Abstract
Exposure to asbestos fibers is associated with non-neoplastic pleural diseases including plaques, fibrosis, and benign effusions, as well as with diffuse malignant pleural mesothelioma. Translocation and retention of fibers are fundamental processes in understanding the interactions between the dose and dimensions of fibers retained at this anatomic site and the subsequent pathological reactions. The initial interaction of fibers with target cells in the pleura has been studied in cellular models in vitro and in experimental studies in vivo. The proposed biological mechanisms responsible for non-neoplastic and neoplastic pleural diseases and the physical and chemical properties of asbestos fibers relevant to these mechanisms are critically reviewed. Understanding mechanisms of asbestos fiber toxicity may help us anticipate the problems from future exposures both to asbestos and to novel fibrous materials such as nanotubes. Gaps in our understanding have been outlined as guides for future research.
TRANSLOCATION AND RETENTION OF FIBERS IN THE PLEURA
Anatomy and Physiology of the Pleura
The parietal pleura lines the chest wall and the superior surface of the diaphragm and the visceral pleura covers the lungs (Figure 1). The pleural space in humans contains a small amount of fluid (0.1–0.2 ml/kg body weight) that is a filtrate from the underlying systemic circulation (Owens & Milligan, 1995; Broaddus, 2008). This space (10–20 μm wide) is lined by a single layer of mesothelial cells resting on a basement membrane and underlying connective tissue and blood vessels. The major routes of drainage of fluid, protein, particulates, and cells from the pleural space are through the lymphatic stomata that open between mesothelial cells on the parietal pleural lining (Hammar, 1994; Wang, 1975; Broaddus et al., 1988).
FIGURE 1.
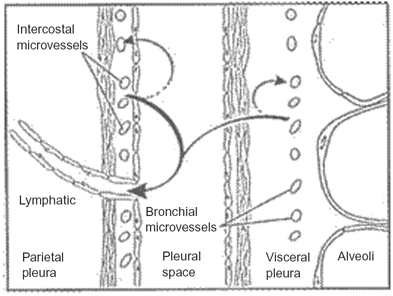
Fluid turnover and lymphatic drainage from the pleural space. In the normal pleural space (shown here), as in other interstitial spaces of the body, liquid slowly filters from systemic capillaries and is absorbed via lymphatics (solid arrows). In the pleural space, the capillary filtrate from systemic capillaries moves across a permeable pleural membrane toward the lower pressure pleural space and is absorbed via the parietal pleural lymphatics. From there, liquid moves via lymphatic propulsion to the central veins. When interstitial edema forms in the adjacent lung, some of that excess liquid moves across the visceral pleura into the pleural space. Asbestos fibers may follow similar routes from the lung to the pleura and are thought to lodge in the parietal pleura preferentially at sites of lymphatic drainage.
Effusions, an accumulation of excess liquid in the pleural space, are common features of a multitude of diseases. Transudative effusions, those not associated with inflammation or injury, usually develop due to increased hydrostatic pressure. In congestive heart failure, the most common cause of transudative effusions, increased pulmonary venous pressure leads to fluid accumulation in the interstitial spaces of the lung; the fluid then moves toward the lower pressure pleural space and leaks across the visceral pleura into the pleural space (Broaddus et al., 1990; Owens & Milligan, 1995). In the setting of inflammation or injury of the lung, pleura, or other organs, exudative effusions may form; these effusions contain elevated levels of protein due to the increased leakage across capillaries with increased permeability (Mutsaers et al., 2004). Excess fluid in any part of the body may find its way to the pleural space via the interstitial tissues along pressure gradients and by moving across the permeable pleural membranes. The normal and pathological paths by which liquid, cells, and particles enter and exit the pleural space suggest pathways by which asbestos fibers may also enter and exit or fail to exit the pleural space. The study of the physiology of the pleural space is challenging; even when using laboratory animal studies, analyses of the pleural space are limited by the narrowness of the space and the difficulty in sampling without inducing inflammation or injury.
Pathways Leading to Translocation of Fibers to the Pleura
The route of translocation of fibers from the lungs to the visceral pleura, into the pleural space, and to the parietal pleura is unknown. It is postulated that asbestos fibers may migrate to the lung interstitium and visceral pleura by a paracellular route or by direct penetration across injured alveolar epithelial cells (Miserocchi et al., 2008). Fibers may be transported to the pleural space via the lymphatics and bloodstream (Oberdörster et al., 1983). Fibers may translocate by themselves or within macrophages. Although studies of asbestos fiber movement have not been possible due to technical limitations, it is likely that asbestos fibers translocate to the pleural space passively in the same manner as interstitial fluid. This process may be enhanced by lung inflammation induced by asbestos fibers or by mixed dust exposures that increase interstitial fluid accumulation and thus fluid movement along the interstitial spaces to the pleural space (Miserocchi et al., 2008).
There are thus few studies that investigated the translocation of fibers from the lung into the pleural space. Even in the few existing studies, data from animal studies may have limited relevance for humans because of the different visceral pleural anatomy in rodents. In the rodent, the visceral pleura is “thin,” consisting mostly of a mesothelial layer and basement membrane lying directly over the alveoli. There is little submesothelial connective tissue and no pleural vasculature. In sheep and humans and other large mammals, the visceral pleura is “thick” and has a significant submesothelial connective tissue space, containing nerves and systemic blood vessels (Figure 1). In contrast to the visceral pleura, the parietal pleura in different species has a constant and similar anatomy (Figure 1; Light & Broaddus, 2010). Thus, due to differences in the visceral pleura, one might postulate a difference between rodents and humans in the movement of the fibers into the pleural space. Due to similarities in the parietal pleura, one might suggest that the localization, accumulation, and actions of fibers in the parietal pleura might be similar.
These questions have been almost impossible to address using current technology but it is hoped that new tools and imaging techniques such as nuclear magnetic resonance (NMR) spectroscopy or two-photon microscopy can be developed to provide data on (1) how fibers distribute in the lungs and pleura, (2) the ultimate destination of fibers, (3) how fiber movement is enhanced, and (4) whether fibers are translocated and retained differently in animals and humans. Such techniques could be invasive, using labeled fibers that could be traced, for use in animal studies, and noninvasive for clinical studies of those exposed to asbestos. This imaging information on fiber localization would enhance diagnosis and follow-up of subjects exposed to asbestos, such as in directing where to sample tissues to assess fiber dosimetry, and how to determine preneoplastic biomarkers (Greillier et al., 2008) that might lead to intervention and prevention of non-neoplastic and neoplastic pleural diseases.
Pleural Fiber Dosimetry in Rodents
Translocation and retention of fibrous particulates from initial sites of pulmonary deposition to extrapulmonary sites are believed to be important aspects of their potential toxicity (Dodson et al., 2003; Suzuki & Kohyama, 1991). Pathologic tissue responses such as edema, inflammation, or fibrosis might potentially affect translocation and retention of particulates in the body, as well as properties of particles themselves including dose, dimensions and biopersistence. Although similarities exist between animal models and humans concerning physiological processes such as interstitial fluid dynamics and lymphatic flow, there are also anatomical differences such as in visceral pleural thickness (Tyler, 1983), as well as physiological differences such as of macrophage size and function, that need to be taken into account when comparing across animal species and when extrapolating from animals to humans (Jarabek et al., 2005; Maxim & McConnell, 2001). Rodents and humans also differ in particle respirability (Mossman et al., 2011) and this limits the use of rodent models for human risk assessment based on fiber dimensions (Lippmann & Schlesinger, 1984; Lippmann et al., 1980).
Biopersistence of fibers in the lung parenchyma also influences the fiber dose that is ultimately translocated to the pleura. Biopersistence in the lung is dependent on (1) site and rate of deposition, (2) pulmonary clearance parameters, (3) solubility in lung fluids, (4) breakage rate and patterns, and (5) rates of fiber translocation and retention. Surface chemistry and diameter are important determinants of solubility. Much of the knowledge base concerning the role of biopersistence is actually derived from studies of synthetic vitreous fibers (Bernstein, 2007; Oberdörster, 2000).
Fiber characteristics also affect clearance from the lung and translocation to the pleura. Macrophage-mediated particle clearance in the lung is likely to influence translocation of particles to interstitial sites. There are important interspecies differences in particle clearance, as well as in biological effects of high pulmonary concentrations of particles, in humans and in different animal species (Bermudez et al., 2002; Oberdörster, 2002). In addition, the method of dose administration in experimental animals is shown to influence pleural pathology outcomes following particle exposure. In silica-exposed rats, pleural granulomas developed in animals following inhalation, but not after instillation, and the different response was likely due to differences in kinetics of particle delivery and lymphatic clearance (Henderson et al., 1995).
The effects of asbestos may be altered when asbestos is mixed with other particulates, a situation common in occupational and environmental exposures. Studies by Davis and colleagues (1991) showed that coexposure of rats to amosite asbestos and to quartz increased the incidence of amosite-induced pleural mesothelioma, presumably by elevation in fiber dosimetry and translocation through the visceral pleura. Recent studies by Bernstein and colleagues (2008) demonstrated that coexposure of chrysotile asbestos together with nonfibrous particulates decreased fiber retention in the lungs of rats, perhaps by increasing macrophage recruitment and macrophage-mediated clearance or by inducing more inflammatory fluid movement to the pleura.
Fiber translocation in rodents appears to be rapid and may be responsible in some cases in particular for pathologic outcomes. In rats, short chrysotile asbestos fibers are found in the pleural space within a week following intratracheal instillation (Viallat et al., 1986). Similarly, crocidolite fibers were detected in the pleural space 1 wk following inhalation (Choe et al., 1997). In another study in rats, short fibers (<5 μm length) were found 5 d after inhalation exposure to a synthetic vitreous fiber (refractory ceramic fiber) aerosol (Gelzleichter et al., 1996). In studies in rats and hamsters involving chronic inhalation of synthetic vitreous fibers as well as of amosite and chrysotile asbestos used as reference materials, significant inter-species differences in pleural pathology were seen (Mast et al., 1994; McConnell, 1994). Subsequent short-term mechanistic studies of translocation showed that the Syrian golden hamster, a species prone to development of pleural fibrosis and mesothelioma following synthetic vitreous fiber exposure, displayed greater translocation of fibers to the pleura than did similarly exposed rats (Gelzleichter et al., 1999). The greater translocation in the Syrian golden hamster may thus have accounted for its greater susceptibility to fiber-induced toxicity.
Pleural Fiber Dosimetry in Humans
There is virtually no knowledge of the kinetics of fiber translocation and retention in the human pleura and there are few pleural fiber burden studies in occupationally exposed workers. In addition, due to loss of anatomical orientation after ashing or digestion of target tissues, it is not known where fibers reside intracellularly or extracellularly. Fibers are identified within mesothelial cells (Davis, 1974; Fasske, 1986; Lee et al., 1993) but, due to technical limitations, no comprehensive studies have quantified intracellular fiber burden at the microscopic level. Although analytical transmission electron microscopy (TEM) with x-ray energy-dispersive analysis is the gold standard for quantitation and identification of asbestos fibers in tissue, in vivo studies would be enhanced greatly by nondestructive imaging approaches that could detect the presence of fibers, their chemical composition, or even the cellular response without destroying anatomical relationships.
A major data gap in understanding mechanisms of asbestos-related pleural disease is the paucity of information available to determine the dose of asbestos fibers that is deposited and retained in the pleural membranes. This overview describes the technical complexity and limitations associated with quantitation of human lung and pleural fiber burdens, as well as summarizing available data.
Roggli (1990, 1992), Roggli and Sharma (2004), and Dodson and Atkinson (2006) reviewed the numerous variables and technical considerations associated with quantitation of tissue fiber burdens in general. Their major conclusions and caveats include:
-
1.
The source of tissue samples ranged from pleural biopsies obtained during diagnostic thoracoscopy (Boutin & Rey, 1993); to surgical specimens including needle biopsies, wedge biopsies, or pneumonectomy or pleural decortication samples; and to pleural and lung tissues obtained during autopsy examination (Roggli & Sharma, 2004).
-
2.
Regardless of the source of tissue, sampling is a potential source of error since there is significant variation in anatomical distribution of fibers, especially in the parietal pleura (Roggli, 1992; Boutin et al., 1996; Mitchev, et al., 2002).
-
3.
Tissues may be contaminated during surgical resection or at autopsy due to fibers present in fixatives, in specimen containers, on surgical gloves, or on dissecting instruments (Roggli & Sharma, 2004).
-
4.
Light microscopy is inadequate for identification and counting of asbestos fibers. Dodson and Atkinson (2006) recommend analytical transmission electron microscopy in combination with x-ray energy-dispersive analysis and selected area diffraction techniques for specific mineralogical identification. Both coated and uncoated fibers, as well as particulates, should be analyzed and quantitated (Dodson & Atkinson, 2006).
-
5.
A systematic approach to counting fibers of all dimensions and analysis of lung fiber burdens needs to be used, as described by the European Respiratory Society (DeVuyst et al., 1988).
-
6.
