Abstract
Asbestos and related fibers are associated with a number of adverse health effects, including malignant mesothelioma (MM), an aggressive cancer that generally develops in the surface serosal cells of the pleural, pericardial, and peritoneal cavities. Although approximately 80% of individuals with MM are exposed to asbestos, fewer than 5% of asbestos workers develop MM. In addition to asbestos, other mineralogical, environmental, genetic, and possibly viral factors might contribute to MM susceptibility. Given this complex etiology of MM, understanding susceptibility to MM needs to be a priority for investigators in order to reduce exposure of those most at risk to known environmental carcinogens. In this review, the current body of literature related to fiber-associated disease susceptibility including age, sex, nutrition, genetics, asbestos, and other mineral exposure is addressed with a focus on MM, and critical areas for further study are recommended.
This review is a post-meeting white paper summarizing the role of factors that influence susceptibility to mineral fiber-induced adverse health effects for the meeting entitled “Asbestos: A Science-Based Examination of the Mode of Action of Asbestos and Related Mineral Fibers,” sponsored by the National Institute of Environmental Health Sciences (NIEHS), Agency for Toxic Substances and Disease Registry (ATSDR), and U.S. Environmental Protection Agency (EPA). The meeting was held December 16-18, 2009, in Research Triangle Park, NC, and the overall goal of the workshop was to review the literature on various areas related to asbestos exposure with some key questions in mind. These questions relate to the current state of asbestos research, the level of consensus in the field, and the areas that require more investigations. Part of this effort is to stimulate research in these areas, as well as to determine the current state of the science particular to the area of susceptibility to fiber-induced disease.
The team addressing susceptibility to asbestos was assigned to consider the main factors that impact susceptibility to fiber-induced adverse health effects, including age, gender and disease status, genetics, and nutrition, as summarized in the following. It is noteworthy that the term “asbestos” often refers to a family of six different fiber types (the amphiboles amosite, crocidolite, tremolite, actinolite, and anthophyllite, and the serpentine chrysotile), each with markedly different mineralogical characteristics and associated adverse health effects. The complexity of defining asbestos minerals, and fibers in general, has led to inconsistencies in the scientific literature (Addison and McConnell 2008). For the purposes of this review, the discussions include studies of asbestos minerals and asbestoslike fibers beyond those listed in this definition. A number of epidemiological studies have primarily associated malignant mesothelioma (MM) with exposure to amphibole-type asbestos fibers, which generally are more persistent and durable in lung than chrysotile; however, to varying degrees, all fiber types have been associated with cancer and MM susceptibility (Berman and Crump 2008; Hodgson and Darnton 2000). Although fiber-induced adverse health effects extend beyond MM susceptibility, this review, reflective of the literature, primarily focuses on MM.
AGE AND SEX IN FIBER-RELATED DISEASE
Although it is well known that males and females display significant differences in responses to environmental insults, gender effects have received relatively little attention in molecular epidemiological studies on environmentally induced diseases. Often gender has been viewed as a confounder rather than a factor of primary importance. Indeed, practically no data exist on the possible role of factors such as age, gender, and disease status in the development of asbestos-related nonmalignant disorders or lung cancer. In contrast, there are extensive data indicating that both occupational and environmental exposures play an important role in the onset of and survival from MM.
MM was shown to occur more frequently in men than in women (Antman et al., 2001). The wide disparity between males and females in the incidence of MM is clearly illustrated by a recent analysis of cases in the United States from 1975 to 2004 based on the Surveillance, Epidemiology and End Results (SEER) Program (Cook et al., 2009). In the analysis, MM was ranked fourth in the list of 10 cancers with the largest male-to-female incidence rate ratios (IRR). The IRR for MM was 4.9, and was preceded only by Kaposi sarcoma (28.7), lip (7.2), and larynx (5.2). Occupational exposure to asbestos in male-dominated professions, such as pipe fitting, construction, shipyard work, and asbestos mining, is strongly associated with risk (Astoul, 2003), and a recent analysis of cancer risk in 15 million people in 5 Nordic countries found MM to have the largest relative differences between the occupations (Pukkala et al., 2009). Thus, the observed gender-related differences in MM incidence may well be claimed to be largely due to occupational reasons; however, since low levels of exposure to MM-associated carcinogens are sufficient to lead to tumorigenesis, it has been difficult to assess susceptibility differences between genders (Astoul, 2003). The question stills remains, how do males and females differ in regard to onset of disease and outcome if both have experienced same equivalent exposures? Understanding the potential mechanisms underlying gender-related differences in response to asbestos-exposure are therefore critical to the development of improved primary prevention strategies and possibly to more adequate therapies.
The genetic variations responsible for the gender-related differences in sensitivity to environmental agents may result from different hormonal environments and/or be evident in sex-specific organs. As for chromosomal changes, it is known that X-chromosome loss occurs with age in women. This was demonstrated in several epidemiological studies assessing micronuclei (MN) frequencies in women versus men (Hando et al., 1994; Surralles et al., 1996; 1999). MN are small amounts of DNA that arise in the cytoplasm when chromatid/chromosomal fragments are not incorporated into daughter nuclei during mitosis, often because these fragments do not possess a centromere (Schmid, 1975). It is also known that the inactive heterochromatic X-chromosome is lost preferentially, and thus the few genes transcribed from this heterochromatic X-chromosome might contribute to sex effects. The importance of loss of the X-chromosome to gender-related differences in sensitivity to environmental carcinogens is uncertain, as loss of the Y-chromosome occurs with age in males.
Although no clear evidence is available that differences exist between females and males in the susceptibility to genotoxic exposure or in the mechanisms underlying disease etiology and pathogenesis, differences between genders in hormonal status and reproductive factors have well-established influences on some cancers (Higginson et al., 1992), in addition to tobacco smoking and job tasks and associated occupational exposures.
Moreover, epigenetic mechanisms, which have not yet been well studied in relation to asbestos-induced susceptibility, may also contribute to the differential susceptibility to MM between men and women (Kaminsky et al., 2006); although the methylation difference of a large number of CpGs analyzed on three human chromosomes identified a relatively small mean methylation difference (0.1%) between males and females (Eckhardt et al., 2006), these small differences in methylation patterns, if present at critical regulatory genes, may exert significant impact on cellular response to environmental exposure. DNA methylation changes in sex hormone genes and/or the gene targets of sex hormones may be considered as a potential epigenetic mechanism underlying gender-specific response to environmental insults. Sex hormones are known to be potent modulators of specific genes and genomic functions, and increasing evidence suggests that sex hormones modify gene expression through local reconfiguration of epigenetic states (Sawan et al., 2008). Different epigenetic mechanisms also appear to cross-influence and reinforce each other in the orchestration of cellular response to environmental stimuli and endogenous cues (Sawan et al., 2008; Vaissiere et al., 2008).
In addition to higher incidence of MM, male gender has also been associated with poor prognosis of MM. Moreover, age at diagnosis, work exposure, age at first exposure, histological subtype, and stage of disease are considered to define a phenotype that sets MM patients with a shortened survival apart from other asbestos-exposed individuals (Boutin et al., 1993; De Pangher Manzini et al., 1993; Van Gelder et al., 1994; Ceresoli et al., 2001; Neumann et al., 2004; BTS statement on malignant mesothelioma in the UK, 2007; Ohar et al., 2007; Montanaro et al., 2009). Recently, the prognostic significance of lymph nodal involvement in this disease was also defined; absence of lymph node metastasis was strongly associated with improved survival (Yan et al., 2006).
However, there appears to be a great deal of conflicting data on the importance of some of these prognostic factors. For instance, the value of patient age in predicting a poor outcome has been challenged (Ohar et al., 2007); at least four studies showed that age is not of importance in this context (Samson et al., 1987; Rusch et al., 1991; Tammilehto, 1992; Boutin et al., 1993), whereas several others suggested that age is an important predictor (Chahinian et al., 1982; De Pangher Manzini et al., 1993; Antman et al., 1988; Calavrezos et al., 1988; Spirtas et al., 1988; Ruffie et al., 1989).
Somewhat surprisingly, there have been several conflicting reports with regard to the importance of stage as a prognostic marker (Samson et al., 1987; Antman et al., 1988; Curran et al., 1998). The main reason for this may be the problems with the existing staging systems. To obtain complete clinical information, patients need to have had cytoreductive surgery such as an extrapleural pneumonectomy However, most patients are not able to have this operation. Consequently, staging data based on radiological findings that are reported in nonsurgical series are likely to be less reliable estimates. As expected, two large surgical series (Rusch & Venkatraman, 1999; Sugarbaker et al., 1999) confirmed that patients staged surgically as stage I or II survive longer than those with advanced stages of MM.
IONIZING RADIATION IN MM
Goodman et al. (2009) published a review of the literature relating the effects of ionizing radiation with MM risk. Ionizing radiation is known to be a human carcinogen and linked to several cancer types (Abbatt, 1979). A review of the literature supports multiple types of ionizing radiation as MM risk factors, finding MM cases among individuals exposed to α- and β-emitters in Thorotrast treatment, γ treatment (60Co and X-ray radiotherapy), and occupational γ radiation (Goodman et al., 2009). However, it is difficult to exclude the possibility of asbestos exposure in radiation-exposed individuals, and not all studies reviewed reached a statistically significant level of association. The role of ionizing radiation in MM remains suggestive and warrants further investigation.
ENVIRONMENTAL EXPOSURES IN MM
MM is a rare, aggressive cancer that is generally found in populations exposed to asbestos (Pass et al., 2004; Astoul, 2003). Although the primary cause of MM is exposure to asbestos fibers (Price, 1997; Carbone et al., 2002; Wagner et al., 1960), other mineralogical (Bertino et al., 2007), radiological (Goodman et al., 2009), and viral exposures (Carbone et al., 2008; Gazdar et al., 2002; Rivera et al., 2008), as well as genetic factors, are thought to contribute to MM susceptibility (Huncharek et al., 1996; Huncharek, 1995; 2002; Ascoli et al., 1998; Dogan et al., 2006; Roushdy-Hammady et al., 2001). Risks associated with exposure to specific types of minerals are difficult to parse; even given environmental and occupational measures of fiber concentration and phase-contrast light microscopy of bronchoalveolar lavage fluid, it can be difficult to address fiber-specific risks given differential alveolar clearance of fibers by type and dimension.
BIOMARKERS IN MM
Due to the rapid progression and high mortality rate associated with MM, identification of a predictive biomarker to screen for individuals at greater risk of developing MM would be useful in studies of tumorigenesis and treatment. Because of recent advances in chemotherapeutic agents that may prolong survival and the potential benefits of biomarkers as diagnostic, monitoring, and screening tools, there has been strong interest in identifying a marker having both high specificity and high sensitivity (Scherpereel & Lee, 2007). In 2003, Robinson et al. (2003) reported that in 7 of 40 asbestos-exposed individuals who tested positive for elevated soluble mesothelin-related peptide (SMRP)/megakaryocyte potentiating factor (both encoded by the same gene), 3 were diagnosed with MM within 3 yr. None of the 33 subjects with normal levels of SMRP developed MM within 8 yr of follow-up study (Pass et al., 2008; Scherpereel & Lee, 2007; Cristaudo et al., 2007). In addition to SMRP (Creaney et al., 2007; Robinson et al., 2003) osteopontin (Pass et al., 2005, 2008; Scherpereel & Lee, 2007; Cristaudo et al., 2006, 2007), and MN/CA9 (Li et al., 2007) are all promising biomarkers for MM. However, no one marker is specific for MM. For example, elevated SMRP levels are sometimes observed in lung cancer patients and healthy, asbestos-exposed individuals. Genetic factors may play a role in population variation of biomarker levels; such potentially confounding factors need to be identified to improve the clinical utility of biomarkers for MM (Cristaudo et al., 2010). A well-characterized biomarker for MM may also be useful in identification of a genetic susceptibility factor by providing an additional quantitative phenotype for use in association and linkage analyses as well as increasing the number of samples that meet inclusion criteria.
