Abstract
The AKT family, comprising three highly homologous kinases, is an essential mediator of the PTEN/PI3K pathway, which is deregulated in many human cancers. A thorough understanding of the specific activities of each isoform in normal and disease tissues is lacking. We evaluated the role of each Akt isoform in gliomagenesis using a model system driven by common glioma abnormalities, loss of function of p53 and Pten, and expression of EGFRvIII. Pten deletion and EGFRvIII expression both accelerated the proliferation of p53-null primary murine astrocytes. All three Akt isoforms were expressed and phosphorylated in astrocytes, with significantly higher activation in Pten-null cells. Despite substantial compensation in many contexts when individual Akt isoforms were inhibited, isoform-specific effects were also identified. Specifically, loss of Akt1 or Akt2 decreased proliferation of Pten wild-type astrocytes, while combined loss of multiple isoforms was needed to inhibit proliferation of Pten-null astrocytes. In addition, Akt3 was required for anchorage-independent growth of transformed astrocytes and human glioma cells, and Akt3 loss inhibited invasion of transformed astrocytes. EGFRvIII expression transformed p53-null astrocytes with or without Pten deletion, causing rapid development of high-grade astrocytoma upon intracranial transplantation. Furthermore, tumorigenesis of Pten;p53-null astrocytes expressing EGFRvIII was delayed by Akt1 loss and accelerated by Akt2 loss. Taken together, these results indicate context-dependent roles for individual Akt isoforms and suggest that there may be heterogeneous tumor response to isoform-specific inhibitors.
Keywords: Akt, Pten, astrocyte, glioblastoma
Introduction
Glioblastomas are the most common malignant brain tumors and the most aggressive of the astrocytic tumors (WHO Grade IV) (1). Virtually all glioblastomas share disruption of three major pathways, namely the receptor tyrosine kinase (RTK)/phosphatidylinositol 3′-kinase (PI3K), p53 and retinoblastoma (Rb)-signaling pathways (2, 3).
The epidermal growth factor receptor (EGFR) is the most commonly mutated RTK in glioblastoma, including the recurrent activating EGFRvIII mutation (4). Activated EGFR signals to multiple effectors including phosphatidylinositol-3-kinase (PI3K) (5). The tumor suppressor PTEN is the sole direct negative regulator of PI3K signaling (6), and PTEN loss of function is frequent in glioblastomas (2, 3). Furthermore, simultaneous mutations in EGFR, PTEN and the p53 pathway have been reported in human glioblastomas (2). The AKT serine/threonine kinases are critical downstream mediators of PI3K signaling, and are among the most frequently hyperactivated kinases in human cancer (7). They not only suppress apoptosis and stimulate proliferation, but also influence invasion, metastasis and cellular metabolism (8). There are three closely related AKT isoforms (AKT1/PKBα, AKT2/PKBβ and AKT3/PKBγ) encoded by separate genes. All three are activated similarly by phosphorylation at two sites: a threonine within the activation loop of the kinase domain (T308, T309, T305 in AKT1, AKT2 and AKT3, respectively; herein referred to as T308) and a serine within the hydrophobic domain (S473, S474, S472 in AKT1, AKT2 and AKT3, respectively, herein referred to as S473). Active AKT phosphorylates multiple targets to mediate its effects on cellular function, including the Forkhead box class O (FOXO) factors, GSK3 isoforms and tuberous sclerosis complex 2 (TSC2), which regulates mTORC1 activity (8). The multiple substrates of AKT are not all targeted in every setting. It is likely that AKT differentially phosphorylates certain substrates depending on the stimulus and cellular context.
While the AKT isoforms have many overlapping substrates and functions, the ablation of individual isoforms in mice reveals distinct physiological roles. Akt1 knockout mice have reduced body size and cell size, Akt2 deletion causes a diabetic phenotype and Akt3 knockouts have reduced brain size (9–14). These isoform-specific phenotypes are likely to be due in part to tissue-specific expression of the isozymes and further influenced by isoform-specific substrates. Sequestration into different subcellular compartments may also regulate isoform specificity (15).
Cancer-associated mutations targeting factors upstream of AKT, such as PTEN loss of function, RTK/PI3K activation or RAS mutation should result in activation of all three isoforms, however the relative contribution of each isoform to oncogenic signaling remains unclear. Expression of myristoylated AKT1, which is constitutively active, induced glioma in mice when combined with oncogenic RAS (16). In tissues outside the central nervous system, Akt1 deficiency was sufficient to inhibit tumorigenesis in Pten heterozygous mice suggesting a predominant role for Akt1 in cancer (17, 18). However, in glioma there are reports of mutations of each individual AKT isoform indicating that they may all have oncogenic potential in gliomagenesis. For example, AKT1 or AKT3 amplifications with concurrent EGFR amplification were identified in human glioblastomas and increased AKT2 expression was reported in high-grade, compared to lower grade, gliomas (2, 19). The biological specificity of the different AKT isoforms is poorly understood.
In the present study, we evaluated the unique contributions of each Akt family member to proliferation, transformation and tumorigenicity in primary murine astrocytes (PMAs) containing mutations in EGFR, Pten and/or p53. This allows analysis of gene function in a genetically defined system with relevance to human glioma.
Methods
Transgenic and knockout mouse lines
Mouse experiments were approved by the Institutional Animal Care and Use Committee. GFAP-cre transgenic mice (20) were used to drive expression of cre recombinase in astrocytes, and were intercrossed with PtenloxP/loxP mice (21), Trp53loxP/loxP mice (22) and Akt1+/− mice (9) to generate GFAPcre;Pten+/+;Trp53loxP/loxP (p53cKO), GFAPcre;PtenloxP/loxP;Trp53loxP/loxP (PtencKO;p53cKO ), GFAPcre;Pten+/+;Trp53loxP/loxP; Akt1−/−(p53cKO;Akt1KO) or GFAPcre;PtenloxP/loxP;Trp53loxP/loxP; Akt1−/− (PtencKO;p53cKO;Akt1KO) mice from which PMA cultures were made.
Cell culture and proliferation assays
PMA cultures were established from 2 day old mice as described (23) and used before passage 10. For growth curves, 2×106 cells were plated per 100 mm dish, then trypsinized, counted and replated every 2 days.
Human glioblastoma cell lines T98G and U87MG were obtained from ATCC more than five years ago, and authenticated in November, 2010 at the Genetics Resources Core facility at The Johns Hopkins University using the PowerPlex 1.2 system as described (http://faf.grcf.jhmi.edu/str.html).
Retroviral and lentiviral production and infection
The cDNA encoding EGFRvIII was cloned into the MSCV-IRES-GFP (MIG) retroviral vector (24). Akt1 and Akt3 open reading frames (ORFs) were amplified from NIH3T3 cDNA. Relevant mutations to generate kinase-dead Akt3 (K177A) and shRNA-insensitive (rescue) constructs were generated by PCR, and Akt ORFs were cloned into MSCV-IRES-YFP (MIY). Retrovirus was produced by transfected 293T cells and used to transduce early passage PMAs in 4 μg/mL polybrene. Lentiviral vectors expressing Akt isoform-specific short-hairpin RNAs (shRNAs) and empty vector (pLKO.1) were from Open Biosystems. Lentivirus was produced as described (25). PMAs were transduced as above, and after 48 hours cells were selected with 5 μg/mL puromycin for 5 days.
