mask σ1 sites, or with the σ2 selective ligand [3H]RHM-1, alone.24,25 The affinity at σ1 receptors varied from 150 to >20,000 nM. All of the compounds in this study bound with low affinity (>200 nM) at σ2 receptors. The affinity of the two compounds with the greatest D2 receptor selectivity (6 and 7) bound to sigma receptors with low affinity (Ki values >500 nM).
6. Discussion
The dopaminergic system has been the most thoroughly studies CNS receptor system using the functional imaging technique, PET. By-and-large, most imaging studies have been conducted using the radiotracer [11C]raclopride, which binds with similar affinity to dopamine D2 and D3 receptors. Therefore, PET imaging studies with this radiotracer are typically reported as dopamine D2/3 receptor binding potential, which is a measure of the density of D2 and D3 receptors in regions of interest such as the caudate and putamen. High affinity, low nonspecific binding radioligands such as [18F]fallypride and [11C]FLB 45726,27 D2 and D3 antagonists have been developed for measuring the density of extrastriatal dopamine D2/3 receptors in the CNS.
A number of studies have suggested that there is a differential regulation of dopamine D2 and D3 receptors in a variety of CNS disorders. For example, using in vitro quantitative autoradiography, Ryoo et al. reported a 45% reduction in D3 receptors in the ventral striatum, and a 15% increase in D2 receptors in the caudate/putamen of parkinsonian brain.28 These data were consistent with 6-OHDA and MPTP lesioning studies in animal models of PD.29,30 Therefore, D2 and D3 receptors appear to be regulated in an opposing manner in response to the loss of dopaminergic input in PD. In addition, PET studies of human subjects with a chronic history of cocaine abuse have revealed a reduction in D2/3 receptors relative to age-matched controls.31,32 Similar results have shown that D2/3 receptors are reduced in autoradiography33 and PET34 imaging studies of rhesus monkeys that have self-administered cocaine. However, autoradiography studies conducted by Staley and Mash reported an upregulation of D3 receptors in human cocaine overdose victims when compared with age-matched controls.35 More recently, Volkow et al. reported a difference in [11C]raclopride binding in the striatum versus thalamus in cocaine abusers versus normal controls.36 Their data demonstrated an increased binding of raclopride in the thalamus, a region of brain having a high density of D3 receptors, whereas a decrease in raclopride uptake was observed in the striatum, a brain region rich in both D2 and D3 receptors. These data suggest that chronic exposure to cocaine may result in a differential regulation of D2 and D3 receptors. That is, chronic cocaine abuse leads to an increase in D3 receptors and a decrease in density of D2 receptors. The above studies highlight the need to develop PET radiotracers which are capable of imaging D3 versus D2 receptors and vice versa.
Over the past decade, our group has focused on the development of ligands having a high affinity and selectivity for D3 versus D2 and D2 versus D3 receptors as useful probes for studying the behavioral pharmacology of these receptors, and for studying the differential expression of D2 and D3 receptors in the CNS with PET. We have recently reported the development of a series of conformationally-flexible benzamide analogs which have the potential for imaging D3 versus D2 receptors in the CNS.37,38 The current study is a continuation of our effort to develop ligands having a high affinity and selectivity for D2 versus D3 receptors which could serves a useful D2-selective behavioral probes and lead compounds for developing D2-selective PET radiotracers. In these earlier studies, we prepared a number of structural analogs of the classical D2-like dopamine receptor antagonists, haloperidol, having a high affinity for D2 versus D3 receptors.15,16
In the current study, we focused on the atypical antipsychotic aripiprazole because this compound was reported to have a modest selectivity for D2 versus D3.39 We explored the possibility of preparing aripiprazole analogs that might have higher affinity and selectivity for D2 versus D3 receptors than that of aripiprazole itself. First, the N-2,3-dichlorophenyl piperazine ring of aripiprazole was replaced with amine groups present in typical antipsychotics such as haloperidol, benperidol, domperidone, and N-methylspiperone. The results of this study revealed that replacing the N-2,3-dichlorophenyl piperazine ring of aripiprazole with these amine groups did not result in useful ligands since compounds 2–5 had low binding affinity at D2, D3, and D4 receptors. Second, we replaced the N-2,3-dichlorophenyl group of aripiprazole with N-2-methoxyphenyl group (compound 6) or N-2-(2-fluoroethoxy)phenyl group (compound 7). Compounds 6 and 7 bind at D2 receptors with nanomolar affinity and have >50-fold selectivity for human D2 receptors compared to human D3 dopamine receptor subtype. These two analogs also bind with low affinity at the D4 dopamine receptor subtype, as well as σ1 and σ2 receptors. These two analogs were evaluated for intrinsic efficacy and found to be partial agonists at both D2 and D3 receptors. Third, we changed the position of the methoxy group from the 2-position to the 4-position of the piperazine moiety. Compound 8 (with N-4-methoxyphenyl group) lost the binding affinity to both D2 and D3 receptors. Substitution on both 2-and 4-positions (compounds 9 and 10) decrease D3 receptor affinity but had little effect on D2 receptor affinity. Fourth, we also introduced a double bond into the tetrahydroisoquinoline ring of 6 and 7. Compounds 11 and 12 maintain a similar affinity for D2 receptors with a slight increase in affinity for D3 receptors. Finally, we changed the length of the carbon spacer in the chain between two ring systems of 6 and 11 from 4-carbon atoms to 3-carbon atoms (compounds 13 and 14) or 5-carbon chain (compounds 15 and 16). This change resulted in compounds with nanomolar affinity at both D2 and D3 receptors, resulting in a low selectivity for the two receptor subtypes.
