Abstract
Herein we report a new type of in vivo fluorogenic probe that enables simultaneous and active targeting of overexpressed receptors, αvβ3 integrins, and extracellular proteases, matrix metalloproteinases (MMPs), in the tumor regions. This c(RGDyK)-conjugated MMP fluorogenic probe efficiently targets the tumor regions with high retention time while maintaining receptor binding affinity and substrate activity. The probe minimizes nonspecific accumulation, and thus demonstrating improved tumor-to-background signal ratio (T/N) in both αvβ3 integrin- and MMP-overexpressing U87MG tumor-bearing mouse model. This strategy can be easily tuned for a wide array of applications targeting various receptors and extracellular proteases in vivo.
Fluorogenic substrates, consisting of protease substrates labeled with a fluorescent reporter and a quencher dye, are highly sensitive and sequence specific that have long been used in in vitro diagnostic assays. Recent advances in optical imaging instrumentation and development of various near-infrared (NIR) fluorescent dyes and NIR quenchers enabled the use of conventional fluorogenic substrates for in vivo imaging applications. Any reported fluorogenic substrate with known sequences can be easily modified to NIR fluorogenic probes, so called peptide-based activatable probes,1–6 for in vivo imaging by replacing conventional dye molecules to a NIR fluorescent dye/quencher pair. Although reported NIR fluorogenic probes showed potential in in vivo imaging, short half-life, poor pharmacokinetic profiles, instability, and high background induced by a nonspecific degradation of fluorogenic substrates still hamper its application.
Development of novel techniques for noninvasive imaging of specific protease’s activity is critical and urgently needed. Proteases are known as extremely important signaling molecules that are involved in numerous pathological processes including cancer, inflammatory, neurological, and cardiovascular diseases.7–9 Successful protease imaging techniques can be used for studying the role of protease expressions in protease-associated disease animal models or monitoring therapeutic efficacy of a number of newly developed protease inhibitors in vivo. Various unique forms of protease-activatable probes have been designed in combination with fluorogenic substrates and materials including targeting ligands, polymers, and inorganic metals such as gold nanoparticles and quantum dots.1–6, 10–18 Although the use of activatable probes seems to be popular, surprisingly, very limited platforms of activatable probes are available which can clearly image proteases’ activity in vivo after systemic administration. Unfortunately, the majority of reported systems demonstrate their proof-of-concept only in vitro or in ex vivo conditions such as after intratumoral injection in tumor models.
Ideal activatable probes can be designed by increasing in vivo stability and/or target (i.e. tumor) specificity of the fluorogenic probe while maintaining biological activity against specific proteases. Chemically, these improvements can be achieved by modifying fluorogenic peptides with water-soluble biomacromolecules such as PEGylated poly-L-lysine11, 19 or tumor-homing polymeric nanoparticles10 since both polymers can increase the circulation half-life and efficiently deliver fluorogenic probes to the tumor mainly by the enhanced permeability retention (EPR) effect.20 Indeed, these concepts showed promising results in many in vivo models, however, there are some drawbacks. For example, such probes take a long time for full activation due to the decreased substrate sensitivity from the conjugated high molecular weight polymer backbone and more importantly, their EPR effect may cause nonspecific uptake causing false fluorescence signals in vivo.
In this study, we report a fluorogenic probe with an active targeting ligand that can be used as an efficient optical imaging probe to monitor extracellular protease expressions. We hypothesize that a targeting ligand will efficiently increase the retention time of the probe in the target region and maintain substrate activity, minimizing nonspecific accumulation and enhancing tumor-to-background signal ratio. To test the rationale of our strategy, we adopted a cyclic Arg-Gly-Asp peptide, c(RGDyK), a well-known αvβ3 integrin ligand that is overexpressed on the surface of angiogenic endothelial cells that mediates cell adhesion.21–23 We synthesized c(RGDyK)-labeled matrix metalloproteinases (MMPs) specific fluorogenic probes, confirmed their enzyme and receptor activity in vitro and demonstrated enhanced imaging of MMPs in both an αvβ3 integrin- and MMP-overexpressing tumor-bearing mouse model. MMPs are a large family of zinc-dependent proteases and play a crucial role in a variety of pathological disorders including cancer and inflammation diseases.24 Accordingly, MMPs are important pharmaceutical and diagnostic targets for treatment of these diseases.
