Abstract
The bladder and distal colon are innervated by lumbar splanchnic (LSN) and pelvic nerves (PN) whose axons arise from dorsal root ganglia (DRG) neurons at thoracolumbar (TL) and lumbosacral (LS) spinal levels, respectively. In an attempt to understand the molecular basis of differences between LSN and PN mechanosensitive afferents, we analyzed the gene expression of two potentially counteracting ion channel groups involved in mechanosensation, transient receptor potential channels (TRPV1 and TRPA1) and mechanosensitive K2P channels (TREK-1, TREK-2 and TRAAK), in TL and LS DRG neurons innervating mouse bladder or distal colon. The proportion of TRPV1-expressing cells (41~61%) did not differ between TL and LS neurons innervating bladder or colon. TRPA1 was seldom detected in bladder LS neurons whereas it was expressed in 64~66% of bladder TL, colon TL and colon LS neurons. Coexpression of TRPV1 and TRPA1 was frequent. TREK-1-expressing cells were more prevalent in LS than TL ganglia in both bladder- and colon-DRG neurons. All three K2P channels were detected more frequently in TRPV1-positive neurons in TL ganglia. More than half of TL neurons expressing only TRPA1 were devoid of any of the three K2P channels whereas all TL neurons expressing both TRPA1 and TRPV1 expressed at least one of the K2P channels. These results reveal clear differences between LSN and PN sensory pathways in TRPA1 and TREK-1 gene expression and in the expression of K2P channels in TRPV1-expressing neurons. This study further documents heterogeneity of visceral afferents based on combinations of the five channels examined.
Keywords: TRPV1, TRPA1, mechanosensitive K2P channel, lumbar splanchnic nerve, pelvic nerve
Sensory neurons innervating lower abdominal organs such as urinary bladder and distal colon are contained in two neural pathways: lumbar splanchnic (LSN) and pelvic nerves (PN) that convey sensory information to thoracolumbar and lumbosacral spinal segments, respectively (Ness and Gebhart, 1990). These two sensory pathways are not only anatomically different in their spinal level of origin, but also physiologically distinct in their relative proportions of mechanically sensitive nerve fiber types. Specifically, in the LSN innervation of the mouse bladder, 67% of mechanically sensitive afferents are of the serosal class that respond only to blunt probing of the urothelial surface, whereas in the PN, mechanosensitive muscular class afferents that respond to both probing and bladder wall stretch predominate (63%) (Xu and Gebhart, 2008). In comparison, in the LSN innervation of the mouse distal colon, ~50% of mechanically sensitive afferents are of the mesenteric class that responds to probing on the mesenteric attachments. In the PN, in contrast, mesenteric afferents are absent and four classes of mechanosensitive afferents (serosal, muscular, mucosal and muscular/mucosal) are found in relatively equal proportions (Brierley et al., 2004). The LSN and PN also differ from each other in the degree of mechanosensitivity of the same classes of afferents. For instance, the magnitude of responses to mechanical stimuli of serosal and muscular afferents in the distal colon is smaller in LSN afferents than in PN afferents of the same classes.
It is poorly understood, however, what underlies the differences in proportions of mechanosensitive afferents and in the degree of their mechanosensitivity between the LSN and PN innervations, and more fundamentally, how these nerve fibers manifest heterogeneity in their responses to varying types of mechanical stimulation. One approach to begin to address these questions is to determine the expression profiles of multiple ion channels involved in mechanosensation in sensory neurons comprising the LSN or PN pathways. In this regard, it is particularly interesting that genetic deletion of transient receptor potential channel V1 (TRPV1) or TRPA1 in mice attenuates visceromotor responses to colorectal distension in vivo and action potential firing of bladder and colon PN afferents to mechanical stimulation in vitro (Brierley et al., 2009; Daly et al., 2007; Jones, III et al., 2005). In Trpv1−/− mice, low-threshold bladder PN afferents show diminished response to high intensity bladder distension, and the muscular/mucosal class of colon PN afferents is less sensitive to colon wall stretch than its wild type counterparts. In Trpa1−/− mice, the responses of colon PN afferents to blunt probing and mucosal stroking are decreased. On the contrary, mice lacking TREK-1 or/and TRAAK, mechanosensitive two pore-domain K+ (K2P) channels, exhibit an increase in response to mechanical probing of the plantar surface of their hindpaw (Alloui et al., 2006; Noel et al., 2009). This suggests that varying combinations/coexpression of these two counteracting ion channel groups (TRPs and TREKs) may contribute, at least in part, to a molecular basis that underlies differences between the LSN and PN sensory pathways and the heterogeneity in mechanosensitivity of these innervations. This idea prompted us to examine the expression of TRPV1, TRPA1 and three mechanosensitive K2P channels (TREK-1, TREK-2 and TRAAK) in thoracolumbar (representing LSN) and lumbosacral (PN) dorsal root ganglia (DRG) neurons. Because of the lack of reliable antibodies to detect several of these channels in mice immunohistochemically and technical issues associated with using multiple probes for in situ hybridization on limited numbers of DRG sections containing traced neurons, here we examined the mRNA expression of the five channels in single neurons retrogradely labeled from urinary bladder or distal colon using the single cell reverse transcription-polymerase chain reaction (RT-PCR) technique.
Experimental Procedures
Animal
Adult male C57BL/6 (Taconic, Germantown, NY) mice (25–30 g) were used in this study. Mice were housed under 12/12-hr light/dark cycle. Water and food were provided ad libitum. All procedures were approved by and in accordance with the guidelines of the Institutional Animal Care and Use Committee, University of Pittsburgh.
Cell labeling
The urinary bladder or distal colon was surgically exposed under 2% isoflurane anesthesia (Hospira Inc., Lake Forest, IL). A 20 mg/mL (in absolute dimethyl sulfoxide) solution of retrograde tracer, 1,1′- dioctadecyl-3,3,3′,3- tetramethylindocarbocyanine perchlorate (DiI; Invitrogen, Carlsbad, CA) was injected into the organ wall using a 30-gauge needle (3~5 sites, each in a volume of ~5 μL). Mice were used for experiments 2-3 weeks after DiI injection.