GENETIC SUSCEPTIBILITY IN FAMILIAL MM
Although many have considered MM the archetype of environmentally induced cancers, familial incidence and low rate among even those most heavily exposed to known MM-specific carcinogens point to a more complex etiology. Given the low incidence of MM, the likelihood of observing multiple cases of this disease in an unexposed family in the absence of a genetic susceptibility factor is exceedingly rare. Recent reviews of the literature available in the PubMed database (PubMed, National Center for Biotechnology Information, U.S. National Library of Medicine) have come to contrasting conclusions on the likely effect of a genetic susceptibility factor. Ascoli et al. (2007) reviewed data from 33 publications spanning 1968–2005 in Medline, Toxline, and Oshline/Hseline databases, including 51 family clusters and 120 cases of MM among blood relatives. In addition, Ascoli et al. (2007) noted 11 newly identified family clusters from three Italian mesothelioma registries. The observation of MM in spouses as well as blood relations and lack of recurrent chromosomal aberrations in these families led them to conclude that there is a lack of evidence to support an influential genetic susceptibility factor. Ugolini et al. (2008) summarized 20 reports of MM among blood relations in the PubMed database from 1960 to 2007, finding that shared exposures were not sufficient to account for the clustering, and concluded that a polygenic factor was likely at play. In fact, in some instances, in one or more cases within the family no direct exposure to a toxic fiber was identified (Bisconti et al., 2000; Ugolini et al., 2008; Lynch et al., 1985). While the conclusions of Ascoli et al. (2007) and Ugolini et al. (2008) differ, in fact both suggest that there is insufficient evidence to implicate a single genetic susceptibility locus.
It is likely that reviews of MM cases in other exposed populations may reveal additional familial clusters of the disease. Genetic LINKAGE analysis on germline DNA in families has the potential to elucidate genetic regions contributing to risk. An extensive collaboration and databank are necessary to aggregate information and DNA samples for large-scale cross-family LINKAGE analyses.
FIBER-RELATED DISEASE SUSCEPTIBILITY IN LIBBY, MT
Vermiculite from a mine near Libby, MT, is contaminated with tremolite asbestos and other amphibole fibers. Asbestos-contaminated Libby vermiculite was used in loose fill attic insulation that remains in millions of homes in the United States, Canada, and other countries, and a historical cohort mortality study revealed that Libby workers were significantly more likely to die from asbestosis, lung cancer, and MM (Sullivan, 2007). To date, more than 30 cases of MM resulting from exposure to asbestos-containing vermiculite have been identified from Libby, MT (Whitehouse et al., 2008). Included in this group are 11 in non-occupationally exposed people, appearing to have developed disease from exposure to contamination of the community and surrounding areas in proximity to the railroad tracks used to haul vermiculite. The cumulative exposures in this area are considered to be similar to those in Western Australia's crocidolite mine at Wittenoom Gorge.
ERIONITE IN AN MM EPIDEMIC
In Cappadocia, Turkey, a region of Central Anatolia, there are 3 villages (Karain: population approximately 600, Tuzkoy: population approximately 1400, and the abandoned village “Old” Sarihidir: population approximately 250 in the 1960s prior to abandonment) in which 50% or more of all deaths are caused by MM (Baris et al., 1978; 1996; Carbone et al., 2002; Baris & Grandjean, 2006). An international collaboration has undertaken a major research initiative to characterize susceptibility and disease etiology in this region in an effort to understand and curtail this epidemic (Carbone et al., 2007). Although some asbestos was found in the region (Rohl et al., 1982), it was determined that asbestos could not account for the MM epidemic, since neighboring villages with similar levels of asbestos did not experience the high incidence of MM (Baris et al., 1987; Carbone et al., 2002). Indeed, nearby villages such as Karlik, which is only 1.5 miles away from Karain, are not experiencing an excess incidence of MM (Baris et al., 1981). The causative agent was identified as erionite (Baris et al., 1979, 1987; Sebastien et al., 1981; Coplu et al., 1996), a member of the zeolite family of mineral fibers originating from a local volcanic tuff that is quarried by villagers for use in the construction of their homes (Carbone et al., 2002; Baris et al., 1987, 1996). This association is strongly supported by pathologic findings similar to those found in asbestos-exposed populations such as plaques, calcifications (Baris et al., 1978; Baris, 1991), and ferruginous bodies which contained erionite fibers (Sebastien et al., 1981; Dumortier et al., 2001).
Erionite was found to induce MM in rodents both by injection into the pleural or peritoneal cavities and by inhalation (Wagner et al., 1985; Johnson et al., 1984; Maltoni et al., 1982; Van der Meeren et al., 1992). Further investigations in rodents showed that erionite disrupts the balance between cell proliferation and apoptosis toward cell proliferation. In addition, only small amounts of erionite (compared to crocidolite asbestos) are necessary to stimulate c-jun proto-oncogene expression, a known mechanism of fiber carcinogenesis (Timblin et al., 1998). Most recently, erionite was found to transform human mesothelial cells into highly proliferating tumor cells (Bertino et al., 2007). The incidence of all other tumor types is similar in these villages and the rest of Turkey, suggesting that erionite acts specifically on MM carcinogenesis (Carbone et al., 2002; Emri et al., 2002).
Because erionite is present within villages, such as Karlik, in measurable quantities in air samples taken above the streets (Baris et al., 1981) and all homes contained similar amounts of erionite (Roushdy-Hammady et al., 2001), it is reasonable to expect all residents within a village to have similar risk of MM. Yet within towns experiencing the epidemic, MM occurs predominantly in specific families, with no development of the disease in neighboring families (Carbone et al., 2002; Roushdy-Hammady et al., 2001).
Evidence of familial MM is not limited to rural Turkey nor to erionite exposure (Risberg et al., 1980; Lynch et al., 1985; Munoz et al., 1988; Otte et al., 1990; Martensson et al., 1984; Hammar et al., 1989; Dawson et al., 1992; Li et al., 1978, 1989; Huncharek, 1995; Huncharek et al., 1996; Ascoli et al., 1998). Furthermore, erionite, now considered to be the most potent known chemical carcinogen in humans by the International Agency for Research on Cancer, is present in the United States, including Arizona, California, Idaho, Nevada, New Mexico, North Dakota, Oregon, Texas, Utah, and Wyoming (Virta, 2006). Although few North American cases of erionite-associated MM have been reported in the literature (Kliment et al., 2009), research to understand the impact of these exposures to susceptibility in areas within the United States is necessary.
GENETIC FACTORS IN AN MM EPIDEMIC
Preliminary pedigree analysis demonstrates that in these villages, MM is common in some families (Roushdy-Hammady et al., 2001) but not in others, consistent with a genetic contribution to susceptibility. A 6-generation extended pedigree (in which MM showed clear familial clustering) of 526 individuals from Tuzkoy was constructed from kinship maps. Analysis revealed that when high-risk MM family members marry into a low-risk family, MM appears in approximately 50% of the descendents. Further, 41 of 87 children with at least 1 affected parent had MM. Both of these findings are suggestive of an autosomal dominant mode of transmission with reduced penetrance (Figure 1), due perhaps to gene-environment interaction (Carbone et al., 2002; Roushdy-Hammady et al., 2001), although there is debate over the methods and interpretation of the study (Saracci & Simonato, 2001; Ascoli et al., 2001). Interestingly, MM does not seem to develop in members of high-risk families born and raised outside these villages (Carbone et al., 2002), supporting the postulation that an environmental trigger may be necessary to induce susceptibility. These observations support a model of interacting genetic and environmental factors in the susceptibility of Cappadocian villagers to MM.
FIGURE 1.
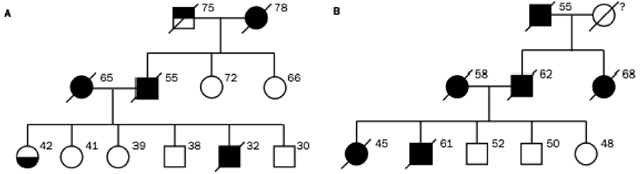
(A, B) Representative three-generation pedigrees taken from a single large pedigree of villagers from Cappadoccia, showing the vertical transmission pattern of MM. Black circles/squares represent affected females/males, respectively. Top-filled square represents liver cancer; bottom-filled circle represents pleural thickening. Ages of individuals in 2001 or ages at death are shown next to each symbol, when known (Roushdy-Hammady et al., 2001).
The level of disease complexity places MM in the group of diseases that present the greatest challenges currently in the field of disease genetics, such as type 2 diabetes, asthma, or cardiovascular disease. MM in Cappadocia is uniquely situated among complex diseases because of the large pedigrees, extensive genotyping, and careful measurements of known environmental susceptibility factors. In addition, this epidemiological story stands alone because of the characteristics of the population; for example, there is likely allelic and environmental homogeneity within this isolated and potentially inbred population, as well as many related affected individuals. These data pave the path to new analytical approaches, building upon and extending methods widely used in the field of computational genetics.
The identification of the putative MM susceptibility gene(s) in a single population, such as Cappadocian villagers, will prompt studies to determine whether the same genes confer susceptibility to familial MM elsewhere in the world and/or contribute to tumor onset or progression in sporadic cancers. The same gene product(s) may act in a common pathway with the environmental susceptibility factors: asbestos, and potentially SV40. In addition, identification of a genetic defect leading to predisposition to MM might lead to the development of preventative and therapeutic approaches that may benefit many patients across industrialized nations.
RECURRENT CHROMOSOMAL ABBERATIONS IN FIBER-RELATED TUMOR TISSUE
There is a long latency between exposure and MM development. A meta-analysis of 21 studies of 1690 cases of MM found that 96% of cancers occurred at least 20 yr after initial exposure, with a mean latency of 32 yr (Lanphear & Buncher, 1992). Murthy and Testa (1999) suggested that such a long latency from the time of exposure to the onset of disease may indicate that multiple genetic events are required for mesothelial cell transformation. Indeed, while no single chromosomal change is shared by all MM, several sites of recurrent deletion were noted that harbor known tumor suppressor genes, and karyotypic analyses of tumors revealed multiple clonal cytogenetic changes, including aneuploidy, deletions, and rearrangements such as unbalanced translocations (Murthy and Testa, 1999; Musti et al., 2006; Testa and Jhanwar, 2003; Gibas et al., 1986; Popescu et al., 1988; Tiainen et al., 1988; Flejter et al., 1989; Hagemeijer et al., 1990; Taguchi et al., 1993; Serio et al., 2009; Neragi-Miandoab & Sugarbaker, 2009; Factor et al., 2009; Ivanov et al., 2009; Taniguchi et al., 2007). Using chromosome banding, fluorescence in situ hybridization, and metaphase-based comparative genomic hybridization analysis, recurring alterations were observed on multiple chromosomes, including chromosomes 1, 3, 4, 6, 9, 11, 13, 14, 15, 17, and 22; moreover, several critical regions, including 1p22, 3p21, 6q, 9p21, 15q11.1-15, and 22q12, were identified and explored as sites harboring putative tumor suppressor genes (Murthy & Testa, 1999).