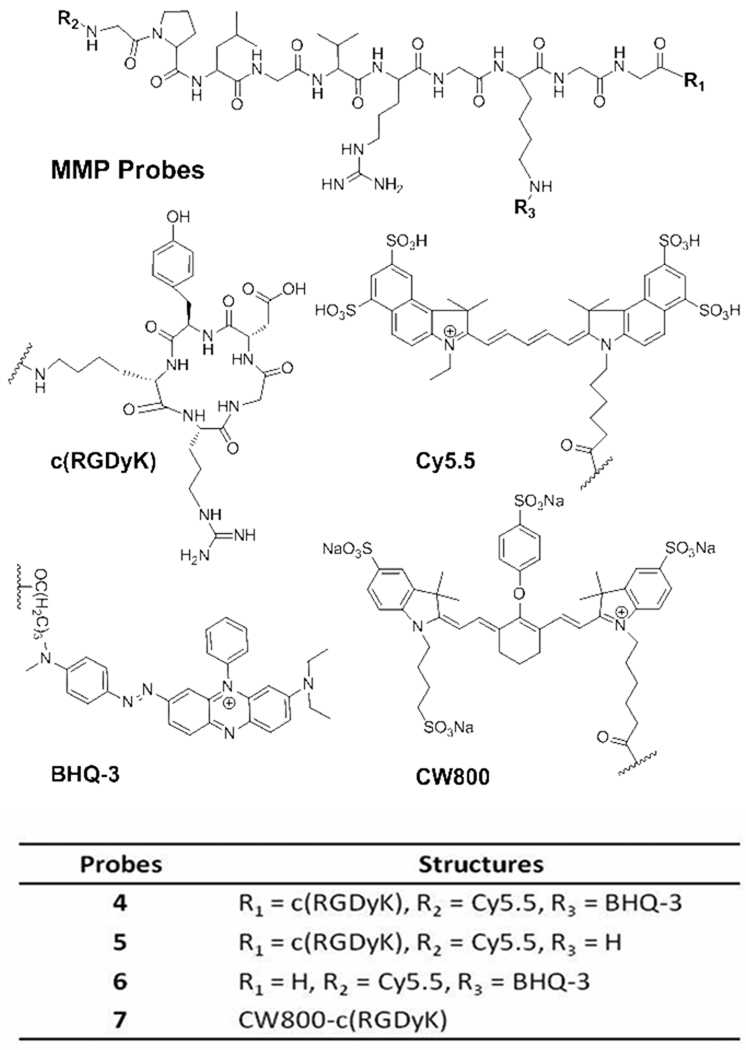
Our lead compound, MMP-P-RGD conjugate 4, is composed of: i) Cy5.5 as a NIR dye (ex/em; 675/695 nm), ii) the MMP-cleavable substrate GPLGVRGKGG as a recognition site indicated by italics with the cleavage site between Gly and Val, iii) NIR dark quencher BHQ-325 (abs. 650 nm), and iv) c(RGDyK) as a targeting ligand. Two Gly residues were incorporated as a spacer between the MMPs-activatable fluorogenic peptide and the targeting ligand. Figure 1 shows the chemical structure of 4. The multi-step synthetic route involves three parts: i) conjugation of c(RGDyK) to NHS ester activated C-terminal carboxyl group in fully protected MMP substrate 1, Fmoc-GPLGVR(Pbf)GK(tboc)GG; ii) labeling NHS ester of NIR Cy5.5 dye to the Fmoc-deprotected N-terminal α-amino group of 2, GPLGVR(Pbf)GK(tboc)GG-c(RGDyK); iii) conjugation of NHS ester of NIR dark quencher BHQ-3 to the ε-amino group of lysine followed by TFA cleavage of 3, Cy5.5-GPLGVRGKGG-c(RGDyK). The peptide sequence of 1 was synthesized by standard solid-phase Fmoc peptide chemistry. In addition, only Cy5.5-labeled analog, Cy5.5-MMP-RGD 5 was prepared for cellular imaging. As controls, an MMP fluorogenic peptide without c(RGDyK) ligand, MMP-P 6 and CW800 dye (ex/em; 775/790 nm)-labeled c(RGDyK), CW800-RGD 7, were also synthesized. Serial conjugation and deprotection of the peptide was performed in solution followed by preparative HPLC purifications. Purified products were confirmed by analytical HPLC and LC/MS (see the Supporting Information for details).
