Abstract
BACKGROUND
Multiple alterations in circadian rhythms have been observed in cancer patients, including the diurnal rhythm of the adrenal hormone cortisol. Diurnal cortisol alterations have been associated with cancer-related physiological processes as well as psychological stress. Here we investigate alterations in diurnal cortisol rhythm in ovarian cancer patients, and potential links with depression, life stress, and functional disability.
METHODS
Women (n = 177) with suspected ovarian cancer completed questionnaires and collected salivary cortisol 3× daily for 3 consecutive days before surgery. One hundred women were subsequently diagnosed with ovarian cancer and 77 with benign disease. In addition, healthy women (n = 33) not scheduled for surgery collected salivary cortisol at the same time points.
RESULTS
Ovarian cancer patients demonstrated significantly elevated nocturnal cortisol (P = .022) and diminished cortisol variability (P = .023) compared with women with benign disease and with healthy women (all P values <.0001). Among ovarian cancer patients, higher levels of nocturnal cortisol and less cortisol variability were significantly associated with greater functional disability, fatigue, and vegetative depression, but not with stress, distress, or depressed affect. There were no significant associations between functional or psychological variables and diurnal cortisol in women with benign disease.
CONCLUSIONS
Nocturnal cortisol and cortisol variability show significant dysregulation in ovarian cancer patients, and this dysregulation was associated with greater functional disability, fatigue, and vegetative depression. These findings suggest potential hypothalamic-pituitary-adrenal involvement in functional disability in ovarian cancer, and may have implications for disease progression.
Keywords: cortisol, ovarian cancer, diurnal rhythm, fatigue, functional disability, depression, stress
Many physiological processes are governed by circadian rhythms in healthy humans and animals. However, in tumor-bearing organisms, the integrity of circadian rhythms is frequently compromised.1 Profound alterations in serum cortisol patterns have been reported in 15 of 20 ovarian cancer patients, including unusually high values of cortisol across the 24-hour cycle, erratic peaks and troughs, and flattened profiles.2 Abnormal diurnal cortisol profiles have been linked with poor performance status,3 persistent fatigue,4 and with poorer survival in breast cancer patients.5
Circadian rhythms align the organism with the light-dark cycle of the earth.6 Cortisol levels rise in the early morning, followed by a decrease over the course of the day, and reach a nadir during sleep.7 Morning light triggers the suprachiasmatic nucleus (SCN) of the hypothalamus to activate the hypothalamic-pituitary-adrenal (HPA) axis,6 leading to cortisol secretion by the adrenal cortex, with subsequent release of energy for daily activities.8
Cortisol also provides energy during the stress response.8 Acute cortisol elevations are observed in response to brief stressors.9,10 With chronic stress, the negative feedback system regulating cortisol becomes impaired,11 leading to chronically elevated cortisol, particularly at night.12 Depressed individuals also have impaired cortisol feedback and chronically elevated nocturnal cortisol.13 For example, depression has been associated with restricted diurnal cortisol variation in patients with advanced meta-static cancers of various types,14 and poor quality social support is associated with higher mean daily cortisol in breast cancer.15
Age-related and experimentally induced impairments in cortisol feedback have been shown to lead to greater tumor growth in animal models.16 For example, implanted tumors grow faster in mice with SCN lesions and consequently altered corticosterone rhythms.17 In humans, nocturnal shift work, with accompanying changes in diurnal rhythms, is associated with increased breast cancer risk.18 Alternatively, tumor tissues may influence circadian regulation in the host. For example tumor-bearing mice show alterations in corticosterone rhythms.19
Salivary cortisol is considered a reliable measure of the amount of unbound, biologically active cortisol in the blood. Repeated cortisol sampling during the day can provide information on circadian rhythm alterations and can suggest possible dysregulation of HPA feedback mechanisms.9
We have previously reported associations between elevated nocturnal cortisol and depression in a small sample of ovarian cancer patients.20 Depression has multiple components, including fatigue, vegetative symptoms, and distressed mood, which can be difficult to tease apart in medical patients. To better understand these relationships in the context of ovarian cancer, we examined 1) disturbances in the cortisol circadian rhythm in ovarian cancer patients, and 2) whether circadian cortisol alterations are associated with psychological factors such as stress or depressed mood and/or with functional disability and fatigue. To control for the stress of anticipated cancer surgery, we included a comparison group of women who underwent surgery for suspected ovarian cancer but were found to have benign disease. A reference group of healthy women not undergoing surgery was also included.
This approach enabled examination of whether dys-regulated cortisol is associated with psychological factors per se (such as distressed mood or perceived stress), and whether vegetative and potentially disease-related symptoms (such as fatigue and functional disability) are the primary correlates of dysregulated cortisol. These questions are of particular significance for understanding the etiology of cortisol dysregulation and vegetative symptoms in ovarian cancer.
On the basis of previous findings2,5 we hypothesized that women with ovarian cancer would demonstrate altered cortisol rhythms, including lower morning cortisol and elevations in afternoon and evening cortisol, resulting in reduced cortisol variability. Furthermore, we hypothesized that altered diurnal cortisol rhythms in ovarian cancer patients would be associated with functional disability, fatigue, and psychosocial factors such as stress and depressed mood.
MATERIALS AND METHODS
Participants
Gynecologic oncology patients with suspected ovarian cancer were prospectively recruited during their presurgical clinic appointment. All were undergoing the stress of anticipated surgery, diagnosis, and possible cancer treatment. Histological diagnosis and inclusion in the study were confirmed by pathology. Patients with primary epithelial ovarian, peritoneal, or fallopian tube carcinomas and benign ovarian neoplasms were eligible. Patients with nonepithelial or metastatic malignancies, low malignant potential tumors, endometriosis or pelvic inflammatory disease, a personal history of cancer, immune disorder, use of systemic corticosteroids or antidepressants,21 or age < 18 years were excluded.
