Abstract
Objective
Somatotroph adenomas are typically recognized when they secrete GH excessively and cause acromegaly. Both ‘silent’ somatotroph adenomas (immunohistochemical evidence of GH excess without biochemical or clinical evidence) and ‘clinically silent’ somatotroph adenomas (immunohistochemical and biochemical evidence but no clinical evidence) have occasionally been reported. The relative frequency of each presentation is unknown. The goal of this study was, therefore, to determine the frequency of clinically silent somatotroph adenomas, a group that is potentially recognizable in vivo.
Design
We retrospectively identified 100 consecutive patients who had surgically excised and histologically confirmed pituitary adenomas.
Methods
Each pituitary adenoma was classified immunohistochemically by pituitary cell type. Somatotroph adenomas were further classified as ‘classic’ (obvious clinical features of acromegaly and elevated serum IGF1), ‘subtle’ (subtle clinical features of acromegaly and elevated IGF1), ‘clinically silent’ (no clinical features of acromegaly but elevated IGF1), and ‘silent’ (no clinical features of acromegaly and normal IGF1).
Results
Of the 100 consecutive pituitary adenomas, 29% were gonadotroph/glycoprotein, 24% somatotroph, 18% null cell, 15% corticotroph, 6% lactotroph, 2% thyrotroph, and 6% not classifiable. Of the 24 patients with somatotroph adenomas, classic accounted for 45.8%, subtle 16.7%, clinically silent 33.3%, and silent 4.2%.
Conclusions
Clinically silent somatotroph adenomas are more common than previously appreciated, representing one-third of all somatotroph adenomas. IGF1 should be measured in all patients with a sellar mass, because identification of a mass as a somatotroph adenoma expands the therapeutic options and provides a tumor marker to monitor treatment.
Introduction
Somatotroph adenomas (GH producing adenomas, somatotropinomas) are typically recognized when they secrete GH excessively and cause the clinical syndrome of acromegaly. This recognition not only identifies a sellar mass as a somatotroph adenoma but also expands the therapeutic options. Occasional reports in the literature also describe ‘silent somatotroph adenomas,’ referring to adenomas that can be identified as somatotroph adenomas by positive immunohistochemical staining for GH, but are not associated with clinical evidence of GH excess. Some of these adenomas are totally silent, in that they are not associated with either clinical manifestations of GH excess or elevated serum concentrations of GH or IGF1 (1–5). Others are ‘clinically silent’, in that GH and/or IGF1 serum concentrations are elevated, even though they are not associated with clinical manifestations of GH excess (5–14). The goal of this study was to determine the frequency of clinically silent somatotroph adenomas, a group that is potentially recognizable biochemically in vivo. To do so, we reviewed the records of 100 consecutive surgically excised pituitary adenomas. Of those identified immunohistologically as somatotroph adenomas, we reviewed the clinical records and categorized the patients according to a spectrum of GH expression as ‘classic’, ‘subtle’, ‘clinically silent’, or ‘silent’.
Subjects and methods
Patient identification
We retrospectively identified 100 consecutive patients who underwent resection of pituitary adenomas at the Hospital of the University of Pennsylvania between June 1, 2007 and November 2, 2009 by performing a query of the laboratory information system (Cerner Millenium, North Kansas, City, MO, USA). Patients were included if our review of the excised tissue confirmed the diagnosis of a pituitary adenoma. The Institutional Review Board of the University of Pennsylvania approved the study.