Anchorage-independent growth and invasion assays
For soft agar assays, PMAs (106) or GBM cell lines (T98G or U87-MG, 105) were resuspended in media containing 0.3% noble agar and plated onto a layer of 0.6% agarose-containing medium in a 35 mm dish. After 10–14 days, colonies greater than 50 μm were counted in 50 random fields corresponding to an area of 30 mm2.
Cell invasion assays were performed using cell culture inserts containing 8 μm pores coated with matrigel (BD Biosciences). PMAs were starved overnight and seeded in DMEM/F12 containing 0.1% FBS with DMEM/F12 containing 10% FBS and 20 ng/mL EGF in the lower chamber. 17 hours later, noninvading cells were removed and invading cells were stained and counted. Percentage of invaded cells compared to control wells without matrigel are presented.
Protein analyses
Protein lysates were prepared in RIPA buffer containing protease and phosphatase inhibitor tablets (Roche). Phospho-Akt S473 immunoprecipitations (IPs) were performed using 100 μg total protein with 2 μL antibody (4051, Cell Signaling) and protein-G agarose (Pierce). IPs or whole cell extracts (20 μg) were separated using precast gels and transferred to PVDF (Invitrogen). Antibodies for phospho-Akt S473 (9271), phospho-Akt T308 (9275), Akt1 (2938), Akt2 (2964), pan Akt (4691), Pten (9559) and EGFR (2235) were from Cell Signaling. Akt3-specific antibody (07-383) was from Upstate and anti-Actin (A5441) was from Sigma. Chemiluminescent detection was performed using horseradish peroxidase-coupled secondary antibodies with ECL (GE) or SuperSignal West Dura (Pierce).
Intracranial implantation of astrocytes
PMA for injection (105 cells) were suspended in matrigel (BD), and implanted into the parietal lobe of six to eight week old athymic mice (CD-1 nude, Charles River Laboratories). Kaplan-Meier analyses were measured as the number of days post-implantation before tumors caused morbidity requiring euthanasia using Prism software.
Tissue collection and immunohistochemistry
Mice were anesthetized and perfused with PBS. Tumor was dissected, with part collected for protein analyses and the remainder fixed in 4% paraformaldehyde in PBS, processed, embedded in paraffin and cut into 5 μm sections. Hematoxylin and Eosin (H&E) stained sections were evaluated for histopathological features and graded using WHO criteria (DWE) (1). Immunohistochemistry (IHC) was with antibodies for EGFR (VP-E605, Vector Laboratories), Pten (9559, Cell Signaling), phospho-Akt S473 (9271, Cell Signaling), Gfap (G3893, Sigma), Nestin (MAB5326, Chemicon) and Ki67 (NCL-Ki67p, Novocastra). For quantification of Ki67, four randomly selected fields (400×) were analyzed from at least three tumors per group. The percentage of positive nuclei were counted manually using Image J software.
Results
Compensatory regulation among Akt isoforms in astrocytes
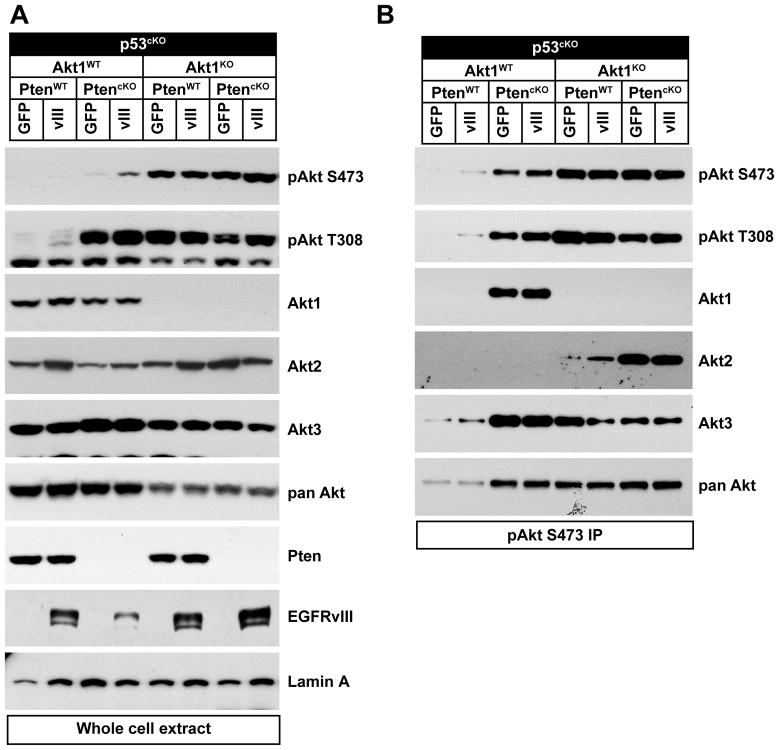
Due to its central role in the PI3K pathway, Akt activation is likely to mediate many effects of Pten deletion in glioma, however the contribution of individual Akt isoforms remains to be defined. PMAs were isolated from the cortex of p53cKO or PtencKO;p53cKO mice. All three Akt isoforms were expressed in PMAs as shown by immunoblotting with isoform specific antibodies (Figure 1A), consistent with previous reports of AKT isoform expression in human GBM samples and cell lines (19, 26). To study the contribution of Akt1 signaling in PMAs, PtencKO;p53cKO mice were bred onto an Akt1 knockout background (9). PMAs were transduced with retrovirus that drove expression of both EGFRvIII and GFP (herein referred to as EGFRvIII), or with a control retrovirus expressing only GFP to examine the role of each Akt isoform in a context of oncogenic signaling relevant to glioma. Phospho-specific antibodies that distinguish each Akt isoform are not available, therefore S473-phosphorylated Akt immunoprecipitates were probed using isoform-specific antibodies. Pten deletion induced elevated phospho-Akt levels as expected, and all three Akt isoforms were phosphorylated (Figure 1B and 2B). The antibody for Akt2 was relatively less sensitive than for other isoforms, so phosphorylation of Akt2 in Akt1 wt PMAs was only seen upon longer exposure (see Fig. 2B). p53 deletion did not induce any difference in Akt expression or activation compared to wild-type PMAs (not shown). Unexpectedly, PMAs deficient for Akt1 had increased levels of phosphorylated Akt compared to Akt1 wild-type cells due to increased phosphorylation of Akt2 without compensatory increase in Akt3 (Fig. 1A,B).
Figure 1. All Akt isoforms were expressed in primary astrocytes.
PMAs were isolated from p53cKO or PtencKO;p53cKO mice with or without Akt1 deletion (Akt1 KO) and transduced with control (GFP) or EGFRvIII (vIII) expressing retrovirus. (A) Immunoblots were performed with the indicated antibodies. (B) Phospho-Akt (S473) immunoprecipitates were immunoblotted as indicated.
