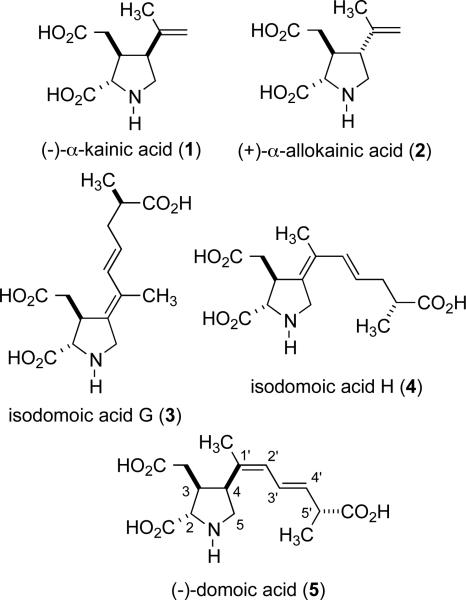
The kainoid family of marine natural products has attracted considerable interest due to the potent pharmacological properties of compounds in this class.[1] By serving as conformationally restricted analogues of glutamate, this group of natural products displays potent neuroexcitatory activity, and the more complex members of the domoic acid type have been responsible for outbreaks of poisioning from mussel toxins.[2] The kainic acid and domoic acid families of natural products include a diverse range of structures that possess highly functionalized pyrrolidine di- or triacid motifs (Scheme 1).[3] Kainic and allokainic acids possess a simple C4 propenyl substituent, whereas domoic acid and its isomers possess more complex C4 functionality. Our interest in the synthesis of members of this class of compounds has led to the development of a number of nickel-catalyzed processes, including the alkylative cyclization of enoate-alkyne and enoate-allene substrates. These processes have served as key steps in the total syntheses of kainic acid (1), allokainic acid (2), and isodomoic acids G (3) and H (4).[4-6] We envisioned that the development of a related cyclization method involving cyclization of an enoate tethered with a chiral allene would allow synthesis of the remaining members of the domoic acid class, including domoic acid (5), that do not possess the C4 exocyclic functionality seen in isodomoic acids G and H. Herein, we describe an initial investigation of this strategy, and the synthesis of a model substructure of domoic acid.
Scheme 1.
Representative kainoid amino acids.
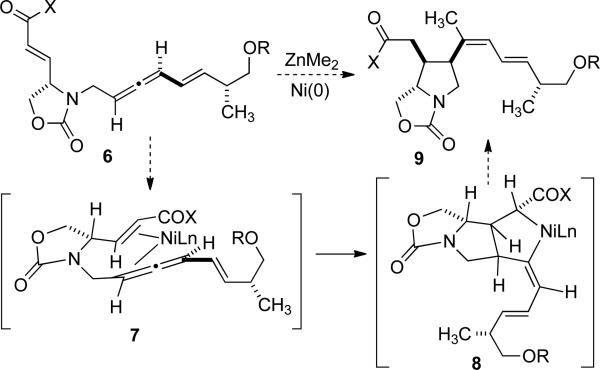
Our first-generation strategy for the synthesis of domoic acid (5) features an ambitious dimethyl zinc-mediated cyclization of enoate-allene 6.[7] As depicted (Scheme 2), we anticipated that the allene chirality of 6 would ultimately establish stereochemistry in the generation of the C3 and C4 stereocenters and the C1' trisubstituted alkene of 9 during the cyclization.[8] Our mechanistic hypothesis involves formation of a Ni(0) π-complex 7 with an eclipsed orientation of the reactive π-systems, followed by the formation of metallacycle 8, where all of the key stereochemical features are established.[9] The prediction that the proximal π-system of the allene will undergo coupling draws from our earlier observations made in the synthesis of kainic acid,[4b] as well as related five-membered aldehyde-allene alkylative cyclization processes.[7d]
Scheme 2.
First-generation strategy.