Figure 1.
Chemical structures of relevant compounds.
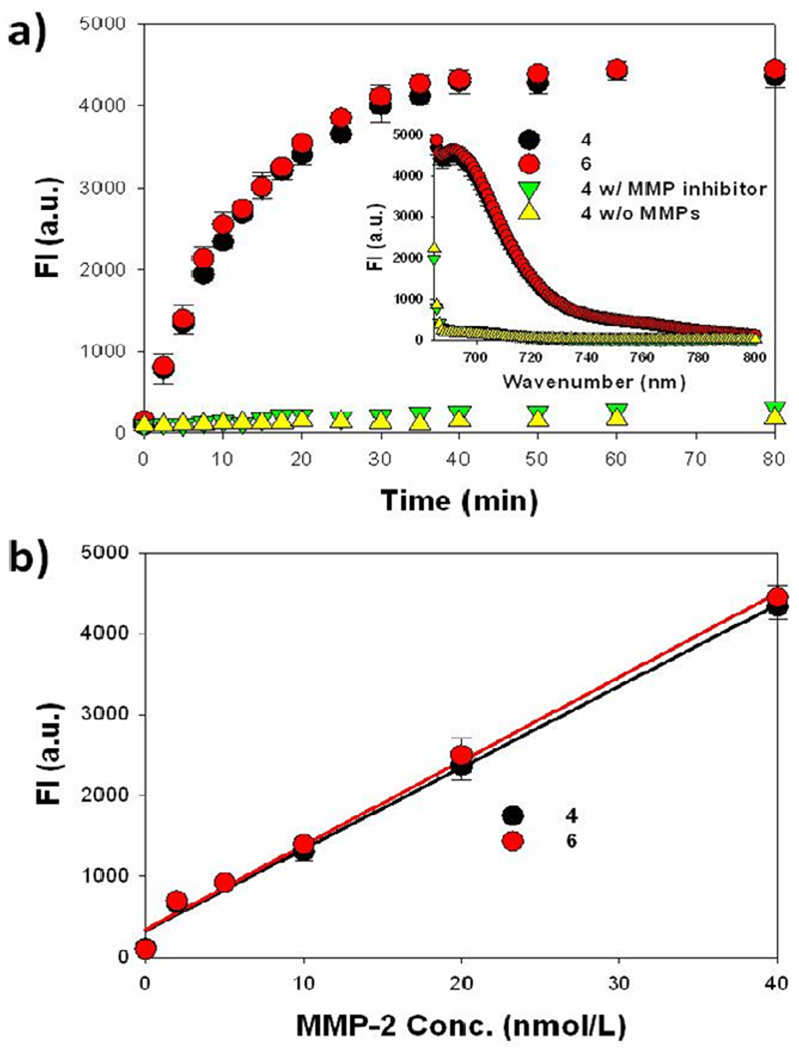
Firstly, the MMP specificity of 4 was compared to that of 6 to confirm whether conjugation of the targeting ligand affects its enzyme specificity. In vitro enzyme specificity was measured in the reaction buffer (100 mM Tris, 200 mM NaCl, 5 mM CaCl2, 0.1% Brij, pH 7.5) containing activated MMP-2 with and without a homophenylalanine-hydroxamine acid based broad spectrum MMP inhibitor (EMD Bioscience) using a spectrofluorometer. The MMP-2 was activated by incubation of 2.5 mM of p-aminophenyl mercuric acid (APMA) in the reaction buffer for 2 h at 37 °C prior to use. As shown in Figure 2a, MMP-2 (40 nM) treatment of 4 and 6 (15 nM) resulted in an increased recovery of the NIR fluorescence signal and showed greater than 20-fold increase relative to the control sample without MMP-2. The fluorescence recovery of 4 was inhibited in the presence of the MMP inhibitor indicating probe selectivity. Similar experiments were performed by incubating 4 in the presence of different concentrations of MMP-2 (0, 2, 5, 10, 20, 40 nM) and the recovery of fluorescence was dependent on the MMP-2 concentration (Figure 2b). The overall MMP specificity of 4 against MMP-2 was similar to that of 6. These results indicate that conjugation of the targeting ligand c(RGDyK) did not alter the MMP specificity of the probe.
