Abstract
Chronic alcohol abuse is often co-morbid with depression symptoms and in many cases it appears to induce major depressive disorder. Structural and functional neuroimaging has provided evidence supporting some degree of neuropathological convergence of alcoholism and mood disorders. In order to understand the cellular neuropathology of alcohol dependence and mood disorders, postmortem morphometric studies have tested the possibility of alterations in the number and size of cells in the prefrontal cortex and other brain regions. The present review compares the cell pathology in the prefrontal cortex between alcohol dependence and depression, and reveals both similarities and differences. One of the most striking similarities is that, although pathology affects both neuronal and glial cells, effects on glia are more dramatic than on neurons in both alcohol dependence comorbid with depression and idiopathic depression. Moreover, prefrontal cortical regions are commonly affected in both depression and alcoholism. However, the cellular changes are more prominent and spread across cortical layers in alcohol dependent subjects than in subjects with mood disorders, and changes in glial nucleus size are opposite in alcoholism and depression. It could be argued that one defining factor in the manifestation of the depressive pathology is a reduction in the glial distribution in the dlPFC that is reflected in a reduced glial density. In alcoholism reduced glial nuclear size might be related to the cytotoxic effects of prolonged alcohol exposure, while in MDD, in the absence of alcohol abuse, other processes might be responsible for the increase in average size of glial nuclei. In either case abnormal function related to glial reduction would be associated with depression due to insufficient glial support to the surrounding neurons.
Keywords: Prefrontal cortex, Postmortem, Morphometry, Alcoholism, Major depressive disorder, Comorbidity
1. Introduction
Chronic alcohol abuse is often co-morbid with depressive symptoms and in many cases it appears to induce major depressive disorder (Hasin & Grant, 2002; Kessler et al., 1997; Schuckit et al., 1997). Conversely, some research supports the perception that human subjects suffering from major depression and other mood disorders are more likely to abuse alcoholic beverages than psychiatrically uncompromised subjects (Regier et al., 1990). Both alcohol dependence and affective disorders increase dramatically the attempt and completion of suicides (Cornelius et al., 1995, 1996; Salloum et al., 1995; Weiss & Hufford, 1999). The multifaceted association between alcoholism and depression outlined above suggests that, regardless of the particular pathophysiology for each of these disorders, specific brain regions and their associated functions might be commonly affected in both disorders. In the present review we will first provide the evidence for convergent pathology in the prefrontal cortex and the hippocampus reported in structural and functional neuroimaging studies. Secondly, we will summarize the findings of postmortem cell counting studies of glial cells in the prefrontal cortex in alcohol dependence, major depressive disorder, and depression co-morbid with alcohol dependence. Thirdly, we will present the evidence for neuronal pathology in those three psychiatric conditions. We will conclude with a discussion on similarities and differences between alcohol-related and depression-related cell pathology.
2. Neuroimaging studies
Structural neuroimaging studies provide direct evidence supporting some degree of neuropathological convergence between alcoholism and depression. For example, similar gross morphological changes are reported in fronto-limbic brain regions in alcohol dependence and depression. In alcohol-dependent subjects structural neuroimaging studies found dilated forebrain ventricles and enlarged cortical sulci throughout the cerebral cortex (Haubek & Lee, 1979; Lishman, 1990; Pfefferbaum et al., 1988; Ron et al., 1982). Enlargement of the lateral and third ventricles or increased sulcal prominence is also reported in depressed subjects (Elkis et al., 1995, 1996; Videbech et al., 2001). However, these enlargements in depression are less pervasive than those reported in alcoholism and not consistently found in all studies of depressed subjects (Elkis et al., 1995). Ventricular and sulcal enlargement are signs of volumetric reductions in the underlying gray or white matter. In alcohol dependent subjects the volumes of both gray and white matter are significantly reduced as compared with age and gender matched normal control subjects. Interestingly, this shrinkage of the forebrain observed in alcoholics (Kril & Halliday, 1999) is more prominent in the frontal and temporal lobes than in other cortical areas (Harper & Kril, 1990b; Kril et al., 1997; Pfefferbaum et al., 1997). Frontal and temporal lobe structures are also reported to be reduced in volume in major depressive disorder (MDD). Substantial reductions in the volume of gray matter are found in the temporal lobe as a whole (Altshuler et al., 1991; Hauser et al., 1989), in the subgenual cortex (Drevets et al., 1997), and in the amygdala (Pearlson et al., 1997; Sheline et al., 1998) in MDD and bipolar disorder (BPD). Moderate reductions in the total volume of the frontal white and gray matter have been described in MDD (Coffey et al., 1993). Moreover, shrinkage in the volume of the hippocampus has been observed in both alcoholism (Agartz et al., 1999; Sullivan et al., 1995) and MDD (Bremner et al., 2000; Sheline et al., 1996).
Functional neuroimaging studies also demonstrate that specific regions of the prefrontal cortex show reduced metabolism in both alcohol dependent and MDD subjects. In chronic alcoholics, performance on behavioral tests that activate specific prefrontal regions shows abnormal reduced regional glucose metabolism or blood flow in medial, dorsolateral and orbitofrontal regions of the prefrontal cortex (George et al., 1999; Hoaken et al., 1998; Pihl et al., 1980; Sullivan et al., 2000; Volkow et al., 1992; Weingartner et al., 1994). Moreover, a significant correlation between the behavioral and cognitive abnormalities and the haemodynamic and metabolic alterations have been demonstrated in the temporal and prefrontal cortical areas in alcoholics (Adams et al., 1993, 1995; Dally et al., 1988; Nicolas et al., 1993), further indicating an abnormal functioning of these cortical regions. Similarly, in mood disorders, dysregulation of glucose metabolism and blood flow also preferentially affects the prefrontal cortex, temporal cortex and amygdala. For example, functional neuroimaging studies of MDD subjects have shown reduced blood flow and glucose metabolism in dorsolateral prefrontal cortex (Austin et al., 1992; Baxter et al., 1989; Bench et al., 1992; Biver et al., 1994; Buchsbaum et al., 1984; Ebert & Ebmeier, 1996; Hurwitz et al., 1990; Mann et al., 1996; Martinot et al., 1990; Mayberg, 1994), medial frontal cortex (Bench et al., 1992; Drevets et al., 1997; George et al., 1997; Mayberg et al., 1997), and orbitofrontal cortex (Mayberg et al., 1990, 1992; Ring et al., 1994).
In summary, it appears that broad changes in structural and functional parameters determined with neuroimaging techniques occur in both alcohol dependence and mood disorders and that the prefrontal cortex is particularly involved in this pathology in both disorders. On the other hand, some differences in the localization of brain volumetric changes and in the temporal progression of the neuropathological changes are also found between alcohol dependence and depression. For instance, several studies in alcoholism have consistently demonstrated that the volume of white matter in the frontal lobe is reduced in chronic alcohol abusers (Harper, 1998).
In contrast, in MDD or BPD subjects, the available studies did not find differences in white matter volumes (reviewed in Soares and Mann, 1997). Despite the absence of changes in white matter volume, structural MRI studies have revealed changes in the optical density of the white matter (also called hyperintensities) in the frontal lobe of elderly MDD subjects (Tupler et al., 2002). These hyperintensities are considered indicative of microvascular disturbances, and their numbers appear to be correlated with the severity of depression (Drevets et al., 1999). Moreover, in alcoholism volumetric reductions of both gray and white matter revert at least partially towards normal values after periods of abstinence as short as one month (Pfefferbaum et al., 1995, 1998; Zipursky et al., 1989), while such reversion in gray matter volumes has not yet been described in depressed subjects. Progression of pathological changes such as reduction of hippocampal volume appears more likely in subjects with recurrent depressive episodes and this reduction is correlated with the number of depressive episodes (Sheline et al., 1996, 1999).