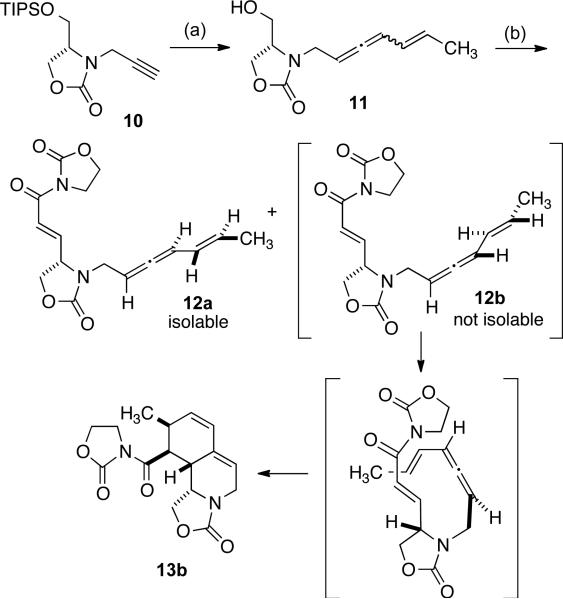
For our initial examination of this approach, the requisite substrate 12 was first prepared (for ease of synthesis) as a mixture of allene epimers beginning from alkyne 10 (Scheme 3). Our earlier studies had illustrated that unsaturated acyl oxazolidinones were the most effective enoate derivatives in nickel cyclization processes.[4] Upon conversion of alcohol 11 to enoate 12, one of the diastereomers of 12 was found to be unstable, based on facility of an undesired Diels Alder cycloaddition to afford product 13b along with a single diastereomer of the desired enoate 12a. The facility of this cycloaddition is notable given the comparatively harsh conditions required for other types of vinyl allene Diels Alder reactions.[10] Based on the stereochemical outcome of the generation of product 13b, allene 12b can be assumed to be the unstable allene diastereomer that undergoes rapid cycloaddition to 13b, and allene 12a is therefore the diastereomer that is isolable.
Scheme 3.
Examination of vinyl allene cyclizations. (a) i. 10, nBuLi, then crotonadehyde, THF, -50 °C, 65 %, ii. Bu3SnH, AIBN, 90 °C, then MsCl, Et3N, CH2Cl2, then (nBu)4NF, THF, 45 %; (b) oxalyl chloride, DMSO, Et3N, CH2Cl2, -78 to -20 °C, then ((2-oxooxazolidin-3-yl)methyl)triphenylphosphonium bromide, DMAP, -20 °C to rt, 14 % of 12a, 31 % of 13b.
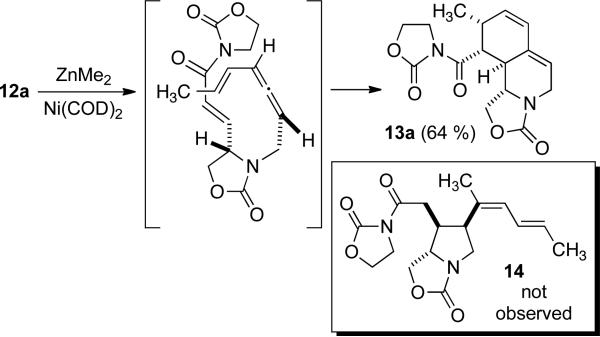
As structure 12a is the diastereomer anticipated to be required for the nickel-catalyzed cyclization, its participation in an alkylative cyclization reaction was examined (Scheme 4). However, we were unable to access the desired product 14. Instead, Diels Alder cycloaddition predominated to afford structure 13a, providing further proof of the structure of diastereomer 12a. It is unclear if the cycloaddition is catalyzed by Ni(COD)2 or ZnMe2, and due to the limited material available, this question was not explored.[11,12] Instead, we opted to redesign the strategy by removing the C3'-C4' unsaturation, thus removing the complexity of the competing Diels Alder cycloaddition.
Scheme 4.
Attempted vinyl allene alkylative cyclization.