Figure 2.
In vitro MMP specificy of MMP-P-RGD 4 and MMP-P 6. (a) Fluorescence emission kinetic spectra of the probes in the presence of MMP-2 with and without a broad spectrum MMP inhibitor. Inset: Fluorescence emission spectra of the probes at 80 min. (b) Fluorescence intensities of the probes in the presence of various concentrations of activated MMP-2 (0, 2, 5, 10, 20, and 40 nM) following an 80 min incubation. Means ± SD (n = 3).
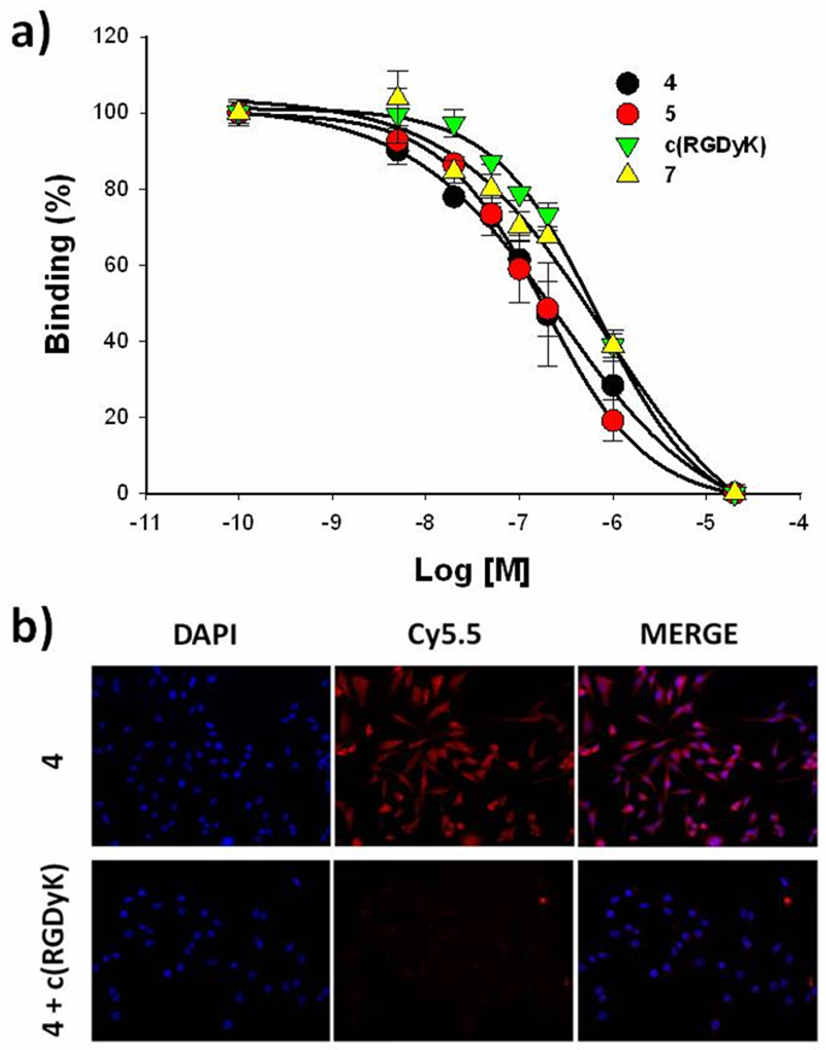
Secondly, the effect of a bulky fluorogenic peptide on the αvβ3 binding affinity of c(RGDyK) was measured by a competitive cell-binding assay in U87MG cells. 125I-eschistatin was used as a specific radio-ligand for competitive displacement. The U87MG cell is known to have high αvβ3 integrin density on the cell surface. All analogs containing c(RGDyK) ligand maintained reasonable binding affinities to its receptor. The IC50 values of c(RGDyK), 4, 5, and 7 were 592, 267, 184, and 456 nM, respectively (Figure 3a). Next, receptor specificity of the probes in cell culture was verified by fluorescent microscopic studies. The probes were incubated in fixed U87MG cells with and without a blocking dose of c(RGDyK) (10 µM). Since 5, the non-quenched form of 4, showed similar αvβ3 binding to 4, 5 was used for in vitro cellular imaging studies. As shown in Figure 3b, 5 showed strong positive fluorescent signals on the cell membranes after 30 min of incubation at 37 °C. Since receptor blocking with an excess c(RGDyK) significantly decreased binding of 5, the probe exhibits significant receptor specificity. Taken together, a NIR fluorogenic probe containing c(RGDyK) ligand shows specific activities against both MMP-2 and αvβ3 signifying 4 could be used as an αvβ3 receptor-targeted MMP-specific molecular beacon.
Figure 3.
(a) Competitive αvβ3 integrin receptor assay of c(RGDyK), 4, 5, and 7 to U87MG human glioma cells. Means ± SD. (n = 3). (b) Specific αvβ3 integrin receptor binding of 4. NIR fluorescence images of U87MG cells were taken after 30 min incubation at 37 °C in the presence of 4 with and without a blocking dose of c(RGDyK) (10 µM).