DRG neuron culture
Mice were sacrificed with overdose of pentobarbital sodium (Nembutal®, Ovation Pharmaceutical, Deerfield, IL) followed by decapitation. Thoracolumbar (TL, T10–L1) or lumbosacral (LS, L6–S1) DRG were collected, enzymatically digested with a mixture of collagenase IV (200 U/ml, Worthington Biochemical, Lakewood, NJ) and dispase (7.5 U/ml, Worthington) in serum-free, advanced Dulbecco’s modified eagle medium (DMEM)/F12 containing 1% penicillin/streptomycin (Invitrogen) and triturated with a fire-polished, large-bore glass Pasteur pipette. Cells were plated on poly-D-lysine-coated coverslips (Becton Dickinson Labware, Bedford, MA) and incubated overnight at 37°C in 5% CO2.
Single cell reverse transcriptase-polymerase chain reaction (RT-PCR)
DiI-labeled neurons in culture were collected individually under a microscope (DMI6000B, Leica Microsystems, Bannockburn, IL) with glass pipettes and expelled into microcentrifuge tubes containing lysis buffer. Reverse transcription was performed using oligo (dT)12-18 primer (Invitrogen) through a series of incubations: 1.5 min at 65°C, 2 min at room temperature, 20 min at 37°C after adding 20 U SuperScript II (Invitrogen) and 10 min at 65°C. Successfully processed cells were screened by examining a transcript of the housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Cells processed without SuperScript II and a cell-free bath aspirate were included as reverse transcription-negative controls in every session of sample collection. The original first-strand cDNA sample was divided in two (2 μL each) and each was used for amplification of either TRPV1 and TRPA1 transcripts or TREK-1, TREK-2 and TRAAK transcripts using a multiplex PCR strategy.
The first round multiplex PCR was performed in a 25 μL solution containing 1×GoTaq reaction buffer (Promega, Madison, WI), 0.4 μM external primers mix, 0.2 mM dNTPs, and 0.2 μL GoTaq DNA polymerase (Promega). Primer sequences are listed in table 1. PCR consisted of initialization at 95°C for 10 min, 26 (for TRP channels) or 35 (for TREK channels) cycles at 94°C for 30 s, 52°C for 30 s and 72°C for 30 s before a final extension step at 72°C for 10 min. Each first round PCR product served as template in the second round PCR using a channel specific internal primer pair. The second round PCR product was electrophoresed on 2% agarose gel, stained with ethidium bromide, digitally photographed (LAS 3000 imaging system, Fujifilm, Japan) and analyzed using ImageJ (version 1.42q, Wayne Rasband, NIH).
Table 1.
Primer pairs used for PCR (5′→3′)
| Gene (expected size) |
External Primers | Internal Primers | Accession No. |
|---|---|---|---|
| GAPDH (493, 242 bp) |
GCTGAGTATGTCGTGGAGTCTA CATACTTGGCAGGTTTCTCCAG |
GTTTGTGATGGGTGTGAACCAC TGGATGCAGGGATGATGTTCTG |
NM_008084 |
| TRPV1 (486, 191 bp) |
GGGAAGAATAACTCACTGCCTGTG TGGGTCCTCGTTGATGATGC |
GGCGAGACTGTCAACAAGATTGC TCATCCACCCTGAAGCACCAC |
NM_001001445.1 |
| TRPA1 (495, 224 bp) |
CTTCCTGGATTACAACAATGCTCTG ATGTCCCCAACCGCCAAGC |
CAGTGGCAATGTGGAGCAATAG AAGGAAAGCAATGGGGTGC |
NM_177781.4 |
| TREK-1 (557, 229 bp) |
CACATGGAGAGATACAGACTGC GAGATGGGTGGAGCTTTCTTTG |
GAGATACAGACTGCTGGCATAG GTAGATGTAAGTACGGGCACAG |
NM_010607 |
| TREK-2 (438, 254 bp) |
CAGTGGCAACGCTATAGTTCTC CCACCTACACTACCTATCCCAT |
GTGATAGGTGGTGCACAGATAG GCCATTGGTTAAGGAGATAGCC |
NM_029911 |
| TRAAK (427, 109 bp) |
CAGTGAGAATCTGGCCTTCATC TTTGAGGCACAGTTGTGAGG |
ATTTGACCAAAGAGCCGTCC TTGGATGTGAAGAACCAGCC |
NM_008431 |
Data analysis
N indicates the number of mice and n, the number of cells. The Chi-square (χ2) test was used for analysis of contingency tables with more than 3 categories and Fisher’s exact test (FET) for subsequent pairwise comparisons. Sets of comparison pairs were chosen a priori. Results were considered statistically significant when P<0.05.
Results
Proportions of TRPV1 and TRPA1 mRNA-expressing TL or LS DRG neurons innervating bladder or distal colon
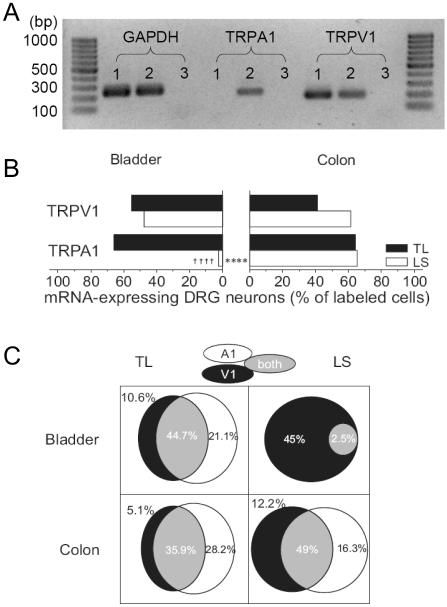
In bladder-DRG neurons, as shown in Fig 1B, 55% (21/38, N=4) of TL and 48% (19/40, N=4) of LS neurons expressed TRPV1 channel gene transcripts. Similar proportions of TRPV1 mRNA-expressing neurons were found in distal colon-DRG neurons (41% of 39 TL and 61% of 49 LS neurons, N=4); no statistical difference was detected between TL and LS DRG neurons innervating the bladder or colon (P>0.25 by χ2 test). However, there was a striking difference in TRPA1 mRNA expression in these same neurons. Whereas 66% (25/38) of bladder TL neurons expressed the TRPA1 gene transcript, virtually no bladder LS neurons expressed this gene transcript (2.5%, 1/40, P<0.0001 by FET). This relative absence of TRPA1 mRNA in bladder LS neurons was an unexpected finding. To evaluate further, we found that the number of cells showing a [Ca2+]i increase in response to 100 μM mustard oil, a TRPA1 agonist, was similarly limited in LS bladder neurons (3 of 19 cells) but comparable to mRNA results in TL bladder neurons (10 of 17 cells). In colon-DRG neurons (Fig. 1B), on the other hand, both TL and LS neurons expressed TRPA1 mRNA to a similar extent (64% in TL and 65% in LS).