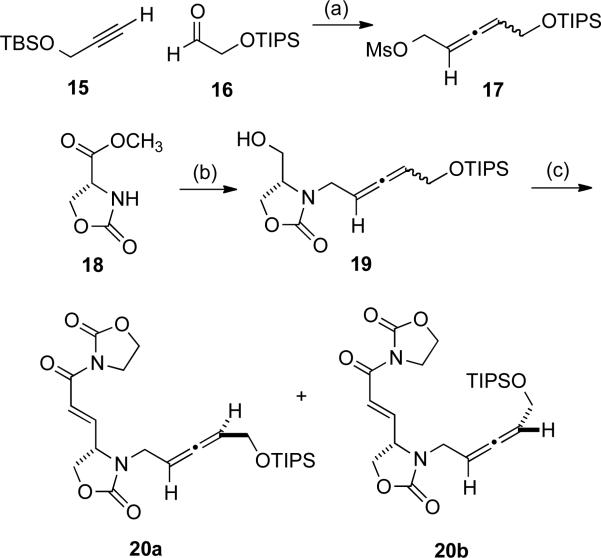
In order to pursue this second-generation strategy, racemic allenyl mesylate 17 was prepared by a straightforward route from alkyne 15 and aldehyde 16 (Scheme 5). N-Alkylation of 18 with mesylate 17 followed by ester reduction afforded 19 as a mixture of allene epimers. Oxidation state adjustment and olefination of 19 provided diastereomers 20a and 20b, which were separated by preparative HPLC. This route was attractive in that both diastereomers of 20 were made available for investigation, and the stereochemistry of 20a was later confirmed by independent asymmetric synthesis (vide infra).
Scheme 5.
Preparation of cyclization substrates. (a) i. 15, nBuLi, THF, -78 °C, then 16, 65 %, ii. PPh3, DEAD, o-nitrobenzene sulfonyl hydrazine, THF, 15 °C to rt, 61 %, iii. PPTS, EtOH, 55 °C, 85 %, iv. MsCl, Et3N, CH2Cl2, 0 °C, 98 %; (b) i. KHMDS, 17, THF, 0 °C, 41 %, ii. NaBH4, EtOH, 0 °C, 80 %; (c) oxalyl chloride, DMSO, Et3N, CH2Cl2, -78 to -20 °C, then ((2-oxooxazolidin-3-yl)methyl)triphenylphosphonium bromide, DMAP, -20 °C to rt, 69 % combined of 20a and 20b (1:1, separated by HPLC).
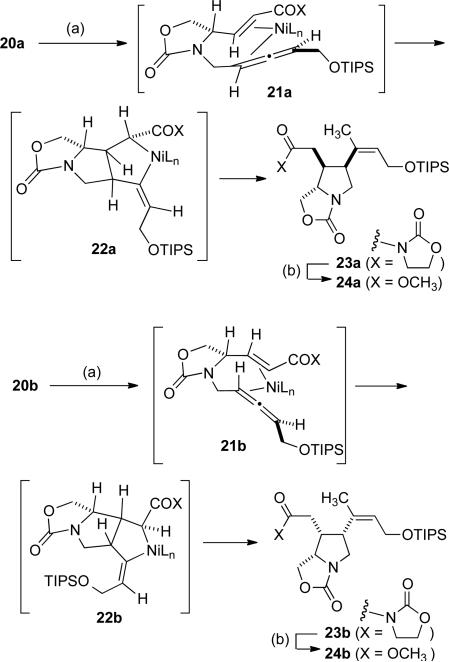
With allene epimers 20a and 20b both in hand, nickel-catalyzed dimethyl zinc-promoted cyclization was investigated (Scheme 6). Gratifyingly, as anticipated from our strategic plan, cyclization of 20a cleanly afforded desired compound 23a, whereas cyclization of 20b under identical conditions afforded diastereomer 23b in a less-efficient and lower-yielding cyclization. The π-complex 21a, followed by cyclization to metallacycle 22a, likely explains the stereochemical outcome of this key cyclization process in the formation of 23a.[9] Alternatively, by utilizing the opposite allene chirality, modified orientation of substrate 20b in π-complex 21b, followed by cyclization to metallacycle 22b, provides diasteromer 23b. This outcome illustrates that the allene chirality is essential in establishing the desired C3 and C4 stereochemical arrangement. Oxazolidinones 23a and 23b were cleanly converted to methyl esters 24a and 24b in straightforward fashion.
