Abstract
Phosphate-solubilizing bacteria (PSB) function in soil phosphorus cycle, increasing the bioavailability of soil phosphorus for plants. Isolation and application of salt-tolerant or halophilic PSB will facilitate the development of saline-alkali soil-based agriculture. A moderately halophilic bacterium was isolated from the sediment of Daqiao saltern on the eastern coast of China, which also performs phosphate-solubilizing ability. The bacterium was assigned to genus Kushneria according to its 16S rRNA gene sequence, and accordingly named as Kushneria sp. YCWA18. The fastest growth was observed when the culturing temperature was 28°C and the concentration of NaCl was 6% (w/v). It was founds that the bacterium can survive at a concentration of NaCl up to 20%. At the optimum condition, the bacterium solubilized 283.16 μg/mL phosphorus in 11 days after being inoculated in 200 mL Ca3(PO4)2 containing liquid medium, and 47.52 μg/mL phosphorus in 8 days after being inoculated in 200 mL lecithin-containing liquid medium. The growth of the bacterium was concomitant with a significant decrease of acidity of the medium.
1. Introduction
Phosphorus (P) is one of the major essential macronutrients for plants, which is applied to the soil in the form of phosphatic manure. However, a large portion of the applied phosphorus is rapidly immobilized, being unavailable to plants [1]. In average, the content of phosphorus of soil is about 0.05% (w/w); however, only 0.1% of them are usable for plants [2]. Saline-alkali soil-based agriculture develops quickly in recent years. Similar to the fertile soil-based agriculture, the intensive culturing of salt-tolerant and even salt-resistant plants has dramatically decreased the availability of phosphorus in saline-alkali soil. The free phosphatic ion in soil plays a crucial role; the orthophosphatic ion is the only ion which can be assimilated in an appreciable amount by plants [3]. Soil microorganisms involve in a wide range of biological processes including the transformation of soil phosphorus. They solubilize soil phosphorus for the growth of plants [4].
The growth of phosphate-solubilizing bacteria (PSB) often causes soil acidification, playing a key role in phosphorus solubilization [5]. Therefore, PSB are considered the important solubilizers of insoluble inorganic phosphate. In turn, plants reimburse PSB with carbohydrates [6]. Since the beginning of last century, many PSB have been isolated including, for example, those in Bacillus, Pseudomonas, Erwinia, Agrobacterium, Serratia, Flavobacterium, Enterobacter, Micrococcus, Azotobacter, Bradyrhizobium, Salmonella, Alcaligenes, Chromobacterium, Arthrobacter, Streptomyces, Thiobacillus, and Escherichia [7]. The microorganisms functioning similarly also include some fungi in genus Penicillium, Aspergillus, Rhizopus, Fusarium, and Sclerotium [7]. Unfortunately, most PSB isolated previously performed relatively low salinity tolerance, being less appropriate for saline-alkali soil-based agriculture. It is urgently needed to isolate highly halophilic PSB for the development of saline-alkali soil-based agriculture. In this study, a moderately halophilic, phosphate-solubilizing bacterium YCWA18 was isolated and characterized.
2. Materials and Methods
2.1. Bacterial Isolation
The sediment was sampled at Daqiao saltern, Jimo, Qingdao, on the eastern coast of China (E 120°49′12′′, N 36°30′00′′). Approximately 1 g sediment was suspended in 100 mL sterilized seawater and vortexed for 10 min. The isolates were obtained by plating a serial of 10-fold dilutions of sediment suspension onto a modified marine agar medium (2216E, one liter of seawater contains 5 g tryptone, 1 g yeast extract and 15 g agar, pH 7.5) [8] and incubating at 28°C for 7 days. The isolates were purified by restreaking on 2216E agar plate and microscopic confirmation.
The isolates were screened for their phosphorus-solubilizing ability by culturing at 28°C on the media supplemented either lecithin or Ca3(PO4)2. When the colonies appeared in one week, those causing a clear phosphate-solubilizing zone were selected out for further characterization. The size of phosphate-solubilizing zone was determined for each colony.