The high affinity and good selectivity of compounds 6 and 7 for D2 versus D3 receptors indicate that they should be useful probes for studying the behavioral pharmacology of the D2 receptor. Furthermore, these compounds can be easily radiolabeled with either carbon-11 (t1/2 = 20.4 min) and fluorine-18 (t1/2 = 109.7 min) and serve as useful PET radiotracers for measuring the density of D2 receptors in the CNS without interference from labeling dopamine D3 receptors. The lipophilicities (log P) of 6 and 7 (Table 1) also suggest that they will readily cross the blood brain barrier and are good candidates for the development of D2 receptor selective imaging agents for the functional imaging technique, PET. PET imaging studies with [11C]6 and [18F]7 are currently ongoing in our group and will be published separately.
In summary, a series of structural congeners of aripiprazole were synthesized and evaluated for their affinities and efficacies atD2 and D3 receptors. The results of the in vitro binding studies have identified two compounds, 6 and 7, which will be useful probes for studying the function of the dopamine D2 versus D3 receptor in the CNS.
7. Experimental
7.1. Chemical analysis
1H NMR spectra were recorded on a Varian 300 MHz NMR spectrometer. Chemical shifts are reported in δ values (parts per million, ppm) relative to an internal standard of tetramethylsilane (TMS). The following abbreviations are used for multiplicity of NMR signals: br s = broad singlet, d = doublet, dd = doublet of doublets, dt = doublet of triplets, m = multiplet, s = singlet, t = triplets. Melting points were determined on an electrothermal melting point apparatus and are uncorrected. Elemental analyses were performed by Atlantic Microlab, Inc., Norcross, GA and were within ±0.4% of the calculated values. Mass spectrometry was provided by the Washington University Mass Spectrometry Resource, an NIH Research Resource (Grant No. P41RR0954). All reactions were carried out under an inert atmosphere of nitrogen. Lipophilicity measurements of the compounds were estimated using the computational program, C log P (Advanced Chemistry Development, Inc., Toronto, Canada).
7.2. 7-(4-(4-(4-Chlorophenyl)-4-hydroxypiperidin-1-yl)butoxy)-3,4-dihydroquinolin-2(1H)-one oxalate (2)
A mixture of 7-(4-bromobutoxy)-3,4-dihydroquinolin-2(1H)-one (20, 1.7 mmol), 4-(chlorophenyl)-4-hydroxypiperidine (1.7 m mol), potassium carbonate (5 equiv) and potassium iodide (1 equiv) in acetonitrile (20 mL) was stirred at reflux overnight. The reaction mixture was evaporated. The resulting residue was suspended in water (25 mL) and filtered. The solid was washed with water and air dried to give the product as white powder (96% yield). The oxalate salt was prepared using 1 equiv of oxalic acid in ethanol and recrystallized from ethanol to give 2 as an off-white powder, mp 178–179 °C; 1H NMR (free base, CDCl3 + DMSO-d6) δ 9.08 (br s, 1H), 7.47 (d, J = 8.7 Hz, 2H), 7.29 (d, J = 8.7 Hz, 2H), 7.02 (d, J = 8.2 Hz, 1H), 6.49 (dd, J = 8.7 and 2.5 Hz, 1H), 6.42 (d, J = 2.5 Hz, 1H), 3.95 (t, J = 6.0 Hz, 2H), 3.50–3.60 (m, 1H), 2.78–2.89 (m, 4H), 2.43–2.62 (m, 6H), 2.02–2.10 (m, 2H), 1.70–1.80 (m, 6H). Anal. (C24H29ClN2-O3·C2H2O4·0.5H2O) C, H, N.
7.3. 7-(4-(4-(2-Oxo-2,3-dihydro-1H-benzo[d]imidazol-1-yl)-piperidin-1-yl)butoxy)-3,4-dihydroquinolin-2(1H)-one oxalate (3)
Compound 3 was synthesized as described for 2, from 4-(2-keto-1-benzimidazolinyl)piperidine in 67% yield. Conversion to the oxalate salt gave 3 as an off-white powder, mp 187–188 °C; 1H NMR (free base, CDCl3) δ 9.88 (br s, 1H), 9.37 (s, 1H), 7.21–7.25 (m, 1H), 7.02–7.10 (m, 4H), 6.52–6.55 (m, 2H), 4.24–4.33 (m, 1H), 4.06 (t, J = 6.6 Hz, 2H), 3.11–3.15 (m, 2H), 2.87–2.92 (m, 2H), 2.50–2.64 (m, 6H), 2.17–2.24 (m, 2H), 1.71–1.89 (m, 6H). Anal. (C25H30N4O3·C2H2O4·1.25H2O) C, H, N.
7.4. 7-(4-(4-(5-Chloro-2-oxo-2,3-dihydro-1H-benzo[d]imidazol-1-yl)piperidin-1-yl)butoxy)-3,4-dihydroquinolin-2(1H)-one oxalate (4)
Compound 4 was synthesized as described for 2, from 5-chloro-1-(4-piperidyl)-2-benzimidazolinone in 70% yield. Conversion to the oxalate salt gave 4 as an off-white powder, mp 184–185 °C; 1H NMR (free base, CDCl3) δ 10.10 (br s, 1H), 9.26 (s, 1H), 6.99–7.10 (m, 4H), 6.53–6.77 (m, 2H), 4.15–4.23 (m, 1H), 4.07 (t, J = 6.6 Hz, 2H), 3.07–3.11 (m, 2H), 2.87–2.92 (m, 2H), 2.45–2.64 (m, 6H), 2.10–2.17 (m, 2H), 1.68–1.92 (m, 6H). Anal. (C25H29ClN4O3·C2H2O4) C, H, N.