In summary, neuroimaging studies support the concept that the same prefrontal and temporal cortical areas might be the site of pathology in both depression related to alcohol dependence and depressive symptoms in idiopathic mood disorders. On the other hand, the differences in white matter pathology and pathological reversibility between alcohol dependence and MDD pointed above indicate that depression related to alcohol and idiopathic depression might obey to different pathophysiological mechanisms at the cellular and functional circuits levels.
3. Prefrontal cell pathology in depression and alcoholism
Postmortem morphometric studies during the last 15 years have tested the possibility of alterations in the numbers and size of neurons and glial cells in the prefrontal cortex (Fig. 1) and other regions in postmortem brain tissue from alcoholics (Harper & Corbett, 1990a; Harper & Kril, 1990b; Jensen & Pakkenberg, 1993; Korbo, 1999; Kril et al., 1997; Kril & Harper, 1989). More recently similar studies have also been undertaken in mood disorders (Cotter et al., 2002a; Miguel-Hidalgo et al., 2000; Öngür et al., 1998; Rajkowska et al., 1999, 2001). One of the most recent studies reveals prefrontal cell pathology in comorbid of alcohol dependence and depression (Miguel-Hidalgo et al., in press). One of the most striking findings reported in this study is that, although pathology affects both neuronal and glial cells, effects on glia are more dramatic than on neurons in alcohol dependence comorbid with depression.
Fig. 1.
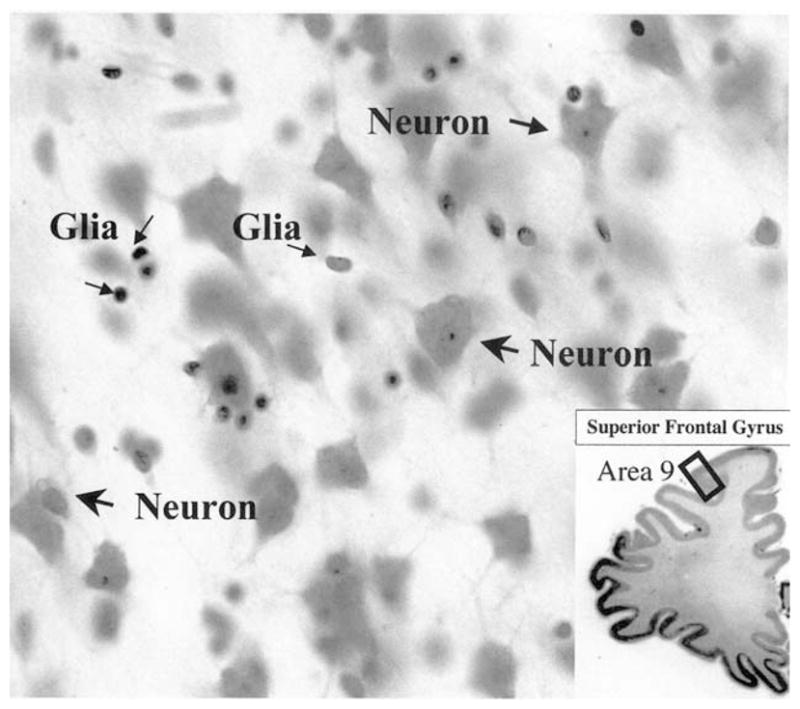
High power micrograph of neurons and glial cells as they appear in the cortical layers of area 9 in coronal section through the prefrontal cortex stained with cresyl violet. Note that both the nucleus and cytoplasm are visible in neurons, while only the nucleus is visible in glial cells. The inset at the bottom right is a low power image of the cresyl violet-stained section containing the spot (black rectangle in area 9) where the high power micrograph was taken. High power: 40× objective; low power: 2× objective.
3.1. Glial pathology of the prefrontal cortexin depression
Recent morphometric studies provide consistent evidence for previously unrecognized reductions in glial cell density and number. Glial cell counts and size measurements in the dorsolateral prefrontal cortex (dlPFC, Brodmann’s area 9) and orbitofrontal cortex (ORB, Brodmann’s area 47) in subjects with MDD reveal marked 11–30% decreases in cell packing densities in both regions as compared with controls (Rajkowska et al., 1999; Cotter et al., 2002b). The degree of glia pathology in MDD differs among prefrontal regions studied. Glial reductions are most striking in more caudally located regions of the prefrontal cortex, the caudal ORB and dlPFC, and very mild in the rostral part of the ORB cortex. Similar reductions in glial cell density are also reported in the dlPFC (Rajkowska et al., 2001) and more recently in ORB (Rajkowska, 2001) in bipolar disorder. Reductions in the density of glial cells in dlPFC in both mood disorders are accompanied by enlargement in the size of glial nuclei (Rajkowska et al., 1999, 2001) suggesting some compensatory mechanisms.
Parallel morphometric analyses of the anterior cingulate cortex conducted by two independent laboratories in postmortem tissues from MDD subjects provide consistent evidence for reductions in a number (Öngür et al., 1998) and density (Cotter et al., 2001a) of glial cells. Striking 24% reductions in number of glial cells were found in the subgenual region of the anterior cingulate cortex (ventral part of Brodmann’s area 24) in patients with familial MDD (Öngür et al., 1998). Similar 41% reductions were observed in the same region in a small subgroup of subjects with familial BPD, as compared with controls (Öngür et al., 1998). However, when familial and unfamilial subgroups of patients were combined the reductions were not found. Evaluation of glial cell density in the supracallosal region of the anterior cingulate cortex (dorsal part of BA24; Cotter et al., 2001a) confirmed findings on glial reductions (22%) in MDD. Analysis of glial cells in the same region in BPD subjects resulted in negative findings (Cotter et al., 2001a). However, in the study by Cotter et al. (2002a), unlike that of Öngür et al. (1998), the studied BPD group was not subdivided according to the family history, which could account for the negative results.
The involvement of specific glial cell types in the general glial pathology of the dorsolateral prefrontal cortex in MDD has been recently approached by immuno-histochemical techniques using antibodies against glial fibrillary acidic protein (GFAP), a specific cytoskeletal marker of astroglia (Miguel-Hidalgo et al., 2000). In this study a decrease in the extent of GFAP immunoreactive structures and in the packing density of GFAP immunoreactive cells was found in a subgroup of MDD subjects under 45 years of age as compared with non-psychiatric controls and to a subgroup of older MDD subjects. These findings indicate that the GFAP-immunoreactive astroglia is differentially involved in the pathology of MDD in younger as compared to older adults.
