Abstract
The macrolactonization of peptide thioester to yield cyclic depsipeptides was developed using imidazole as a catalyst. This strategy was applied to the synthesis of kahalalide B and its analogues.
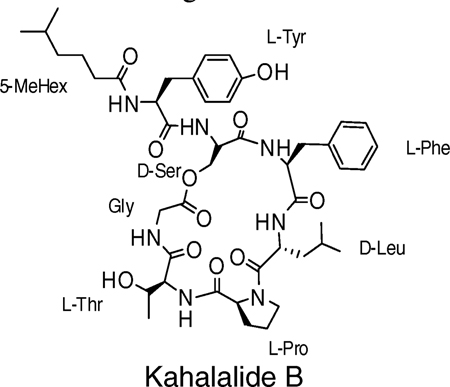
Bioactive cyclic peptides and depsipeptides that are isolated from natural sources provide a range of lead structures for the design of new drugs.1 Kahalalide compounds, A-F, are cyclic depsipeptides isolated from a sacoglossan mollusk, Elysia rufescens, and its algal diet Bryopsis sp. They range from a C31 tetrapeptide to a C75 octapeptide.2 Kahalalide compounds have similar structures with an endocyclic depsipeptide bond and fatty acids at the N-termini. Kahalalides exhibit a diverse spectrum of biological activities, including antiviral, antimicrobial, and antitumor activity.3 Though the pharmaceutical potential of these natural compounds is high, isolation and purification of larger quantities of this class of products is difficult. The development of synthetic strategies for natural compounds and their analogues is essential for the discovery of lead compounds of this type for use in drug discovery.4
The most commonly used methodology for the synthesis of cyclic depsipeptide involes cyclization of a linear depsipeptide containing an internal ester bond. The intramolecular macrolactamization can be carried out either in solution or on resin.5 Problematically, the endoester bond may break during the acidic cleavage when a typical solid-phase synthetic strategy is applied to make the linear precursor and/or cyclic product. The choice of resins is thus limited, and some of the widely used resins, which require strong acidic cleavage conditions, cannot be successfully used. To overcome this limitation, macrolactonization is a favorable alternative. Since the lactonization is much less efficient than the lactamization under conventional coupling conditions, synthesis of cyclic depsipeptides by this strategy is rarely reported and remains a challenge.6
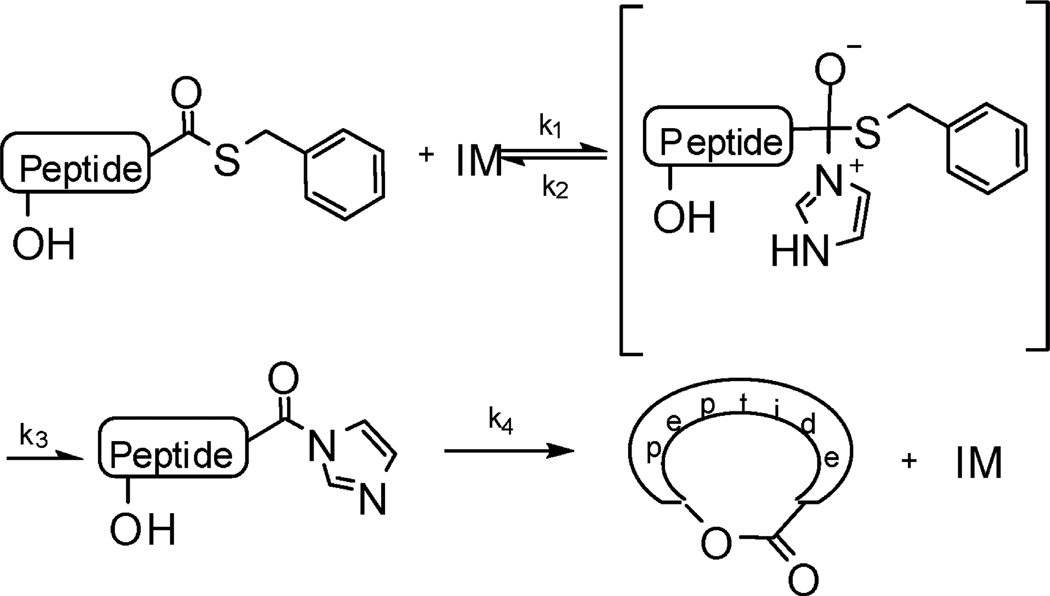
Biosynthesis of cyclic depsipeptides through peptide synthetase has been reported by using peptide thioester as substrate.7 Studies of different peptide synthetase systems suggest the possibility that a histidine residue functions as a catalyst of condensation/elongation/cyclization reaction in the peptide synthesis.8 Imidazole has been reported as a catalyst mimicking the histidine residues of enzymes hydrolyzing the ester bond and the thioester bond.9 Our recent studies have shown that a cyclic peptide can be formed through direct aminolysis of a peptide benzylthioester catalyzed by imidazole.10 The generally accepted mechanism of the imidazole catalysis involves the reaction intermediates of acyl imidazole and the acyl imidazolium cation, which are formed by the direct attack of imidazole on the carbonyl group. This mechanism can also be envisioned for the formation of a cyclic depsipeptide by macrolactonization. The mild imidazole-catalytic condition may also eliminate the risk of racemization during acyl activation. Herein, we report a imidazole-catalytic approach for the synthesis of cyclic depsipeptides by macrolactonization of peptide thioesters and the use of this application in the synthesis of kahalalide B and its analogues.
