Abstract
Objectives
To identify children at risk for in-hospital mortality following tracheotomy.
Design
Retrospective cohort study.
Setting
25 746 876 US hospitalisations for children within the Kids’ Inpatient Database 1997, 2000, 2003 and 2006.
Participants
18 806 hospitalisations of children ages 0–18 years undergoing tracheotomy, identified from ICD-9-CM tracheotomy procedure codes.
Main outcome measure
Mortality during the initial hospitalisation when tracheotomy was performed in relation to patient demographic and clinical characteristics (neuromuscular impairment (NI), chronic lung disease, upper airway anomaly, prematurity, congenital heart disease, upper airway infection and trauma) identified with ICD-9-CM codes.
Results
Between 1997 and 2006, mortality following tracheotomy ranged from 7.7% to 8.5%. In each year, higher mortality was observed in children undergoing tracheotomy who were aged <1 year compared with children aged 1–4 years (mortality range: 10.2–13.1% vs 1.1–4.2%); in children with congenital heart disease, compared with children without congenital heart disease (13.1–18.7% vs 6.2–7.1%) and in children with prematurity, compared with children who were not premature (13.0–19.4% vs 6.8–7.3%). Lower mortality was observed in children with an upper airway anomaly compared with children without an upper airway anomaly (1.5–5.1% vs 9.1–10.3%). In 2006, the highest mortality (40.0%) was observed in premature children with NI and congenital heart disease, who did not have an upper airway anomaly.
Conclusions
Congenital heart disease, prematurity, the absence of an upper airway anomaly and age <1 year were characteristics associated with higher mortality in children following tracheotomy. These findings may assist provider communication with children and families regarding early prognosis following tracheotomy.
INTRODUCTION
Children experiencing potential life-limiting respiratory compromise may require tracheotomy for survival. Children have a substantial risk of early mortality following tracheotomy. Recent studies report that 7–8% of children do not survive the hospitalisation when tracheotomy is performed.1–3 It is believed the vast majority of deaths following tracheotomy are not tracheotomy-related, but rather are due to the child’s underlying diagnoses and clinical characteristics.4–11
Over time, tracheotomy is being performed less in previously healthy children with acute upper airway compromise due to infection, and more often in children with existing chronic health conditions.12–15 Guidance to help physicians inform and advise families facing decisions regarding tracheotomy in children with chronic health conditions has been difficult to develop, in part because these children have a heterogeneous array of underlying diagnoses.2,3,12–16 Up to 60% of children undergoing tracheotomy have multiple chronic diagnoses affecting different organ systems that makes counselling of families with good measures of risk and benefit even more difficult.3
Most single-institutional tracheotomy outcome studies in children have sample sizes that limit the ability to analyse how these diagnoses impact the risk of mortality.10,17–19 To our knowledge, previous studies have not evaluated the mortality risk in children who have multiple, chronic diagnoses. The objectives of this study were to (1) describe the clinical characteristics of children undergoing tracheotomy from a nationally representative cohort of hospitalised children, and (2) identify which characteristics, individually and in combination, were associated with mortality during the initial hospitalisation when the tracheotomy was performed.
METHODS
Study design and setting
This is a retrospective cohort analysis of the Healthcare Cost and Utilization Project Kids’ Inpatient Database (KID). KID is a national database of hospitalisations for children for the years 1997, 2000, 2003 and 2006.20,21 From 1997 to 2006, the KID sample increased from 1.9 million hospital discharges from 2521 hospitals in 22 states to 3.1 million hospital discharges from 3739 hospitals in 38 states.20,21
The KID sampling design includes 10% of uncomplicated births and 80% of all other paediatric discharges from each hospital.20 The dataset includes a weight variable for each observation so that weighted analyses may produce national estimates of total US discharges for specific diagnoses and procedures. Diagnoses and procedures are identified from the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) coding.
Study participants and main outcome measure
Children age 0–18 years undergoing tracheotomy in 1997, 2000, 2003 and 2006 were identified with ICD-9-CM procedural coding (31.1 (temporary tracheotomy) or 31.2 (permanent tracheotomy)) used in previous studies.1–3 The main outcome measure was in-hospital mortality, defined as mortality occurring during the hospitalisation when the tracheotomy was performed.
Patient characteristics
Patient demographics included age at admission for tracheotomy, race/ethnicity (white, black, Hispanic, other or missing) and insurance type (Medicaid, health maintenance organisation/private or self-pay).20,21 We also described hospital discharge disposition: (1) to home, (2) to home with home nursing, or (3) to a skilled nursing or rehabilitation or long-term care facility.
Clinical characteristics evaluated were neuromuscular impairment (NI), upper airway anomaly, chronic lung disease, prematurity, upper airway infection, congenital heart disease, head and neck trauma and ‘other,’ (defined below) as categorised in previous studies in children with tracheotomy.11,22 These characteristics were identified by ICD-9-CM codes for each child, based on previous studies and the tabular index of ICD-9-CM diagnostic coding.23–26
NI included static and progressive, central and peripheral neuromuscular diagnoses such as cerebral palsy and muscular dystrophy. Upper airway anomalies included congenital craniofacial abnormalities, diagnoses such as vocal cord paralysis and laryngotracheomalacia. Chronic lung disease included diagnoses such as bronchopulmonary dysplasia and cystic fibrosis. Prematurity was defined as gestational age ≤36 weeks at delivery. Upper airway infection included diagnoses such as epiglottitis and tracheitis. Congenital heart disease included bulbus cordis, cardiac septal closure, congenital valvular and great vessel anomalies. The ‘other’ category included children without a diagnosis within the remaining categories. Aside from ‘other’, the categories were not mutually exclusive.