3.2. Glial pathology of the prefrontal cortexin alcoholism
In spite of cumulating evidence indicating crucial roles of glial cells in regulating activity in the CNS (Laming et al., 2000), and their recent implication in mood disorders (reviewed by Cotter et al., 2001b; Rajkowska, 2000), only a few studies have addressed the putative involvement of glial cells in the histopathology of alcoholism in the human cerebral cortex. For example, the density of glial cells was increased in the superior frontal gyrus in a group of alcoholics that included individuals with Wernicke’s encephalopathy (Harper et al., 1987; Kril & Harper, 1989), although these studies did not distinguish cortical layers and used two-dimensional rather than the more precise three-dimensional cell counting technique. In contrast, a recent study in the hippocampus of alcoholics with neither Wernicke’s encephalopathy nor Korsakoff psychosis reported dramatic 37% reductions in the number of glial cells and a preferential reduction in the number of astrocytes in alcohol dependent subjects (Korbo, 1999). One study in severe alcoholism reports a patchy loss of GFAP immunoreactive structures, a beading of astrocytic fibers, and soma swelling in individual astrocytes in some regions of the brain (Cullen & Halliday, 1994). Thus, until recently it was unclear whether the putative glial pathology in alcoholism is accompanied by a decrease or an increase in the density of glial cells and their subcellular components, and whether there is a differential involvement of particular cortical layers in this pathology. To ascertain whether alcohol dependence in subjects without major neurological damage (i.e. absence of Wernicke’s encephalopathy, Korsakoff psychosis, or signs of gross neurological damage to the brain) affects significantly the number of glial cells in the dorsolateral prefrontal cortex we studied recently the packing density and the size of glial cell nuclei in that cortical region in 21 control and 17 alcohol dependent subjects (Miguel-Hidalgo et al., in press, Fig. 1). We found that the average density of glial cells in all cortical layers combined was significantly reduced by 11% in the alcohol-dependent group as compared with controls. Analysis of the density of glial cells considering the values for individual layers also revealed a significant difference between alcohol-dependent subjects and control subjects. Density of glial cells was significantly lower in the lower cortical layers V (12%), and VI (14%) in the alcohol-dependent group as compared to controls. In order to determine whether depression in alcohol dependent subjects might be associated with differential glial pathology further analyses were performed after dividing the group of alcohol-dependent subjects into those with depressive symptoms (Alc-D, n=8), and those without reported symptoms of depression (Alc-ND, n=9) (Miguel-Hidalgo et al., 2002). Although glial densities did not significantly differ between Alc-D and Alc-ND, in most measures the highest values of average glial packing density were found in the control group, the lowest values were observed in the Alc-D group and intermediate values appeared in the Alc-ND group. When considering the glial density in all layers combined of the three groups (control, Alc-D, Alc-ND) significant reductions were found in the Alc-ND (8.8%) and in the Alc-D group (13%), as compared with the control group (Fig. 2). In addition, significantly lower glial densities were found in layers I (13.5%) and VI (15.3%) in the Alc-ND group and in layers II (19.4%), V(16.5%) and VI (15.3%) in the Alc-D group as compared with controls. Analysis of glial cell nucleus size revealed a significant difference in the average size of glial nuclei in all layers pooled together, with the average size in the alcohol dependent subjects being significantly smaller by 3.2% than in the control group (Fig. 3). However, no significant difference was detected between the Alc-D and Alc-ND groups in the average size of glial cell nuclei. Nevertheless, the average values of glial size in the Alc-D group were still the lowest of the three groups examined.
Fig. 2.

Plots showing the distribution of individual values for packing density of glial cells in all layers combined of the dorsolateral prefrontal cortex in the four diagnostic groups of subjects: non-psychiatric controls (CONTROL), alcohol-dependent without depression (ALC. NON-DEPR), alcohol-dependent with depression (ALC. DEPR.), and subjects with major depressive disorder (MAJOR DEPR. DIS.). A horizontal bar indicates the median value. Note that a significant reduction in glial density is found in all three groups of patients as compared with the control group (ANOVA F=4.57, P<0.006) however, the most prominent reductions (P<0.006) are found in the group of subjects with comorbid alcohol dependence and depression. For further details see (Miguel-Hidalgo et al., 2002).
Fig. 3.
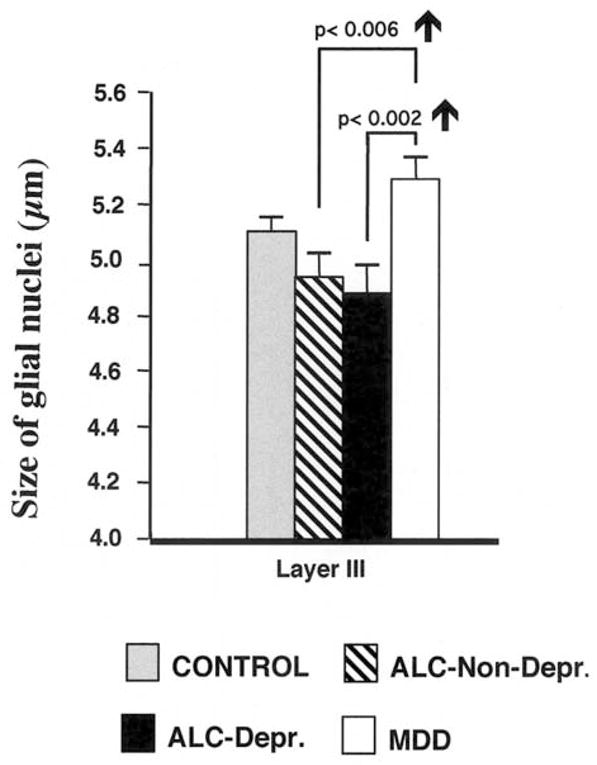
Graph comparing the average size of glial cell nuclei in cortical layer III of the dorsolateral prefrontal cortex in the four diagnostic groups of subjects: non-psychiatric controls (CONTROL), alcohol-dependent without depression (ALC. NON-DEPR), alcohol-dependent with depression (ALC. DEPr.), and subjects with major depressive disorder (MAJORDEPR. DIS.). Error bars represent the standard error of the mean. Note that glial nuclei are larger in the group of subjects with major depressive disorder as compared to the group of alcohol-dependent without depression and alcohol dependent with depression. For further details see (Miguel-Hidalgo et al., 2002).
3.3. Comparison of glial cell pathology between depression and alcohol dependence
To better understand the possible link between depressive symptoms and the glial pathology in alcohol dependent subjects we compared the data on glial cell density and size obtained from the alcohol-dependent subjects (Miguel-Hidalgo et al., 2002) to those previously published from a group of 12 MDD subjects (Rajkowska et al., 1999). These data from the MDD subjects were obtained from the same cortical region (prefrontal area 9) and using the same histological procedures and cell counting methodology as in our study on alcoholism. The comparison between the four groups, MDD, Alc-ND, Alc-D and controls revealed significant differences in the overall glial density as well as in nuclear glial size between the groups (Figs. 2 and 3). The overall packing density in the MDD group was significantly smaller than in the control group but not different from the packing density either in the Alc-ND or the Alc-D group (Fig. 2). Only glial density in layer VI of the MDD subjects was significantly higher by 14% than in the Alc-D group. However, we observed a remarkable difference between MDD subjects and the other groups when we compared the nuclear size in particular layers of the dlPFC. In the MDD group the average nuclear size was increased in the upper cortical layers II, III and IV as compared with the Alc-D group, and in layer III as compared with the Alc-ND group (Fig. 3). The size of glial nuclei in the MDD subjects was also larger when compared with that of controls. Therefore, a reduction in the average nuclear size of glial cells is characteristic for the brains of alcoholics regardless of the occurrence of comorbid depressive symptoms. By contrast, the average glial nuclear size is increased in MDD subjects as compared to controls or alcohol-dependent subjects.
3.4. Neuronal pathology in major depression
3.4.1. Neocortex
Morphometric analysis of neuronal density in the ORB cortex in MDD revealed the most prominent reductions in the rostral part of this region, whereas the caudal part exhibited mostly reductions in the density of glial cells rather than neurons (Rajkowska et al., 1999). These neuronal reductions in the rostral ORB were confined to upper cortical layers with most dramatic changes in layer II cells (mostly corresponding to non-pyramidal, inhibitory local-circuit neurons). More recently the density of neurons positive for another calcium binding protein, calbindin 28 kD, was also found to be reduced in layer II in MDD as compared to controls (Rajkowska et al., 2002). The reductions in neuronal densities in the ORB cortex are paralleled by smaller sizes (5–9%) of neuronal somata and significant, 12–15% decreases in cortical thickness observed in rostral and middle ORB cortex in MDD groups (Rajkowska et al., 1999).