7.5. 7-(4-(3Methyl-4-oxo-1-phenyl-1,3,8-triazaspiro[4.5]decan-8-yl)butoxy)-3,4-dihydro-quinolin-2(1H)-one oxalate (5)
Compound 5 was synthesized as described for 2, from 3-methyl-1-phenyl-1,3,8-triazaspiro[4.5]decan-4-one in 72% yield. Conversion to the oxalate salt gave 5 as an off-white powder, mp 241–242 °C; 1H NMR (free base, CDCl3) δ 7.96 (br s, 1H), 7.25–7.30 (m, 2H), 7.04 (d, J = 8.2 Hz, 1H), 6.83–6.92 (m, 3H), 6.52 (dd, J = 8.2 and 2.2 Hz, 1H), 6.33 (d, J = 2.2 Hz, 1H), 4.67 (s, 2H), 3.97 (t, J = 6.2 Hz, 2H), 3.00 (s, 3H), 2.86–2.91 (m, 6H), 2.54–2.78 (m, 6H), 1.65–1.85 (m, 6H). Anal. (C27H34N4O3·C2H2O4) C, H,N.
7.6. 7-(4-(4-(2-Methoxyphenyl)piperazin-1-yl)butoxy)-3,4-dihydroquinolin-2(1H)-one oxalate (6)
Compound 6 was synthesized as described for 2, from 1-(2-methoxyphenyl)piperazine hydrochloride in 96% yield. Conversion to the oxalate salt gave 6 as an off-white powder, mp 116–117 °C; 1H NMR (free base, CDCl3) δ 7.96 (br s, 1H), 7.04 (d, J = 8.2 Hz, 1H), 6.85–7.01 (m, 4H), 6.53 (dd, J = 8.2 and 2.4 Hz, 1H), 6.32 (d, J = 2.4 Hz, 1H), 3.96 (t, J = 6.2 Hz, 2H), 3.86 (s, 3H), 3.10 (br s, 4H), 2.87–2.92 (m, 2H), 2.59–2.67 (m, 4H), 2.45–2.50 (m, 2H), 1.70–1.84 (m, 6H). Anal. (C24H31N3O3·C2H2O4·0.5H2O) C, H, N.
7.7. 7-(4-(4-(2-Hydroxyphenyl)piperazin-1-yl)butoxy)-3,4-dihydroquinolin-2(1H)-one (17)
Compound 17 was synthesized as described for 2, from 1-(2-hydroxyphenyl)piperazine in 84% yield as an off-white powder; mp 107–108 °C; 1H NMR (free base, CDCl3) δ 8.15 (br s, 1H), 7.15–7.18 (m, 1H), 7.05–7.10 (m, 1H), 7.04 (d, J = 8.2 Hz, 1H), 6.83–6.96 (m, 2H), 6.53 (dd, J = 8.2 and 2.3 Hz, 1H), 6.33 (d, J = 2.3 Hz, 1H), 3.97 (t, J = 6.0 Hz, 2H), 2.87–2.93 (m, 6H), 2.59–2.64 (m, 6H), 2.48 (t, J = 7.4 Hz, 2H), 1.66–1.87 (m, 5H). Anal. (C23H29N3O3) C, H, N.
7.8. 7-(4-(4-(2-(2-Fluoroethoxy)phenyl)piperazin-1-yl)butoxy)-3,4-dihydroquinolin-2(1H)-one oxalate (7)
1-Bromo-2-fluoroethane (5.0 mmol) and potassium carbonate (4 equiv) were added into the solution of 17 (1.5 mmol) in acetone (15 mL). The reaction mixture was heated at 65–70 °C for 48 h. The solid was filtered off and washed with acetone. The filtrate was evaporated. The resulting residue was purified by silica gel column chromatography (5% methanol in dichloromethane) to give the product (59% yield). Conversion to the oxalate salt gave 7 as an off-white powder, mp 147–148 °C; 1H NMR (free base, CDCl3) δ 8.07 (br s, 1H), 7.04 (d, J = 8.2 Hz, 1H), 6.95–6.99 (m, 3H), 6.85–6.88 (m, 1H), 6.53 (dd, J = 8.2 and 2.4 Hz, 1H), 6.32 (d, J = 2.4 Hz, 1H), 4.85–4.88 (m, 1H), 4.69–4.72 (m, 1H), 4.29–4.32 (m, 1H), 4.20–4.23 (m, 1H), 3.96 (t, J = 6.2 Hz, 2H), 3.14 (br s, 4H), 2.87–2.92 (m, 2H), 2.59–2.64 (m, 6H), 2.45–2.50 (m, 2H), 1.68–1.84 (m, 4H). Anal. (C25H32FN3O3·C2H2O4·0.5H2O) C, H, N.
7.9. 7-(4-(4-(4-Methoxyphenyl)piperazin-1-yl)butoxy)-3,4-dihydroquinolin-2(1H)-one oxalate (8)
Compound 8 was synthesized as described for 2, from 1-(4-methoxyphenyl)piperazine hydrochloride in 93% yield. Conversion to the oxalate salt gave 8 as an off-white powder, mp 152–153 °C; 1H NMR (free base, CDCl3) δ 7.61 (br s, 1H), 7.05 (d, J = 8.4 Hz, 1H), 6.91 (d, J = 7.5 Hz, 2H), 6.84 (d, J = 7.5 Hz, 2H), 6.53 (d, J = 8.4 Hz, 1H), 6.29 (m, 1H), 3.93–3.98 (m, 2H), 3.77 (s, 3H), 3.10 (br s, 4H), 2.88–2.90 (m, 2H), 2.60–2.64 (m, 6H), 2.45–2.49 (m, 2H), 1.62–1.82 (m, 4H). Anal. (C24H31N3O3·C2H2O4·0.5H2O) C, H, N.
