Abstract
Nicotinic acid (NA) has been used as a lipid drug for five decades. The lipid-lowering effects of NA are attributed to its ability to suppress lipolysis in adipocytes and lower plasma FFA levels. However, plasma FFA levels often rebound during NA treatment, offsetting some of the lipid-lowering effects of NA and/or causing insulin resistance, but the underlying mechanisms are unclear. The present study was designed to determine whether a prolonged, continuous NA infusion in rats produces a FFA rebound and/or insulin resistance. NA infusion rapidly lowered plasma FFA levels (>60%, P < 0.01), and this effect was maintained for ≥5 h. However, when this infusion was extended to 24 h, plasma FFA levels rebounded to the levels of saline-infused control rats. This was not due to a downregulation of NA action, because when the NA infusion was stopped, plasma FFA levels rapidly increased more than twofold (P < 0.01), indicating that basal lipolysis was increased. Microarray analysis revealed many changes in gene expression in adipose tissue, which would contribute to the increase in basal lipolysis. In particular, phosphodiesterase-3B gene expression decreased significantly, which would increase cAMP levels and thus lipolysis. Hyperinsulinemic glucose clamps showed that insulin's action on glucose metabolism was improved during 24-h NA infusion but became impaired with increased plasma FFA levels after cessation of NA infusion. In conclusion, a 24-h continuous NA infusion in rats resulted in an FFA rebound, which appeared to be due to altered gene expression and increased basal lipolysis in adipose tissue. In addition, our data support a previous suggestion that insulin resistance develops as a result of FFA rebound during NA treatment. Thus, the present study provides an animal model and potential molecular mechanisms of FFA rebound and insulin resistance, observed in clinical studies with chronic NA treatment.
Keywords: hypolipidemic drug, free fatty acids, perilipin, phosphodiesterase, triglyceride synthesis, insulin resistance, nicotinic acid receptor, microarray analysis
nicotinic acid (NA; or niacin) is a B-group vitamin. In addition to its function as a vitamin, NA, in high doses, has been used as a lipid drug for five decades (6, 21); it produces very desirable effects such as decreasing plasma triglycerides (TG), VLDL, and LDL-cholesterol levels and increasing HDL-cholesterol levels (14, 38). Major clinical trials have demonstrated that NA treatment reduces the progression of atherosclerotic cardiovascular disease (9, 18). The lipid-lowering effects of NA have traditionally been attributed to its antilipolytic effect in adipocytes (10). NA binds to and stimulates a G protein-coupled receptor [i.e., GPR109A or HM74A (37, 41)] in the plasma membrane of adipocytes to decrease cAMP and lipolysis, which in turn reduces the plasma levels of FFA, substrates for hepatic TG synthesis, and VLDL formation. However, this classic view may need revision, because circulating FFA levels, which initially decrease during treatment, rebound after long-term NA treatment (see below), whereas its lipid-lowering effects persist (38). When this occurs, the hypolipidemic effects of NA cannot be explained by reduced FFA delivery to the liver. There is evidence that NA directly inhibits diacylglycerol O-acyltransferase-2 in the liver to decrease TG synthesis and VLDL formation (20). Moreover, NA may lower circulating lipid levels by increasing the lipoprotein lipase activity, thereby enhancing the TG-VLDL removal rate (8, 14). Thus, despite a long history of clinical use, the precise mechanism by which NA lowers circulating lipids remains unclear.