Scheme 6.
Alkylative cyclization of an enoate / chiral allene. (a) ZnCl2, MeLi, Ti(O-iPr)4, Ni(COD)2 (20 mol %), -20 °C to rt, 77 % (in the case of 23a), 24 % (in the case of 23b); (b) MeOMgBr, toluene/THF, 0 °C to rt, 75 % (in the case of 24a), 79 % (in the case of 24b).
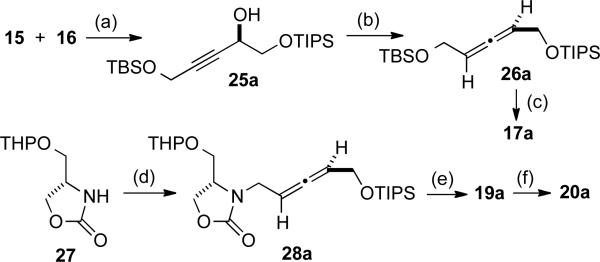
Lastly, an improved route to compound 24a involving asymmetric synthesis of the requisite allene fragment and an improved entry to a pyrrolidine precursor was developed (Scheme 7). Enantioselective addition of alkyne 15 to aldehyde 16 following the method of Carreira provided alcohol 25a in 94% ee,[13 and subjecting this structure to the allylic diazene method of Myers provided allene 26a in 92% ee.[14] Conversion to mesylate 17a and N-alkylation of 27 provided access to diastereomerically pure 28a. Conversion of 28a to 19a and then 20a completed the stereoselective approach to 24a and confirmed the structures of diastereomers 20a and 20b. In this route, precursor 27 was utilized rather than N-alkylation of 18 in order to avoid a problematic epimerization that had been observed in our parallel efforts to synthesize isodomoic acids G and H. This improved entry to compound 24a avoids the tedious separation of 20a and 20b and provides a selective entry to the substructure that directly corresponds to that seen in domoic acid and a range of naturally occurring isodomic acid structures.
Scheme 7.
Enantioselective preparation of a cyclization precursor. (a) zinc trifluoromethane sulfonate, . (1R, 2S)-(-)-N-methylephedrine, 15, then 16, toluene, rt, 58 % (94 % ee); (b) PPh3, DEAD, o-nitrobenzene sulfonyl hydrazine, THF, -15 °C to rt, 61 % (92 % ee); (c) i. PPTS, EtOH, 55 °C, 85 %, ii. MsCl, Et3N, CH2Cl2, 0 °C, 98 %; (d) 17a, 27, benzyltriethylammonium bromide, 50 % NaOH, CH2Cl2, 50 %, (e) PPTS, EtOH, 55 °C, 76 %. (f) oxalyl chloride, DMSO, Et3N, CH2Cl2, -78 to -20 °C, then ((2-oxooxazolidin-3-yl)methyl)triphenyl-phosphonium bromide, DMAP, -20 °C to rt, 74 %.
In summary, the first examples of enoate-chiral allene alkylative cyclizations have been demonstrated. The process is effective in the synthesis of functionalized pyrrolidine derivatives that possess stereodefined alkenyl substituents. An advanced intermediate towards the synthesis of domoic acid was prepared using this method. The efficient synthesis of structure 24a may be useful in future synthetic approaches to domoic acid and several naturally occurring isodomoic acids. A notable feature of this approach is that the C3-C4 bond, the C3 and C4 stereogenic centers, and the C1'-C2' trisubstututed alkene are introduced efficiently and with high selectivity in this new catalytic process.
Experimental Section
Preparation of compound 23a
To a 0.5 mL 0 °C THF solution of anhydrous ZnCl2 (68 mg, 0.5 mmol), 0.67 mL of MeLi (0.66 mL, 1.0 mmol, 1.5 M in diethyl ether) was added dropwise. After stirring for 10 min at 0 °C, the mixture was transferred by cannula to a 5 mL 0 °C THF solution of Ni(COD)2 (12 mg, 0.04 mmol). The mixture was cooled to -20 °C, then an 8 mL THF solution of 20a (100 mg, 0.2 mmol) and titanium isopropoxide (60 mg, 60 μL, 0.2 mmol) was added dropwise. The mixture was slowly warmed to rt, and stirred for 2 h, quenched with sat. NH4Cl, extracted with ethyl acetate, and the combined extracts were washed with brine and dried over MgSO4. After filtration, the solvent was removed under vacuum, and the crude product was purified by flash column chromatography (hexane/ethyl acetate, 2:1 to 1:2) to yield 78 mg (77%, 94:6 dr) of 23a as a thick oil.