Appropriate control populations need to be used because there is significant variability in human lung fiber burdens (Roggli, 1990). A systematic analysis of lung asbestos fiber burdens in workers with asbestos-related disease, people with asbestos exposure in households or in buildings, and control cases revealed a wide range of counts with considerable overlap between workers, other asbestos-exposed cases, and controls (Roggli & Sharma, 2004).
-
7.
The criteria used to define and count asbestos fibers need to be stated explicitly. Some investigators only count fibers longer than 5 μm; however, the majority of asbestos fibers in human tissue samples are shorter than 5 μm (Dodson & Atkinson, 2006).
-
8.
Tissue preparation techniques may introduce artifacts due to tissue drying or traumatic disruption of fiber bundles (Dodson & Atkinson, 2006).
Finally, although quantitation of human lung and pleural asbestos fiber burden is the only technique available to assess the dose delivered to and retained at the target tissue, there are additional considerations in interpretation of these data. Tissue fiber burden depends on the time since cessation of exposure. In addition, the fiber burden and the types of fibers in the lung may not reflect the fiber burden in the pleura. For example, shorter uncoated fibers are more readily cleared from the lungs; however, while these fibers may be decreasing in the lungs, they may be accumulating in the pleura and extrapulmonary sites (Holt, 1981) and be associated with development of disease at these sites (Dodson & Hammer, 2006; Dodson & Atkinson, 2006).
It is important to note that the lungs of normal control cases evaluated at autopsy contain significant numbers of commercial and noncommercial asbestos fibers, as well as other particulate and fibrous minerals. This is noteworthy especially in lungs from those who resided in urban settings (Table 1). By comparing lung fiber burdens between those with pleural mesothelioma and those without, investigators showed that, although there is overlap, there is an increased risk for mesothelioma, with an elevated lung burden of certain fibers, such as crocidolite, amosite, and tremolite; due to its lower biopersistence, chrysotile may not be reliably analyzed by autopsy studies (Table 2).
TABLE 1.
Asbestos Fiber Content in Lung Tissue of an Urban Population
| Fiber type | Fiber number/g wet lung |
| Chrysotile asbestos fibers | 130.0 × 103 |
| Antigorite | 2.5 × 103 |
| Noncommercial amphiboles: | |
| Tremolite | 15.0 × 103 |
| Actinolite | 5.1 × 103 |
| Anthophyllite | 3.7 × 103 |
| Commercial amphiboles: | |
| Amosite and crocidolite | 1.1 × 103 |
Note. Analysis of 21 urban cases using analytical transmission electron microscopy with analysis of all fibers longer than 1 μm revealed these average fiber numbers/g wet lung. (Churg & Warnock, 1980).
TABLE 2.
Lung Fiber Burdens in Malignant Mesothelioma Patients
| Fiber type | Percent of patients with fibers | Percent of controls with fibers |
| Chrysotile | 80 | 67 |
| Tremolite | 20 | 11 |
| Crocidolite | 59 | 16 |
| Amosite | 81 | 40 |
| Other: | ||
| Mullite | 98 | 98 |
| Iron | 88 | 65 |
| Rutile | 83 | 79 |
| Muscovite | 61 | 65 |
| Silica | 55 | 65 |
Note. In a study of young persons (age 50 yr or less at the time of diagnosis), the lungs of 69 patients who had died with malignant pleural mesothelioma and 57 controls selected from the national work-related disease surveillance system in the United Kingdom were analyzed by electron microscopy for fiber distribution. Increased odds ratios for mesothelioma were found for crocidolite, amosite, and tremolite; the contribution of chrysotile was less clear due to low biopersistence. Nonasbestos fibers probably made no contribution to mesothelioma in this study (McDonald et al., 2001).
In contrast to these and other studies of fiber burdens in the lung, only a few studies have reported asbestos fiber burdens in the pleura. In those few studies that analyzed pleural fiber burden, the results from lung and pleura differed, perhaps due to the technical problems described earlier, and appeared to indicate that pleura has a predominance of short chrysotile fibers. Sebastien et al. (1980) concluded that lung fiber burden could not be used as an accurate reflection of pleural fiber burden. In their parietal pleural samples, most of the asbestos fibers were short chrysotile fibers. Gibbs et al. (1991) also reported lower asbestos counts in the visceral pleura than in matched lung samples from the same patients and found mostly short chrysotile asbestos fibers.
Dodson et al. (1990) analyzed lung tissue, lymph nodes, and pleural plaques obtained at autopsy from eight shipyard workers in Italy. Data showed both chrysotile and amphibole asbestos fibers in the lungs; however, chrysotile asbestos fibers were the most frequent type of asbestos found in pleural plaques. Most fibers in the lymph nodes and pleural plaques were shorter than 5 μm, although some fibers longer than 8 μm were present at these sites. More recently, Suzuki and his coworkers (2005) compared asbestos fiber burdens of human mesothelioma tissues obtained following bulk tissue digestion or ashing of 25-μm tissue sections using high-resolution analytical electron microscopy. The majority of fibers were ≤5 μm long and 92.7% were ≤0.25 μm wide. Chrysotile asbestos fibers were identified most frequently in a total of 168 cases of human malignant mesotheliomas obtained from biopsy or autopsy specimens (Suzuki et al., 2005). In an earlier study, Suzuki and Yuen (2001) detected only short, thin chrysotile asbestos fibers in 25.7% of the lungs and in 77.4% of the mesothelial tissues of patients with malignant mesothelioma. These tissue samples were obtained from cases throughout the United States that were sent to Dr. Suzuki for pathological review and were systematically analyzed using histology, immunohistochemistry, and electron microscopy, in some cases, over a 15-yr period. As summarized succinctly by Dumortier et al. (1998) in a letter to the editor in 1998, the size and type of asbestos fibers associated with development of diffuse malignant pleural mesothelioma remain controversial (Mossman et al., 2011; Case et al., 2011; Aust et al., 2011).
One possible explanation for the confusion in pleural sampling came from a pioneering study carried out by Boutin et al. (1996). Using video-assisted fiber-optic thoracoscopy in eight asbestos-exposed patients and six unexposed cases, Dr. Boutin and colleagues (1996) sampled specific anatomic regions of the parietal pleura identified as collecting spots for inorganic particulates and fibers that translocate to the pleural spaces. These regions are called “black spots” due to localized accumulation of carbon particles and are sites of lymphatic drainage located in the lower coastal regions of the parietal pleura and on the superior dome of the diaphragm. Using transmission electron microscopy, Boutin et al. (1996) identified numerous amphibole as well as chrysotile fibers at black spots, and 22.5% were ≥5 μm long. The mean asbestos fiber concentration in the 8 exposed cases was 12.4 ± 9.8 × 106 fibers/g dry lung tissue, 4.1 ± 1.9 × 106 fibers/g black spots on the parietal pleura, and 0.5 ± 0.2 × 106 fibers/g normal parietal pleura, using bleach digestion of lung tissue and low-temperature ashing of pleural tissue samples. Evidence indicated that asbestos fibers accumulate in focal areas of the parietal pleura and that these “black spots” are the most likely anatomic origin of diffuse malignant mesothelioma. In a subsequent study of black spots analyzed from 150 consecutive autopsies of urban residents in Brussels, Belgium, the histopathological appearance of black spots showed chronic inflammation with lymphocytes, plasma cells, and macrophages with a variety of particulates and fibers both intracellularly and extracellularly (Mitchev et al., 2002). Of note, there was no anatomic relationship between black spots and parietal pleural plaques. Black spots were present in 92.7% of these cases; these lesions were more numerous in older cases and in males. Evidence indicated that these cases may have had greater exposure to coal dust used for home heating and in industry. In this case series, asbestos bodies >1000/g dry lung were found in 15 of 97 cases studied; unfortunately, pleural samples were not analyzed for the presence of asbestos fibers (Mitchev et al., 2002). The discrepancy between studies of pleural fiber burden and distribution may thus be explained by the inhomogeneity of fiber deposition in the parietal pleura. Since this important observation of the localization of pleural fibers in black spots, almost no studies addressed pleural fiber burden to clarify which fibers are present and which fibers are associated most closely with asbestos-induced pleural disease, whether neoplastic or non-neoplastic.
Knowledge and Data Gaps in Fiber Translocation and Dosimetry
In considering the data existing on the subject of fiber translocation and dosimetry, there are numerous gaps in the knowledge base that may be amenable to newer methods.
-
(a)
There is a significant lack of understanding of the contributions of the various potential routes of fiber translocation, including direct interstitial transport, macrophage-mediated transport, lymphatic transport, and hematogenous transport. There is little known about which fibers move out from the lung to the pleura and at what rate, and which accumulate in the pleura. There is a need to improve our understanding of the kinetics of fiber translocation and pathogenic pleural responses following mixed asbestos fiber exposure and with coexposure to other particulates.
-
(b)
There are gaps in understanding the role of fiber dose, dimension, and type in the induction of pleural lesions. There is a lack of understanding of the relationship between pleural fiber burden and disease in mixed fiber dust exposure. To date, experimental animal studies only examined relatively limited size fractions of fibers, due to limited respirability in rodent studies (Lippmann & Schlesinger, 1984; Lippmann et al., 1980).
-
(c)
There is a need to study the role of short, thin fibers in the induction of pleural lesions. Most pleural disease is believed to be due to amphibole exposure (Churg, 1982; Roggli et al., 2002), and most disease was ascribed to long, thin fibers, but there is still much uncertainty concerning the contributions to disease of short, thin fibers that predominate in pleural fiber burden studies (Dodson et al., 2003; Suzuki et al., 2005; Mossman et al., 2011; Aust et al., 2011; Case et al., 2011). While the preponderance of evidence shows that long, thin fibers are the most pathogenic, there is little understanding of how dose, surface properties, and biopersistence of short, thin fibers affect the exposure-response relationship with respect to non-neoplastic pleural outcomes in mixed exposures. This need is made more urgent with recent findings concerning the pleural effects of engineered fibrous nanomaterials such as instilled (Poland et al., 2008) and inhaled (Ryman-Rasmussen et al., 2009) carbon nanotubes in mice.
-
(d)
There is a significant lack of information correlating kinetics with pathological outcomes in the pleura following experimental fiber exposures in laboratory animals. Maxim and McConnell (2001) suggested that humans and rats are similar in pathological responses to fibers with respect to pulmonary fibrosis outcomes; as yet, there are no comparable data for pleural fibrosis.
-
(e)
There is need to develop fiber size separation methods to enable mechanistic studies of characterized fiber preparations. This will allow an understanding of the role of fiber size and dimension on cellular targets of pleural disease.
-
(f)
In general, it is not understood how inhalation of fibers leads ultimately to pleural disease. To date there have been few inhalation studies with well-characterized aerosols of different asbestos fiber types in experimental animals. Most inhalation bioassays have been long-term hazard assessment studies in animal models; otherwise, studies have relied on short-term instillation studies in rodents or in vitro studies. The cost, complexity, and specialized requirements of inhalation studies with fibers have not allowed routine state-of-the-art fiber inhalation exposures in support of mechanistic studies.
INTERACTION OF FIBERS WITH TARGET CELLS IN THE PLEURA
Cellular Interactions
For reasons yet to be fully elucidated, fibrous particulates have an unusual affinity for the visceral and parietal pleura, and these tissues are sites for inflammatory, fibroproliferative, and neoplastic diseases in humans and in experimental animals. Non-neoplastic asbestos-associated diseases of the pleura in humans include benign asbestos-related pleural effusion, pleural plaques, diffuse pleural thickening, and rounded atelectasis (Chapman et al., 2003; Nishimura & Broaddus, 1998). Pleural fibrotic and inflammatory lesions develop in rodents in response to inhaled fibers, but these have not been categorized into separate lesion types as is the case in humans and often have not been described separately from pulmonary parenchymal fibrosis by toxicologic pathologists. It is noteworthy that significant pleural lesions similar to human pleural fibrotic lesions were found in chronic rodent inhalation bioassays with synthetic vitreous fibers as well as with asbestos fibers (McConnell et al., 1999).
Mesothelial cells, resident and elicited inflammatory cells, and pleural fibroblasts are believed to be important effector cells in the pathogenesis of asbestos-induced non-neoplastic pleural diseases. Mesothelial progenitor cells may also participate in pleural repair and disease (Herrick & Mutsaers, 2004). There have been numerous studies both in vivo in experimental animals and in vitro using human and animal cell culture systems that review the cellular interactions in pleural tissues and in the pleural space (Chapman et al., 2003; Mutsaers et al., 2004, 2006; Robledo & Mossman, 1999). It is known that following inhalation and instillation of asbestos fibers into the lung, there are rapid alterations in both resident and elicited populations of pleural inflammatory cells and mesothelial cells. Pleural inflammatory cell changes were produced in rats in association with translocation of asbestos fibers (Choe et al., 1997) or following particulate-induced pulmonary inflammation itself (Lehnert et al., 1985). Changes were noted in mesothelial cells of the visceral pleura at early time points following asbestos instillation and inhalation (Adamson, 1997; Dodson & Ford, 1985), and interactions between pleural inflammatory cells and mesothelial cells are believed to be important in the development of fiber-induced pleural injury and disease. Rat and rabbit pleural mesothelial cells are known to release chemoattractants for inflammatory cells following exposure to asbestos (Boylan et al., 1992; Hill et al., 2003; Tanaka et al., 2000), and pleural macrophage-derived mediators can modulate mesothelial cell function (Baumann et al., 1993, 1996).