Figure 1. Gene expression of TRPV1 and TRPA1 in DRG neurons innervating the mouse bladder or distal colon.
(A) Representative photographs of RT-PCR products of GAPDH, TRPA1 and TRPV1 from 3 bladder DRG neurons (lanes 1~3, no RT control in lane 3). The expected amplicon sizes were 242 bp (GAPDH), 224 bp (TRPA1) and 191 bp (TRPV1). (B) The proportions of neurons expressing TRPV1 mRNA did not differ either between bladder- and colon-DRG neurons or between TL and LS ganglia. However, TRPA1 mRNA-expressing neurons were virtually absent in bladder LS neurons. †††† P<0.0001 vs. TL. **** P<0.0001 between bladder- and colon-DRG neurons by FET. (C) The majority of bladder- and colon-DRG neurons expressed both TRPV1 and TRPA1 mRNA. The proportions of TRP mRNAs-positive neurons are expressed as percentages of labeled neurons.
Co-expression analysis of these two TRP channels showed a large overlap (36~49% of traced neurons) between neurons expressing TRPV1 and TRPA1 mRNAs with the exception of bladder LS neurons, where the TRPA1 gene transcript was seldom detected (Fig 1C). In bladder TL neurons, 81% of TRPV1 mRNA-positive cells expressed TRPA1 gene transcript and 68% of TRPA1 mRNA-expressing cells were positive to TRPV1 gene transcript. In colon TL and LS neurons, 88% and 80% of TRPV1 mRNA-positive cells expressed TRPA1 gene transcript, and 56% and 75% of TRPA1 mRNA-expressing cells were positive to TRPV1 mRNA, respectively.
Proportions of TREK-1, TREK-2 and TRAAK mRNA-expressing TL or LS DRG neurons innervating bladder or distal colon
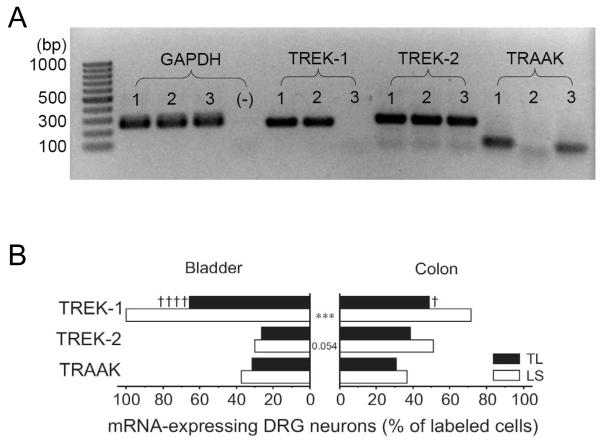
In both bladder- and colon-DRG neurons, all three mechanosensitive K2P channel gene transcripts were detected with a higher prevalence of TREK-1 than TREK-2 or TRAAK (Fig 2). The proportion of TREK-1 mRNA-expressing neurons was greater in LS than in TL ganglia in both bladder- and colon-DRG neurons (P<0.0001 in bladder- and P<0.05 in colon-DRG neurons by FET), and all bladder LS neurons were positive to TREK-1 gene transcript, which was statistically different from its colon counterpart (P<0.001 by FET). The proportion of neurons expressing TREK-2 or TRAAK mRNA did not differ either between LS and TL ganglia or between bladder- and colon-DRG neurons.
Figure 2. Gene expression of mechanosensitive K2P channels in DRG neurons innervating the mouse bladder or distal colon.
(A) Representative photographs of RT-PCR products of GAPDH, TREK-1, TREK-2 and TRAAK from 3 colon DRG neurons (lanes 1~3, (-) indicates no template control for GAPDH screening). The expected amplicon sizes were 242 bp (GAPDH), 229 bp (TREK-1), 254 bp (TREK-2) and 109 bp (TRAAK). (B) The gene transcripts of all three mechanosensitive K2P channels, TREK-1, TREK-2 and TRAAK, were detected in bladder- and colon-DRG neurons in both TL and LS ganglia. TREK-1 mRNA was most prevalent. The proportion of neurons expressing TREK-1 mRNA was less in TL than in LS ganglia in both organs († P<0.05 and †††† P<0.0001 vs. LS) and in colon- than in bladder-DRG neurons (*** P<0.001 by FET).
We further analyzed the expression of these three K2P channel gene transcripts by dividing the traced neurons into four subgroups, TRPV1 or TRPA1 mRNA-positive and –negative neurons, which revealed clear differences in the gene expression pattern of the three mechanosensitive K2P channels, not only between TRP channel mRNA-positive and –negative neurons, but also between TL and LS DRG neurons.
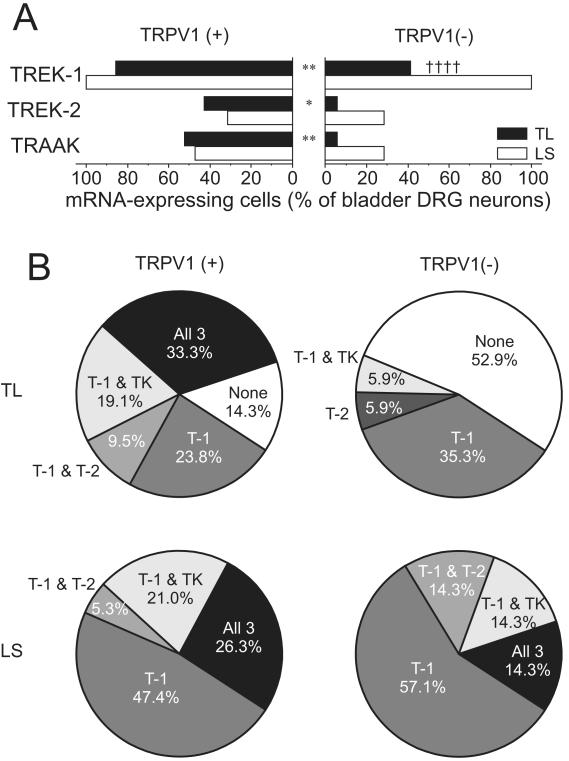
In bladder TL DRG neurons (Fig.3), all three K2P channel gene transcripts were detected more frequently in TRPV1 mRNA-expressing neurons whereas no difference was detected between the two subgroups in LS neurons (Fig 3A). One-third of TRPV1 mRNA-positive bladder TL neurons expressed all three K2P channel gene transcripts, which was never observed in TRPV mRNA-negative neurons where, moreover, 53% were devoid of any of the three K2P channel gene transcripts (Fig 3B).