Supplementary Material
Acknowledgements
The authors wish to acknowledge receipt of NIH grant GM57014 in support of this work The authors thank colleagues at Wayne State University, where early portions of this work were conducted. JM thanks the faculty and students of the Institute of Chemical Research of Catalonia (ICIQ) for hospitality during a sabbaticalstay.
Footnotes
Supporting information for this article is available on the WWW under http://www.chemeurj.org/ or from the author.
References
- 1.a Monaghan DT, Bridges RJ, Cotman CW. Annu. Rev. Pharmacol. Toxicol. 1989;29:365–402. doi: 10.1146/annurev.pa.29.040189.002053. [DOI] [PubMed] [Google Scholar]; b Moloney MG. Nat. Prod. Rep. 1998;15:205–219. doi: 10.1039/a815205y. [DOI] [PubMed] [Google Scholar]; c Chamberlin R, Bridges R. In: Drug Design For Neuroscience. Kozikowski AP, editor. Raven Press, Ltd.; New York: 1993. pp. 231–259. (c) [Google Scholar]; d McGeer EG, Olney JW, McGeer PL. Kainic Acid as a Tool in Neurobiology. Raven Press; New York: 1978. [Google Scholar]
- 2.a Lefebvre KA, Robertson A. Toxicon. 2010;56:218–230. doi: 10.1016/j.toxicon.2009.05.034. [DOI] [PubMed] [Google Scholar]; b Wright JLC, et al. Can. J. Chem. Rev. Can. Chim. 1989;67:481–490. [Google Scholar]
- 3.a Parsons AF. Tetrahedron. 1996;52:4149–4174. [Google Scholar]; b Moloney MG. Nat. Prod. Rep. 1999;16:485–498. doi: 10.1039/a800247i. [DOI] [PubMed] [Google Scholar]; c Moloney MG. Nat. Prod. Rep. 2002;19:597–616. doi: 10.1039/b103777n. [DOI] [PubMed] [Google Scholar]; d Takemoto T, Daigo K. Chem. Pharm. Bull. 1958;6:578. [Google Scholar]
- 4.a Chevliakov MV, Montgomery J. Angew. Chem. Int. Ed. 1998;37:3144–3146. doi: 10.1002/(SICI)1521-3773(19981204)37:22<3144::AID-ANIE3144>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]; b Chevliakov MV, Montgomery J. J. Am. Chem. Soc. 1999;121:11139–11143. [Google Scholar]; c Ni Y, Amarasinghe KKD, Ksebati B, Montgomery J. Org. Lett. 2003;5:3771–3773. doi: 10.1021/ol0355783. [DOI] [PubMed] [Google Scholar]; d Ni Y, Kassab RM, Chevliakov MV, Montgomery J. J. Am. Chem. Soc. 2009;131:17714–17718. doi: 10.1021/ja907931u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.For other approaches to kainic and allokainic acid, see reference 3 and the following representative examples: Oppolzer W, Thirring K. J. Am. Chem. Soc. 1982;104:4978–4979.Sakaguchi H, Tokuyama H, Fukuyama T. Org. Lett. 2008;10:1711–1714. doi: 10.1021/ol800328q.Xia Q, Ganem B. Org. Lett. 2001;3:485–487. doi: 10.1021/ol007009q.Martinez MM, Hoppe D. Org. Lett. 2004;6:3743–3746. doi: 10.1021/ol0485666.Yoo SE, Lee SH. J. Org. Chem. 1994;59:6968–6972.Farwick A, Helmchen G. Org. Lett. 2010;12:1108–1111. doi: 10.1021/ol1001076.
