Abstract
Background & Aims
The distinct role of portosystemic shunting (PSS) in the pathogenesis of sarcopenia (skeletal muscle loss) that occurs commonly in cirrhosis is unclear. We have previously shown increased expression of myostatin (inhibitor of skeletal muscle mass) in the portacaval anastamosis (PCA) rat model of sarcopenia of PSS. The present study was performed to examine the mechanisms of sarcopenia following PCA.
Methods
In PCA and sham operated pair fed control rats, the phenylalanine flooding dose method was used to quantify the fractional and absolute protein synthesis rates in the skeletal muscle over time and in response to follistatin, a myostatin antagonist. The expression of myostatin and markers of satellite cell (myocyte precursors) proliferation and differentiation were quantified by real-time PCR and Western blot analyses.
Results
The absolute synthesis rate (ASR) was lower at 2, 4, and 6 weeks (p <0.05) and the fractional synthesis rate (FSR) of skeletal muscle protein was significantly lower (p <0.05) at week 2 in the PCA rats compared to control rats. Expression of myostatin was elevated while markers of satellite cell proliferation and differentiation were lower at 4 and 6 weeks after PCA. Follistatin increased skeletal muscle mass, muscle FSR and ASR, decreased expression of myostatin protein, and an increased expression of markers of satellite cell function.
Conclusions
Sarcopenia associated with PSS is caused by impaired protein synthesis and reduced satellite cell function due to increased myostatin expression. Confirming these alterations in human patients with cirrhosis will provide novel therapeutic targets for sarcopenia of liver disease.
Keywords: Skeletal muscle loss, Liver disease, Portacaval anastamosis, Myostatin
Introduction
Sarcopenia or loss of skeletal muscle mass is the most common and potentially reversible complication of cirrhosis with porto-systemic shunting (PSS) and adversely affects prognosis [1]. However, the distinct role of PSS in the etiology of sarcopenia is unclear. Sarcopenia results from varying contributions of reduced protein synthesis, increased protein breakdown, and an impaired proliferation and differentiation of skeletal muscle progenitor satellite cells [2]. Satellite cells are myogenically committed precursor cells that contribute to recovery from injury as well as the regenerative response in adult skeletal muscle cells [3]. The portacaval anastamosis (PCA) rat reproduces the nutritional, metabolic, biochemical, hormonal, and behavioral changes that occur in cirrhosis and permits the consequences of PSS to be examined independent of any inflammatory and cellular injury responses of cirrhosis [2,4,5]. Hepatic atrophy develops following PSS and despite the above described changes, cirrhosis, ascites or varices do not develop. We have previously shown that in the initial two weeks following PCA, there is an increase in expression of genes regulating protein breakdown. Subsequently, there is an increased expression of myostatin accompanied by impaired satellite cell function after this initial two week period [6].
Myostatin, a member of the TGFβ superfamily, has been implicated in the pathogenesis of diminished muscle mass by impaired satellite cell function (in vitro studies) as well as reduced protein synthesis (both cell culture and in vivo animal studies) [7–11]. The relation between changes in myostatin expression and skeletal muscle protein synthesis following portosystemic shunting has not been examined [6,12]. Therefore, we quantified both the fractional synthesis rate (FSR) and the absolute synthesis rate (ASR) of the skeletal muscle protein at 2, 4, and 6 weeks following PCA and in sham operated, pair fed, and control rats. We also studied the response to follistatin, a functional antagonist of myostatin, in order to establish the causal role of myostatin in sarcopenia of PSS [13,14].
Materials and methods
Animals
Male Sprague–Dawley rats (post pubertal, 9 weeks old) with an end to side PCA or sham surgery (Charles River, Inc.; Wilmington, DE) were housed individually in a 12 h light/dark cycle. Pair feeding was done by providing the sham operated control rats with the same quantity of standard rat chow (Harland Teklad rodent chow #8604; protein 24.5%, fat 4.4%, 3.93 kcal/g) as had been ingested by a paired PCA rat fed ad libitum.
Food and water intake were measured daily, and animals were weighed every 3 days. Lean body mass and grip strength were measured weekly until the animals were killed. Shunt patency and size were established by our previous criteria of elevated plasma ammonia, low spleen weight, and plasma amino acid concentrations and anatomic evaluation at necropsy [2]. Rates of protein synthesis were measured at 2, 4, and 6 weeks after surgery in PCA and control rats (n = 5 each).
A separate group of male Sprague–Dawley rats (8–9 weeks age) subjected to PCA or sham surgery (n = 8 each) were randomized to receive either follistatin in phosphate buffered saline in the dose of 10 μg/100 g or vehicle alone. Follistatin or vehicle was administered three times a week intraperitoneally at weeks 3 and 4 after surgery. This time interval was chosen based on our previous observations that the skeletal muscle expression of myostatin is elevated at weeks 3 and 4 after PCA [6]. The dose was based on previously published data [14–16]. Food, water intake, lean body mass and forelimb grip strength were measured. In a subgroup (n = 5 in each of the four groups) of these rats, skeletal muscle protein synthesis rate was quantified after completion of follistatin administration. All studies were approved by the Institutional Animal Care and Use Committee.