Figure 3. Gene expression of mechanosensitive K2P channels in TRPV1 mRNA-positive or –negative DRG neurons innervating the mouse bladder.
(A) The proportion of TREK-1, TREK-2 or TRAAK mRNA expressing neurons was greater in TRPV1 mRNA-positive than in TRPV1 mRNA-negative bladder TL neurons (* P<0.05 and ** P<0.01). TREK-1 expression in TRPV1 mRNA-negative bladder LS neurons was greater than in TL neurons (†††† P<0.0001 by FET). (B) Subgroups of these neurons were identified based on the K2P channel mRNA expression profile. In TRPV1 mRNA-negative bladder TL neurons, about half of them did not express any of the three K2P channel mRNAs. T-1, T-2 and TK represent TREK-1, TREK-2 and TRAAK, respectively.
A similar trend was observed in colon-DRG neurons (Fig. 4). The proportion of TREK-1, TREK-2 or TRAAK mRNA-expressing cells was greater in TRPV1 mRNA-positive neurons in TL but not in LS ganglia (Fig 4A). All TRPV1 mRNA-positive colon TL neurons expressed at least one of the three mechanosensitive K2P channel gene transcripts while more than half of TRPV1 mRNA-negative neurons did not express any of them (Fig 4B).
Figure 4. Gene expression of mechanosensitive K2P channels in TRPV1 mRNA-positive or –negative DRG neurons innervating the mouse distal colon.
(A) The proportion of TREK-1, TREK-2 or TRAAK mRNA expressing neurons was greater in TRPV1 mRNA-positive than in TRPV1 mRNA-negative colon TL neurons (** P<0.01 and *** P<0.001). TREK-1 expression in TRPV1 mRNA-negative colon LS neurons was greater than in TL neurons (†† P<0.01 by FET). (B) Subgroups of these neurons were identified based on the K2P channel mRNA expression profile. All of the TRPV1 mRNA-positive colon TL neurons expressed at least one of the three K2P channel mRNAs whereas more than half of their TRPV1 mRNA-negative TL counterparts did not express any of the three K2P channel mRNAs. T-1, T-2 and TK represent TREK-1, TREK-2 and TRAAK, respectively.
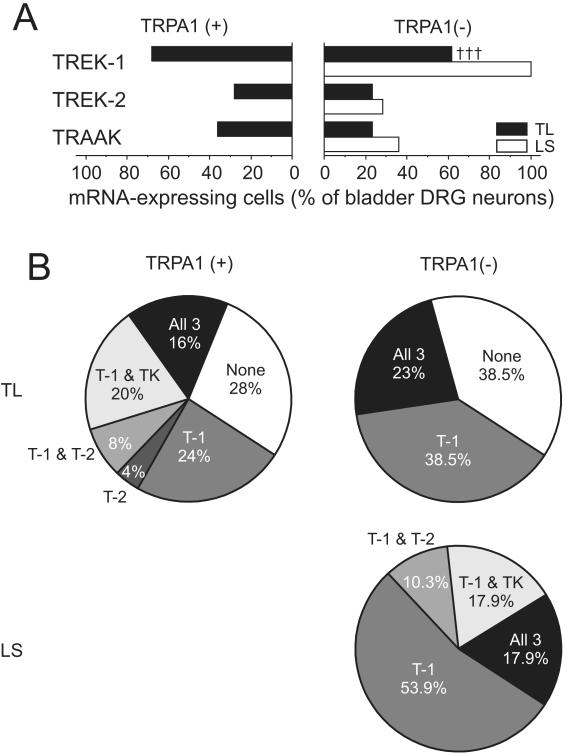
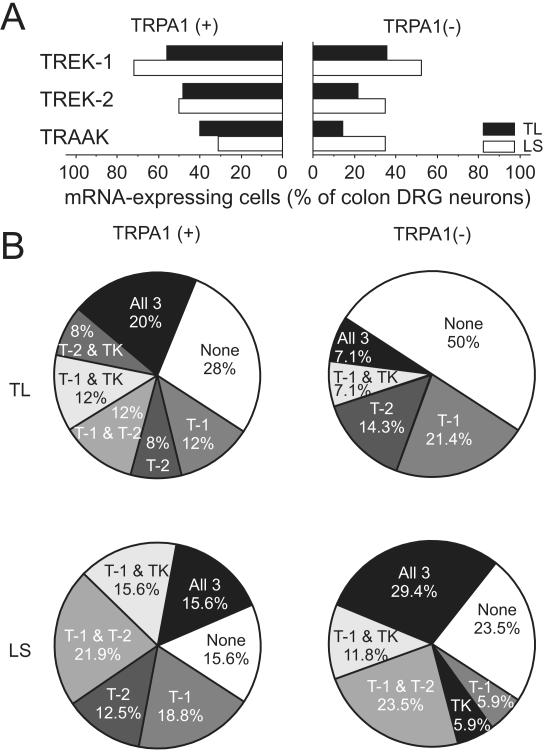
Unlike in TRPV1 mRNA-positive and -negative neurons, the proportion of TREK-1, TREK-2 or TRAAK mRNA-expressing cells did not differ between TRPA1 mRNA-positive and –negative subgroups in bladder TL, colon TL or colon LS neurons (Figs 5 & 6). However, the overall heterogeneity in the gene expression pattern of the three K2P channels was less in TRPA1 mRNA-negative TL neurons. For instance, in bladder TL neurons that did not express TRPA1 mRNA, only three subgroups could be identified (cells expressing TREK-1 mRNA only, cells expressing all three K2P channel mRNAs and cells expressing no K2P channel mRNAs) whereas TRPA1 mRNA-expressing neurons could be categorized into six different subgroups (Fig 5B). Further characterization of bladder- or colon-DRG neurons expressing TRPA1 mRNA is summarized in Table 2. TL neurons that expressed only TRPA1 mRNA had significantly larger proportion of cells devoid of the three K2P channel gene transcripts than those expressing both TRPA1 and TRPV1 mRNAs.
Figure 5. Gene expression of mechanosensitive K2P channels in TRPA1 mRNA-positive or –negative DRG neurons innervating the mouse bladder.