The modified Ca3(PO4)2 culture medium contained with the following ingredients (l−1) [9]: glucose 10 g, (NH4)2SO4 0.5 g, NaCl 30 g, KCl 0.3 g, FeSO4·7H2O 0.03 g, MnSO4·4H2O 0.03 g, MgSO4·7H2O 0.3 g, Ca3(PO4)2 10 g, agar 20 g, H20 1000 mL, pH 7.0–7.5. The lecithin culture medium was composed of (l−1) [10]: glucose 10 g, (NH4)2SO4 0.5 g, NaCl 30 g, KCl 0.3 g, FeSO4·7H2O 0.03 g, MnSO4·4H2O 0.03 g, MgSO4·7H2O 0.3 g, lecithin 0.2 g, CaCO3 5 g, yeast extract 0.4 g, agar 20 g, H20 1000 mL, pH 7.0–7.5.
The ingredients were prepared and sterilized by an autoclave for 20 min at 115°C without lecithin. Lecithin was prepared by diluting in sterile water and was added to the medium before inoculation.
Bacterial isolate was freeze-stored in 2216E medium supplemented with 30% (v/v) glycerol at −80°C.
2.2. Bacterial Characterization
The isolate was phenotypically characterized following the minimal standards for describing the new taxa of family Halomonadaceae recommended previously [11]. Gram-staining was carried out with the method described by Dussault [12]. Anaerobic growth performance was determined by inoculating the semisolid 2216E (0.6% agar, w/v) at the bottom of tube and sealing with 2 mL agar (2%, w/v) and 2 mL paraffin. Acidification in liquid medium was observed using bromocresol purple supplemented with 1% of carbohydrate. Other morphological, physiological, and biochemical characterizations were done as described by Mata et al. [13].
2.3. Tests of Salt, pH, and Temperature Tolerance
NaCl tolerance was determined in 2216E medium containing 0, 0.5, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 12.5, 15, 20, 25, and 28% (w/v) total salts. The pH range for growth was tested at pH 2.0–11.0 in increments of pH 1.0. Growth was determined at A600. Growth at 0, 6, 10, 15, 20, 24, 28, 32, 37, 42, and 45°C was also determined.
2.4. Taxonomical Assignment
DNA was extracted with the method of Hiraishi [14] and used as the template for the amplification of 16S rRNA gene with universal primers 27F and 1492R [15]. The sequence obtained was aligned with its orthologs retrieved from both GenBank and TYP16S databases. The phylogenetic assignment was performed using MEGA software with bootstrap percentages calculated with 1000 replications.
2.5. Determination of Phosphorus-Solubilizing Ability
The bacterium was inoculated into 200 mL liquid media supplemented with either Ca3(PO4)2 or lecithin and cultured at 28°C for 12 days with continuous agitation (150 r/min). 10 ml culture was sampled aseptically every 24 hours for the determination of acidity and available phosphorus. The acidity was assayed simply by reading on a pH meter, and the phosphorus availability was determined with Mo-blue method [16]. Optimum pH and temperature for P-solubilization in liquid Ca3(PO4)2 medium were determined following the above method.
3. Results
3.1. Characterization of the Isolate
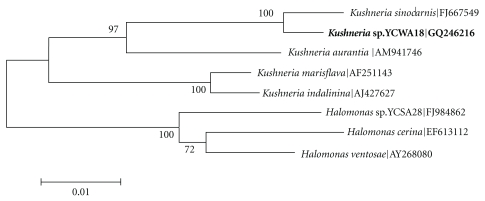
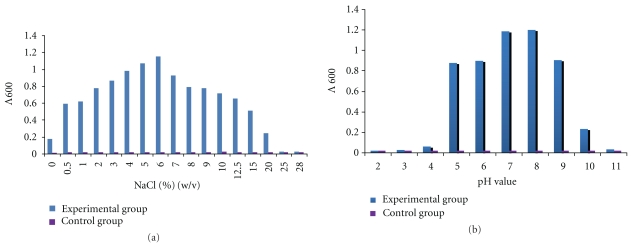
The closest species of the isolate was Kushneria sinocarnis; the similarity between their 16S rRNA genes is 98.57%. As shown in Figure 1, the isolate was assigned to the clade of K. sinocarnis with 100% bootstrap support. The isolate was named Kushneria sp. YCWA18. It is a Gram-negative bacterium and grows aerobically. Its colony is yellow and round with a diameter between 1.2–2.5 mm after growing on 2216E for 48 hours. The fastest growth is observed at 6% NaCl (w/v), 28°C and pH 7.0–8.0 (Figure 2). The isolate can survive at high concentrations of NaCl (up to 20%) and pH range of 4.0–10.0 (Figure 2). It reduces nitrate and hydrolyzes aesculin but not urea, Tween20 and Tween80. The isolate produces acid from carbohydrates including D-fructose, α-D-glucose, D-mannose, maltose, D-sorbitol, succinamic acid, adonitol and L-alanyl-glycine but not D-psicose, D- glucosaminic acid, and inosine.