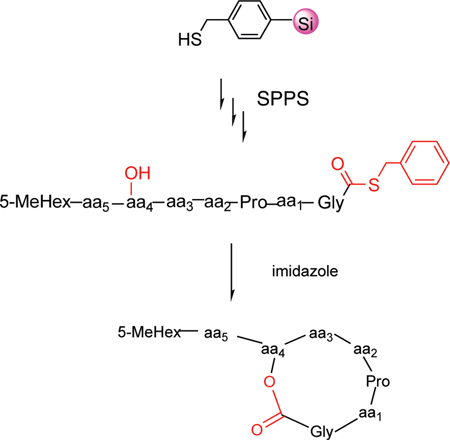
The stepwise synthesis of the linear peptides starts with functionalized mercaptomethylphenyl silica gel 1 as the “volatilizable” support (Scheme 1).11 PyBOP/DIEA was used as the protected amino acid activation reagent to generate resin bound 2. Boc-glycine was coupled on the resin as the C-terminal residue based on the structure of kahalalide B. After removal of the Boc group with 55% TFA, threonine, proline, d-leucine, phenylalanine, d-serine, tyrosine, and 5-methylhexanoic acid were coupled stepwise to form the on-resin synthetic precursor of kahalalide B 3a. The resin-bound 3a was then treated with anhydrous HF for 2 h at 0 °C. Following evaporation of the anhydrous HF with a gaseous nitrogen stream, the unprotected peptide thioester 4a was obtained following lyophlization.
Scheme 1.
Synthesis of Kahalalide B and Its Analogues through Macrolactonization of Peptide Thioester Catalyzed by Imidazole
The cyclization to the depsipeptide was performed by macrolactonization in acetonitrile using imidazole as a catalyst. The effect of imidazole on catalytic esterification and cyclization was first tested using an N-acetyl pentapeptide thioester Ac-Xxx-Ala-Phe-Tyr-Gly-SCH2Ph, where Xxx was Ser or Thr. It was found the concentration of imidazole significantly accelerated the macrolactonization. Macrolactonization by the hydroxyl on the serine residue was complete after a 24 h reaction at room temperature gave a yield over 95% when the concentration of imidazole was 1.5 M, but failed even after reacting for 10 days when the concentration was 0.15 M. The effect of the concentration of imidazole on macrolactonization by peptide threonine had a similar result but a lower yield of 70% after reacting for 24 h. The need for a high concentration of imidazole likely suggests that the formation of the imidazolyl intermediate was rate-limiting (Scheme 2). Increasing the concentration of imidazole thus drives the formation of the imidazolyl intermediate. The formation of kahalalide B (5a) was tested by dissolving 4a to a concentration of 1 mM in 1.5 M imidazole in acetonitrile at room temperature for 24 h. A portion of the reaction mixture was examined by LC–MS. It was found that the cyclic depsipeptide of kahalalide B was quantitatively formed. When compared to the reported strategy of PyBOP-DIEA activation for macrolactonization, the yield of cyclization of the same compound is much higher using the present experimental approach (reported, 28%; current, quantitative).12 To accecelarate the cyclization, microwave reaction was tested under the same concentrations but at 65 °C in a separate experiment. It was found that the cyclization was complete within 2 h with a quantitative conversion.