Statistical analyses
All analyses were weighted to achieve national estimates of tracheotomy operations using the KID hospital stratification algorithm with SAS proc survey commands.21,27 We present the variance of the weighted estimates with SE. We compared our calculated national estimates for tracheotomy with those available from the Agency for Healthcare Research and Quality for accuracy.28 We clustered the data by individual hospital, and stratified the data by hospital type and geographical region to account for institutional and regional effects. Race/ethnicity data were missing in 16.4–26.4% of tracheotomy hospitalisations from each year. We therefore grouped children with missing rate/ethnicity data in a separate category for analysis. Missing discharge disposition and insurance data from each year (0% to 1.2%) were excluded from analyses.
We evaluated patient characteristic trends over time in children undergoing tracheotomy using a χ2 trend test. We used logistic regression to evaluate the association of each patent characteristic with in-hospital mortality for each year. Many children undergoing tracheotomy may have more than one of the patient characteristics under study.3 Therefore, in the year 2006 we evaluated the impact of characteristic combinations on mortality using partition, classification and regression tree (CART) modelling. We determined which characteristic combinations were associated with the highest and lowest mortality following tracheotomy using CART binary split and postpruning, goodness of fit rules.29 Statistical Analysis Software version 9.1.3 and JMP (SAS Institute, Inc, Cary, North Carolina, USA) were used for all analyses.
RESULTS
There were 25 746 876 hospitalisations for children ages 0–18 years identified from the KID 1997, 2000, 2003 and 2006 combined. Of these, tracheotomy was performed in 18 806 hospitalisations (1 tracheotomy per 1369 hospitalisations). Between 1997 and 2006, in-hospital mortality following tracheotomy ranged from 7.7% to 8.5%. There was no trend in mortality observed over time (p=0.7). 30.9% (1997) to 48.2% (2000) of deaths occurred within 14 days of tracheotomy placement. Median age at admission for tracheotomy decreased from 4.0 years (IQR 0–15) in 1997 to 1.0 years (IQR 0–14) in 2006 (p<0.001). The percentage of children undergoing tracheotomy who utilised public insurance increased from 43.1% in 1997 to 51.1% in 2006, p<0.001 (table 1).
Table 1.
In-hospital mortality, demographic and hospital discharge characteristics of infants and children undergoing tracheotomy, Kids’ Inpatient Database 1997, 2000, 2003 and 2006
| Characteristic | Year
|
||||
|---|---|---|---|---|---|
| 1997 | 2000 | 2003 | 2006 | p Value* | |
| Number of tracheotomy operations (SE)† | 4322 (232) | 4511 (228) | 4502 (232) | 4751 (247) | 0.7 |
| In-hospital mortality | |||||
| % Of patients who died during the hospitalisation when tracheotomy was performed (SE) | 7.7% (0.7) | 7.9% (0.7) | 7.8% (0.5) | 8.5% (0.5) | 0.7 |
| Median number of days between tracheotomy and death (IQR)‡ | 27 (9–56) | 17 (4–47) | 22 (6–55) | 26 (9–59) | <0.001 |
| % Of all deaths within 14 days of tracheotomy placement (SE)‡ | 30.9% (3.7) | 48.2% (4.2) | 36.4% (3.4) | 36.5% (3.3) | <0.001 |
| Age at admission for tracheotomy | |||||
| Median age in years (IQR) | 4.0 (0–15) | 2.0 (0–14) | 2.0 (0–14) | 1.0 (0–14) | <0.001 |
| Insurance use and gender | |||||
| Public insurance use (SE) | 43.1% (1.7) | 41.0% (1.8) | 48.4% (1.6) | 51.1% (1.5) | <0.001 |
| Male gender (SE) | 63.1% (1.2) | 58.7% (1.2) | 60.7% (1.0) | 58.2% (1.0) | 0.008 |
| Race/ethnicity | |||||
| White (SE) | 41.0% (2.8) | 45.3% (2.2) | 36.1% (2.1) | 37.6% (2.1) | <0.01 |
| Non-Hispanic black (SE) | 16.4% (1.4) | 15.8% (1.3) | 15.8% (1.4) | 16.2% (1.3) | |
| Hispanic (SE) | 10.5% (1.5) | 14.5% (1.7) | 14.9% (1.6) | 15.8% (1.6) | |
| Other (SE) | 5.7% (0.7) | 8.0% (1.0) | 7.5% (0.8) | 8.2% (0.9%) | |
| Missing (SE) | 26.4% (3.5) | 16.4% (2.5) | 25.6% (3.3) | 22.1% (3.1%) | |
| Discharge disposition | |||||
| To home with home health nursing (SE) | 14.3% (1.8) | 15.8% (1.6) | 18.7% (1.5) | 21.4% (1.6) | <0.001 |
| Transfer to short-term hospital, skilled nursing or intermediate care facility (SE) | 35.1% (1.7) | 35.6% (1.7) | 34.0% (1.5) | 36.2% (1.6) | |
| To home without home health nursing (SE) | 42.8% (2.0) | 40.4% (1.9) | 38.3% (2.0) | 33.4% (1.8) | |
| Missing (SE) | 0% | <0.01% | 1.2% (0.1) | 0% | |
p Value represents significance for a trend over time for each characteristic.
SE, standard error of the weighted estimate.
Tracheotomy placement date was available for 53% (1997), 73% (2000), 65% (2003) and 68% (2006) of children who died during the hospitalisation.
Clinical characteristics
Between 1997 and 2006, there was a significant increase in the percentage of children undergoing tracheotomy with one or more of the following clinical characteristics: NI (36.9–46.4%, p<0.01), chronic lung disease (31.9–44.4%, p<0.01), congenital heart disease (10.2–18.6%, p<0.01) and prematurity (7.2–12.3%, p<0.01). The percentage of children undergoing tracheotomy with an upper airway anomaly (27.1–27.6%, p>0.1) or upper airway infection (9.1–8.6%, p>0.1) stayed constant. There was a decrease in the percentage of children undergoing tracheotomy with trauma (29.2–25.1%, p<0.01) and in children with the ‘other’ category (9.1% vs 6.4%, p<0.01) (table 2).
Table 2.