Figure 1.
Neighbor-joining phylogenetic tree based on 16S rRNA gene sequences, showing the position of strain YCWA18 with respect to related species.
Figure 2.
Test of NaCl and pH tolerance of the isolate ((a): NaCl; (b): pH).
3.2. Growth on Ca3(PO4)2 and Lecithin Containing Solid Media
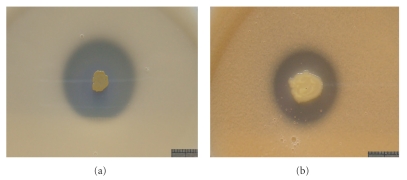
The isolate grows well at 28°C on lecithin and Ca3(PO4)2 containing solid media. In 3 days (lecithin containing medium) or 5 days (Ca3(PO4)2 containing medium), clear phosphate-solubilizing zone forms. In 10 days, the phosphate-solubilizing zone expanded to the biggest (about 2.5–3.0 cm on Ca3(PO4)2 containing plate, Figure 3(a) and about 1.7–2.0 cm on lecithin containing medium, Figure 3(b)).
Figure 3.
The phosphate-solubilizing zone formed on medium containing Ca3(PO4)2 (a) and lecithin (b). Bar: 1 cm.
3.3. Growth in Ca3(PO4)2 and Lecithin-Containing Liquid Media
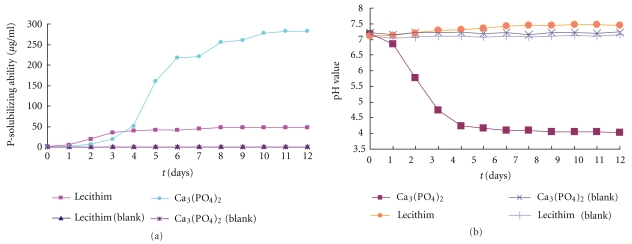
As showed in Figure 4, Ca3(PO4)2 solubilization is slow in the first two days and then becomes fast, reaching the highest (283.16 μg/mL) in about 11 days. Lecithin solubilization starts to increases in 1 day, reaching the highest 47.52 μg/mL in 8 days. It was found that the growth of the isolate caused a significant increase of acidity in Ca3(PO4)2 containing medium. In about 4 days, the acidity increased from pH 7.21 to pH 4.24. In contrast, the acidity decreased from pH 7.1 to pH 7.46 in lecithin-containing medium.
Figure 4.
Phosphorus-solubilizing performance of the isolate ((a) P-solubilizing ability; (b) pH value of the medium).
3.4. The Influence of Temperature and Acidity on Phosphorus Solubilization in Ca3(PO4)2-Containing Medium
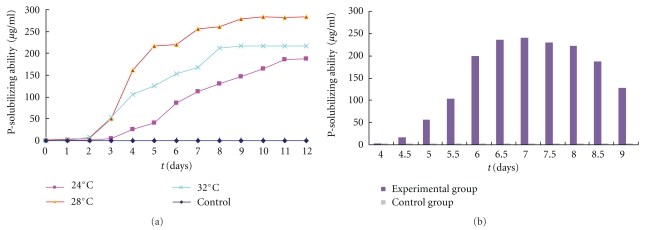
As showed in Figure 5, the concentration of soluble phosphorus in Ca3(PO4)2 containing medium starts to increases in 2 days when the isolate was cultured at 28°C and 32°C, reaching the highest in 7-8 days, faster than the increment achieved at 24°C. At 28°C, the bacterium obtained the highest solubilizing ability of Ca3(PO4)2, about 283.16 μg/mL in 200 mL Ca3(PO4)2 containing medium at 28°C, while at 32°C and 24°C, it obtained the ability of 217.58 μg/mL, and 187 μg/mL, respectively, and it was found that phosphorus solubilization reached the maximum when the pH value of Ca3(PO4)2 containing medium is 7.0.
Figure 5.
The influence of temperature (a) and acidity (b) on the phosphorus solubilization in Ca3(PO4)2 containing medium.