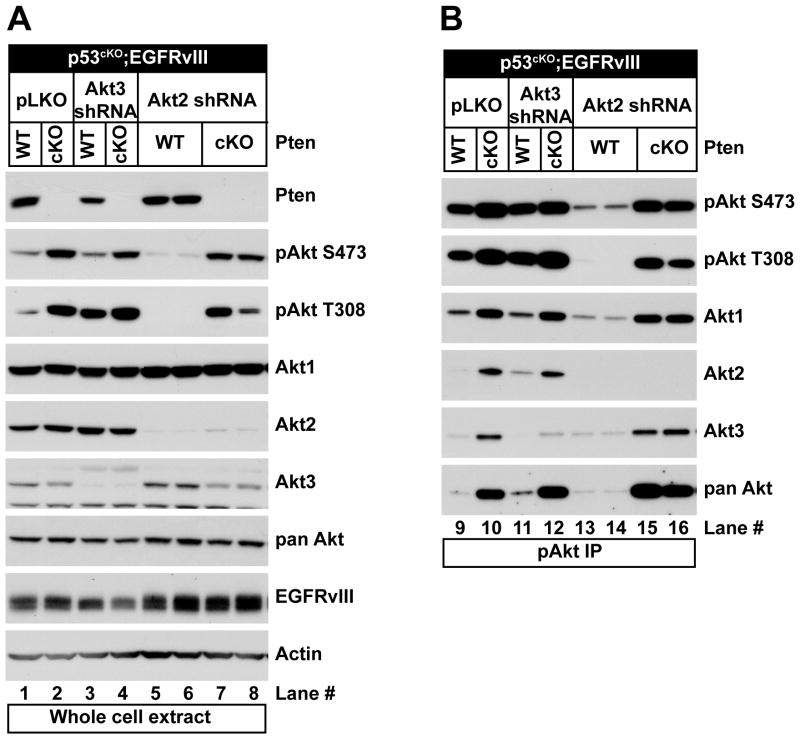
Figure 2. Redundancy of Akt isoforms phosphorylated in primary astrocytes.
(A) Western blot analyses using the indicated antibodies of PMAs isolated from p53cKO or PtencKO;p53cKO mice expressing EGFRvIII (vIII) that were transduced with control lentivirus (pLKO) or lentivirus expressing Akt3-specific or Akt2-specific shRNA. (B) Phospho-Akt (S473) immunoprecipitates of the same lysates were immunoblotted as indicated.
To investigate the roles of Akt2 and Akt3 in astrocytes, we transduced cells with lentivirus expressing isoform-specific shRNAs. Knock-down of Akt3 caused a consistent reduction in Pten expression in Pten wild-type PMAs (Figure 2A, compare lanes 1 and 3) that was associated with an increase in levels of Akt2 phosphorylation (Figure 2B compare lanes 9 and 11), but caused minimal effects on total phospho-Akt levels compared to empty lentivirus (pLKO) controls. In contrast, Akt2 knock-down resulted in a reduction of S473 and T308 phosphorylation in Pten wild-type cells, and there was no compensatory increase in phosphorylation of Akt1 or Akt3 (Figure 2B, compare lane 9 to 13 and 14). Thus, Akt2 phosphorylation increased to compensate for loss of Akt1 or Akt3, but there was no significant compensation for the loss of Akt2.
Gene expression data from the Cancer Genome Atlas (cancergenome.nih.gov) was used to evaluate the expression of all AKT isoforms in human glioblastomas with genomic amplification of EGFR, analogous to our model system with EGFRvIII overexpression. There was a variable range of expression for all three AKT isoforms in human glioblastomas, with AKT2 showing the lowest level of expression. EGFR amplification was not associated with overexpression of any one isoform, but was found in tumors with a variety of combined Akt isoform expression patterns (Figure S1).
Akt inhibition impacts proliferation of PMAs
Deletion of Pten in astrocytes enhanced the proliferation of wild-type and p53-deficient PMAs ((23) and Figure S2A,B). Expression of EGFRvIII further enhanced proliferation of PtencKO cells in the presence or absence of p53 (Figure S2C,D).
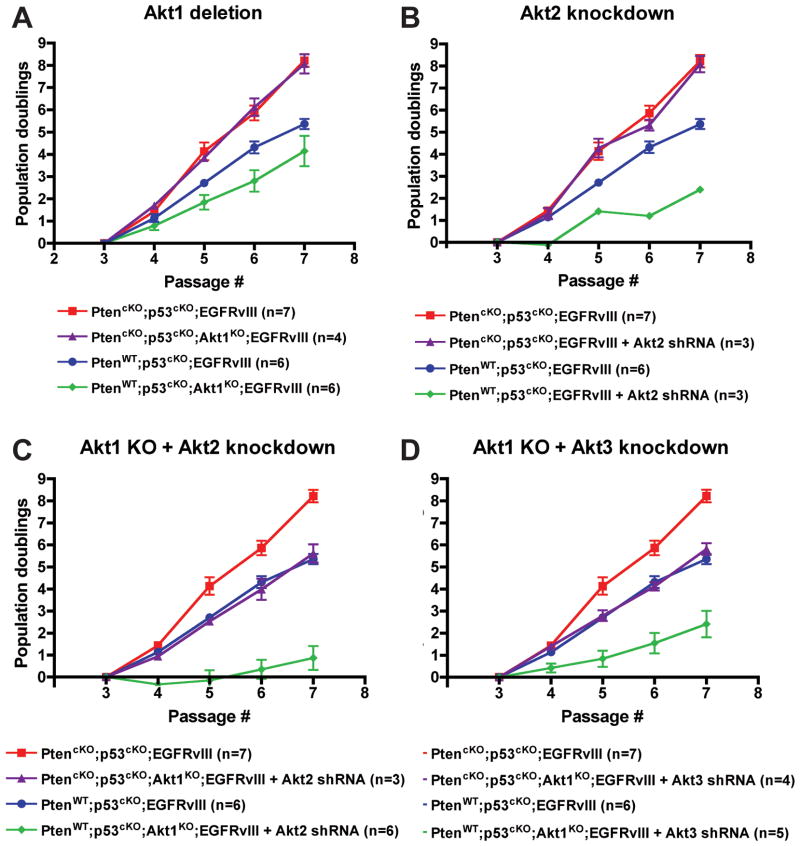
To determine the functional role of Akt isoforms in astrocytes, we evaluated PMA proliferation after loss of each isoform (Figure 3A-D). The proliferation of p53cKO;EGFRvIII PMAs was inhibited upon Akt1 deletion and Akt2 knock-down, and markedly more delayed upon combined inhibition of both isoforms (Figure 3A-C). Akt3 knock-down alone had no effect on the proliferation of these cells (Figure S2E), however it further enhanced the inhibition observed with Akt1 deletion (Figure 3D). In contrast, the proliferation of PtencKO;p53cKO;EGFRvIII PMAs was completely insensitive to inhibition of each Akt isoform individually (Figure 3A,B and S2E). However, the combined inhibition of Akt1 with Akt2 or Akt3 decreased proliferation of PtencKO;p53cKO;EGFRvIII PMA to rates comparable to Pten wild-type (p53cKO;EGFRvIII) cells (Figure 3C,D). Thus, there was greater functional redundancy among Akt isoforms in a Pten-null context, but this could be compromised by decreasing multiple Akt isoforms. Notably, Akt isoform deletion or knock-down did not significantly induce apoptosis (not shown). We also found that Akt1 deletion had no effect on the neuronal hypertrophy of Pten-deficient granule neurons in vivo (Figure S3) (27), demonstrating redundancy for Akt1 function in both astrocytes and neurons.