Diagnoses of infants and children undergoing tracheotomy, Kids’ Inpatient Database 1997, 2000, 2003 and 2006*
| Characteristic | Year
|
||||
|---|---|---|---|---|---|
| 1997 | 2000 | 2003 | 2006 | p Value† | |
| Total number of tracheotomy operations | 4322 | 4511 | 4502 | 4751 | 0.7 |
| Neuromuscular impairment | 1593 (36.9%) | 1795 (39.8%) | 1876 (41.7%) | 2204 (46.4%) | <0.01 |
| Epilepsy | 644 (14.9%) | 782 (17.3%) | 822 (18.3%) | 953 (20.1%) | |
| Cerebral palsy | 629 (14.5%) | 523 (11.6%) | 583 (13.0%) | 638 (13.4%) | |
| Brain or spinal cord malformation | 282 (6.5%) | 319 (7.1%) | 312 (6.9%) | 349 (7.4%) | |
| Hydrocephalus | 234 (5.4%) | 236 (5.2%) | 275 (6.1%) | 308 (6.5%) | |
| Central nervous system infection | 116 (2.7%) | 97 (2.1%) | 99 (2.2%) | 169 (3.5%) | |
| Muscular dystrophy or myopathy | 104 (2.4%) | 112 (2.5%) | 122 (2.7%) | 104 (2.2%) | |
| Chronic lung disease | 1378 (31.9%) | 1635 (36.2%) | 1988 (44.2%) | 2108 (44.3%) | <0.01 |
| Arising in the neonatal period‡ | 543 (12.7%) | 723 (16.0%) | 1045 (23.2%) | 1121 (23.6%) | |
| Chronic respiratory failure | 678 (15.7%) | 708 (15.7%) | 753 (16.7%) | 722 (15.1%) | |
| Respiratory congenital anomalies | 99 (2.3%) | 142 (3.2%) | 132 (2.9%) | 148 (3.1%) | |
| Cystic fibrosis, other pulmonary fibrosis | 47 (1.2%) | 42 (1.0%) | 35 (0.8%) | 92 (2.0%) | |
| Diaphragm paralysis | 11 (0.3%) | 14 (0.3%) | 27 (0.6%) | 25 (0.5%) | |
| Trauma | 1264 (29.2%) | 1296 (28.7%) | 1138 (25.3%) | 1194 (25.1%) | <0.01 |
| Skull fracture or intracranial injury | 876 (20.2%) | 895 (19.9%) | 804 (17.9%) | 816 (17.1%) | |
| Cervical injury | 215 (5.0%) | 243 (5.4%) | 286 (6.4%) | 362 (7.6%) | |
| Other head and neck injury | 173 (4.0%) | 158 (3.5%) | 48 (1.1%) | 16 (<0.1%) | |
| Upper airway anomaly | 1171 (27.1%) | 1280 (28.3%) | 1237 (27.4%) | 1308 (27.6%) | >0.1 |
| Atresia, agenesis or other anomaly of the larynx or trachea | 490 (11.3%) | 539 (11.9%) | 572 (12.7%) | 580 (12.2%) | |
| Laryngeal stenosis | 357 (8.2%) | 372 (8.2%) | 364 (8.1%) | 332 (7.0%) | |
| Vocal cord paralysis or dysfunction | 220 (5.1%) | 216 (4.8%) | 194 (4.3%) | 290 (6.1%) | |
| Choanal atresia | 66 (1.5%) | 59 (1.3%) | 35 (0.8%) | 52 (1.1%) | |
| Cervical haemangioma, lymphangioma | 57 (1.3%) | 100 (2.2%) | 68 (1.5%) | 54 (1.1%) | |
| Congenital heart disease | 441 (10.2%) | 600 (13.2%) | 717 (15.9%) | 883 (18.6%) | <0.01 |
| Atrial or ventricular septal defect | 185 (4.3%) | 250 (5.5%) | 204 (4.5%) | 241 (5.1%) | |
| Patent ductus arteriosus | 131 (3.0%) | 188 (4.2%) | 194 (4.3%) | 294 (6.2%) | |
| Single ventricle, tetralogy of Fallot, transposition of great arteries, truncus arteriosus | 100 (2.3%) | 101 (2.2%) | 139 (3.1%) | 143 (3.0%) | |
| Aorta, pulmonary artery or pulmonary vein anomaly | 63 (1.5%) | 65 (1.5%) | 68 (1.6%) | 88 (2.0%) | |
| Aortic, pulmonary, mitral or trileptal valve anomaly | 40 (0.9%) | 87 (1.9%) | 122 (2.7%) | 160 (3.4%) | |
| Upper airway infection | 393 (9.0%) | 317 (7.0%) | 346 (7.7%) | 411 (8.6%) | >0.1 |
| Tracheitis | 243 (5.6%) | 229 (5.1%) | 251 (5.6%) | 333 (7.0%) | |
| Laryngeal, tonsil or other upper respiratory infection | 122 (2.8%) | 68 (1.5%) | 54 (1.2%) | 48 (1.0%) | |
| Epiglottitis or laryngotracheitis | 28 (0.7%) | 19 (0.4%) | 36 (0.8%) | 28 (0.6%) | |
| Prematurity (<36 weeks’ gestation) | 311 (7.2%) | 494 (10.9%) | 521 (11.6%) | 606 (12.8%) | <0.01 |
| Other | 389 (9.0%) | 339 (7.5%) | 318 (7.1%) | 304 (6.4%) | <0.01 |
No and % of total tracheotomy operations are displayed.
p Value represents a trend over time for each characteristic category.
Includes bronchopulmonary dysplasia, hyaline membrane disease, interstitial pulmonary fibrosis of prematurity and idiopathic respiratory distress syndrome of the newborn.
Between 1997 and 2006, there was a significant increase in the percentage of children undergoing tracheotomy with two or more clinical characteristics (55.3–67.1%, p<0.01), including NI and chronic lung disease (11.8–22.2%, p<0.01), NI and upper airway anomaly (11.7–15.4%, p<0.01), prematurity and chronic lung disease (5.5–11.4%, p<0.01), congenital heart disease and chronic lung disease (4.8–12.2%, p<0.01).