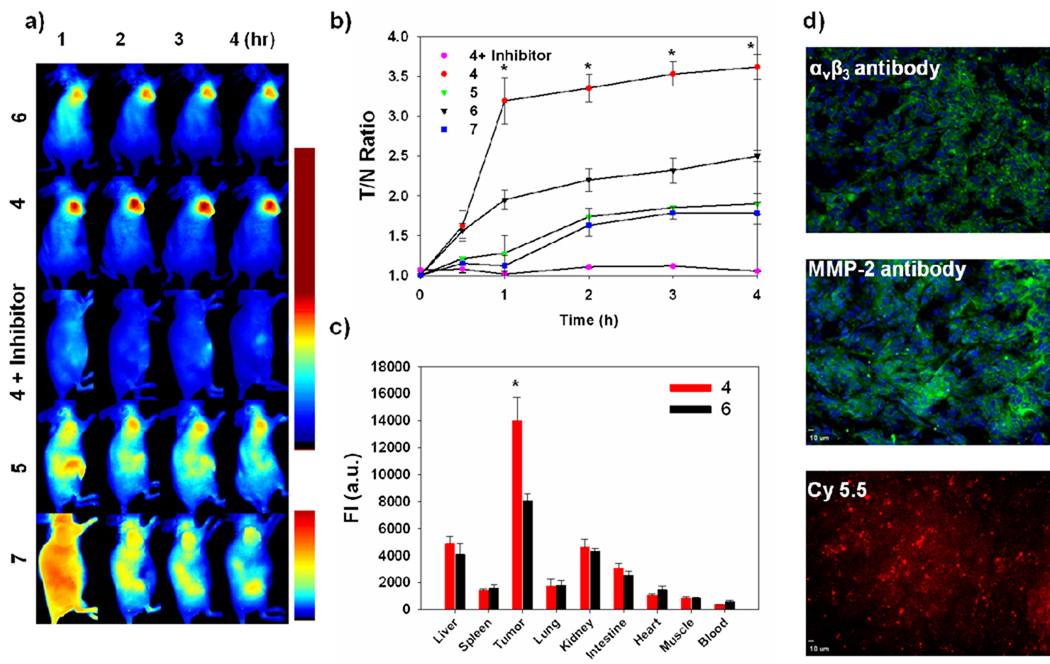
To investigate whether the receptor-targeting ligand can enhance the visualization of extracellular protease expression in vivo, 3 nmol of 4 was intravenously injected into U87MG tumor-bearing mice treated with and without an MMP inhibitor. In order to inhibit the activity of MMPs in U87MG tumor, MMP inhibitor was intratumorally injected 30 min before the injection of 4. To demonstrate the superiority of this new receptor-targeted fluorogenic probe over existing NIR dye-labeled receptor-targeted peptides in tumor imaging, 5 and 7 (0.5 nmol) were each also injected into an U87MG tumor model. It should be noted that only a limited dose of dye-labeled peptide probe like 5 and 7 can be injected for whole body imaging because of its high fluorescent background from the dye. In contrast, since fluorogenic probes are strongly quenched and a majority of the probe is activated at the target site, higher doses of the probe can be injected to enhance the ratio of region-of-interest (ROI) signal of tumor to normal tissue, muscle, (T/N) at very early times. After injection, in vivo optical imaging was performed for 4 h using a small animal imaging system (Maestro 2) equipped with appropriate filters for Cy5.5 and CW800. Figure 4a shows representative serial images of mice at selected time points. Both 4 and 6 enabled visualization of MMP expression in an MMP-positive tumor but 4 provided brighter NIR fluorescent images compared to 6 from 1 h post-injection. As expected, when MMP expression was inhibited by MMP inhibitors, the NIR fluorescence signal from the tumor was significantly reduced. In addition, tumor uptake was effectively blocked at 30 min post-injection with excess amount of unlabeled c(RGDyK) (200 nmol) with 4 demonstrating the c(RGDyK)-mediated specific binding of 