Quantitative examination of another prefrontal regions, dlPFC in MDD did not reveal significant reductions in overall or laminar neuronal density when the entire population of the Nissl stained neurons was analyzed (Rajkowska et al., 1999). Nonetheless, when all neurons were subdivided into four separate subtypes based on the size of their cell bodies, significant reductions (22–37%) in the density of neurons with large (20–36 μm in diameter) body size were found in layers II, III and VI of the dlPFC. These large pyramidal neurons, the size of which is smaller in MDD subjects than in controls, are thought to be excitatory pyramidal neurons. However, final identification of this cell type has yet to be confirmed by neurochemical methods. The reductions in the density of large neurons were accompanied by 6–27% increases in the density of the smallest neurons (5–11 μm in diameter) and reductions in the mean neuronal size in layers III and VI. The above suggests that either neuronal shrinkage or a developmental deficiency, rather than neuronal loss, accounts for the overall smaller sizes of neuronal soma in those cortical layers. Comparable reductions (23%) in the size of neuronal cell bodies have been recently found in layer VI of the supracallosal region of the anterior cingulate cortex in MDD but not BPD (Cotter et al., 2001a).
Laminar analysis of neuron density in the dlPFC region in bipolar disorder revealed significant 16–22% reductions in the mean density of all neurons in layer III (Rajkowska et al., 2001). In addition, significant decreases in the density of large pyramidal neurons were found in layers III and V. Measurements of laminar thickness in these groups indicated a 16% increase in the width of deep layer III in BPD subjects, where glial size was increased as well. In light of the increased width of layer III, one possibility is that an increase in inter-neuronal neuropil, perhaps including the processes of hypertrophied glial cells, might account for the reduction in neuronal and glial densities in this layer in BPD patients. The cortical width in the dlPFC region, unlike that in rostral parts of ORB region remained unchanged in both MDD and BPD as compared with control subjects (Rajkowska et al., 1999, 2001).
3.5. Neuronal pathology in alcoholism
Two studies in chronic alcoholics report loss or reduced density of neurons in the cortex of the superior frontal gyrus (part of the dorsolateral prefrontal cortex, dlPFC) (Kril & Harper, 1989; Kril et al., 1997). It seems, however that in the brain of alcoholics without Wernicke’s encephalopathy or Korsakoff psychosis, so-called uncomplicated alcoholics, neuronal loss does not extend to other cortical areas and is rather specific to the prefrontal cortex (Harper, 1998; Jensen & Pakkenberg, 1993; Kril & Halliday, 1999). In the superior frontal gyrus the reductions in neuronal number appear to be accompanied by a loss of basal dendrites in pyramidal neurons of cortical layer III (Harper & Corbett, 1990a) suggesting that a reduction of in neuronal number might be preceded by elimination of synaptic inputs to layer III neurons. Recently, we have found in a preliminary study that the packing density of neurons in all cortical layers combined was reduced in the dlPFC in alcoholics as compared to non-psychiatric controls (unpublished results). In addition, we have observed that cortical layer III had the most prominent reductions of neuronal density. In alcohol dependence we have also observed that the glial density/neuronal density ratio is reduced significantly in cortical layer VI of alcohol dependent subjects as compared to controls, but not in the other cortical layers. However, there was no difference between controls and MDD subjects. These results on the cell packing density of neurons in the dlPFC are in line with the results of Kril & Harper (1989; Kril et al., 1997) in the superior frontal gyrus. The pathological changes in the brain in alcoholism may be related to neuronal alterations not directly leading to cell death. In the hippocampus early reports indicated a loss of neurons in chronic alcoholics (Bengoechea & Gonzalo, 1990, 1991). However, more recent studies using unbiased stereological techniques have demonstrated that the number of neurons in the hippocampus of alcoholics is unchanged as compared with controls (Korbo, 1999; Kril et al., 1997). These findings are consistent with the absence of a generalized neuronal loss in the cortex of chronic alcohol abusers. By contrast, these same findings underscore the particular vulnerability of neurons in the prefrontal cortex of alcoholics, which so far appears to be the only cortical region significantly affected by neuronal loss (Harper, 1998: Harper et al., 1987).
4. Discussion
In summary, the available evidence of cellular changes in depression and in alcoholism indicates that some prefrontal cortical regions are commonly affected in depressed and alcohol-dependent subjects. However, the changes are more prominent and more spread across cortical layers in alcohol dependent subjects than in subjects with mood disorders.
Reductions in the density of neurons are found in both alcohol dependence and mood disorders. In MDD and BPD these reductions in neuronal density seem subtler since they are only found in specific cortical layers in any of the prefrontal regions studied, but not when all layers are combined together. By contrast, in alcoholics all cortical layers are affected by the reduction in the number of neurons of the dorsolateral prefrontal cortex (Harper, 1998). This pronounced loss of neurons in a particular cortical region in alcoholics might reflect an especial neuronal susceptibility to the toxic effects of ethanol. In addition, reductions of neuronal size occur in the prefrontal cortex in both depression and alcoholism and in both instances these reductions are observed in all cortical layers combined. In MDD the reduction in size is prominent in layers II and III in dlPFC and orbitofrontal cortex and appears to affect mainly the larger pyramidal cells. Interestingly, in alcoholism a reduction in the dendritic arbor of layer III pyramidal neurons has been described (Harper & Corbett, 1990a). In our studies on alcoholism in the dlPFC this cortical layer presents also the largest reduction in neuronal density. A preferential involvement of layer III pyramidal neurons in the pathology of MDD and alcoholism suggests that an abnormal functioning of cortico-cortical connections, which are mostly established by layer III neurons (Arikuni et al., 1988; Cavada et al., 2000; Cavada & Reinoso-Suarez, 1981; Vogt & Pandya, 1987), may be one of the main components of the cortical physiopathology of MDD and alcohol dependence.
The cell counting data indicate that reduction in the density or number of glial cells in the dlPFC accompanies both depression and alcohol dependence. Regarding individual cortical layers a common reduction in glial density in layer VI of the dlPFC is found in both disorders. Glial pathology, however, appears otherwise to be more differentiated between MDD and alcoholism than neuronal pathology. For instance, there is a reduction in glial density in layer V in alcoholics with depression, which was not observed in MDD subjects. In addition, while in alcoholics, as compared to controls, there is a reduction in glial nuclear size across most layers, in MDD subjects the average size of glial cells in layer III was larger than the corresponding layer of controls subjects.
Another striking difference between glial pathology in MDD and in alcoholism resides in the hippocampus. In uncomplicated alcoholics Korbo (1999) found a dramatic decrease in the number of glial cells in the hippocampus. In contrast, Stockmeier et al. (2002) recently found a significant increase in the density of glial cells in the hippocampus of MDD subjects as compared to controls (Stockmeier et al., 2002). These differences in glial pathology between alcohol dependence and MDD may contribute to explain the cellular etiology of depressive symptoms according to different mechanisms. One possibility is that glial pathology is directly caused by chronic alcohol abuse, but that this glial pathology is not related to the alcohol-induced neuronal (and behavioral) dysfunction. In this case we would be obligated to propose that glia-independent neuronal responses to pathological processes are common in alcoholism and MDD. This would explain why in some prefrontal regions there appears to be more similarities between these two diseases in neuronal pathology than in glial pathology. On the contrary, if we think that glial alterations are primary and consequently crucial to explain the immediate causes of depressive symptoms, then those differentiated glial alterations would somehow converge into causing the neuronal dysfunction that is ultimately responsible of depression (for further discussion see Cotter et al., 2002a; Miguel-Hidalgo et al., 2002; Rajkowska, 2000).