Bivariate analysis of patient characteristics and mortality
Over time, the age distribution of children who died following tracheotomy changed significantly. The percentage of deaths attributable to children less than 1 year of age increased from 54.5% in 1997 to 68.9% in 2006 (p<0.01). In all years, children less than 1 year of age at tracheotomy experienced higher mortality compared with children age 1–4 years (12.1% vs 1.1% (1997); 10.2% vs 5.4% (2000); 10.6% vs 3.4% (2003); 13.1% vs 4.2% (2006)), p<0.01. Gender, race/ethnicity, insurance status and hospital region did not correlate with in-hospital mortality in bivariate analysis (p>0.1).
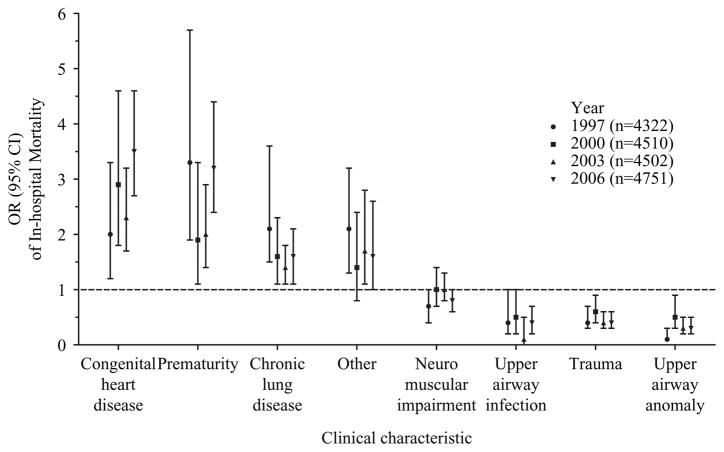
In all years, higher mortality was observed in children undergoing tracheotomy with congenital heart disease, compared with children without congenital heart disease (13.1% vs 7.1% (1997); 16.8% vs 6.6% (2000); 13.9% vs 6.6% (2003); 18.7% vs 6.2% (2006)), p<0.01; prematurity, compared with children without prematurity (19.4% vs 6.8% (1997); 13.2% vs 7.3% (2000); 13.0% vs 7.0% (2003); 19.4% vs 6.9% (2006)), p=0.02; and chronic lung disease, compared with children without chronic lung disease (12.3% vs 5.6% (1997); 10.2% vs 6.6% (2000); 9.1% vs 6.7% (2003); 10.7% vs 6.8% (2006)), p=0.05 (figure 1).
Figure 1.
The bivariate OR (95% CI) of in-hospital mortality associated with each clinical characteristic from each year. The ORs represent the odds of mortality in the presence versus the absence of each characteristic.
In all years, lower mortality was observed in children undergoing tracheotomy with trauma, compared with children who did not experience trauma (4.2% vs 9.2% (1997); 5.4% vs 9.0% (2000); 3.9% vs 9.0% (2003); 4.6% vs 9.8% (2006)), p≤0.02; and an upper airway anomaly compared with children who did not have an upper airway anomaly (1.5% vs 10.0% (1997); 5.1% vs 9.1% (2000); 2.9% vs 9.6% (2003); 3.8% vs 10.3% (2006)), p≤0.02 (figure 1).
Partition regression analysis of patient characteristic combinations and mortality
We performed three partition regression models to evaluate patient characteristic combinations and in-hospital mortality during the hospitalisation when tracheotomy was performed. We performed this analysis for the year 2006, the most recent KID data available. Model 1 was performed on all patients (n=4751). Model 2 was limited to children with NI, the most prevalent clinical characteristic among children undergoing tracheotomy in 2006 (n=2204). Model 3 was limited to infants less than 1 year of age, representing 45% (n=2103) of all children undergoing tracheotomy in 2006.
Model 1
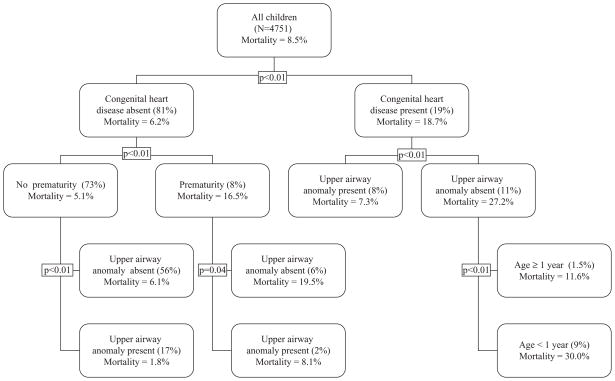
In 2006, congenital heart disease was the characteristic most significantly associated with in-hospital mortality among all children undergoing tracheotomy. In-hospital mortality in children with congenital heart disease was 18.7% compared with 6.2% for children without congenital heart disease (p<0.01). The highest in-hospital mortality (30.0%) among children with congenital heart disease was observed in infants less than 1 year of age who did not have an upper airway anomaly; 9.1% of all children undergoing tracheotomy in 2006 had this combination of characteristics. In-hospital mortality was significantly lower (1.8% vs 30.0%, p<0.01) in children without congenital heart disease, who were not premature and who had an upper airway anomaly; 17.1% of all children undergoing tracheotomy in 2006 had this combination of characteristics (figure 2).
Figure 2.
In-hospital mortality for each node in the classification and regression tree (CART) partition analyses for tracheotomy operations performed in the year 2006. The p values indicate the significance of differences in mortality observed at each node.
Model 2
In 2006, prematurity was the characteristic most significantly associated with mortality in children with NI. Mortality in children with NI and prematurity was 23.5% compared with 5.4% in children with NI who were not premature (p<0.01). The highest in-hospital mortality (40.0%) following tracheotomy in children with NI and prematurity was observed in those children who also had congenital heart disease, but did not have an upper airway anomaly. The lowest in-hospital mortality was significantly lower (1.4% vs 40.0%, p<0.01) following tracheotomy in children with NI was observed in those children with an upper airway anomaly who did not have prematurity or congenital heart disease (data not shown).