(A) The proportion of TREK-1, TREK-2 or TRAAK mRNA expressing bladder TL neurons did not differ between TRPA1 mRNA-positive and –negative groups. The one bladder LS neuron expressing TRPA1 gene transcript was positive to all three K2P channel mRNAs (not plotted). TREK-1 gene expression in TRPA1 mRNA-negative bladder LS neurons was greater than in TL neurons (††† P<0.001 vs. LS by FET). (B) Subgroups of these neurons were identified based on the K2P channel mRNA expression profile. Note the less heterogeneity in the TRPA1 mRNA-negative bladder-DRG neurons compared with the TRPA1 mRNA-positive counterpart. T-1, T-2 and TK represent TREK-1, TREK-2 and TRAAK, respectively.
Figure 6. Gene expression of mechanosensitive K2P channels in TRPA1 mRNA-positive or –negative DRG neurons innervating the mouse distal colon.
(A) The proportion of TREK-1, TREK-2 or TRAAK mRNA expressing colon-DRG neurons did not differ between TRPA1 mRNA-positive and –negative groups in either TL or LS ganglia. (B) Subgroups of these neurons were identified based on the K2P channel mRNA expression profile. No difference in the relative proportion of each subgroup was detected between TRPA1 mRNA-positive and –negative colon-DRG neurons. T-1, T-2 and TK represent TREK-1, TREK-2 and TRAAK, respectively.
Table 2.
Differences in relative proportions (%) of cells positive to K2P channel mRNAs between DRG neurons expressing only TRPA1 or TRPV1 and both TRPA1 and TRPV1 mRNAs
| Bladder TL | Colon TL | Colon LS | |||||||
|---|---|---|---|---|---|---|---|---|---|
| A1 (n=8) |
V1 (n=4) |
A1 + V1 (n=17) |
A1 (n=11) |
V1 (n=2) |
A1 + V1 (n=14) |
A1 (n=8) |
V1 (n=6) |
A1 + V1 (n=24) |
|
| TREK-1 (T-1) | 12.5 | 0 | 29.4 | 9.1 | 0 | 14.3 | 37.5 | 0 | 12.5 |
| TREK-2 (T-2) | 12.5 | 0 | 0 | 9.1 | 50 | 7.1 | 12.5 | 0 | 12.5 |
| TRAAK (TK) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| T-1 + T-2 | 0 | 0 | 11.8 | 0 | 0 | 21.4 | 0 | 16.7 | 29.2 |
| T-1 + TK | 12.5 | 0 | 23.5 | 9.1 | 0 | 14.3 | 12.5 | 16.7 | 16.7 |
| T-2 + TK | 0 | 0 | 23.5 | 9.1 | 0 | 7.1 | 0 | 0 | 0 |
| All 3 | 0 | 75.0 * | 11.8 | 0 * | 50 | 35.7 | 12.5 | 50.0 | 16.7 |
| None | 62.5 ** | 25.0 | 0 | 63.6 *** | 0 | 0 | 25.0 | 16.7 | 12.5 |
P<0.05
P<0.01
P<0.001 vs. A1+V1 by FET.
Discussion
This study documents significant differences between LSN and PN sensory pathways and a complex heterogeneity in the expression of TRPV1, TRPA1, TREK-1, TREK-2 and TRAAK in sensory neurons that innervate the mouse urinary bladder and distal colon. As knowledge of such differences accumulates, it contributes to understanding functional differences between LSN and PN afferents that innervate these organs.
Although there are some inconsistent findings regarding the role of TRPV1 and TRPA1 as mechanotransducer channels (Bautista et al., 2006; Kwan et al., 2006; Liedtke, 2007; Vilceanu and Stucky, 2010), these two channels are essential for mechanosensation in both bladder and colon under normal as well as pathological conditions in which mechanical hypersensitivity develops (Brierley et al., 2009; Cattaruzza et al., 2010; Christianson et al., 2010; Daly et al., 2007; Jones, III et al., 2005; Miranda et al., 2007; Yang et al., 2008; Yu et al., 2010). Therefore, when expressed in TRPV1- and/or TRPA1-expressing bladder- and colon-DRG neurons, the three mechanosensitive K2P channels TREK-1, TREK-2 and TRAAK may counterbalance neuronal excitation produced by mechanical stimulation by mediating hyperpolarizing outward K+ currents and hence contribute to tuning the degree/pattern of mechanosensitivity. Considering the desensitization characteristics of the three K2P channels to mechanical stimulation, one would expect that the fast-desensitizing TREK-1 and TRAAK channels may have more important inhibitory roles in setting the response onset and TREK-2, which exhibits little or no desensitization, in shaping the encoding properties (Honore et al., 2006).
TRPV1 and TRPA1 gene expression in LSN and PN sensory pathways
Consistent with previous immunohistochemical studies of visceral sensory neurons, we found that the majority of DRG neurons innervating the mouse bladder or colon expressed TRPV1 and TRPA1 gene transcripts with frequent co-localization. We found similar (Brierley et al., 2005; Cho and Valtschanoff, 2008) to relatively smaller (Christianson et al., 2006) proportions of neurons expressing TRPV1 mRNA (41% of colon TL, 61% of colon LS, 55% of bladder TL and 48% of bladder LS neurons) than what has been reported in immunostained DRG sections: 58% of mouse bladder DRG neurons in L5 ganglia (Cho and Valtschanoff, 2008) and ~82% and 50~65% of mouse colon DRG neurons in TL and LS ganglia, respectively (Brierley et al., 2005; Christianson et al., 2006). Comparatively, the proportions of TRPA1 mRNA-expressing colon DRG neurons in this study (64~66%) are slightly higher than the 54~58% of mouse colon DRG neurons reported as TRPA1-immunoreactive in DRG sections (Brierley et al., 2009). In mouse LS ganglia, however, we found only 1 of 40 bladder DRG neurons positive to TRPA1 mRNA (discussed below in detail), which is in stark contrast to previous findings in rats where 51% of bladder LS neurons were TRPA1-immunoreactive (Du et al., 2007). The difference in TRPA1 expression between these two rodent species was also noted in urothelial cells. In mice, TRPA1 expression was not detected in urothelium or was noted as extremely low in cultured urothelial cells whereas in rat urothelial cells the presence of TRPA1 was confirmed by immunohistochemistry and quantitative real-time PCR (Everaerts et al., 2010; Streng et al., 2008).