The NF2 tumor suppressor gene, located at 22q12, is inactivated in about 40-50% of human MMs (Bianchi et al., 1995; Sekido et al., 1995; Cheng et al., 1999). Moreover, several cases of MM were reported in patients with neurofibromatosis 2 (Baser et al., 2002, 2005), an autosomal dominant disorder produced by germline mutation of the NF2 gene. In animal models, evidence suggests that disruption of the NF2 signaling pathway plays an important role in MM development (Altomare et al., 2005, 2009; Fleury-Feith et al., 2003; Jongsma et al., 2008), and several groups found altered expression of NF2 in human MM cells (Guled et al., 2009; Thurneysen et al., 2009). The NF2 product, Merlin, represses cyclin D1 expression, and loss/inactivation of NF2 in MM leads to cell cycle progression in connection with upregulation of cyclin D1 (Xiao et al., 2005). Merlin also inhibits Rac/Pak and focal adhesion kinase (FAK) signaling, which play a role in cell migration and spreading, respectively, and inactivation of NF2 in MM promotes cell invasiveness and spreading (Xiao et al., 2002; Poulikakos et al., 2006).
Homozygous deletion at CDKN2A/ARF, a locus encoding the tumor suppressors p16(INK4A) and p14(ARF), in the shortest region of overlapping deletions in 9p21, is frequently observed in MM (Cheng et al., 1994; Ladanyi, 2005). Inhibitory binding may occur between p16(INK4A) and the cyclin-dependent kinase CDK4, reducing the catalytic activity of the CDK4/cyclin D enzymes (Serrano et al., 1993). The high rates of homozygous deletions of the p16(INK4A) gene in cell lines from numerous tumor types provided support for the postulation that it is the tumor suppressor gene at 9p21 (Kamb et al., 1994; Nobori et al., 1994). Functional studies demonstrated that reexpression of p16(INK4A) in MM cells results in cell cycle arrest, cell death, tumor suppression, and tumor regression (Frizelle et al., 1998). Currently, p16(INK4A) inactivation is considered to be the most common genetic abnormality in MM (Cheng et al., 1994; Ladanyi, 2005), and it may have prognostic significance (Illei et al., 2003). Dacic et al. (2008) observed decreased frequency of homozygous deletion of 9p21 and loss of p16(INK4α) in patients with long-term survival of greater than 3 yr, in contrast to patients with rapidly fatal MM.
While a pathogenic role for loss of p14(ARF) function is less well documented in MM, adenovirus-mediated transfer of p14(ARF) in cultured human MM cell lines was found to induce G1-phase cell cycle arrest and apoptotic cell death (Yang et al., 2000), suggesting that p14(ARF), like p16(INK4A), is an important target of 9p21 deletions in MM. Moreover, while inactivation of p14(ARF) is implicated as a tumor suppressor mainly in association with the simultaneous inactivation of p16(INK4A), heterozygous p19(Arf) mice were recently shown to have increased susceptibility to asbestos-induced MMs compared to wild-type littermates, and tumor formation did not require inactivation of p16Ink4a (Altomare et al., 2009). However, deletions of the CDKN2A/ARF locus inducing loss of both gene products may result in a synergistic effect with regard to MM tumorigenesis, by deregulating both the Rb and p53 pathways via loss of p16(INK4A) and p19(ARF), respectively. Despite the identification of recurrent genomic imbalances in MM, cytogenetics and in situ hybridization have been explored for use as diagnostic and prognostic measures with only modest success (Dacic et al., 2008; Chiosea et al., 2008; Factor et al., 2009; Flores-Staino et al., 2010).
Analyses of common regions of genomic aberrations for patterns of allele-specific hemizygosity need to be explored in populations affected with MM. Some studies identified increased risk associated with hemizygosity at NF2 in mice, and MM arising in these mice usually displayed loss of the remaining wild-type allele (Fleury-Feith et al., 2003). However beyond simple gene-dosage related changes, in sites of recurrent loss, it is possible that the remaining hemizygous segment, uncovered by recurrent deletions in tumor tissue, contains a single common underlying haplotype contributing to disease etiology. Since it is likely that the disease susceptibility locus in a single affected population may have been inherited identically by decent (IBD), genome-wide single-nucleotide polymorphism (SNP) data in available samples from Turkey and Italy are particularly well suited to this kind of analysis. The statistical significance of any haplotype in tumor tissue shared across individuals can be determined either by permutation test or analytically, based on the haplotype frequencies observed in the population. Patients who are homozygous for the same underlying haplotypes in these regions may also contribute to the significance of the association with disease; combining these data may improve power to detect important disease-associated haplotypes. Such methods provide an additional avenue for detection by extending the coverage of the genome-wide marker data.
GENETIC ANALYSES
Genetic research on MM has progressed slowly due to the rarity of the disease as well as the difficulty of sample collection in such a late-onset and highly malignant cancer. As sample collection progresses in populations and families disproportionately affected by MM, and collaborations develop across continents, data sets will emerge with sufficient size and richness to identify novel susceptibility loci and further explore the genetic pathways that have emerged from past studies of recurrent chromosomal imbalances and differential expression in human MM and mouse models of the disease. Because genetic risk factors may be specific to different asbestos exposures, such studies are likely to require carefully documented fiber-specific exposure levels, as well as documentation of activities and behaviors that may affect susceptibility.
Gene Expression in Tumor Cell Lines
Cancer results from an accumulation of genomic alterations that affect gene expression and lead to loss of cell cycle control. With the advances in expression arrays, new avenues are available to search genome-wide for common effects of heterogeneous chromosomal imbalances. Although several studies attempted genome-wide expression analyses on data sets, these have been too few to yield conclusive results. Integrated, high-throughput microarray-based genomic copy number and expression analysis of normal and tumor tissues from MM patients may offer a wealth of opportunity to conduct extension and validation studies.
Genetic Association Analyses of SNP and CNV
To date, association studies for MM focused on candidate genes. Recently, Gemignani et al. (2009) examined genes involved in xenobiotic and oxidative metabolism and found a marginally significant association with a SNP in the NAT2 gene, replicating the findings of several other groups; however, the results are controversial. Polymorphisms in NAT2 impact N-acetylation, which in human populations segregate into fast, intermediate, and slow acetylator phenotypes, have been associated with several types of cancer (Roemer et al., 2008; Weistenhofer et al., 2008; Guey et al., 2010; Malik et al., 2009; Sobti et al., 2009). A study of occupationally asbestos-exposed Finnish MM patients demonstrated an increased risk in individuals with the NAT2 slow acetylator phenotype (Hirvonen et al., 1995, 1996) as did Gemignani et al. (2009), while studies of Italian patients found an increased risk of MM with the NAT2 fast acetylator phenotype (Neri et al., 2005). Variants of the CYP1A1 (Neri et al., 2005, 2006), GSTM1 (Hirvonen et al., 1995; Landi et al., 2007), GSTT1 (Neri et al., 2006; Landi et al., 2007), EPHX (Neri et al., 2005; 2006), XRCC1 (Dianzani et al., 2006), and XRCC3 (Dianzani et al., 2006) genes have also been implicated with borderline statistical significance in candidate gene studies.
Studies of MM have suffered from poor statistical power due to small sample size, population structure, and poorly characterized environmental exposures. Genome-wide association studies (GWAS) in a large, unrelated sample set with rich data on environmental exposures are likely to reveal common genetic susceptibility factors segregating in affected populations. In addition, results of GWAS in cancer (and other complex disorders) suggest that the genes involved with MM susceptibility may have quite modest effects. Therefore, to acquire the necessary sample size for a well-powered study, a large international collaboration is required.
Spans of homozygosity may also arise in MM patients from deletions or mitotic nondisjunction (in which case, they are truly spans of hemizygosity, and can be identified as spans of reduced copy number) or through copy-neutral events such as gene conversion, nondisjunction followed by duplication of a parental chromosome, mitotic recombination, or disease association under a recessive model. Shared haplotypes within spans of homo- or hemizygosity in whole blood or tumor tissue among MM patients may reveal genes important in MM carcinogenesis, such as tumor suppressor genes. These data provide an exciting opportunity to develop new methods for detection and analysis of a combination of homozygosity and hemizygosity among individuals affected by MM.
Because somatic changes in DNA accompany tumor formation and metastasis, DNA copy number variants (CNV) need to be assessed in tumor tissue as well as whole blood from the same affected individuals to be genotyped with high-density arrays, thus providing data on paired tumor and germline DNA from a subset of affected individuals.
Linkage Analyses in MM-Enriched Families
Several families in the United States have a high incidence of MM but little or no known asbestos exposure. Risk of MM among even the most heavily asbestos-exposed workers is only about 5%, indicating that a factor, perhaps genetic, other than asbestos accounts for the susceptibility within such families. Linkage analyses in families with many cases of MM as well as analyses in numerous families with small numbers of cases are powered to identify rare variants on shared haplotypes contributing to disease risk (Kruglyak et al., 1996). Such analyses can complement association studies in large data sets that are powered to detect common variation by identifying differential allele frequencies between unrelated cases and controls.
NUTRITION AND ASBESTOS-RELATED DISEASE
There is a paucity of studies examining the relationship between diet and asbestos-related diseases. On the other hand, consumers worldwide have become increasingly interested in how diet influences their health and about the nutritional value and safety of the food they eat. Evidence continues to mount that dietary components not only influence the ability to achieve one's genetic potential but also influence physical and cognitive function and the risk of a number of diseases. Unfortunately, there are numerous inconsistencies in the available data, and few, if any, effective strategies to identify those who might benefit from dietary intervention have been identified. Nevertheless, support for the Wynder and Gori (1977) suggestion more than 30 yr ago that diet was fundamental to determining cancer risk has continued, and recent epidemiological studies clarified the influence of specific food constituents on cancer risk and tumor behavior. The primary health effects of asbestos exposure are related to the lung, ranging from fibrosis to lung cancer and MM. Therefore, the focus of this section is on what is known (or not known) about nutrients and lung disease, and on the little that is known about nutrients and asbestos-related diseases.
In an early case-control study of diet and MM in Louisiana, Schiffman et al. (1988) reported that consumption of vegetables may have a protective effect on developing this disease, citing an inverse relationship between MM and vegetable intake although a specific food could not be implicated nor could the mechanism be explained. A subsequent report on 94 men and women with MM suggested that tomato or carrot consumption may decrease the risk for MM (Muscat & Huncharek, 1996). The significant reduction in risk of MM was associated with carrot intake but not other carotene- or vitamin A-containing foods. The decreased risk for MM was associated with consumption of tomatoes or tomato juice but the association did not achieve statistical significance. Nevertheless, Muscat and Huncharek (1996) indicated that consumption of many vegetables and fruits might have protective effects against MM. No other studies have been identified that further examined the relationship between dietary components and MM. In contrast, interest in the broader relationship between diet and cancer increased over the last two decades, and since lung carcinoma continues to be the most common cancer globally, followed by breast and colorectal cancers, research has focused on the impact of diet and its components on specific types of cancer.
Fortes et al. (2003) reported on the relationship between components of the Mediterranean diet and lung cancer in a hospital-based, case-control study of patients admitted between 1993 and 1996. Basing nutrient intake on self-administered food frequency questionnaires and controlling for smoking, data demonstrated the protective effects for high consumption of carrots, tomatoes, white meat, exclusive use of olive oil, and regular consumption of sage. Data supported claims that foods rich in antioxidants may reduce the risk for lung cancer. The mechanisms implicated included the protection against oxidative damage to DNA and polyphenolic compounds that prevented interaction of benzo[a]pyrene (BaP) with DNA. Despite this and many other suggestive reports, the effects of diet on cancer development or progression remain contradictory and likely to reflect variation in the ability of food components to reach and/or modify critical cellular targets (Milner, 2006).