Model 3
In 2006, the absence of an upper airway anomaly was the characteristic most significantly associated with mortality in infants less than 1 year of age. Mortality in infants with an upper airway anomaly was 5.4% compared with 18.9% in infants without an upper airway anomaly (p<0.01). The highest in-hospital mortality (34.0%) was observed in infants without an upper airway anomaly, who were premature and also had congenital heart disease. In-hospital mortality was significantly lower (2.9% vs 34.0%, p<0.001) in infants with an upper airway anomaly, who were not premature, and did not have congenital heart disease (data not shown).
DISCUSSION
This study complements existing literature by reporting important findings regarding patient characteristics that are associated with in-hospital mortality following tracheotomy in children. In both bivariate and partition regression analyses, prematurity, congenital heart disease and age less than 1 year were associated with higher mortality. The presence of an upper airway anomaly was consistently associated with lower mortality. Up to 30-fold differences in mortality were observed among children with different combinations of these characteristics.
We are grateful for the large tracheotomy sample size available from the KID that permitted the analysis of mortality by different patient demographic and clinical characteristics. Even when aggregating patient data across multiple decades, most single institutional studies achieve a sample size of a few hundred patients at best, and these studies do not report mortality differences by underlying diagnosis.10,17,19 Previous studies that utilised large administrative hospital databases reported mortality during the hospitalisation when tracheotomy was performed for all children, children with NI and infants age less than 1 year of age that are congruent with the present study.1–3
The present study adds to this previous work by presenting mortality based on combinations of different patient characteristics within these three cohorts. Combinations of co-morbid illnesses are often contemplated when evaluating prognosis and mortality in both paediatric and adult critically ill patients.30–32 Over or underestimating mortality following tracheotomy may occur if multiple conditions are not considered. For instance, in 2006 the overall mortality for children with NI undergoing tracheotomy was 7.6%. However, mortality for these children ranged from 1.4% to 40.0%, depending on which additional characteristics were present or absent.
One interesting finding from this study was that in-hospital mortality following tracheotomy remained statistically constant over time, despite an increasing prevalence of patient characteristics associated with higher mortality (table 1). Additional changes in demographics may have counterbalanced the impact of these characteristics and stabilised mortality over time. For example, there was a non-significant trend (p=0.1) of decreasing mortality in children undergoing tracheotomy with chronic lung disease and the ‘other’ category from 1997 to 2006 (figure 1). Similar to previous studies, a trend towards younger age at tracheotomy was observed.33,34 This trend may have kept mortality constant over time owing to the simultaneous increase in prevalence of infants age less than 1 year and children ages 1–4 years undergoing tracheotomy. These age cohorts were associated with the highest and lowest mortality, respectively, of all age groups following tracheotomy.
Lower mortality in children with an upper airway anomaly following tracheotomy is reported previously.3 It is possible that the risks associated with tracheotomy in children with an upper airway anomaly may be outweighed by lowering the mortality risk conferred by alleviating the upper airway obstruction.3 Under certain circumstances, the anomaly itself may be alleviated by medical or surgical intervention following tracheotomy, which may further decrease its mortality risk. The mortality risk may be even lower when there are no additional co-morbidities likely to compromise the child’s health. For instance, we observed the lowest mortality in children following tracheotomy with upper airway anomaly when both prematurity and congenital heart disease were absent (figure 2).
Tracheotomy in the absence of an upper airway anomaly may indicate situations where the underlying aetiology of respiratory insufficiency may remain following tracheotomy and contribute substantially to the risk of mortality. This situation may be especially relevant in children with congenital heart disease. The absence of an upper airway anomaly was the characteristic most significantly associated with higher mortality in children with congenital heart disease (figure 1). Upper airway anomaly and congenital heart disease were consistently found to be arranged side-by-side within each mortality partition model, suggesting that the pairing of these characteristics is important to consider when projecting mortality following tracheotomy. It is possible that congenital heart disease and an upper airway anomaly together are manifestations of an underlying genetic disorder or other congenital syndrome.35
Increased prevalence of children undergoing tracheotomy with congenital heart disease, and higher mortality in these children has not been described previously. Children with congenital heart disease may be predisposed to upper airway abnormalities from frequent intubation, prolonged ventilation support and recurrent laryngeal nerve injury.36 Improved survival of children with congenital heart disease may have permitted the development of longer-term restrictive or obstructive lower respiratory tract insufficiency requiring tracheotomy in some children.37,38 This may help explain the observed increase in tracheotomy performed in children with both congenital heart disease and chronic lung disease. These factors along with the intrinsic risk of morbidity associated with the congenital heart disease itself, may help explain the high mortality following tracheotomy experienced by these children.39
The association of prematurity with higher in-hospital mortality following tracheotomy has not been reported in previous studies. There are several potential reasons to explain this finding. Premature infants with very low birth weight or complex underlying conditions are at higher risk for mortality even without tracheotomy.40,41 Some infants may encounter acute life-threatening respiratory events and require tracheotomy to avoid immediate death.40,42,43 Care may be subsequently withdrawn or redirected following tracheotomy in these infants due to poor prognosis, failure of improved health status or other reasons.44–47 Care withdrawal may be occurring more frequently in premature infants following tracheotomy than in children with other chronic conditions when facing similar situations.48
Limitations
We could not determine the true reason for mortality from the administrative data available in KID. Important clinical data such as the number of failed extubations, total number of ventilator days, degree of long-term oxygenation or ventilation support and haemodynamic instability were unavailable.31,49 We were unable to follow patients over time after hospital discharge. Therefore, the in-hospital mortality we observed may underestimate longer-term death in these children.