Scheme 2.
Mechanism of Imidazole Catalysis of Macrolactonization
Because there are also hydroxyl groups on the residue of serine or threonine at position aa1 and tyrosine at position aa5, the cyclic product identified in LC–MS analysis could be either the expected kahalalide B or the esterification product of C-terminal thioester by the hydroxyl groups of these residues. To distinguish these possible products, semipreparative reverse-phase HPLC was used to purify the cyclic product. The purified product was analyzed by NMR spectroscopy and found to be identical to the natural kahalalide B reported earlier.12 In addition, linear peptides CH3CO-Tyr-Ala-Phe-Tyr-Gly-SCH2Ph, CH3CO-Ser-Gly-SCH2Ph, and CH3CO-Thr-Gly-SCH2Ph were also synthesized, and cyclizations were attempted under the same conditions as the cyclization of kahalalide B. No cyclization products were detected in the three cases even after reacting for 3 days.
Analogues of kahalalide B (5b–e) were synthesized by selectively changing residues at positions aa1, aa2, aa3, aa4, and aa5. Residues of glycine and proline were reserved in the cyclic product. The yield and purity of kahalalide B and its analogues are shown in Table 1.
Table 1.
Kahalalide B and Analogues Synthesized by Macrolactonization
| linear peptide thioesters |
cyclic products |
||||||
|---|---|---|---|---|---|---|---|
| entry | yield (%)a |
yield (%)b |
MW (calcd)c |
MW (obsd)d |
yield (%)e |
MW (calcd)c |
MW (obsd)d |
| a | 90 | 42 | 1002.5 | 1002.5 | 98 | 878.5 | 878.5 |
| b | 92 | 40 | 1004.5 | 1004.5 | 90 | 880.4 | 880.4 |
| c | 90 | 57 | 930.4 | 930.4 | 85 | 806.4 | 806.4 |
| d | 94 | 51 | 960.5 | 960.5 | 80 | 836.4 | 836.4 |
| e | 90 | 46 | 1016.5 | 1016.5 | 95 | 892.5 | 892.5 |
Yield is based on the weight of crude product and the amount of resin used.
Yield is based on the weight of purified product (purity >95%) and the amount of resin used.
Caclulated from [M + H].
[M + H]+ by ESI-MS.
Yield is based on the weights of purified cyclic products and their purified linear peptide thioesters.
In summary, we present here a novel method for the synthesis of cyclic depsipeptides by macrolactonization of peptide thioesters. The esterifaction/cycliczation was greatly faciliated with catalysis by imidazole. Kahalalide B and its analogues were obtained in high yields and purities by this method. This strategy is promising for the synthesis of other natural cyclic depsipeptides.
Supplementary Material
Acknowledgment
This work was supported by the State of Florida, Executive Officer of the Governor’s Office of Tourism, Trade and Economic Development, National Science Foundation (RAH CHE 0455072), 1P41GM079590, 1P41GM081261, and U54HG03916-MLSCN.