- 6.For other approaches to domoic acid and naturally occurring isodomoic acids: Ohfune Y, Tomita M. J. Am. Chem. Soc. 1982;104:3511–3513.Clayden J, Knowles FE, Baldwin IR. J. Am. Chem. Soc. 2005;127:2412–2413. doi: 10.1021/ja042415g.Denmark SE, Liu JHC, Muhuhi JM. J. Am. Chem. Soc. 2009;131:14188–14189. doi: 10.1021/ja9063475.
- 7.For development of related cyclization methods: Montgomery J, Savchenko AV. J. Am. Chem. Soc. 1996;118:2099–2100.Montgomery J, Chevliakov MV, Brielmann HL. Tetrahedron. 1997;53:16449–16462.Montgomery J, Oblinger E, Savchenko AV. J. Am. Chem. Soc. 1997;119:4911–4920.Song M, Montgomery J. Tetrahedron. 2005;61:11440–11448.Ikeda S, Sato Y. J. Am. Chem. Soc. 1994;116:5975–5976.Ikeda S, Yamamoto H, Kondo K, Sato Y. Organometallics. 1995;14:5015–5016.Kang SK, Yoon SK. Chem. Commun. 2002:2634–2635. doi: 10.1039/b207620a.
- 8.For representative transition metal-catalyzed couplings involving chiral allenes: Brummond KM, Kerekes AD, Wan HH. J. Org. Chem. 2002;67:5156–5163. doi: 10.1021/jo016305t.Ng SS, Jamison TF. J. Am. Chem. Soc. 2005;127:7320–7321. doi: 10.1021/ja0521831.Urabe H, Takeda T, Hideura D, Sato F. J. Am. Chem. Soc. 1997;119:11295–11305.Wender PA, Glorius F, Husfeld CO, Langkopf E, Love JA. J. Am. Chem. Soc. 1999;121:5348–5349.
- 9.While this simplified model serves as a useful stereochemical predictor, computational studies suggested a more complex role of the organozinc in promoting the cyclization. See: Hratchian HP, Chowdhury SK, Gutierrez-Garcia VM, Amarasinghe KKD, Heeg MJ, Schlegel HB, Montgomery J. Organometallics. 2004;23:4636–4646.
- 10.Deutsch EA, Snider BB. J. Org. Chem. 1982;47:2682–2684.Keck GE, Kachensky DF. J. Org. Chem. 1986;51:2487–2493. For facile ene reaction – Diels Alder cascades involving vinyl allenes: Robinson JM, Sakai T, Okano K, Kitawaki T, Danheiser RL. J. Am. Chem. Soc. 2010;132:11039–11041. doi: 10.1021/ja1053829.Sakai T, Danheiser RL. J. Am. Chem. Soc. 2010;132:13203–13205. doi: 10.1021/ja106901u. For a review on vinyl allene cycloadditions: Murakami M, Matsuda T. Cycloadditions of Allenes. In: Krause N, Hashmi ASK, editors. Modern Allene Chemistry. Vol. 2. Wiley-VCH; Weinheim: 2004. pp. 727–815. For an overview of cyclizations and cycloadditions of allenes: Wei LL, Xiong H, Hsung RP. Acc. Chem. Res. 2003;36:773–782. doi: 10.1021/ar030029i.
- 11.For nickel-catalyzed [4+2] reactions: Wender PA, Smith TE. J. Org. Chem. 1995;60:2962–2963.Wender PA, Smith TE. J. Org. Chem. 1996;61:824–825.
- 12.For other metal-catalyzed processes of vinyl allenes: Murakami M, Itami K, Ito Y. J. Am. Chem. Soc. 1999;121:4130–4135.Ma SM. Chem. Rev. 2005;105:2829–2871. doi: 10.1021/cr020024j.Hashmi ASK. Angew. Chem. Int. Ed. 2000;39:3590–3593.
- 13.El-Sayed E, Anand NK, Carreira EM. Org. Lett. 2001;3:3017–3020. doi: 10.1021/ol016431j. [DOI] [PubMed] [Google Scholar]
- 14.Myers AG, Zheng B. J. Am. Chem. Soc. 1996;118:4492–4493. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.