Mesothelial Cell Biology and Fiber Interactions
During the past two decades, there has been a great increase in our knowledge of the importance of the mesothelial cell in fiber-induced pleural disease. It has become apparent that these cells play a central dynamic role in the control of injury and repair processes that take place in the pleural and other serosal tissues (Mutsaers et al., 2004). Mesothelial cells are a unique cell type originating from mesoderm and vested with a number of important specialized functions including release of pro- and anti-inflammatory and other immunomodulatory mediators; secretion of factors that promote deposition and clearance of fibrin; and synthesis of growth factors and extracellular matrix proteins that aid in serosal repair (Jantz & Antony, 2008). Asbestos may injure pleural mesothelial cells either by direct or indirect mechanisms, including (1) injury by free radicals, (2) inflammasome activation, (3) alterations of intracellular signaling pathways, (4) release of cytokines and chemokines, (5) physical disruption of chromosomes, (6) alterations in growth factors, and (7) changes in coagulation and fibrinolysis pathways (Manning et al., 2002; Mutsaers et al., 2004; Robledo & Mossman, 1999). Mesothelial cells internalize the fibers via integrins or other receptors, and uptake of the fibers was found in some studies to be necessary for adverse effects of the fibers such as reactive oxygen species (ROS) generation, DNA damage, and apoptosis (Liu et al., 2000). Reactive oxygen species derived directly from the surface chemistry of fibers themselves (Fubini, 1997) as well as from cellular responses are believed to be important in both neo-plastic and non-neoplastic asbestos-associated pleural disease (Janssen-Heininger et al., 2008; Shukla et al., 2003). Although the limitations of cell culture systems for particulate studies have been well described, it is recognized that much of the mechanistic understanding of how fibers interact with mesothelial cells and produce fiber-induced effects derives from in vitro experiments (Donaldson et al., 2009).
Knowledge and Data Gaps Concerning Pleural Cell Biology and Asbestos Fiber Exposure
-
(a)
Fibers may translocate to the pleural space and are postulated to induce pleural disease by direct interaction with pleural cells. However, some pleural conditions such as inflammation or fibrosis may be influenced by fibers and their actions in the neighboring lung. The relative contribution of direct fiber exposure versus indirect signaling effects on mesothelial cells is not known.
-
(b)
There is still much to know about how fiber mineralogy, dimensions, physicochemical properties, and biopersistence contribute to induction and progression of pleural lesions.
-
(c)
There is need for better development of biomarkers of pleural disease and assessment of pleural changes in experimental animal models. Although bron-choalveolar lavage fluid (BALF) analysis has been routinely utilized in experimental studies of the lung, pleural lavage has not been routinely used to assess changes in the pleural space. Advancing understanding of pleural disease will require better use of pleural endpoints in acute and chronic studies of fiber exposure.
-
(d)
The major target of asbestos in the pleural space is thought to be the mesothelial cell. The contribution of the inflammatory pleural cells including macrophages is less well understood. In addition, there is a need to develop additional understanding of possible mesothelial progenitor cells in asbestos-associated injury and disease (Herrick & Mutsaers, 2004).
-
(e)
The thoracoscopic study by Boutin et al. (1996) demonstrating the focal accumulation of fibers in “black spots” of the parietal pleura provided important new insights. Similar studies might advance the understanding of pleural fiber burden and disease. By obtaining biopsy samples from those undergoing thoracoscopy or thoracotomy, one could determine the locations of fibers and the genetic changes at sites of asbestos deposition, and investigate biomarkers of asbestos toxicity. A systematic analysis using analytical transmission electron microscopy could quantitate the dimensions and types of mineral particles and fibers that are translocated to and retained in the pleura (both visceral and parietal) of control individuals and patients with asbestos-related pleural diseases. Such studies may also help identify whether fibers are located predominately intracellularly or extracellularly, and identify the target cells.
-
(f)
The role of the specific arms of the inflammatory response can now be studied using mice with genetically engineered deletion of specific cell types or inflammatory cytokines. Such studies can be used to indicate the role of inflammation in producing the various fiber-induced pleural diseases and whether particular inflammatory mechanisms might be a therapeutic target.
-
(g)
Noninvasive techniques for assessing fiber burden or the tissue reaction to fibers would be of inestimable value in investigating the natural history of pleural reactions in animals and in humans over the decades of tumor development. Novel imaging techniques could ultimately serve as a tool for following those at risk and testing strategies for intervention.
Biological Mechanisms Responsible for Non-Neoplastic Pleural Disease
It has generally been accepted from studies of animal models of asbestos fiber exposure that inflammatory changes in the lung and pleura precede subsequent fibroproliferative and mesothelial cell proliferative responses. The early pioneering intracavitary instillation and implantation studies of Freidrich Pott and coworkers (Pott, 1980; Pott et al., 1974) and Merle Stanton and colleagues (Stanton et al., 1969, 1977, 1981) revealed that the physical properties of fibers such as dimension are important in the pathogenesis of asbestos-associated disease of the serosal tissues (Case et al., 2011; Aust et al., 2011).
Since those initial studies, much has been learned about other physicochemical properties of inhaled particles believed to be important in their disease-inducing abilities such as the surface properties relevant for oxidant generation (Fubini, 1997) and the chemical properties relevant for biopersistence (Bernstein et al., 2001, 2005; Bernstein, 2007; Mossman et al., 2011).
It is noteworthy that up until now biopersistence studies focused on the lung parenchyma. There are few pleural fiber burden or pleural biopersistence investigations in either experimental animals or humans.
Fiber Type and Potency
In rodent studies in which high concentrations of fibers were instilled or implanted in the pleural space, all mineralogical forms of asbestos fibers were produced pleural fibrosis and malignant mesothelioma. In an inhalation study in rats using well-characterized aerosols and state-of-the-art methods to assess retained lung burdens, Bernstein et al. (1995) found that chrysotile exposure failed to induce pleural lesions despite producing severe pulmonary fibrosis (asbestosis) and lung tumors (Mast et al., 1994). This appears to correlate with human epidemiologic studies because recent analysis suggests that most asbestos-associated mesotheliomas are due to amphibole exposure (Berman & Crump, 2008; Mossman et al., 2011).
While there is a general lack of understanding of comparative pleural potency of different asbestos fiber types, a reanalysis of previous asbestos fiber inhalation studies in rats compared size, shape, and mineralogy with lung tumor and mesothelioma outcomes (Berman et al., 1995). In this study, multivariate measures of exposure were identified that described the lung tumor responses in 13 previous asbestos (chrysotile, amosite, crocidolite, tremolite) inhalation experiments in AF/HAN rats. Due to limitations in the characterization of asbestos fiber dimensions in the original studies, new exposure measures were developed from samples of the original dusts that were regenerated and analyzed by transmission electron microscopy using a direct transfer technique. Structures contributing to lung cancer risk appeared to be long (≥20 μm) and thin (≤0.4 μm) fibers. The analysis did not find significant mineralogical differences in potency across asbestos types for pulmonary tumors but noted that amphibole asbestos was more potent than chrysotile in induction of malignant mesothelioma.
Proposed Mechanisms for Asbestos-Associated Non-Neoplastic Pleural Lesions in Rodents
Although the rodent visceral pleura differs markedly from that of humans in thickness and anatomy (Tyler, 1983), the rodent parietal pleura and the resident pleural inflammatory cells are similar to those of humans (Everitt et al., 1997; Gelzleichter et al., 1996). In rodents, asbestos and synthetic vitreous fiber exposure by inhalation or instillation resulted in pleural inflammatory and fibrotic changes (McConnell et al., 1999). In animal models of asbestos-induced lung cancer and mesothelioma, inflammation and fibrosis always preceded the development of oncogenic outcomes (Greim et al., 2001), and these processes may share some mechanistic underpinnings. Similarly, it is worthy of note that in the rodent fiber inhalation bioassays conducted to date, pleural inflammatory and fibroproliferative lesions were accompanied by pulmonary parenchymal changes. Recently, there have been a number of chronic studies that suggested that the Syrian golden hamster may be particularly susceptible to the development of pleural mesothelioma as well as of pleural fibroproliferative changes following asbestos and synthetic vitreous fiber inhalation (Everitt et al., 1997; Gelzleichter et al., 1999; Mast et al., 1994; McConnell et al., 1999).
As described earlier, mesothelial cell responses to translocated fibers and/or responses to inflammatory mediators released from lung parenchymal and pleural cells are believed to be important in the pathogenesis of pleural fibrosis and asbestos-associated pleurisy (Mutsaers et al., 2004; Robledo & Mossman, 1994). Mesothelial cells are known to phagocytize asbestos fibers (Boylan et al., 1995), a step that may then lead to oxidant-induced signaling pathways, altered cell proliferation, apoptosis (Liu et al., 2000; Shukla et al., 2003b), necrosis (Yang et al., 2010), and release of chemokines and cytokines that mediate pleural inflammation (Jantz & Antony, 2008). A variety of growth factors are associated with pleural fibrosis, especially transforming growth factor (TGF)-β1 (Decologne et al., 2007; Mutsaers et al., 2006).
Proposed Mechanisms for Asbestos-Associated Non-Neoplastic Pleural Disease in Humans
The nonmalignant manifestations of asbestos in the pleura include benign asbestos pleurisy, pleural plaques, diffuse pleural fibrosis, and rounded atelectasis (ATS Official Statement, 2004; Nishimura & Broaddus 1998). Although these nonmalignant pleural diseases may themselves produce symptoms, especially diffuse pleural fibrosis, these are important clinically because the symptoms identify people who have had significant exposure to asbestos and often mimic and require diagnostic workups to exclude malignancy. These diseases are also important for research by giving insight into fiber toxicology and pathogenesis and by identifying groups for which development of biomarkers and early intervention for diagnosis or treatment are warranted.
Benign asbestos pleurisy may develop as early as 10 yr after exposure (ATS, 2004). Because it usually produces no apparent symptoms and is often detected incidentally, the incidence is unclear. The benign pleural effusion may be bloody, thus leading to concern for underlying malignancy. The effusion may last for months, may be unilateral or bilateral, and may recur. The effusion may antedate diffuse pleural fibrosis (Lillis et al., 1988), although the reason for this association is not known.
Pleural plaques are the most common pleural manifestations of asbestos exposure and represent evidence of clinically significant exposure, retention, and biologic response to fibers. In general, plaques develop 20-30 yr after initial exposure (Nishimura & Broaddus, 1998; ATS, 2004). Plaques are usually located on the parietal pleura or on the dome of the diaphragm and appear as circumscribed areas of collagen deposition without inflammation. Plaques may be associated with decreases in lung function and symptoms of dyspnea, but most individuals with pleural plaques alone display no apparent symptoms and no obvious impaired lung function. Although localized and unilateral pleural thickening may have other causes such as prior tuberculosis, trauma, or talc instillation, multiple and bilateral pleural plaques, particularly when calcified, are considered to be pathognomonic for asbestos or erionite exposure (Nishimura & Broaddus, 1998). Of note, those subjects without plaques may also have significant asbestos exposure; it is not known why some exposed individuals form plaques and others do not.
Plaques are biomarkers for asbestos or erionite exposure and of elevated fiber burden in the lung (Churg, 1982; Kishimoto et al., 1989; Roggli & Sanders, 2000). It is not known how pleural plaques correlate with pleural fiber burden. Different fiber types may play a role in plaque formation: Plaques have been associated with the presence of high aspect ratio amphiboles in the lung (Churg, 1982, 1983, 1994), but in at least one study, only chrysotile fibers were found in the plaques themselves (Churg, 1982).
The biologic response to asbestos fibers in individuals with pleural plaques may differ from those without plaques. In animal studies pleural plaques were found to be a consequence of the cellular inflammatory response to asbestos (Sahn & Antony, 1984). In any case, pleural plaques represent a marker of exposure to asbestos and therefore a marker of increased risk for asbestos-related disease, and perhaps may be used to select patients for focused clinical trials to assess other biomarkers of risk and to identify prevention strategies. In general, understanding more about the formation of pleural plaques and their significance as a biomarker of exposure and of enhanced risk would provide insight into pathological mechanisms and suggest possible interventions in exposed populations.
Rounded atelectasis is thought to be a consequence of any type of pleuritis and pleural fibrosis and represents a folding of the lung within a region of pleural thickening. It may resemble a mass and thus raise concern for lung cancer. Little is known regarding the pathogenesis of this entity (Hillerdal, 1989).