Figure 3. Akt1 and Akt2 predominantly affected PMA proliferation.
Growth of p53cKO;EGFRvIII PMAs was measured in Pten wildtype (WT) or deficient (cKO) cells that were (A) Akt1 WT or KO (B) Akt1 WT expressing Akt2-specific shRNA, or Akt1 KO expressing (C) Akt2-specific or (D) Akt3-specific shRNA. Plotted are the mean cumulative population doublings ± SEM. n= number of individual cultures per group.
Akt3 is uniquely required for anchorage-independent growth of Pten-deficient PMA and regulates cell invasion
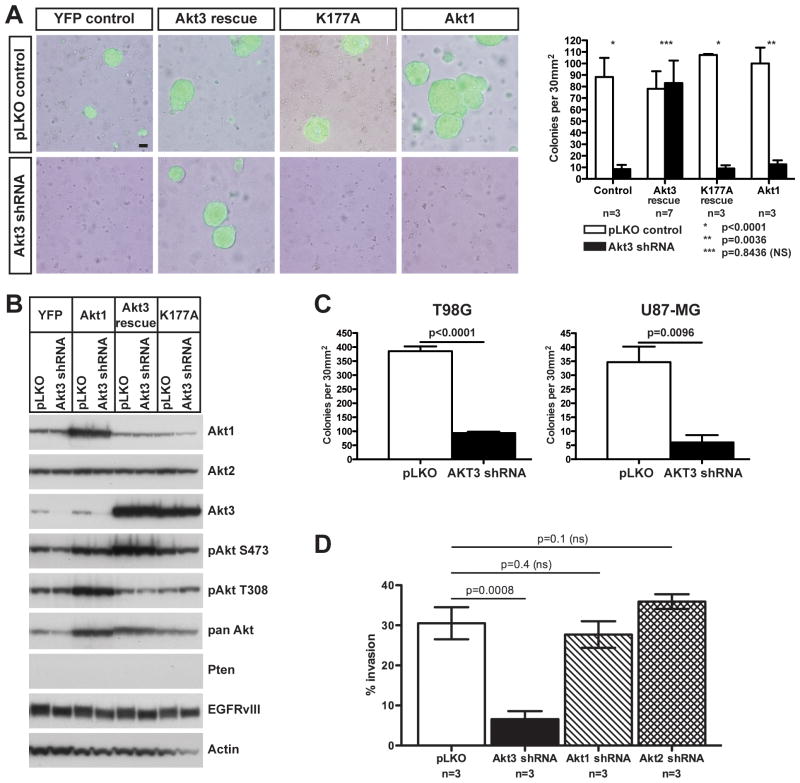
We assessed whether the increased proliferation conferred by Pten deletion and EGFRvIII expression was also associated with anchorage-independent growth, a hallmark of neoplastic transformation. Wild-type, PtencKO, p53cKO, PtencKO;p53cKO and p53cKO;EGFRvIII PMAs all failed to form colonies in soft agar. Colony formation was only observed with PtencKO;p53cKO;EGFRvIII PMAs (Figure S4). Akt3 knock-down significantly inhibited the ability of PtencKO;p53cKO;EGFRvIII PMAs to form colonies in soft agar (Figure 4A), while genetic deletion of Akt1 or Akt2 knock-down individually or in combination had no effect on colony formation or size (Figure S5).
Figure 4. Akt3 regulated anchorage-independent growth and invasiveness of PMAs and GBM cells.
(A) PtencKO;p53cKO;EGFRvIII PMAs were transduced with control (pLKO) or Akt3 shRNA-expressing lentivirus along with control (YFP) or retrovirus over-expressing Akt3 or kinase-dead Akt3 transcripts resistant to the shRNA (Akt3 rescue and K177A) or Akt1. Anchorage-independent growth was assessed by plating in soft agar. Left: Overlays of phase contrast and GFP fluorescence of representative colonies after 10 days. Scale bar = 50 μm. Right: Quantification, n= number of individual cultures per group. (B) Western analyses of cells in (A) using the indicated antibodies prior to plating in soft agar. (C) Quantification of colonies formed after human GBM cell lines T98G and U87-MG were transduced with control (pLKO) or AKT3-specific shRNA lentivirus and plated in agar from three independent experiments. (D) Akt3, but not Akt1 or Akt2, knockdown decreased invasion of PtencKO;p53cKO;EGFRvIII PMAs. Quantification of cell invasion through matrigel in a Boyden chamber from three independently isolated cultures. Graphs in A, C and D indicate mean ± SEM and significance values (p) were calculated using unpaired t-tests.
Loss of anchorage-independent growth was specifically caused by Akt3 knock-down and not off-target effects of the shRNA, because expression of a mutated Akt3 transcript that was resistant to the shRNA rescued anchorage-independent growth (Figure 4A). Akt3 kinase activity was essential, since an shRNA-resistant, kinase-dead mutant of Akt3 (K177A) was unable to restore colony formation. Over-expression of Akt1 also failed to rescue colony formation in the presence of the Akt3 shRNA, showing that the effect was specific for Akt3. Western blot analysis confirmed the overexpression of the Akt3 rescue, K177A and Akt1 proteins (Figure 4B).
The unique requirement of Akt3 for anchorage-independent growth of transformed PMAs was unexpected. To determine if the same isoform specificity was required in human glioma cells, we investigated the influence of AKT3 knock-down on the ability of U87-MG (PTEN null with wild-type p53) and T98G (PTEN mutant (L42R) and p53 mutant) to grow in soft agar. Both of these glioma cell lines, like PMAs, express all three AKT isoforms (19). In both cell lines, AKT3 knock-down significantly reduced the number of colonies formed in agar (Figure 4C) demonstrating a non-redundant function for AKT3 in anchorage-independent growth of mouse and human glioma cells.
Glioblastomas are highly invasive tumors and anchorage-independent growth can be associated with tumor cell invasion. We found that PtencKO;p53cKO;EGFRvIII PMAs were also highly invasive as assayed by invasion through matrigel in a Boyden chamber. Knock-down of Akt3, but not Akt1 or Akt2, strongly inhibited invasion compared to cells transduced with control lentivirus (Figure 4D). Therefore, Akt3 mediates anchorage-independent growth as well as astrocyte invasion, and thus may contribute in part to the malignant nature of gliomas.