4. Discussions
Phosphorus is an important limiting factor in agriculture production, and microbial activation seems to be an effective way to solve the solidified phosphorus in soil. Many bacterial strains with P-solubilizing abilities have been examined in previous studies, but few of them can function well at high NaCl concentration, and reports on isolation of halophilic PSB strain have not been found yet. The result obtained in this study shows that the isolate YCWA18 has a broad growth range: it can survive at high concentrations of NaCl (up to 20%) and pH range of 4.0–10.0, and it can solubilize both inorganic phosphorus and organophosphorus, and result also shows that its P-solubility for Ca3(PO4)2 is higher than for lecithin (Figure 3).
The formation of the clear zones is concerned with the P-solubilization of the strain (Figure 3). It may secrete some substances into surroundings in the course of growing, which can solubilize phosphate or organophosphate. P-solubilization result may vary depending on kinds of the metabolin, how quickly it releases, and also its spread degree on the medium. Therefore, observational method of P-solubilizing zone can only be used to qualitative assays [17].
Phosphorus solubilization is a complex process, which is influenced by diverse factors such as nutritional richness and physiological and growing status of the bacterium [18]. A number of theories have been proposed to explain the mechanism of phosphate solubilization, and the most important among them are acid production theory and enzyme theory. According to the acid production, the process of phosphate solubilization by PSB is due to the production of low molecular weight organic acids that was accompanied by the acidification of the medium [6, 19, 20], and those organic acids can chelate the cation with their hydroxyl and carboxyl groups [21]. The analysis of culture filtrates of PSBs has shown the presence of number of organic acids such as malic, glyoxalic, succinic, fumaric, tartaric, alpha keto butyric, oxalic, citric, 2-ketogluconic, and gluconic acid [22–24]. A decrease in the pH of the medium from the initial value of 7.0 to a final value of 2.0 was recorded by many workers [25, 26], and in our study, the decrease of the pH of Ca3(PO4)2-containing medium from pH 7.21 to pH 4.24 also supported this theory.
Enzymolysis is the possible mechanism of lecithin solubilization [27]. The acidity of lecithin-containing medium during culturing is a little higher than that of the control (Figure 4(b)). We believe that phosphorus solubilization from lecithin is not through acidification. It is possible that the increase of lecithin-containing medium during culturing is caused by enzymes which act on lecithin and produce choline.
The association among bacterial growth, supernatant acidity, and the concentration of solubilized phosphorus has been found [28]. As a well-developed strategy, microorganisms have been recruited to transform insoluble phosphorus of mineral sources into the soluble one [29]. As a particular case, Gram-negative bacteria mobilize insoluble phosphate very efficiently; they produce gluconic acid during the extracellular oxidation of glucose catalyzed by quinoprotein glucose dehydrogenase [30]. Microbial phosphorus mobilization should be the only way of increasing plant-available phosphorus in soil [31].
Diverse microorganisms are able to change phosphorus forms; however, their transforming ability may associate with the ecological conditions including soil characteristics and vegetation. It has been found that the performance of phosphorus-solubilizing microorganisms is severely affected by environmental factor, especially stress ones [32, 33]. In order to obtain the bacteria with high phosphorus-solubilizing ability, it is important to use samples of environmental extremes, for example, saline-alkali soil. In this work, a high phosphorus-solubilizing bacterium was isolated from the sediment if a saltern. It can grow on the solid media containing 20% (w/v) of sodium chloride, being halotolerant according to the suggested standards [34]. The biological characteristics and factors influencing its phosphate-solubilizing ability need to be studied further. Our trials paved an effective avenue of isolating halophilic and phosphorus-solubilizing bacteria from marine environment in order to promote saline-alkali soil-based agriculture.
Acknowledgment
This work was supported financially by National Natural Science Foundation of China (41006103), Public science and technology research funds projects of ocean (201105007-01), the National Infrastructure of Natural Resources for Science and Technology Program of China (2004DKA30640), and the Scientific Research Foundation of the First Institute of Oceanography, SOA (2011G24).