Acute NA administration in humans (39) or rodents (29) results in a rapid decrease in the plasma FFA level followed by a rebound and an overshoot above preinfusion levels. The rebound or overshoot of the plasma FFA level is probably due to the combination of waning of antilipolytic effects of ingested or injected NA and stimulation of lipolysis by lipolytic hormones, such as epinephrine and cortisol, which increase following NA administration (28, 29, 40). In contrast, chronic treatment with NA is associated with increased basal (or fasting) FFA levels (32, 33). This may occur as a result of an overshoot of plasma FFA following the last NA administration the previous evening (42). However, when type 2 diabetic patients were treated extensively (i.e., with high and frequent dosages) for 3 days with acipimox, a long-acting antilipolytic drug derived from NA, plasma FFA levels remained suppressed during the treatment period, but mean FFA levels gradually increased from day 1 to day 3 (42). These data suggest that slowly developing changes occur in adipocytes that increase lipolysis during repetitive or continuous exposure to acipimox (or NA). Consistent with this idea, fasting plasma FFA levels decreased in healthy subjects during the first week of NA treatment but rebounded to control levels after 2 wk (2), and increased fasting FFA levels have been observed after 2 wk or more of NA treatment (32, 33). Because FFA rebound may offset some of the lipid-lowering effects of NA and/or cause insulin resistance (2, 22, 32, 42), it is important to understand the underlying mechanisms.
The mechanisms underlying FFA rebound during chronic NA treatment have been unclear, in part due to a lack of appropriate animal models. One goal of the present study was to develop an animal model of FFA rebound during continuous NA administration. Our recent study (12) showed that the ability of NA to lower plasma FFA was maintained for up to 7 h when NA was constantly infused in rats. In the present study, we extended this infusion to 24 h and tested whether this prolonged exposure to NA would cause an FFA rebound. In addition, we evaluated the molecular mechanisms underlying this phenomenon. Our recent study (12) demonstrated that NA exerts widespread effects to alter gene expression in major tissues involved in lipid metabolism, including adipose tissue. Therefore, we were particularly interested in assessing changes in gene expression in adipose tissue after 24-h NA infusion using a microarray analysis. Finally, we investigated whether the FFA rebound was accompanied by impaired insulin's action to suppress lipolysis and/or to stimulate glucose metabolism.
METHODS
Animals and Catheterization
Male Wistar rats weighing 280–300 g were obtained from Simonsen (Gilroy, CA) and studied at least 5 days after arrival. Animals were housed under controlled temperature (22 ± 2°C) and lighting (12-h light, 6 AM-6 PM; 12-h dark, 6 PM-6 AM) with free access to water and standard rat chow. At least 4 days before the experiment, the animals were placed in individual cages with tail restraints as previously described (11, 12, 24), which was required to protect tail blood vessel catheters during the experiments. The animals were free to move about and were allowed unrestricted access to food and water. One tail vein infusion catheter was placed the day before the experiment, and one tail artery blood sampling catheter was placed the morning of the experiment (∼7 AM). All procedures involving animals were approved by the Institutional Animal Care and Use Committee at the University of Southern California.
Experimental Protocols
Short (5-h) infusions.
Experiments were conducted in the conscious state after an overnight fast; food was removed at 5 PM on the day before the experiment. Animals received a constant infusion of saline or NA (30 μmol/h) for 5 h starting at 1 PM. NA infusate (60 mM) was prepared in saline, and the pH was adjusted to 7.4. During the NA infusion, blood samples were collected at 10-min intervals for immediate measurement of the plasma glucose level. Exogenous glucose was infused at varying rates to maintain plasma glucose at basal levels (∼5.5 mM). Small amounts of glucose needed to be infused during the first 2 h of the NA infusion by exogenous glucose infusion (12). Additional blood samples were collected at various times for measurements of plasma insulin and FFA.
24-h infusions.
Saline or NA (30 μmol/h) was infused for 24 h starting at ∼1 PM on day 1 and continuing until the same time on day 2. Food was removed at 5 PM on day 1. Blood samples were collected at the end of the 24-h infusions for measurements of plasma glucose, insulin, FFA, glycerol, catecholamine, and corticosterone levels. After the blood sampling, the animals were anesthetized with pentobarbital sodium, and epididymal adipose tissue samples were rapidly dissected out, frozen immediately using liquid N2 cooled aluminum blocks, and stored at −80°C for later analysis.
24-h NA infusion followed by 3-h saline infusion.