Methods
Protein synthesis rates were measured using the flooding dose technique by an intravenous injection of L-[4-3H] phenylalanine [17]. In brief, L-[4-3H] phenylalanine with unlabeled phenylalanine (150 mM in sterile water) 50 μCi/ml was injected (1.0 ml/100 g body weight) through the tail vein. Twenty minutes later the rats were killed with intraperitoneal pentobarbital and the gastrocnemius muscle and liver rapidly harvested, blotted dry of blood, weighed wet, and frozen in liquid nitrogen.
A precisely weighed amount (~0.1 g) of frozen muscle was pulverized, precipitated using ice cold 2% (w/w) perchloric acid, and centrifuged. Free amino acids in the supernatant that formed the precursors for protein synthesis were separated using HPLC (Agilent Technologies, Santa Clara, CA) and the specific radioactivity (SA) of phenylalanine measured. The precipitate containing labeled phenylalanine bound to muscle protein was quantified by hydrolyzing the protein in 5 ml of 6 M HCl for 24 h at 110 °C.
Fractional rate of protein synthesis
The FSR of protein (Ks) was obtained by
where t is the incorporation time in hours, SB is the specific radioactivity of phenylalanine in the protein, SA the specific radioactivity of phenylalanine in the tissue, and the unit of Ks is % per hour.
The ASR was calculated as the FSR × total protein content of the tissue and expressed as mg/h. The total protein in the skeletal muscle was measured using the BioRad DC assay (BioRad, Hercules, CA). Food efficiency was calculated as previously described [2,18].
Generation of follistatin
Follistatin is a potent antagonist of myostatin and at least three isoforms of follistatin have been identified [19]. The FST303 expression construct was prepared from the FST315 cDNA by deleting the sequence 3′ to codon 303 up to the start of the Myc-His tag using standard PCR methods. The resulting C-terminal Myc-His-tagged cDNA constructs were transfected into HEK-293-F cell suspension cultures in freestyle serum-free medium (Invitrogen). Recombinant proteins were purified by nickel–Sepharose affinity chromatography (QIAGEN, Valencia, CA) and concentrated by centrifugal dialysis into Dulbecco’s PBS.
Measurement of lean body mass
Lean body mass was obtained weekly using the TOBEC® body analyzer for small animals (SA-3000) fitted with the 114 × 318 mm measuring chamber (SA-3114) (EC Systems, Inc., Springfield IL) as described by us [6].
Grip strength
Forelimb grip strength was measured by a single operator using a computerized rat grip strength meter model 1067CSX (Columbus Instruments, Columbus, OH) as described earlier [6].
Tissue extraction and processing
Real-time PCR and Western blot assays were done using protocols previously described [6,12].
Quantitative real-time PCR
Real time polymerase chain reaction for quantification of mRNA was performed on a Stratagene Mx 3000 P (Stratagene Inc.; La Jolla, CA) using a SYBR® protocol on the fluorescence temperature cycler by methods as described previously [6,12]. Expression of genes regulating skeletal muscle mass: components of the ubiquitin proteasome pathway, myostatin, its receptor activin 2br, intracellular signaling protein, cyclin dependant kinase inhibitor p21, markers of satellite cell proliferation (proliferating cell nuclear antigen) and differentiation (myoD, myf5, myogenin), and insulin like growth factor 1 (IGF1) were quantified and the results expressed using a relative quantification method [6,12]. The primer sequences have been published by us earlier [6,12]. Real-time PCR products were then separated by electrophoresis to confirm product presence and size.
Western blot analysis
Protein expression of genes regulating skeletal muscle mass was quantified by Western blot assays by methods described earlier [12]. To identify the mechanism by which myostatin inhibits components of the signaling pathway that regulate protein synthesis, phosphorylated Akt (both Ser and Thr), phosphorylated mTOR, and p70s6k were quantified. Additionally, phospho AMP kinase-α Thr172 was quantified to examine the crosstalk between myostatin and mTOR. All primary and secondary antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA) except antimyostatin antibody (Bethyl Laboratories; Montgomery, TX).
Proteasome 20S activity
The activity of the ubiquitin proteasome pathway was quantified by measuring the total proteasome 20S activity in skeletal muscle by the Chemicon Proteasome 20S activity assay kit (Chemicon International, Temecula, CA) based on detection of the fluorophore 7-amino-4-methylcoumarin (AMC) after cleavage from the labeled substrate LLVY-AMC. The free AMC fluorescence was quantified using a 380/460 nm filter set in a Varian Eclipse Fluorometer (Varian Inc., Palo Alto, CA). Enzyme activity of the 20S proteasome was expressed as relative fluorescence units (RFU/μg protein).