Differences between LSN and PN sensory pathways innervating the mouse bladder
The most striking differences between the two sensory pathways innervating the mouse bladder were (1) the virtual absence of TRPA1 mRNA-expressing neurons in LS ganglia and (2) the higher prevalence of TREK-1 mRNA-expressing neurons in LS ganglia (all bladder LS neurons contained the TREK-1 gene transcript). The relative absence of TRPA1 mRNA and abundance of inhibitory TREK-1 mRNA in bladder LS neurons suggest that PN bladder afferents may have lower mechanosensitivity than LSN afferents. Indeed, PN muscular and serosal afferents appeared to be less sensitive to probing at lower forces than LSN counterparts in single nerve fiber recordings in vitro (Xu and Gebhart, 2008). Xu and Gebhart (2008) also observed that bladder PN muscular afferents could be divided into low- (64%) and high-threshold (36%) afferents, and low-threshold afferents could be further categorized into two groups based on their ability to encode high intensity stretch stimulation. The extremely low TRPA1 gene expression in bladder LS neurons suggest that this ion channel does not determine either the mechanical threshold or encoding properties in the PN sensory pathway innervating the mouse bladder.
This finding also raises an interesting question about the mechanism of PN pathway sensitization by TRPA1 agonist acrolein, an environmental irritant and the metabolite of the antineoplastic chemical cyclophosphamide (Chen and Gebhart, 2010; Dang et al., 2008; Yoshimura and de Groat, 1999). Considering the relative absence of TRPA1 mRNA in the PN sensory pathway innervating the mouse bladder, sensitization of this sensory pathway by systemic cyclophosphamide treatment in the mouse would thus be expected not to be due to a direct action of its metabolite acrolein on bladder PN sensory afferents. Rather, in mice, inflammatory mediators produced during cyclophosphamide-induced cystitis may sensitize the bladder PN sensory pathway. Xu and Gebhart (2008) reported that both low- and high-threshold bladder PN afferents of muscular class were sensitized after exposed to cocktail of inflammatory mediators (5 μM of bradykinin, serotonin, histamine and prostaglandin E2, pH 6.0). Alternatively, cystitis and inflammatory mediators may up-regulate TRPA1 gene transcription in bladder LS neurons, which has yet to be examined.
In the present study, we found no differences in the gene expression pattern of TREK-1, TREK-2 and TRAAK between TRPV1 mRNA-positive and –negative bladder LS neurons. In the absence of both TRPA1 and TRPV1 mRNA and presence of inhibitory K2P channel gene transcripts, TRPV1 mRNA-negative bladder LS neurons may be less sensitive to low intensity mechanical stimulation and hence contribute to a high-threshold afferent phenotype. This notion is supported by previous findings that only 1 of 15 high-threshold in contrast to 67% of low-threshold mouse bladder PN afferents was capsaicin-sensitive (Daly et al., 2007). It should be also noted that the proportions of low- and high-threshold afferents did not differ in TRPV1 knockout mice from those in wild type mice. The most interesting feature of TRPV1 knockout mice was that low-threshold bladder PN afferents failed to encode high intensity distension, whereas high-threshold afferents remained unaffected (Daly et al., 2007). Together with the present findings, these observations indicate that there are molecules other than TRPV1 and TRPA1 that define the high-threshold mechanosensitive bladder afferent phenotype and render the low-threshold afferents responsive to low intensity mechanical stimulation; TRPV1 appears to confer the ability to encode high intensity mechanical stimulation to the low-threshold afferent class.
Unlike LS neurons, TL bladder neurons showed remarkable differences between TRPV1 mRNA-positive and -negative groups in K2P channel gene expression (Fig 3). First, the proportion of TREK-1, TREK-2 or TRAAK mRNA-expressing neurons was significantly greater in TRPV1 mRNA-positive neurons. Second, about half of TRPV1 mRNA-negative neurons did not express any of the three K2P channel gene transcripts whereas more than 80% of TRPV1 mRNA-positive neurons expressed at least one type of K2P channel mRNA. Interestingly, this relative abundance of inhibitory K2P channel mRNAs was observed only in TRPV1 mRNA-expressing neurons and not in TRPA1 mRNA-expressing neurons even though the majority (81%) of TRPV1 mRNA-expressing neurons also expressed TRPA1 gene transcript (Fig 1B and 5A). Accordingly, bladder TL neurons that express only TRPA1 mRNA differ in their expression of TREK-1, TREK-2 and TRAAK mRNAs from those neurons expressing both TRPA1 and TRPV1 gene transcripts. While all 17 TL neurons expressing both TRPA1 and TRPV1 mRNAs were positive to at least one of the three K2P channel gene transcripts (12 of them expressed more than two types of K2P channel mRNAs), 5 of 8 TL neurons that expressed only TRPA1 mRNA did not express any of the three K2P channel gene transcripts. Thus, it would be interesting to examine in the future whether mechanosensitive bladder LSN afferents that respond either only to mustard oil or both to mustard oil and capsaicin differ from each other in their mechanical threshold and/or encoding properties.
In electrophysiological recordings in vitro, three classes of mechanosensitive afferents were identified in the LSN innervation of the mouse bladder (serosal 67%, muscular 30% and muscular/urothelial 3%) and four different classes in the PN pathway (serosal 14%, muscular 63%, muscular/urothelial 14%, and urothelial 9%). In the present study, however, both bladder TL and LS DRG neurons showed greater heterogeneity because of their complex gene expression patterns of TRPV1 and/or TRPA1 with the three mechanosensitive K2P channels, which indicates that a simple correlation of functional afferent classes with the expression patterns of five channels mRNAs studied here is impracticable.
Differences between LSN and PN sensory pathways innervating the mouse distal colon
In the proportions of colon-DRG neurons expressing TRPV1, TRPA1, TREK-2 or TRAAK gene transcripts, there was no difference between LSN and PN pathways; TREK-1 mRNA-expressing neurons, however, were more prevalent in the PN pathway. As in bladder DRG neurons, we found that the proportion of TREK-1, TREK-2 or TRAAK mRNA-expressing neurons was greater in TRPV1 mRNA-positive colon TL neurons than in their TRPV mRNA-negative counterparts despite the robust co-expression of TRPA1 mRNA in TRPV1 mRNA-expressing neurons (88%), which again indicates that colon (like bladder) TL neurons that express both TRPV1 and TRPA1 gene transcripts differ in their K2P channel gene expression from those expressing only TRPA1 mRNA. We found that ~70% of colon TL neurons that expressed only TRPA1 mRNA did not express any of the three K2P channel gene transcripts whereas all of the 14 neurons that expressed both TRPA1 and TRPV1 mRNAs were positive to at least one of the K2P channel mRNAs (11 of 14 expressed more than two types of K2P channel gene transcripts). In this regard, colon LS neurons differed from their TL counterparts because they showed no statistically significant difference in the K2P channel gene expression profile between neurons expressing only TRPA1 mRNA and those expressing both TRPA1 and TRPV1 mRNAs. The majority of colon LS neurons expressing TRPA1 mRNA were positive to at least one of the three K2P channel gene transcripts. These findings suggest that the two TRPA1 mRNA-expressing groups (i.e., only TRPA1 vs. both TRPA1 and TRPV1) may have different mechanosensitive properties from each other only in the LSN pathway innervating the mouse distal colon.