EGFRvIII synergizes with p53 and Pten loss to render PMAs tumorigenic
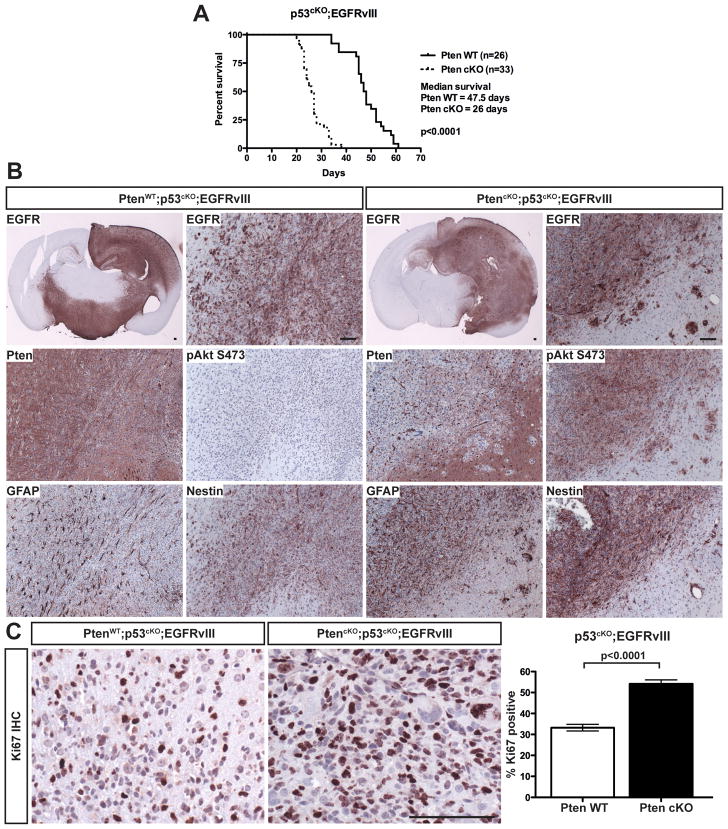
Intracranial implantation of PMAs into immunocompromised mice was used to test synergy of mutations in gliomagenesis. The combined deletion of Pten and p53 in astrocytes weakly synergized to induce tumors in a subset of recipient mice, with prolonged latency (Table S1). The addition of EGFRvIII induced rapid tumor growth in 100% of recipient mice, regardless of Pten status. Deletion of Pten significantly accelerated tumor onset (Figure 5A). p53 deletion was essential in the transformation of PMAs as EGFRvIII expressing cells that retained p53 failed to generate tumors in the presence or absence of Pten (Table S1).
Figure 5. Transformed PMAs formed high-grade astrocytoma following orthotopic transplantation.
(A) Kaplan-Meier curves of mice following implantation of p53cKO;EGFRvIII PMAs with or without Pten deletion. (B) IHC analysis of representative tumors from (A). Sections were stained for human EGFR (shown at low and high magnification), Pten, pAkt (S473), Gfap and Nestin. (C) Left: IHC for Ki67 in p53cKO;EGFRvIII tumors with or without Pten. Right: Quantification of Ki67 positive cells. Shown is the mean ± SEM. Bars in A and C are 100 μm.
Most tumors had cytological features of high-grade glioma (HGG). They appeared relatively undifferentiated with some signs of astrocytic differentiation (anaplastic astrocytoma; WHO grade III). A few cases showed a focal oligodendroglial phenotype or occasional regions with cytological features of a primitive neuroectodermal tumor. Several tumors exhibited necrosis and/or hemorrhage, the presence of necrosis elevating the grade (WHO grade IV; glioblastoma). The tumors were also invasive, with frequent perivascular and leptomeningeal spread in addition to direct invasion of the parenchyma and white matter tracts. Furthermore, all tumors expressed markers expected in HGG, such as Gfap, and also expressed Nestin, a feature observed in many human glioblastomas (Figure 5B). As expected, all tumors expressed high levels of EGFRvIII. Pten was absent in tumors from PtencKO;p53cKO;EGFRvIII PMAs, and was present in tumors from Pten wild-type (p53cKO;EGFRvIII) PMAs, indicating that loss of Pten was not required to render PMAs tumorigenic (Figure 5B). Phosphorylated Akt (S473) was significantly elevated only in Pten-deficient tumors, consistent with the expectation that Pten loss enhances PI3K signaling (Figure 5B).
Tumors were highly proliferative, as shown by IHC for Ki67. Consistent with the in vitro analyses, Pten deletion caused a significant increase in proliferation in vivo (Figure 5C). Apoptosis, measured by IHC for activated caspase 3, was minimal in all tumors analyzed (not shown), therefore Pten deletion accelerated tumor formation through increased tumor cell proliferation, without substantial effects on apoptosis.
Akt isoforms have differential roles in gliomagenesis
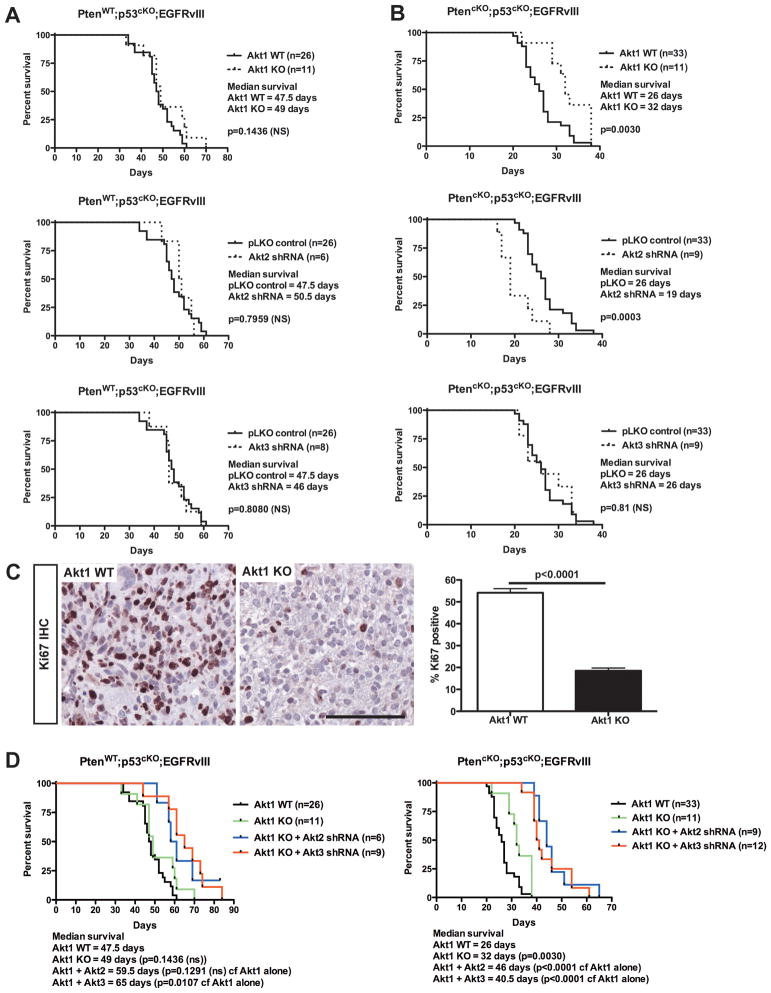
We assessed the contribution of each Akt isoform to tumor establishment and growth in vivo of p53cKO;EGFRvIII or PtencKO;p53cKO;EGFRvIII PMAs. Tumors derived from Pten wild-type cells were insensitive to inhibition of any individual Akt isoform (Figure 6A). Akt1 deletion did not affect the proliferation of tumor cells from p53cKO;EGFRvIII PMAs in vivo despite the decreased proliferation observed in vitro (Figure S6).