Assays
Plasma amino acid concentrations were measured using an HPLC equipped with a fluorescence detector using o-phthaldehyde/2-mercaptoethanol derivative and pre-column derivatization [20].
Statistical analyses
The difference in protein synthesis in skeletal muscle between groups was expected to be 50% based on the differences in the skeletal muscle mass. With a two tail test, α of 0.05 and power of 90%, four rats were needed in each group. Accounting for technical limitations i.e., failed studies and blocked shunts, an additional animal was used in each group (n = 5 in each group). Qualitative variables were compared using the Chi squared test. Quantitative and rating variables were compared using the analysis of variance for data with normal distribution and by the Kruskall–Wallis test for skewed data. A p value of <0.05 was considered statistically significant.
Results
The characteristics of the PCA and control animals at 2, 4, and 6 weeks after surgery are shown in Table 1. There were no significant differences between the groups prior to surgery. At all time points examined, the PCA rats had lower body weight, lean body mass, skeletal muscle weight and grip strength compared to the control rats (at least p <0.05). Weight gain in a simultaneous ad lib fed group of sham operated rats was 439.8 ± 10.8 g (p <0.01 compared to pair fed sham operated rats). Spleen weight was significantly lower (p <0.05) in PCA (0.73 ± 0.03 g) compared to sham (1.10 ± 0.02 g) with a low variance.
Table 1.
Characteristics of PCA and sham rats.
| Preoperative Weight (g) | Weight at kill (g) | Lean body mass (g) | Gastrocnemius Weight (g) | Grip Strength (lb) | Arterial Ammonia (μmol) | |
|---|---|---|---|---|---|---|
| PCA | ||||||
| Week 2 | 259.2 ± 11.8 | 261.2 ± 36.2a | 234.9 ± 25a | 0.6 ± 0.1a | 0.65 ± 0.002 | 396.0 ± 35.6a |
| Week 4 | 261.0 ± 7.6 | 282.6 ± 45.8a | 248.5 ± 29.6a | 0.7 ± 0.1a | 0.79 ± 0.002 | 308.6 ± 14.3a |
| Week 6 | 260.8 ± 10.6 | 298.6 ± 57.4a | 263.3 ± 39.8a | 0.5 ± 0.1a | 1.28 ± 0.01 | 246.6 ± 12.4a |
| SHAM | ||||||
| Week 2 | 261.0 ± 8.3 | 344 ± 10.7b | 290.3 ± 16.7b | 0.8 ± 0.1b | 1.7 ± .004 | 109.8 ± 14.4d |
| Week 4 | 258.0 ± 7.2 | 388.0 ± 12.0c | 317.1 ± 8.2c | 1.0 ± 0.2c | 2.06 ± 0.003 | 80.8 ± 6.6d |
| Week 6 | 262.3 ± 9.3 | 452 ± 26.3d | 369.3 ± 20.3d | 1.2 ± 0.2e | 5.16 ± 0.008 | 80.7 ± 4.3d |
Mean ± SEM n = 5 in each group.
: p <0.05.
: p <0.01.
: p <0.001.
Table 2 shows the FSR, ASR, and synthesis of protein per gram of skeletal muscle in the PCA and control rats measured at 2, 4, and 6 weeks. The ASR of skeletal muscle protein in PCA rats was significantly lower (p <0.05) than in control animals for all time points while the FSR in PCA was only decreased at week 2 (p <0.05).
Table 2.
Protein synthesis rate in skeletal muscle.
| FSR %/hr | ASR mg/hr | Protein Synthesis/ gm muscle mg/hr/gm | |
|---|---|---|---|
| PCA | |||
| Week 2 | 0.2 ± 0.02 | 11.2 ± 2.4 | 18.2 ± 4.9 |
| Week 4 | 0.18 ± 0.03 | 11.9 ± 2.6 | 17.7 ± 7.4 |
| Week 6 | 0.13 ± 0.02 | 6.3 ± 1.3 | 12.0 ± 6.5 |
| SHAM | |||
| Week 2 | 0.36 ± 0.1a | 34.1 ± 11.9c | 39.4 ± 23.2d |
| Week 4 | 0.17 ± 0.02b | 24.7 ± 3.3c | 25.3 ± 7.6d |
| Week 6 | 0.13 ± 0.0b | 23.5 ± 4.2c | 19.4 ± 6.0d |
Mean ± SEM. FSR, fractional synthesis rate; ASR, absolute synthesis rate.
p <0.05 – PCA vs. sham FSR;
p <0.05 – FSR in sham at week 2 compared to weeks 4 and 6;
p < 0.01 – PCA vs. sham ASR;
p <0.01 – PCA vs. sham protein synthesis/g muscle.
