Abstract
Activation of the stress protein response (SPR) inhibits iNOS-dependent release of NO from alveolar macrophages by blocking the activation of the STAT1 pathway in response to IFN-γ, a major cytokine present in the airspace of patients with acute lung injury. Inhibition of STAT1 phosphorylation after heat stress was associated with detergent insolubilization of the STAT1 protein and its proteasomal degradation which was reversed with the pretreatment of cells with glycerol, a chemical chaperone that reduces the extent of heat-induced protein denaturation. This early effect of SPR is the result of the disruption of the Hsp90 binding to the STAT1 protein. Our results also demonstrated that a late effect of SPR activation involves the regulation of iNOS function by inducible Hsp70 (Hsp70i). The STAT1 signaling pathway recovered function within 12 hours post-SPR activation and synthesis of the iNOS protein, however, NO production did not occur until 72 hours later. Inhibiting Hsp70i expression after heat stress recovered iNOS function whereas overexpressing Hsp70i in the absence of heat stress inhibited iNOS function. In summary, heat stress-induced transient inhibition of STAT1 following its dissociation from Hsp90, and the later transient inhibition of iNOS activity by inducible Hsp70, represent novel mechanisms by which the activation of the stress protein response inhibits the IFN-γ signaling pathway in alveolar macrophages. Since Hsp90 inhibitors have been shown to be safe in humans, these results also highlight a potential clinical application for this class of drugs in modulating NO signaling during the early phase of acute lung injury.
Keywords: Lung, Chaperones, Macrophages, Heat Shock, Signal Transduction
Introduction
Acute lung injury (ALI) is a devastating clinical syndrome manifested by an inflammatory response that leads to respiratory failure with an overall mortality rate of 30–40 percent 1. Clinical studies have shown that the vectorial fluid transport across the alveolar epithelium critical for maintaining fluid balance in the airspace is impaired in most patients with ALI 1. Furthermore, there is a direct correlation between the impairment of the fluid removal from the airspace and mortality in patients with ALI 2. Recent experimental evidence indicates that alveolar fluid clearance is impaired by iNOS/NO-dependent mechanisms 3. Under those circumstances, large amounts of NO are released by alveolar macrophages and epithelial cells and cause modification of ENaC, CFTR and other ion channels involved in alveolar epithelial ion transport rendering these ion channels nonfunctional 4, 5. We have previously reported that activation of the stress protein response (SPR), a highly conserved cellular defense mechanism characterized by the increased expression of heat shock proteins, restores the vectorial lung fluid transport in part by reducing inducible nitric oxide synthase (iNOS) synthesis 6. However, the mechanisms by which SPR activation affects the iNOS-dependent NO release are still not completely understood.
The heat shock or stress protein response (SPR) classically defined as a highly conserved cellular defense mechanism is characterized by the increased expression of stress proteins. This allows the cell to withstand a subsequent insult that would otherwise be lethal, a phenomenon referred as “thermotolerance” or “preconditioning”. The activation of SPR is characterized by an early phase defined by the inhibition of proinflammatory cell signaling pathways within minutes after onset of SPR and a delayed phase in which the development of tolerance to inflammatory stimuli takes several hours and requires the de novo synthesis of proteins. Heat shock proteins not only function as molecular chaperones for newly synthesized proteins, but are also essential factors in the cell signaling pathways activated by proinflammatory mediators 7, 8.
The delayed phase of SPR is associated with the expression of inducible heat shock proteins including Hsp70 and Hsp27. Several studies including our own work have shown that lung injury due to ischemia-reperfusion or hemorrhagic shock is inhibited in animals that have been preconditioned with stress 6, 9, 10 In some of these studies, the inhibitory effect of SPR could be reproduced by the adenoviral gene transfer of Hsp70 into the distal airspaces of the lung, suggesting that Hsp70 may participate in the anti-inflammatory effect induced by SPR 11
During the course of ALI, interferon-gamma (IFN-γ) an activator of the STAT1 pathway is found in high levels in the pulmonary edema fluid of these patients 12. The STAT proteins are members of a family of transcription factors that transmit signals from the extracellular surface of the cell to the nucleus where they activate transcription of various genes including iNOS 13. While there is considerable information about the activation of STAT proteins and their role in transcription, much less is known about the regulation of the STAT1 signaling pathway and how the activation of SPR effects STAT1 and iNOS function. Thus, the objective of this study was to determine the mechanisms by which heat stress regulates the STAT1 pathway, the expression of iNOS and the release of NO by alveolar macrophages after exposure to IFN-γ.
Material and Methods
Cells
An alveolar macrophage cell line, MH-S, were used in these studies.
SPR activation using heat (Heat Shock
MH-S cells were incubated at 43°C for 30 minutes and allowed to recover at 37°C for the hours indicated in the text prior to further analysis.
Western Blotting
Cells were seeded and cultured for 24 hours prior to any treatment. After treatment with IFN-γ (10 ng/ml) for the indicated time, the cells were washed three times with phosphate buffered saline (PBS) on ice, lysed in 1x LSB, electrophoresed and transferred to nitrocellulose. STAT1 antibody (1:1,000), HRP-GAM (1:2,000).
Measurement of extracellular nitric oxide
MH-S cells were stimulated with IFN-γ for 24 hours. The extracellular medium was used for analysis of the presence of nitrite, a stable end-product of nitric oxide using the Griess reagent.
Measurement of iNOS or inducible Hsp70 mRNA
Real-time RT-PCR primers and probes are designed using Primer Express software (PE-Applied Biosystems (PE-ABI), Warrington, UK). The real-time RT-PCR probes are labelled with a fluorophore reporter dye (6-carboxy-fluorescein, FAM) at the 5′ end and a quencher dye (BHQ, Biosearch Technologies, Inc., Novato CA) at the 3′ end. Quantitative real time RT-PCR is performed as previously published 14. Mouse RT-PCR primers: iNOS TAGF: GAGCATCCCAAGTACGAGTGGT, iNOS TAGR: GGCCACGGCAGGCAG, iNOS TAGP: CCAGGAGCTCGGGTTGAAGTGGTATG. Mouse RT-PCR primers: Hsp70i TAGF: CTGTAGGAAGGATTTGTACACTTTAAACTC, Hsp70i TAGR: TTAAGGGTCAGCTCCTGAAGGT, Hsp70i TAGP: TCTGAGTCCCACACTCTCACCACCCA.
EMSA
Nuclear protein isolation and EMSA was performed as described previously 7. TheSTAT1 consensu s oligonucleotide probe: 5′-ATG TGA GGG GAC TTT CCC AGG C-3′.
Isolation of membrane enriched fraction
MH-S cells were scraped into a hypotonic buffer (10 mM HEPES, 10 mM NaCl, 5 mM MgCl2, 1 mM DTT, protease inhibitors, phosphatase inhibitors) and processed as previously described 15 .
Immunoprecipitations
MH-S cells were lysed as described for western blots with the addition of 40mM sodium molybdate for the immunoprecipitation of STAT1/Hsp90 complexes.
siRNA transfection
siRNA to Hsp70i was transfected into MH-S cells using X-tremeGene® according to manufactures protocol using a ratio of 10:2 of transfection reagent in microliters to micrograms of siRNA.
Adenovirus infection
Adenovirus infection of macrophages was performed as previously described 16 .