References
- 1.Goldstein AH. Bacterial solubilization of mineral phosphates: historical perspectives and future prospects. American Journal of Alternative Agriculture. 1986;1:57–65. [Google Scholar]
- 2.Scheffer F, Schachtschabel P. Lehrbuch der Bodenkunde. Stuttgart, Germany: Ferdinand Enke Verlag; 1992. [Google Scholar]
- 3.Beever RE, Burns DJW. Phosphorus uptake, storage and utilization by fungi. Advances in Botanical Research. 1980;8:121–219. [Google Scholar]
- 4.Rodríguez H, Fraga R. Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnology Advances. 1999;17(4–5):319–359. doi: 10.1016/s0734-9750(99)00014-2. [DOI] [PubMed] [Google Scholar]
- 5.Abd-Alla MH. Phosphatases and the utilization of organic phosphorus by Rhizobium leguminosarum biovar viceae. Letters in Applied Microbiology. 1994;18(5):294–296. [Google Scholar]
- 6.Goldstein AH. Recent progress in understanding the molecular genetics and biochemistry of calcium phosphate solubilization by Gram negative bacteria. Biological Agriculture and Horticulture. 1995;12(2):185–193. [Google Scholar]
- 7.Zhao XR, Lin QM. A review of phosphate—dissolving microorganisms. Soil Fertilizer. 2001;3:7–11. [Google Scholar]
- 8.Hou YH, Wang QF, Shen JH, Miao JL, Li GY. Molecular identification of a halotolerant bacterium NJ82 from Antarctic Sea ice and preliminary study on its salt tolerance. Microbiology. 2008;35(4):486–490. [Google Scholar]
- 9.Nautiyal CS. An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiology Letters. 1999;170(1):265–270. doi: 10.1111/j.1574-6968.1999.tb13383.x. [DOI] [PubMed] [Google Scholar]
- 10.Niewolak S. Occurrence of microorganisms in fertilized lakes. II. Lecithin- mineralizing microorganisms. Polskie Archiwum Hydrobiologii. 1980;27:53–71. [Google Scholar]
- 11.Arahal DR, Vreeland RH, Litchfield CD, et al. Recommended minimal standards for describing new taxa of the family Halomonadaceae. International Journal of Systematic and Evolutionary Microbiology. 2007;57(10):2436–2446. doi: 10.1099/ijs.0.65430-0. [DOI] [PubMed] [Google Scholar]
- 12.Dussault HP. An improved technique for staining red halophilic bacteria. Journal of bacteriology. 1955;70(4):484–485. doi: 10.1128/jb.70.4.484-485.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mata JA, Martínez-Cánovas J, Quesada E, Béjar V. A detailed phenotypic characterisation of the type strains of Halomonas species. Systematic and Applied Microbiology. 2002;25(3):360–375. doi: 10.1078/0723-2020-00122. [DOI] [PubMed] [Google Scholar]
- 14.Hiraishi A. Direct automated sequencing of 16S rDNA amplified by polymerase chain reaction from bacterial cultures without DNA purification. Letters in Applied Microbiology. 1992;15(5):210–213. doi: 10.1111/j.1472-765x.1992.tb00765.x. [DOI] [PubMed] [Google Scholar]
- 15.Lane DJ. Nucleic Acid Techniques in Bacterial Systematics. New York, NY, USA: John Wiley and Sons; 1991. 16S/23S rRNA sequencing; pp. 115–175. [Google Scholar]
- 16.Watanabe FS, Olsen SR. Test of an ascorbic acid method for determining phosphorous in water and NaHCO3 extracts from soil. Soil Science Society of America Proceedings. 1965;29:677–678. [Google Scholar]
- 17.Piccini D, Azcon R. Effect of phosphate-solubilizing bacteria and vesicular-arbuscular mycorrhizal fungi on the utilization of Bayovar rock phosphate by alfalfa plants using a sand-vermiculite medium. Plant and Soil. 1987;101(1):45–50. [Google Scholar]
- 18.Reyes I, Bernier L, Simard RR, Antoun H. Effect of nitrogen source on the solubilization of different inorganic phosphates by an isolate of Penicillium rugulosum and two UV-induced mutants. FEMS Microbiology Ecology. 1999;28(3):281–290. [Google Scholar]
- 19.Puente ME, Bashan Y, Li CY, Lebsky VK. Microbial populations and activities in the rhizoplane of rock-weathering desert plants. I. Root colonization and weathering of igneous rocks. Plant Biology. 2004;6(5):629–642. doi: 10.1055/s-2004-821100. [DOI] [PubMed] [Google Scholar]