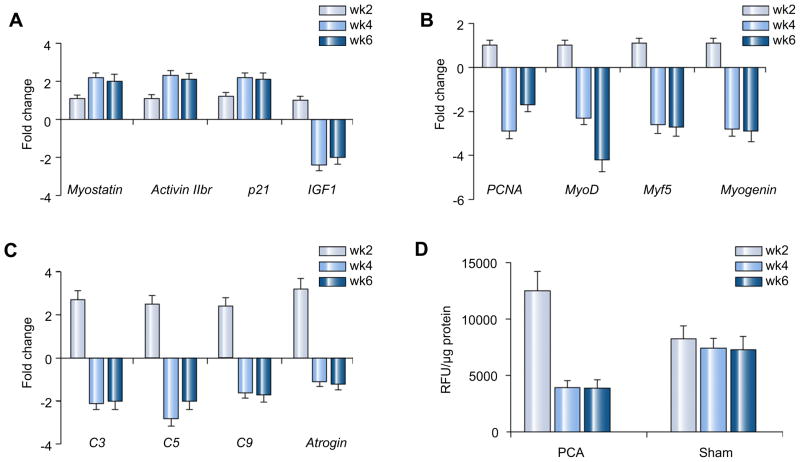
As shown in Fig. 1, skeletal muscle expression of myostatin mRNA, its receptor activin 2br and intracellular signal cyclin dependant kinase inhibitor p21 (p21) were higher in PCA while the expression of IGF1 mRNA was lower in the PCA than in controls at weeks 4 and 6 (A). These alterations were accompanied by lower expression of markers of satellite cell proliferation (PCNA) and differentiation (myoD, myf5 and myogenin) in the skeletal muscle of PCA rats compared to that in sham rats (B). We also observed that in the initial 2 weeks following PCA, the expression of genes in the ubiquitin proteasome pathway (C) and proteasome activity (D) was higher at 2 weeks but by 4 weeks it was lower than that in control rats.
Fig. 1. Histograms (A–C) showing relative expression of mRNA by real-time PCR of genes regulating skeletal muscle protein synthesis, expressed as fold change in PCA compared to control rats.
Bars extending above the baseline show an increased expression and bars extending below the baseline show a lower expression in PCA compared with sham operated control rats. (A) Expression of myostatin, its receptor activin 2br, and intracellular signaling protein, p21 was significantly elevated (p <0.01) while that of insulin like growth factor 1 was lower in the PCA compared to that in the control rats at weeks 4 and 6 (p <0.01). (B) Relative expression of mRNA by real-time PCR of the markers of satellite cell function – the proliferating cell nuclear antigen (satellite cell proliferation) and myoD, myf 5, and myogenin (satellite cell differentiation), was significantly lower in PCA compared to control rats at weeks 4 and 6 (p <0.01). (C) The expression of proteasome C3, C5, C9, and atrogin was significantly higher at week 2 and subsequently at weeks 4 and 6, their expression was lower in PCA rats compared to the control rats (p <0.01). (D) Proteasome 20S activity assay showed higher activity at 2 weeks after PCA (p <0.05) while subsequently the activity was lower (p < 0.05) than that in the control rats.
Response to follistatin
Administration of follistatin resulted in increases in whole body weight, lean body mass, muscle weight, grip strength, and food efficiency in the PCA rats to levels that were higher compared to the group administered vehicle alone and were similar to the sham operated controls (Table 3). Plasma testosterone levels were lower in the PCA rats than in sham rats and did not change in response to follistatin in either group. Plasma ammonia concentrations were not altered in response to follistatin.
Table 3.
Animal characteristics in response to follistatin.*
| PCA PBS | PCA FSTN | Sham PBS | Sham FSTN | |
|---|---|---|---|---|
| Preoperative weight (g) | 269.3 ± 2.3 | 265 ± 3.2 | 268.8 ± 1.6 | 268.1 ± 1.7 |
| Weight at kill (g) | 274.5 ± 15.2 | 372.6 ± 10.6a | 384.1 ± 7.1a | 388.8 ± 10.7a |
| LBM (g) | 243.7 ± 9.4 | 294.4 ± 5.2b | 302.3 ± 6.7b | 307 ± 7.3b |
| Gastrocnemius(g) | 0.93 ± 0.33 | 1.5 ± 0.3a | 1.64 ± 0.3a | 1.5 ± 0.3a |
| Extensor digitorum longus (g) | 0.67 ± 0.4 | 1.93 ± 0.51a | 1.90 ± 0.5a | 1.43 ± 0.4a |
| Food efficiency (g/g) | 0.04 ± 0.02 | 0.20 ± 0.01c | 0.3 ± 0.01d | 0.3 ± 0.01d |
| Grip strength at 4 weeks (lbs) | 1.57 ± 0.08 | 2.62 ± 0.05b | 2.94 ± 0.11b | 3.06 ± 0.09b |
| Plasma testosterone (ng/dl) | 149.1 ± 24.2 | 125.7 ± 20.5 | 998.193 ± 193.3b | 948.6 ± 182.7b |
| Arterial ammonia (μmol) | 300.1 ± 15.3 | 333.6 ± 18.2 | 82.9 ± 5.4d | 85.1 ± 7.9d |
Mean ± SEM. PBS, phosphate buffered saline; FSTN, follistatin; PCA, portacaval anastamosis.