Results
SPR activation inhibits IFN-γ-mediated NO production
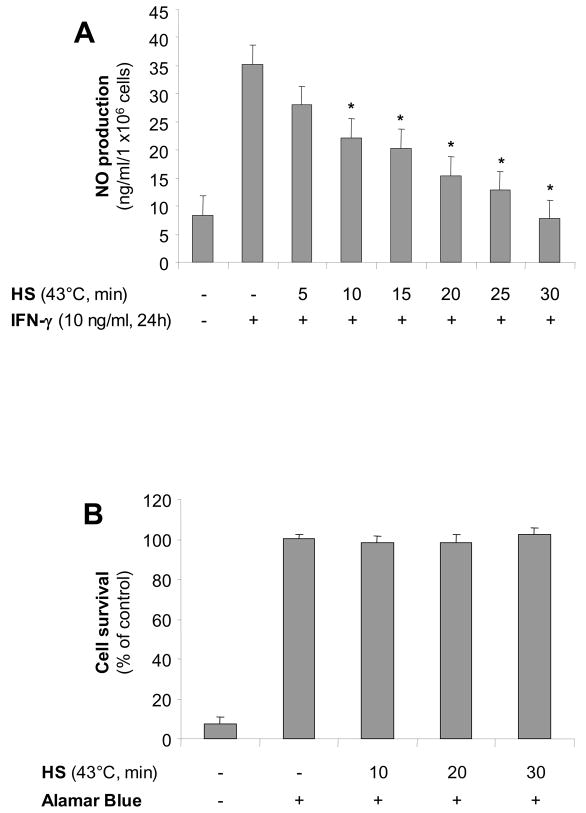
Alveolar macrophages exposed to increasing times of heat stress at 43°C and recovered at 37°C for 1 hour before IFN-γ stimulation for 24h, released decreasing amounts of NO (nitrite) measured in the extracellular medium (Figure 1A). This decrease in NO production was not due to cell death as determined by alamar blue on the same cells (Figure 1B).
Figure 1. SPR activation inhibits IFN-γ-mediated NO production, iNOS mRNA and protein in alveolar macrophages.
Panel A. Heat stress inhibits IFN-γ-mediated NO release in the extracellular medium in a time-dependent manner. MH-S cells were incubated at 43°C for increasing times, then allowed to recover at 37°C for 1 hour prior to IFN-γ stimulation (10 ng/ml) for 24 hours. Extracellular medium was assayed for nitrite using the Griess reagent. Panel B Heat stress does not affect the viability of MH-S cells. MH-S cells were incubated in 10% Alamar Blue reagent diluted in cell medium and incubated at 37°C for 2 hours. Color change was determined spectrophotometrically. For both panels, results are the mean ± SD of 4 separate experiments with each condition carried out in triplicate. Panel C. Heat stress inhibits IFN-γmediated iNOS mRNA expression. MH-S cells were either untreated or were incubated at 43°C and allowed to recover for 1 hour prior to IFN-γ stimulation (10 ng/ml for 2h) and analysis for iNOS mRNA by real-time RT-PCR normalized with GAPDH mRNA levels. Results are the mean ± SD of three experiments done in triplicate; *p < 0.05 from control cells; **p < 0.05 from cells treated with INF-γ alone. Panel D. Heat stress inhibits IFN-γ-mediated iNOS protein expression in MH-S cells. MH-S cells were either untreated or were incubated at 43°C and allowed to recover for 1 hour prior to IFN-γ stimulation (10 ng/ml for 7h) and analysis for iNOS protein by Western blot; one representative experiment is shown, three additional experiments gave comparable results.
SPR activation prevents IFN-γ-mediated iNOS mRNA and protein expression as well as STAT1 function
SPR activation followed by a one hour recovery at 37°C and subsequent stimulation with IFN-γ completely inhibited iNOS mRNA (Figure 1C) and protein expression (Figure 1D).
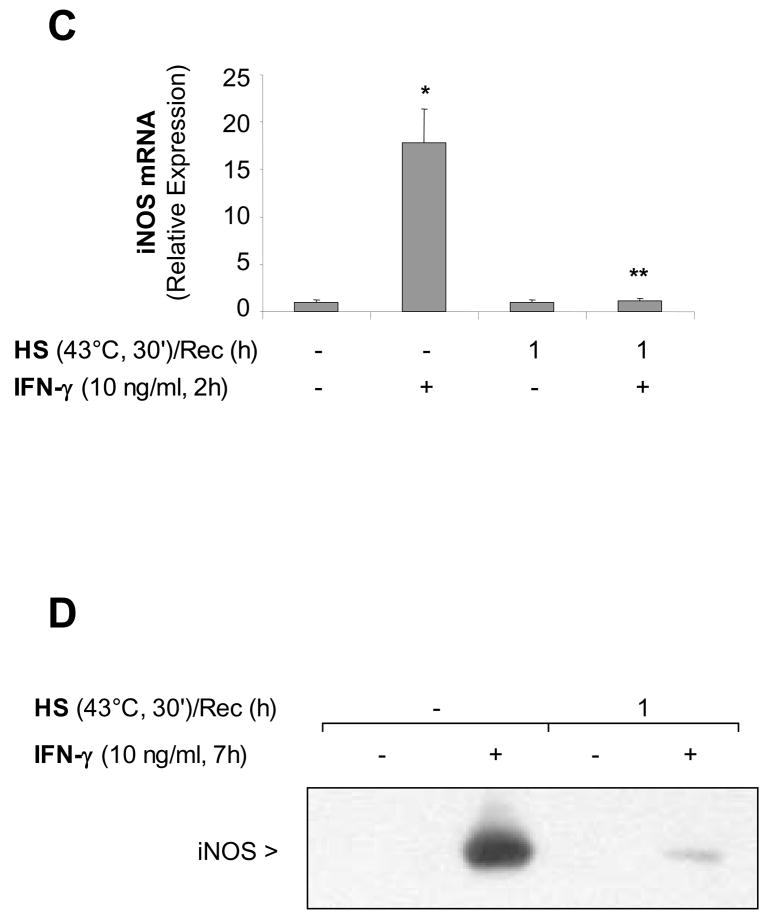
STAT1 activated by IFN-γ is an essential activator of iNOS transcription 17. After one-hour recovery from heat stress, the non-phosphorylated STAT1 protein was degraded (Figure 2A). Some cells were pretreated with the proteasome inhibitor MG132 for 1 hour prior to heat stress and exposure to IFN-γ. MG132 inhibited STAT1 degradation, however there was no phosphorylation of STAT1 on tyrosine 701 (Figure 2A). Thus, proteosomal degradation of the STAT1 protein was not the only mechanism of attenuation of the IFN-γ-mediated STAT1 activation by SPR. Further analysis showed that STAT1 did not translocate to the nucleus. As shown in Figure 2B, in the absence of heat stress, STAT1 protein extracted from the nucleus after IFN-γstimulation, bound to the STAT1 consensus oligonucleotide whereas after heat stress, a 1 hour recovery and IFN-γ stimulation, no binding was observed. Finally, cell fractionation experiments confirmed that heat stress caused the partition of STAT1 into the triton detergent insoluble fraction prior to its proteosomal degradation and that STAT1 insolubilization was not affected by IFN-γstimulation (Figure 2C).
Figure 2. SPR activation prevents IFN-γ activation of the STAT1 signaling pathway, promoting STAT1 detergent insolubility and degradation. Both time and glycerol restore STAT1 signaling after SPR activation.