Diffuse pleural thickening is a diffuse, not circumscribed, thickening of the pleura that develops approximately 30 yr following exposure (Nishimura & Broaddus, 1998; ATS, 2004). Unlike pleural plaques, diffuse thickening mostly affects the visceral pleura. Diffuse pleural thickening is associated with clinically significant ventilatory impairment, pulmonary restriction, and low lung volumes. Diffuse pleural thickening may coexist with pleural plaques, and may be associated with a higher fiber burden than is found with pleural plaques alone (Stephens et al., 1987). The relationship of diffuse pleural thickening to asbestos-induced fibrosis of the lungs is not known. The types of asbestos fibers likely to produce diffuse pleural thickening are not known. In one study, the fibers found in the pleura were short chrysotile fibers, while the fibers in the lungs were longer and thinner amphiboles (Gibbs et al., 1991). As with other non-neoplastic asbestos-induced pleural disease, diffuse pleural thickening raises concerns for underlying mesothelioma. If lung function is severely compromised, the patient may undergo decortication or removal of the pleura; nevertheless, removal of the thickened pleura may not improve lung function due to accompanying fibrosis of the underlying lung.
These non-neoplastic pleural pathologies are particularly common in those exposed to amphibole fibers in Libby, MT (Peipins et al., 2003), suggesting that these fibers may exert unique toxicity for the pleura. Libby amphibole is a mixture of winchite, richterite, and tremolite, in decreasing order of abundance (Meeker et al., 2003). In 1980, a morbidity study was carried out on workers who had used Libby vermiculite as an inert carrier for various types of lawn-care products (Lockey et al., 1984). Libby vermiculite was found to contain asbestiform minerals. In the workers exposed to Libby vermiculite, workplace exposures were associated with bloody pleural effusions and localized pleural thickening. A follow-up study of the worker cohort 25 years after discontinuation of Libby vermiculite mining in 1980 demonstrated an elevated prevalence of pleural changes, increasing from 2% in 1980 to 29% (80 of 280 workers) in 2005 (Rohs et al., 2005). Of workers with a low lifetime cumulative fiber exposure (CFE) of only <2.2 fibers/cc-yr, as many as 20% displayed pleural changes. A significant CFE response relationship was demonstrated between percent pleural changes, which ranged from 7 to 54%, and the lowest to the highest CFE quartile. The mean CFE (SD) related to localized pleural thickening, diffuse pleural thickening, and interstitial fibrosis in vermiculite workers with no historical exposure to commercial asbestos was 3.45 (4.95), 8 (5.32), and 11.37 (6.82) fiber-cc/yr, respectively (Rohs et al., 2005). This relationship was confirmed by Whitehouse (2004), who demonstrated progressive loss of lung function in Libby residents with and without reported occupational exposure who had predominantly pleural changes. Studies of Libby miners and millers demonstrated an association between Libby amphibole exposure and increased incidence of nonmalignant respiratory disease mortality at a CFE of less than 4.5 fiber/cc-yr (Sullivan, 2007). In summary, studies of workers exposed to the Libby amphibole indicate the propensity for these amphiboles to induce pleural disease and nonmalignant respiratory morbidity and mortality at relatively low lifetime cumulative fiber exposure levels.
Knowledge and Data Gaps in Nonmalignant Pleural Disease in Humans
Unanswered questions regarding nonmalignant pleural disease include:
-
(a)
Pleural plaques have been associated with long amphibole fibers in the lung and with short chrysotile fibers in the pleura. Thus, it is not known which types of asbestos fibers induce pleural plaques and how pleural plaques correlate with pleural (not lung) fiber burden.
-
(b)
The fiber burden and fiber types in the pleura of those with pleural disease have not been documented. Fiber burden in the lung may not correlate with that in the pleura: Low counts in the lung may be associated with high counts in the pleura if fibers have translocated to the pleura; fiber types found in the lung may be the ones that are retained at this site, whereas different fibers may translocate to the pleura and induce disease there. Autopsy studies might be used to compare lung and pleural fiber burdens and relative distribution of different fiber types and sizes in these different locations.
-
(c)
Most studies of pleural fiber burden reported the presence of short chrysotile fibers, and yet the role of these short chrysotile fibers in pleural disease has not been established. Because most pleural disease has been attributed to high aspect ratio amphibole fibers, it is not known whether the short chrysotile fibers are pathogenic, either alone or by enhancing the toxicity of longer amphibole fibers, or whether they are acting as bystanders. These fibers may be located outside the area of interest, corresponding to the “black spots” where pathogenic fibers are located. Further animal studies using well-characterized short chrysotile fibers in the pleural space would be valuable in addressing this important issue.
-
(d)
Although the incidence and severity of pleural disease following exposure to Libby amphibole is high, it is not yet known whether it is actually higher than after exposure to other asbestos types. If Libby amphibole is particularly toxic for the human pleura, the mechanism is not known; perhaps Libby fibers are more readily translocated and retained in the pleura or the Libby fibers that reach the pleura are particularly toxic. In vitro and in vivo studies using Libby amphibole can address these important questions.
-
(e)
The genetic determinants of the individual responses to asbestos are not known-genetic factors may determine susceptibility to non-neoplastic and neoplastic pleural disease following asbestos exposure. Similarly, it is not known whether genetic differences explain why some individuals develop a fibrotic response while others have a neoplastic response. Genetic studies of affected individuals and their families would be valuable to address this issue.
-
(f)
New mechanistically oriented, short-term testing strategies need to be developed to assess pathogenicity of fiber and particulate preparations, not only with respect to car-cinogenicity but also for fibrotic and inflammatory changes in the pleura. The reason for the propensity of the Syrian golden hamster to develop pleural disease needs further investigation.
-
(g)
There is a need to compare nanoparticle-induced lung and pleural changes with asbestos-associated pleural diseases to identify specific physicochemical determinants of toxicity.
-
(h)
The role of inflammation in the development of pleural fibrosis is not understood. Studies are needed in the role of inflammatory cells and of profibrotic cytokines such as TGF-beta. The role of specific receptors such as the Nalp3 inflammasome and its contribution to chronic inflammatory states (Dostert et al., 2008) need additional investigation.
BIOLOGICAL MECHANISMS RESPONSIBLE FOR NEOPLASTIC PLEURAL DISEASE
Fiber Type and Potency
The most potent risk factors for diffuse malignant mesothelioma are environmental or occupational exposure to erionite, asbestos fibers, and vermiculite that contains noncommercial amphiboles (Institute of Medicine, 2006). Based on a recent meta-analysis of the epidemiologic evidence (Berman & Crump, 2008), amphibole asbestos is more potent than chrysotile asbestos in inducing diffuse malignant mesothelioma. This difference in potency was attributed to the greater biopersistence of amphibole asbestos in lungs in comparison with chrysotile asbestos (Bernstein & Hoskins, 2006). Development of diffuse malignant mesothelioma following exposure to chrysotile asbestos is attributed to contamination of some chrysotile deposits with tremolite, a naturally occurring amphibole (Institute of Medicine, 2006).
Biopersistence in the lungs is a key physicochemical property of crystalline mineral fibers and is associated with induction of fibrosis, lung cancer, and malignant mesothelioma in rodent models (ILSI, 2005). Biopersistence in the pleura has not been studied extensively. Boutin and Rey (1993) recovered asbestos fibers in parietal pleural samples of asbestos workers during thoracoscopy. It is likely that long asbestos fibers accumulate at the parietal pleural membrane because they cannot be efficiently cleared through lymphatic stomata. Earlier studies reported low pleural asbestos fiber burdens in asbestos workers (Gibbs et al., 1991). More recent studies recovered large numbers of short asbestos fibers from the lungs and pleural tissues of asbestos-exposed patients (Dodson et al., 2003, 2005, 2007; Suzuki et al., 2005). Mechanistic studies conducted in cell culture associated exposure to long asbestos fibers with activation of the EGF receptor and intracellular signaling pathways leading to cell proliferation (Pache et al., 1998; Mossman et al., 2011). Asbestos fibers were also found to interfere physically with the mitotic apparatus (Hei et al., 2000; Huang et al., 2011).
Fibers may be altered secondarily in the lungs or pleura. Depending on their chemical composition, surface area, and crystalline structure, asbestos fibers may leach, split, or break (ILSI, 2005). The kinetics of fiber alteration and clearance from the pleural space has not been investigated. Secondary modification of surface properties including binding of phospholipids, acquisition or depletion of cations, and protein adsorption in the pleura may also modify toxicity (Fubini & Mollo, 1995).
Proposed Mechanisms for Asbestos-Induced Mesothelioma
Asbestos and erionite fibers were shown to induce genotoxicity directly. Chronic rodent studies established an association between persistent inflammation and carcinogenicity induced by inhalation of crystalline mineral fibers (ILSI, 2005). Chronic inflammation triggered in response to biopersistent fibers may amplify the genotoxicity of asbestos fibers in pleural target cells (Figure 2). Following internalization by phagocytosis, asbestos fibers trigger macrophage activation and generation of reactive oxygen and nitrogen species, leading to tissue injury. Recent studies in genetically engineered mice suggest a central role for the NALP3 inflammasome in rapid release of active IL-1β (Cassel et al., 2008; Dostert et al., 2008), a cytokine that triggers recruitment of additional inflammatory cells and release of cytokines (tumor necrosis factor [TNF]-α, inter-leukin [IL]-6, IL-8) that perpetuate inflammation in response to biopersistent asbestos fibers (Shukla et al., 2003a). TNF-α also activates the nuclear factor (NF)-κB pathway in mesothelial cells, allowing these cells to survive and proliferate in the presence of asbestos-induced DNA damage (Yang et al., 2006).
FIGURE 2.
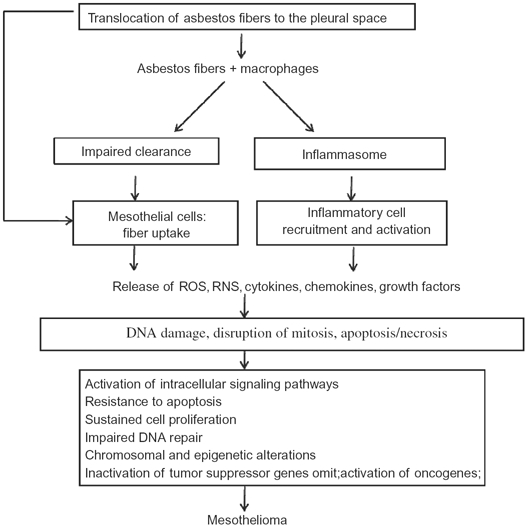
Proposed mechanisms for asbestos-induced mesothelioma. Asbestos fibers are thought to lead to mesothelioma via mechanisms as outlined in this algorithm. Asbestos fibers enter the pleural space, where they interact with pleural macrophages and mesothelial cells and induce an influx of inflammatory cells. These early interactions result in release of reactive oxygen and nitrogen species (ROS, RNS), cytokines, and growth factors that may mediate indirect effects on mesothelial cells. The fibers may also act directly on mesothelial cells by inducing DNA damage, interrupting chromosomal segregation, or inducing apoptosis or necrosis. Such direct and indirect actions lead to chronic stimulation and injury of the mesothelium that may proceed over decades by a multistep path to cancer. Key steps in the development of cancer include genetic and epigenetic alterations leading to sustained cell proliferation, resistance to apoptosis, and inactivation of tumor suppressor genes.
Amphibole asbestos fibers contain surface redox-active iron (Fe) that generates ROS leading to lipid peroxidation, protein oxidation, and DNA damage in lung and pleural target cells (Manning et al., 2002). Erionite fibers may secondarily acquire Fe that catalyzes generation of ROS (Hardy & Aust, 1995; Aust et al., 2011). Secondary deposition of endogenous Fe may enhance redox activity or disrupt Fe homeostasis in the lungs or pleura producing oxidative stress (Ghio et al., 2008). In response to chronic oxidative stress, intracellular signaling pathways trigger activation of transcription factors, stimulation of cell proliferation, and resistance to apoptosis (Albrecht et al., 2004; Mossman et al., 2011).
Asbestos in cell culture (Broaddus et al., 1996; Berube et al., 1996) and in some animal studies (Marchi et al., 2000) was found to induce apoptosis in mesothelial cells, and ROS may contribute to this early apoptosis (Broaddus et al., 1996); at later times, DNA or chromosomal damage may also trigger apoptosis. Presumably, it is the cells that have inherent resistance to apoptosis or acquire resistance that will survive the initial and ongoing damage to initiate multistep acquisition of genetic abnormalities that characterize tumor development (Broaddus, 1997). Mesothelial cells (or other progenitor cells) may acquire resistance by inherent overexpression of anti-apoptotic molecules or more likely by upregulation of these molecules, e.g., the Bcl-2 or the IAP family. Mesothelial cells with other preexisting abnormalities in DNA damage-induced signaling or in mitochondrial function may not undergo apoptosis and may persist despite asbestos-induced toxicity (Upadhyay & Kamp, 2003).