Figure 6. Loss of Akt1 and Akt2 had opposing effects on latency of Pten deficient tumors.
Kaplan-Meier curves of mice following intracranial implantation of (A) PtenWT;p53cKO;EGFRvIII or (B) PtencKO;p53cKO;EGFRvIII PMAs that were (upper) Akt1 WT or KO, or treated with (middle) Akt2- or (lower) Akt3-shRNAs. (C) Left: IHC for Ki67 (brown) was performed on PtencKO;p53cKO;EGFRvIII tumors from (B, upper) and counterstained with hematoxylin (blue). Bar is 100 μm. Right: Quantification of Ki67 positive cells. Shown is the mean ± SEM. The significance value (p) was determined using an unpaired t test. (D) Kaplan-Meier curves of mice following intracranial implantation of (left) PtenWT;p53cKO;EGFRvIII or (right) PtencKO;p53cKO;EGFRvIII PMAs that were Akt1WT (black), Akt1KO (green) or Akt1KO expressing (blue) Akt2 or (red) Akt3 shRNAs. For A, B and D, n=number of mice per group and p values were calculated using the logrank test.
In contrast, Pten-deficient (PtencKO;p53cKO;EGFRvIII) tumors displayed opposing effects to Akt inhibition. Deletion of Akt1 delayed tumor onset in recipient mice (Figure 6B, upper). In contrast, Akt2 shRNA accelerated tumor development from PtencKO;p53cKO;EGFRvIII PMAs (Figure 6B, middle). Unlike Akt1 and Akt2, Akt3 knockdown had no effect on the survival of recipient mice (Figure 6B, lower), despite the significant effect of Akt3 knock-down on anchorage-independent growth. Akt1-deficient tumors had a significant reduction of tumor cell proliferation (Figure 6C); however, the overall levels of phosphorylated Akt were not changed in these tumors (Figure S7A). Importantly, Akt2 and Akt3 knock-down was maintained in the tumors as shown by Western blotting (Figure S7B,C). The downstream phosphorylation of Akt substrates Gsk3β, Foxo1 or PRAS40 correlated with the overall levels of phospho-Akt, and did not show any isoform-specific selectivity (data not shown), suggesting that there may be other isoform-specific substrates. Strikingly the differences in recipient mice survival correlated with the specific Akt isoforms present, and not with the levels of phosphorylated Akt (S473).
The effects of simultaneous inhibition of two Akt isoforms were also investigated. Akt1 deletion with Akt3 shRNA significantly delayed onset of Pten WT tumors (Figure 6D, left), but more significant effects were observed in Pten deficient tumors upon combined deletion of Akt1 with either Akt2 or Akt3 shRNA (Figure 6D, right).
Discussion
A unique model to study Akt activity in glioma
Up-regulated AKT activity is a common feature of human high-grade gliomas and is associated with poor prognosis (28). We evaluated the unique and redundant contributions of the different Akt isoforms in the context of astrocyte growth and gliomagenesis. Our model system provides a tool to study the effects of Pten deletion in tumors with identical initiating mutations (in this case Trp53 deletion and EGFRvIII expression) and minimal additional alterations. These three mutations are frequently observed in human HGG (2) and they synergized to render PMAs highly tumorigenic in a rapid and reproducible manner.
Redundant and specific activities of Akt isoforms in brain
Pten deletion and EGFRvIII expression each contributed to increased activation of all three Akt isoforms, and further increases in proliferation. Deletion or knock-down of the Akt isoforms individually or in combination revealed a complicated system of compensation such that overall levels of Akt phosphorylation were not ablated by disruption of any one Akt family member. However, loss of individual Akt isoforms revealed distinct consequences in different functional assays.
Previous studies suggested that Akt1 is the predominant Akt isoform driving the growth of multiple tumor types, since its deletion is sufficient to suppress tumor formation in the cancer prone Pten heterozygous mice (17, 18). Akt3 is expressed in a more tissue-restricted pattern than Akt1. Furthermore, in a mouse model of breast cancer with detectable Akt3 expression, Akt3 deletion had no significant influence on tumor progression (29).
However, the brain-specific developmental defects in the Akt3 knock-out mouse indicated that it may play a more important role in brain than the other isoforms. Indeed, Akt3 was specifically required for anchorage-independent growth of PtencKO;p53cKO;EGFRvIII astrocytes, whereas even the combined deletion of Akt1 with Akt2 knock-down had no effect on colony formation. Moreover, exogenous Akt1 expression was unable to substitute for Akt3 loss in this context despite increased levels of phospho-Akt. Therefore, there is a distinct function for Akt3 in this process. In contrast, Akt1 plays a key role in anchorage-independent growth of transformed mouse embryonic fibroblasts and multiple other cancer cell lines (30–32). However, Akt3 is not expressed in many of these lines, highlighting the importance of elucidating the context-specific roles of the Akt isoforms.
There is emerging evidence that despite many common substrates, Akt isoforms may direct distinct outcomes through regulation of unique substrates. For example, the actin-bundling protein palladin is a substrate for Akt1, but not Akt2, in breast cancer cells. Palladin-mediated effects on cell motility may underlie the distinct effects of Akt1 and Akt2 on breast cancer cell invasion (33). While Akt3 is less broadly expressed, its important role in brain development indicates that there may be isoform-specific substrates for Akt3 in PMAs. Akt3 inhibition also significantly reduced the ability of PMAs to invade through matrigel. These data suggest that while Akt3 inhibition may not result in a cytotoxic or cytostatic tumor response, it has potential to prevent tumor infiltration. The potential role for Akt3 in astrocyte transformation may also be relevant to other tumors where Akt3 is the predominantly active isoform such as malignant melanoma (34).
Surprisingly, Akt1 and Akt2 had opposing effects on tumorigenesis of Pten knock-out PMAs, with Akt1 loss causing a delay, and Akt2 loss causing an acceleration of tumor growth in vivo. Notably, the levels of total Akt phosphorylation were not predictive of the functional consequences following loss of individual isoforms. Paradoxically, the more rapid tumor growth of Akt2 knock-down cells was associated with decreased levels of phospho-Akt. Akt2 ablation has also been shown to accelerate the development of tumors in two different mouse models of breast cancer (29). The reasons for this are not clear. It is possible that there are unknown isoform-specific substrates that may mediate these effects. Alternatively, isoform-specific inhibition may disrupt the normal balance between the activities of the three family members altering downstream signaling, perhaps through modulation of microRNAs (35). Finally, it is possible that an alternative pathway is engaged when the balance of Akt activity is disrupted. For example, PKC can act as an alternative effector downstream of EGFR and Pten (36).