4 in vivo (see the Supporting Information, Figure S3). Like other conventional types of NIR dye-labeled peptide probes, 5 and 7 showed gradually increased fluorescent signals in the tumor after the probe was slowly cleared from the blood and other organs. T/N ratios are illustrated in Figure 4b. 4 showed enhanced T/N and were up to 2.3, 1.5, 2.0, 3.4-fold higher than 5, 6, 7 and 4 treated with an inhibitor, respectively. After whole-mouse imaging at 4 h time point, tumors and organs were carefully removed and the biodistribution of 4 and 6 were evaluated (Figure 4c). Ex vivo biodistribution studies also indicated that 4 was predominantly activated in the tumors among other organs and compared to other groups. Selective tumor tissues were also analyzed by immunohistochemistry and NIR fluorescent microscopy to confirm the expression of biomarkers and accumulation of the probe. As shown in Figure 4d, αvβ3 integrin and MMP-2 antibody staining revealed strong expression of biomarkers in U87MG tumors and NIR fluorescence microscopy images showed a strong Cy5.5 signal, consistent with in vivo imaging. Overall, our in vivo results markedly supported the role of c(RGDyK) as a receptor-targeting ligand.
Figure 4.
(a) Representative serial in vivo NIR fluorescence images of MMP- and αvβ3 integrin receptor-positive U87MG tumor-bearing mice injected intravenously with 4, 5, 6, 4 with MMP inhibitor, and 7. Images were acquired at the indicated time points and were normalized by the maximum average value. The color bar indicates radiant efficiency (upper bar; for Cy5.5, low 0, high 0.111, lower bar; for CW800, low 0, high 0.03 scaled counts/sec). (b) T/N ratio analysis of NIR fluorescence intensity of U87MG tumor in vivo. (c) Biodistribution of 4 and 6. Means ± SD. (n = 3), * P < 0.05. (d) Tumor section counterstained with FITC-labeled antibody for αvβ3 integrin receptor (upper) and MMP-2 (middle). NIR fluorescence microscopy of tumor section injected with 4 (lower).