Cruciferous vegetables (broccoli, cabbage, cauliflower, Brussels sprouts, kale) that are rich in isothiocyanates were postulated to reduce the risk for lung cancer. A recently published systematic review of the epidemiologic literature through December 2007 identified 30 studies on the association between lung cancer and either total cruciferous vegetable consumption or specific cruciferous vegetables and found that the risk for lung cancer among those in the highest category of total cruciferous vegetable intake was 22% lower in case-control studies compared to those in the lowest category of intake (Lam et al., 2009). Moreover, the strongest inverse relationship was seen among those who were homozygous for GSTM1 and GSTT1 deletions. Of interest is that a review of the relationship between asbestos-related diseases and genetic variability in genes involved in xenobiotic and oxidative metabolism or in DNA repair processes identified increased risk with the GSTM1-null genotype (Neri et al., 2008). Taken together, these data suggest that components of cruciferous vegetables may modify the risk for asbestos-related disease in individuals with genetic susceptibility, warranting further investigation.
The chemopreventive potential of β-carotene, a provitamin A carotenoid, was supported by a number of observational studies, leading to the initiation of several large-scale randomized chemoprevention trials to determine whether supplementation with β-carotene protected against lung cancer, with disappointing results (Omenn et al., 1996; Virtamo et al., 2003). In fact, these trials found that β-carotene supplementation elevated the risk of lung cancer in high-risk populations. Given the importance of clarifying the potential role of carotenoids in the development of lung cancer and the diverse body of evidence, Gallicchio et al. (2008) conducted a systematic and quantitative review of randomized clinical trials and prospective observational studies and demonstrated that of β-carotene supplementation was not associated with a decrease in the risk of developing lung cancer. The lack of association between lung cancer risk and carotenoid intake was attributed to the likelihood that carotenoid levels were more of a marker of a healthier lifestyle (higher intake of fruits and vegetables) or of residual confounding by smoking. Studies to further examine the effects of carotenoids on lung cancer are not likely to be fruitful.
Another component of food with potential anticancer effects is the phytosterols or plant sterols. Phytoestrols are structurally similar to cholesterol, exist in several forms in plants, and are found in grain legumes (sesame, chickpeas, lentils, and peas), cereal grains (wheat, corn, millet, rye and barley), vegetable oils (e.g., corn), and nuts (pecans, pine, pistachio, peanuts, cashew, and almonds) (Woyengo et al., 2009). Woyengo et al. (2009) concluded that phytosterols appear to inhibit the development of various cancers by inhibiting proliferation and promoting apoptosis of cancer cells through the activation of caspase enzymes and may reduce cancer development by lowering blood cholesterol and consequently altering cell membranes. Further study of the potential role of phytosterols in humans is required.
A number of other foods or food components have been studied and not shown to influence cancer risk, including garlic (Kim and Kwon, 2009) and vitamins A, E, C, and folate (Cho et al., 2006). Flavonoids, a group of potentially chemoprotective compounds widely distributed in fruit, vegetables, and beverages of plant origin, have also been studied as the potential active ingredient responsible for the reduction of cancer risk. In their analysis of data from the Nurses’ Health Study, Wang et al. (2009) did not find a significant role for five common flavonols and flavones or selected flavonoid-rich foods in cancer prevention. In a small case-control study, dietary zinc and copper, but not selenium, appeared to be associated with lower risk for lung cancer (Mahabir et al., 2007).
Finally, a few words about nutrition in early life: It is well accepted that environmental exposures play a significant role in the etiology of disease. Such exposures usually precede the appearance of clinical disease, often by a prolonged period of time. Besides the role of genetic mutations, epigenetic processes such as DNA methylation and covalent modification of histones are likely to play a role in alterations in gene activity that may contribute to risk for some types of cancer. Burdge et al. (2009) provided a compelling hypothesis that increased susceptibility to certain disease, including cancer, may have a common origin in developmental changes induced by epigenetic changes induced by environmental exposures, which includes nutrition. The maternal diet, including nutrients known to affect methylation reactions such as folate, vitamin B12, and sulfur amino acids, may impact the developing fetus and contribute to disease susceptibility later in life. This is an important area of future investigation as interest increases with regard to how nutrients influence the development or susceptibility to multiple diseases.
In summary, in the area of the impact of nutrients on asbestos-related diseases, little is known and even less is being investigated. However, because of the increasing awareness that nutrients play an important role in the onset and development of disease and because of the ever-changing food supply as we face a global economy, it seems highly worthwhile to begin to support active research in this area.
Footnotes
#This author has disclosed a potential conflict of interest as described by one or more of the following: He/She may have also received (and may also apply in future for) competitive-funding research grants from U.S. publicly financed, peer-reviewed grant approval process agencies concerning asbestos exposure and disease, including topics covered in all aspects of the workshop, including but not limited to research support from NIEHS.
Meeting support was provided by NIEHS, ATSDR and US-EPA. We thank Dr. Maureen Gwinn for advice during the preparation of the article.
REFERENCES
- Abbatt J. D. History of the use and toxicity of thorotrast. Environ. Res. 1979;18:6–12. [PubMed] [Google Scholar]
- Addison J., McConnell E. E. A review of carcinogenicity studies of asbestos and non-asbestos tremolite and other amphiboles. Regul. Toxicol. Pharmacol. 2008;52:S187–S199. doi: 10.1016/j.yrtph.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Altomare D. A., Menges C. W., Pei J., Zhang L., Skele-Stump K. L., Carbone M., Kane A. B., Testa J. R. Activated TNF-alpha/NF-kappaB signaling via downregulation of Fas-associated factor 1 in asbestos-induced mesotheliomas from Arf knockout mice. Proc. Natl. Acad. Sci. USA. 2009;106:3420–3425. doi: 10.1073/pnas.0808816106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altomare D. A., Vaslet C. A., Skele K. L., De Rienzo A., Devarajan K., Jhanwar S. C., McClatchey A. I., Kane A. B., Testa J. R. A mouse model recapitulating molecular features of human mesothelioma. Cancer Res. 2005;65:8090–8095. doi: 10.1158/0008-5472.CAN-05-2312. [DOI] [PubMed] [Google Scholar]
- Antman K. H., Pass H. I., Schiff P. B. Management of mesothelioma. In: DeVita V. T. J., Hellman S., Rosenberg S. A., editors. Cancer: Principles and practice of oncology. 6th ed. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 1943–1969. [Google Scholar]
- Antman K., Shemin R., Ryan L., Klegar K., Osteen R., Herman T., Lederman G., Corson J. Malignant mesothelioma: Prognostic variables in a registry of 180 patients, the Dana-Farber Cancer Institute and Brigham and Women's Hospital experience over two decades, 1965–1985. J. Clin. Oncol. 1988;6:147–153. doi: 10.1200/JCO.1988.6.1.147. [DOI] [PubMed] [Google Scholar]
- Ascoli V., Cavone D., Merler E., Barbieri P. G., Romeo L., Nardi F., Musti M. Mesothelioma in blood related subjects: report of 11 clusters among 1954 Italy cases and review of the literature. Am. J. Ind. Med. 2007;50:357–369. doi: 10.1002/ajim.20451. [DOI] [PubMed] [Google Scholar]
- Ascoli V., Mecucci C., Knuutila S. Genetic susceptibility and familial malignant mesothelioma. Lancet. 2001;357:1804. doi: 10.1016/S0140-6736(00)04922-9. [DOI] [PubMed] [Google Scholar]
- Ascoli V., Scalzo C. C., Bruno C., Facciolo F., Lopergolo M., Granone P., Nardi F. Familial pleural malignant mesothelioma: Clustering in three sisters and one cousin. Cancer Lett. 1998;130:203–207. doi: 10.1016/s0304-3835(98)00142-6. [DOI] [PubMed] [Google Scholar]
- Astoul P. In: Textbook of pleural diseases. Light R. W., Lee Y. C. G., editors. New York: Arnold; 2003. [Google Scholar]
- Baris B., Demir A. U., Shehu V., Karakoca Y., Kisacik G., Baris Y. I. Environmental fibrous zeolite (erionite) exposure and malignant tumors other than mesothelioma. J. Environ. Pathol. Toxicol. Oncol. 1996;15:183–189. [PubMed] [Google Scholar]
- Baris I., Simonato L., Artvinli M., Pooley F., Saracci R., Skidmore J., Wagner C. Epidemiological and environmental evidence of the health effects of exposure to erionite fibres: A four-year study in the Cappadocian region of Turkey. Int. J. Cancer. 1987;39:10–17. doi: 10.1002/ijc.2910390104. [DOI] [PubMed] [Google Scholar]
- Baris Y. I. Fibrous zeolite (erionite)-related diseases in Turkey. Am. J. Ind. Med. 1991;19:374–378. doi: 10.1002/ajim.4700190310. [DOI] [PubMed] [Google Scholar]
- Baris Y. I., Artvinli M., Sahin A. A. Environmental mesothelioma in Turkey. Ann. NY Acad. Sci. 1979;330:423–432. doi: 10.1111/j.1749-6632.1979.tb18744.x. [DOI] [PubMed] [Google Scholar]
- Baris Y. I., Grandjean P. Prospective study of mesothelioma mortality in Turkish villages with exposure to fibrous zeolite. JNCI. 2006;98:414–417. doi: 10.1093/jnci/djj106. [DOI] [PubMed] [Google Scholar]