The existence of common sites of histopathology in MDD and alcohol-dependence might explain why there is a high incidence of depression in chronic alcohol abusers. On the one hand, different factors and mechanisms may be operating in mood disorders and alcohol-dependence to induce differential abnormal cellular pathology in circuits that regulate emotional and cognitive aspects of depression. On the other hand, regardless of different mechanisms producing alterations in those common circuits it would be the malfunction of those circuits that is the immediate cause for depressive symptoms. At this respect it is important to note that while in idiopathic MDD and other mood disorders the etiology remains obscure, in alcohol-dependence ethanol is obviously a candidate for main causal factor, because ethanol is known to produce functional and structural alterations in neurons and glial cells (Diamond & Gordon, 1997; Fadda & Rossetti, 1998; Snyder, 1996). This explanation is supported by clinical studies showing that after a sufficient period of abstinence there is remission of depressive symptoms in many alcoholics (Brown et al., 1995; Brown & Schuckit, 1988; Davidson, 1995; Golding et al., 1993; Nakamura et al., 1983). In MDD, in the absence of a defined physiological culprit it is not possible to use the withdrawal technique to ascertain their influence in the behavioral, neuroimaging (structural and functional) and cellular changes associated with depression. Furthermore, while in alcoholism there have been longitudinal studies (before and after abstinence) that reveal the possibility of morphological and functional recovery (Pfefferbaum et al., 1995, 1998; Volkow et al., 1994; Zipursky et al., 1989), there have been considerably fewer studies in depression addressing the possibility of reversion of structural or functional changes (Kennedy et al., 2001; Mayberg et al., 1997).
The reduction in the density of glial cells figs. prominently in the pathological changes of the dlPFC in both depression and alcohol dependence. In the latter disorder the average reduction in the density of glial cells is even greater than in MDD. Accordingly, it could be argued that one defining factor in the manifestation of the depressive pathology is a reduction in the glial distribution in the dlPFC that is reflected in a reduced glial density. In alcoholism reduced glial nuclear size might be related to the cytotoxic effects of prolonged alcohol exposure, while in MDD, in the absence of alcohol abuse, other processes might be responsible for the increase in average size of glial nuclei. In either case abnormal function due to or related to glial reduction would be associated with depression. It cannot be ruled out that subjects that are prone to alcohol dependence do share some glial alterations with MDD and BPD subjects prior to onset of protracted alcohol abuse. Long-term exposure to alcohol may just complicate a predisposition to glial alterations. Alternatively, alcohol may directly and independently affect those glial cells in the prefrontal cortex that are involved in the pathology of depression. Consistent with this suggestion is the association between prior alcohol dependence and MDD that has been recently reported in a large epidemiological study (Hasin and Grant, 2002). In either case, it should be expected that the glial alterations in alcohol-dependent subjects with depressive pathology are more marked than in uncomplicated alcoholics without depressive symptoms. In fact, in our studies, the subgroup of alcoholics with depression did display a larger reduction in glial packing density than the subgroup of alcoholics without depressive symptoms. Future molecular and genetic studies will establish whether individuals with alcohol dependence and those with mood disorders share genetic loci for specific brain pathology.
Acknowledgments
Work by the authors discussed in the present article was supported by a grant from ABMRF (JJMH) and by grants MH60451 and MH61578 (GR).
References
- Adams KM, Gilman S, Koeppe B, Kluin K, Junck L, Lohman M, et al. Correlation of neuropsychological function with cerebral metabolic rate in subdivisions of the frontal cortex of older alcoholics patients measured with [18F]fluorodeoxyglucose and positron emision tomography. Neuropsychology. 1995;9:275–80. [Google Scholar]
- Adams KM, Gilman S, Koeppe RA, Kluin KJ, Brunberg JA, Dede D, et al. Neuropsychological deficits are correlated with frontal hypometabolism in positron emission tomography studies of older alcoholic patients. Alcohol Clinical and Experimental Research. 1993;17:205–10. doi: 10.1111/j.1530-0277.1993.tb00750.x. [DOI] [PubMed] [Google Scholar]
- Agartz I, Momenan R, Rawlings RR, Kerich MJ, Hommer DW. Hippocampal volume in patients with alcohol dependence. Archives of General Psychiatry. 1999;56:356–63. doi: 10.1001/archpsyc.56.4.356. [DOI] [PubMed] [Google Scholar]
- Altshuler LL, Conrad A, Hauser P, Li XM, Guze BH, et al. Reduction of temporal lobe volume in bipolar disorder: a preliminary report of magnetic resonance imaging. Archives of General Psychiatry. 1991;48:482–3. doi: 10.1001/archpsyc.1991.01810290094018. [DOI] [PubMed] [Google Scholar]
- Arikuni T, Watanabe K, Kubota K. Connections of area 8 with area 6 in the brain of the macaque monkey. The Journal of Comparative Neurology. 1988;277:21–40. doi: 10.1002/cne.902770103. [DOI] [PubMed] [Google Scholar]
- Austin MP, Dougall N, Ross M, Murray C, O’Carroll RE, Moffoot A, et al. Single photon emission tomography with 99mTc-exametazime in major depression and the pattern of brain activity underlying the psychotic/neurotic continuum. Journal of Affective Disorders. 1992;26:31–43. doi: 10.1016/0165-0327(92)90032-2. [DOI] [PubMed] [Google Scholar]
- Baxter LR, Schwartz JM, Phelps ME, Mazziotta JC, Guze BM, Selin CE, et al. Reduction of prefrontal cortex glucose metabolism common to three types of depression. Archives of General Psychiatry. 1989;46:243–50. doi: 10.1001/archpsyc.1989.01810030049007. [DOI] [PubMed] [Google Scholar]
- Bench CJ, Friston KJ, Brown RG, Scott LC, Frackowiak RS, Dolan RJ. The anatomy of melancholia: focal abnormalities of cerebral blood flow in major depression. Psychological Medicine. 1992;22:607–15. doi: 10.1017/s003329170003806x. [DOI] [PubMed] [Google Scholar]
- Bengoechea O, Gonzalo LM. Effect of chronic alcoholism on the human hippocampus. Histology and Histopathology. 1990;5:349–57. [PubMed] [Google Scholar]
- Bengoechea O, Gonzalo LM. Effects of alcoholization on the rat hippocampus. Neuroscience Letters. 1991;123:112–4. doi: 10.1016/0304-3940(91)90170-x. [DOI] [PubMed] [Google Scholar]
- Biver F, Goldman S, Delvenne V, Luxen A, Maertelaer VD, Hubain P, Mendlewicz J, Lotstra F. Frontal and parietal metabolic disturbances in unipolar depression. Biological Psychiatry. 1994;36:381–8. doi: 10.1016/0006-3223(94)91213-0. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Narayan M, Anderson ER, Staib LH, Miller HL, Charney DS. Hippocampal volume reduction in major depression. The American Journal of Psychiatry. 2000;157:115–8. doi: 10.1176/ajp.157.1.115. [DOI] [PubMed] [Google Scholar]