Animals were administered follistatin for 2 weeks and killed at 4 weeks after surgery.
p <0.05 compared to PCA on PBS;
p <0.01compared to PCA on PBS.
p <0.001 compared to PCA on PBS;
p <0.01 compared to PCA on PBS.
Both fractional and absolute synthesis of skeletal muscle protein increased in response to follistatin in the PCA animals to levels that did not differ from sham controls (Table 4).
Table 4.
Protein synthesis in response to follistatin.
| PCA PBS | PCA FSTN | Sham PBS | Sham FSTN | |
|---|---|---|---|---|
| Skeletal muscle | ||||
| Fractional synthesis rate (%/h) | 0.14 ± 0.01* | 0.76 ± 0.22 | 0.51 ± 0.09 | 0.62 ± 0.25 |
| Absolute synthesis rate (mg/h) | 6.1 ± 1.4* | 31.3 ± 2.4 | 31.7 ± 5.6 | 30.4 ± 6.6 |
p <0.001 compared to other groups;
p <0.0001 compared to other groups.
PCA, portacaval anastamosis.
PBS, phosphate buffered saline.
FSTN, follistatin.
All measurements were made following follistatin administered intraperitoneally in the dose of 10 μg/100 g tiw for 2 weeks, beginning 2 weeks after surgery.
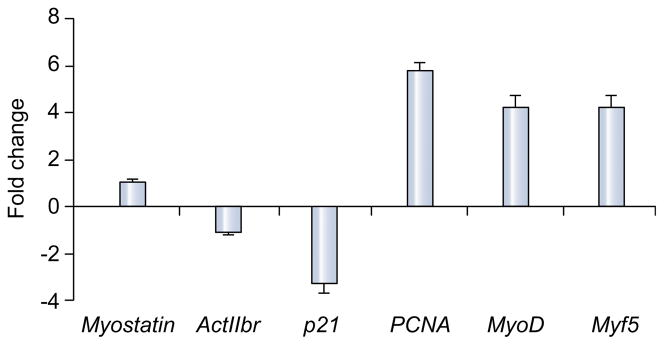
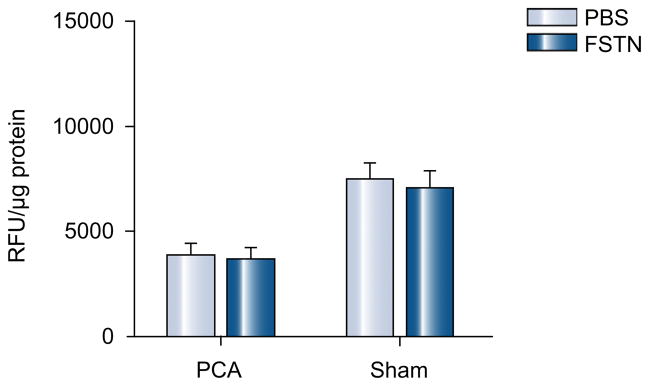
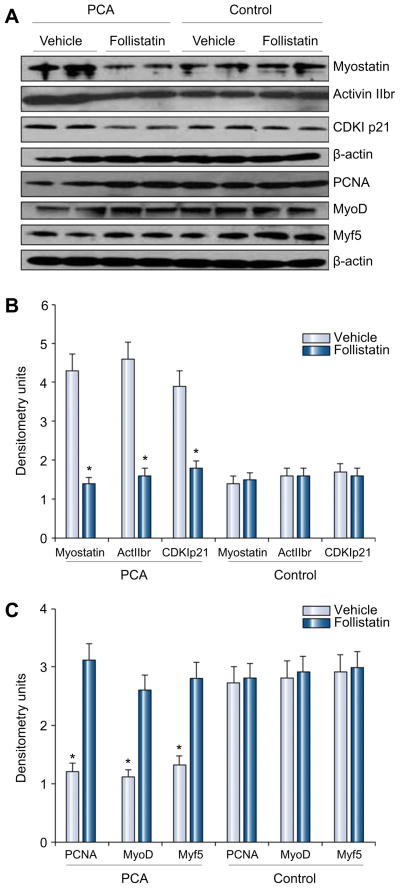
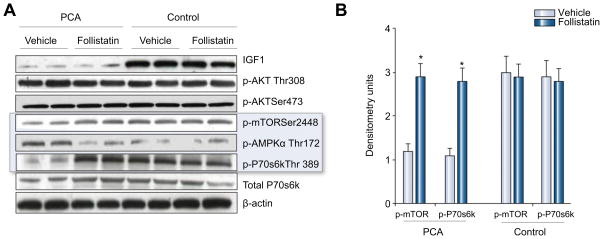
As shown in Fig. 2 expression of myostatin mRNA was not significantly altered in the PCA animals treated with follistatin. There was, however, a significantly lower expression of activin 2br (myostatin receptor), its intracellular signal, cyclin dependant kinase p21 (p21), and a higher expression of markers of satellite cell proliferation (PCNA) and differentiation (myoD, myogenin) in the PCA animals treated with follistatin compared with those treated with vehicle alone (Fig. 2). There was no significant alteration in proteasome activity in response to follistatin (Fig. 3). The expression of genes regulating components in the ubiquitin proteasome pathway (proteasome C3, C5, C9 and atrogin) also did not show any significant change in response to follistatin (data not shown). As shown in the Western blots in Fig. 4(A), there was lower expression of myostatin, activin 2br, and p21 and an increased expression of markers of satellite cell function proteins in response to follistatin in the PCA animals. Follistatin had no significant effect on any of the measured proteins in control animals (Fig. 4B and C). Furthermore, following PCA, as shown in Fig. 5A and B, there was an impaired phosphorylation of skeletal muscle mTOR and its downstream target, p70s6k, a critical regulator of protein synthesis without any change in phosphorylation of Akt. This was accompanied by an increased phosphor AMPK Thr172, a direct inhibitor of mTOR. In response to follistatin these alterations were reversed suggesting AMP kinase as the regulatory protein between myostatin and mTOR following PCA.