Footnotes
Supporting Information Available: Experimental detail, compound characterization, copies of 1H NMR, 13C NMR spectra, HRMS, and LC–MS for cyclic compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Xiao Q, Pei D. J. Med. Chem. 2007;50:3132. doi: 10.1021/jm070282e. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Qin C, Bu X, Zhong X, Ng NLJ, Guo Z. J. Comb. Chem. 2004;6:398. doi: 10.1021/cc030117u. [DOI] [PubMed] [Google Scholar]; (c) Mahlert C, Sieber SA, Grünewald J, Marahiel MA. J. Am. Chem. Soc. 2005;127:9571. doi: 10.1021/ja051254t. [DOI] [PubMed] [Google Scholar]; (d) Liu S, Gu W, Lo D, Ding X-Z, Ujiki M, Adrian TE, Soff GA, Silivermann RB. J. Med. Chem. 2005;48:3630. doi: 10.1021/jm048952t. [DOI] [PubMed] [Google Scholar]; (e) Gulavita NK, Wright AE, McCarthy PJ, Pomponi SA, Longley RE. J. Nat. Toxins. 1996;5:225. [Google Scholar]
- 2.Hamann MT, Otto CS, Scheuer P. J. Org. Chem. 1996;61:6594. doi: 10.1021/jo960877+. [DOI] [PubMed] [Google Scholar]
- 3.(a) Rawat DS, Joshi MC, Joshi P, Atheaya H. Anticancer Agents Med. Chem. 2006;6:33. doi: 10.2174/187152006774755519. [DOI] [PubMed] [Google Scholar]; (b) Cruz LJ, Luque-Ortega JR, Rivas L, Albericio F. Mol. Pharm. 2009;6:813. doi: 10.1021/mp8001039. [DOI] [PubMed] [Google Scholar]; (c) Hamann MT. Curr. Opin. Mol. Ther. 2004;6:657. [PMC free article] [PubMed] [Google Scholar]; (d) García-Rocha M, Bonay P, Avila J. Cancer Lett. 1996;99:43. doi: 10.1016/0304-3835(95)04036-6. [DOI] [PubMed] [Google Scholar]; (e) Copp BR. Nat. Prod. Rep. 2003;6:535. doi: 10.1039/b212154a. [DOI] [PubMed] [Google Scholar]
- 4.(a) López-Macià À, Jiménez JC, Royo M, Giralt E, Albericio F. Tetrahedron Lett. 2000;41:9765. [Google Scholar]; (b) Bourel-Bonnet L, Rao KV, Hamann MT, Ganesan A. J. Med. Chem. 2005;48:1330. doi: 10.1021/jm049841x. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Jiménez JC, Chavarría B, López-Macià À, Royo M, Giralt E, Albericio F. Org. Lett. 2003;5:2115. doi: 10.1021/ol0345273. [DOI] [PubMed] [Google Scholar]; (d) López-Macià À, Jiménez JC, Royo M, Giralt E, Albericio F. J. Am. Chem. Soc. 2001;123:11398. doi: 10.1021/ja0116728. [DOI] [PubMed] [Google Scholar]; (e) Gracia C, Isidro-Llobet A, Cruz LJ, Acosta GA, Álvarez M, Cuevas C, Giralt E, Albericio F. J. Org. Chem. 2006;71:7196. doi: 10.1021/jo060976f. [DOI] [PubMed] [Google Scholar]
- 5.(a) Coin I, Beerbaum M, Schmieder P, Bienert M, Beyermann M. Org. Lett. 2008;10:3857. doi: 10.1021/ol800855p. [DOI] [PubMed] [Google Scholar]; (b) Bisek N, Wetzel S, Arndt H-D, Waldmann H. Chem.—Eur. J. 2008;14:8847. doi: 10.1002/chem.200800692. [DOI] [PubMed] [Google Scholar]; (c) Bowers A, West N, Taunton J, Schreiber SL, Bradner JE, Williams RM. J. Am. Chem. Soc. 2008;130:11219. doi: 10.1021/ja8033763. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Xie W, Ding D, Zi W, Li G, Ma D. Angew. Chem., Int. Ed. 2008;47:2844. doi: 10.1002/anie.200705557. [DOI] [PubMed] [Google Scholar]; (e) Tan L, Ma D. Angew. Chem., Int. Ed. 2008;47:3614. doi: 10.1002/anie.200800397. [DOI] [PubMed] [Google Scholar]; (f) Ruiz-Rodríguez J, Spengler J, Albericio F. Angew. Chem., Int. Ed. 2009;48:1. doi: 10.1002/anie.200904135. [DOI] [PubMed] [Google Scholar]; (G) Wen S, Packham G, Ganesan A. J. Org. Chem. 2008;73:9353. doi: 10.1021/jo801866z. [DOI] [PubMed] [Google Scholar]; (h) Lee Y, Silverman RB. Org. Lett. 2000;2:3743. doi: 10.1021/ol0002830. [DOI] [PubMed] [Google Scholar]