- Brown SA, Inaba RK, Gillin JC, Schuckit MA, Stewart MA, Irwin MR. Alcoholism and affective disorder: clinical course of depressive symptoms. The American Journal of Psychiatry. 1995;152:45–52. doi: 10.1176/ajp.152.1.45. [DOI] [PubMed] [Google Scholar]
- Brown SA, Schuckit MA. Changes in depression among abstinent alcoholics. Journal of Studies On Alcohol. 1988;49:412–7. doi: 10.15288/jsa.1988.49.412. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS, DeLisi LE, Holcomb H, Cappelletti J, King AC, Johnson J, et al. Anteroposterior gradients in cerebral glucose use in schizophrenia and affective disorders. Archives of General Psychiatry. 1984;41:1159–66. doi: 10.1001/archpsyc.1984.01790230045007. [DOI] [PubMed] [Google Scholar]
- Cavada C, Company T, Tejedor J, Cruz-Rizzolo RJ, Reinoso-Suarez F. The anatomical connections of the macaque monkey orbitofrontal cortex. A review Cerebral Cortex. 2000;10:220–42. doi: 10.1093/cercor/10.3.220. [DOI] [PubMed] [Google Scholar]
- Cavada C, Reinoso-Suarez F. Intrahemispheric cortico-cortical afferent connections of the prefrontal cortex in the cat. Brain Research. 1981;223:128–33. doi: 10.1016/0006-8993(81)90811-8. [DOI] [PubMed] [Google Scholar]
- Coffey CE, Wilkinson WI, Weiner RD, Parashos IA, Djang WT, Webb MC, et al. Quantitative cerebral anatomy in depression. Archives of General Psychiatry. 1993;50:7–16. doi: 10.1001/archpsyc.1993.01820130009002. [DOI] [PubMed] [Google Scholar]
- Cornelius JR, Salloum IM, Mezzich J, Cornelius MD, Fabrega H, Jr, Ehler JG, et al. Disproportionate suicidality in patients with comorbid major depression and alcoholism. American Journal of Psychiatry. 1995;152:358–64. doi: 10.1176/ajp.152.3.358. [DOI] [PubMed] [Google Scholar]
- Cornelius JR, Salloum IM, Day NL, Thase ME, Mann JJ. Patterns of suicidality and alcohol use in alcoholics with major depression. Alcohol Clinical and Experimental Research. 1996;20:1451–5. doi: 10.1111/j.1530-0277.1996.tb01148.x. [DOI] [PubMed] [Google Scholar]
- Cotter D, Mackay D, Chana G, Beasley C, Landau S, Everall IP. Reduced neuonal size and glial cell density in area 9 of the dorsolateral prefrontal cortex in subjects with major depressive disorder. Cerebral Cortex. 2002a;12:386–94. doi: 10.1093/cercor/12.4.386. [DOI] [PubMed] [Google Scholar]
- Cotter D, Mackay D, Landau S, Kerwin R, Everall I. Reduced glial cell density and neuronal size in the anterior cingulate cortex in major depressive disorder. Archives of General Psychiatry. 2001a;58:545–53. doi: 10.1001/archpsyc.58.6.545. [DOI] [PubMed] [Google Scholar]
- Cotter DR, Pariante CM, Everall IP. Glial cell abnormalities in major psychiatric disorders: the evidence and implications. Brain Research Bulletin. 2001b;55:585–95. doi: 10.1016/s0361-9230(01)00527-5. [DOI] [PubMed] [Google Scholar]
- Cotter DR, Pariante CM, Rajkowska G. Glial pathology and major psychiatric disorders. In: Agam G, Everall IP, Belmaker RH, editors. The postmortem brain in psychiatric research. Boston: Kluwer Academic Publishers; 2002b. [Google Scholar]
- Cullen KM, Halliday GM. Chronic alcoholics have substantial glial pathology in the forebrain and diencephalon. Alcohol and Alcoholism Supplement. 1994;2:253–7. [PubMed] [Google Scholar]
- Dally S, Luft A, Ponsin JC, Girre C, Mamo H, Fournier E. Abnormal pattern of cerebral blood flow distribution in young alcohol addicts. British Journal of Addiction. 1988;83:105–9. doi: 10.1111/j.1360-0443.1988.tb00458.x. [DOI] [PubMed] [Google Scholar]
- Davidson KM. Diagnosis of depression in alcohol dependence: changes in prevalence with drinking status. The British Journal of Psychiatry. 1995;166:199–204. doi: 10.1192/bjp.166.2.199. [DOI] [PubMed] [Google Scholar]
- Diamond I, Gordon AS. Cellular and molecular neuroscience of alcoholism. Physiological Reviews. 1997;77:1–20. doi: 10.1152/physrev.1997.77.1.1. [DOI] [PubMed] [Google Scholar]
- Drevets W, Price J, Simpson JRJ, Todd R, Reich T, Vannier M, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–7. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Kishore MG, Krishnan RKR. Neuroimaging studies of mood disorders. In: Charney DS, Nestler EJ, Bunney BS, editors. Neurobiology of mental illness. New York: Oxford University Press; 1999. [Google Scholar]
- Ebert D, Ebmeier KP. The role of the cingulate gyrus in depression: from functional anatomy to neurochemistry. Biological Psychiatry. 1996;39:1044–50. doi: 10.1016/0006-3223(95)00320-7. [DOI] [PubMed] [Google Scholar]
- Elkis H, Friedman L, Buckley PF, Lee HS, Lys C, Kaufman B, et al. Increased prefrontal sulcal prominence in relatively young patients with unipolar major depression. Psychiatry Research. 1996;67:123–34. doi: 10.1016/0925-4927(96)02744-8. [DOI] [PubMed] [Google Scholar]
- Elkis H, Friedman L, Wise A, Meltzer HY. Meta-analyses of studies of ventricular enlargement and cortical sulcal prominence in mood disorders. Comparisons with controls or patients with schizophrenia. Archives of General Psychiatry. 1995;52:735–46. doi: 10.1001/archpsyc.1995.03950210029008. [DOI] [PubMed] [Google Scholar]
- Fadda F, Rossetti ZL. Chronic ethanol consumption: from neuroadaptation to neurodegeneration. Progress in Neurobiology. 1998;56:385–431. doi: 10.1016/s0301-0082(98)00032-x. [DOI] [PubMed] [Google Scholar]
- George M, Ketter T, Parekh P, Rosinsky N, Ring H, Pazzaglia P, et al. Blunted left cingulate activation in mood disorder subjects during a response interference task (the Stroop) Journal of Neuropsychiatry and Clinical Neuroscience. 1997;9:55–63. doi: 10.1176/jnp.9.1.55. [DOI] [PubMed] [Google Scholar]
- George MS, Teneback CC, Bloomer CW, Horner MD, Anton RF. Using neuroimaging to understand the alcohol’s brain effects. CNS spectrums. 1999;4:88–92. [Google Scholar]
- Golding JM, Burnam MA, Benjamin B, Wells KB. Risk factors for secondary depression among Mexican Americans and non-Hispanic whites. Alcohol use, alcohol dependence, and reasons for drinking. The Journal of Nervous and Mental Disease. 1993;181:166–75. doi: 10.1097/00005053-199303000-00004. [DOI] [PubMed] [Google Scholar]
- Harper C. The neuropathology of alcohol-specific brain damage, or does alcohol damage the brain? Journal of Neuropathology and Experimental Neurology. 1998;57:101–10. doi: 10.1097/00005072-199802000-00001. [DOI] [PubMed] [Google Scholar]
- Harper C, Corbett D. Changes in the basal dendrites of cortical pyramidal cells from alcoholic patients—a quantitative Golgi study. Journal of Neurology, Neurosurgery and Psychiatry. 1990a;53:856–61. doi: 10.1136/jnnp.53.10.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper C, Kril J, Daly J. Are we drinking our neurones away? Britsh Medical Journal (Clinical Research Edition) 1987;294:534–6. doi: 10.1136/bmj.294.6571.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper CG, Kril J. Neuropathology of alcoholism. Alcohol and Alcoholism. 1990b;25:207–16. doi: 10.1093/oxfordjournals.alcalc.a044994. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Grant BF. Major depression in 6050 former drinkers: association with past alcohol dependence. Archives of General Psychiatry. 2002;59:794–800. doi: 10.1001/archpsyc.59.9.794. [DOI] [PubMed] [Google Scholar]