Panel A. SPR activation prevents phosphorylation of STAT1 in response to IFN-γby causing STAT1 degradation via the proteasome. MH-S cells were either untreated or were incubated at 43°C and allowed to recover for one hour prior to IFN-γ stimulation (10 ng/ml for 30 min) and then analyzed for STAT1 and phosphorylated STAT1 by western blot. Some cells were pretreated with a proteasome inhibitor MG132 (10 μM) one hour prior to exposure to IFN-γ. Panel B. SPR activation prevents the translocation of IFN-γmediated STAT1 to the nucleus. MH-S cells were either untreated or were incubated at 43°C and allowed to recover for 1 hour prior to IFN-γ stimulation (10 ng/ml for 30 min). Electrophoretic mobility shift assay (EMSA) analysis was used to examine STAT1 activity in IFN-γ-treated cells. Nuclear extracts were prepared (see methods) and 5 μg of nuclear protein were incubated with a 32P-labeled DNA containing STAT1 binding consensus sequence (GAS). In some reactions, a 100-fold excess of the unlabeled oligonucleotide was added (indicated as cold probe in the figure). The experiment was performed 5 times. One representative experiment is shown. Panel C. SPR activation causes STAT1 partition into the detergent insoluble cell fraction. MH-S cells were either untreated or were incubated at 43°C and allowed to recover for one hour. Some cells were the treated by IFN-γ (10 ng/ml) or its vehicle for 30 min. Afterwards, the cells were harvested in phosphate buffered saline supplemented with 0.1% Triton X-100, 5 mM MgCl2 and protease inhibitors. The lysates were clarified by centrifugation at 16,000 x g for 10 minutes at 4 C. The supernatant was removed and adjusted to 1x Laemmli sample buffer and the material present within the detergent insoluble pellet was similarly resuspended in 1x Laemmli sample buffer. Aliquots of the detergent insoluble material (I) and the detergent soluble material (S) were analyzed for the presence of STAT1 antibody by Western blot. In each case, the same amount of total protein was applied to the gel. Panel D. Pretreatment with glycerol blocks the heat stress-dependent inhibition of STAT1 phosphorylation in response to IFN-γ . MH-S cells were left untreated or treated with glycerol (1M) for one hour. MH-S cells were either untreated or were incubated at 43°C and allowed to recover for one hour. Cells were the treated by IFN-γ (10 ng/ml) or its vehicle for 30 min and then analyzed for phospho-STAT1 by Western blot.
We examined whether insolubilization of the STAT1 complex might be prevented by pretreating the cell monolayers with glycerol, a chemical chaperone that reduces thermal denaturation of proteins 18. The results showing the triton-soluble fraction of the cell lysates indicate that pretreatment with glycerol not only prevents the degradation of some of the STAT1 protein, but also restores phosphorylation of STAT1 in cells that have been exposed to heat stress and IFN-γ( Figure 2D).
STAT1 function requires Hsp90
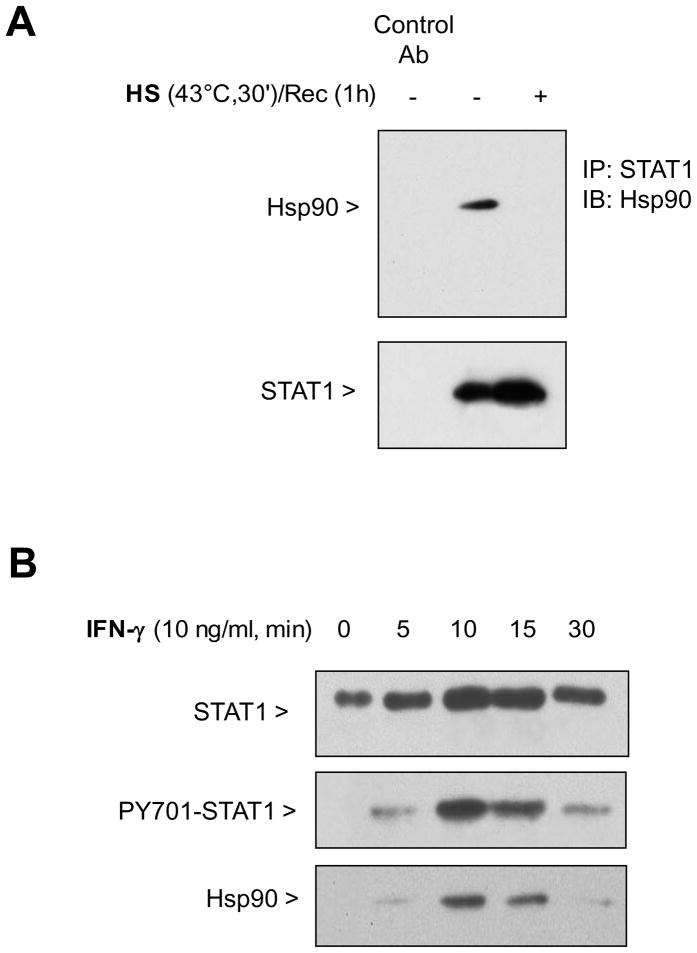
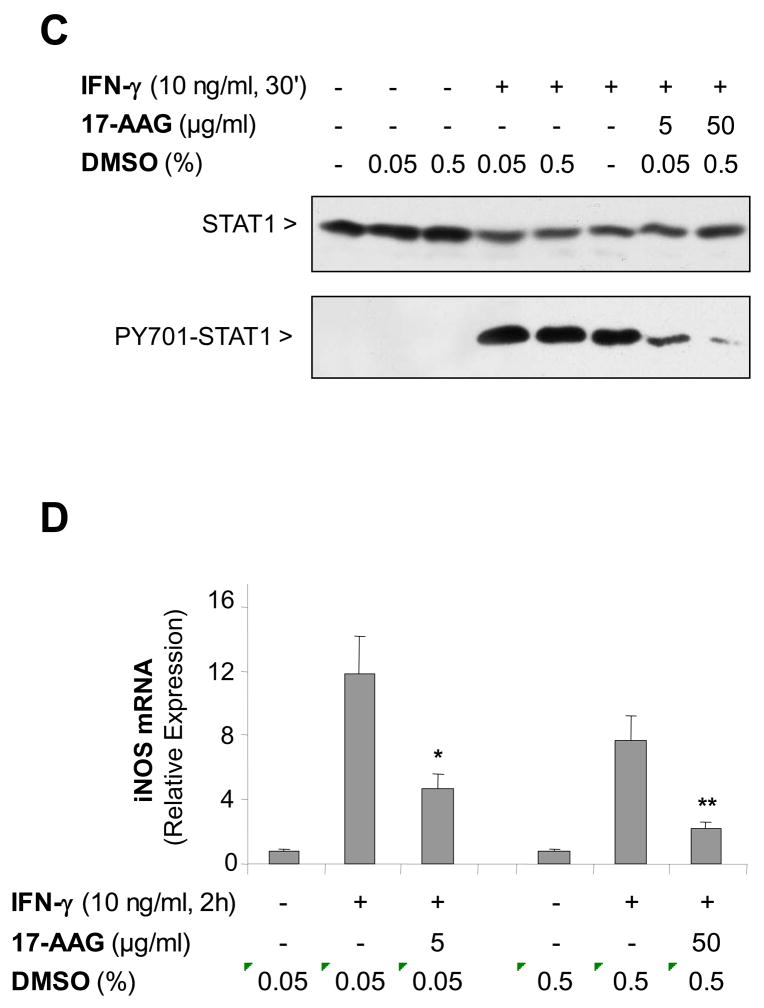
Hsp90 is known to bind various proteins to maintain protein conformation necessary for function. We tested whether STAT1 was an Hsp90 client protein. Hsp90 co-immunoprecipitated with STAT1 under non-stimulated conditions in MH-S cells, but not after SPR activation with heat (Figure 3A). In a second series of experiments, we performed a differential centrifugation to isolate the membrane-enriched fraction of MH-S cells in the absence of IFN-γ stimulation and after increasing times of IFN-γ stimulation. Both STAT1 and Hsp90 were recruited to this membrane enriched cell fraction 10–15 min after exposure to IFN-γ. This recruitment was associated with an increase in the level of phosphorylated STAT1 on tyrosine 701 (Figure 3B). Further analysis of the Hsp90/STAT1 association showed that treatment of macrophages for 1 hour with 17-AAG, an inhibitor of Hsp90 binding, resulted in the loss of STAT1 phosphorylation on tyrosine 701 after IFN-γ stimulation (Figure 3C). This loss of activated STAT1 by 17-AAG correlated with the absence of iNOS gene transcription (Figure 3D).