In electrophysiological recordings in vitro, five different classes of mechanosensitive colon afferents were found in both LSN and PN pathways. Serosal, mucosal (responsive to mucosal stroking) and muscular classes were present in both pathways whereas a mesenteric class was found only in the LSN and a muscular/mucosal class only in the PN pathway. When the degree of mechanosensitivity was compared within the same classes of afferents between the two pathways, the PN pathway was more sensitive to mechanical stimulation (Brierley et al., 2004). Interestingly, characterization of mechanosensitivity of these five colon afferent classes in transgenic mice lacking either TRPV1 or TRPA1 revealed that (1) the relative proportions of the five classes were not altered in either transgenic genotype, (2) the mechanosensitivities of PN serosal and mucosal classes were not changed in TRPV1 knockout mice, but decreased in TRPA1 knockout mice, (3) the response of the PN muscular/mucosal class to mucosal stroking was not changed in TRPV1 knockout mice, but decreased in TRPA1 knockout mice, (4) the response of the PN muscular/mucosal class to colon wall stretch was decreased in TRPV1 knockout mice, but not altered in TRPA1 knockout mice, and (5) the mechanosensitivity of the PN muscular class was not significantly changed either in TRPV1 or TRPA1 knockout mice (Brierley et al., 2009; Jones, III et al., 2005). These findings indicate that TRPV1 and TRPA1 are both expressed in at least a subset of muscular/mucosal afferents, and involved in detecting different sensory modalities - TRPV1 for detecting colon wall stretch and TRPA1 mucosal deformation. Likewise, both TRP channels appear to be expressed in a subset of serosal afferents because 47% of PN serosal afferents are capsaicin-sensitive (Brierley et al., 2005) and their mechanosensitivity is decreased in TRPA1 knockout mice (Brierley et al., 2009). However, the mechanosensitivity of serosal afferents appears to be largely dependent on TRPA1 rather than TRPV1. It is intriguing that the same channel, TRPA1 in this case, enables some afferent classes (mucosal and muscular/mucosal) to respond to low-intensity stimulation (mucosal stroking) but not another class (serosal). This apparent differential role of TRPA1 could be simply due to different degrees of its expression, or different locations of afferent endings within the layers of the colon wall. Alternatively, one can hypothesize a diverse degree/pattern of TRPA1 co-expression with inhibitory K2P channels in different afferent classes, for example that serosal afferents express higher levels of K2P channel(s) than mucosal and muscular/mucosal afferents. In the present study, we found six subgroups of colon LS neurons expressing both TRPV1 and TRPA1 mRNAs (representing at least a subset of PN muscular/mucosal and serosal classes) based on their K2P channel gene expression profile, 59% of which expressed gene transcript of TREK-2, the little or no-desensitizing channel to mechanical stimulation.
Brierley et al (2004) distinguished two subgroups in PN muscular/mucosal afferents based on their stretch encoding properties. One group (54%, low-responding group) showed a lower frequency of action potential firing and gradual slope of the stimulus-response function than the other (high-responding group), which was similar to muscular afferents in their stretch encoding properties. Malin et al (2009) also noted two distinct stretch-responsive neuronal groups in L6 DRG that exhibited different action potential firing frequencies in response to colorectal distension (a mechanical stimulus likely activating both muscular and muscular/mucosal afferent endings). They found that the vast majority of low-firing frequency afferents were sensitized by capsaicin and mustard oil and had a relatively higher mechanical threshold than the high-firing frequency afferent group that was seldom TRPV-immunoreactive and not generally sensitized by either capsaicin or mustard oil. Although these two studies used different methods to characterize mechanosensitive colon-DRG neurons, it seems likely that the high-responding PN muscular/mucosal class corresponds to the high-firing frequency group of L6 DRG neurons, suggesting that most are colon LS neurons which do not express either of the two TRP channels studied here, 27% (3/11) of which also did not express any of the three inhibitory K2P channels.
Conclusions
We found clear differences in the proportions of DRG neurons expressing TRPA1 and TREK-1 mRNA between LSN and PN sensory pathways innervating mouse bladder and colon. In the LSN pathway, TREK-1, TREK-2 or TRAAK gene transcripts were more prevalent in TRPV1 mRNA-positive DRG neurons, suggesting potential differences in their mechanosensitivity from their TRPV1 mRNA-negative counterparts. Similarly, the relative proportions of mechanosensitive K2P channel gene-expressing neurons in the LSN pathway were greater in DRG neurons expressing both TRPV1 and TRPA1 mRNAs than those expressing only TRPA1 mRNA. By examining the co-expression of TRPV1 and/or TRPA1 gene transcripts with the three mechanosensitive K2P channel mRNAs, we revealed a complex heterogeneity of visceral sensory neurons, which prompts future studies to correlate these (and other) patterns of channel expression with functionally identified visceral afferent endings.