Of 592 potentially eligible patients who consented to participate in the study, 48 had tumors of nonovarian pathology, 52 had ovarian tumors of low malignant potential, 41 had inflammatory disorders, 70 were taking antidepressants, and 5 were taking corticosteroids and were excluded. Thirty-nine patients withdrew from the study, mostly because of time limitations before surgery or emotional distress, and an additional 22 did not have surgery. Of eligible patients, 43 did not have adequate time for cortisol collection because of the immediacy of their surgeries, and 95 chose not to collect cortisol data, usually because of time constraints or emotional distress. Therefore, the final sample included 177 patients. After surgery, 100 women were diagnosed with ovarian cancer, and 77 were diagnosed with benign disease. Cortisol and demographic data were also collected from a reference group of 33 healthy women not undergoing surgery.
Procedure
Study procedures were approved by institutional review boards at the Universities of Iowa and Miami. During the presurgical visit, informed consent was obtained, and participants received questionnaires and instructions for home collection of salivary cortisol. Samples were collected upon awakening, between 4:00 and 6:30 PM, and at bedtime for the 3 days before surgery. A research assistant called each participant before sample collection to repeat instructions for maximum compliance. Samples were refrigerated until brought to the clinic at the time of surgery, where they were stored at −80°C until analysis. Before surgery, patients did not have a definitive cancer diagnosis and generally did not know their prognosis.
Measures
Quality of life: physical well-being
The Functional Assessment of Cancer Therapy22 is designed to assess health-related quality of life in ovarian cancer patients over the past week. The subscale of interest in the current study was the physical well-being subscale, consisting of 7 items designed to assess physical symptoms associated with cancer or its treatment, with higher scores indicating better functioning.
Performance status
Before surgery, both patients and oncologists completed a measure of the patient’s performance status.23 Functional status is rated from 0 (fully active) to 4 (completely disabled), with higher scores indicating greater impairment.
Fatigue and distress
The Profile of Mood States,24 a 37-item scale assessing mood over the past week, is frequently used to assess distress in patients with cancer.25 To provide a distress score without a vegetative component, the anxiety and depression subscales were made into a composite. The fatigue subscale was examined separately.
Depressive symptoms
The Center for Epidemiological Studies Depression scale includes 20 items measuring frequency of depressive symptoms over the previous week.26 A 4-factor structure has been identified, including depressed affect, vegetative depression, positive affect, and interpersonal relationships.27 The vegetative and depressed affect subscales were the subscales of interest in this study.
Perceived stress
The Perceived Stress Scale (PSS) includes 14 items measuring the perception of situations appraised as unpredictable or uncontrollable28 over the previous week. The PSS has previously been used in studies of a variety of patients with chronic illness.29
Stressful life events
A modified form of the Life Experiences Survey30,31 was used, asking participants to identify number and severity of stressful events that have occurred over the previous 12 months. Stressful events included marital conflict, death of a family member, and loss of employment.
Medical data
Medical data, including histology, stage, and grade of tumor, were abstracted from patients’ medical records. Demographic data and information about average hours of sleep over the past week were obtained by self-report.
Cortisol
Collection time of salivary cortisol samples was written on the Salivette by participants at the time of sampling, a procedure that has been found to be highly reliable and accurate in previous studies.32 Salivary cortisol samples are stable at room temperature.9 Assays were performed using a commercial chemiluminescence immunoassay (IBL, Hamburg, Germany). The lower detection limit is 0.41 nmol/L, and interassay and intra-assay coefficients of variance are <10%.
Data Analysis
Data reduction strategies
Cortisol data were first examined for time of sampling outliers. Morning cortisol rises occur in conjunction with personal awakening time.7 Therefore, we asked patients to take their first cortisol sample with reference to personal time of awakening.32 Because patients awake at different times in the morning, acceptable ranges of sampling times were determined to fit the maximum number of participants, while retaining a certain amount of homogeneity. Samples were retained if they were taken between 4:00 and 9:00 AM for morning cortisol, 4:00 and 6:30 PM for afternoon cortisol, and 8:00 PM and 12:00 AM for nocturnal cortisol. Previous research has indicated that aggregation across multiple cortisol assessments increases reliability of measurement.9,32,33 Mean cortisol values at each time point were calculated, and values were transformed using the natural logarithm (ln) to normalize their distribution. Two cortisol values that were >10 standard deviations above the mean were removed. Diurnal cortisol variability was defined for our analyses as ln(nocturnal cortisol/morning cortisol). This was used rather than a straight proportion (AM cortisol – nocturnal cortisol/AM cortisol) × 100 (which is equal to 1–nocturnal cortisol/AM cortisol) as suggested by Jehn et al.14 to normalize the distribution. Note that a sign reversal in interpreting the direction of the relationship is necessary because high values of 1–nocturnal cortisol/AM cortisol correspond to low values of nocturnal cortisol/AM cortisol and vice versa. For clarity of interpretation in Figure 1, mean cortisol variability was back-transformed (ie, [1–eln(nocturnal cortisol/AM cortisol)] × 100). As sleep disturbance is associated with alterations in circadian rhythms, sleep duration was also tested as a potential covariate. Secondary analyses removing patients taking anxiolytic medications were also conducted.
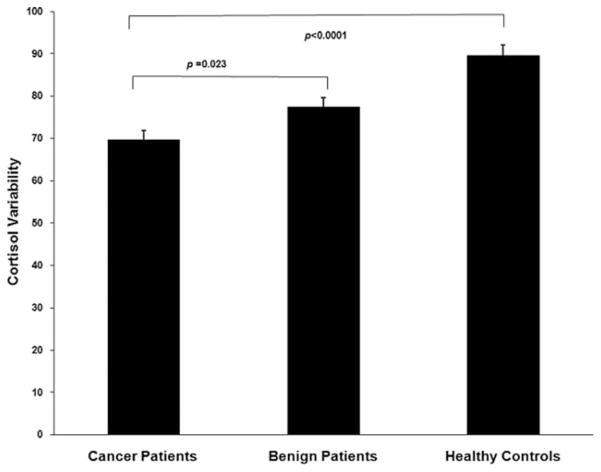
Figure 1.