Tissue handling, histology and immunohistochemistry
Fresh tissue from each adenoma was fixed in 10% formalin for a period of 6–72 h. Routine processing and embedding into paraffin were performed according to standard protocols. Sections (4 μm thick) were stained with hematoxylin and eosin (H&E) or were used for immunohistochemical analysis. Each adenoma was immunohistochemically stained for six pituitary hormones: GH, prolactin, ACTH, FSH, LH, and TSH. The antibodies (Dako, Carpinteria, CA, USA) used were rabbit polyclonal anti-GH at 1:700 dilution (Dako, A0570), rabbit polyclonal anti-prolactin at 1:250 (Dako, A0569), mouse monoclonal anti-ACTH at 1:2000 (Dako, M3501, clone 02A3), mouse monoclonal anti-FSHβ subunit at 1:40 (Dako, M3504), mouse monoclonal anti-LHβ subunit at 1:300 (Dako, M3502, clone C93), and mouse monoclonal anti-TSHβ subunit at 1:400 (Dako, M3503, clone 0042). Staining was performed on a Bond Max Autostainer (Leica Microsystems, Buffalo Grove, IL, USA) after antigen retrieval. Hematoxylin (blue) counterstaining was performed to allow the visualization of cell nuclei. The substrate chromogen, 3′,3-diamobenzidine, was used to visualize the targeted complex via a brown precipitate.
All somatotroph adenomas were further analyzed by cytokeratin staining with mouse monoclonal anti-CAM5.2 undiluted (BD Pharmagen 349205, Franklin Lakes, NJ, USA), using the same immunohistochemical protocol.
Adenoma classification
A neuropathologist (J B) reviewed H&E and immunohistochemical stains and categorized the adenomas based on the type, intensity, and distribution of hormone expression. Adenomas that expressed GH were categorized as somatotrophs; prolactin as lactotrophs; ACTH as corticotrophs; FSH and/or LH as gonadotrophs; FSH, LH, and TSH as glycoprotein; TSH as thyrotrophs; and, if there was no staining, null cell. Staining was evaluated in a semi-quantitative manner, using three grades of positivity: strong, moderate, and weak. The distribution of staining was graded as diffuse (present throughout the adenoma), scattered (present in a small subset of adenoma cells, distributed throughout the adenoma), and focal (present in the majority of cells in one or more geographic areas of the adenoma). Adenomas that showed strong or moderate staining (diffuse, scattered or focal) for one hormone, but no staining for the other five hormones, were classified in the category associated with the expressed hormone (e.g. somatotroph for adenomas that showed positivity only for GH). Adenomas that showed not only strong staining for one hormone but also weak staining (diffuse or scattered) for one or more other hormones were classified by the dominant hormone. Glycoprotein adenomas (concurrent positivity for FSH, LH, and TSH), and in some cases gonadotroph adenomas (those showing positivity for both FSH and LH), were identified by the positive staining of the characteristic set of multiple hormones, and negative staining for the rest of the immunohistochemical panel. Adenomas that did not exhibit staining for any of the hormones, or only weak scattered staining, were classified as null cell adenomas. Lack of expression of all hormones was confirmed at the time of review by repeat immunohistochemical staining of the tissue. It was observed that two adenomas were almost entirely necrotic, precluding immunohistochemical categorization and one adenoma, which showed strong diffuse staining for ACTH and GH and moderate scattered staining of LH and TSH, was considered indeterminate by immunohistochemical classification. Adenomas that strongly expressed both GH and a second hormone were classified as somatotrophs for the purposes of this study.
The cytokeratin staining pattern of the somatotroph adenomas was evaluated in 300 cells of each somatotroph adenoma. The individual cells were classified as having a perinuclear pattern, dot pattern, or transitional pattern, as described in Obari et al. (15). The adenomas were then classified as densely granulated, sparsely granulated, and transitional based on the proportions of the three staining types. Adenomas were classified as densely granulated if >70% of cells exhibited a perinuclear pattern or <8% exhibited a dot pattern, irrespective of the percentage of cells that exhibited a transitional pattern. Adenomas were classified as sparsely granulated if >70% of cells exhibited a dot pattern, irrespective of the percentage of cells with a transitional pattern. Adenomas were classified as intermediate if they did not fit into either the densely or the sparsely granulated categories.