- Baris Y. I., Sahin A. A., Ozesmi M., Kerse I., Ozen E., Kolacan B., Altinors M., Goktepeli A. An outbreak of pleural mesothelioma and chronic fibrosing pleurisy in the village of Karain/Urgup in Anatolia. Thorax. 1978;33:181–192. doi: 10.1136/thx.33.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baris Y. I., Saracci R., Simonato L., Skidmore J. W., Artvinli M. Malignant mesothelioma and radiological chest abnormalities in two villages in Central Turkey. An epidemiological and environmental investigation. Lancet. 1981;1:984–987. doi: 10.1016/s0140-6736(81)91742-6. [DOI] [PubMed] [Google Scholar]
- Baser M. E., De Rienzo A., Altomare D., Balsara B. R., Hedrick N. M., Gutmann D. H., Pitts L. H., Jackler R. K., Testa J. R. Neurofibromatosis 2 and malignant mesothelioma. Neurology. 2002;59:290–291. doi: 10.1212/wnl.59.2.290. [DOI] [PubMed] [Google Scholar]
- Baser M. E., Rai H., Wallace A. J., Evans D. G. Neurofibromatosis 2 (NF2) and malignant mesothelioma in a man with a constitutional NF2 missense mutation. Family Cancer. 2005;4:321–322. doi: 10.1007/s10689-005-0659-8. [DOI] [PubMed] [Google Scholar]
- Berman D. W., Crump K. S. A meta-analysis of asbestos-related cancer risk that addresses fiber size and mineral type. Crit. Rev. Toxicol. 2008;38(suppl. 1):49–73. doi: 10.1080/10408440802273156. [DOI] [PubMed] [Google Scholar]
- Bertino P., Marconi A., Palumbo L., Bruni B. M., Barbone D., Germano S., Dogan A. U., Tassi G. F., Porta C., Mutti L., Gaudino G. Erionite and asbestos differently cause transformation of human mesothelial cells. Int. J. Cancer. 2007;121:12–20. doi: 10.1002/ijc.22687. [DOI] [PubMed] [Google Scholar]
- Bianchi A. B., Mitsunaga S. I., Cheng J. Q., Klein W. M., Jhanwar S. C., Seizinger B., Kley N., Klein-Szanto A. J., Testa J. R. High frequency of inactivating mutations in the neurofibromatosis type 2 gene (NF2) in primary malignant mesotheliomas. Proc. Natl. Acad. Sci. USA. 1995;92:10854–10858. doi: 10.1073/pnas.92.24.10854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisconti M., Bisetti A., Bidoli P. Malignant mesothelioma in subjects with Marfan's syndrome and Ehlers-Danlos syndrome: Only an apparent association? Respiration. 2000;67:223–228. doi: 10.1159/000029493. [DOI] [PubMed] [Google Scholar]
- Boutin C., Rey F., Gouvernet J., Viallat J. R., Astoul P., Ledoray V. Thoracoscopy in pleural malignant mesothelioma: A prospective study of 188 consecutive patients. Part 2: Prognosis and staging. Cancer. 1993;72:394–404. doi: 10.1002/1097-0142(19930715)72:2<394::aid-cncr2820720214>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- BTS statement on malignant mesothelioma in the UK. Thorax. 2007;62(suppl. 2):ii1–ii19. doi: 10.1136/thx.2007.087619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdge G. C., Lillycrop K. A., Jackson A. A. Nutrition in early life, and risk of cancer and metabolic disease: Alternative endings in an epigenetic tale? Br. J. Nutr. 2009;101:619–630. doi: 10.1017/S0007114508145883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calavrezos A., Koschel G., Husselmann H., Taylessani A., Heilmann H. P., Fabel H., Schmoll H. J., Dietrich H., Hain E. Malignant mesothelioma of the pleura. A prospective therapeutic study of 132 patients from 1981-1985. Klin. Wochenschr. 1988;66:607–613. doi: 10.1007/BF01728801. [DOI] [PubMed] [Google Scholar]
- Carbone M., Emri S., Dogan A. U., Steele I., Tuncer M., Pass H. I., Baris Y. I. A mesothelioma epidemic in Cappadocia: Scientific developments and unexpected social outcomes. Nat. Rev. Cancer. 2007;7:147–154. doi: 10.1038/nrc2068. [DOI] [PubMed] [Google Scholar]
- Carbone M., Kratzke R. A., Testa J. R. The pathogenesis of mesothelioma. Semin. Oncol. 2002;29:217. doi: 10.1053/sonc.2002.30227. [DOI] [PubMed] [Google Scholar]
- Carbone M., Pannuti A., Zhang L., Testa J. R., Bocchetta M. A novel mechanism of late gene silencing drives SV40 transformation of human mesothelial cells. Cancer Res. 2008;68:9488–9496. doi: 10.1158/0008-5472.CAN-08-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceresoli G. L., Locati L. D., Ferreri A. J., Cozzarini C., Passoni P., Melloni G., Zannini P., Bolognesi A., Villa E. Therapeutic outcome according to histologic subtype in 121 patients with malignant pleural mesothelioma. Lung Cancer. 2001;3:279–287. doi: 10.1016/s0169-5002(01)00257-4. [DOI] [PubMed] [Google Scholar]
- Chahinian A. P., Pajak T. F., Holland J. F., Norton L., Ambinder R. M., Mandel E. M. Diffuse malignant mesothelioma. Prospective evaluation of 69 patients. Ann. Intern. Med. 1982;96:746–755. doi: 10.7326/0003-4819-96-6-746. [DOI] [PubMed] [Google Scholar]
- Cheng J. Q., Jhanwar S. C., Klein W. M., Bell D. W., Lee W. C., Altomare D. A., Nobori T., Olopade O. I., Buckler A. J., Testa J. R. p16 Alterations and deletion mapping of 9p21-p22 in malignant mesothelioma. Cancer Res. 1994;5:5547–5551. [PubMed] [Google Scholar]
- Cheng J. Q., Lee W. C., Klein M. A., Cheng G. Z., Jhanwar S. C., Testa J. R. Frequent mutations of NF2 and allelic loss from chromosome band 22q12 in malignant mesothelioma: Evidence for a twohit mechanism of NF2 inactivation. Genes Chromosomes Cancer. 1999;24:238–242. [PubMed] [Google Scholar]
- Chiosea S., Krasinskas A., Cagle P. T., Mitchell K. A., Zander D. S., Dacic S. Diagnostic importance of 9p21 homozygous deletion in malignant mesotheliomas. Mod. Pathol. 2008;21:742–747. doi: 10.1038/modpathol.2008.45. [DOI] [PubMed] [Google Scholar]
- Cho E., Hunter D. J., Spiegelman D., Albanes D., Beeson W. L., van den Brandt P. A., Colditz G. A., Feskanich D., Folsom A. R., Fraser G. E., Freudenheim J. L., Giovannucci E., Goldbohm R. A., Graham S., Miller A. B., Rohan T. E., Sellers T. A., Virtamo J., Willett W. C., Smith-Warner S. A. Intakes of vitamins A, C and E and folate and multivitamins and lung cancer: A pooled analysis of 8 prospective studies. Int. J. Cancer. 2006;118:970–978. doi: 10.1002/ijc.21441. [DOI] [PubMed] [Google Scholar]
- Cook M. B., Dawsey S. M., Freedman N. D., Inskip P. D., Wichner S. M., Quraishi S. M., Devesa S. S., McGlynn K. A. Sex disparities in cancer incidence by period and age. Cancer Epidemiol. Biomarkers Prev. 2009;18:1174–1182. doi: 10.1158/1055-9965.EPI-08-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coplu L., Dumortier P., Demir A. U., Selcuk Z. T., Kalyoncu F., Kisacik G., DeVuyst P., Sahin A. A., Baris Y. I. An epidemiological study in an Anatolian village in Turkey environmentally exposed to tremolite asbestos. J. Environ. Pathol. Toxicol. Oncol. 1996;15:177–182. [PubMed] [Google Scholar]
- Creaney J., Yeoman D., Naumoff L. K., Hof M., Segal A., Musk A. W., De Klerk N., Horick N., Skates S. J., Robinson B. W. Soluble mesothelin in effusions: A useful tool for the diagnosis of malignant mesothelioma. Thorax. 2007;62:569–576. doi: 10.1136/thx.2006.068114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristaudo A., Foddis R., Bonotti A., Simonini S., Vivaldi A., Guglielmi G., Bruno R., Landi D., Gemignani F., Landi S. Polymorphisms in the putative micro-RNA-binding sites of mesothelin gene are associated with serum levels of mesothelin-related protein. Occup. Environ. Med. 2010;67:233–236. doi: 10.1136/oem.2009.049205. [DOI] [PubMed] [Google Scholar]
- Cristaudo A., Foddis R., Buselli R., Gattini V., Di Palma N., Guglielmi G. Medical surveillance of workers previously exposed to asbestos. Med. Lav. 2006;97:475–481. [PubMed] [Google Scholar]
- Cristaudo A., Foddis R., Vivaldi A., Guglielmi G., Dipalma N., Filiberti R., Neri M., Ceppi M., Paganuzzi M., Ivaldi G. P., Mencoboni M., Canessa P. A., Ambrosino N., Chella A., Mutti L., Puntoni R. Clinical significance of serum mesothelin in patients with mesothelioma and lung cancer. Clin. Cancer Res. 2007;13:5076–5081. doi: 10.1158/1078-0432.CCR-07-0629. [DOI] [PubMed] [Google Scholar]
- Curran D., Sahmoud T., Therasse P., van Meerbeeck J., Postmus P. E., Giaccone G. Prognostic factors in patients with pleural mesothelioma: The European Organization for Research and Treatment of Cancer experience. J. Clin. Oncol. 1998;16:145–152. doi: 10.1200/JCO.1998.16.1.145. [DOI] [PubMed] [Google Scholar]
- Dacic S., Kothmaier H., Land S., Shuai Y., Halbwedl I., Morbini P., Murer B., Comin C., Galateau-Salle F., Demirag F., Zeren H., Attanoos R., Gibbs A., Cagle P., Popper H. Prognostic significance of p16/cdkn2a loss in pleural malignant mesotheliomas. Virchows Arch. 2008;453:627–635. doi: 10.1007/s00428-008-0689-3. [DOI] [PubMed] [Google Scholar]
- Dawson A., Gibbs A., Browne K., Pooley F., Griffiths M. Familial mesothelioma. Details of 17 cases with histopathologic findings and mineral analysis. Cancer. 1992;70:1183–1187. doi: 10.1002/1097-0142(19920901)70:5<1183::aid-cncr2820700526>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- De Pangher Manzini V., Brollo A., Franceschi S., DeMatthaeis M., Talamini R., Bianchi C. Prognostic factors of malignant mesothelioma of the pleura. Cancer. 1993;72:410–417. doi: 10.1002/1097-0142(19930715)72:2<410::aid-cncr2820720216>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Dianzani I., Gibello L., Biava A., Giordano M., Bertolotti M., Betti M., Ferrante D., Guarrera S., Betta G. P., Mirabelli D., Matullo G., Magnani C. Polymorphisms in DNA repair genes as risk factors for asbestos-related malignant mesothelioma in a general population study. Mutat. Res. 2006;599:124–134. doi: 10.1016/j.mrfmmm.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Dogan A. U., Baris Y. I., Dogan M., Emri S., Steele I., Elmishad A. G., Carbone M. Genetic predisposition to fiber carcinogenesis causes a mesothelioma epidemic in Turkey. Cancer Res. 2006;66:5063–5068. doi: 10.1158/0008-5472.CAN-05-4642. [DOI] [PubMed] [Google Scholar]