Diurnal cortisol variability is shown among ovarian cancer patients, patients with benign pelvic disease, and healthy women. Ovarian cancer patients demonstrated significantly lower cortisol variability than the other groups. P values were Bonferroni corrected.
Mixed models analyses
Linear mixed model analysis for repeated measures was used to examine the differences in mean cortisol level at each time point in the 3 groups. Fixed effects included subject group (ovarian cancer, benign disease, and healthy control), time (morning waking, afternoon, and night), and group by time interaction. The test of significance for group-time interaction examines whether the presence of ovarian cancer affects the pattern of change in cortisol level during the day. As age has been associated with alterations in the HPA axis,34 the model included age as a covariate. As a secondary analysis, we examined differences in cortisol among the groups based on time of collection (hourly intervals) and individual cortisol data points rather than the mean over 3 days. This analysis used the generalized estimating equation method with SAS Proc Genmod.35 For each time period (waking, afternoon, night), we examined whether the group differences stayed consistent across hourly time intervals within each time period (group × hour interaction). In the absence of a group × hour interaction (indicating group differences are similar across hourly intervals), the main effect of group (group differences averaged across the hourly intervals) within each time period was examined. To examine pair-wise comparisons between groups, tests of mean contrast based on estimates from the fitted model were performed, with P values adjusted using Bonferroni’s method to account for the number of tests performed.
Correlational analyses
Impaired negative feedback of cortisol was expected to be most noteworthy at the nocturnal time point12; accordingly, Pearson correlations were conducted to examine the associations between nocturnal cortisol, cortisol variability, and behavioral factors, first in ovarian cancer patients, and then in patients with benign disease. All correlations were adjusted for age; for analyses including only ovarian cancer patients, disease stage was also controlled.
RESULTS
Participant Characteristics
Demographic information is presented in Table 1. Cancer patients were significantly older than patients with benign disease (P < .001), but did not differ significantly in age from healthy women (P = .10). More advanced cancer stages were associated with significantly higher morning (r = 0.23, P = .02) and afternoon (r = 0.32, P = .002), but not nocturnal (r = 0.13, P = 33) cortisol levels, reflecting elevated cortisol levels in patients of all stages at night. Dividing patients into those with early (stage 1 and 2) and advanced (stage 3 and 4) stage disease, we found no stage by time interaction for cortisol, (P = .77), suggesting that the overall diurnal rhythm was not significantly different between patients with early and advanced stage disease.
Table 1.
Participant Characteristics
| Group | Ovarian Cancer Patients, n = 100 | Patients With Benign Disease, n = 77 | Healthy Women, n = 33 |
|---|---|---|---|
| Age y, mean (SD) | 58.19 (11.78) | 51.04 (12.09) | 52.79 (16.06) |
| Ethnicity | |||
| Non-Hispanic | 93.0% | 84.4% | 93.9% |
| Hispanic | 7.0% | 15.6% | 6.1% |
| Race | |||
| American Indian or Alaskan Native | 2.0% | 0.0% | 0.0% |
| Asian or Pacific Islander | 1.0% | 1.3% | 6.1% |
| Black/African American | 2.0% | 11.7% | 0.0% |
| Caucasian | 95.0% | 87.0% | 90.9% |
| Other | 0.0% | 0.0% | 3.0% |
| Income, $ | |||
| <10,000 | 9.3% | 10.0% | 6.1% |
| 10,001–20,000 | 8.0% | 18.6% | 9.1% |
| 20,001–30,000 | 19.5% | 18.6% | 27.3% |
| 30,001–40,000 | 12.6% | 8.6% | 18.1% |
| 40,001–60,000 | 24.2% | 27.0% | 24.2% |
| 60,001–80,000 | 12.6% | 8.6% | 6.1% |
| >80,000 | 13.8% | 8.6% | 9.1% |
| Average sleep/night in last week, h | 6.74 (1.53) | 6.76 (1.47) | 7.19 (0.74) |
| Stage | |||
| I | 18.2% | ||
| II | 8.1% | ||
| III | 61.6% | ||
| IV | 12.1% | ||
| Grade | |||
| 1 | 9.0% | ||
| 2 | 10.0% | ||
| 3 | 60.0% | ||
| Unavailable | 21.0% | ||
| Tumor histology | |||
| Serous | 69.0% | ||
| Endometrioid | 13.0% | ||
| Mucinous | 5.0% | ||
| Other | 4.0% | ||
| Unavailable | 9.0% | ||
| Residual disease | |||
| Yes | 24.5% | ||
| No | 75.5% | ||
SD indicates standard deviation.
Age was positively associated with cortisol levels at all time points (all P values <.015). Average sleep duration during the last week was not significantly associated with cortisol levels at any time point (P values >.46) and did not differ significantly between groups (P = .27); thus, sleep was not used as a covariate.