7.10. 7-(4-(4-(2,4-Dimethoxyphenyl)piperazin-1-yl)butoxy)-3,4-dihydroquinolin-2(1H)-one oxalate (9)
Compound 9 was synthesized as described for 2, from 1-(2,4-dimethoxyphenyl)piperazine hydrochloride in 71% yield. Conversion to the oxalate salt gave 9 as an off-white powder, mp 167–168 °C; 1H NMR (free base, CDCl3) δ 7.80 (br s, 1H), 7.05 (d, J = 8.5 Hz, 1H), 6.87 (d, J = 8.5 Hz, 1H), 6.41–6.54 (m, 3H), 6.30 (s, 1H), 3.96 (t, J = 6.1 Hz, 2H), 3.84 (s, 3H), 3.78 (s, 3H), 3.03 (br s, 4H), 2.87–2.92 (m, 2H), 2.60–2.66 (m, 6H), 2.45–2.50 (m, 2H), 1.68–1.84 (m, 4H). Anal. (C25H33N3O4·C2H2O4·0.5H2O) C, H, N.
7.11. 7-(4-(4-(2-Methoxy-4-methylphenyl)piperazin-1-yl)butoxy)-3,4-dihydroquinolin-2(1H)-one oxalate (10)
Compound 10 was synthesized as described for 2, from 1-(2-methoxy-4-methylphenyl)piperazine hydrochloride (19) in 95% yield. Conversion to the oxalate salt gave 10 as an off-white powder, mp 146–147 °C; 1H NMR (free base, CDCl3) δ 7.83 (br s, 1H), 6.97 (d, J = 8.1 Hz, 1H), 6.77 (d, J = 7.8 Hz, 1H), 6.64 (d, J = 8.1 Hz, 1H), 6.61 (s, 1H), 6.45 (d, J = 8.4 Hz, 1H), 6.24 (s, 1H), 3.88 (t, J = 6.1 Hz, 2H), 3.77 (s, 3H), 2.99 (br s, 4H), 2.80–2.85 (m, 2H), 2.50–2.61 (m, 6H), 2.37–2.42 (m, 2H), 2.23 (s, 3H), 1.60–1.74 (m, 4H). Anal. (C25H33N3O3·C2H2O4·1.5H2O) C, H, N.
7.12. 1-(2-Methoxy-4-methylphenyl)piperazine hydrochloride (19)19
A solution of 2-methoxy-4-methylaniline (18, 10.9 mmol), bis-(2-chloroethyl)amine hydrochloride (12.0 mmol), potassium carbonate (15.2 mmol) in 1-butanol (5 mL) was refluxed under nitrogen overnight. The hot reaction mixture was filtered and the filtrate was concentrated under vacuum. The resulting residue was triturated with acetone and filtered to give 19 as an off-white powder (14% yield), mp 212–213 °C (dec); 1H NMR (free base, CDCl3) δ 9.14 (s, 1H), 6.68–6.82 (m, 3H), 3.77 (s, 3H), 3.11–3.19 (m, 8H), 2.25 (s, 3H).
7.13. 7-(4-(4-(2-Methoxyphenyl)piperazin-1-yl)butoxy)quinolin-2(1H)-one oxalate (11)
A mixture of 7-(4-bromobutoxy)quinolin-2(1H)-one17 (21, 0.78 mmol), 1-(2-methoxyphenyl)piperazine hydrochloride (1.00 mmol), and triethylamine (0.5 mL) in acetonitrile (15 mL) was refluxed overnight. The solvent was evaporated under reduced pressure. The residue was dissolved in dichloromethane, washed with saturated NaHCO3 solution, dried over Na2SO4, concentrated in vacuo. The resulting residue was purified by silica gel column chromatography (5% methanol in dichloromethane) to give the product as an oil (62% yield). Conversion to the oxalate salt gave 11 as an off-white powder, mp 180–182 °C; 1H NMR (free base, CDCl3) δ 12.30 (s, 1H), 7.71–7.74 (m, 1H), 7.42–7.45 (m, 1H), 6.78–7.02 (m, 6H), 6.53–6.56 (m, 1H), 4.07–4.10 (m, 2H), 3.86 (s, 3H), 3.11 (br s, 4H), 2.68 (br s, 4H), 2.47–2.52 (m, 2H), 1.72–1.90 (m, 4H). HRMS calcd for C24H30N3O3 [M+H]+ 408.2287, found: 408.2290.
7.14. 7-(4-(4-(2-(2-Fluoroethoxy)phenyl)piperazin-1-yl)butoxy)quinolin-2(1H)-one oxalate (12)
Compound 12 was synthesized as described for 11 from 21 and 1-(2-(2-fluoroethoxy)phenyl)piperazine20 in 65% yield. Conversion to oxalate salt gave 12 as a white powder, mp 152–154 °C; 1H NMR (free base, CDCl3) δ 12.02 (s, 1H), 7.72 (d, J = 9.4 Hz, 1H), 7.44 (d, J = 9.4 Hz, 1H), 6.79–6.96 (m, 6H), 6.53 (d, J = 9.4 Hz, 1H), 4.78 (dt, J = 47.7 and 3.9 Hz, 2H), 4.25 (dt, J = 27.6 and 3.9 Hz, 2H), 4.08–4.13 (m, 2H), 3.14 (br s, 4H), 2.67 (br s, 4H), 2.49 (t, J = 7.5 Hz, 2H), 1.72–1.90 (m, 4H). HRMS calcd for C25H31FN3O3 [M+H]+ 440.2349, found: 440.2345.