- Dumortier P., Coplu L., Broucke I., Emri S., Selcuk T., de Maertelaer V., De Vuyst P., Baris I. Erionite bodies and fibres in bronchoalveolar lavage fluid (BALF) of residents from Tuzkoy, Cappadocia, Turkey. Occup. Environ. Med. 2001;58:261–266. doi: 10.1136/oem.58.4.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhardt F., Lewin J., Cortese R., Rakyan V. K., Attwood J., Burger M., Burton J., Cox T. V., Davies R., Down T. A., Haefliger C., Horton R., Howe K., Jackson D. K., Kunde J., Koenig C., Liddle J., Niblett D., Otto T., Pettett R., Seemann S., Thompson C., West T., Rogers J., Olek A., Berlin K., Beck S. DNA methylation profiling of human chromosomes 6, 20 and 22. Nat Genet. 2006;38:1378–1385. doi: 10.1038/ng1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emri S., Demir A., Dogan M., Akay H., Bozkurt B., Carbone M., Baris I. Lung diseases due to environmental exposures to erionite and asbestos in Turkey. Toxicol. Lett. 2002;127:251–257. doi: 10.1016/s0378-4274(01)00507-0. [DOI] [PubMed] [Google Scholar]
- Factor R. E., Dal Cin P., Fletcher J. A., Cibas E. S. Cytogenetics and fluorescence in situ hybridization as adjuncts to cytology in the diagnosis of malignant mesothelioma. Cancer Cytopathol. 2009;117:247–253. doi: 10.1002/cncy.20036. [DOI] [PubMed] [Google Scholar]
- Flejter W. L., Li F. P., Antman K. H., Testa J. R. Recurring loss involving chromosomes 1, 3, and 22 in malignant mesothelioma: Possible sites of tumor suppressor genes. Genes Chromosomes Cancer. 1989;1:148–154. doi: 10.1002/gcc.2870010207. [DOI] [PubMed] [Google Scholar]
- Fleury-Feith J., Lecomte C., Renier A., Matrat M., Kheuang L., Abramowski V., Levy F., Janin A., Giovannini M., Jaurand M. C. Hemizygosity of Nf2 is associated with increased susceptibility to asbestos-induced peritoneal tumours. Oncogene. 2003;22:3799–3805. doi: 10.1038/sj.onc.1206593. [DOI] [PubMed] [Google Scholar]
- Flores-Staino C., Darai-Ramqvist E., Dobra K., Hjerpe A. Adaptation of a commercial fluorescent in situ hybridization test to the diagnosis of malignant cells in effusions. Lung Cancer. 2010;68:39–43. doi: 10.1016/j.lungcan.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Fortes C., Forastiere F., Farchi S., Mallone S., Trequattrinni T., Anatra F., Schmid G., Perucci C. A. The protective effect of the Mediterranean diet on lung cancer. Nutr. Cancer. 2003;46:30–37. doi: 10.1207/S15327914NC4601_04. [DOI] [PubMed] [Google Scholar]
- Frizelle S. P., Grim J., Zhou J., Gupta P., Curiel D. T., Geradts J., Kratzke R. A. Re-expression of p16INK4a in mesothelioma cells results in cell cycle arrest, cell death, tumor suppression and tumor regression. Oncogene. 1998;16:3087–3095. doi: 10.1038/sj.onc.1201870. [DOI] [PubMed] [Google Scholar]
- Gallicchio L., Boyd K., Matanoski G., Tao X. G., Chen L., Lam T. K., Shiels M., Hammond E., Robinson K. A., Caulfield L. E., Herman J. G., Guallar E., Alberg A. J. Carotenoids and the risk of developing lung cancer: A systematic review. Am. J. Clin. Nutr. 2008;88:372–383. doi: 10.1093/ajcn/88.2.372. [DOI] [PubMed] [Google Scholar]
- Gazdar A. F., Butel J. S., Carbone M. SV40 and human tumours: myth, association or causality? Nat. Rev. Cancer. 2002;2:957–964. doi: 10.1038/nrc947. [DOI] [PubMed] [Google Scholar]
- Gemignani F., Neri M., Bottari F., Barale R., Canessa P. A., Canzian F., Ceppi M., Spitaleri I., Cipollini M., Ivaldi G. P., Mencoboni M., Scaruffi P., Tonini G. P., Ugolini D., Mutti L., Bonassi S., Landi S. Risk of malignant pleural mesothelioma and polymorphisms in genes involved in the genome stability and xenobiotics metabolism. Mutat. Res. 2009;671:76–83. doi: 10.1016/j.mrfmmm.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Gibas Z., Li F. P., Antman K. H., Bernal S., Stahel R., Sandberg A. A. Chromosome changes in malignant mesothelioma. Cancer Genet. Cytogenet. 1986;20:191–201. doi: 10.1016/0165-4608(86)90074-9. [DOI] [PubMed] [Google Scholar]
- Goodman J. E., Nascarella M. A., Valberg P. A. Ionizing radiation: A risk factor for mesothelioma. Cancer Causes Control. 2009;20:1237–1254. doi: 10.1007/s10552-009-9357-4. [DOI] [PubMed] [Google Scholar]
- Guey L. T., Garcia-Closas M., Murta-Nascimento C., Lloreta J., Palencia L., Kogevinas M., Rothman N., Vellalta G., Calle M. L., Marenne G., Tardon A., Carrato A., Garcia-Closas R., Serra C., Silverman D. T., Chanock S., Real F. X., Malats N. Genetic susceptibility to distinct bladder cancer subphenotypes. Eur. Urol. 2010;57:283–292. doi: 10.1016/j.eururo.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guled M., Lahti L., Lindholm P. M., Salmenkivi K., Bagwan I., Nicholson A. G., Knuutila S. CDKN2A, NF2, and JUN are dysregulated among other genes by miRNAs in malignant mesothelioma—A miRNA microarray analysis. Genes Chromosomes Cancer. 2009;48:615–623. doi: 10.1002/gcc.20669. [DOI] [PubMed] [Google Scholar]
- Hagemeijer A., Versnel M. A., Van Drunen E., Moret M., Bouts M. J., van der Kwast T. H., Hoogsteden H. C. Cytogenetic analysis of malignant mesothelioma. Cancer Genet. Cytogenet. 1990;47:1–28. doi: 10.1016/0165-4608(90)90258-c. [DOI] [PubMed] [Google Scholar]
- Hammar S. P., Bockus D., Remington F., Freidman S., LaZerte G. Familial mesothelioma: A report of two families. Hum. Pathol. 1989;20:107–112. doi: 10.1016/0046-8177(89)90173-1. [DOI] [PubMed] [Google Scholar]
- Hando J. C., Nath J., Tucker J. D. Sex chromosomes, micronuclei and aging in women. Chromosoma. 1994;103:186–192. doi: 10.1007/BF00368011. [DOI] [PubMed] [Google Scholar]
- Higginson J., Muir C. S., Muñoz N. Cambridge monographs on cancer research. New York: Cambridge University Press; 1992. Human cancer: Epidemiology and environmental causes. [Google Scholar]
- Hirvonen A., Pelin K., Tammilehto L., Karjalainen A., Mattson K., Linnainmaa K. Inherited GSTM1 and NAT2 defects as concurrent risk modifiers in asbestos-related human malignant mesothelioma. Cancer Res. 1995;55:2981–2983. [PubMed] [Google Scholar]
- Hirvonen A., Saarikoski S. T., Linnainmaa K., Koskinen K., Husgafvel-Pursiainen K., Mattson K., Vainio H. Glutathione S-transferase and N-acetyltransferase genotypes and asbestosassociated pulmonary disorders. JNCI. 1996;88:1853–1856. doi: 10.1093/jnci/88.24.1853. [DOI] [PubMed] [Google Scholar]
- Hodgson J. T., Darnton A. The quantitative risks of mesothelioma and lung cancer in relation to asbestos exposure. Ann. Occup. Hyg. 2000;44:565–601. [PubMed] [Google Scholar]
- Huncharek M. Genetic factors in the aetiology of malignant mesothelioma. Eur. J. Cancer. 1995;31A:1741–1747. doi: 10.1016/0959-8049(95)00365-p. [DOI] [PubMed] [Google Scholar]
- Huncharek M. Non-asbestos related diffuse malignant mesothelioma. Tumori. 2002;88:1–9. [PubMed] [Google Scholar]
- Huncharek M., Kelsey K., Muscat J., Christiani D. Parental cancer and genetic predisposition in malignant pleural mesothelioma: A case-control study. Cancer Lett. 1996;102:205–208. doi: 10.1016/0304-3835(96)04172-9. [DOI] [PubMed] [Google Scholar]
- Illei P. B., Ladanyi M., Rusch V. W., Zakowski M. F. The use of CDKN2A deletion as a diagnostic marker for malignant mesothelioma in body cavity effusions. Cancer. 2003;99:51–56. doi: 10.1002/cncr.10923. [DOI] [PubMed] [Google Scholar]
- Ivanov S. V., Miller J., Lucito R., Tang C., Ivanova A. V., Pei J., Carbone M., Cruz C., Beck A., Webb C., Nonaka D., Testa J. R., Pass H. I. Genomic events associated with progression of pleural malignant mesothelioma. Int. J. Cancer. 2009;124:589–599. doi: 10.1002/ijc.23949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson N. F., Edwards R. E., Munday D. E., Rowe N., Wagner J. C. Pluripotential nature of mesotheliomata induced by inhalation of erionite in rats. Br. J. Exp. Pathol. 1984;65:377–388. [PMC free article] [PubMed] [Google Scholar]
- Jongsma J., van Montfort E., Vooijs M., Zevenhoven J., Krimpenfort P., van der Valk M., van de Vijver M., Berns A. A conditional mouse model for malignant mesothelioma. Cancer Cell. 2008;13:261–271. doi: 10.1016/j.ccr.2008.01.030. [DOI] [PubMed] [Google Scholar]
- Kamb A., Gruis N. A., Weaver-Feldhaus J., Liu Q., Harshman K., Tavtigian S. V., Stockert E., Day R. S., 3rd, Johnson B. E., Skolnick M. H. A cell cycle regulator potentially involved in genesis of many tumor types. Science. 1994;264:436–440. doi: 10.1126/science.8153634. [DOI] [PubMed] [Google Scholar]
- Kaminsky Z., Wang S. C., Petronis A. Complex disease, gender and epigenetics. Ann. Med. 2006;38:530–544. doi: 10.1080/07853890600989211. [DOI] [PubMed] [Google Scholar]
- Kim J. Y., Kwon O. Garlic intake and cancer risk: An analysis using the Food and Drug Administration's evidence-based review system for the scientific evaluation of health claims. Am. J. Clin. Nutr. 2009;89:257–264. doi: 10.3945/ajcn.2008.26142. [DOI] [PubMed] [Google Scholar]
- Kliment C. R., Clemens K., Oury T. D. North american erionite-associated mesothelioma with pleural plaques and pulmonary fibrosis: A case report. Int. J. Clin. Exp. Pathol. 2009;2:407–410. [PMC free article] [PubMed] [Google Scholar]
- Kruglyak L., Daly M. J., Reeve-Daly M. P., Lander E. S. Parametric and nonparametric linkage analysis: A unified multipoint approach. Am J Human Genet. 1996;58:1347–1363. [PMC free article] [PubMed] [Google Scholar]
- Ladanyi M. Implications of P16/CDKN2A deletion in pleural mesotheliomas. Lung Cancer. 2005;49:S95–S98. doi: 10.1016/j.lungcan.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Lam T. K., Gallicchio L., Lindsley K., Shiels M., Hammond E., Tao X. G., Chen L., Robinson K. A., Caulfield L. E., Herman J. G., Guallar E., Alberg A. J. Cruciferous vegetable consumption and lung cancer risk: A systematic review. Cancer Epidemiol. Biomarkers Prev. 2009;18:184–195. doi: 10.1158/1055-9965.EPI-08-0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landi S., Gemignani F., Neri M., Barale R., Bonassi S., Bottari F., Canessa P. A., Canzian F., Ceppi M., Filiberti R., Ivaldi G. P., Mencoboni M., Scaruffi P., Tonini G. P., Mutti L., Puntoni R. Polymorphisms of glutathione-S-transferase M1 and manganese superoxide dismutase are associated with the risk of malignant pleural mesothelioma. Int. J. Cancer. 2007;120:2739–2743. doi: 10.1002/ijc.22590. [DOI] [PubMed] [Google Scholar]