Mesothelial cells may have inherent or acquired activation of prosurvival pathways, from either the exposure to asbestos with upregulation of growth factor receptors (Pache et al., 1998) or downstream pathways (MAPK, ERK, Akt/mTOR) or another survival mechanism (Jimenez et al., 1997; Altomare et al., 2005), as proposed with SV40 infection (Kroczynska et al., 2006). Inflammation may also initiate an environment that itself promotes prosurvival mechanisms. Inflammation may also be induced by the fibers themselves, by an influx of cells of the innate immune system or by asbestos-induced necrosis (Yang et al., 2010). The microenvironment including the presence of inflammatory cells, endothelial cells, and fibroblasts, along with the formation of a three-dimensional shape itself, supports the resistance to apoptosis (Barbone et al., 2008; Daubriac et al., 2009). Critically important survival mechanisms that inhibit apoptosis, if understood, might be a target for intervention (Heintz et al., 2010).
This chronic inflammatory environment may contribute to acquired, heritable genetic, or epigenetic alterations leading to inactivation of tumor suppressor genes, activation of oncogenes, and altered regulation of cell cycle and DNA repair pathways (Kratzke and Gazdar, 2005). Specific genetic, epigenetic, and chromosomal alterations are characteristic of diffuse malignant mesothelioma (Murthy and Testa, 1999; Apostolou et al., 2005). Oxidants generated directly by redox-active asbestos fibers or indirectly following phagocytosis may also induce DNA and chromosomal damage (Jaurand, 1996; Hei et al., 2000; Huang et al., 2011). An indirect mechanism associated with persistent inflammation was proposed for altered gene methylation profiles characteristic of human malignant pleural mesotheliomas (Christensen et al., 2009). These genetic and epigenetic alterations may select for mesothelial cells that are able to survive and proliferate in a chronic inflammatory environment (Huang et al., 2011).
KNOWLEDGE AND DATA GAPS FOR THE BIOLOGIC MECHANISMS FOR NEOPLASTIC PLEURAL DISEASE
-
(a)
Workers are usually exposed to mixed dusts contaminated with asbestos fibers. It is unknown whether asbestos-related pleural diseases are potentiated by exposure to other dusts such as vermiculite, crystalline silica, or metals in the occupational environment.
-
(b)
The role of SV40 virus as a cofactor with asbestos fibers in the development of diffuse malignant mesothelioma is controversial (Gazdar et al., 2002). Mechanistic studies in cell cultures and in rodents suggest that SV40 viral oncoproteins induce mesothelial cell transformation and diffuse malignant mesothelioma, although human epidemiological studies do not support a causal association (Weiner & Neragi-Miandoab, 2009). Additional epidemiological studies using specific serological markers for SV40 virus infections are needed (Kean et al., 2009).
-
(c)
The physical and chemical properties of mineral fibers associated with carcinogenicity include surface chemistry and reactivity, surface area, fiber dimensions, and biopersistence. The relative importance of these different properties with respect to carcinogenic potency is uncertain and may depend on the geological source of the fibrous mineral and its associated contaminants. Commercial asbestos fibers and erionite fibers have been most widely studied. Noncommercial amphibole fibers, other naturally occurring asbestiform fibers, and newly engineered fibrous nanomaterials (Jaurand et al., 2009; Sanchez et al., 2009) need to be well characterized and their potential for translocation and persistence in the pleura must be determined.
-
(d)
The potential for any natural or engineered fibrous material with physicochemical properties similar to asbestos fibers to induce persistent inflammation and promote the development of diffuse malignant mesothelioma needs to be investigated before the fibers are widely used.
-
(e)
Various direct and indirect mechanisms were proposed for the induction of diffuse malignant mesothelioma by asbestos fibers. These mechanisms may interact at multiple stages during the long latent period associated with this malignancy. The relative importance of these different mechanisms in tumor development and progression is unknown. The ability of different fiber types to induce specific genetic and epigenetic alterations characteristic of diffuse malignant mesothelioma needs to be determined (Andujar et al., 2007).
-
(f)
Chronic rodent inhalation assays are expensive, technically demanding, and not suitable for mechanistic studies because only a minority of rats develop diffuse malignant mesothelioma following inhalation. Current screening assays for fiber toxicity use short-term in vitro or in vivo assays; however, it is difficult to extrapolate from acute, high-dose exposures to chronic or repeated, low-dose exposures in vivo. A new toxicologic screening strategy needs to be developed and validated to assess potential carcinogenicity of naturally occurring mineral fibers and engineered fibrous nanomaterials.
-
(g)
Mesothelial cells are mobile and appear to be able to detach and relocate at other sites in the pleural space (Foley-Comer et al., 2002). Mesothelioma is also associated with mobile spheroids, clumps of malignant cells floating in the pleural fluid; such spheroids appear to remain viable and resistant to apoptosis (Barbone et al., 2008; Daubriac et al., 2009). It is not known whether this mobility may allow preneoplastic cells to move from areas of asbestos accumulation to other areas where mesothelioma may develop. If so, and if new technologies are developed that will allow one to distinguish malignant mesothelial cells from reactive benign mesothelial cells, pleural fluid could be sampled for preneoplastic mesothelial cells in order to identify individuals at risk for developing diffuse malignant mesothelioma.
-
(h)
New immunohistochemical and molecular markers of preneoplastic and neoplastic lesions would improve early diagnosis and therapy of diffuse malignant mesothelioma (Husain et al., 2009).
-
(i)
Populations exposed to asbestos or asbestiform fibers from Libby MT, workers in certain trades, those exposed on 9/11 at Ground Zero, or those with known high exposure (e.g., those with bilateral pleural plaques) need to be followed in epidemiologic studies that include noninvasive studies of biomarkers and imaging with the potential for more invasive studies using pleuroscopy or pleural lavage. In this way, knowledge can be gained about the natural history of asbestos-induced pleural disease in order to understand preneoplastic changes and ultimately permit early diagnosis or prevention studies.
Footnotes
*This author has disclosed a potential conflict of interest as described by one or more of the following: He/She has acted and/or is currently acting as an expert witness or consultant for law firms representing plaintiffs and/or defendants in asbestos litigation and compensation board proceedings, and has been a paid or unpaid consultant to regulatory and medical agencies and compensation boards in North America, including but not limited to NIEHS, EPA, ATSDR, ATS, NGOs and individual and collective citizen groups concerned with asbestos exposure and disease.
#This author has disclosed a potential conflict of interest as described by one or more of the following: He/She may have also received (and may also apply in future for) competitive-funding research grants from U.S. publicly financed, peer-reviewed grant approval process agencies concerning asbestos exposure and disease, including topics covered in all aspects of the workshop, including but not limited to research support from NIEHS.
Dr. Broaddus acknowledges research support from the Department of Defense (PR 080717) and Dr. Kane acknowledges research support from the National Institute of Environmental Health Sciences (RO1 ES016178 and P42 ES013660).
REFERENCES
- Adamson I. Y. Early mesothelial cell proliferation after asbestos exposure: in vivo and in vitro studies. Environ. Health Perspect. 1997;105:1205–1208. doi: 10.1289/ehp.97105s51205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht C., Borm P. J., Unfried K. Signal transduction pathways relevant for neoplastic effects of fibrous and non-fibrous particles. Mutat Res. 2004;553:23–35. doi: 10.1016/j.mrfmmm.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Altomare D. A., You H., Xiao G. H., Ramos-Nino M. E., Skele K. L., De Rienzo A., Jhanwar S. C., Mossman B. T., Kane A. B., Testa J. R. Human and mouse mesotheliomas exhibit elevated AKT/PKB activity, which can be targeted pharmacologically to inhibit tumor cell growth. Oncogene. 2005;24:6080–6089. doi: 10.1038/sj.onc.1208744. [DOI] [PubMed] [Google Scholar]
- American Thoracic Society. (ATS) Diagnosis and initial management of non-malignant diseases related to asbestos. Am. J. Respir. Crit. Care Med. 2004;170:691–715. doi: 10.1164/rccm.200310-1436ST. [DOI] [PubMed] [Google Scholar]
- Andujar P., Lecomte C., Renier A., Fleury-Feith J., Kheuang L., Daubriac J., Janin A., Jaurand M. C. Clinico-pathological features and somatic gene alterations in refractory ceramic fibre-induced murine mesothelioma reveal mineral fibre-induced mesothelioma identities. Carcinogenesis. 2007;28:1599–1605. doi: 10.1093/carcin/bgm023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolou S., Balsara B. R., Testa J. R. Cytogenetics of malignant mesothelioma. Malignant mesothelioma. In: Pass H. I., Vogelzang N. J., Carbone M., editors. Advances in pathogenesis, diagnosis and translational therapies. Vol. 6. New York: Springer Science and Business Media; 2005. pp. 101–111. [Google Scholar]
- Aust A. E., Cook P. M., Dodson R. F. Morphological and chemical mechanisms of elongated mineral particle toxicities. J. Toxicol. Environ. Health B, 2011;14:40–75. doi: 10.1080/10937404.2011.556046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbone D., Yang T. M., Morgan J. R., Gaudino G., Broaddus V. C. Mammalian target of rapamycin contributes to the acquired apoptotic resistance of human mesothelioma multicellular spheroids. J. Biol. Chem. 2008;283:13021–13030. doi: 10.1074/jbc.M709698200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann M. H., Heinrich K., Sahn S. A., Strange C. Pleural macrophages differentially alter mesothelial cell growth and collagen production. Inflammation. 1993;17:1–12. doi: 10.1007/BF00916387. [DOI] [PubMed] [Google Scholar]
- Baumann M. H., Strange C., Sahn S. A., Kinasewitz G. T. Pleural macrophages differentially alter pleural mesothelial cell glycosaminoglycan production. Exp. Lung Res. 1996;22:101–111. doi: 10.3109/01902149609074020. [DOI] [PubMed] [Google Scholar]
- Berman D. W., Crump K. S. A meta-analysis of asbestos-related cancer risk that addresses fiber size and mineral type. Crit. Rev. Toxicol. 2008;38:49–73. doi: 10.1080/10408440802273156. [DOI] [PubMed] [Google Scholar]
- Berman D. W., Crump K. S., Chatfield E. J., Davis J. M., Jones A. D. The sizes, shapes, and mineralogy of asbestos structures that induce lung tumors or mesothelioma in AF/HAN rats following inhalation. Risk Anal. 1995;15:181–195. doi: 10.1111/j.1539-6924.1995.tb00312.x. [DOI] [PubMed] [Google Scholar]
- Bermudez E., Mangum J. B., Asgharian B., Wong B. A., Reverdy E. E., Janszen D. B., Hext P. M., Warheit D. B., Everitt J. I. Long-term pulmonary responses of three laboratory rodent species to sub-chronic inhalation of pigmentary titanium dioxide particles. Toxicol. Sci. 2002;70:86–97. doi: 10.1093/toxsci/70.1.86. [DOI] [PubMed] [Google Scholar]
- Bernstein D. M. Synthetic vitreous fibers: A review toxicology, epidemiology and regulations. Crit. Rev. Toxicol. 2007;37:839–886. doi: 10.1080/10408440701524592. [DOI] [PubMed] [Google Scholar]