Fig. 2. Histogram showing relative expression of mRNA by real-time PCR of genes regulating skeletal muscle protein synthesis and satellite cell function expressed as fold change in PCA rats at the end of treatment with follistatin compared with those treated with vehicle alone.
The expression of myostatin was unchanged, while its receptor activin 2br, and its intracellular signal, cyclin dependant kinase p21 were significantly lower (p <0.01) in the PCA rats treated with follistatin compared to those treated with vehicle. In contrast, expression of markers of satellite cell function – proliferating cell nuclear antigen, myoD and myogenin were significantly higher in the PCA rats administered follistatin (p <0.01).
Fig. 3.
Follistatin did not result in an alteration in proteasome activity in either PCA or control rats (p > 0.1).
Fig. 4. (A) Representative Western blots of proteins regulating skeletal muscle mass, markers of protein synthesis and satellite cell function.
β-Actin was used as a loading control in all experiments. (B) Densitometry (n = 8 each) showed that the expression of myostatin, its receptor activin 2br and intracellular signal, CDKI p21 were significantly lower in PCA rats administered follistatin. The expression of markers of satellite cell function – proliferating cell nuclear antigen (PCNA), and markers of differentiation (myoD and myogenin) were significantly higher in the PCA rats administered follistatin. Follistatin did not have any significant effect in control rats.
Fig. 5. (A) Representative Western blots of IGF1, phospho-Akt ( ), phospho-mTOR, phospho-AMPKα, total and phospho-p70s6k in the PCA and sham rats treated with vehicle and follistatin.
(B) Densitometry of these blots showed lower expression of IGF1 in the PCA rats compared to control animals and did not change in response to follistatin in either group. Elevated phospho AMPkinase, a direct inhibitor of mTOR, resulted in lower expression of both phospho mTOR and phospho-p70s6k in the PCA rats treated with vehicle and were reversed in response to blocking myostatin. Follistatin did not have any significant effect in control rats.
Discussion
The present study demonstrates that increased myostatin expression is responsible for the diminished skeletal muscle mass due to an impaired protein synthesis response and attenuated satellite cell function following portosystemic shunting. Lower body weight, lean body mass, skeletal muscle weight, grip strength, and altered expression of genes regulating skeletal muscle mass in the PCA compared to pair fed sham rats shows that these alterations are a direct effect of the shunt and not due to restricted food intake in the control animals. These observations were similar to those reported by us earlier [2,6]. Our data also show that the initial response in both groups is an increase in muscle proteolysis. This was evidenced by an increase in components of the ubiquitin proteasome pathway as well as direct measurement of proteasome activity that was greater in the PCA rat compared to control rats. Our observations suggest that the initial period of proteolysis results in a lower skeletal muscle mass. Subsequent recovery of skeletal muscle mass requires an increase in protein synthesis. Failure to increase muscle mass following the initial proteolysis in the PCA rat was accompanied by an inability to increase both FSR and ASR unlike that in the sham control group. Administration of follistatin, a myostatin antagonist, resulted in a lower expression of skeletal muscle myostatin protein and consequent increase in markers of satellite cell function, ASR, and FSR of skeletal muscle protein synthesis with near complete reversal of sarcopenia following PCA.
The FSR of skeletal muscle protein in the sham rats but not in the PCA rats was higher at week 2 than at subsequent weeks when the FSR was similar in the two groups. It is important to underscore the absence of an increase in FSR of the skeletal muscle protein during the 6 weeks of observation in the PCA rats. Quantifying the FSR of protein alone does not take into consideration the protein pool size that is decreased in conditions with lower muscle mass [8,17]. The initial increase in FSR at week 2 in control rats is responsible for the growth in these animals. Once the muscle mass is increased, the total mass of protein synthesized in the muscle reaches a steady state and the FSR decreases in response to the increased total protein mass in the muscle. This interpretation is supported by our observation that ASR in the sham rats was not significantly different throughout the study. In contrast, in the PCA rat, increased protein synthesis did not occur and reflected impaired protein synthesis required for recovery.