Figure 3. STAT1 forms a complex with Hsp90 enabling activation of STAT1 and is disrupted by SPR activation in alveolar macrophages.
Panel A. Heat stress causes a dissociation of the complex formed between STAT1 and Hsp90. MH-S cells were either untreated or were incubated at 43°C and allowed to recover for one hour. The cells were then lysed in a buffer containing 0.1% NP-40. Following centrifugation, the resultant supernatants were used for immunoprecipitation reactions using an antibody specific for STAT1. The resultant immunoprecipitates were examined via Western blotting for Hsp90 and STAT1. Panel B. Both STAT1 and Hsp90 are recruited to the plasma membrane upon stimulation with IFN-γ . MH-S cells were stimulated with IFN-γ (10 ng/ml) for the indicated times. Plasma membranes were isolated using dounce homogenization and differential centrifugation. The presence of total STAT1, phosphorylated STAT1 and Hsp90 was determined in the cell membrane fraction by Western blot. Panel C. Pretreatment with 17-AAG an Hsp90 inhibitor, inhibits phosphorylation of STAT1 in response to IFN-γ. MH-S cells were pretreated with 17-AAG an Hsp90 inhibitor , (5 or 50 μg/ml), one hour prior to IFN-γ stimulation (10 ng/ml for 30 min) and then analyzed for STAT1 and phospho-STAT1 by Western blot. Each set of experiments has been performed with the same final concentration of DMSO (solvent for 17-AAG). Panel D. Pretreatment with 17-AAG an Hsp90 inhibitor, inhibits IFN-γ-mediated iNOS mRNA expression. MH-S cells were pretreated with 17-AAG (5 and 50 μg/ml) one hour prior to IFN-γ stimulation (10 ng/ml for 2h) and analyzed for iNOS mRNA by real-time RT-PCR normalized with GAPDH mRNA levels. Each set of experiments has been performed with the same final concentration of DMSO (solvent for 17-AAG). Results are the mean ± SD of three experiments done in triplicate; *p < 0.05 from cells exposed to IFN-γ with 0.05% DMSO; **p < 0.05 from cells exposed to IFN-γ with 0.5% DMSO.
Production of nitric oxide after SPR activation and recovery of IFN-γ stimulated STAT1 signaling
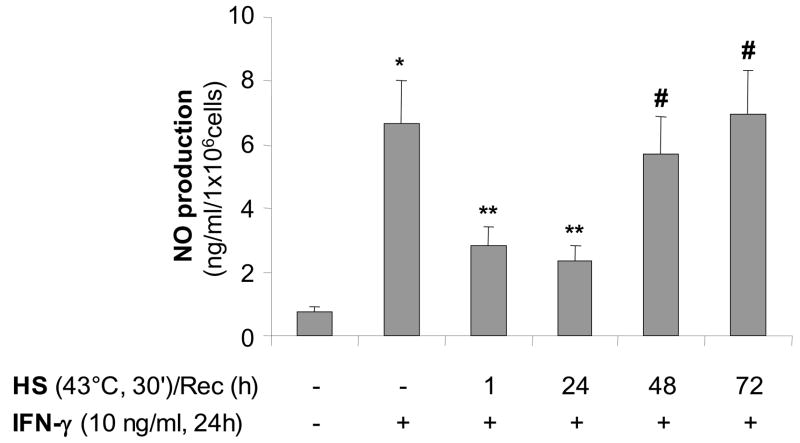
We next determined the time-course of the recovery of iNOS function after heat stress. Nitric oxide production was observed only after 48 hours following heat stress and reached normal values after 72 hours recovery post heat stress (Figure 4).
Figure 4. Recovery of iNOS function after SPR activation correlates with the decrease in SPR-mediated synthesis of the inducible Hsp70 protein in alveolar macrophages.
iNOS function recovers 48 hours after SPR activation. MH-S cells were incubated at 43°C for 30 min, then allowed to recover at 37°C for the indicated times prior to IFN-γ stimulation (10 ng/ml) for 24 hours. Extracellular medium was assayed for nitrite using the Griess reagent. Results are the mean ± SD of 4 separate experiments with each condition carried out in triplicate; *p < 0.05 from control cells; **p < 0.05 from cells treated with IFN-γ alone; #p < 0.05 from cells heat stressed/recovered 1 hour and treated with IFN-γ.
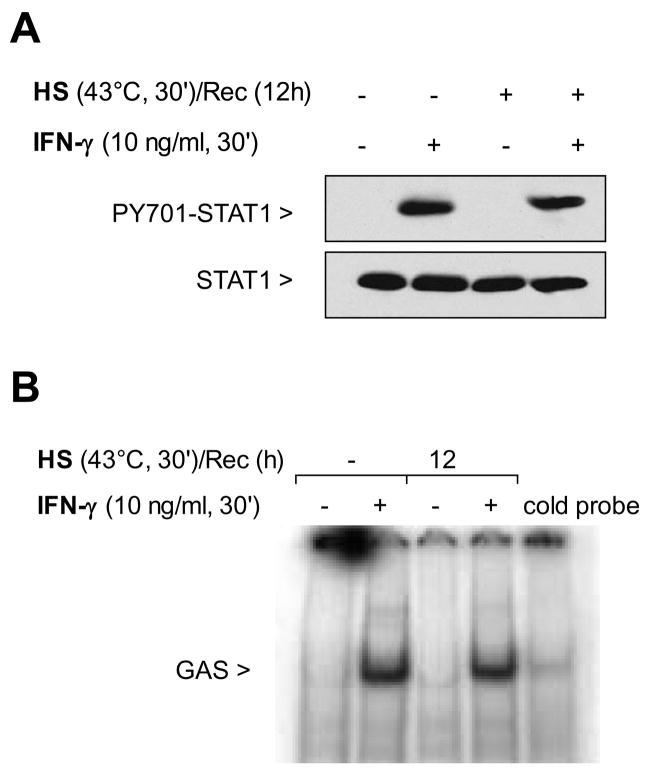
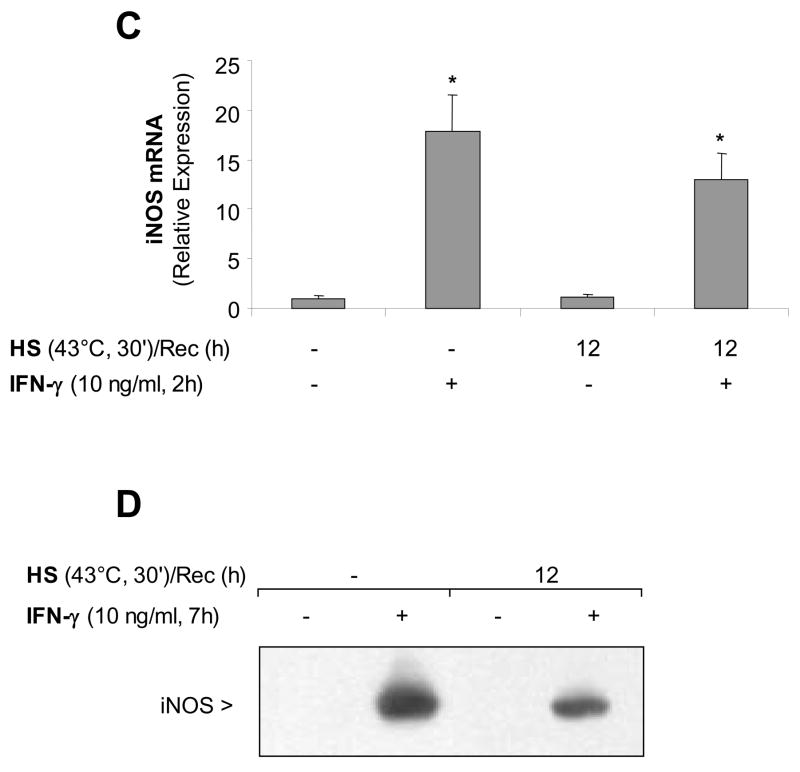
We determined whether the slow recovery of the IFN-γ-mediated NO production after SPR activation was caused by a prolonged inhibition of the STAT1 pathway. Figure 5A shows that with a 12 hour recovery post-heat stress, the STAT1 protein was phosphorylated. Since the STAT1 protein was phosphorylated, the presence of nuclear STAT1 protein and its DNA binding function after a 12 hour recovery from heat stress was tested. As shown in Figure 5B, in the absence of heat stress, STAT1 protein extracted from the nucleus after IFN-γ stimulation, bound to the STAT1 consensus oligonucleotide. Likewise, after heat stress, 12 hours recovery and IFN-γ stimulation, STAT1 binding was observed. Further analysis confirmed that the IFN-γdependent activation of the STAT1 signaling pathway fully recovered after 12 hours recovery from heat stress as iNOS mRNA was detected in these cells using real-time RT-PCR (Figure 5C) as well as the iNOS protein as determined by western blot (Figure 5D). These results show that after a 12-hour recovery from heat stress, alveolar macrophages stimulated with IFN-γ are able to activate the STAT1 pathway resulting in synthesis of both iNOS mRNA and protein. In contrast, the release of NO from these cells after SPR activation and subsequent IFN-γ stimulation did not follow the same temporal recovery. Indeed, there was an inhibition of NO production 12 hours after SPR activation even though the iNOS mRNA and protein was synthesized. Thus, SPR regulates iNOS-dependent NO release by mechanisms that are independent of iNOS gene and protein expression.