Clinical and biochemical characterization of patients with somatotroph adenomas
We reviewed the clinical records of patients with somatotroph adenomas and extracted demographic data, clinical characteristics, and serum IGF1 concentrations. We determined the presence of what we defined as ‘highly specific’ and ‘associated’ features of acromegaly. We defined highly specific features as those usually seen only in patients with acromegaly (growth of hands, feet, head or jaw in adulthood, frontal bossing, prognathism, large tongue, and wide hands and feet) and defined associated features as those seen in the general population but more commonly in acromegaly (carpal tunnel syndrome, obstructive sleep apnea, type 2 diabetes, hypertension, and colonic polyps). We then classified patients as having typical clinical features of acromegaly if they exhibited two or more highly specific features; mild features if they exhibited one highly specific feature or three associated features; and no features if they exhibited no highly specific features and not more than two associated features.
Finally, we defined the spectrum of GH expression of somatotroph adenomas by classifying each adenoma (Table 1) as ‘classic’ (typical clinical features of acromegaly and elevated serum IGF1), ‘subtle’ (mild clinical features of acromegaly and elevated IGF1), ‘clinically silent’ (no clinical features of acromegaly but elevated IGF1), or ‘silent’ (no clinical features of acromegaly and normal IGF1) based on the combination of clinical characteristics, serum IGF1 concentration, and immunohistochemical staining.
Table 1.
Spectrum of GH expression by somatotroph adenomas.
| Classification | Acromegalic features | Serum IGF1 | Immunohistochemical staining |
|---|---|---|---|
| Classic | Typical | Elevated | Positive |
| Subtle | Mild | Elevated | Positive |
| Clinically silent | None | Elevated | Positive |
| Silent | None | Normal | Positive |
Results
Classification of pituitary adenomas
Of the 100 consecutive pituitary adenomas, the cell type by immunohistochemistry was gonadotroph/glycoprotein in 29%, somatotroph in 24%, null cell in 18%, corticotroph in 15%, lactotroph in 6%, thyrotroph in 2%, and not classifiable in 6% (Table 2). The two adenomas that stained strongly for both GH and prolactin (somatomammotroph adenomas) and one adenoma that stained strongly for both GH and ACTH were classified as somatotroph adenomas for the purpose of this study.
Table 2.
Immunohistochemical classification of 100 consecutive surgically excised pituitary adenomas.
| Adenoma type | Number |
|---|---|
| Gonadotroph/glycoprotein | 29 |
| Somatotroph | 24 |
| Null cell | 18 |
| Corticotroph | 15 |
| Lactotroph | 6 |
| Thyrotroph | 2 |
| Not classifiable | 6 |
Classification of somatotroph adenomas
Of the 24 patients who had somatotroph adenomas, 11 (45.8%) were considered to have classic somatotroph adenomas, four (16.7%) subtle somatotroph adenomas, eight (33.3%) clinically silent somatotroph adenomas, and one (4.2%) a silent somatotroph adenoma (Table 3). The clinical assessment of each of the eight patients categorized as having a clinically silent somatotroph adenoma was made by an experienced endocrinologist. The mean serum IGF1 concentrations were 768 ng/ml in the classic, 533 ng/ml in the subtle, and 451 ng/ml in the clinically silent adenomas.
Table 3.
Frequencies of types of somatotroph adenomas.
| Somatotroph adenoma typea | n | % |
|---|---|---|
| Classic | 11 | 45.8 |
| Subtle | 4 | 16.7 |
| Clinically silent | 8 | 33.3 |
| Silent | 1 | 4.2 |
| Total | 24 | 100 |
Definitions of adenoma types given in text.
Two of the classic somatotroph adenomas co-secreted prolactin and one of the clinically silent somatotroph adenomas co-secreted ACTH.