Although the inhibition of myostatin by follistatin has been reported previously [14,21–23], this is the first study to demonstrate the causal role of myostatin in the reduced skeletal muscle mass in PSS that commonly accompanies cirrhosis. Follistatin increased muscle mass, protein synthesis, and in vivo markers of satellite cell function in the PCA rat. Reversal of impaired protein synthesis response, phosphorylation of mTOR, and ribosomal p70s6k by follistatin provides direct evidence that myostatin impairs skeletal muscle protein synthesis by inhibiting mTOR in the PCA rat. These observations confirm reports by previous authors that myostatin inhibits protein synthesis in C2C12 cells in vitro [7] as well as indirect evidence that skeletal muscle protein synthesis was increased in myostatin knockout mice [8].
The mechanisms by which follistatin regulates skeletal muscle mass include a direct binding to myostatin and/or by binding to the myostatin receptor, activin 2bR [22,23]. The present observations suggest that follistatin has a direct effect on lowering myostatin protein and its intracellular consequences without significantly altering expression of myostatin mRNA. In vitro studies have shown that follistatin binds to myostatin protein and may explain lower myostatin protein expression without significant alteration in myostatin mRNA in the PCA rats administered follistatin [13]. Since we did not observe a myostatin-follistatin complex on our Western blots, it is likely that this may be related to an extracellular location of this binding.
The lower expression of myostatin protein following follistatin resulted in an increase in both the fractional and absolute synthesis rate of skeletal muscle protein in the PCA group. These data show that the impaired protein synthesis response is reversed by reducing myostatin in these animals. Follistatin reversed impaired phosphorylation of mTOR and p70s6 kinase, critical components of the signaling pathway for protein synthesis. This suggests a novel direct crosstalk between myostatin and mTOR. AMPK is a direct inhibitor of mTOR phosphorylation and the elevated expression of AMPK following PCA was reversed by follistatin. This suggests that myostatin regulates protein synthesis by inhibiting mTOR phosphorylation via AMP kinase.
We conclude that impaired skeletal muscle protein synthesis in portosystemic shunting is the primary mechanism for low muscle mass and is due to an increased expression of myostatin. Demonstration of similar alterations in PSS in human cirrhosis will potentially result in a paradigm shift in our understanding of the mechanisms of skeletal muscle loss in liver disease. Understanding the mechanisms that regulate the expression and effects of myostatin overexpression has the potential to identify novel targets in the therapy of reduced muscle mass in cirrhotic patients with PSS.
References
- 1.Selberg O, Bottcher J, Tusch G, Pichlmayr R, Henkel E, Muller MJ. Identification of high- and low-risk patients before liver transplantation: a prospective cohort study of nutritional and metabolic parameters in 150 patients. Hepatology. 1997;25 (3):652–657. doi: 10.1002/hep.510250327. [DOI] [PubMed] [Google Scholar]
- 2.Dasarathy S, Mullen KD, Conjeevaram HS, Kaminsky-Russ K, Wills LA, McCullough AJ. Preservation of portal pressure improves growth and metabolic profile in the male portacaval-shunted rat. Dig Dis Sci. 2002;47 (9):1936–1942. doi: 10.1023/a:1019683703951. [DOI] [PubMed] [Google Scholar]
- 3.Kuang S, Rudnicki MA. The emerging biology of satellite cells and their therapeutic potential. Trends Mol Med. 2008;14 (2):82–91. doi: 10.1016/j.molmed.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Lundborg H, Hamberger A. Effects of portacaval anastomosis on liver and brain protein synthesis in rats. Surgery. 1977;82 (5):643–647. [PubMed] [Google Scholar]
- 5.Dunlop DS, Kaufman H, Zanchin G, Lajtha A. Protein synthesis rates in rats with portacaval shunts. J Neurochem. 1984;43 (5):1487–1489. doi: 10.1111/j.1471-4159.1984.tb05413.x. [DOI] [PubMed] [Google Scholar]
- 6.Dasarathy S, Muc S, Hisamuddin K, Edmison JM, Dodig M, McCullough AJ, et al. Altered expression of genes regulating skeletal muscle mass in portacaval anastamosis rat. Am J Physiol Gastrointest Liver Physiol. 2006;292 (4):G1105–G1113. doi: 10.1152/ajpgi.00529.2006. [DOI] [PubMed] [Google Scholar]