Diurnal Cortisol Rhythm in the 3 Groups
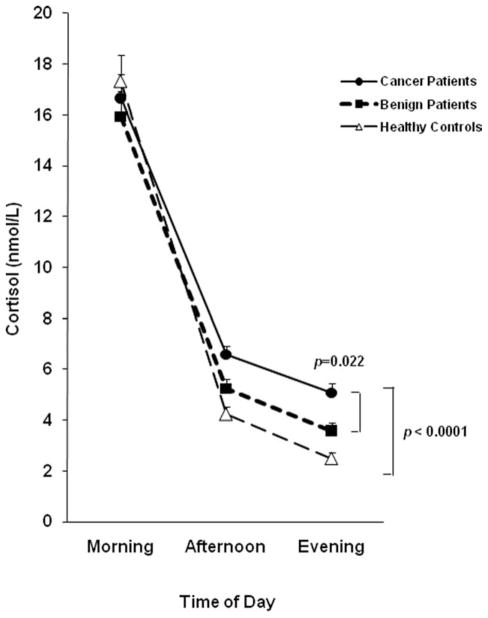
The mixed model analysis adjusting for age revealed a significant group by time interaction, F4,109 = 10.08, P < .0001, indicating that the 3 groups differed in their pattern of cortisol secretion over the course of the day (Fig. 2). Follow-up between group comparisons indicated that ovarian cancer patients did not differ from the other 2 groups in morning cortisol levels (Bonferroni adjusted P’s >.90). Mean afternoon cortisol levels of ovarian cancer patients were 55% higher than those of healthy women (P<.0001), but did not differ significantly from those of women with benign disease (P = .07). Nocturnal cortisol levels among women with ovarian cancer (mean = 5.07 nmol/L; standard error [SE] = 0.39) were 41.5% higher than those among women with benign disease (mean = 3.58 nmol/L; SE = 0.31; P = .022) and 103% higher than those among healthy women (mean = 2.49 nmol/L; SE = 0.23; P < .0001). Because of concerns that anxiolytic medications might affect the HPA axis,36,37 these analyses were repeated eliminating patients taking anxiolytic agents, and results were essentially identical (PM cortisol: ovarian vs healthy, P < .0001; ovarian vs benign, P = .08; nocturnal cortisol: ovarian vs healthy, P < .0001; ovarian vs benign, P = .024). When secondary analyses were performed using the generalized estimating equation model, between-group differences for nocturnal cortisol were even more pronounced, with cortisol of ovarian cancer patients on average 67% (SE = 25.2%) higher than that of women with benign disease (P = .004) and 137% (SE = 32.7%) higher than among healthy women (P < .0001). However, using the generalized estimating equation model, statistically significant differences between groups did not emerge for afternoon cortisol (P values > 0.2), and thus afternoon cortisol findings should be interpreted with caution. Cortisol variability of ovarian cancer patients was significantly lower than that of both benign patients (P = .023) and healthy women (P < .0001), indicating a less marked diurnal rhythm in cancer patients (Fig. 1).
Figure 2.
Diurnal cortisol levels are shown among ovarian cancer patients (n = 100), patients with benign pelvic disease (n = 77), and healthy women (n = 33). Cortisol means were age-adjusted and back-transformed from the natural log values. Ovarian cancer patients demonstrated significantly higher afternoon and nocturnal cortisol than healthy controls (P < .0001), and significantly higher nocturnal cortisol than women with benign disease (P = .022). Error bars represent the standard error. All P values were Bonferroni corrected for multiple comparisons.
Cortisol and Quality of Life Among Ovarian Cancer Patients
Means and standard deviations of psychosocial measures are shown in Table 2 and correlations in Table 3. Correlations are adjusted for age and disease stage. Among women with ovarian cancer, greater diurnal cortisol dysregulation was associated with indicators of more severe functional disability. Specifically, patients with higher levels of nocturnal cortisol had significantly greater fatigue (P = .005), and poorer physician-rated (P = .043) and patient-rated performance status (P = .035). Lower cortisol variability was also associated with greater fatigue (P = .01), poorer physician-rated (P = .010) and patient-rated performance status (P = .004), and poorer physical well-being (P = .007). The Center for Epidemiological Studies Depression scale total depression score was associated with higher nocturnal cortisol (P = .059) and lower cortisol variability (P = .028). It was specifically the vegetative component of depression (nocturnal cortisol: r = 0.30, P = .004; cortisol variability: r = 0.30, P = .005) rather than the affective component (nocturnal cortisol: r = 0.12, P = .28; cortisol variability: r = 0.16, P = .14) that showed significant links to cortisol. Otherwise, no measure of stress or distress was significantly associated with cortisol (all P values >.12).
Table 2.
Means and Standard Deviations for Psychosocial Measures
| Measure | Ovarian Cancer, n=100, Mean (SD) | Benign Disease, n=77, Mean (SD) |
|---|---|---|
| FACT physical well-beinga | 20.77 (5.66) | 22.20 (5.83) |
| Physician’s rating of functioningb | 0.41 (0.55) | 0.21 (0.44) |
| Patient’s rating of functioningc | 0.81 (0.89) | 0.47 (0.81) |
| POMS fatigue | 7.71 (5.47) | 6.70 (5.56) |
| CES-D | 15.58 (8.66) | 15.04 (10.68) |
| CES-D vegetative | 6.50 (3.80) | 6.26 (4.71) |
| CES-D depressed mood | 3.55 (3.18) | 3.70 (3.47) |
| POMS distress | 16.46 (10.67) | 16.45 (12.61) |
| Perceived Stress Scale | 20.65 (6.07) | 22.15 (8.00) |
| No. of life events (LES)b | 2.23 (2.18) | 3.04 (2.65) |
| Severity of life events (LES) | 4.24 (4.26) | 5.30 (5.03) |
SD indicates standard deviation; FACT, Functional Assessment of Cancer Therapy; POMS, Profile of Mood States; CES-D, Center for Epidemiological Studies Depression scale; LES, Life Experiences Survey.
P <.001.
P <.05.
P <.01.
Table 3.