Figure 5. STAT1 signaling pathway is functional after 12 hours recovery post-heat stress induction and subsequent IFN-γ stimulation.
Panel A: MH-S cells were either untreated or were incubated at 43°C and allowed to recover for 12 hours prior to IFN-γ stimulation (10 ng/ml for 30 min) and then analyzed for STAT1 and phosphorylated STAT1 by western blot. Panel B: MH-S cells were either untreated or were incubated at 43°C and allowed to recover for 12 hours prior to IFN-γ stimulation (10 ng/ml for 30 min). Electrophoretic mobility shift assay (EMSA) analysis was used to examine STAT1 activity in IFN-γ-treated cells. Nuclear extracts were prepared (see methods) and 5 μg of nuclear protein were incubated with a 32P-labeled DNA containing STAT1 binding consensus sequence (GAS). In some reactions, a 100-fold excess of the unlabeled oligonucleotide was added (indicated as cold probe in the figure). The experiment was performed 5 times. One representative experiment is shown. Panel C: MH-S cells were either untreated or were incubated at 43°C and allowed to recover for 12 hours prior to IFN-γ stimulation (10 ng/ml for 2h) and analysis for iNOS mRNA by real-time RT-PCR normalized with GAPDH mRNA levels. Results are the mean ± SD of three experiments done in triplicate; *p < 0.05 from control cells. Panel D: MH-S cells were either untreated or were incubated at 43°C and allowed to recover for 1 hour prior to IFN-γ stimulation (10 ng/ml for 7h) and analysis for iNOS protein by Western blot; one representative experiment is shown, three additional experiments gave comparable results.
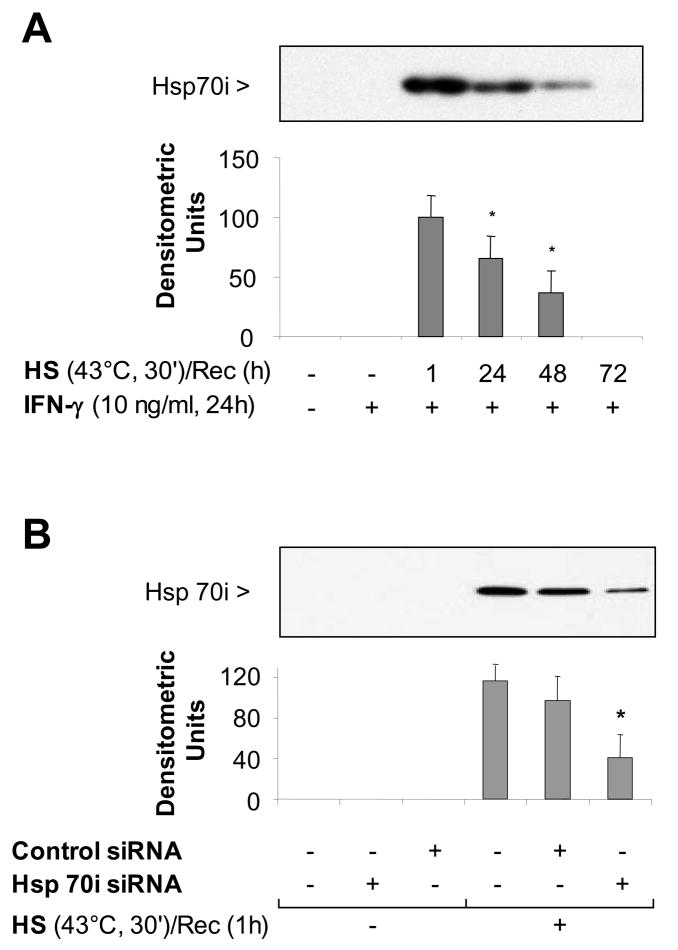
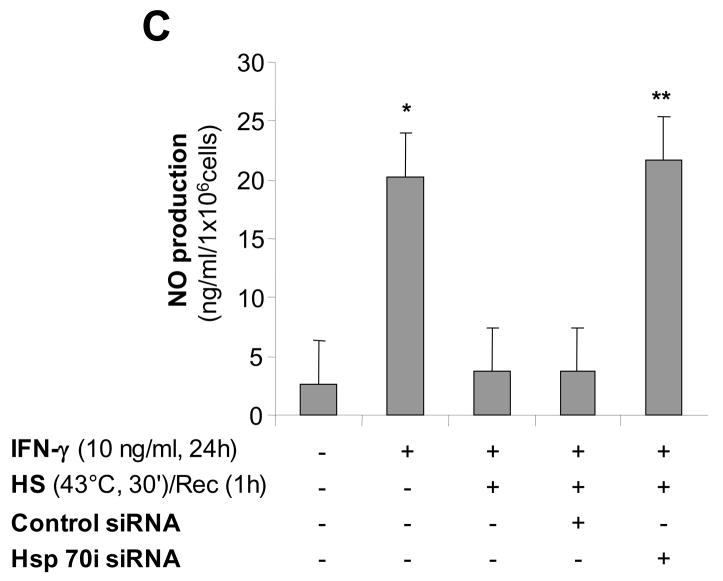
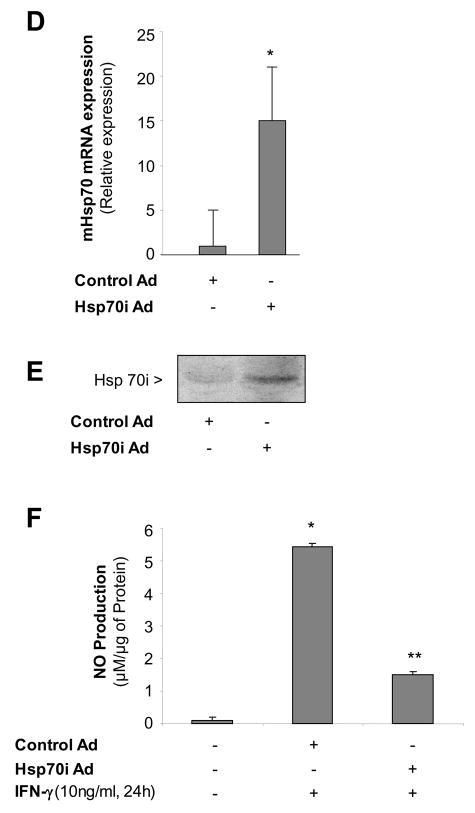
Since the inducible form of Hsp70 is expressed at high levels after heat stress, we hypothesized that in the late phase of SPR activation, Hsp70i expression transiently inhibits iNOS function. First, we determined the temporal expression of Hsp70i. As shown in Figure 6A, Hsp70i expression was expressed at high levels after 1 hour recovery and these levels decreased by 25% after 24 hours recovery and by more than 50% after 48 hours recovery. There was an inverse correlation between the SPR-induced Hsp70 protein expression and NO release by alveolar macrophages (Compare Figures 4A&6A). To further explore the importance of SPR-induced Hsp70 expression on iNOS function, Hsp70 expression was inhibited using a specific siRNA. Alveolar macrophages were transfected with a siRNA control or a specific siRNA to Hsp70 after 30 minutes of heat stress. The cells were allowed to recover for 24 hours and then stimulated with IFN-γ. As seen in Figure 6B, siRNA transfection after heat stress resulted in a 65 to 70% reduction in Hsp70 expression. This reduction in Hsp70i expression resulting from siRNA inhibition was sufficient to allow NO release by alveolar macrophages stimulated with IFN-γ (Figure 6C). Control and Hsp70i siRNA transfections had no effect on NO production (data not shown). Lastly, we determined whether overexpression of the inducible Hsp70i protein would prevent the release of NO from non-heat stressed alveolar macrophages when stimulated with IFN-γ. Infection of MH-S cells with a adenovirus encoding Hsp70i, but not with a control adenovirus, significantly increased the expression of both Hsp70i mRNA and protein (Figure 6D&E) and decreased the release of NO from these cells after stimulation with IFN-γ( Figure 6F).