- Haubek A, Lee K. Computed tomography in alcoholic cerebellar atrophy. Neuroradiology. 1979;18:77–9. doi: 10.1007/BF00344826. [DOI] [PubMed] [Google Scholar]
- Hauser P, Altshuler LL, Berrettini W, Dauphinais ID, Gelernter J, Post RM. Temporal lobe measurement in primary affective disorder by magnetic resonance imaging. Journal of Neuropsychiatry and Clinical Neuroscience. 1989;1:128–34. doi: 10.1176/jnp.1.2.128. [DOI] [PubMed] [Google Scholar]
- Hoaken PN, Giancola PR, Pihl RO. Executive cognitive functions as mediators of alcohol-related aggression. Alcohol and Alcoholism. 1998;33:47–54. doi: 10.1093/oxfordjournals.alcalc.a008347. [DOI] [PubMed] [Google Scholar]
- Hurwitz TA, Clark C, Murphy E, Klonoff H, Martin WR, Pate BD. Regional cerebral glucose metabolism in major depressive disorder. Canadian Journal of Psychiatry. 1990;35:684–8. doi: 10.1177/070674379003500807. [DOI] [PubMed] [Google Scholar]
- Jensen GB, Pakkenberg B. Do alcoholics drink their neurons away? Lancet. 1993;342:1201–4. doi: 10.1016/0140-6736(93)92185-v. [DOI] [PubMed] [Google Scholar]
- Kennedy SH, Evans KR, Kruger S, Mayberg HS, Meyer JH, McCann S, et al. Changes in regional brain glucose metabolism measured with positron emission tomography after paroxetine treatment of major depression. American Journal of Psychiatry. 2001;158:899–905. doi: 10.1176/appi.ajp.158.6.899. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Crum RM, Warner LA, Nelson CB, Schulenberg J, Anthony JC. Lifetime co-occurrence of DSM-III-Ralcohol abuse and dependence with other psychiatric disorders in the national comorbidity survey. Archives of General Psychiatry. 1997;54:313–21. doi: 10.1001/archpsyc.1997.01830160031005. [DOI] [PubMed] [Google Scholar]
- Korbo L. Glial cell loss in the hippocampus of alcoholics. Alcohol Clin Exp Res. 1999;23:164–8. [PubMed] [Google Scholar]
- Kril JJ, Halliday GM. Brain shrinkage in alcoholics: a decade on and what have we learned? Progress in Neurobiology. 1999;58:381–7. doi: 10.1016/s0301-0082(98)00091-4. [DOI] [PubMed] [Google Scholar]
- Kril JJ, Halliday GM, Svoboda MD, Cartwright H. The cerebral cortex is damaged in chronic alcoholics. Neuroscience. 1997;79:983–98. doi: 10.1016/s0306-4522(97)00083-3. [DOI] [PubMed] [Google Scholar]
- Kril JJ, Harper CG. Neuronal counts from four cortical regions of alcoholic brains. Acta Neuropathologica. 1989;79:200–4. doi: 10.1007/BF00294379. [DOI] [PubMed] [Google Scholar]
- Laming PR, Kimelberg H, Robinson S, Salm A, Hawrylak N, Muller C, et al. Neuronal-glial interactions and behaviour. Neuroscience and Biobehavioral Reviews. 2000;24:295–340. doi: 10.1016/s0149-7634(99)00080-9. [DOI] [PubMed] [Google Scholar]
- Lishman WA. Alcohol and the brain. British Journal of Psychiatry. 1990;156:635–44. doi: 10.1192/bjp.156.5.635. [DOI] [PubMed] [Google Scholar]
- Mann JJ, Malone KM, Diehl DJ, Perel J, Cooper TB, Mintun MA. Demonstration of vivo of reduced serotonin reponsivity in the brain of untreated depressed patients. American Journal Psychiatry. 1996;153:174–82. doi: 10.1176/ajp.153.2.174. [DOI] [PubMed] [Google Scholar]
- Martinot JL, Hardy P, Feline A, Huret J, Mazoyer B, Attar-Levy D, et al. Left prefrontal glucose hypometabolism in the depressed state: a confirmation. American Journal of Psychiatry. 1990;147:1313–7. doi: 10.1176/ajp.147.10.1313. [DOI] [PubMed] [Google Scholar]
- Mayberg HS. Frontal lobe dysfunction in secondary depression. Journal of Neuropsychiatry and Clinical Neuroscience. 1994;6:428–42. doi: 10.1176/jnp.6.4.428. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Brannan SK, Mahurin RK, Jerabek PA, Brickman JS, Tekell JL, Silva JA, et al. Cingulate function in depression: a potential predictor of treatment response. Neuroreport. 1997;8:1057–61. doi: 10.1097/00001756-199703030-00048. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Starkstein SE, Peyser CE, Brandt J, Dannals RF, Folstein SE. Paralimbic frontal lobe hypometabolism in depression associated with Huntington’s disease. Neurology. 1992;42:1791–7. doi: 10.1212/wnl.42.9.1791. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Starkstein SE, Sadzot B, Preziosi T, Andrezejewski PL, Dannals RF, et al. Selective hypometabolism in the inferior frontal lobe in depressed patients with Parkinson’s disease. Annals of Neurology. 1990;28:57–64. doi: 10.1002/ana.410280111. [DOI] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ, Baucom C, Dilley G, Overholser JC, Meltzer HY, Stockmeier CA, et al. GFAP-immunoreactivity in the prefrontal cortex distinguishes young from old adults in major depressive disorder. Biological Psychiatry. 2000;48:860–72. doi: 10.1016/s0006-3223(00)00999-9. [DOI] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ, Wei J, Andrew M, Overholser JC, Jurjus GJ, Stockmeier CA, et al. Glial pathology in dorsolateral prefrontal cortex in alcohol dependence with and without depressive symptoms. Biological Psychiatry. 2002;52:1121–33. doi: 10.1016/s0006-3223(02)01439-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura MM, Overall JE, Hollister LE, Radcliffe E. Factors affecting outcome of depressive symptoms in alcoholics. Alcoholism, Clinical And Experimental Research. 1983;7:188–93. doi: 10.1111/j.1530-0277.1983.tb05437.x. [DOI] [PubMed] [Google Scholar]
- Nicolas JM, Catafau AM, Estruch R, Lomena FJ, Salamero M, Herranz R, et al. Regional cerebral blood flow-SPECT in chronic alcoholism: relation to neuropsychological testing. Journal of Nuclear Medicine. 1993;34:1452–9. [PubMed] [Google Scholar]
- Öngür D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proceedings of the National Academy of Sciences USA. 1998;95:13290–5. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlson GD, Barta PE, Powers RE, Menon RR, Richards SS, Aylward EH, et al. Ziskind-Somerfeld Research Award 1996. Medial and superior temporal gyral volumes and cerebral asymmetry in schizophrenia versus bipolar disorder. Biological Psychiatry. 1997;41:1–14. doi: 10.1016/s0006-3223(96)00373-3. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom M, Crusan K, Jernigan TL. Brain CT changes in alcoholics: effects of age and alcohol consumption. Alcohol Clinical Experimental Research. 1988;12:81–7. doi: 10.1111/j.1530-0277.1988.tb00137.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Mathalon DH, Lim KO. Frontal lobe volume loss observed with magnetic resonance imaging in older chronic alcoholics. Alcohol Clinical and Experimental Research. 1997;21:521–9. doi: 10.1111/j.1530-0277.1997.tb03798.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Mathalon DH, Shear PK, Rosenbloom MJ, Lim KO. Longitudinal changes in magnetic resonance imaging brain volumes in abstinent and relapsed alcoholics. Alcoholism Clinical and Experimental Research. 1995;19:1177–91. doi: 10.1111/j.1530-0277.1995.tb01598.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Rosenbloom MJ, Mathalon DH, Lim KO. A controlled study of cortical gray matter and ventricular changes in alcoholic men over a 5-year interval. Archives of General Psychiatry. 1998;55:905–12. doi: 10.1001/archpsyc.55.10.905. [DOI] [PubMed] [Google Scholar]