The selective advantage for Akt2 loss in tumorigenesis of transformed Pten-deficient PMAs contrasts with a report suggesting that AKT2 activity is important for driving tumor growth in brain (19). Furthermore, that study described elevated levels of AKT2 in high-grade gliomas relative to low-grade gliomas, and showed that siRNA-mediated inhibition of AKT2 induced apoptosis in glioma cell lines in vitro. Inhibition of AKT2 delayed tumor development in vivo using an orthotopic transplantation model utilizing U87-MG glioblastoma cells that overexpress EGFRvIII, but only when used in combination with siRNA targeting EGFRvIII (26). There are several important differences to consider in the interpretation of the present in vivo study and these earlier reports. Notably, the relative activity of the different Akt isoforms (especially Akt2) is different in these cells compared to what we observed in PMAs (19). Further, the spectrum of other mutations within a tumor is likely to influence the role of specific isoforms. For example, Akt2 knock-down did not alter tumor growth of p53cKO;EGFRvIII PMAs in vivo.
Implications for therapies targeting Akt
Although individual Akt isoform inhibition had no influence on the latency of Pten wild-type tumors, our data suggest that better outcomes may be achieved by concurrently targeting multiple isozymes in tumors with an intact PI3K signaling pathway. Importantly, the data with respect to Akt2 inhibition and enhanced tumor growth suggests that context-specific activities of AKT isoforms in different tumors may contribute to unexpected outcomes in response to selective inhibitors. Despite the tumor promoting effects of Akt2 knockdown in Pten null cells, combined loss of Akt1 and 2, or Akt1 and 3 significantly delayed tumor onset, suggesting that depletion of multiple isoforms may disrupt a necessary threshold level of Akt signaling leading to growth inhibition.
Why do the functional consequences of Akt isoform ablation differ between experimental systems? Importantly, cell culture conditions provide multiple growth factors that stimulate PI3K signaling as well as other growth regulatory pathways. These conditions may reveal differences in isoform contribution in the context of strong pathway activation, and may be analogous to tumors that contain multiple mutations that hyperactivate cellular signaling pathways. Phenotypes apparent in vivo, but not in vitro, may reflect cellular response to an environment with a different repertoire of growth signals, and may also be influenced by paracrine stimuli that are absent in vitro. In vitro growth properties are not always concordant with in vivo tumorigenicity. There are examples of aggressive malignancies such as glioblastomas that can be established as cell lines but fail to form xenografts (37), as well as oncogenes that render transformed cells tumorigenic, but fail to induce growth of the same cells in soft agar (38). It is noteworthy that the p53cKO;EGFRvIII PMAs were highly tumorigenic in vivo, but failed to display anchorage-independent growth in vitro. Additional loss of Pten conferred effective anchorage independent growth. Interestingly, it was recently shown that PTEN deficiency correlated with the ability of primary human glioblastoma samples to form neurospheres in culture, a form of anchorage-independent growth (39). Thus, each experimental paradigm provides a different challenge for tumor cell proliferation, survival and invasion, and reveals different selective advantages conferred by mutation.
Combined deletion of Akt1 and Akt2 in human colon cancer cell lines almost completely blocked their ability to grow in soft agar. In vivo, the cells were tumorigenic when injected subcutaneously, but were profoundly deficient in the ability to metastasize when delivered by intrasplenic injection. This highlights the ability of different tumor microenvironments to reveal unique Akt isoform-dependent phenotypes (40). In our study, Akt3 inhibition potently and selectively decreased colony growth in agar and in vitro invasion, without detectable effects in intracranial tumor growth. This likely indicates that the microenvironment at the site of intracranial implantation did not apply the required selective pressure to reveal the Akt3-dependent effects in vivo. For these experiments, transformed PMAs were injected into the cortex adjacent to the corpus callosum containing white matter tracts that are common routes for glioblastoma invasion. It is possible that an alternative site might have revealed greater differences in tumor cell invasion in vivo. The varied consequences of ablation of individual Akt isoforms in different assays demonstrates the complexity of signaling through this pathway and indicates that the optimal isoform for therapeutic inhibition may vary depending on the spectrum of mutations and the tumor microenvironment.
Supplementary Material
Acknowledgments
We thank Jovan Mitchell and Kristen Cox for genotyping, Dr. Christopher Calabrese, Melissa Johnson and John Killmar for mouse surgical assistance, Dr. Chunxu Qu for assistance with gene expression analysis and Dr. John Gray and the Vector Core Lab for lentivirus production. We thank Dr. Abhijit Guha for EGFRvIII cDNA, Dr. Tak Mak for PtenloxP mice, Dr. David Gutmann for GFAP-cre mice and Dr. Anton Berns and the Mouse Models of Human Cancer Consortium repository for p53loxP mice.
This work was supported by NIH grants CA135554 and CA096832 and ALSAC.
Footnotes
The authors declare no conflict of interest.
References
- 1.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, editors. WHO classification of tumours of the central nervous system. 4. Lyon: International agency for research on cancer; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The Cancer Genome Atlas. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–8. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parsons DW, Jones S, Zhang X, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–12. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong AJ, Bigner SH, Bigner DD, Kinzler KW, Hamilton SR, Vogelstein B. Increased expression of the epidermal growth factor receptor gene in malignant gliomas is invariably associated with gene amplification. Proc Natl Acad Sci U S A. 1987;84:6899–903. doi: 10.1073/pnas.84.19.6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moscatello DK, Holgado-Madruga M, Emlet DR, Montgomery RB, Wong AJ. Constitutive activation of phosphatidylinositol 3-kinase by a naturally occurring mutant epidermal growth factor receptor. J Biol Chem. 1998;273:200–6. doi: 10.1074/jbc.273.1.200. [DOI] [PubMed] [Google Scholar]
- 6.Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:13375–8. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- 7.Altomare DA, Testa JR. Perturbations of the AKT signaling pathway in human cancer. Oncogene. 2005;24:7455–64. doi: 10.1038/sj.onc.1209085. [DOI] [PubMed] [Google Scholar]
- 8.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–74. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen WS, Xu PZ, Gottlob K, et al. Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene. Genes Dev. 2001;15:2203–8. doi: 10.1101/gad.913901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cho H, Mu J, Kim JK, et al. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta) Science. 2001;292:1728–31. doi: 10.1126/science.292.5522.1728. [DOI] [PubMed] [Google Scholar]