- 6.(a) Li KW, Wu J, Xing W, Simon JA. J. Am. Chem. Soc. 1996;118:7237. [Google Scholar]; (b) Sarabia F, García-Castro M, Chammaa S. Tetrahedron Lett. 2005;46:7695. [Google Scholar]
- 7.(a) Tseng CC, Bruner SD, Kohli RM, Marahiel MA, Walsh CT, Sieber SA. Biochemistry. 2002;41:13350. doi: 10.1021/bi026592a. [DOI] [PubMed] [Google Scholar]; (b) Grnewald J, Sieber SA, Marahiel MA. Biochemistry. 2004;43:2915. doi: 10.1021/bi036140d. [DOI] [PubMed] [Google Scholar]; (c) Mahlert C, Sieber SA, Grnewald J, Marahiel MA. J. Am. Chem. Soc. 2005;127:9571. doi: 10.1021/ja051254t. [DOI] [PubMed] [Google Scholar]
- 8.(a) Kohli RM, Walsh CT, Burke MD. Nature. 2002;418:658. doi: 10.1038/nature00907. [DOI] [PubMed] [Google Scholar]; (b) Marahiel MA, Stachelhaus T, Mootz HD. Chem. ReV. 1997;97:2651. doi: 10.1021/cr960029e. [DOI] [PubMed] [Google Scholar]; (c) Sieber S, Tao J, Walsh CT, Marahiel MA. Angew. Chem., Int. Ed. 2004;43:493. doi: 10.1002/anie.200352787. [DOI] [PubMed] [Google Scholar]; (d) Trauger JW, Kohli RM, Mootz HD, Marahiel MA, Walsh CT. Nature. 2000;407:215. doi: 10.1038/35025116. [DOI] [PubMed] [Google Scholar]; (e) Kohli RM, Trauger JW, Schwarzer D, Marahiel MA, Walsh CT. Biochemistry. 2001;40:7099. doi: 10.1021/bi010036j. [DOI] [PubMed] [Google Scholar]; (f) Trauger JW, Kohli RM, Walsh CT. Biochemistry. 2001;40:7092. doi: 10.1021/bi010035r. [DOI] [PubMed] [Google Scholar]; (g) Stachelhaus T, Mootz HD, Bergendahl V, Marahieli MA. J. Bio. Chem. 1998;273:22773. doi: 10.1074/jbc.273.35.22773. [DOI] [PubMed] [Google Scholar]
- 9.(a) Bruice TC, Schmir GL. J. Am. Chem. Soc. 1957;79:1663. [Google Scholar]; (b) Bruice TC, Schmir GL. J. Am. Chem. Soc. 1958;80:148. [Google Scholar]; (c) Bruice TC, Schmir GL. J. Am. Chem. Soc. 1958;80:2265. [Google Scholar]; (d) Kirsch JF, Jencks WP. J. Am. Chem. Soc. 1964;86:833. [Google Scholar]; (e) Yamada H, Kuroki R, Hirata M, Imoto T. Biochemistry. 1983;22:4551. doi: 10.1021/bi00288a031. [DOI] [PubMed] [Google Scholar]; (f) Bender ML, Turnqest BW. J. Am. Chem. Soc. 1957;79:1652. [Google Scholar]
- 10.Li Y, Yongye A, Giulianotti M, Martinez-Mayorga K, Yu Y, Houghten RA. J. Comb. Chem. 2009;11:1066. doi: 10.1021/cc900100z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.(a) Li Y, Yu Y, Giulianotti M, Houghten RA. J. Comb. Chem. 2008;10:613. doi: 10.1021/cc800076b. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Houghten RA, Yu Y. J. Am. Chem. Soc. 2005;127:8582. doi: 10.1021/ja0402396. [DOI] [PubMed] [Google Scholar]
- 12.López-Macià À, Jiménez JC, Royo M, Giralt E, Albericio F. Tetrahedron Lett. 2000;41:9765. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.