- 20.Kim KY, Jordan D, Krishnan HB. Rahnella aquatilis, a bacterium isolated from soybean rhizosphere, can solubilize hydroxyapatite. FEMS Microbiology Letters. 1997;153(2):273–277. [Google Scholar]
- 21.Kpomblekou K, Tabatabai MA. Effect of organic acids on release of phosphorus from phosphate rocks. Soil Science. 1994;158(6):442–453. [Google Scholar]
- 22.Lapeyrie F, Ranger J, Vairelles D. Phosphate-solubilizing activity of ectomycorrhizal fungi in vitro. Canadian Journal of Botany. 1991;69(2):342–346. [Google Scholar]
- 23.Cuningham JE, Kuiak C. Production of citric and oxalic acid and solubilization of calcium phosphate by Penicillium billai . Applied and Environmental Microbiology. 1992;58:1451–1458. doi: 10.1128/aem.58.5.1451-1458.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fasim F, Ahmed N, Parsons R, Gadd GM. Solubilization of zinc salts by a bacterium isolated from the air environment of a tannery. FEMS Microbiology Letters. 2002;213(1):1–6. doi: 10.1111/j.1574-6968.2002.tb11277.x. [DOI] [PubMed] [Google Scholar]
- 25.Gaur AC, Sachar S. Effect of rock phosphate and glucose concentration on phosphate solubilization by Aspergillus awamori . Current Science. 1980;49:553–554. [Google Scholar]
- 26.Illmer P, Schineer F. Solubilization of insoluble phosphates by micro- organisms isolated from forest soils. Soil Biology and Biochemistry. 1992;24:389–395. [Google Scholar]
- 27.Karl DM. Cellular nucleotide measurements and applications in microbial ecology. Microbiological Reviews. 1980;44(4):758–760. doi: 10.1128/mr.44.4.739-796.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodríguez H, Fraga R. Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnology Advances. 1999;17(4–5):319–339. doi: 10.1016/s0734-9750(99)00014-2. [DOI] [PubMed] [Google Scholar]
- 29.Halder AK, Mishra AK, Bhattacharyya P, Chakrabartty PK. Solubilization of rock phosphate by Rhizobium and Bradyrhizobium. Journal of General and Applied Microbiology. 1990;36(2):81–92. [Google Scholar]
- 30.Goldstein AH. Involvement of the quinoprotein glucose dehydrogenase in the solubilization of exogenous phosphates by Gram-negative bacteria. In: Torriani-Gorini A, Yagil E, Silver S, editors. Phosphate in Microorganisms: Cellular and Molecular Biology. Washington, DC, USA: ASM Press; 1996. pp. 197–203. [Google Scholar]
- 31.Illmer P, Schinner F. Solubilization of inorganic calcium phosphates—solubilization mechanisms. Soil Biology and Biochemistry. 1995;27(3):257–263. [Google Scholar]
- 32.Sánchez-Porro C, de la Haba RR, Soto-Ramírez N, Márquez MC, Montalvo-Rodríguez R, Ventosa A. Description of Kushneria aurantia gen. nov., sp. nov., a novel member of the family Halomonadaceae, and a proposal for reclassification of Halomonas marisflavi as Kushneria marisflavi comb. nov., of Halomonas indalinina as Kushneria indalinina comb. nov. and of Halomonas avicenniae as Kushneria avicenniae comb. nov. International Journal of Systematic and Evolutionary Microbiology. 2009;59(2):397–405. doi: 10.1099/ijs.0.001461-0. [DOI] [PubMed] [Google Scholar]
- 33.Yoon JH, Choi SH, Lee KC, Kho YH, Kang KH, Park YH. Halomonas marisflavae sp. nov., a halophilic bacterium isolated from the Yellow Sea in Korea. International Journal of Systematic and Evolutionary Microbiology. 2001;51(3):1171–1177. doi: 10.1099/00207713-51-3-1171. [DOI] [PubMed] [Google Scholar]
- 34.Cabrera A, Aguilera M, Fuentes S, Incerti C, Russell NJ, Ramos-Cormenzana A. Halomonas indalinina sp. nov., a moderately halophilic bacterium isolated from a solar saltern in Cabo de Gata, Almería, southern Spain. International Journal of Systematic and Evolutionary Microbiology. 2007;57(2):376–380. doi: 10.1099/ijs.0.64702-0. [DOI] [PubMed] [Google Scholar]