Characteristics of patients with clinically silent somatotroph adenomas
The characteristics of the eight patients with clinically silent somatotroph adenomas are shown in Table 4. There was no clear predilection for gender or age. Of the eight patients, four had no associated clinical features of acromegaly, two had only hypertension, and two had hypertension and another associated feature. All patients, by definition, had serum IGF1 concentrations above the age- and gender-adjusted normal range; three had concentrations more than twice the upper limit of normal. Duplicate determinations of IGF1 were preoperatively available for three patients and in each confirmed the elevation. Serum IGF1 values were postoperatively available for seven patients and had decreased to normal in five patients; one patient whose value was elevated postoperatively demonstrated a decrease in IGF1 after administration of lanreotide. Each of the eight patients had either confirmation of the elevated IGF1 value preoperatively and/or a decrease in IGF1 to normal in response to surgery or lanreotide. All patients had macroadenomas. Of the eight patients, two underwent surgery because of neurological symptoms, two because of an enlarging mass by serial imaging, and four because of the patients’ anxiety about the presence of a sellar mass in the absence of symptoms.
Table 4.
Characteristics of patients with clinically silent somatotroph adenomas.
| Serum IGF1 | |||||||
|---|---|---|---|---|---|---|---|
| Patient no. | Age (years) | Gender | Associated features | Preoperative (ng/ml) | Postoperative (mm) | Normal rangea | Dimensions adenomab |
| 17 | 53 | F | HTN | 470c | 130c | 92–190 | 10×10×9 |
| 40 | 25 | F | None | 442d, 464d | 205d | 89–397 | 34×25×17 |
| 45 | 55 | M | HTN | 264c, 309c | – | 87–225 | ‘Macroadenoma’ |
| 48 | 77 | M | HTN, T2DM | 362d | 66d | 35–213 | 28×24×24 |
| 52 | 35 | F | None | 371c | 230c | 126–291 | 10×9×12 |
| 54 | 22 | M | None | 812c, 835c | 735c | 126–382 | 15×25×21 |
| 62 | 37 | F | None | – | 341c, 326c,e, 194c,f | 126–291 | 22×28×20 |
| 70 | 74 | F | HTN, polyps | 487c | 246c | 25–171 | 18×12×17 |
HTN, hypertension and T2DM, type 2 diabetes mellitus.
Normal ranges for serum IGF1 concentration are specific for age and gender.
Dimensions: cephalocaudad×transverse×anterio–posterior.
Performed at quest diagnostics laboratories.
Performed at ARUP laboratories.
Postoperative.
IFG-1 value post-administration of lanreotide.
Immunohistochemical staining of clinically silent somatotroph adenomas
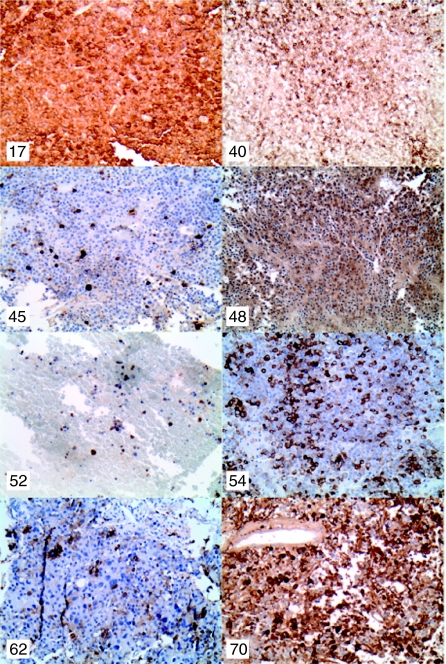
Figure 1 shows representative sections of the eight clinically silent somatotroph adenomas and illustrates that all express GH strongly, some diffusely, and others scattered. Adenomas from patients 17, 40, 48, and 70 show a strong, diffuse staining pattern, and adenomas from patients 45, 54, and 62 show a strong scattered pattern. The tissue available for histological analysis from patient 52 consisted primarily of blood, but the limited number of viable adenoma cells shows strong GH staining in the majority. The range of the strength and patterns of GH staining in the adenomas from patients who were classified as clinically silent was not obviously different from those of the patients classified as classic.
Figure 1.
Representative images of immunohistochemical staining for GH of adenomas from eight patients with clinically silent somatotroph adenomas (200× magnification). All show strong staining, some diffuse, and others scattered. Subject numbers in the upper left corners correspond to those in Table 4.