- Bernstein D. M., Chevalier J., Smith P. Comparison of Calidria chrysotile asbestos to pure tremolite: Final results of the inhalation biopersistence and histopathology examination following short-term exposure. Inhal. Toxicol. 2005;17:427–449. doi: 10.1080/08958370591002012. [DOI] [PubMed] [Google Scholar]
- Bernstein D. M., Donaldson K., Decker U., Gaering S., Kunzendorf P., Chevalier J., Holm S. E. A biopersistence study following exposure to chrysotile asbestos alone or in combination with fine particles. Inhal. Toxicol. 2008;20:1009–1028. doi: 10.1080/08958370802259053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein D. M., Hoskins J. A. The health effects of chrysotile: current perspective based upon recent data. Regul. Toxicol. Pharmacol. 2006;45:252–264. doi: 10.1016/j.yrtph.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Bernstein D. M., Riego Sintes J. M., Ersboell B. K., Kunert J. Biopersistence of synthetic mineral fibers as a predictor of chronic inhalation toxicity in rats. Inhal. Toxicol. 2001;13:823–849. doi: 10.1080/089583701752378133. [DOI] [PubMed] [Google Scholar]
- Bernstein D. M., Thevenaz P., Fleissner H., Anderson R., Hesterberg T. W., Mast R. Evaluation of the oncogenic potential of man-made vitreous fibres: The inhalation model. Ann. Occup. Hyg. 1995;39:661–672. doi: 10.1016/0003-4878(94)00108-d. [DOI] [PubMed] [Google Scholar]
- BéruBé K. A., Quinlan T. R., Fung H., Magae J., Vacek P., Taatjes D. J., Mossman B. T. Apoptosis is observed in mesothelial cells after exposure to crocidolite asbestos. Am. J. Respir. Cell Mol. Biol. 1996;15:141–147. doi: 10.1165/ajrcmb.15.1.8679218. [DOI] [PubMed] [Google Scholar]
- Boutin C., Dumortier P., Rey F., Viallat J. R., DeVuyst P. Black spots concentrate oncogenic asbestos fibers in the parietal pleura: Thoracoscopic and mineralogic study. Am. J. Respir. Crit. Care Med. 1996;153:444–449. doi: 10.1164/ajrccm.153.1.8542156. [DOI] [PubMed] [Google Scholar]
- Boutin C., Rey F. Thoracoscopy in pleural malignant mesothelioma: A prospective study of 188 consecutive patients: Part I. Diagnosis. Cancer. 1993;72:389–393. doi: 10.1002/1097-0142(19930715)72:2<389::aid-cncr2820720213>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Boutin C., Schlesser M., Frenay G., Germain S. Malignant mesothelioma. Diagnosis and treatment. Rev. Pract. 1997;47:1333–1339. [PubMed] [Google Scholar]
- Boylan A. M., Ruegg C., Kim K. J., Hebert C. A., Hoeffel J. M., Pytela R., Sheppard D., Goldstein I. M., Broaddus V. C. Evidence of a role for mesothelial cell-derived interleukin-8 in the pathogenesis of asbestos-induced pleurisy in rabbits. J. Clin. Invest. 1992;89:1257–1267. doi: 10.1172/JCI115710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boylan A. M., Sanan D. A., Sheppard D., Broaddus V. C. Vitronectin enhances internalization of crocidolite asbestos by rabbit pleural mesothelial cells via the integrin alpha v beta 5. J. Clin. Invest. 1995;96:1987–2001. doi: 10.1172/JCI118246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broaddus V. C. Asbestos, the mesothelial cell and malignancy: A matter of life or death. Am. J. Respir. Cell Mol. Biol. 1997;17:657–659. doi: 10.1165/ajrcmb.17.6.f141. [DOI] [PubMed] [Google Scholar]
- Broaddus V. C. Transudative pleural effusions. In: Loddenkemper R., Anthony V., editors. Pleural diseases, European Respiratory Monograph 22. Sheffield, UK: European Respiratory Society Journals; 2002. pp. 157–176. [Google Scholar]
- Broaddus V. C. Physiology: fluid and solute exchange in normal physiological states. In: Light R., Lee Y., editors. Textbook of pleural diseases. London: Hodder Arnold; 2008. pp. 43–48. [Google Scholar]
- Broaddus V. C., Wiener-Kronish J. P., Berthiaume Y., Staub N. C. Removal of pleural liquid and protein by lymphatics in awake sheep. J. Appl. Physiol. 1988;64:384–390. doi: 10.1152/jappl.1988.64.1.384. [DOI] [PubMed] [Google Scholar]
- Broaddus V. C., Wiener-Kronish J. P., Staub N. C. Clearance of lung edema into the pleural space of volumeloaded, anesthetized sheep. J. Appl. Physiol. 1990;68:2623–2630. doi: 10.1152/jappl.1990.68.6.2623. [DOI] [PubMed] [Google Scholar]
- Broaddus V. C., Yang L., Scavo L. M., Ernest J. D., Boylan A. M. Asbestos induces apoptosis of human and rabbit pleural mesothelial cells via reactive oxygen species. J. Clin. Invest. 1996;98:2050–2059. doi: 10.1172/JCI119010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case B. W., Abraham J., Meeker G., Pooley F. D., Pinkerton K. E. Applying definitions of “asbestos” to environmental and “low-dose” exposure levels and health effects, particularly malignant mesothelioma. J. Toxicol. Environ. Health B. 2011;14:3–39. doi: 10.1080/10937404.2011.556045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassel S. L., Eisenbarth S. C., Iyer S. S., Sadler J. J., Colegio O. R., Tephly L. A., Carter A. B., Rotham P. B., Flavell R. A., Sutterwala F. S. The Nalp3 inflammasome is essential for the development of silicosis. Proc. Natl. Acad. Sci. USA. 2008;105:9035–9040. doi: 10.1073/pnas.0803933105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman S. J., Cookson W. O. C., Musk A. W., Lee Y. C. Benign asbestos pleural diseases. Curr. Opin. Pulm. Med. 2003;9:266–271. doi: 10.1097/00063198-200307000-00004. [DOI] [PubMed] [Google Scholar]
- Choe N., Tanaka S., Xia W., Hemenway D. R., Roggli V. L., Kagan E. Pleural macrophage recruitment and activation in asbestos-induced pleural injury. Environ. Health Perspect. 1997;105:1257–1260. doi: 10.1289/ehp.97105s51257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen B. C., Marsit C. J., Houseman E. A., Godleski J. J., Longacker J. L., Zheng S., Yeh R. F., Wrensch M. R., Wiemels J. L., Karagas M. R., Bueno R., Sugarbaker D. J., Nelson H. H., Wiencke J. K., Kelsey K. T. Differentiation of lung adenocarcinoma, pleural mesothelioma, and nonmalignant pulmonary tissues using DNA methylation profiles. Cancer Res. 2009;69:6315–6321. doi: 10.1158/0008-5472.CAN-09-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churg A. Asbestos fibers and pleural plaques in a general autopsy population. Am. J. Pathol. 1982;109:88–96. [PMC free article] [PubMed] [Google Scholar]
- Churg A. Current issues in the pathologic and mineralogic diagnosis of asbestosinduced disease. Chest. 1983;84:275–280. doi: 10.1378/chest.84.3.275. [DOI] [PubMed] [Google Scholar]
- Churg A. Deposition and clearance of chrysotile asbestos. Ann. Occup. Hyg. 1994;38:625–633. doi: 10.1093/annhyg/38.4.625. [DOI] [PubMed] [Google Scholar]
- Churg A., Warnock M. L. Asbestos fibers in the general population. Am. Rev. Respir. Dis. 1980;122:669–678. doi: 10.1164/arrd.1980.122.5.669. [DOI] [PubMed] [Google Scholar]
- Daubriac J., Fleury-Feith J., Kheuang L., Galipon J., Saint-Albin A., Renier A., Giovannini M., Galateau-Sallé F., Jaurand M. C. Malignant pleural mesothelioma cells resist anoikis as quiescent pluricellular aggregates. Cell Death Differ. 2009;16:1146–1155. doi: 10.1038/cdd.2009.32. [DOI] [PubMed] [Google Scholar]
- Davis J. M. G. An electron microscope study of the response of mesothelial cells to the intrapleural injection of asbestos dust. Br. J. Exp. Pathol. 1974;55:64–70. [PMC free article] [PubMed] [Google Scholar]
- Davis J. M., Jones A. D., Miller B. G. Experimental studies in rats on the effects of asbestos inhalation coupled with the inhalation of titanium dioxide or quartz. Int. J. Exp. Pathol. 1991;72:501–525. [PMC free article] [PubMed] [Google Scholar]
- Decologne N., Kolb M., Margetts P. J., Menetrier F., Artur Y., Garrido C., Gauldie J., Camus P., Bonniaud P. TGF-beta1 induces progressive pleural scarring and subpleural fibrosis. J. Immunol. 2007;179:6043–6051. doi: 10.4049/jimmunol.179.9.6043. [DOI] [PubMed] [Google Scholar]
- DeVuyst P., Karjalainen A., Dumortier P., Pairon J., Monso E., Brochard P., Teschlet H., Tossavainen A., Gibbs A. Guidelines for mineral fibre analyses in biological samples: Report of the ERS Working Group. Eur. Respir. J. 1998;11:1416–1426. doi: 10.1183/09031936.98.11061416. [DOI] [PubMed] [Google Scholar]
- Dodson R. F., Atkinson M. A. L. Measurements of asbestos burden in tissues. Ann. NY Acad. Sci. 2006;1076:281–291. doi: 10.1196/annals.1371.015. [DOI] [PubMed] [Google Scholar]
- Dodson R. F., Hammar S. P. Pleural mesothelioma in a woman whose documented past exposure to asbestos was from smoking asbestos-containing filtered cigarettes: The comparative value of analytical transmission electron microscopic analysis of lung and lymph node tissue. Inhal. Toxicol. 2006;18:679–684. doi: 10.1080/08958370600743068. [DOI] [PubMed] [Google Scholar]
- Dodson R. F., Atkinson M. A., Levin J. L. Asbestos fiber length as related to potential pathogenicity: a critical review. Am. J. Ind. Med. 2003;44:291–297. doi: 10.1002/ajim.10263. [DOI] [PubMed] [Google Scholar]
- Dodson R. F., Graef R., Shepherd S., O'Sullivan M., Levin J. Asbestos burden in cases of mesothelioma from individuals from various regions of the United States. Ultrastruct. Pathol. 2005;29:415–433. doi: 10.1080/019131290945682. [DOI] [PubMed] [Google Scholar]
- Dodson R. F., Shepherd S., Levin J., Hammar S. P. Characteristics of asbestos concentration in lung as compared to asbestos concentration in various levels of lymph nodes that collect drainage from the lung. Ultrastruct. Pathol. 2007;31:95–133. doi: 10.1080/01913120701423907. [DOI] [PubMed] [Google Scholar]
- Dodson R. F., Williams M. G., Jr., Corn C. J., Brollo A., Bianchi C. Asbestos content of lung tissue, lymph nodes, and pleural plaques from former shipyard workers. Am. Rev. Respir Dis. 1990;142:843–847. doi: 10.1164/ajrccm/142.4.843. [DOI] [PubMed] [Google Scholar]
- Dodson R. F., Ford J. O. Early response of the visceral pleura following asbestos exposure: An ultrastructural study. J. Toxicol. Environ. Health. 1985;15:673–686. doi: 10.1080/15287398509530695. [DOI] [PubMed] [Google Scholar]
- Dodson R. F., O'Sullivan M. F., Huang J., Holiday D. B., Hammar S. P. Asbestos in extrapulmonary sites: omentum and mesentery. Chest. 2000;117:486–493. doi: 10.1378/chest.117.2.486. [DOI] [PubMed] [Google Scholar]
- Donaldson K., Borm P. J., Castranova V., Gulumian M. The limits of testing particle-mediated oxidative stress in vitro in predicting diverse pathologies; Relevance for testing of nanoparticles. Part. Fibre Toxicol. 2009;6:13. doi: 10.1186/1743-8977-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dostert C., Pétrilli V., Van Bruggen R., Steele C., Mossman B. T., Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumortier P., De Vuyst P., Rey F. Letter to the editor: RE main asbestos type in pleural mesothelioma. Am. J. Ind. Med. 1998;33:94–95. doi: 10.1002/(sici)1097-0274(199801)33:1<94::aid-ajim12>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Everitt J. I., Gelzleichter T. R., Bermudez E., Mangum J. B., Wong B. A., Janszen D. B., Moss O. R. Comparison of pleural responses of rats and hamsters to subchronic inhalation of refractory ceramic fibers. Environ. Health Perspect. 1997;105:1209–1213. doi: 10.1289/ehp.97105s51209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasske E. Pathogenesis of pulmonary fibrosis induced by chrysotile asbestos. Virchows Arch. 1986;408:329–346. doi: 10.1007/BF00707692. [DOI] [PubMed] [Google Scholar]
- Foley-Comer A. J., Herrick S. E., Al-Mishlab T., Prêle C. M., Laurent G. J., Mutsaers S. E. Evidence for incorporation of free-floating mesothelial cells as a mechanism of serosal healing. J. Cell. Sci. 2002;115:1383–1389. doi: 10.1242/jcs.115.7.1383. [DOI] [PubMed] [Google Scholar]
- Fubini B. Surface reactivity in the pathogenic response to particulates. Environ. Health Perspect. 1997;105:1013–1020. doi: 10.1289/ehp.97105s51013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fubini B., Mollo L. Role of iron in the reactivity of mineral fibers. Toxicol. Lett. 1995;82-83:951–960. doi: 10.1016/0378-4274(95)03531-1. [DOI] [PubMed] [Google Scholar]