- Pihl RO, Segal Z, Yankofsky L. The effect of alcohol and placebo on affective reactions of social drinkers to a procedure designed to induce depressive affect anxiety and hostility. Journal of Clinical Psychology. 1980;36:337–42. doi: 10.1002/1097-4679(198001)36:1<337::aid-jclp2270360148>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Rajkowska G. Postmortem studies in mood disorders indicate altered numbers of neurons and glial cells. Biological Psychiatry. 2000;48:766–77. doi: 10.1016/s0006-3223(00)00950-1. [DOI] [PubMed] [Google Scholar]
- Rajkowska G. Similarities in glial pathology in orbitofrontal cortex in bipolar disorder and schizophrenia. 40th American College of Neuropsychopharmacology Annual Meeting [abstracts]; p. 414. [Google Scholar]
- Rajkowska G, O’Dwyer G, Shao Q, Stockmeier C, Miguel-Hidalgo JJ. Calbindin-immunoreactive non-pyramidal neurons are reduced in the dorsolateral prefrontal cortex in major depression and schizophrenia. Society for Neuroscience Abstracts. 2002:28. [Google Scholar]
- Rajkowska G, Halaris A, Selemon L. Reductions in neuronal and glial density characterize the dorsolateral prefrontal cortex in bipolar disorder. Biological Psychiatry. 2001;49:741–52. doi: 10.1016/s0006-3223(01)01080-0. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Miguel-Hidalgo JJ, Wei J, Dilley G, Pittman SD, Meltzer HY, et al. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biological Psychiatry. 1999;45:1085–98. doi: 10.1016/s0006-3223(99)00041-4. [DOI] [PubMed] [Google Scholar]
- Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, et al. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study [see comments] JAMA. 1990;264:2511–8. [PubMed] [Google Scholar]
- Ring HA, Bench CJ, Trimble MR, Brooks DJ, Frackowiak RS, Dolan RJ. Depression in Parkinson’s disease. A positron emission study. British Journal of Psychiatry. 1994;165:333–9. doi: 10.1192/bjp.165.3.333. [DOI] [PubMed] [Google Scholar]
- Ron MA, Acker W, Shaw GK, Lishman WA. Computerized tomography of the brain in chronic alcoholism: a Survey and follow-up study. Brain. 1982;105:497–514. doi: 10.1093/brain/105.3.497. [DOI] [PubMed] [Google Scholar]
- Salloum IM, Mezzich JE, Cornelius J, Day NL, Daley D, Kirisci L. Clinical profile of comorbid major depression and alcohol use disorders in an initial psychiatric evaluation. Comprehensive Psychiatry. 1995;36:260–6. doi: 10.1016/s0010-440x(95)90070-5. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Tipp JE, Bergman M, Reich W, Hesselbrock VM, Smith TL. Comparison of induced and independent major depressive disorders in 2,945 alcoholics. American Journal of Psychiatry. 1997;154:948–57. doi: 10.1176/ajp.154.7.948. [DOI] [PubMed] [Google Scholar]
- Sheline Y, Wang P, Gado M, Csernansky J, Vannier M. Hippocampal atrophy in recurrent major depression. Proceedings of the National Academy of Sciences USA. 1996;93:3908–13. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Gado MH, Price JL. Amygdala core nuclei volumes are decreased in recurrent major depression. Neuroreport. 1998;9:2023–8. doi: 10.1097/00001756-199806220-00021. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Sanghavi M, Mintun MA, Gado MH. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. Journal of Neuroscience. 1999;19:5034–43. doi: 10.1523/JNEUROSCI.19-12-05034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder A. Responses of glia to alcohol. In: Aschner N, Kimelberg H, editors. The role of glia in neurotoxicity. Boca Raton: CRC Press; 1996. [Google Scholar]
- Soares J, Mann J. The anatomy of mood disorders—review of structural neuroimaging studies. Biological Psychiatry. 1997;41:86–106. doi: 10.1016/s0006-3223(96)00006-6. [DOI] [PubMed] [Google Scholar]
- Stockmeier CA, Mahajan GJ, Konick LC, Overholser JC, Jurjus GJ, Meltzer HY, et al. Preliminary evidence that neuronal and glial density is increased and neuronal size is decreased in hippocampus in major depressive disorder. Society for Neuroscience Abstracts. 2002:28. [Google Scholar]
- Sullivan EV, Marsh L, Mathalon DH, Lim KO, Pfefferbaum A. Anterior hippocampal volume deficits in nonamnesic, aging chronic alcoholics. Alcohol Clinical and Experimental Research. 1995;19:110–22. doi: 10.1111/j.1530-0277.1995.tb01478.x. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom MJ, Pfefferbaum A. Pattern of motor and cognitive deficits in detoxified alcoholic men. Alcoholism, Clinical and Experimental Research. 2000;24:611–21. [PubMed] [Google Scholar]
- Tupler L, Krishnan K, McDonald W, Dombeck C, D’Souza S, Steffens D. Anatomic location and laterality of MRI signal hyperintensities in late-life depression. Journal of Psychosomatic Research. 2002;53:665–76. doi: 10.1016/s0022-3999(02)00425-7. [DOI] [PubMed] [Google Scholar]
- Videbech P, Ravnkilde B, Fiirgaard B, Clemmensen K, Egander A, Rasmussen NA, et al. Structural brain abnormalities in unselected in-patients with major depression. Acta Psychiatrica Scandinavica. 2001;103:282–6. doi: 10.1034/j.1600-0447.2001.00305.x. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Pandya DN. Cingulate cortex of the rhesus monkey: II. Cortical afferents. The Journal of Comparative Neurology. 1987;262:271–89. doi: 10.1002/cne.902620208. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Hitzemann R, Wang GJ, Fowler JS, Burr G, et al. Decreased brain metabolism in neurologically intact healthy alcoholics. American Journal of Psychiatry. 1992;149:1016–22. doi: 10.1176/ajp.149.8.1016. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Hitzemann R, Fowler JS, Overall JE, Burr G, et al. Recovery of brain glucose metabolism in detoxified alcoholics. American Journal of Psychiatry. 1994;151:178–83. doi: 10.1176/ajp.151.2.178. [DOI] [PubMed] [Google Scholar]
- Weingartner HJ, Eckardt MJ, Hommer D, Johnson DN. Impairments in reflective cognitive functions in alcoholics: a neuropharmacological model. Alcohol and Alcoholism. 1994;2(Supp):291–8. [PubMed] [Google Scholar]
- Weiss RD, Hufford MR. Substance abuse and suicide. In: Jacobs DG, editor. The Harvard Medical School guide to suicide assessment and intervention. San Francisco: Jossey Bass; 1999. [Google Scholar]
- Zipursky RB, Lim KC, Pfefferbaum A. MRI study of brain changes with short-term abstinence from alcohol. Alcohol Clinical and Experimental Research. 1989;13:664–6. doi: 10.1111/j.1530-0277.1989.tb00401.x. [DOI] [PubMed] [Google Scholar]