- Lanphear B. P., Buncher C. R. Latent period for malignant mesothelioma of occupational origin. J. Occup. Med. 1992;34:718–721. [PubMed] [Google Scholar]
- Li F. P., Dreyfus M. G., Antman K. H. Asbestos-contaminated nappies and familial mesothelioma. Lancet. 1989;1:909–910. doi: 10.1016/s0140-6736(89)92916-4. [DOI] [PubMed] [Google Scholar]
- Li F. P., Lokich J., Lapey J., Neptune W. B., Wilkins E. W., Jr. Familial mesothelioma after intense asbestos exposure at home. J. Am. Med. Assoc. 1978;240:467. [PubMed] [Google Scholar]
- Li G., Passebosc-Faure K., Feng G., Lambert C., Cottier M., Gentil-Perret A., Fournel P., Perol M., Genin C. MN/CA9: A potential gene marker for detection of malignant cells in effusions. Biomarkers. 2007;12:214–220. doi: 10.1080/13547500601068192. [DOI] [PubMed] [Google Scholar]
- Lynch H. T., Katz D., Markvicka S. E. Familial mesothelioma: Review and family study. Cancer Genet. Cytogenet. 1985;15:25–35. doi: 10.1016/0165-4608(85)90128-1. [DOI] [PubMed] [Google Scholar]
- Mahabir S., Spitz M. R., Barrera S. L., Beaver S. H., Etzel C., Forman M. R. Dietary zinc, copper and selenium, and risk of lung cancer. Int. J. Cancer. 2007;120:1108–1115. doi: 10.1002/ijc.22451. [DOI] [PubMed] [Google Scholar]
- Malik M. A., Upadhyay R., Modi D. R., Zargar S. A., Mittal B. Association of NAT2 gene polymorphisms with susceptibility to esophageal and gastric cancers in the Kashmir Valley. Arch. Med. Res. 2009;40:416–423. doi: 10.1016/j.arcmed.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Maltoni C., Minardi F., Morisi L. Pleural mesotheliomas in Sprague-Dawley rats by erionite: First experimental evidence. Environ. Res. 1982;29:238–244. doi: 10.1016/0013-9351(82)90024-x. [DOI] [PubMed] [Google Scholar]
- Martensson G., Larsson S., Zettergren L. Malignant mesothelioma in two pairs of siblings: Is there a hereditary predisposing factor? Eur. J. Respir. Dis. 1984;65:179–184. [PubMed] [Google Scholar]
- Milner J. A. Diet and cancer: facts and controversies. Nutr Cancer. 2006;56:216–224. doi: 10.1207/s15327914nc5602_13. [DOI] [PubMed] [Google Scholar]
- Montanaro F., Rosato R., Gangemi M., Roberti S., Ricceri F., Merler E., Gennaro V., Romanelli A., Chellini E., Pascucci C., Musti M., Nicita C., Barbieri P. G., Marinaccio A., Magnani C., Mirabelli D. Survival of pleural malignant mesothelioma in Italy: A population-based study. Int. J. Cancer. 2009;124:201–207. doi: 10.1002/ijc.23874. [DOI] [PubMed] [Google Scholar]
- Munoz L., Guzman J., Ponce de Leon S., Mutchinick O., Arista J., Vazquez J. Familial malignant pleural mesothelioma. Report of 3 cases. Rev. Invest. Clin. 1988;40:413–417. [PubMed] [Google Scholar]
- Murthy S. S., Testa J. R. Asbestos, chromosomal deletions, and tumor suppressor gene alterations in human malignant mesothelioma. J. Cell Physiol. 1999;180:150–157. doi: 10.1002/(SICI)1097-4652(199908)180:2<150::AID-JCP2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Muscat J. E., Huncharek M. Dietary intake and the risk of malignant mesothelioma. Br. J. Cancer. 1996;73:1122–1125. doi: 10.1038/bjc.1996.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musti M., Kettunen E., Dragonieri S., Lindholm P., Cavone D., Serio G., Knuutila S. Cytogenetic and molecular genetic changes in malignant mesothelioma. Cancer Genet. Cytogenet. 2006;170:9–15. doi: 10.1016/j.cancergencyto.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Neragi-Miandoab S., Sugarbaker D. J. Chromosomal deletion in patients with malignant pleural mesothelioma. Interact. Cardiovasc. Thorac. Surg. 2009;9:42–44. doi: 10.1510/icvts.2008.201509. [DOI] [PubMed] [Google Scholar]
- Neri M., Filiberti R., Taioli E., Garte S., Paracchini V., Bolognesi C., Canessa P. A., Fontana V., Ivaldi G. P., Verna A., Bonassi S., Puntoni R. Pleural malignant mesothelioma, genetic susceptibility and asbestos exposure. Mutat. Res. 2005;59:36–44. doi: 10.1016/j.mrfmmm.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Neri M., Taioli E., Filiberti R., Paolo Ivaldi G., Aldo Canessa P., Verna A., Marroni P., Puntoni R., Hirvonen A., Garte S. Metabolic genotypes as modulators of asbestos-related pleural malignant mesothelioma risk: A comparison of Finnish and Italian populations. Int. J. Hyg Environ Health. 2006;209:393–398. doi: 10.1016/j.ijheh.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Neri M., Ugolini D., Dianzani I., Gemignani F., Landi S., Cesario A., Magnani C., Mutti L., Puntoni R., Bonassi S. Genetic susceptibility to malignant pleural mesothelioma and other asbestos-associated diseases. Mutat. Res. 2008;659:126–136. doi: 10.1016/j.mrrev.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Neumann V., Rutten A., Scharmach M., Muller K. M., Fischer M. Factors influencing long-term survival in mesothelioma patients—Results of the German mesothelioma register. Int. Arch. Occup. Environ. Health. 2004;77:191–199. doi: 10.1007/s00420-003-0498-6. [DOI] [PubMed] [Google Scholar]
- Nobori T., Miura K., Wu D. J., Lois A., Takabayashi K., Carson D. A. Deletions of the cyclin-dependent kinase-4 inhibitor gene in multiple human cancers. Nature. 1994;368:753–756. doi: 10.1038/368753a0. [DOI] [PubMed] [Google Scholar]
- Ohar J. A., Ampleford E. J., Howard S. E., Sterling D. A. Identification of a mesothelioma phenotype. Respir. Med. 2007;101:503–509. doi: 10.1016/j.rmed.2006.06.028. [DOI] [PubMed] [Google Scholar]
- Omenn G. S., Goodman G. E., Thornquist M. D., Balmes J., Cullen M. R., Glass A., Keogh J. P., Meyskens F. L., Jr., Valanis B., Williams J. H., Jr., Barnhart S., Cherniack M. G., Brodkin C. A., Hammar S. Risk factors for lung cancer and for intervention effects in CARET, the Beta-Carotene and Retinol Efficacy Trial. JNCI. 1996;88:1550–1559. doi: 10.1093/jnci/88.21.1550. [DOI] [PubMed] [Google Scholar]
- Otte K. E., Sigsgaard T. I., Kjaerulff J. Massive exposure to asbestos and malignant mesothelioma, familial accumulation. Ugeskr Laeger. 1990;152:3013–3014. [PubMed] [Google Scholar]
- Pass H. I., Lott D., Lonardo F., Harbut M., Liu Z., Tang N., Carbone M., Webb C., Wali A. Asbestos exposure, pleural mesothelioma, and serum osteopontin levels. N. Engl. J. Med. 2005;353:1564–1573. doi: 10.1056/NEJMoa051185. [DOI] [PubMed] [Google Scholar]
- Pass H. I., Vogelzang N., Hahn S., Carbone M. Malignant pleural mesothelioma. Curr. Problem Cancer. 2004;28:93–174. doi: 10.1016/j.currproblcancer.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Pass H. I., Wali A., Tang N., Ivanova A., Ivanov S., Harbut M., Carbone M., Allard J. Soluble mesothelin-related peptide level elevation in mesothelioma serum and pleural effusions. Ann. Thorac. Surg. 2008;85:265–272. doi: 10.1016/j.athoracsur.2007.07.042. discussion 272. [DOI] [PubMed] [Google Scholar]
- Popescu N. C., Chahinian A. P., DiPaolo J. A. Nonrandom chromosome alterations in human malignant mesothelioma. Cancer Res. 1988;48:142–147. [PubMed] [Google Scholar]
- Poulikakos P. I., Xiao G. H., Gallagher R., Jablonski S., Jhanwar S. C., Testa J. R. Re-expression of the tumor suppressor NF2/merlin inhibits invasiveness in mesothelioma cells and negatively regulates FAK. Oncogene. 2006;25:5960–5968. doi: 10.1038/sj.onc.1209587. [DOI] [PubMed] [Google Scholar]
- Price B. Analysis of current trends in United States mesothelioma incidence. Am. J. Epidemiol. 1997;145:211–218. doi: 10.1093/oxfordjournals.aje.a009093. [DOI] [PubMed] [Google Scholar]
- Pukkala E., Martinsen J. I., Lynge E., Gunnarsdottir H. K., Sparen P., Tryggvadottir L., Weiderpass E., Kjaerheim K. Occupation and cancer—Follow-up of 15 million people in five Nordic countries. Acta Oncol. 2009;48:646–790. doi: 10.1080/02841860902913546. [DOI] [PubMed] [Google Scholar]
- Risberg B., Nickels J., Wagermark J. Familial clustering of malignant mesothelioma. Cancer. 1980;45:2422–2427. doi: 10.1002/1097-0142(19800501)45:9<2422::aid-cncr2820450930>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Rivera Z., Strianese O., Bertino P., Yang H., Pass H., Carbone M. The relationship between simian virus 40 and mesothelioma. Curr. Opin. Pulm. Med. 2008;14:316–321. doi: 10.1097/MCP.0b013e3283018220. [DOI] [PubMed] [Google Scholar]
- Robinson B. W., Creaney J., Lake R., Nowak A., Musk A. W., de Klerk N., Winzell P., Hellstrom K. E., Hellstrom I. Mesothelin-family proteins and diagnosis of mesothelioma. Lancet. 2003;362:1612–1616. doi: 10.1016/S0140-6736(03)14794-0. [DOI] [PubMed] [Google Scholar]
- Roemer H. C., Weistenhofer W., Lohlein D., Geller F., Blomeke B., Golka K. N-Acetyltransferase 1 in colon and rectal cancer cases from an industrialized area. J. Toxicol. Environ Health A. 2008;71:902–905. doi: 10.1080/15287390801988582. [DOI] [PubMed] [Google Scholar]
- Rohl A. N., Langer A. M., Moncure G., Selikoff I. J., Fischbein A. Endemic pleural disease associated with exposure to mixed fibrous dust in Turkey. Science. 1982;216:518–520. doi: 10.1126/science.7071597. [DOI] [PubMed] [Google Scholar]
- Roushdy-Hammady I., Siegel J., Emri S., Testa J. R., Carbone M. Geneticsusceptibility factor and malignant mesothelioma in the Cappadocian region of Turkey. Lancet. 2001;357:444–445. doi: 10.1016/S0140-6736(00)04013-7. [DOI] [PubMed] [Google Scholar]
- Ruffie P., Feld R., Minkin S., Cormier Y., Boutan-Laroze A., Ginsberg R., Ayoub J., Shepherd F. A., Evans W. K., Figueredo A. Diffuse malignant mesothelioma of the pleura in Ontario and Quebec: A retrospective study of 332 patients. J. Clin. Oncol. 1989;7:1157–1168. doi: 10.1200/JCO.1989.7.8.1157. [DOI] [PubMed] [Google Scholar]
- Rusch V. W., Piantadosi S., Holmes E. C. The role of extrapleural pneumonectomy in malignant pleural mesothelioma. A Lung Cancer Study Group trial. J. Thorac. Cardiovasc. Surg. 1991;102:1–9. [PubMed] [Google Scholar]
- Rusch V. W., Venkatraman E. S. Important prognostic factors in patients with malignant pleural mesothelioma, managed surgically. Ann. Thorac. Surg. 1999;68:1799–1804. doi: 10.1016/s0003-4975(99)01038-3. [DOI] [PubMed] [Google Scholar]