- Gazdar A. F., Butel J. S., Carbone M. SV40 and human tumours: myth, association or causality? Nat. Rev. Cancer. 2002;2:957–964. doi: 10.1038/nrc947. [DOI] [PubMed] [Google Scholar]
- Gelzleichter T. R., Bermudez E., Mangum J. B., Wong B. A., Everitt J. I., Moss O. R. Pulmonary and pleural responses in Fischer 344 rats following short-term inhalation of a synthetic vitreous fiber. I. Quantitation of lung and pleural fiber burdens. Fundam. Appl. Toxicol. 1996;30:31–38. doi: 10.1006/faat.1996.0040. [DOI] [PubMed] [Google Scholar]
- Gelzleichter T. R., Bermudez E., Mangum J. B., Wong B. A., Janszen D. B., Moss O. R., Everitt J. I. Comparison of pulmonary and pleural responses of rats and hamsters to inhaled refractory ceramic fibers. Toxicol. Sci. 1999;49:93–101. doi: 10.1093/toxsci/49.1.93. [DOI] [PubMed] [Google Scholar]
- Gelzleichter T. R., Bermudez E., Mangum J. B., Wong B. A., Moss O. R., Everitt J. I. Pulmonary and pleural responses in Fischer 344 rats following shortterm inhalation of a synthetic vitreous fiber. II. Pathobiologic responses. Fundam. Appl. Toxicol. 1996;30:39–46. doi: 10.1006/faat.1996.0041. [DOI] [PubMed] [Google Scholar]
- Ghio A. J., Stonehuerner J. G., Richards J. H., Crissman K. M., Roggli V. L., Piantadosi C. A., Carraway M. S. Iron homeostasis and oxidative stress in idiopathic pulmonary alveolar proteinosis: A case-control study. Respir Res. 2008;9:10–18. doi: 10.1186/1465-9921-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs A. R., Stephen M., Griffith D. M., Blight B. J., Pooley F. D. Fibre distribution in the lungs and pleura of subjects with asbestos related diffuse fibrosis. Br. J. Ind. Med. 1991;48:762–770. doi: 10.1136/oem.48.11.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greillier L., Baas P., Welch J. J., Hasan B., Passioukov A. Biomarkers for malignant pleural mesothelioma: current status. Mol. Diagn. Ther. 2008;12:375–390. doi: 10.1007/BF03256303. [DOI] [PubMed] [Google Scholar]
- Greim H., Borm P., Schins R., Donaldson K., Driscoll K., Hartwig A., Kuempel E., Oberdörster G., Speit G. Toxicity of fibers and particles. Report of the workshop held in Munich, Germany, 26-27 October 2000. Inhal. Toxicol. 2001;13:737–754. doi: 10.1080/08958370118273. [DOI] [PubMed] [Google Scholar]
- Hammar S. P. The pathology of benign and malignant pleural disease. Chest Surg. Clin. North Am. 1994;4:405–430. [PubMed] [Google Scholar]
- Hardy J.A., Aust A. E. The effect of iron binding on the ability of crocidolite asbestos to catalyze DNA single-strand breaks. Carcinogenesis. 1995;16:319–325. doi: 10.1093/carcin/16.2.319. [DOI] [PubMed] [Google Scholar]
- Hei T. K., Xu A., Louie D., Zhou Y. L. Genotoxicity versus carcinogenicity: Implications from fiber toxicity studies. Inhal. Toxicol. 2000;12(suppl. 3):141–147. doi: 10.1080/08958378.2000.11463207. [DOI] [PubMed] [Google Scholar]
- Heintz N. H., Janssen-Heininger Y. M., Mossman B. T. Asbestos, lung cancers, and mesotheliomas: from molecular approaches to targeting tumor survival pathways. Am. J. Respir. Cell Mol. Biol. 2010;42:133–139. doi: 10.1165/rcmb.2009-0206TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson R. F., Driscoll K. E., Harkema J. R., Lindenschmidt R. C., Chang I. Y., Maples K. R., Barr E. B. A comparison of the inflammatory response of the lung to inhaled versus instilled particles in F344 rats. Fundam. Appl. Toxicol. 1995;24:183–197. doi: 10.1006/faat.1995.1022. [DOI] [PubMed] [Google Scholar]
- Herrick S. E., Mutsaers S. E. Mesothelial progenitor cells and their potential in tissue engineering. Int. J. Biochem. Cell Biol. 2004;36:621–642. doi: 10.1016/j.biocel.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Hill G. D., Mangum J. B., Moss O. R., Everitt J. I. Soluble ICAM-1, MCP-1, and MIP-2 protein secretion by rat pleural mesothelial cells following exposure to amosite asbestos. Exp. Lung Res. 2003;29:277–290. doi: 10.1080/01902140303788. [DOI] [PubMed] [Google Scholar]
- Hillerdal G. Rounded atelectasis. Clinical experience with 74 patients. Chest. 1989;95:836–841. doi: 10.1378/chest.95.4.836. [DOI] [PubMed] [Google Scholar]
- Holt P. F. Transport of inhaled dust extrapulmonary sites. J. Pathol. 1981;133:123–129. doi: 10.1002/path.1711330204. [DOI] [PubMed] [Google Scholar]
- Huang S. X. L., Jaurand M. C., Kamp D. W., Whysner J., Hei T. K. Role of mutagenicity in mineral fiber-induced carcinogenicity and other diseases. J. Toxicol. Environ. Health B. 2011;14:179–245. doi: 10.1080/10937404.2011.556051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain A. N., Colby T. V., Ordonez N. G., Krausz T., Borczuk A., Cagle P. T., Chirieac L. R., Chur A., Galateau-Salle F., Gibbs A. R., Gown A. M., Hammar S. P., Litzky L. A., Roggli V. L., Travis W. D., Wick M. R. Guidelines for pathologic diagnosis of malignant mesothelioma: A consensus statement from the International Mesothelioma Interest Group. Arch. Pathol. Lab. Med. 2009;133:1317–1331. doi: 10.5858/133.8.1317. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine. Asbestos: Selected cancers. Washington, DC: National Academies Press; 2006. [PubMed] [Google Scholar]
- International Life Sciences Institute. Testing of fibrous particles: Short-term assays and strategies. Report of an ILSI Risk Science Institute Working Group. Inhal. Toxicol. 2005;17:497–537. doi: 10.1080/08958370591001121. [DOI] [PubMed] [Google Scholar]
- Janssen-Heininger Y. M., Mossman B. T., Heintz N. H., Forman H. J., Kalyanaraman B., Finkel T., Stamler J. S., Rhee S. G., van der Vliet A. Redox-based regulation of signal transduction: principles, pitfalls, and promises. Free Radical Biol. Med. 2008;45:1–17. doi: 10.1016/j.freeradbiomed.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantz M. A., Antony V. B. Pathophysiology of the pleura. Respiration. 2008;75:121–133. doi: 10.1159/000113629. [DOI] [PubMed] [Google Scholar]
- Jarabek A. M., Asgharian B., Miller F. J. Dosimetric adjustments for interspecies extrapolation of inhaled poorly soluble particles (PSP) Inhal. Toxicol. 2005;17:317–334. doi: 10.1080/08958370590929394. [DOI] [PubMed] [Google Scholar]
- Jaurand M. C. Use of in-vitro genotoxicity and cell transformation assays to evaluate the potential carcinogenicity of fibres. IARC Sci. Publ. 1996;140:55–72. [PubMed] [Google Scholar]
- Jaurand M. C., Renier A., Daubriac J. Mesothelioma: Do asbestos and carbon nanotubes pose the same health risk? Part. Fibre Toxicol. 2009;6:16–30. doi: 10.1186/1743-8977-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez L. A., Zanella C., Fung H., Janssen Y. M., Vacek P., Charland C., Goldberg J., Mossman B. T. Role of extracellular signal-regulated protein kinases in apoptosis by asbestos and H2O2. Am. J. Physiol. 1997;273:L1029–L1035. doi: 10.1152/ajplung.1997.273.5.L1029. [DOI] [PubMed] [Google Scholar]
- Kean J. M., Rao S., Wang M., Garcea R. L. Seroepidemiology of human polyomaviruses. 2009. PLoS Pathol. doi: 10.1371/journal.ppat.1000363. [DOI] [PMC free article] [PubMed]
- Kishomoto T., Ono T., Okada K., Ito H. Relationship between number of asbestos bodies in autopsy lung and pleural plaques on chest X-ray film. Chest. 1989;95:549–552. doi: 10.1378/chest.95.3.549. [DOI] [PubMed] [Google Scholar]
- Kratzke R. A., Gazdar A. F. Oncogenes and tumur suppressor genes in malignant mesothelioma. In: Pass H. I., Vogelzang N. J., Carbone M., editors. Advances in pathogenesis, diagnosis, and translational therapies. Vol. 8. New York: Springer Science & Business Media; 2005. pp. 124–140. [Google Scholar]
- Kroczynska B., Cutrone R., Bocchetta M., Yang H., Elmishad A. G., Vacek P., Ramos-Nino M., Mossman B. T., Pass H. I., Carbone M. Crocidolite asbestos and SV40 are cocarcinogens in human mesothelial cells and in causing mesothelioma in hamsters. Proc. Natl. Acad. Sci. USA. 2006;103:14128–4133. doi: 10.1073/pnas.0604544103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. M., Green F. H., Demetrick D. J., Jiang X. X., Schürch S. A study of surface property changes in rat mesothelial cells induced by asbestos using aqueous two-phase polymer solutions. Biochim. Biophys. Acta. 1993;1181:223–232. doi: 10.1016/0925-4439(93)90025-v. [DOI] [PubMed] [Google Scholar]
- Lehnert B. E., Valdez Y. E., Bomalaski S. H. Lung and pleural free-cell responses to the intrapulmonary deposition of particles in the rat. J. Toxicol. Environ. Health. 1985;16:823–839. doi: 10.1080/15287398509530791. [DOI] [PubMed] [Google Scholar]
- Light R. W., Broaddus V. C. Pleural Effusion and Pleural Tumors. In: Mason J. R., Broaddus V. C., Martin T. R., King T. E. Jr., Schraufnagel D. E., Murray J. F., Nadel J. A., editors. Murray and Nadel's Textbook of Respiratory Medicine. 5th ed. Philadelphia: Elsevier Saunders; 2010. pp. 1719–1763.pp. 1792–1813. 2. [Google Scholar]
- Lillis R., Lerman Y., Selikoff I. J. Symptomatic benign pleural effusions among asbestos insulation workers: Residual radiographic abnormalities. Br. J. Ind. Med. 1988;45:443–449. doi: 10.1136/oem.45.7.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippmann M., Schlesinger R. B. Interspecies comparisons of particle deposition and mucociliary clearance in tracheobronchial airways. J. Toxicol. Environ. Health. 1984;13:441–469. doi: 10.1080/15287398409530509. [DOI] [PubMed] [Google Scholar]
- Lippmann M., Yeates D. B., Albert R. E. Deposition, retention, and clearance of inhaled particles. Br. J. Ind. Med. 1980;37:337–362. doi: 10.1136/oem.37.4.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Ernest J. D., Broaddus V. C. Phagocytosis of crocidolite asbestos induces oxidative stress, DNA damage and apoptosis in mesothelial cells. Am. J. Respir. Cell Mol. Biol. 2000;23:371–378. doi: 10.1165/ajrcmb.23.3.4094. [DOI] [PubMed] [Google Scholar]
- Lockey J. E., Brooks S. M., Jarabek A. M., Khoury P. R., McKay R. T., Carson A., Morrison J. A., Wiot J. F., Spitz H. B. Pulmonary changes after exposure to vermiculite contaminated with fibrous tremolite. Am. Rev. Respir. Dis. 1984;129:952–958. doi: 10.1164/arrd.1984.129.6.952. [DOI] [PubMed] [Google Scholar]
- Manning C. B., Vallyathan V., Mossman B. T. Diseases caused by asbestos: mechanisms of injury and disease development. Int. Immunopharmacol. 2002;2:191–200. doi: 10.1016/s1567-5769(01)00172-2. [DOI] [PubMed] [Google Scholar]
- Marchi E., Liu W., Broaddus V. C. Mesothelial cell apoptosis is confirmed in vivo by morphological change in cytokeratin distribution. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000;278:L528–L535. doi: 10.1152/ajplung.2000.278.3.L528. [DOI] [PubMed] [Google Scholar]
- Mast R. W., Hesterberg T. W., Glass L. R., McConnell E. E., Anderson R., Bernstein D. M. Chronic inhalation and biopersistence of refractory ceramic fiber in rats and hamsters. Environ. Health Perspect. 1994;102:207–209. doi: 10.1289/ehp.94102s5207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxim L. D., McConnell E. E. Interspecies comparisons of the toxicity of asbestos and synthetic vitreous fibers: A weight-of-the-evidence approach. Regul. Toxicol. Pharmacol. 2001;33:319–342. doi: 10.1006/rtph.2001.1467. [DOI] [PubMed] [Google Scholar]
- Meeker G., Bern A. M., Brownfield I. K., Lowers H. A., Sutley S. J., Hoefen T. M., Vance J. S. The composition and morphology of amphiboles from the Rainy Creek complex, near Libby, Montana. Am. Mineral. 2003;88:1955–1969. [Google Scholar]
- McConnell E. E. Synthetic vitreous fibers—Inhalation studies. Regul. Toxicol. Pharmacol. 1994;20:S22–S34. [PubMed] [Google Scholar]
- McConnell E. E., Axten C., Hesterberg T. W., Chevalier J., Miller W. C., Everitt J., Oberdörster G., Chase G. R., Thevenaz P., Kotin P. Studies on the inhalation toxicology of two fiberglasses and amosite asbestos in the Syrian golden hamster. Part II. Results of chronic exposure. Inhal. Toxicol. 1999;11:785–835. doi: 10.1080/089583799196754. [DOI] [PubMed] [Google Scholar]