Adjusteda Pearson Correlations Between Cortisol Values and Psychosocial Measures
| Measure | Ovarian Cancer Patients | Benign Patients | ||
|---|---|---|---|---|
| Nocturnal Cortisol | Cortisol Variability | Nocturnal Cortisol | Cortisol Variability | |
| FACT physical well-being | −0.17 | 0.29b | −0.02 | 0.21 |
| Physician’s rating of functioning | 0.21c | −0.28b | −0.10 | −0.01 |
| Patient’s rating of functioning | 0.22c | −0.31b | 0.14 | −0.10 |
| POMS fatigue | 0.29b | −0.28b | 0.02 | −0.08 |
| CES-D | 0.20 | −0.26c | −0.14 | 0.07 |
| CES-D vegetative subscale | 0.30b | −0.30b | −0.19 | 0.14 |
| CES-D depression subscale | 0.11 | −0.16 | −0.17 | 0.15 |
| POMS distress | 0.07 | −0.14 | −0.03 | 0.02 |
| Perceived Stress Scale | 0.03 | −0.16 | −0.10 | 0.11 |
| No. of life events (LES) | −0.02 | −0.15 | −0.01 | −0.01 |
| Severity of life events (LES) | −0.04 | −0.10 | −0.01 | 0.06 |
FACT indicates Functional Assessment of Cancer Therapy; POMS, Profile of Mood States; CES-D, Center for Epidemiological Studies Depression scale; LES, Life Experiences Survey.
Ovarian cancer patient correlations are adjusted for stage and age; correlations for patients with benign disease are adjusted for age.
P ≤ .01.
P <.05.
Cortisol and Quality of Life Among Patients With Benign Disease
In benign patients, relationships with cortisol variables were less robust, with no associations observed between functional, depression, or stress variables and nocturnal cortisol or cortisol variability (all P’s >.09), adjusting for age.
DISCUSSION
The key findings of this study are that ovarian cancer patients demonstrate substantially altered diurnal cortisol rhythms, with significantly higher nocturnal cortisol levels and blunted cortisol variability compared with patients with benign disease or healthy women. These results extend previous research reporting altered diurnal cortisol profiles in patients with a variety of cancers.1,14,38 Both morning and afternoon cortisol levels increased with increasing cancer stage; nocturnal cortisol levels were substantially elevated in all stages.
Cortisol dysregulation was associated with poorer physical well-being and performance status, and higher levels of fatigue, overall depression, and vegetative depression in women with ovarian cancer. In women with benign disease, relationships between stress or functional variables and cortisol were not observed. These findings extend our previous report of a relation between total depression, vegetative depression, and nocturnal cortisol elevations in ovarian cancer,20 and are consistent with previous reports linking depression with hypercortisolemia, down-regulation of glucocorticoid receptors, and dysregulation of the HPA axis.39 HPA alterations in breast cancer survivors have also been linked with fatigue, with the highest levels of fatigue observed in those survivors with the flattest cortisol slopes.4 Several syndromes characterized by pervasive and persistent fatigue are associated with diurnal cortisol alterations, including fibromyalgia40 and chronic fatigue syndrome.41 It is possible that HPA dysregulation may have direct effects on fatigue and functional disturbances, as cortisol is intimately linked to energy production and regulation, along with many other vital bodily functions.8,42
Although it has been proposed that stress may be a contributing factor to dysregulation of diurnal cortisol patterns in cancer patients,1 in the current study, stress was not associated with cortisol levels in either group of patients. Previous findings have shown metastatic breast cancer patients to have flatter diurnal cortisol profiles than healthy women, with no association between cortisol slope and stress. In contrast, among healthy women, flatter cortisol slopes were associated with greater perceived stress.38 In metastatic breast cancer patients, flatter diurnal cortisol slopes were also linked with escape from dexamethasone suppression but not with acute stress reactivity, suggesting a role for dysregulated feedback inhibition rather than heightened stress responsiveness in these patients.43 These findings, in combination with the current results, suggest that alterations in diurnal cortisol rhythm may have different physiological underpinnings in women with cancer than in healthy women. It may be that in patients with advanced disease, such as the majority of the ovarian cancer patients in this study, the contribution of disease processes overshadows the contribution of stress to cortisol regulation.
Several disease-related mechanisms may underlie disrupted cortisol feedback inhibition in ovarian cancer. Ovarian cancer cells release high levels of proinflammatory cytokines, such as interleukin-6 (IL-6),44 which, along with other proinflammatory cytokines, have potent effects on the HPA axis and may stimulate the HPA axis to the point that its regulatory mechanisms cannot contain cortisol levels within normal limits, particularly at night when negative feedback should be most potent.45 Consistent with this possibility, we have previously noted positive correlations between IL-6 and nocturnal cortisol in ovarian cancer patients.20 Proinflammatory cytokines have been implicated in vegetative behavioral responses, including fatigue, changes in appetite and sleep, anhedonia, and behavioral deactivation; these encompass several of the disabilities noted here.46 Thus, disease-related processes may set off an inflammatory cascade that both modulates the HPA axis and results in vegetative sequelae, including fatigue and functional disturbances as well as affective symptoms. Depression may also potentiate inflammatory processes in the tumor microenvironment,47,48 an effect that would ultimately contribute to greater HPA dysregulation. Further investigation is needed to specify both direct and indirect mechanisms relating HPA alterations to fatigue and functional disabilities.
There are other pathways by which disease-related processes may lead to changes in the HPA axis. Alterations in diurnal cortisol could be the result of overall changes in circadian rhythms that are associated with cancer progression,1 although relevant mechanisms are not known. Notably, proinflammatory cytokines such as interleukin-1β and tumor necrosis factor-α decrease the expression of mRNAs for clock genes in animal models, leading to effects such as the flattening of the diurnal rhythm of activity.49,50
Alterations in diurnal cortisol may have implications for a variety of outcomes in cancer patients. For example, dysregulated diurnal endocrine rhythms have been associated with alterations in circadian patterns of immune cell trafficking.51 Higher mean diurnal cortisol has been associated with suppressed cellular immunity in advanced breast cancer patients.52 Another line of research has suggested that patients with altered endocrine rhythms may not derive full benefit from chemotherapy.53,54 There are also direct relationships between glucocorticoids and cancer growth. For example, glucocorticoids directly enhance a survival pathway and inhibit apoptosis of a mammary tumor cell line,55 down-regulate expression of DNA repair genes including BRCA1,56 and decrease paclitaxel-induced apoptosis in a mammary cancer cell line.57 Thus, alterations in diurnal cortisol may have long-term implications for immune function, response to treatment, and survival.