7.15. 7-(3-(4-(2-Methoxyphenyl)piperazin-1-yl)propoxy)-3,4-dihydroquinolin-2(1H)-one oxalate (13)
1-(3-Chloropropyl)-4-(2-methoxyphenyl)piperazine22 (2.0 mmol) was added to a solution of 7-hydroxy-3,4-dihydroquinolin-2(1H)-one (22a, 2.0 mmol), sodium methoxide (25% in methanol, 2.0 mmol) in ethanol (10 mL). The mixture was refluxed for 5 h. The solvent was removed in vacuo. The residue was diluted with dichloromethane and washed with water. The organic phase was dried and concentrated. The residue was purified by column chromatography (5% methanol in dichloromethane) to give product as a free base in 67% yield. Conversion to oxalate salt gave 13 as a white powder, mp 115–116 °C; 1H NMR (free base, CDCl3) δ 8.07 (br s, 1H), 6.85–7.05 (m, 5H), 6.53 (dd, J = 8.1, and 2.4 Hz, 1H), 6.33 (d, J = 2.4 Hz, 1H), 4.00 (t, J = 6.3 Hz, 2H), 3.86 (s, 3H), 3.11 (br s, 4H), 2.89 (t, J = 7.2 Hz, 2H), 2.57–2.69 (m, 8H), 1.97–2.02 (m, 2H). HRMS calcd for C23H30N3O3 [M+H]+ 396.2287, found: 396.2284.
7.16. 7-(3-(4-(2-Methoxyphenyl)piperazin-1-yl)propoxy)quinolin-2(1H)-one oxalate (14)
Compound 14 was synthesized as described for 13 from 7-hydroxyquinolin-2(1H)-one (22b) and 1-(3-chloropropyl)-4-(2-methoxyphenyl)piperazine22 in 52% yield. Conversion to oxalate salt gave 14 as a white powder, mp 143–145 °C; 1H NMR (free base, CDCl3) δ 12.13 (s, 1H), 7.72 (d, J = 9.3 Hz, 1H), 7.45 (d, J = 9.3 Hz, 1H), 6.81–7.02 (m, 6H), 6.55 (d, J = 9.6 Hz, 1H), 4.15 (t, J = 6.2 Hz, 2H), 3.87 (s, 3H), 3.13 (br s, 4H), 2.71 (br s, 4H), 2.63 (t, J = 7.4 Hz, 2H), 2.03–2.08 (m, 2H). Anal. (C23H27N3O3·H2C2O4·H2O) C, H, N.
7.17. 7-(5-(4-(2-Methoxyphenyl)piperazin-1-yl)pentyloxy)-3,4-dihydroquinolin-2(1H)-one oxalate (15)
Compound 15 was synthesized as described for 2 from 7-(5-bromopentyloxy)-3,4-dihydroquinolin-2(1H)-one (23)21 and 1-(2-methoxyphenyl)piperazine hydrochloride in 47% yield. Conversion to oxalate salt gave 15 as a white powder, mp 198–200 °C; 1H NMR (free base, CDCl3) δ 7.90 (br s, 1H), 6.85–7.06 (m, 5H), 6.52 (dd, J = 8.4 and 2.4 Hz, 1H), 6.31 (d, J = 2.4 Hz, 1H), 3.93 (t, J = 6.5 Hz, 2H), 3.87 (s, 3H), 3.11 (br s, 4H), 2.90 (t, J = 7.5 Hz, 2H), 2.59–2.66 (m, 6H), 2.44 (t, J = 7.5 Hz, 2H), 1.50–1.83 (m, 6H). Anal. (C25H33N3O3·H2C2O4) C, H, N.
7.18. 7-(5-(4-(2-Methoxyphenyl)piperazin-1-yl)pentyloxy)quinolin-2(1H)-one oxalate (16)
A mixture of 15 (0.28 mmol), and DDQ (0.34 mmol) in dichloromethane (5 mL) was stirred at room temperature overnight. The mixture was washed with saturated NaHCO3 solution, dried, and concentrated in vacuo. The residue was purified by column chromatography (5% methanol in dichloromethane) to give the product as a free base in 75% yield. Conversion to oxalate salt gave 16 as a white powder; mp 229–230 °C. 1H NMR (free base, CDCl3) δ 12.05 (s, 1H), 7.72 (d, J = 9.3 Hz, 1H), 7.44 (d, J = 9.3 Hz, 1H), 6.79–7.10 (m, 6H), 6.53 (d, J = 9.3 Hz, 1H), 4.07 (t, J = 6.3 Hz, 2H), 3.86 (s, 3H), 3.11 (br s, 4H), 2.68 (br s, 4H), 2.46 (t, J = 6.6 Hz, 2H), 1.82–1.90 (m, 2H), 1.53–1.70 (m, 4H). Anal. (C25H31N3O3·H2C2O4) C, H, N.