Tumor-targeting fluorogenic probes and dye-labeled peptide ligands have different targets; fluorogenic probes target overexpressed enzymes around the tumor site and peptide ligands target overexpressed receptors in the tumor. Yet, both probes can be used in combination to target the entire tumor area. Our results demonstrate the advantages of receptor-targeted fluorogenic probes for in vivo imaging applications. We anticipate that the combination of NIR fluorogenic probes and peptide ligands becomes applicable to a wide variety of target extracellular proteases and receptors, offering a series of receptor-targeted fluorogenic probes as novel imaging probes with improved efficacy in vivo.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Intramural Research Program (IRP) of the National Institute of Biomedical Imaging and Bioengineering (NIBIB), National Institutes of Health (NIH) and the International Cooperative Program of National Science Foundation of China (NSFC) (81028009).
Footnotes
ASSOCIATED CONTENT
Supporting Information. Details of synthesis and experimental methods. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Lee S, Park K, Kim K, Choi K, Kwon IC. Activatable imaging probes with amplified fluorescent signals. Chem. Commun. (Camb) 2008:4250–4260. doi: 10.1039/b806854m. [DOI] [PubMed] [Google Scholar]
- 2.Kobayashi H, Choyke PL. Target-cancer-cell-specific activatable fluorescence imaging probes: rational design and in vivo applications. Acc. Chem. Res. 2011;44:83–90. doi: 10.1021/ar1000633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee S, Park K, Lee SY, Ryu JH, Park JW, Ahn HJ, Kwon IC, Youn IC, Kim K, Choi K. Dark quenched matrix metalloproteinase fluorogenic probe for imaging osteoarthritis development in vivo. Bioconjug. Chem. 2008;19:1743–1747. doi: 10.1021/bc800264z. [DOI] [PubMed] [Google Scholar]
- 4.Zhu L, Xie J, Swierczewska M, Zhang F, Quan Q, Ma Y, Fang X, Kim K, Lee S, Chen X. Real-time video imaging of protease expression in vivo. Theranostics. 2011;1:18–27. doi: 10.7150/thno/v01p0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee S, Xie J, Chen X. Activatable molecular probes for cancer imaging. Curr. Top. Med. Chem. 2010;10:1135–1144. doi: 10.2174/156802610791384270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee S, Xie J, Chen X. Peptides and peptide hormones for molecular imaging and disease diagnosis. Chem. Rev. 2010;110:3087–3111. doi: 10.1021/cr900361p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drag M, Salvesen GS. Emerging principles in protease-based drug discovery. Nat. Rev. Drug Discov. 2010;9:690–701. doi: 10.1038/nrd3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turk B. Targeting proteases: successes, failures and future prospects. Nat. Rev. Drug Discov. 2006;5:785–799. doi: 10.1038/nrd2092. [DOI] [PubMed] [Google Scholar]
- 9.Cudic M, Fields GB. Extracellular proteases as targets for drug development. Curr. Protein Pept. Sci. 2009;10:297–307. doi: 10.2174/138920309788922207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee S, Cha EJ, Park K, Lee SY, Hong JK, Sun IC, Kim SY, Choi K, Kwon IC, Kim K, Ahn CH. A near-infrared-fluorescence-quenched gold-nanoparticle imaging probe for in vivo drug screening and protease activity determination. Angew. Chem. Int. Ed. Engl. 2008;47:2804–2807. doi: 10.1002/anie.200705240. [DOI] [PubMed] [Google Scholar]
- 11.Bremer C, Tung CH, Weissleder R. In vivo molecular target assessment of matrix metalloproteinase inhibition. Nat. Med. 2001;7:743–748. doi: 10.1038/89126. [DOI] [PubMed] [Google Scholar]