We could not determine the primary indication for tracheotomy or the true reason for death from the administrative data. The clinical characteristics evaluated do not include all of the underlying clinical conditions that children with tracheotomy may possess. The characteristics were ascertained from administrative diagnosis codes which may be associated with coding error and bias.50 For instance, premature children may be more likely to receive a prematurity code during a newborn admission compared with an admission later in life.
The trends observed in the co-morbid conditions may have been influenced by ICD-9-CM coding changes.51 We evaluated each individual ICD-9-CM code in the present study for changes in description, coding number, new additions or discontinuation during the study period.51 We accounted for these changes to minimise their impact on the results. For example, ICD-9-CM code 438.50 (other paralytic syndrome including ‘locked-in state’ or ‘quadriplegia’) was used as a NI code. In 1998, the number of this code changed from 438.50 to 438.53. Therefore, for the 1997 analysis, we used 438.50 to capture this code. For the 2000, 2003 and 2006 analyses, we used 438.53.
Heterogeneity of severity of illness and underlying disease pathology exists within the clinical characteristics evaluated in the present study which likely influences mortality. For example, severe forms of congenital heart disease may be associated with higher mortality following tracheotomy than milder forms.39 The clinical course may be different in a child requiring tracheotomy with cystic fibrosis compared with bronchopulmonary dysplasia. Further investigation within each clinical characteristic is required to better understand these associations.
Implications and conclusions
Tracheotomy may be a critical intervention to maintain immediate survival in a child with a chronic medical condition who is experiencing life-threatening respiratory compromise. However, many of these children will not survive the hospitalisation, especially those children with multiple co-morbid conditions that are associated with an increased mortality risk.
The mortality information presented in this study complements a growing body of literature describing the health outcomes that children and their families experience following tracheotomy. Some studies report favourable outcomes,17,19,52–54 whereas others report that children experience repeated, lengthy hospitalisations following tracheotomy,3,18 and their parents experience an immense care giving burden that is associated with impaired quality of life, marital discord and loss of employment.55–57
The outpatient and community healthcare providers of a child under consideration for tracheotomy may be in an ideal position to collaborate with inpatient clinicians and augment patient and family counselling of risks and benefits. When a longitudinal, established relationship with the child exists, outpatient primary and specialty care clinicians, in particular, may offer an important comprehensive assessment of the child’s underlying chronic health conditions, long-term health trajectory and family’s projected ability to cope with the child having a tracheotomy. We hope the findings from the present study encourage paediatric providers of all types to better understand, inform and advise families regarding the factors that influence child health outcomes following tracheotomy.
Acknowledgments
JGB was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development career development award K23 HD058092. Waiver of informed consent was granted owing the retrospective nature of the data. The work contains no identifiable patient information.
Funding NIH
Footnotes
What is already known on this topic
▶ Guidance to help physicians inform and advise families facing decisions regarding tracheotomy in children with chronic health conditions and counsel them with good measures of risk and benefit has been difficult to develop. This is, in part, because these children have a heterogeneous array of underlying diagnoses and previous studies have small sample sizes that limit the ability to analyse how these diagnoses individually and in combination impact the risk of mortality.
What this study adds
▶ Congenital heart disease, prematurity, the absence of an upper airway anomaly and age <1 year were characteristics associated with higher mortality in children following tracheotomy. The highest in-hospital mortality (40.0%) following tracheotomy was observed in premature children who had both neuromuscular impairment and congenital heart disease, but did not have an upper airway anomaly.
Competing interests None.
Ethics approval This study was conducted with the approval of the Children’s Hospital, Boston. This research was approved by the institutional review board (IRB No M08-10-0467).
Provenance and peer review Not commissioned; externally peer reviewed.
References
- 1.Berry JG, Lieu TA, Forbes PW, et al. Hospital volumes for common pediatric specialty operations. Arch Pediatr Adolesc Med. 2007;161:38–43. doi: 10.1001/archpedi.161.1.38. [DOI] [PubMed] [Google Scholar]
- 2.Lewis CW, Carron JD, Perkins JA, et al. Tracheotomy in pediatric patients: a national perspective. Arch Otolaryngol Head Neck Surg. 2003;129:523–9. doi: 10.1001/archotol.129.5.523. [DOI] [PubMed] [Google Scholar]