- 7.Taylor WE, Bhasin S, Artaza J, Byhower F, Azam M, Willard DH, Jr, et al. Myostatin inhibits cell proliferation and protein synthesis in C2Cl2 muscle cells. Am J Physiol Endocrinol Metab. 2001;280 (2):E221–E228. doi: 10.1152/ajpendo.2001.280.2.E221. [DOI] [PubMed] [Google Scholar]
- 8.Welle S, Bhatt K, Pinkert CA. Myofibrillar protein synthesis in myostatin-deficient mice. Am J Physiol Endocrinol Metab. 2006;290 (3):E409–E415. doi: 10.1152/ajpendo.00433.2005. [DOI] [PubMed] [Google Scholar]
- 9.Suryawan A, Frank JW, Nguyen HV, Davis TA. Expression of the TGF-beta family of ligands is developmentally regulated in skeletal muscle of neonatal rats. Pediatr Res. 2006;59 (2):175–179. doi: 10.1203/01.pdr.0000196718.47935.6e. [DOI] [PubMed] [Google Scholar]
- 10.McCroskery S, Thomas M, Maxwell L, Sharma M, Kambadur R. Myostatin negatively regulates satellite cell activation and self-renewal. J Cell Biol. 2003;162 (6):1135–1147. doi: 10.1083/jcb.200207056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McFarland DC, Velleman SG, Pesall JE, Liu C. The role of myostatin in chicken (Gallus domesticus) myogenic satellite cell proliferation and differentiation. Gen Comp Endocrinol. 2007;151 (3):351–357. doi: 10.1016/j.ygcen.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 12.Dasarathy S, Dodig M, Muc SM, Kalhan SC, McCullough AJ. Skeletal muscle atrophy is associated with an increased expression of myostatin and impaired satellite cell function in the portacaval anastamosis rat. Am J Physiol Gastrointest Liver Physiol. 2004;287 (6):G1124–G1130. doi: 10.1152/ajpgi.00202.2004. [DOI] [PubMed] [Google Scholar]
- 13.Amthor H, Nicholas G, McKinnell I, Kemp CF, Sharma M, Kambadur R, et al. Follistatin complexes myostatin and antagonises myostatin-mediated inhibition of myogenesis. Dev Biol. 2004;270 (1):19–30. doi: 10.1016/j.ydbio.2004.01.046. [DOI] [PubMed] [Google Scholar]
- 14.Hosoyama T, Yamanouchi K, Nishihara M. Role of serum myostatin during the lactation period. J Reprod Dev. 2006;52 (4):469–478. doi: 10.1262/jrd.18009. [DOI] [PubMed] [Google Scholar]
- 15.Kogure K, Zhang YQ, Kanzaki M, Omata W, Mine T, Kojima I. Intravenous administration of follistatin: delivery to the liver and effect on liver regeneration after partial hepatectomy. Hepatology. 1996;24 (2):361–366. doi: 10.1002/hep.510240212. [DOI] [PubMed] [Google Scholar]
- 16.Patella S, Phillips DJ, Tchongue J, de Kretser DM, Sievert W. Follistatin attenuates early liver fibrosis: effects on hepatic stellate cell activation and hepatocyte apoptosis. Am J Physiol Gastrointest Liver Physiol. 2006;290 (1):G137–G144. doi: 10.1152/ajpgi.00080.2005. [DOI] [PubMed] [Google Scholar]
- 17.Garlick PJ, McNurlan MA, Preedy VR. A rapid and convenient technique for measuring the rate of protein synthesis in tissues by injection of [3H] phenylalanine. Biochem J. 1980;192 (2):719–723. doi: 10.1042/bj1920719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dasarathy S, Mullen KD, Dodig M, Donofrio B, McCullough AJ. Inhibition of aromatase improves nutritional status following portacaval anastomosis in male rats. J Hepatol. 2006;45 (2):214–220. doi: 10.1016/j.jhep.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 19.Rodino-Klapac LR, Haidet AM, Kota J, Handy C, Kaspar BK, Mendell JR. Inhibition of myostatin with emphasis on follistatin as a therapy for muscle disease. Muscle Nerve. 2009;39 (3):283–296. doi: 10.1002/mus.21244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turnell DC, Cooper JD. Rapid assay for amino acids in serum or urine by pre-column derivatization and reversed-phase liquid chromatography. Clin Chem. 1982;28 (3):527–531. [PubMed] [Google Scholar]
- 21.Lee SJ. Quadrupling muscle mass in mice by targeting TGF-beta signaling pathways. PLoS One. 2007;2 (8):e789. doi: 10.1371/journal.pone.0000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee SJ, McPherron AC. Regulation of myostatin activity and muscle growth. Proc Natl Acad Sci USA. 2001;98 (16):9306–9311. doi: 10.1073/pnas.151270098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hill JJ, Davies MV, Pearson AA, Wang JH, Hewick RM, Wolfman NM, et al. The myostatin propeptide and the follistatin-related gene are inhibitory binding proteins of myostatin in normal serum. J Biol Chem. 2002;277 (43):40735–40741. doi: 10.1074/jbc.M206379200. [DOI] [PubMed] [Google Scholar]