In addition, the cytokeratin staining pattern of the clinically silent adenomas, as an indicator of subtype, was not obviously different from those of the other subtypes. Specifically, three classic somatotroph adenomas were classified as densely granulated, three as sparsely granulated, four as intermediate type, and one had insufficient remaining tissue to stain. Of the subtle somatotroph adenomas, none was densely or sparsely granulated, three were intermediate, and one had insufficient tissue to stain. Of the clinically silent somatotroph adenomas, one was densely granulated, three were sparsely granulated, two were intermediate, and two had too little tissue to stain. The single silent adenoma was intermediately granulated.
Discussion
Of the 100 consecutive pituitary adenomas at a single institution that were excised surgically and confirmed histologically, 24 were identified immunohistochemically as somatotroph adenomas. Review of the clinical and biochemical characteristics of these 24 somatotroph adenomas resulted in characterizing 45.8% as presenting with classic acromegaly, 16.7% as having subtle acromegaly, 33.3% as clinically silent, and 4.2% as totally silent. The surprising finding was the substantial proportion – one-third – that presented as ‘clinically silent,’ in that the patients harboring those adenomas could not be recognized as even subtly acromegalic by experienced endocrinologists.
Somatotroph adenomas in which there is biochemical and immunohistochemical evidence of GH excess in the absence of clinical manifestations of acromegaly were first reported in 1985 (13). It was observed that two women who had no clinical features of acromegaly but who did have non-suppressible serum GH concentrations had pituitary adenomas that, after excision, showed immunohistochemical staining for GH. The term ‘clinically silent somatotroph adenoma’ was first used 2 years later in describing three women with non-suppressible serum GH concentrations and positive GH staining of the excised adenoma tissue (7). The largest previous series of clinically silent somatotroph adenomas described a total of 17 patients who had silent somatotroph adenomas, of which four were clinically silent (5). These four patients had no clinical manifestations of acromegaly but did have elevated basal serum GH concentrations that did not suppress after ingestion of glucose. These four patients represented only 1.6% of the total number of 251 somatotroph adenomas in that series. The reason for the much lower frequency in that study compared with this study is not clear, although it might be the result of different criteria for determining GH excess.
The explanation for the lack of clinical manifestations of some somatotroph adenomas compared with the obvious manifestations in others, even though both exhibit elevated GH and/or IGF1 concentrations, is not certain. Each patient with a clinically silent somatotroph adenoma was examined by an experienced endocrinologist, reducing the likelihood that clinical features were present, but undetected, although even the most experienced endocrinologist may miss subtle features of acromegaly. Another possible explanation is a lesser duration of excessive GH secretion, but some reports describe such patients in whom excessive GH secretion has been documented for several years (9, 10). The possible explanations that inefficient GH secretion or secretion of biologically inactive GH explain lack of clinical manifestations are refuted by finding clear elevation of serum IGF1 in all of the patients presented here. The most likely explanation of the differential GH expression is differential GH secretion, as judged by differential IGF1 concentrations. Although three of the eight patients with clinically silent adenomas reported here had serum IGF1 concentrations more than twice the upper limit of normal, the mean IGF1 concentrations were higher in those patients categorized as classic and subtle than in those classified as clinically silent.
Because previous studies have reported possible associations between sparsely and densely granulated somatotroph adenomas and various biological features, such as size, aggressiveness, and propensity to respond to a somatostatin analog (15, 16), we assessed all of the somatotroph adenomas for this characteristic indirectly by the pattern of cytokeratin immunostaining. We found no obvious differences between the cytokeratin staining pattern of clinically silent adenomas and those of the other clinical subtypes, although the number of somatotroph adenomas was relatively small for subgroup analysis.