- 3.Berry JG, Graham DA, Graham RJ, et al. Predictors of clinical outcomes and hospital resource use of children after tracheotomy. Pediatrics. 2009;124:563–72. doi: 10.1542/peds.2008-3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hawkins DB, Williams EH. Tracheostomy in infants and young children. Laryngoscope. 1976;86:331–40. doi: 10.1288/00005537-197603000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Primuharsa Putra SH, Wong CY, Hazim MY, et al. Paediatric tracheostomy in Hospital University Kebangsaan Malaysia – a changing trend. Med J Malaysia. 2006;61:209–13. [PubMed] [Google Scholar]
- 6.Carter P, Benjamin B. Ten-year review of pediatric tracheotomy. Ann Otol Rhinol Laryngol. 1983;92:398–400. doi: 10.1177/000348948309200422. [DOI] [PubMed] [Google Scholar]
- 7.Prescott CA, Vanlierde MJ. Tracheostomy in children – the Red Cross War Memorial Children’s Hospital experience 1980–1985. Int J Pediatr Otorhinolaryngol. 1989;17:97–107. doi: 10.1016/0165-5876(89)90085-2. [DOI] [PubMed] [Google Scholar]
- 8.Dempster JH, Dykes EH, Brown WC, et al. Tracheostomy in childhood. J R Coll Surg Edinb. 1986;31:359–63. [PubMed] [Google Scholar]
- 9.Rodgers BM, Rooks JJ, Talbert JL. Pediatric tracheostomy: long-term evaluation. J Pediatr Surg. 1979;14:258–63. doi: 10.1016/s0022-3468(79)80481-9. [DOI] [PubMed] [Google Scholar]
- 10.Wetmore RF, Handler SD, Potsic WP. Pediatric tracheostomy. Experience during the past decade. Ann Otol Rhinol Laryngol. 1982;91:628–32. doi: 10.1177/000348948209100623. [DOI] [PubMed] [Google Scholar]
- 11.Kremer B, Botos-Kremer AI, Eckel HE, et al. Indications, complications, and surgical techniques for pediatric tracheostomies – an update. J Pediatr Surg. 2002;37:1556–62. doi: 10.1053/jpsu.2002.36184. [DOI] [PubMed] [Google Scholar]
- 12.Stern AM, Markel H. Formative years: children’s health in the United States, 1880–2000. Ann Arbor, Michigan, USA: University of Michigan Press; 2002. [Google Scholar]
- 13.Seddon PC, Khan Y. Respiratory problems in children with neurological impairment. Arch Dis Child. 2003;88:75–8. doi: 10.1136/adc.88.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karim RM, Momin IA, Lalani II, et al. Aspiration pneumonia in pediatric age group: etiology, predisposing factors and clinical outcome. J Pak Med Assoc. 1999;49:105–8. [PubMed] [Google Scholar]
- 15.Arcand P, Granger J. Pediatric tracheostomies: changing trends. J Otolaryngol. 1988;17:121–4. [PubMed] [Google Scholar]
- 16.Pucher B, Szydlowski J, Steiner I, et al. Tracheotomy in children of the Pediatric ENT Department in years 1995–2005. Otolaryngol Pol. 2006;60:525–8. [PubMed] [Google Scholar]
- 17.Midwinter KI, Carrie S, Bull PD. Paediatric tracheostomy: Sheffield experience 1979–1999. J Laryngol Otol. 2002;116:532–5. doi: 10.1258/002221502760132403. [DOI] [PubMed] [Google Scholar]
- 18.Graf JM, Montagnino BA, Hueckel R, et al. Pediatric tracheostomies: a recent experience from one academic center. Pediatr Crit Care Med. 2008;9:96–100. doi: 10.1097/01.PCC.0000298641.84257.53. [DOI] [PubMed] [Google Scholar]
- 19.Ang AH, Chua DY, Pang KP, et al. Pediatric tracheotomies in an Asian population: the Singapore experience. Otolaryngol Head Neck Surg. 2005;133:246–50. doi: 10.1016/j.otohns.2005.03.085. [DOI] [PubMed] [Google Scholar]
- 20.Agency for Healthcare Research and Quality. Introduction to the HCUP Kids’ Inpatient Database (KID) 2003. Rockville, Maryland: 2005. [Google Scholar]
- 21.Agency for Healthcare Research and Quality. Introduction to the HCUP Kids’ Inpatient Database (KID) 2006. Rockville, Maryland: 2008. [Google Scholar]
- 22.Graham RJ, Fleegler EW, Robinson WM. Chronic ventilator need in the community: a 2005 pediatric census of Massachusetts. Pediatrics. 2007;119:e1280–7. doi: 10.1542/peds.2006-2471. [DOI] [PubMed] [Google Scholar]
- 23.Lasser MS, Liao JG, Burd RS. National trends in the use of antireflux procedures for children. Pediatrics. 2006;118:1828–35. doi: 10.1542/peds.2006-1185. [DOI] [PubMed] [Google Scholar]
- 24.Srivastava R, Downey EC, Feola P, et al. Quality of life of children with neurological impairment who receive a fundoplication for gastroesophageal reflux disease. J Hosp Med. 2007;2:165–73. doi: 10.1002/jhm.167. [DOI] [PubMed] [Google Scholar]
- 25.Schneier AJ, Shields BJ, Hostetler SG, et al. Incidence of pediatric traumatic brain injury and associated hospital resource utilization in the United States. Pediatrics. 2006;118:483–92. doi: 10.1542/peds.2005-2588. [DOI] [PubMed] [Google Scholar]
- 26.Connor JA, Gauvreau K, Jenkins KJ. Factors associated with increased resource utilization for congenital heart disease. Pediatrics. 2005;116:689–95. doi: 10.1542/peds.2004-2071. [DOI] [PubMed] [Google Scholar]
- 27.ChuR, Houchens R, Elixhauser A, et al. Using the KIDS’ Inpatient Database (KID) to Estimate Trends. Agency for Healthcare Research and Quality; 2007. [Google Scholar]
- 28.Healthcare Cost and Utilization Project. HCUPnet. Agency for Healthcare Research and Quality; Rockville, Maryland: 2009. [Google Scholar]
- 29.Brieman L, Freidman JH, Stone C, et al. Classification and regression trees. Belmont, California, USA: Wadsworth International; 1984. [Google Scholar]
- 30.Poses RM, McClish DK, Smith WR, et al. Prediction of survival of critically ill patients by admission comorbidity. J Clin Epidemiol. 1996;49:743–7. doi: 10.1016/0895-4356(96)00021-2. [DOI] [PubMed] [Google Scholar]
- 31.Farias JA, Frutos-Vivar F, Casado Flores J, et al. Grupo Internacional de la Ventilación Mecánica en Niños. Med Intensiva. 2006;30:425–31. doi: 10.1016/s0210-5691(06)74565-x. [Factors associated with the prognosis of mechanically ventilated infants and children. An international study] [DOI] [PubMed] [Google Scholar]