8. Radiological binding and functional assays
8.1. Dopamine receptor binding assay
The method for the iodination of 125I-IABN using peracetic acid has been previously described.23 For radioligand binding studies, membrane homogenates from stably transfected HEK 293 cells expressing either the human D2, D3, or D4 receptors were prepared using a polytron tissue homogenizer (Brinkman Instruments, Westbury, NY). The tissue was suspended in 50 mM Tris–HCl, 150 mM NaCl and 1 mM EDTA at pH 7.5 to approximately 5–20 µg of protein per 50 µL prior to the assay. Assays were performed in a total volume of 150 µL. Binding reactions were carried out for 60 min at 37 °C and the reaction was terminated by rapid filtration over Schleicher and Schuell No. 32 glass fiber filters (Whatman plc, Maidstone, England). After washing filters with buffer, the radioactivity of the 125I-labeled ligand was quantitated using a Packard Cobra gamma counter with an efficiency of 75%. Protein concentrations were determined using a BCA reagent (Pierce, Rockford, Illinois) with bovine serum albumin as the protein standard.
For competition curves using a transfected cell line expressing D2, D3, or D4 dopamine receptors, experiments were performed in triplicate with two concentrations of inhibitor per decade over at least five orders of magnitude. The concentration of the radioligand was approximately equal to the Kd values. Controls containing either no inhibitor or 2 µM(+)-butaclamol were used to define total binding and nonspecific binding, respectively. Competition data for D2-like dopamine receptors were modeled for a single-site fit using the tablecurve program (Jandel Scientific Software, San Rafael, California); the IC50 values for the competitive inhibitors were converted to Ki values using the Cheng and Prusoff corrections.40
8.2. Sigma receptor binding assays
Test compounds were dissolved in N,N-dimethylformamide (DMF), dimethyl sulfoxide (DMSO) or ethanol and then diluted in 50 mM Tris–HCl buffer, pH 7.4, containing 150 mM NaCl and 100 mM EDTA. Membrane homogenates were made from guinea pig brain for σ1 binding assay and rat liver for σ2 binding assay. Membrane homogenates were diluted with 50 mMTris–HCl buffer, pH 8.0, and incubated at 25 °C in a total volume of 150 µL in 96-well plates with the radioligand and test compounds with concentrations ranging from 0.1 nM to 10 µM. After incubation was completed, the reactions were terminated by the addition of 150 µL of ice-cold wash buffer (10 mM Tris–HCl, 150 mM NaCl, pH 7.4) using a 96-channel transfer pipette (Fisher Scientific, Pittsburgh, PA), and the samples harvested and filtered rapidly through 96-well fiber glass filter plate (Millipore, Billerica, MA) that had been presoaked with 100 µL of 50 mMTris–HCl buffer, pH 8.0, for 1 h. Each filter was washed three times with 200 µL of ice-cold wash buffer. AWallac 1450 MicroBeta liquid scintillation counter (Perkin–Elmer, Boston, MA) was used to quantitate the bound radioactivity.
The σ1 receptor binding assay was conducted using guinea pig brain membrane homogenates (~300 µg protein) and ~5 nM [3H](+)-pentazocine (34.9 Ci/mmol, Perkin–Elmer, Boston, MA). The incubation time was 90 min. Nonspecific binding was determined from samples that contained 10 µM of cold haloperidol.
The σ2 receptor binding assays were conducted using rat liver membrane homogenates (~300 µg protein) and ~1 nM [3H]RHM-1 (80 Ci/mmol, American Radiolabeled Chemicals Inc., St. Louis, MO) alone or ~5 nM [3H]DTG (58.1 Ci/mmol, Perkin–Elmer, Boston, MA) in the presence of 1 µM (+)-pentazocine to block σ1 sites. The incubation time was 60 min for [3H]RHM-1 and 120 min for [3H]DTG. Nonspecific binding was determined from samples that contained 10 µM of cold haloperidol.
Data from the competitive inhibition experiments were modeled using nonlinear regression analysis to determine the concentration of inhibitor that inhibits 50% of the specific binding of the radioligand (IC50 value). Competitive curves were best fit to a one-site fit and gave pseudo-Hill coefficients of 0.6–1.0. Ki values were calculated using the method of Cheng and Prusoff23 and represent mean values ± SEM. The Kd value used for [3H](+)-pentazocine with guinea pig brain homogenates was 7.89 nM; a Kd value of 30.73 nM was used for [3H]DTG with rat liver, while 0.66 nM was used for [3H]RHM-1 with rat liver.25
8.3. Whole cell adenylyl cyclase assay
The accumulation of 3H-cyclic AMP in HEK cells was measured by a modification of the method of Shimizu et al.41 as previously described.23 Transfected HEK cells were treated with serum-free medium containing 2,8-3H-adenine (ICN) and cells were incubated at 37 °C for 75 min. Cells and drugs diluted in serum-free media containing 0.1 mM 3-isobutyl-1-methylxanthine (Sigma) were mixed to give a final volume of 500 µL and cells were incubated for 20 min at 37 °C. The reaction was stopped by addition of 500 µL of 10% trichloroacetic acid and 1 mM cyclic AMP. After centrifugation, the supernatants were fractionated using Dowex AG1-X8 and neutral alumina to separate the 3H-ATP and the 3H-cyclic AMP. Individual samples were corrected for column recovery by monitoring the recovery of the cyclic AMP using spectrophotometric analysis at OD 259 nm.23,41
Acknowledgments
This research was funded by MH081281 and DA023957 awarded by the National Institutes of Health.