The current findings should be viewed in light of certain limitations. It is unclear to what extent cortisol levels among ovarian cancer patients may have been influenced by the stress of anticipating surgery. Nocturnal cortisol among ovarian cancer patients was higher than that of patients with benign disease who were also facing surgery; thus, differences between these groups likely reflect differences related to disease processes. Diurnal cortisol levels in noncancer patients anticipating surgery (lumbar disk) were commensurate with our healthy controls, suggesting that the values observed here are not just secondary to surgical stress, (although cortisol of lumbar patients was assayed by radioimmunoassay, which limits interpretations of equivalence to current results).58 In addition, it should be noted that a large proportion of eligible patients did not collect presurgical cortisol. About a third of these had emergent surgery and did not have the necessary presurgical time for cortisol collection. Others declined cortisol collection because of the stressful nature of the presurgical period. Thus, generalizability may be limited. This study is cross-sectional and correlational, and therefore causal interpretations are speculative. Although sampling of diurnal cortisol can shed light on possible dysregulation of HPA feedback mechanisms, greater specificity regarding feedback disturbances requires more specific stimulation studies for confirmation.9 It should also be noted that cortisol signaling can be most fully understood in combination with data on tissue sensitivity to glucocorticoids, which are not available for these patients.
The role of cortisol in the stress response is well known. Given that cancer diagnosis is a major stressor, psychological factors have been posited to play an important role in cortisol dysregulation in cancer patients. An alternative pathway to dysregulated diurnal cortisol may be implicated as well, however, as the current study and several previous investigations suggest that diurnal cortisol dysregulation among cancer patients may have more to do with impaired feedback inhibition and/or inflammatory pathways than stress sensitivity. Further investigation is needed into causes and consequences of the observed diurnal cortisol dysregulation in ovarian cancer patients, as this dysregulation may have implications for functional disability as well as treatment and prognosis.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
Funding for this study was provided by National Institutes of Health (NIH) grants R01CA104825, R21CA88293, and R01CA140933 to Susan Lutgendorf; the NIH had no further role in study design; in collection, analysis, and interpretation of data; in writing of the report; or in the decision to submit the paper for publication.
References
- 1.Sephton S, Spiegel D. Circadian disruption in cancer: a neuroendocrine-immune pathway from stress to disease? Brain Behav Immun. 2003;17:321–328. doi: 10.1016/s0889-1591(03)00078-3. [DOI] [PubMed] [Google Scholar]
- 2.Touitou Y, Bogdan A, Levi F, Benavides M, Auzeby A. Disruption of the circadian patterns of serum cortisol in breast and ovarian cancer patients: relationships with tumour marker antigens. Br J Cancer. 1996;74:1248–1252. doi: 10.1038/bjc.1996.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Touitou Y, Levi F, Bogdan A, Benavides M, Bailleul F, Misser J-L. Rhythm alteration in patients with metastatic breast cancer and poor prognostic factors. J Cancer Res Clin. 1995;121:181–188. doi: 10.1007/BF01198101. [DOI] [PubMed] [Google Scholar]
- 4.Bower JE, Ganz PA, Dickerson SS, Petersen L, Aziz N, Fahey JL. Diurnal cortisol rhythm and fatigue in breast cancer survivors. Psychoneuroendocrinology. 2005;30:92–100. doi: 10.1016/j.psyneuen.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Sephton S, Sapolsky RM, Kraemer HC, Speigel D. Diurnal cortisol rhythm as a predictor of breast cancer survival. J Natl Cancer Inst. 2000;92:994–1000. doi: 10.1093/jnci/92.12.994. [DOI] [PubMed] [Google Scholar]
- 6.Buijs RM, van Eden CG, Goncharuk VD, Kalsbeek A. Circadian and season rhythms: the biological clock tunes the organs of the body: timing by hormones and the autonomic nervous system. J Endocrinol. 2003;177:17–26. doi: 10.1677/joe.0.1770017. [DOI] [PubMed] [Google Scholar]
- 7.Kirschbaum C, Hellhammer DH. Salivary cortisol. In: Fink G, editor. Encyclopedia of Stress. Vol. 3. Burlington, MA: Academic Press; 2000. [Google Scholar]
- 8.Maier S, Watkins L. Cytokines for psychologists: implications of bidirectional immune to brain communication for understanding behavior, mood, and cognition. Psychol Rev. 1998;105:83–107. doi: 10.1037/0033-295x.105.1.83. [DOI] [PubMed] [Google Scholar]
- 9.Kirschbaum C, Hellhammer DH. Salivary cortisol in psychobiological research: an overview. Neuropsychobiology. 1989;22:150–169. doi: 10.1159/000118611. [DOI] [PubMed] [Google Scholar]
- 10.Smyth J, Ockenfels M, Gorin A, et al. Individual differences in the diurnal cycle of cortisol. Psychoneuroendocrinology. 1997;22:89–105. doi: 10.1016/s0306-4530(96)00039-x. [DOI] [PubMed] [Google Scholar]
- 11.Sapolsky RM, Alberts SC, Altmann J. Hypercortisolism associated with social subordinance or social isolation among wild baboons. Arch Gen Psychiatry. 1997;54:1137–1143. doi: 10.1001/archpsyc.1997.01830240097014. [DOI] [PubMed] [Google Scholar]