- 32.Pollack MM, Ruttimann UE, Getson PR. Pediatric risk of mortality (PRISM) score. Crit Care Med. 1988;16:1110–16. doi: 10.1097/00003246-198811000-00006. [DOI] [PubMed] [Google Scholar]
- 33.Wetmore RF, Marsh RR, Thompson ME, et al. Pediatric tracheostomy: a changing procedure? Ann Otol Rhinol Laryngol. 1999;108:695–9. doi: 10.1177/000348949910800714. [DOI] [PubMed] [Google Scholar]
- 34.Zeitouni A, Manoukian J. Tracheotomy in the first year of life. J Otolaryngol. 1993;22:431–4. [PubMed] [Google Scholar]
- 35.Pearl W. Congenital heart disease in the Pierre Robin syndrome. Pediatr Cardiol. 1982;2:307–9. doi: 10.1007/BF02426978. [DOI] [PubMed] [Google Scholar]
- 36.Khariwala SS, Lee WT, Koltai PJ. Laryngotracheal consequences of pediatric cardiac surgery. Arch Otolaryngol Head Neck Surg. 2005;131:336–9. doi: 10.1001/archotol.131.4.336. [DOI] [PubMed] [Google Scholar]
- 37.Fitzgerald DA, Sherwood M. Long-term cardio-respiratory consequences of heart disease in childhood. Paediatr Respir Rev. 2007;8:313–21. doi: 10.1016/j.prrv.2007.08.006. quiz 321–2. [DOI] [PubMed] [Google Scholar]
- 38.LoTempio MM, Shapiro NL. Tracheotomy tube placement in children following cardiothoracic surgery: indications and outcomes. Am J Otolaryngol. 2002;23:337–40. doi: 10.1053/ajot.2002.126854. [DOI] [PubMed] [Google Scholar]
- 39.Jenkins KJ, Gauvreau K, Newburger JW, et al. Consensus-based method for risk adjustment for surgery for congenital heart disease. J Thorac Cardiovasc Surg. 2002;123:110–18. doi: 10.1067/mtc.2002.119064. [DOI] [PubMed] [Google Scholar]
- 40.Berger TM, Hofer A. Causes and circumstances of neonatal deaths in 108 consecutive cases over a 10-year period at the Children’s Hospital of Lucerne, Switzerland. Neonatology. 2009;95:157–63. doi: 10.1159/000153100. [DOI] [PubMed] [Google Scholar]
- 41.Wootten CT, French LC, Thomas RG, et al. Tracheotomy in the first year of life: outcomes in term infants, the Vanderbilt experience. Otolaryngol Head Neck Surg. 2006;134:365–9. doi: 10.1016/j.otohns.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 42.Markestad T, Kaaresen PI, Rønnestad A, et al. Norwegian Extreme Prematurity Study Group. Early death, morbidity, and need of treatment among extremely premature infants. Pediatrics. 2005;115:1289–98. doi: 10.1542/peds.2004-1482. [DOI] [PubMed] [Google Scholar]
- 43.Bancalari E, Claure N, Sosenko IR. Bronchopulmonary dysplasia: changes in pathogenesis, epidemiology and definition. Semin Neonatol. 2003;8:63–71. doi: 10.1016/s1084-2756(02)00192-6. [DOI] [PubMed] [Google Scholar]
- 44.Goc B, Walencka Z, Wloch A, et al. Trisomy 18 in neonates: prenatal diagnosis, clinical features, therapeutic dilemmas and outcome. J Appl Genet. 2006;47:165–70. doi: 10.1007/BF03194617. [DOI] [PubMed] [Google Scholar]
- 45.Moro T, Kavanaugh K, Okuno-Jones S, et al. Neonatal end-of-life care: a review of the research literature. J Perinat Neonatal Nurs. 2006;20:262–73. doi: 10.1097/00005237-200607000-00015. [DOI] [PubMed] [Google Scholar]
- 46.Masri C, Farrell CA, Lacroix J, et al. Decision making and end-of-life care in critically ill children. J Palliat Care. 2000;16(Suppl):S45–52. [PubMed] [Google Scholar]
- 47.Camhi SL, Mercado AF, Morrison RS, et al. Deciding in the dark: advance directives and continuation of treatment in chronic critical illness. Crit Care Med. 2009;37:919–25. doi: 10.1097/CCM.0b013e31819613ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ciais JF. Questions concerning an endotracheal intubation or a tracheotomy in amyotrophic lateral sclerosis. Rev Neurol (Paris) 2006;162(Spec No 2):4S323–4S328. [PubMed] [Google Scholar]
- 49.Overstreet DW, Jackson JC, van Belle G, et al. Estimation of mortality risk in chronically ventilated infants with bronchopulmonary dysplasia. Pediatrics. 1991;88:1153–60. [PubMed] [Google Scholar]
- 50.Miller ML, Wang MC. Accuracy of ICD-9-CM coding of cervical spine fractures: Implications for research using administrative databases. Annu Proc Assoc Adv Automot Med. 2008;52:101–5. [PMC free article] [PubMed] [Google Scholar]
- 51.National Center for Health Statistics, Centers for Medicare and Medicaid Services. Conversion Table of New ICD-9-CM Codes. Oct, 2004. [Google Scholar]
- 52.Gray RF, Todd NW, Jacobs IN. Tracheostomy decannulation in children: approaches and techniques. Laryngoscope. 1998;108:8–12. doi: 10.1097/00005537-199801000-00002. [DOI] [PubMed] [Google Scholar]
- 53.Shinkwin CA, Gibbin KP. Tracheostomy in children. J R Soc Med. 1996;89:188–92. doi: 10.1177/014107689608900404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.French LC, Wootten CT, Thomas RG, et al. Tracheotomy in the preschool population: indications and outcomes. Otolaryngol Head Neck Surg. 2007;137:280–3. doi: 10.1016/j.otohns.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 55.Hartnick CJ, Giambra BK, Bissell C, et al. Final validation of the Pediatric Tracheotomy Health Status Instrument (PTHSI) Otolaryngol Head Neck Surg. 2002;126:228–33. doi: 10.1067/mhn.2002.122634. [DOI] [PubMed] [Google Scholar]
- 56.Thyen U, Kuhlthau K, Perrin JM. Employment, child care, and mental health of mothers caring for children assisted by technology. Pediatrics. 1999;103:1235–42. doi: 10.1542/peds.103.6.1235. [DOI] [PubMed] [Google Scholar]
- 57.Thyen U, Terres NM, Yazdgerdi SR, et al. Impact of long-term care of children assisted by technology on maternal health. J Dev Behav Pediatr. 1998;19:273–82. doi: 10.1097/00004703-199808000-00006. [DOI] [PubMed] [Google Scholar]