- 12.Zheng G, Chen J, Stefflova K, Jarvi M, Li H, Wilson BC. Photodynamic molecular beacon as an activatable photosensitizer based on protease-controlled singlet oxygen quenching and activation. Proc. Natl. Acad. Sci. U. S. A. 2007;104:8989–8994. doi: 10.1073/pnas.0611142104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee S, Ryu JH, Park K, Lee A, Lee SY, Youn IC, Ahn CH, Yoon SM, Myung SJ, Moon DH, Chen X, Choi K, Kwon IC, Kim K. Polymeric nanoparticle-based activatable near-infrared nanosensor for protease determination in vivo. Nano Lett. 2009;9:4412–4416. doi: 10.1021/nl902709m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barnett EM, Zhang X, Maxwell D, Chang Q, Piwnica-Worms D. Single-cell imaging of retinal ganglion cell apoptosis with a cell-penetrating, activatable peptide probe in an in vivo glaucoma model. Proc. Natl. Acad. Sci. U. S. A. 2009;106:9391–9396. doi: 10.1073/pnas.0812884106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Medintz IL, Clapp AR, Brunel FM, Tiefenbrunn T, Uyeda HT, Chang EL, Deschamps JR, Dawson PE, Mattoussi H. Proteolytic activity monitored by fluorescence resonance energy transfer through quantum-dot-peptide conjugates. Nat. Mater. 2006;5:581–589. doi: 10.1038/nmat1676. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen QT, Olson ES, Aguilera TA, Jiang T, Scadeng M, Ellies LG, Tsien RY. Surgery with molecular fluorescence imaging using activatable cell-penetrating peptides decreases residual cancer and improves survival. Proc. Natl. Acad. Sci. U. S. A. 2010;107:4317–4322. doi: 10.1073/pnas.0910261107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park K, Kim JH, Nam YS, Lee S, Nam HY, Kim K, Park JH, Kim IS, Choi K, Kim SY, Kwon IC. Effect of polymer molecular weight on the tumor targeting characteristics of self-assembled glycol chitosan nanoparticles. J. Control. Release. 2007;122:305–314. doi: 10.1016/j.jconrel.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 18.Stefflova K, Li H, Chen J, Zheng G. Peptide-Based Pharmacomodulation of a Cancer-Targeted Optical Imaging and Photodynamic Therapy Agent. Bioconjug. Chem. 2007;18:379–388. doi: 10.1021/bc0602578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nahrendorf M, Waterman P, Thurber G, Groves K, Rajopadhye M, Panizzi P, Marinelli B, Aikawa E, Pittet MJ, Swirski FK, Weissleder R. Hybrid in vivo FMT-CT imaging of protease activity in atherosclerosis with customized nanosensors. Arterioscler. Thromb. Vasc. Biol. 2009;29:1444–1451. doi: 10.1161/ATVBAHA.109.193086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fang J, Nakamura H, Maeda H. The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv Drug Deliv. Rev. 2011 doi: 10.1016/j.addr.2010.04.009. DOI:10.1016/j.addr.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 21.Chen X. Multimodality imaging of tumor integrin alphavbeta3 expression. Mini. Rev. Med. Chem. 2006;6:227–234. doi: 10.2174/138955706775475975. [DOI] [PubMed] [Google Scholar]
- 22.Niu G, Chen X. Why integrin as a primary target for imaging and therapy. Theranostics. 2011;1:30–47. doi: 10.7150/thno/v01p0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen X. Integrin targeted imaging and therapy. Theranostics. 2011;1:28–29. doi: 10.7150/thno/v01p0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johansson MK, Cook RM. Intramolecular dimers: a new design strategy for fluorescence-quenched probes. Chemistry. 2003;9:3466–3471. doi: 10.1002/chem.200304941. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.