- 12.Chrousos GP, Gold PW. A healthy body in a healthy mind-and vice versa-the damaging power of “uncontrollable” stress. J Clin Endocrinol Metab. 1998;83:1842–1845. doi: 10.1210/jcem.83.6.4908. [DOI] [PubMed] [Google Scholar]
- 13.Deuschle M, Schweiger U, Weber B, et al. Diurnal activity and pulsatility of the hypothalamus-pituitary-adrenal system in male depressed patients and healthy controls. J Clin Endocrinol Metab. 1997;82:234–238. doi: 10.1210/jcem.82.1.3689. [DOI] [PubMed] [Google Scholar]
- 14.Jehn CF, Kuehnhardt D, Bartholomae A, et al. Biomarkers of depression in cancer patients. Cancer. 2006;107:2723–2729. doi: 10.1002/cncr.22294. [DOI] [PubMed] [Google Scholar]
- 15.Turner-Cobb J, Sephton S, Koopman C, Blake-Mortimer J, Spiegel D. Social support and salivary cortisol in women with metastatic breast cancer. Psychosom Med. 2000;62:337–345. doi: 10.1097/00006842-200005000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Sapolsky RM, Donnelly TM. Vulnerability to stress-induced tumor growth increases with age in rats: role of glucocorticoids. Endocrinology. 1985;117:662–666. doi: 10.1210/endo-117-2-662. [DOI] [PubMed] [Google Scholar]
- 17.Filipski E, King VM, Li X, et al. Host circadian clock as a control point in tumor progression. J Natl Cancer Inst. 2002;94:690–697. doi: 10.1093/jnci/94.9.690. [DOI] [PubMed] [Google Scholar]
- 18.Schernhammer ES, Laden F, Speizer FE, et al. Rotating night shifts and risk of breast cancer in women participating in nurses’ health study. J Natl Cancer Inst. 2001;93:1563–1568. doi: 10.1093/jnci/93.20.1563. [DOI] [PubMed] [Google Scholar]
- 19.Mormont MC, Levi F. Circadian-system alterations during cancer processes: a review. Int J Cancer. 1997;70:241–247. doi: 10.1002/(sici)1097-0215(19970117)70:2<241::aid-ijc16>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 20.Lutgendorf SK, Weinrib AZ, Penedo F, et al. Interleukin-6, cortisol, and depressive symptoms in ovarian cancer patients. J Clin Oncol. 2008;26:4820–4827. doi: 10.1200/JCO.2007.14.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thakore JH, Barnes C, Joyce J, Medbak S, Dinan TG. Effects of anti-depressant treatment on corticotropin-induced cortisol responses in patients with melancholic depression. Psychiatry Res. 1997;73:27–32. doi: 10.1016/s0165-1781(97)00106-6. [DOI] [PubMed] [Google Scholar]
- 22.Basen-Engquist K, Bodurka-Bevers D, Fitzgerald MA, et al. Reliability and validity of the Functional Assessment of Cancer Therapy-Ovarian. J Clin Oncol. 2001;19:1809–1817. doi: 10.1200/JCO.2001.19.6.1809. [DOI] [PubMed] [Google Scholar]
- 23.Gynecologic Oncology Group. Protocol Procedures Manual. Appendix 10. Nov, 2005. [Google Scholar]
- 24.McNair DM, Lorr M, Droppelman LF. EdiTS Manual for the Profile of Mood States. San Diego, CA: EITS; 1992. [Google Scholar]
- 25.Andersen B, Anderson B, deProsse C. Controlled prospective longitudinal study of women with cancer: I. Psychological functioning and outcomes. J Consult Clin Psychol. 1989;57:683–691. doi: 10.1037//0022-006x.57.6.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Radloff L. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 27.Sheehan TJ, Fifield J, Reisine S, Tennen H. The measurement structure of the Center for Epidemiologic Studies depression scale. J Pers Assess. 1995;64:507–521. doi: 10.1207/s15327752jpa6403_9. [DOI] [PubMed] [Google Scholar]
- 28.Cohen S, Kamarck T, Mermelstein R. Global measure of perceived stress. J Health Hum Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 29.Stone AA, Mezzacappa E, Donatone B, Gonder M. Psychosocial stress and social support are associated with prostate-specific antigen levels in men: results from a community screening program. Health Psychol. 1999;18:482–486. doi: 10.1037//0278-6133.18.5.482. [DOI] [PubMed] [Google Scholar]
- 30.Sarason IG, Johnson JH, Siegel JM. Assessing the impact of life changes: development of the Life Experiences Survey. J Consult Clin Psychol. 1978;46:932–946. doi: 10.1037//0022-006x.46.5.932. [DOI] [PubMed] [Google Scholar]
- 31.Leserman J, Whetten K, Lowe K, Stangl D, Swartz MS, Thielman NM. How trauma, recent stressful events, and PTSD affect functional health status and health utilization in HIV-infected patients in the south. Psychosom Med. 2005;67:500–507. doi: 10.1097/01.psy.0000160459.78182.d9. [DOI] [PubMed] [Google Scholar]
- 32.Kraemer HC, Giese-Davis J, Yutsis M, et al. Design decisions to optimize reliability of daytime cortisol slopes in an older population. Am J Geriatr Psychiatry. 2006;14:325–333. doi: 10.1097/01.JGP.0000201816.26786.5b. [DOI] [PubMed] [Google Scholar]
- 33.Giese-Davis J, Wilhelm FH, Conrad A, et al. Depression and stress reactivity in metastatic breast cancer. Psychosom Med. 2006;68:675–683. doi: 10.1097/01.psy.0000238216.88515.e5. [DOI] [PubMed] [Google Scholar]
- 34.Deuschle M, Gotthardt U, Schweiger U, et al. With aging in humans the activity of the hypothalamus-pituitary-adrenal system increases and its diurnal amplitude flattens. Life Sci. 1997;61:2246–2249. doi: 10.1016/s0024-3205(97)00926-0. [DOI] [PubMed] [Google Scholar]
- 35.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 36.File SE. Interactions of anxiolytic and antidepressant drugs with hormones of the hypothalamic-pituitary-adrenal axis. Pharmacol Ther. 1990;46:357–395. doi: 10.1016/0163-7258(90)90024-v. [DOI] [PubMed] [Google Scholar]