Appendix
Elemental analyses
| Compound | %C | %H | %N | |||
|---|---|---|---|---|---|---|
| Calcd | Found | Calcd | Found | Calcd | Found | |
| 2 | 59.15 | 58.79 | 6.11 | 5.85 | 5.31 | 5.22 |
| 3 | 59.28 | 59.28 | 6.36 | 6.45 | 10.24 | 9.85 |
| 4 | 58.01 | 58.07 | 5.59 | 5.86 | 10.02 | 10.00 |
| 5 | 63.03 | 62.95 | 6.57 | 6.60 | 10.14 | 10.13 |
| 6 | 61.40 | 61.40 | 6.74 | 6.54 | 8.26 | 8.20 |
| 7 | 59.99 | 60.03 | 6.53 | 6.55 | 7.77 | 7.54 |
| 8 | 61.40 | 61.62 | 6.74 | 6.62 | 8.26 | 8.33 |
| 9 | 60.21 | 60.47 | 6.74 | 6.72 | 7.80 | 7.70 |
| 10 | 59.99 | 59.60 | 7.08 | 6.79 | 7.77 | 7.41 |
| 14 | 59.87 | 60.09 | 6.23 | 6.21 | 8.38 | 8.28 |
| 15 | 63.14 | 62.91 | 6.87 | 6.79 | 8.18 | 8.00 |
| 16 | 63.39 | 63.27 | 6.50 | 6.48 | 8.21 | 8.07 |
| 17 | 69.85 | 69.72 | 7.39 | 7.25 | 10.62 | 10.64 |
References and notes
- 1.Liu L-X, Burgess LH, Gonzalez AM, Sibley DR, Chiodo LA. Synapse. 1999;31:108. doi: 10.1002/(SICI)1098-2396(199902)31:2<108::AID-SYN3>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 2.Herve D, Le-Moine C, Corvol JC, Belluscio L, Ledent C, Fienberg AA, Jaber M, Studler JM, Girault JA. J. Neurosci. 2001;21:4390. doi: 10.1523/JNEUROSCI.21-12-04390.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luedtke RR, Mach RH. Curr. Pharm. Des. 2003;9:643. doi: 10.2174/1381612033391199. [DOI] [PubMed] [Google Scholar]
- 4.Sokoloff P, Giros B, Martres MP, Bouthenet ML, Schwartz JC. Nature. 1990;347:146. doi: 10.1038/347146a0. [DOI] [PubMed] [Google Scholar]
- 5.Joyce JN. Pharmacol. Ther. 2001;90:231. doi: 10.1016/s0163-7258(01)00139-5. [DOI] [PubMed] [Google Scholar]
- 6.Xu J, Chu W, Tu Z, Jones LA, Luedtke RR, Perlmutter JS, Mintun MA, Mach RH. Synapse. 2009;63:717. doi: 10.1002/syn.20652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu J, Hassanzadeh B, Chu W, Tu Z, Jones LA, Luedtke RR, Perlmutter JS, Mintun MA, Mach RH. Synapse. 2010;64:449. doi: 10.1002/syn.20748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuzhikandathil EV, Westrich L, Bakhos S, Pasuit J. Mol. Cell. Neurosci. 2004;26:144. doi: 10.1016/j.mcn.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 9.Mach RH, Huang Y, Freeman RA, Wu L, Blair S, Luedtke RR. Bioorg. Med. Chem. 2003;11:225. doi: 10.1016/s0968-0896(02)00341-3. [DOI] [PubMed] [Google Scholar]
- 10.Chu W, Tu Z, McElveen E, Xu J, Taylor M, Luedtke RR, Mach RH. Bioorg. Med. Chem. 2005;13:77. doi: 10.1016/j.bmc.2004.09.054. [DOI] [PubMed] [Google Scholar]
- 11.Grundt P, Carlson EE, Cao J, Bennett CJ, McElveen E, Taylor M, Luedtke RR, Hauck-Newman A. J. Med. Chem. 2005;48:839. doi: 10.1021/jm049465g. [DOI] [PubMed] [Google Scholar]
- 12.Elsinga PH, Hatano K, Ishiwata K. Curr. Med. Chem. 2006;13:2139. doi: 10.2174/092986706777935258. [DOI] [PubMed] [Google Scholar]
- 13.Willeit M, Ginovart N, Kapur S, Houle S, Hussey D, Seeman P, Wilson AA. Biol. Psychiatry. 2006;59:389. doi: 10.1016/j.biopsych.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 14.Egeton A, Hirani E, Ahmad R, Turton DR, Brickute D, Rosso L, Howes OD, Luthra SK, Grasby PM. Synapse. 2010;64:301. doi: 10.1002/syn.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vangveravong S, McElveen E, Taylor M, Xu J, Tu Z, Luedtke RR, Mach RH. Bioorg. Med. Chem. 2006;14:815. doi: 10.1016/j.bmc.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 16.Vangveravong S, Taylor M, Xu J, Cui J, Calvin W, Babic S, Luedtke RR, Mach RH. Bioorg. Med. Chem. 2010;18:5291. doi: 10.1016/j.bmc.2010.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oshiro Y, Sato S, Kurahashi N, Tanaka T, Kikuchi T, Tottori K, Uwahodo Y, Nishi T. J. Med. Chem. 1998;41:658. doi: 10.1021/jm940608g. [DOI] [PubMed] [Google Scholar]
- 18.Bhat L, Mohapatra PP, Bhat SR. U.S. Pat. Appl. Publ. 2008 Nov. 27 20080293736. [Google Scholar]
- 19.Pascal J, Julien I, Pinhas H, Dumez D, Darre L, Poizot A. Eur. J. Med. Chem. 1990;25:291. [Google Scholar]