Figure 6. Inducible Hsp70 inhibits iNOS function due to activation of the stress protein response by heat.
Panel A. Time course of Hsp70i expression after heat stress. MH-S cells were incubated at 43°C for 30 min, then allowed to recover at 37°C for the indicated times prior to IFN-γ stimulation (10 ng/ml) for 24 hours. Cells were analyzed for Hsp70i by Western blot. Panel B. Transfection with a specific siRNA one hour after SPR activation inhibits the expression of the inducible Hsp70. MH-S cells were either transfected with a control siRNA or a specific siRNA to Hsp70 twenty four hours before heat stress (before) or heat stressed, allowed to recover at 37°C for 1 hour and then transfected with a control or a specific siRNA to Hsp70 (after). Twenty four hours post-heat stress, cells were lysed and the expression of Hsp70 protein determined by Western blot. Densitometry analysis results are the mean ± SD of three experiments; *p < 0.05 from cells treated with control siRNA. Panel C. Inhibition of heat stressed induced Hsp70 results in NO production in response to IFN-γ. MH-S cells were incubated at 43°C for 30 minutes allowed to recover at 37°C for 1 hour and then transfected with a control or a specific siRNA to Hsp70 (after). Twenty-four hours post-transfection, cells were exposed to IFN-γ (10 ng/ml) for 24 hours. Extracellular medium was assayed for nitrite using the Griess reagent. Results are the mean ± SD of 3 separate experiments with each condition carried out in triplicate; *p < 0.05 from cells exposed to IFN-γ alone; **p < 0.05 from heat stressed Control Ad infected cells exposed to IFN-γ. Panel D: Cells were infected with a recombinant adenovirus expressing Hsp70i. Forty-eight hours post-infection, cells were lysed and the expression of Hsp70i mRNA determined by real-time RT-PCR normalized with GAPDH mRNA levels. Results are the mean ± SD of three experiments done in triplicate; *p < 0.05 from control cells. Panel E: Cells were infected with a recombinant adenovirus expressing Hsp70i. Forty-eight hours post-infection, cells were lysed and the expression of Hsp70i protein determined by western. Panel F: Cells were infected as described above. Cells were exposed to IFN-γ (10 ng/ml) 24 hours post-infection for 24 hours. Forty-eight hours post-infection, the extracellular medium was assayed for nitrite using the Griess reagent. Results reported as NO production of infected cells. Results are the mean ± SD of 3 separate experiments with each condition carried out in triplicate; *p < 0.05 from control cells; **p < 0.05 from Control Ad infected cells exposed to IFN-γ.
Discussion
In this study, we show for the first time that in mouse alveolar macrophages (1) activation of the stress protein response inhibits the STAT1/iNOS signaling pathway; (2) STAT1 and Hsp90 form a complex in the unstimulated cell while IFN-γ stimulation led to recruitment of both STAT1 and Hsp90 to the plasma membrane; (3) the disruption of STAT1/Hsp90 binding inhibits IFN-γ activation of STAT1 and results in the insolubility and subsequent degradation of STAT1 via the proteosome; and lastly (4) the late inhibition of iNOS function after heat stress is due to the expression of inducible Hsp70 and not additional biochemical changes associated with heat stress 19.
Activation of the stress protein response due to thermal or non-thermal stress results in the increased synthesis of molecular chaperones or heat shock proteins 19. These chaperones bind to proteins that have unfolded due to stress-induced partial denaturation, directing them to refold. During this period, the cell enters a senescent phase and is non-responsive to external stimulation and only after a period of time do the cells return to a “normal” state in which they respond again to stimuli. In this study, we show that a non-lethal heat stress can inhibit IFN-γmediated nitric oxide production without directing the alveolar macrophages into the apoptotic pathway. Under these conditions, the STAT1 signaling pathway is inhibited and refractive to activation by IFN-γ. In the early phase of SPR activation, heat stress resulted in the degradation of the STAT1 protein. Similar to our previous study on heat stress and the NF-κB signaling pathway in which the IKKα and IKKβ become detergent insoluble after heat stress 7, the STAT1 protein also is found first in a nonionic detergent insoluble fraction and subsequently degraded by the proteasome. Interestingly, this insolubility can be reversed by the chemical chaperone glycerol.
What is the mechanism that maintains STAT1 in a phosphorylation competent conformation? Heat shock proteins not only function as molecular chaperones for newly synthesized proteins, but are also essential factors in the cell signaling pathways activated by inflammatory mediators 7, 8. Hsp90 is a highly conserved and essential stress protein that functions as a positive regulator of cell signaling pathways by modifying or maintaining the conformation of its client proteins for active signaling. Moreover, blocking the ATPase site of Hsp90 using geldanamycin or its derivative 17-AAG inhibits the function of these client proteins 20. We previously showed that the binding of Hsp90 to its client protein IKKα/β may be necessary to maintain solubility and thus function of the protein 7. In other words, solubility of NF-κB and now STAT1 directly relates to its conformation and thus its function. Furthermore, a previous study reported that STAT3 is a client protein of Hsp90 and that this interaction is required for STAT3 phosphorylation 15. We found that STAT1 and Hsp90 form a complex under non-stimulated conditions, albeit a fraction of the total STAT1 population, and that this complex is disrupted after heat stress. JAK1/2, Tyk2, STAT1 and STAT3 as well as the cytokine receptors have been found in a complex in the plasma membrane 15, 21-23, we likewise found that STAT1 and Hsp90 were present in a complex under non-stimulated conditions and recruited to the plasma membrane under IFN-γ stimulated conditions. Thus, we postulate that in alveolar macrophages, Hsp90 binding to STAT1 is necessary for STAT1 function.