- 37.Skelton KH, Nemeroff CB, Knight DL, Owens MJ. Chronic administration of the triazolobenzodiazepine alprazolam produces opposite effects on corticotropin-releasing factor and urocortin neuronal systems. J Neurosci. 2000;20:1240–1248. doi: 10.1523/JNEUROSCI.20-03-01240.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abercrombie HC, Giese-Davis J, Sephton S, Epel ES, Turner-Cobb JM, Spiegel D. Flattened cortisol rhythms in metastatic breast cancer patients. Psychoneuroendocrinology. 2004;29:1082–1092. doi: 10.1016/j.psyneuen.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 39.Raison CL, Miller AH. When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry. 2003;160:1554–1565. doi: 10.1176/appi.ajp.160.9.1554. [DOI] [PubMed] [Google Scholar]
- 40.McCain GA, Tilbe KS. Diurnal hormone variation in fibromyalgia syndrome: a comparison with rheumatoid arthritis. J Rheumatol. 1989;(19 suppl):154–157. [PubMed] [Google Scholar]
- 41.Nater UM, Youngblood LS, Jones JF, et al. Alterations in diurnal salivary cortisol rhythm in a population-based sample of cases with Chronic Fatigue Syndrome. Pyschosomatic Medicine. 2008;70:298–305. doi: 10.1097/PSY.0b013e3181651025. [DOI] [PubMed] [Google Scholar]
- 42.Baxter JD, Frohman LA, Felig P. Introduction to the endocrine system. In: Felig P, Baxter JD, Frohman LA, editors. Endocrinology and Metabolism. 3. New York, NY: McGraw-Hill; 1995. pp. 3–20. [Google Scholar]
- 43.Spiegel D, Giese-Davis J, Taylor CB, Kraemer H. Stress sensitivity in metastatic breast cancer: analysis of hypothalamic-pituitary-adrenal axis function. Psychoneuroendocrinology. 2006;31:1231–1244. doi: 10.1016/j.psyneuen.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Watson JM, Sensintaffar JL, Berek JS, Martinez-Maza O. Constitutive production of interleukin 6 by ovarian cancer cell lines and by primary ovarian tumor cultures. Cancer Res. 1990;50:6959–6965. [PubMed] [Google Scholar]
- 45.Chrousos GP. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med. 1995;332:1351–1362. doi: 10.1056/NEJM199505183322008. [DOI] [PubMed] [Google Scholar]
- 46.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–57. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hughes JW, Watkins L, Blumenthal JA, Kuhn C, Sherwood A. Depression and anxiety symptoms are related to increased 24-hour urinary norepinephrine excretion among healthy middle-aged women. J Psychosom Res. 2004;57:353–358. doi: 10.1016/j.jpsychores.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 48.Nilsson MB, Armaiz-Pena G, Takahashi R, et al. Stress hormones regulate IL-6 expression by human ovarian carcinoma cells through a SRC-dependent mechanism. J Biol Chem. 2007;282:29919–29926. doi: 10.1074/jbc.M611539200. [DOI] [PubMed] [Google Scholar]
- 49.Cavadini G, Petrzilka S, Kohler P, et al. TNF-alpha suppresses the expression of clock genes by interfering with E-box-mediated transcription. Proc Natl Acad Sci U S A. 2007;104:12843–12848. doi: 10.1073/pnas.0701466104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ohdo S, Koyanagi S, Suyama H, Higuchi S, Aramaki H. Changing the dosing schedule minimizes the disruptive effects of interferon on clock function. Nat Med. 2001;7:356–360. doi: 10.1038/85507. [DOI] [PubMed] [Google Scholar]
- 51.Kronfol Z, Nair M, Zhang Q, Hill EE, Brown MB. Circadian immune measures in healthy volunteers: relationship to hypothalamic-pituitary-adrenal axis hormones and sympathetic neurotransmitters. Psychosom Med. 1997;59:42–50. doi: 10.1097/00006842-199701000-00006. [DOI] [PubMed] [Google Scholar]
- 52.Sephton SE, Dhabhar FS, Keuroghlian AS, et al. Depression, cortisol, and suppressed cell-mediated immunity in metastatic breast cancer. Brain Behav Immun. 2009;23:1148–1155. doi: 10.1016/j.bbi.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 53.Mormont M-C, Hecquet B, Bogdan A, Benavides M, Touitou Y, Levi F. Non-invasive estimation of the circadian rhythm in serum cortisol in patients with ovarian or colorectal cancer. Int J Cancer. 1998;78:421–424. doi: 10.1002/(sici)1097-0215(19981109)78:4<421::aid-ijc5>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 54.Levi F. Circadian chronotherapy for human cancers. Lancet. 2001;2:307–315. doi: 10.1016/S1470-2045(00)00326-0. [DOI] [PubMed] [Google Scholar]
- 55.Moran TJ, Gray S, Mikosz CA, Conzen SD. The glucocorticoid receptor mediates a survival signal in human mammary epithelial cells. Cancer Res. 2000;60:867–872. [PubMed] [Google Scholar]
- 56.Antonova L, Mueller CR. Hydrocortisone down-regulates the tumor suppressor gene BRCA1 in mammary cells: a possible molecular link between stress and breast cancer. Genes Chromosomes Cancer. 2008;47:341–352. doi: 10.1002/gcc.20538. [DOI] [PubMed] [Google Scholar]
- 57.Pang D, Kocherginsky M, Krausz T, Kim SY, Conzen SD. Dexamethasone decreases xenograft response to Paclitaxel through inhibition of tumor cell apoptosis. Cancer Biol Ther. 2006;5:933–940. doi: 10.4161/cbt.5.8.2875. [DOI] [PubMed] [Google Scholar]
- 58.Johansson AC, Gunnarsson LG, Linton SJ, et al. Pain, disability and coping reflected in the diurnal cortisol variability in patients scheduled for lumbar disc surgery. Eur J Pain. 2008;12:633–640. doi: 10.1016/j.ejpain.2007.10.009. [DOI] [PubMed] [Google Scholar]