- McDonald J. C., Armstrong B. G., Edwards C. W., Gibbs A. R., Lloyd H. M., Pooley F. D., Ross D. J., Rudd R. M. Case-referent survey of young adults with mesothelioma: I. Lung fibre analyses. Ann. Occup. Hyg. 2001;45:513–518. [PubMed] [Google Scholar]
- Miserocchi G., Sancini G., Mantegazza F., Chiappino G. Translocation pathways for inhaled asbestos fibers. Environ. Health. 2008;7:4. doi: 10.1186/1476-069X-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchev K., Dumortier P., De Vuyst P. Black spots and hyaline pleural plaques on the parietal pleura of 150 urban necropsy cases. Am. J. Surg. Pathol. 2002;26:1198–1206. doi: 10.1097/00000478-200209000-00010. [DOI] [PubMed] [Google Scholar]
- Mossman B. T., Lippman M., Hesterberg T. W., Kelsey K. T., Barchowsky A., Bonner J. C. Pulmonary endpoints (lung carcinomas and asbestosis) following inhalation exposure to asbestos. J. Toxicol. Environ. Health B, 2011;14:76–121. doi: 10.1080/10937404.2011.556047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy S. S., Testa J. R. Asbestos, chromosomal deletions, and tumor suppressor gene alterations in human malignant mesothelioma. J. Cell Physiol. 1999;180:150–157. doi: 10.1002/(SICI)1097-4652(199908)180:2<150::AID-JCP2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Mutsaers S. E., Kalomenidis I., Wilson N. A., Lee Y. C. Growth factors in pleural fibrosis. Curr. Opin. Pulm. Med. 2006;12:251–258. doi: 10.1097/01.mcp.0000230627.88386.b9. [DOI] [PubMed] [Google Scholar]
- Mutsaers S. E., Prele C. M., Brody A. R., Idell S. Pathogenesis of pleural fibrosis. Respirology. 2004;9:428–440. doi: 10.1111/j.1440-1843.2004.00633.x. [DOI] [PubMed] [Google Scholar]
- Nishimura S. L., Broaddus V. C. Asbestos-induced pleural disease. Clin. Chest Med. 1998;19:311–329. doi: 10.1016/s0272-5231(05)70079-4. [DOI] [PubMed] [Google Scholar]
- Oberdörster G., Ferin J., Marcello N. L., Meinhold S. H. Effects of intrabronchially instilled amosite on lavagable lung and pleural cells. Environ Health Perspect. 1983;51:41–48. doi: 10.1289/ehp.835141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdörster G. Determinants of the pathogenicity of man-made vitreous fibers (MMVF) Int. Arch. Occup. Environ. Health. 2000;73:S60–S68. doi: 10.1007/pl00014628. [DOI] [PubMed] [Google Scholar]
- Oberdörster G. Toxicokinetics and effects of fibrous and nonfibrous particles. Inhal. Toxicol. 2002;14:29–56. doi: 10.1080/089583701753338622. [DOI] [PubMed] [Google Scholar]
- Owens M. W., Milligan S. A. Pleuritis abd pleural effusions. Curr. Opin. Pulm. Med. 1995;1:318–323. [PubMed] [Google Scholar]
- Pache J. C., Janssen Y. M., Walsh E. S., Quinlan T. R., Zanella C. L., Low R. B., Taatjes D. J., Mossman B. T. Increased epidermal growth factor-receptor protein in a human mesothelial cell line in response to long asbestos fibers. Am. J. Pathol. 1998;152:333–340. [PMC free article] [PubMed] [Google Scholar]
- Peipins L. A., Lewin M., Campolucci S., Lybarger J. A., Miller A., Middleton D., Weis C., Spence M., Black B., Kapil V. Radiographic abnormalities and exposure to asbestos-contaminated vermiculite in the community of Libby, Montana, USA. Environ. Health Perspect. 2003;111:1753–1759. doi: 10.1289/ehp.6346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poland C. A., Duffin R., Kinloch I., Maynard A., Wallace W. A., Seaton A., Stone V., Brown S., Macnee W., Donaldson K. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat. Nanotechnol. 2008;3:423–428. doi: 10.1038/nnano.2008.111. [DOI] [PubMed] [Google Scholar]
- Pott F. Animal experiments on biological effects of mineral fibres. IARC Sci. Publ. 1980;30:261–272. [PubMed] [Google Scholar]
- Pott F., Huth F., Friedrichs K. H. Tumorigenic effect of fibrous dusts in experimental animals. Environ. Health Perspect. 1974;9:313–315. doi: 10.1289/ehp.749313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robledo R., Mossman B. Cellular and molecular mechanisms of asbestosinduced fibrosis. J. Cell Physiol. 1999;180:158–166. doi: 10.1002/(SICI)1097-4652(199908)180:2<158::AID-JCP3>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Roggli V. L., Sharma A., Butnor K. J., Sporn T., Vollmer R. T. Malignant mesothelioma and occupational exposure to asbestos: A clinicopathological correlation of 1445 cases. Ultrastruct. Pathol. 2002;26:55–65. doi: 10.1080/01913120252959227. [DOI] [PubMed] [Google Scholar]
- Roggli V. L. Human disease consequences of fiber exposures: A review of human pathology and fiber burden data. Environ. Health Perspect. 1990;88:295–303. doi: 10.1289/ehp.9088295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roggli V. L. Quantitative and analytical studies in the diagnosis of mesotheliomas. Sem. Diag. Pathol. 1992;9:162–168. [PubMed] [Google Scholar]
- Roggli V. L., Sanders L. L. Asbestos content of lung tissue and carcinoma of the lung: A clinicopathologic correlation and mineral fiber analysis of 234 cases. Ann. Occup. Hyg. 2000;44:109–117. [PubMed] [Google Scholar]
- Roggli V. L., Sharma A. In: Analysis of tissue mineral fiber content In Pathology of asbestos-associated disease. 2nd ed. Roggl V. L., Oury T. D., Spron T. A., editors. New York: Springer-Verlag; 2004. pp. 309–354. [Google Scholar]
- Rohs A. M., Lockey J. E., Dunning K. K., Shukla R., Fan H., Hilbert T., Borton E., Wiot J., Meyer C., Shipley R. T., Lemasters G. K., Kapil V. Low-level fiber-induced radiographic changes caused by Libby vermiculite: A 25-year followup study. Am. J. Respir. Crit. Care Med. 2008;177:630–637. doi: 10.1164/rccm.200706-841OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryman-Rasmussen J. P., Cesta M. F., Brody A. R., Shipley-Phillips J. K., Everitt J. I., Tewksbury E. W., Moss O. R., Wong B. A., Dodd D. E., Andersen M. E., Bonner J. C. Inhaled carbon nanotubes reach the subpleural tissue in mice. Nat. Nanotechnol. 2009;4:747–751. doi: 10.1038/nnano.2009.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahn S. A., Antony V. B. Pathogenesis of pleural plaques. Relationship of early cellular response and pathology. Am. Rev. Respir. Dis. 1984;130:884–887. doi: 10.1164/arrd.1984.130.5.884. [DOI] [PubMed] [Google Scholar]
- Sanchez V. C., Pietruska J. R., Miselis N. R., Hurt R. H., Kane A. B. Biopersistence and potential adverse health impacts of fibrous nanomaterials: What have we learned from asbestos? Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2009;1:511–529. doi: 10.1002/wnan.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastien P., Janson X., Gaudichet A., Hirsch A., Bignon J. Asbestos retention in human respiratory tissues: Comparative measurements in lung parenchyma and in partial pleura. IARC Sci. Publ. 1980;30:237–246. [PubMed] [Google Scholar]
- Shukla A., Gulumian M., Hei T. K., Kamp D., Rahman Q., Mossman B. T. Multiple roles of oxidants in the pathogenesis of asbestos-induced diseases. Free Radical Biol. Med. 2003;34:1117–1129. doi: 10.1016/s0891-5849(03)00060-1. [DOI] [PubMed] [Google Scholar]
- Shukla A., Jung M., Stern M., Fukagawa N. K., Taatjes D. J., Sawyer D., Van Houten B., Mossman B. T. Asbestos induces mitochondrial DNA damage and dysfunction linked to the development of apoptosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003;285:L1018–L1025. doi: 10.1152/ajplung.00038.2003. [DOI] [PubMed] [Google Scholar]
- Stanton M. F., Blackwell R., Miller E. Experimental pulmonary carcinogenesis with asbestos. Am. Ind. Hyg. Assoc. J. 1969;30:236–244. doi: 10.1080/00028896909343117. [DOI] [PubMed] [Google Scholar]
- Stanton M. F., Layard M., Tegeris A., Miller E., May M., Morgan E., Smith A. Relation of particle dimension to carcinogenicity in amphibole asbestoses and other fibrous minerals. JNCI. 1981;67:965–975. [PubMed] [Google Scholar]
- Stanton M. F., Laynard M., Tegeris A., Miller E., May M., Kent E. Carcinogenicity of fibrous glass: Pleural response in the rat in relation to fiber dimension. J. Natl. Cancer Inst. 1977;58:587–603. doi: 10.1093/jnci/58.3.587. [DOI] [PubMed] [Google Scholar]
- Stephens M., Gibbs A. R., Pooley F. D., Wagner J. C. Asbestos induced diffuse pleural fibrosis: pathology and mineralogy. Thorax. 1987;42:583–588. doi: 10.1136/thx.42.8.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan P. A. Vermiculite, respiratory disease, and asbestos exposure in Libby, Montana: update of a cohort mortality study. Environ Health Perspect. 2007;115:579–585. doi: 10.1289/ehp.9481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y., Kohyama N. Translocation of inhaled asbestos fibers from the lung to other tissues. Am. J. Ind. Med. 1991;19:701–704. doi: 10.1002/ajim.4700190603. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Yuen S. R., Ashley R. Short, thin asbestos fibers contribute to the development of human malignant mesothelioma: Pathological evidence. Int. J. Hyg. Environ. Health. 2005;208:201–210. doi: 10.1016/j.ijheh.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Yuen S. R. Asbestos tissue burden study on human malignant mesothelioma. Ind. Health. 2001;39:150–160. doi: 10.2486/indhealth.39.150. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Yuen S. R., Ashley R. Short, thin asbestos fibers contribute to the development of human malignant mesothelioma: Pathological evidence. Int. J. Hyg. Environ. Health. 2005;208:201–210. doi: 10.1016/j.ijheh.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Tanaka S., Choe N., Iwagaki A., Hemenway D. R., Kagan E. Asbestos exposure induces MCP-1 secretion by pleural mesothelial cells. Exp. Lung Res. 2000;26:241–255. doi: 10.1080/019021400404528. [DOI] [PubMed] [Google Scholar]
- Tyler W. S. Comparative subgross anatomy of lungs. Pleurae, interlobular septa, and distal airways. Am. Rev. Respir. Dis. 1983;128:S32–S36. doi: 10.1164/arrd.1983.128.2P2.S32. [DOI] [PubMed] [Google Scholar]
- Upadhyay D., Kamp D. W. Asbestos-induced pulmonary toxicity: role of DNA damage and apoptosis. Exp. Biol. Med. 2003;228:650–659. doi: 10.1177/153537020322800602. [DOI] [PubMed] [Google Scholar]
- Viallat J. R., Raybuad F., Passarel M., Boutin C. Pleural migration of chrysotile fibers after intratracheal injection in rats. Arch. Environ. Health. 1986;41:282–286. doi: 10.1080/00039896.1986.9936697. [DOI] [PubMed] [Google Scholar]
- Wang N. S. The preformed stomas connecting the pleural cavity and the lymphatics in the parietal pleura. Am. Rev. Respir. 1975;110:623–633. doi: 10.1164/arrd.1975.111.1.12. [DOI] [PubMed] [Google Scholar]
- Weiner S. J., Neragi-Miandoab S. Pathogenesis of malignant pleural mesothelioma and the role of environmental and genetic factors. J. Cancer Res. Clin. Oncol. 2009;135:15–27. doi: 10.1007/s00432-008-0444-9. [DOI] [PubMed] [Google Scholar]
- Whitehouse A. C. Asbestos-related pleural disease due to tremolite associated with progressive loss of lung function: Serial observations in 123 miners, family members, and residents of Libby, Montana. Am. J. Ind. Med. 2004;46:219–225. doi: 10.1002/ajim.20053. [DOI] [PubMed] [Google Scholar]
- Yang H., Bocchetta M., Kroczynska B., Elmishad A. G., Chen Y., Liu Z., Bubici C., Mossman B. T., Pass H. I., Testa J. R., Franzoso G., Carbone M. TNFalpha inhibits asbestos-induced cytotoxicity via a NF-kappaB-dependent pathway, a possible mechanism for asbestos-induced oncogenesis. Proc. Natl. Acad. Sci. USA. 2006;103:10397–10402. doi: 10.1073/pnas.0604008103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Rivera Z., Jube S., Nasu M., Bertino P., Goparaju C., Franzoso G., Lotze M. T., Krausz T., Pass H. I., Bianchi M. E., Carbone M. Programmed necrosis induced by asbestos in human mesothelial cells causes high-mobility group box 1 protein release and resultant inflammation. Proc. Natl. Acad. Sci. USA. 2010;107:12611–12616. doi: 10.1073/pnas.1006542107. [DOI] [PMC free article] [PubMed] [Google Scholar]