Studies carried out by Shang and Tomasi recently demonstrated that JAK1, interacts with Hsp90 and disruption of this binding resulted in proteasome degradation of JAK1 24. Our studies do not preclude that Hsp90 can also bind to JAK1 and it is indeed possible that Hsp90, STAT1 and its kinase JAK1 form an active complex at the plasma membrane in response to the binding of IFN-γ to its receptor. Finally, the results of this study show that (1) heat stress disrupts the binding of Hsp90 to its client protein STAT1 and subsequently attenuates STAT1 function and that (2) treatment with an Hsp90 inhibitor in the absence of heat stress and thus Hsp70i expression are consistent with our previous studies on IKKα/β and PDK-1 in which we identified both of these proteins as novel client proteins of Hsp90 and that their function is indeed regulated by Hsp90 binding 7, 25. We therefore can add STAT1 protein to the growing list of Hsp90 client proteins 26.
While the STAT1 signaling pathway recovers function 12 hours post-activation of the stress protein response, the recovery of iNOS function takes 48 hours. The iNOS protein has been shown to be a client protein of Hsp90 27; however, the length of the recovery of iNOS function indicates that another mechanism could also be involved in the inhibition of iNOS after heat stress. Previous studies using overexpression of recombinant Hsp70i have shown that Hsp70i can inhibit iNOS function 28, 29. However, the role of the SPR-mediated Hsp70i expression in modulating iNOS function is unknown. Thus, we tested whether the SPR-mediated induction of Hsp70i was inhibiting iNOS function. Our initial experiment showed that it was a 48-hour recovery from heat stress before alveolar macrophages would release NO in response to IFN-γ stimulation. The NO release inversely correlated with the level of inducible Hsp70 protein. Further investigation using either siRNA to Hsp70i to inhibit the heat stress-induced expression of Hsp70i or recombinant adenovirus to overexpress Hsp70i in the absence of SPR activation showed that in alveolar macrophages, it is Hsp70i expression that modulates iNOS function. What is the mechanism of Hsp70i attenuation of iNOS-dependent NO release in alveolar macrophages? Some studies have shown that Hsp70i can inhibit iNOS expression 28, 30,31. This does not appear to be the mechanism of inhibition of iNOS-dependent NO release in alveolar macrophages since we can detect iNOS protein in the detergent soluble fraction. In support of our findings, one recent study identified iNOS/Hsp70i complex formation in mouse intestinal tissue lysates and suggested that Hsp70i may regulate iNOS function 29. We are currently investigating possible mechanisms of attenuation of iNOS function by Hsp70i in alveolar macrophages. In summary, our working hypothesis is that Hsp90 is a critical part of the cell signaling pathways such as STAT1/iNOS that mediate lung fluid balance abnormalities induced by ALI. SPR activation may protect the integrity of the alveolar-capillary barrier by two mechanisms: (a) first by an immediate dissociation of Hsp90 from its client proteins rendering these proteins nonfunctional, then (b) by a delayed phase involving the de novo synthesis of Hsp70 that binds to and temporarily inhibits the function of some of the Hsp90 client proteins until Hsp90 can re-complex with these proteins.
What are the clinical implications of our findings? The airspace release of iNOS-dependent NO is deleterious during the early phase of acute lung injury. Indeed, the release of NO by alveolar macrophages and lung epithelial cells inhibits ion channel function via post-translational modification, thus preventing removal of edema fluid from the airspace of the lungs 32. Several studies have reported that prior activation of the stress protein response prevents the abnormalities in the lung fluid balance associated with ALI 6, 9, 33. These studies may have an important therapeutic significance in humans. We recently reported that SPR activation occurs in patients with ALI and correlates with the preservation of alveolar fluid clearance 34. Thus, the stress response could be activated as a prophylactic therapy to protect from lung fluid balance abnormalities associated with ALI, using pharmacologic inhibitors of Hsp90 that have been shown to be safe in humans 35.
Supplementary Material
Figure 7. Schematic of heat stress inhibition of STAT1 and iNOS signaling pathways.
Heat stress or treatment of macrophages with 17-AAG results in the disruption of the STAT1-Hsp90 complex preventing STAT1 phosphorylation and translocation to the nucleus. Post-heat stress when the STAT1 signaling pathway is responsive to IFN-γ stimulation, nitric oxide production from iNOS is inhibited due to the inducible form of Hsp70.
Acknowledgments
The authors thank Drs. Kimberly Mace and Nancy Boudreau for critical review of the manuscript. This work was primarily supported by UCSF Academic Senate Grant (M. Howard), NIH Grant GM 62188 (J.F. Pittet), ALA Senior Research Training Fellowship and T32 GM008440 (J. Roux).
Abbreviations
- STAT
signal transducer and activator of transcription
- SPR
stress protein response
- ALI
acute lung injury
- I/R
ischemia-reperfusion
- 17-AAG
17-allyl-amino-demethoxygeldanamycin
References
- 1.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–49. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 2.Ware LB, Matthay MA. Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;163:1376–83. doi: 10.1164/ajrccm.163.6.2004035. [DOI] [PubMed] [Google Scholar]
- 3.Pittet JF, Lu LN, Morris DG, et al. Reactive nitrogen species inhibit alveolar epithelial fluid transport after hemorrhagic shock in rats. J Immunol. 2001;166:6301–10. doi: 10.4049/jimmunol.166.10.6301. [DOI] [PubMed] [Google Scholar]
- 4.Guo Y, DuVall MD, Crow JP, Matalon S. Nitric oxide inhibits Na+ absorption across cultured alveolar type II monolayers. Am J Physiol. 1998;274:L369–77. doi: 10.1152/ajplung.1998.274.3.L369. [DOI] [PubMed] [Google Scholar]
- 5.Bebok Z, Varga K, Hicks JK, et al. Reactive oxygen nitrogen species decrease cystic fibrosis transmembrane conductance regulator expression and cAMP-mediated Cl- secretion inairway epithelia. J Biol Chem. 2002;277:43041–9. doi: 10.1074/jbc.M203154200. [DOI] [PubMed] [Google Scholar]
- 6.Pittet JF, Lu LN, Geiser T, Lee H, Matthay MA, Welch WJ. Stress preconditioning attenuates oxidative injury to the alveolar epithelium of the lung following haemorrhage in rats. J Physiol. 2002;538:583–97. doi: 10.1113/jphysiol.2001.013102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pittet JF, Lee H, Pespeni M, O'Mahony A, Roux J, Welch WJ. Stress-induced inhibition of the NF-kappaB signaling pathway results from the insolubilization of the IkappaB kinase complex following its dissociation from heat shock protein 90. J Immunol. 2005;174:384–94. doi: 10.4049/jimmunol.174.1.384. [DOI] [PubMed] [Google Scholar]
- 8.Zhang H, Burrows F. Targeting multiple signal transduction pathways through inhibition of Hsp90. J Mol Med. 2004;82:488–99. doi: 10.1007/s00109-004-0549-9. [DOI] [PubMed] [Google Scholar]