Acknowledgements
We thank Michael Burcham for preparation of the figures and Timothy McMurray for assistance with RT-PCR. Supported by National Institutes of Health (NIH) awards R01 NS035790 and UL1 RR024153 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health NIH, and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at http://www.ncrr.nih.gov/. Information on Re-engineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Alloui A, Zimmermann K, Mamet J, Duprat F, Noel J, Chemin J, Guy N, Blondeau N, Voilley N, Rubat-Coudert C, Borsotto M, Romey G, Heurteaux C, Reeh P, Eschalier A, Lazdunski M. TREK-1, a K+ channel involved in polymodal pain perception. EMBO J. 2006;25:2368–2376. doi: 10.1038/sj.emboj.7601116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Brierley SM, Carter R, Jones W, III, Xu L, Robinson DR, Hicks GA, Gebhart GF, Blackshaw LA. Differential chemosensory function and receptor expression of splanchnic and pelvic colonic afferents in mice. J Physiol. 2005;567:267–281. doi: 10.1113/jphysiol.2005.089714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brierley SM, Hughes PA, Page AJ, Kwan KY, Martin CM, O’Donnell TA, Cooper NJ, Harrington AM, Adam B, Liebregts T, Holtmann G, Corey DP, Rychkov GY, Blackshaw LA. The ion channel TRPA1 is required for normal mechanosensation and is modulated by algesic stimuli. Gastroenterology. 2009;137:2084–2095. doi: 10.1053/j.gastro.2009.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brierley SM, Jones RC, III, Gebhart GF, Blackshaw LA. Splanchnic and pelvic mechanosensory afferents signal different qualities of colonic stimuli in mice. Gastroenterology. 2004;127:166–178. doi: 10.1053/j.gastro.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Cattaruzza F, Spreadbury I, Miranda-Morales M, Grady EF, Vanner S, Bunnett NW. Transient receptor potential ankyrin-1 has a major role in mediating visceral pain in mice. Am J Physiol Gastrointest Liver Physiol. 2010;298:G81–G91. doi: 10.1152/ajpgi.00221.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Gebhart GF. Differential purinergic signaling in bladder sensory neurons of naive and bladder-inflamed mice. Pain. 2010;148:462–472. doi: 10.1016/j.pain.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho WG, Valtschanoff JG. Vanilloid receptor TRPV1-positive sensory afferents in the mouse ankle and knee joints. Brain Res. 2008;1219:59–65. doi: 10.1016/j.brainres.2008.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson JA, Bielefeldt K, Malin SA, Davis BM. Neonatal colon insult alters growth factor expression and TRPA1 responses in adult mice. Pain. 2010;151:540–549. doi: 10.1016/j.pain.2010.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson JA, Traub RJ, Davis BM. Differences in spinal distribution and neurochemical phenotype of colonic afferents in mouse and rat. J Comp Neurol. 2006;494:246–259. doi: 10.1002/cne.20816. [DOI] [PubMed] [Google Scholar]
- Daly D, Rong W, Chess-Williams R, Chapple C, Grundy D. Bladder afferent sensitivity in wild-type and TRPV1 knockout mice. J Physiol. 2007;583:663–674. doi: 10.1113/jphysiol.2007.139147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang K, Lamb K, Cohen M, Bielefeldt K, Gebhart GF. Cyclophosphamide-induced bladder inflammation sensitizes and enhances P2X receptor function in rat bladder sensory neurons. J Neurophysiol. 2008;99:49–59. doi: 10.1152/jn.00211.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du S, Araki I, Yoshiyama M, Nomura T, Takeda M. Transient receptor potential channel A1 involved in sensory transduction of rat urinary bladder through C-fiber pathway. Urology. 2007;70:826–831. doi: 10.1016/j.urology.2007.06.1110. [DOI] [PubMed] [Google Scholar]
- Everaerts W, Vriens J, Owsianik G, Appendino G, Voets T, De RD, Nilius B. Functional characterization of transient receptor potential channels in mouse urothelial cells. Am J Physiol Renal Physiol. 2010;298:F692–F701. doi: 10.1152/ajprenal.00599.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honore E, Patel AJ, Chemin J, Suchyna T, Sachs F. Desensitization of mechano-gated K2P channels. Proc Natl Acad Sci U S A. 2006;103:6859–6864. doi: 10.1073/pnas.0600463103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RC, III, Xu L, Gebhart GF. The mechanosensitivity of mouse colon afferent fibers and their sensitization by inflammatory mediators require transient receptor potential vanilloid 1 and acid-sensing ion channel 3. J Neurosci. 2005;25:10981–10989. doi: 10.1523/JNEUROSCI.0703-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang DS, Woolf CJ, Corey DP. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron. 2006;50:277–289. doi: 10.1016/j.neuron.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Liedtke WB. TRPV Channels’ Function in Osmo- and Mechanotransduction. In: Liedtke WB, Heller S, editors. TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades. CRC Press; Boca Raton (FL): 2007. pp. 303–318. [PubMed] [Google Scholar]
- Miranda A, Nordstrom E, Mannem A, Smith C, Banerjee B, Sengupta JN. The role of transient receptor potential vanilloid 1 in mechanical and chemical visceral hyperalgesia following experimental colitis. Neuroscience. 2007;148:1021–1032. doi: 10.1016/j.neuroscience.2007.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ness TJ, Gebhart GF. Visceral pain: a review of experimental studies. Pain. 1990;41:167–234. doi: 10.1016/0304-3959(90)90021-5. [DOI] [PubMed] [Google Scholar]
- Noel J, Zimmermann K, Busserolles J, Deval E, Alloui A, Diochot S, Guy N, Borsotto M, Reeh P, Eschalier A, Lazdunski M. The mechano-activated K+ channels TRAAK and TREK-1 control both warm and cold perception. EMBO J. 2009;28:1308–1318. doi: 10.1038/emboj.2009.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streng T, Axelsson HE, Hedlund P, Andersson DA, Jordt SE, Bevan S, Andersson KE, Hogestatt ED, Zygmunt PM. Distribution and function of the hydrogen sulfide-sensitive TRPA1 ion channel in rat urinary bladder. Eur Urol. 2008;53:391–399. doi: 10.1016/j.eururo.2007.10.024. [DOI] [PubMed] [Google Scholar]
- Vilceanu D, Stucky CL. TRPA1 mediates mechanical currents in the plasma membrane of mouse sensory neurons. PLoS One. 2010;5:e12177. doi: 10.1371/journal.pone.0012177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Gebhart GF. Characterization of mouse lumbar splanchnic and pelvic nerve urinary bladder mechanosensory afferents. J Neurophysiol. 2008;99:244–253. doi: 10.1152/jn.01049.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Li Y, Zuo X, Zhen Y, Yu Y, Gao L. Transient receptor potential ankyrin-1 participates in visceral hyperalgesia following experimental colitis. Neurosci Lett. 2008;440:237–241. doi: 10.1016/j.neulet.2008.05.093. [DOI] [PubMed] [Google Scholar]
- Yoshimura N, de Groat WC. Increased excitability of afferent neurons innervating rat urinary bladder after chronic bladder inflammation. J Neurosci. 1999;19:4644–4653. doi: 10.1523/JNEUROSCI.19-11-04644.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu YB, Yang J, Zuo XL, Gao LJ, Wang P, Li YQ. Transient receptor potential vanilloid-1 (TRPV1) and ankyrin-1 (TRPA1) participate in visceral hyperalgesia in chronic water avoidance stress rat model. Neurochem Res. 2010;35:797–803. doi: 10.1007/s11064-010-0137-z. [DOI] [PubMed] [Google Scholar]