This study ascertains systematically the frequency of clinically silent somatotroph adenomas among all somatotroph adenomas and among consecutive pituitary adenomas that were histologically and immunohistochemically characterized. A limitation of the findings is that the entire population of patients was restricted to those who had surgery, a necessity to confirm the diagnosis of a somatotroph adenoma immunohistochemically. Another limitation is that the clinical assignments were retrospectively made, although this limitation is mitigated by specific documentation by experienced endocrinologists of lack of acromegalic features in each of the eight patients with clinically silent adenomas.
Although GH suppressibility was not tested, IGF1 elevation is rarely seen in the presence of normal GH suppressibility (17). Importantly, five of the seven patients had a decrease in IGF1 to normal after resection of their somatotroph adenomas and one patient who had an elevated value postoperatively exhibited a decrease to normal in response to lanreotide. All eight patients had either a confirmation of their elevated IGF1 concentration preoperatively and/or a decrease in IGF1 in response to surgery or lanreotide, strongly suggesting that each adenoma was the cause of that patient’s elevated IGF1. Similar analyses should be performed in other surgical series, however, to determine whether the current results are confirmed.
The clinical significance of finding that a third of all somatotroph adenomas are clinically nonfunctioning is that in this substantial fraction of patients, an otherwise unidentifiable sellar mass can be recognized as a somatotroph adenoma merely by measurement of the serum concentration of IGF1. This recognition expands the therapeutic options to include pharmacological treatment and also provides a tumor marker to follow to monitor the efficacy of treatment. This study supports the recommendation to measure the serum concentration of IGF1 in all patients who have a sellar mass.
Declaration of interest
P J Snyder receives research support from Novartis and Tercica. The other authors have no conflicts of interest to disclose.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Acknowledgements
We thank Drs John Lee and Eric Zager for allowing us to review the records of their patients.
Footnotes
(A N Wade is now at Division of Endocrinology and Metabolism, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa)
(J Baccon is now at Department of Pathology, Penn State Milton S. Hershey Medical Center, Hershey, Pennsylvania, USA)
References
- Abe T, Taniyama M, Xu B, Ozawa H, Kawamura N, Shimazu M, Sasaki K, Izumiyama H, Kushima M, Kuwazawa J, Sano T, Matsumoto K. Silent mixed corticotroph and somatotroph macroadenomas presenting with pituitary apoplexy. Acta Neuropathologica. 2001;102:435–440. doi: 10.1007/s004010100396. [DOI] [PubMed] [Google Scholar]
- Kovacs K, Lloyd R, Horvath E, Asa SL, Stefaneanu L, Killinger DW, Smyth HS. Silent somatotroph adenomas of the human pituitary. A morphologic study of three cases including immunocytochemistry, electron microscopy, in vitro examination, and in situ hybridization. American Journal of Pathology. 1989;134:345–353. [PMC free article] [PubMed] [Google Scholar]
- Mohammed S, Syro L, Abad V, Salehi F, Horvath E, Scheithauer BW, Kovacs K, Cusimano M. Silent somatotroph adenoma of the pituitary in an adolescent. Canadian Journal of Neurological Sciences. 2009;36:123–125. doi: 10.1017/s0317167100006466. [DOI] [PubMed] [Google Scholar]
- Naritaka H, Kameya T, Sato Y, Furuhata S, Otani M, Kawase T. Morphological characterization and subtyping of silent somatotroph adenomas. Pituitary. 1999;1:233–241. doi: 10.1023/A:1009942122673. [DOI] [PubMed] [Google Scholar]