- 11.Cho H, Thorvaldsen JL, Chu Q, Feng F, Birnbaum MJ. Akt1/PKBalpha is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J Biol Chem. 2001;276:38349–52. doi: 10.1074/jbc.C100462200. [DOI] [PubMed] [Google Scholar]
- 12.Garofalo RS, Orena SJ, Rafidi K, et al. Severe diabetes, age-dependent loss of adipose tissue, and mild growth deficiency in mice lacking Akt2/PKB beta. J Clin Invest. 2003;112:197–208. doi: 10.1172/JCI16885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Easton RM, Cho H, Roovers K, et al. Role for Akt3/protein kinase Bgamma in attainment of normal brain size. Mol Cell Biol. 2005;25:1869–78. doi: 10.1128/MCB.25.5.1869-1878.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tschopp O, Yang ZZ, Brodbeck D, et al. Essential role of protein kinase B gamma (PKB gamma/Akt3) in postnatal brain development but not in glucose homeostasis. Development. 2005;132:2943–54. doi: 10.1242/dev.01864. [DOI] [PubMed] [Google Scholar]
- 15.Santi SA, Lee H. The Akt isoforms are present at distinct subcellular locations. Am J Physiol Cell Physiol. 2010;298:C580–91. doi: 10.1152/ajpcell.00375.2009. [DOI] [PubMed] [Google Scholar]
- 16.Holland EC, Celestino J, Dai C, Schaefer L, Sawaya RE, Fuller GN. Combined activation of Ras and Akt in neural progenitors induces glioblastoma formation in mice. Nat Genet. 2000;25:55–7. doi: 10.1038/75596. [DOI] [PubMed] [Google Scholar]
- 17.Chen ML, Xu PZ, Peng XD, et al. The deficiency of Akt1 is sufficient to suppress tumor development in Pten+/− mice. Genes Dev. 2006;20:1569–74. doi: 10.1101/gad.1395006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stiles B, Gilman V, Khanzenzon N, et al. Essential role of AKT-1/protein kinase B alpha in PTEN-controlled tumorigenesis. Mol Cell Biol. 2002;22:3842–51. doi: 10.1128/MCB.22.11.3842-3851.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mure H, Matsuzaki K, Kitazato KT, et al. Akt2 and Akt3 play a pivotal role in malignant gliomas. Neuro Oncol. 2010;12:221–32. doi: 10.1093/neuonc/nop026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bajenaru ML, Zhu Y, Hedrick NM, Donahoe J, Parada LF, Gutmann DH. Astrocyte-specific inactivation of the neurofibromatosis 1 gene (NF1) is insufficient for astrocytoma formation. Mol Cell Biol. 2002;22:5100–13. doi: 10.1128/MCB.22.14.5100-5113.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suzuki A, Yamaguchi MT, Ohteki T, et al. T cell-specific loss of Pten leads to defects in central and peripheral tolerance. Immunity. 2001;14:523–34. doi: 10.1016/s1074-7613(01)00134-0. [DOI] [PubMed] [Google Scholar]
- 22.Jonkers J, Meuwissen R, van der Gulden H, Peterse H, van der Valk M, Berns A. Synergistic tumor suppressor activity of BRCA2 and p53 in a conditional mouse model for breast cancer. Nat Genet. 2001;29:418–25. doi: 10.1038/ng747. [DOI] [PubMed] [Google Scholar]
- 23.Fraser MM, Zhu X, Kwon CH, Uhlmann EJ, Gutmann DH, Baker SJ. Pten loss causes hypertrophy and increased proliferation of astrocytes in vivo. Cancer Res. 2004;64:7773–9. doi: 10.1158/0008-5472.CAN-04-2487. [DOI] [PubMed] [Google Scholar]
- 24.Persons DA, Allay JA, Allay ER, et al. Enforced expression of the GATA-2 transcription factor blocks normal hematopoiesis. Blood. 1999;93:488–99. [PubMed] [Google Scholar]
- 25.Hanawa H, Kelly PF, Nathwani AC, et al. Comparison of various envelope proteins for their ability to pseudotype lentiviral vectors and transduce primitive hematopoietic cells from human blood. Mol Ther. 2002;5:242–51. doi: 10.1006/mthe.2002.0549. [DOI] [PubMed] [Google Scholar]
- 26.Michiue H, Eguchi A, Scadeng M, Dowdy SF. Induction of in vivo synthetic lethal RNAi responses to treat glioblastoma. Cancer Biol Ther. 2009;8:2306–13. doi: 10.4161/cbt.8.23.10271. [DOI] [PubMed] [Google Scholar]
- 27.Kwon CH, Zhu X, Zhang J, et al. Pten regulates neuronal soma size: a mouse model of Lhermitte-Duclos disease. Nat Genet. 2001;29:404–11. doi: 10.1038/ng781. [DOI] [PubMed] [Google Scholar]
- 28.Ermoian RP, Furniss CS, Lamborn KR, et al. Dysregulation of PTEN and protein kinase B is associated with glioma histology and patient survival. Clin Cancer Res. 2002;8:1100–6. [PubMed] [Google Scholar]
- 29.Maroulakou IG, Oemler W, Naber SP, Tsichlis PN. Akt1 ablation inhibits, whereas Akt2 ablation accelerates, the development of mammary adenocarcinomas in mouse mammary tumor virus (MMTV)-ErbB2/neu and MMTV-polyoma middle T transgenic mice. Cancer Res. 2007;67:167–77. doi: 10.1158/0008-5472.CAN-06-3782. [DOI] [PubMed] [Google Scholar]
- 30.Skeen JE, Bhaskar PT, Chen CC, et al. Akt deficiency impairs normal cell proliferation and suppresses oncogenesis in a p53-independent and mTORC1-dependent manner. Cancer Cell. 2006;10:269–80. doi: 10.1016/j.ccr.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 31.Liu X, Shi Y, Han EK, et al. Downregulation of Akt1 inhibits anchorage-independent cell growth and induces apoptosis in cancer cells. Neoplasia. 2001;3:278–86. doi: 10.1038/sj.neo.7900163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vasudevan KM, Barbie DA, Davies MA, et al. AKT-independent signaling downstream of oncogenic PIK3CA mutations in human cancer. Cancer Cell. 2009;16:21–32. doi: 10.1016/j.ccr.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chin YR, Toker A. Function of Akt/PKB signaling to cell motility, invasion and the tumor stroma in cancer. Cell Signal. 2009;21:470–6. doi: 10.1016/j.cellsig.2008.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stahl JM, Sharma A, Cheung M, et al. Deregulated Akt3 activity promotes development of malignant melanoma. Cancer Res. 2004;64:7002–10. doi: 10.1158/0008-5472.CAN-04-1399. [DOI] [PubMed] [Google Scholar]
- 35.Iliopoulos D, Polytarchou C, Hatziapostolou M, et al. MicroRNAs differentially regulated by Akt isoforms control EMT and stem cell renewal in cancer cells. Sci Signal. 2009;2:ra62. doi: 10.1126/scisignal.2000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fan QW, Cheng C, Knight ZA, et al. EGFR signals to mTOR through PKC and independently of Akt in glioma. Sci Signal. 2009;2:ra4. doi: 10.1126/scisignal.2000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rutka JT, Giblin JR, Dougherty DY, et al. Establishment and characterization of five cell lines derived from human malignant gliomas. Acta Neuropathol. 1987;75:92–103. doi: 10.1007/BF00686798. [DOI] [PubMed] [Google Scholar]
- 38.Thompson AD, Teitell MA, Arvand A, Denny CT. Divergent Ewing’s sarcoma EWS/ETS fusions confer a common tumorigenic phenotype on NIH3T3 cells. Oncogene. 1999;18:5506–13. doi: 10.1038/sj.onc.1202928. [DOI] [PubMed] [Google Scholar]
- 39.Chen R, Nishimura MC, Bumbaca SM, et al. A hierarchy of self-renewing tumor-initiating cell types in glioblastoma. Cancer Cell. 2010;17:362–75. doi: 10.1016/j.ccr.2009.12.049. [DOI] [PubMed] [Google Scholar]
- 40.Ericson K, Gan C, Cheong I, et al. Genetic inactivation of AKT1, AKT2, and PDPK1 in human colorectal cancer cells clarifies their roles in tumor growth regulation. Proc Natl Acad Sci U S A. 2010;107:2598–603. doi: 10.1073/pnas.0914018107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.