- 20.Tietze R, Hocke C, Löber S, Hübner H, Kuwert T, Gmeiner P, Prante O. J. Labelled Compd. Radiopharm. 2006;49:55. [Google Scholar]
- 21.Banno K, Fujioka T, Kikuchi T, Oshiro Y, Hiyama T, Nakagawa K. Chem. Pharm. Bull. 1988;36:4377. doi: 10.1248/cpb.36.4377. [DOI] [PubMed] [Google Scholar]
- 22.Leopoldo M, Lacivita E, Passafiume E, Contino M, Colabufo NA, Berardi F, Perrone R. J. Med. Chem. 2007;50:5043. doi: 10.1021/jm070721+. [DOI] [PubMed] [Google Scholar]
- 23.Luedtke RR, Freeman RA, Boundy VA, Martin MW, Mach RH. Synapse. 2000;38:438. doi: 10.1002/1098-2396(20001215)38:4<438::AID-SYN9>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 24.Hellewell SB, Bruce A, Feinstein G, Orringer J, Williams W, Bowen WD. Eur. J. Pharmacol., Mol. Pharmacol. Sec. 1994;268:9. doi: 10.1016/0922-4106(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 25.Xu J, Tu Z, Jones LA, Vangveravong S, Wheeler KT, Mach RH. Eur. J. Pharmacol. 2005;525:8. doi: 10.1016/j.ejphar.2005.09.063. [DOI] [PubMed] [Google Scholar]
- 26.Narendran R, Frankle WG, Mason NS, Rabiner EA, Gunn RN, Searle GE, Vora S, Litschge M, Kendro S, Cooper TB, Mathis CA, Laurelle M. Synapse. 2009;63:447. doi: 10.1002/syn.20628. [DOI] [PubMed] [Google Scholar]
- 27.Narendran R, Mason NS, May MA, Chen CM, Kendro S, Ridler K, Rabiner EA, Laurelle M, Mathis CA, Frankle WG. Synapse. 2011;65:35. doi: 10.1002/syn.20813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ryoo HL, Pierrotti D, Joyce JN. Mov. Disord. 1998;13:788. doi: 10.1002/mds.870130506. [DOI] [PubMed] [Google Scholar]
- 29.Levesque D, Martres MP, Diaz J, Griffon N, Lammers CH, Sokoloff P, Schwartz JC. Proc. Natl. Acad. Sci. U.S.A. 1995;92:1719. doi: 10.1073/pnas.92.5.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morissette M, Goulet M, Grondin R, Blanchet P, Bedard PJ, Di Paolo T, Levesque D. Eur. J. Neurosci. 1998;10:2565. doi: 10.1046/j.1460-9568.1998.00264.x. [DOI] [PubMed] [Google Scholar]
- 31.Volkow ND, Fowler JS, Wang GJ, Hitzemann R, Logan J, Schlyer DJ, Dewey SL, Wolf AP. Synapse. 1993;14:169. doi: 10.1002/syn.890140210. [DOI] [PubMed] [Google Scholar]
- 32.Volkow ND, Fowler JS, Wolf AP, Schlyer D, Shiue CY, Alpert R, Dewey SL, Logan J, Bendriem B, Christman D, Hitzeman R, Henn F. Am. J. Psychiatry. 1990;147:719. doi: 10.1176/ajp.147.6.719. [DOI] [PubMed] [Google Scholar]
- 33.Moore RJ, Vinsant SL, Nader MA, Porrino LJ, Friedman DP. Synapse. 1998;30:88. doi: 10.1002/(SICI)1098-2396(199809)30:1<88::AID-SYN11>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 34.Nader MA, Morgan D, Gage HD, Nader SH, Calhoun TL, Buchheimer N, Ehrenkaufer R, Mach RH. Nat. Neurosci. 2006;9:1050. doi: 10.1038/nn1737. [DOI] [PubMed] [Google Scholar]
- 35.Staley JK, Mash DC. J. Neurosci. 1996;16:6100. doi: 10.1523/JNEUROSCI.16-19-06100.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Volkow ND, Fowler JS, Wang GJ, Swanson JM. Mol. Psychiatry. 2004;9:557. doi: 10.1038/sj.mp.4001507. [DOI] [PubMed] [Google Scholar]
- 37.Mach RH, Tu Z, Xu J, Li S, Jones LA, Taylor M, Luedtke RR, Derdeyn CP, Perlmutter JS, Mintun MA. Synapse. doi: 10.1002/syn.20891. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tu Z, Li S, Xu J, Chu W, Jones LA, Luedtke RR, Mach RH. Nucl. Med. Biol. doi: 10.1016/j.nucmedbio.2011.01.002. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yokoi F, Grunder G, Biziere K, Stephane M, Dogan AS, Dannals RF, Ravert H, Suri A, Bramer S, Wong DF. Neuropsychopharmacology. 2002;27:248. doi: 10.1016/S0893-133X(02)00304-4. [DOI] [PubMed] [Google Scholar]
- 40.Cheng YC, Prusoff WH. Biochem. Pharmacol. 1973;22:3099. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- 41.Shimizu H, Daly JW, Creveling CR. J. Neurochem. 1969;16:1609. doi: 10.1111/j.1471-4159.1969.tb10360.x. [DOI] [PubMed] [Google Scholar]
- 42.Taylor M, Griffin SA, Grundt P, Newman AH, Luedtke RR. Synapse. 2010;64:251. doi: 10.1002/syn.20725. [DOI] [PMC free article] [PubMed] [Google Scholar]