- Trouillas J, Sassolas G, Loras B, Velkeniers B, Raccurt M, Chotard L, Berthezene F, Tourniaire J, Girod C. Somatotropic adenomas without acromegaly. Pathology, Research and Practice. 1991;187:943–949. doi: 10.1016/s0344-0338(11)81065-4. [DOI] [PubMed] [Google Scholar]
- Kalavalapalli S, Reid H, Kane J, Buckler H, Trainer P, Heald AH. Silent growth hormone secreting pituitary adenomas: IGF-1 is not sufficient to exclude growth hormone excess. Annals of Clinical Biochemistry. 2007;44:89–93. doi: 10.1258/000456307779596075. [DOI] [PubMed] [Google Scholar]
- Klibanski A, Zervas NT, Kovacs K, Ridgway EC. Clinically silent hypersecretion of growth hormone in patients with pituitary tumors. Journal of Neurosurgery. 1987;66:806–811. doi: 10.3171/jns.1987.66.6.0806. [DOI] [PubMed] [Google Scholar]
- Matsuno A, Ogino Y, Katayama H, Osamura RY, Nagashima T. Identification of a silent pituitary somatotropic adenoma based on a paradoxic response of growth hormone on a thyrotropin-releasing hormone or gonadotropin-releasing hormone provocation test. American Journal of Obstetrics and Gynaecology. 2001;184:286–288. doi: 10.1067/mob.2001.109396. [DOI] [PubMed] [Google Scholar]
- Pestell R, Herington A, Best J, Boolell M, McKelvie P, Arnott R, Alford F. Growth hormone excess and galactorrhoea without acromegalic features. Case reports. British Journal of Obstetrics and Gynaecology. 1991;98:92–97. doi: 10.1111/j.1471-0528.1991.tb10317.x. [DOI] [PubMed] [Google Scholar]
- Sakharova AA, Dimaraki EV, Chandler WF, Barkan AL. Clinically silent somatotropinomas may be biochemically active. Journal of Clinical Endocrinology and Metabolism. 2005;90:2117–2121. doi: 10.1210/jc.2004-0875. [DOI] [PubMed] [Google Scholar]
- Sen O, Ertorer ME, Aydin MV, Erdogan B, Altinors N, Zorludemir S, Guvener N. Silent pituitary macroadenoma co-secreting growth hormone and thyroid stimulating hormone. Journal of Clinical Neuroscience. 2005;12:318–320. doi: 10.1016/j.jocn.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Sidhaye A, Burger P, Rigamonti D, Salvatori R. Giant somatotrophinoma without acromegalic features: more “quiet” than “silent”: case report. Neurosurgery. 2005;56:E1154. doi: 10.1227/01.NEU.0000157961.67867.E5. [DOI] [PubMed] [Google Scholar]
- Tourniaire J, Trouillas J, Chalendar D, Bonneton-Emptoz A, Goutelle A, Girod C. Somatotropic adenoma manifested by galactorrhea without acromegaly. Journal of Clinical Endocrinology and Metabolism. 1985;61:451–453. doi: 10.1210/jcem-61-3-451. [DOI] [PubMed] [Google Scholar]
- Yamada S, Sano T, Stefaneanu L, Kovacs K, Aiba T, Sawano S, Shishiba Y. Endocrine and morphological study of a clinically silent somatotroph adenoma of the human pituitary. Journal of Clinical Endocrinology and Metabolism. 1993;76:352–356. doi: 10.1210/jc.76.2.352. [DOI] [PubMed] [Google Scholar]
- Obari A, Sano T, Ohyama K, Kudo E, Qian ZR, Yoneda A, Rayhan N, Mustafizur Rahman M, Yamada S. Clinicopathological features of growth hormone-producing pituitary adenomas: difference among various types defined by cytokeratin distribution pattern including a transitional form. Endocrine Pathology. 2008;19:82–91. doi: 10.1007/s12022-008-9029-z. [DOI] [PubMed] [Google Scholar]
- Bhayana S, Booth GL, Asa SL, Kovacs K, Ezzat S. The implication of somatotroph adenoma phenotype to somatostatin analog responsiveness in acromegaly. Journal of Clinical Endocrinology and Metabolism. 2005;90:6290–6295. doi: 10.1210/jc.2005-0998. [DOI] [PubMed] [Google Scholar]
- Freda PU. Monitoring of acromegaly: what should be performed when GH and IGF-1 levels are discrepant? Clinical Endocrinology. 2009;71:166–170. doi: 10.1111/j.1365-2265.2009.03556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]