- Samson M. K., Wasser L. P., Borden E. C., Wanebo H. J., Creech R. H., Phillips M., Baker L. H. Randomized comparison of cyclophosphamide, imidazole carboxamide, and adriamycin versus cyclophosphamide and adriamycin in patients with advanced stage malignant mesothelioma: A Sarcoma Intergroup Study. J. Clin. Oncol. 1987;5:86–91. doi: 10.1200/JCO.1987.5.1.86. [DOI] [PubMed] [Google Scholar]
- Saracci R., Simonato L. Familial malignant mesothelioma. Lancet. 2001;358:1813–1814. doi: 10.1016/S0140-6736(01)06816-7. [DOI] [PubMed] [Google Scholar]
- Sawan C., Vaissiere T., Murr R., Herceg Z. Epigenetic drivers and genetic passengers on the road to cancer. Mutat. Res. 2008;642:1–13. doi: 10.1016/j.mrfmmm.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Scherpereel A., Lee Y. G. Biomarkers for mesothelioma. Curr. Opin. Pulm. Med. 2007;13:339–443. doi: 10.1097/MCP.0b013e32812144bb. [DOI] [PubMed] [Google Scholar]
- Schiffman M. H., Pickle L. W., Fontham E., Zahm S. H., Falk R., Mele J., Correa P., Fraumeni J. F., Jr. Case-control study of diet and mesothelioma in Louisiana. Cancer Res. 1988;48:2911–2915. [PubMed] [Google Scholar]
- Schmid W. The micronucleus test. Mutat. Res. 1975;31:9–15. doi: 10.1016/0165-1161(75)90058-8. [DOI] [PubMed] [Google Scholar]
- Sebastien P., Gaudichet A., Bignon J., Baris Y. I. Zeolite bodies in human lungs from Turkey. Lab. Invest. 1981;44:420–425. [PubMed] [Google Scholar]
- Sekido Y., Pass H. I., Bader S., Mew D. J., Christman M. F., Gazdar A. F., Minna J. D. Neurofibromatosis type 2 (NF2) gene is somatically mutated in mesothelioma but not in lung cancer. Cancer Res. 1995;55:1227–1231. [PubMed] [Google Scholar]
- Serio G., Gentile M., Pennella A., Marzullo A., Buonadonna A. L., Nazzaro P., Testini M., Musti M., Scattone A. Characterization of a complex chromosome aberration in two cases of peritoneal mesothelioma arising primarily in the hernial sac. Pathol. Int. 2009;59:415–421. doi: 10.1111/j.1440-1827.2009.02387.x. [DOI] [PubMed] [Google Scholar]
- Serrano M., Hannon G. J., Beach D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature. 1993;366:704–707. doi: 10.1038/366704a0. [DOI] [PubMed] [Google Scholar]
- Sobti R. C., Kaur P., Kaur S., Janmeja A. K., Jindal S. K., Kishan J., Raimondi S. Impact of interaction of polymorphic forms of p53 codon 72 and N-acetylation gene (NAT2) on the risk of lung cancer in the North Indian population. DNA Cell Biol. 2009;28:443–449. doi: 10.1089/dna.2008.0797. [DOI] [PubMed] [Google Scholar]
- Spirtas R., Connelly R. R., Tucker M. A. Survival patterns for malignant mesothelioma: The SEER experience. Int. J. Cancer. 1988;41:525–530. doi: 10.1002/ijc.2910410409. [DOI] [PubMed] [Google Scholar]
- Sugarbaker D. J., Flores R. M., Jaklitsch M. T., Richards W. G., Strauss G. M., Corson J. M., DeCamp M. M., Jr., Swanson S. J., Bueno R., Lukanich J. M., Baldini E. H., Mentzer S. J. Resection margins, extrapleural nodal status, and cell type determine postoperative long-term survival in trimodality therapy of malignant pleural mesothelioma: Results in 183 patients. J Thorac Cardiovasc Surg. 1999;117:54–63. doi: 10.1016/s0022-5223(99)70469-1. discussion 63–65. [DOI] [PubMed] [Google Scholar]
- Sullivan P. A. Vermiculite, respiratory disease, and asbestos exposure in Libby, Montana: Update of a cohort mortality study. Environ. Health Perspect. 2007;115:579–585. doi: 10.1289/ehp.9481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surralles J., Hande M. P., Marcos R., Lansdorp P. M. Accelerated telomere shortening in the human inactive X chromosome. Am. J. Hum. Genet. 1999;65:1617–1622. doi: 10.1086/302665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surralles J., Jeppesen P., Morrison H., Natarajan A. T. Analysis of loss of inactive X chromosomes in interphase cells. Am. J. Hum. Genet. 1996;59:1091–1096. [PMC free article] [PubMed] [Google Scholar]
- Taguchi T., Jhanwar S. C., Siegfried J. M., Keller S. M., Testa J. R. Recurrent deletions of specific chromosomal sites in 1p, 3p, 6q, and 9p in human malignant mesothelioma. Cancer Res. 1993;53:4349–4355. [PubMed] [Google Scholar]
- Tammilehto L. Malignant mesothelioma: Prognostic factors in a prospective study of 98 patients. Lung Cancer. 1992;8:175–184. [Google Scholar]
- Taniguchi T., Karnan S., Fukui T., Yokoyama Y., Tagawa H., Yokoi K., Ueda Y., Mitsudomi T., Horio Y., Hida T., Yatabe Y., Seto M., Sekido Y. Genomic profiling of malignant pleural mesothelioma with array-based comparative genomic hybridization shows frequent non-random chromosomal alteration regions including JUN amplification on 1p32. Cancer Sci. 2007;9:438–446. doi: 10.1111/j.1349-7006.2006.00386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa J. R., Jhanwar S. C. In: Textbook of pleural diseases. Light R. W., Lee Y. C. G., editors. London, New York: Arnold; 2003. [Google Scholar]
- Thurneysen C., Opitz I., Kurtz S., Weder W., Stahel R. A., Felley-Bosco E. Functional inactivation of NF2/merlin in human mesothelioma. Lung Cancer. 2009;64:140–147. doi: 10.1016/j.lungcan.2008.08.014. [DOI] [PubMed] [Google Scholar]
- Tiainen M., Tammilehto L., Mattson K., Knuutila S. Nonrandom chromosomal abnormalities in malignant pleural mesothelioma. Cancer Genet. Cytogenet. 1988;33:251–274. doi: 10.1016/0165-4608(88)90035-0. [DOI] [PubMed] [Google Scholar]
- Timblin C. R., Guthrie G. D., Janssen Y. W., Walsh E. S., Vacek P., Mossman B. T. Patterns of c-fos and c-jun protooncogene expression, apoptosis, and proliferation in rat pleural mesothelial cells exposed to erionite or asbestos fibers. Toxicol. Appl. Pharmacol. 1998;151:88–97. doi: 10.1006/taap.1998.8450. [DOI] [PubMed] [Google Scholar]
- Ugolini D., Neri M., Ceppi M., Cesario A., Dianzani I., Filiberti R., Gemignani F., Landi S., Magnani C., Mutti L., Puntoni R., Bonassi S. Genetic susceptibility to malignant mesothelioma and exposure to asbestos: The influence of the familial factor. Mutat. Res. 2008;658:162–171. doi: 10.1016/j.mrrev.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Vaissiere T., Sawan C., Herceg Z. Epigenetic interplay between histone modifications and DNA methylation in gene silencing. Mutat. Res. 2008;659:40–48. doi: 10.1016/j.mrrev.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Van der Meeren A., Fleury J., Nebut M., Monchaux G., Janson X., Jaurand M. C. Mesothelioma in rats following intrapleural injection of chrysotile and phosphorylated chrysotile (chrysophosphate) Int. J. Cancer. 1992;50:937–942. doi: 10.1002/ijc.2910500620. [DOI] [PubMed] [Google Scholar]
- Van Gelder T., Damhuis R. A., Hoogsteden H. C. Prognostic factors and survival in malignant pleural mesothelioma. Eur. Respir. J. 1994;7:1035–1038. [PubMed] [Google Scholar]
- Virta R. L. U.S. Department of the Interior. Zeolites. Washington, DC: U.S. Geological Survey; 2006. [Google Scholar]
- Virtamo J., Pietinen P., Huttunen J. K., Korhonen P., Malila N., Virtanen M. J., Albanes D., Taylor P. R., Albert P. Incidence of cancer and mortality following alpha-tocopherol and beta-carotene supplementation: A postintervention follow-up. J. Am. Med. Assoc. 2003;290:476–485. doi: 10.1001/jama.290.4.476. [DOI] [PubMed] [Google Scholar]
- Wagner J. C., Skidmore J. W., Hill R. J., Griffiths D. M. Erionite exposure and mesotheliomas in rats. Br. J. Cancer. 1985;51:727–730. doi: 10.1038/bjc.1985.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner J. C., Sleggs C. A., Marchand P. Diffuse pleural mesothelioma and asbestos exposure in the North Western Cape Province. Br. J. Ind. Med. 1960;17:260–271. doi: 10.1136/oem.17.4.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Lee I. M., Zhang S. M., Blumberg J. B., Buring J. E., Sesso H. D. Dietary intake of selected flavonols, flavones, and flavonoid-rich foods and risk of cancer in middle-aged and older women. Am. J. Clin. Nutr. 2009;89:905–912. doi: 10.3945/ajcn.2008.26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weistenhoer W., Blaszkewicz M., Bolt H.M., Golka K. NAcetyltransferase 2 and medical history in bladder cancer cases with a suspected occupational disease (BK 1301) in Germany. J. Toxicol. Environ. Health A. 2008;71:906–910. doi: 10.1080/15287390801988681. [DOI] [PubMed] [Google Scholar]
- Whitehouse A. C., Black C. B., Heppe M. S., Ruckdeschel J., Levin S. M. Environmental exposure to Libby Asbestos and mesotheliomas. Am. J. Ind. Med. 2008;51:877–880. doi: 10.1002/ajim.20620. [DOI] [PubMed] [Google Scholar]
- Woyengo T. A., Ramprasath V. R., Jones P. J. Anticancer effects of phytosterols. Eur. J. Clin. Nutr. 2009;63:813–820. doi: 10.1038/ejcn.2009.29. [DOI] [PubMed] [Google Scholar]
- Wynder E. L., Gori G. B. Contribution of the environment to cancer incidence: An epidemiologic exercise. J. Natl. Cancer Inst. 1977;58:825–832. doi: 10.1093/jnci/58.4.825. [DOI] [PubMed] [Google Scholar]
- Xiao G. H., Beeser A., Chernoff J., Testa J. R. p21-Activated kinase links Rac/Cdc42 signaling to merlin. J. Biol. Chem. 2002;277:883–886. doi: 10.1074/jbc.C100553200. [DOI] [PubMed] [Google Scholar]
- Xiao G. H., Gallagher R., Shetler J., Skele K., Altomare D. A., Pestell R. G., Jhanwar S., Testa J. R. The NF2 tumor suppressor gene product, merlin, inhibits cell proliferation and cell cycle progression by repressing cyclin D1 expression. Mol. Cell. Biol. 2005;25:2384–2394. doi: 10.1128/MCB.25.6.2384-2394.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan T. D., Yoo D., Sugarbaker P. H. Significance of lymph node metastasis in patients with diffuse malignant peritoneal mesothelioma. Eur. J. Surg. Oncol. 2006;32:948–953. doi: 10.1016/j.ejso.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Yang C. T., You L., Yeh C. C., Chang J. W., Zhang F., McCormick F., Jablons D. M. Adenovirus-mediated p14(ARF) gene transfer in human mesothelioma cells. JNCI. 2000;92:636–641. doi: 10.1093/jnci/92.8.636. [DOI] [PubMed] [Google Scholar]