- 9.Hiratsuka M, Yano M, Mora BN, Nagahiro I, Cooper JD, Patterson GA. Heat shock pretreatment protects pulmonaryisografts from subsequent ischemia -reperfusion injury. J Heart Lung Transplant. 1998;17:1238–46. [PubMed] [Google Scholar]
- 10.Javadpour M, Kelly CJ, Chen G, Stokes K, Leahy A, Bouchier-Hayes DJ. Thermotolerance induces heat shock protein 72 expression and protects against ischaemia-reperfusion-induced lung injury. Br J Surg. 1998;85:943–6. doi: 10.1046/j.1365-2168.1998.00722.x. [DOI] [PubMed] [Google Scholar]
- 11.Weiss YG, Maloyan A, Tazelaar J, Raj N, Deutschman CS. Adenoviral transfer of HSP-70 into pulmonary epithelium ameliorates experimental acute respiratory distress syndrome. J Clin Invest. 2002;110:801–6. doi: 10.1172/JCI15888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arndt PG, Fantuzzi G, Abraham E. Expression of interleukin-18 in the lung after endotoxemia or hemorrhage-induced acute lung injury. Am J Respir Cell Mol Biol. 2000;22:708–13. doi: 10.1165/ajrcmb.22.6.3832. [DOI] [PubMed] [Google Scholar]
- 13.Schindler C, Darnell JE., Jr Transcriptional responses to polypeptide ligands: the JAK-STAT pathway. Annu Rev Biochem. 1995;64:621–51. doi: 10.1146/annurev.bi.64.070195.003201. [DOI] [PubMed] [Google Scholar]
- 14.Roux J, Kawakatsu H, Gartland B, et al. Interleukin-1beta decreases expression of the epithelial sodium channel alpha-subunit in alveolar epithelial cells via a p38 MAPK-dependent signaling pathway. J Biol Chem. 2005;280:18579–89. doi: 10.1074/jbc.M410561200. [DOI] [PubMed] [Google Scholar]
- 15.Shah M, Patel K, Fried VA, Sehgal PB. Interactions of STAT3 with caveolin-1 and heat shock protein 90 in plasma membrane raft and cytosolic complexes. Preservation of cytokine signaling during fever. J Biol Chem. 2002;277:45662–9. doi: 10.1074/jbc.M205935200. [DOI] [PubMed] [Google Scholar]
- 16.Fasbender A, Lee JL, Walters RW, Moninger TO, Zabner J, Welsh MJ. Incorporation of Adenovirus in Calcium Phosphate Precipitates Enhances Gene Transfer to Airway Epithelia In Vitro and In Vivo. J Clin Invest. 1998;102:184–193. doi: 10.1172/JCI2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blanchette J, Jaramillo M, Olivier M. Signalling events involved in interferon-gamma-inducible macrophage nitric oxide generation. Immunology. 2003;108:513–22. doi: 10.1046/j.1365-2567.2003.01620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pinto M, Morange M, Bensaude O. Denaturation of proteins during heatshock. In vivo recovery of solubility and activity of reporter enzymes. J Biol Chem. 1991;266:13941–6. [PubMed] [Google Scholar]
- 19.Welch WJ. Mammalian stress response: cell physiology, structure/function of stress proteins, and implications for medicine and disease. Physiol Rev. 1992;72:1063–81. doi: 10.1152/physrev.1992.72.4.1063. [DOI] [PubMed] [Google Scholar]
- 20.Kamal A, Boehm MF, Burrows FJ. Therapeutic and diagnostic implications of Hsp90 activation. Trends Mol Med. 2004;10:283–90. doi: 10.1016/j.molmed.2004.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koshelnick Y, Ehart M, Hufnagl P, Heinrich PC, Binder BR. Urokinase receptor is associated with the components of the JAK1/STAT1 signaling pathway and leads to activation of this pathway upon receptor clustering in the human kidney epithelial tumor cell line TCL-598. J Biol Chem. 1997;272:28563–7. doi: 10.1074/jbc.272.45.28563. [DOI] [PubMed] [Google Scholar]
- 22.Ju H, Venema VJ, Liang H, Harris MB, Zou R, Venema RC. Bradykinin activates the Janus-activated kinase/signal transducers and activators of transcription (JAK/STAT) pathway in vascular endothelial cells: localization of JAK/STAT signalling proteins in plasmalemmal caveolae. Biochem J. 2000;351:257–64. doi: 10.1042/0264-6021:3510257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takaoka A, Mitani Y, Suemori H, et al. Cross talk between interferon-gamma and -alpha/beta signaling components in caveolar membrane domains. Science. 2000;288:2357–60. doi: 10.1126/science.288.5475.2357. [DOI] [PubMed] [Google Scholar]
- 24.Shang L, Tomasi TB. The heat shock protein 90-CDC37 chaperone complex is required for signaling by types I and II interferons. J Biol Chem. 2006;281:1876–84. doi: 10.1074/jbc.M509901200. [DOI] [PubMed] [Google Scholar]
- 25.Pespeni MH, Hodnett M, Abayasiriwardana KS, et al. Sensitization of mesothelioma cells to tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis by heat stress via the inhibition of the 3-phosphoinositide-dependent kinase 1/Akt pathway. Cancer Res. 2007;67:2865–71. doi: 10.1158/0008-5472.CAN-06-3871. [DOI] [PubMed] [Google Scholar]
- 26.Pratt WB, Toft DO. Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp Biol Med (Maywood) 2003;228:111–33. doi: 10.1177/153537020322800201. [DOI] [PubMed] [Google Scholar]
- 27.Yoshida M, Xia Y. Heat shock protein 90 as an endogenous protein enhancer of inducible nitric-oxide synthase. J Biol Chem. 2003;278:36953–8. doi: 10.1074/jbc.M305214200. [DOI] [PubMed] [Google Scholar]
- 28.Lau SS, Griffin TM, Mestril R. Protection against endotoxemia by HSP70 in rodent cardiomyocytes. Am J Physiol Heart Circ Physiol. 2000;278:H1439 –45. doi: 10.1152/ajpheart.2000.278.5.H1439. [DOI] [PubMed] [Google Scholar]
- 29.Kiang JG, Bowman PD, Wu BW, et al. Geldanamycin treatment inhibits hemorrhage-induced increases in KLF6 and iNOS expression in unresuscitated mouse organs: role of inducible HSP70. J Appl Physiol. 2004;97:564–9. doi: 10.1152/japplphysiol.00194.2004. [DOI] [PubMed] [Google Scholar]
- 30.Murphy P, Sharp A, Shin J, et al. Suppressive effects of ansamycins on inducible nitric oxide synthase expression and the development of experimental autoimmune encephalomyelitis. J Neurosci Res. 2002;67:461–70. doi: 10.1002/jnr.10139. [DOI] [PubMed] [Google Scholar]
- 31.Yeo M, Park HK, Kim DK, et al. Restoration of heat shock protein 70 suppresses gastric mucosal inducible nitric oxide synthase expression induced by Helicobacter pylori. Proteomics. 2004;4:3335–42. doi: 10.1002/pmic.200400951. [DOI] [PubMed] [Google Scholar]
- 32.Zhu S, Ware LB, Geiser T, Matthay MA, Matalon S. Increased levels of nitrate and surfactant protein a nitration in the pulmonary edema fluid of patients with acute lung injury. Am J Respir Crit Care Med. 2001;163:166–72. doi: 10.1164/ajrccm.163.1.2005068. [DOI] [PubMed] [Google Scholar]
- 33.Lee H, Pespeni M, Roux J, Dennery PA, Matthay MA, Pittet JF. HO-1 induction restores c-AMP-dependent lung epithelial fluid transport following severe hemorrhage in rats. Faseb J. 2005;19:287–9. doi: 10.1096/fj.04-2254fje. [DOI] [PubMed] [Google Scholar]
- 34.Ganter MT, Ware LB, Howard M, et al. Extracellular heat shock protein 72 is a marker of the stress protein response in acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2006;291:L354–61. doi: 10.1152/ajplung.00405.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sausville EA, Tomaszewski JE, Ivy P. Clinical development of 17-allylamino, 17-demethoxygeldanamycin. Curr Cancer Drug Targets. 2003;3:377–83. doi: 10.2174/1568009033481831. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.