In separate experiments, the 24-h NA (30 μmol/h) infusion was followed by a 3-h saline infusion. The purpose of this experiment was to evaluate effects of the washout of NA on plasma FFA and glycerol levels. A control group continued to receive the NA infusion during the 3-h follow-up period. Blood samples were collected at various times before (2 h) and during the 3-h follow-up period for measurements of plasma FFA and glycerol levels.
Hyperinsulinemic euglycemic clamp.
In separate experiments, the 24-h saline and NA infusions were followed by a 2-h hyperinsulinemic euglycemic clamp. To accomplish this, insulin was infused at a constant rate of 1 or 5 mU·kg−1·min−1, and blood samples were taken at 10-min intervals for immediate measurement of the plasma glucose level. Exogenous glucose was infused at varying rates to maintain the plasma glucose at basal levels. The glucose infusion rate (GINF) required to clamp plasma glucose during insulin infusion was used as an index of insulin's ability to suppress hepatic glucose production and to increase peripheral glucose uptake. Additional blood samples were taken at various time points for measurements of plasma insulin and FFA levels.
Gene Expression Microarray Analysis
Total RNA was extracted from frozen adipose tissue samples using Tri Reagent obtained from the Molecular Research Center (Cincinnati, OH) according to the manufacturer's instructions. The RNA samples (n = 3 for each group) were subjected to DNA microarray analysis. First-strand cDNA was synthesized from 3 μg of total RNA using T7-oligo(dT) primer and SuperScript II (Invitrogen Life Technologies), while second-strand cDNA was synthesized with second-strand buffer (Invitrogen Life Technologies), DNA polymerase I (New England Biolabs), DNA ligase (NEB), and RNase H (Invitrogen Life Technologies). The cDNA was used as a template, and in vitro transcription was conducted using an RNA Transcript Labeling Kit (Enzo Diagnostics) to produce hybridizable biotin-labeled RNA targets. After in vitro transcription, cRNA was purified using an RNeasy Mini Kit (Qiagen). The cRNA was fragmented by incubation at 94°C for 35 min, after which it was applied to the Affymetrix GeneChip Rat Gene 1.0 ST Array and hybridized at 40°C for 16 h. The GeneChip was then washed several times and stained with streptavidin-conjugated phycoerythrin in a GeneChip Fluidics Station 400 (Affymetrix). Finally, the chips were scanned using an Agilent Scanner (Agilent Technologies) and analyzed with GeneChip Analysis Suite 5.0 (Affymetrix).
Western Blot Analysis
Frozen tissues (∼100 mg) were homogenized using a Tekmar homogenizer (Cincinnati, OH) at half-maximum speed (1 min, on ice) in 500 μl of buffer (50 mM Tris, pH 7.4, 150 mM NaCl, 5 mM Na4P2O7, 50 mM NaF, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, 0.1 mM PMSF, 1% NP-40, 2% protease inhibitor cocktail) (11). Tissue lysates were further solubilized by incubation at 4°C for 1 h with continuous rotation, after which they were centrifuged at 14,000 g and 4°C for 30 min. The supernatants (25 μg of protein) were then resolved by SDS-PAGE followed by electrophoretic transfer of the proteins onto nitrocellulose membranes (Bio-Rad). Next, the membranes were probed with antibodies against hormone-sensitive lipase (HSL, rabbit polyclonal; a generous gift from Dr. Andrew Bicknell, University of Reading, UK), phospho-HSL (Ser563, rabbit polyclonal; Cell Signaling Technology, Beverly, MA), diacylglycerol acyltransferase (DGAT2, rabbit polyclonal; GeneTex, San Diego, CA), phosphodiesterase (PDE)-3B (rabbit polyclonal, kindly provided by Dr. Vincent Manganiello at NIH/NHLBI), and perilipin (goat polyclonal; AbCam, Cambridge, MA). After incubation with the primary antibodies, the membranes were incubated with Alexa 680-labeled goat anti-rabbit or Alexa 680-labeled donkey anti-goat secondary antibody (Molecular Probes). The signals were then detected and quantitated on an Odyssey Infrared Imaging System (Li-COR, Lincoln, NE).
RT-PCR
Semiquantitative RT-PCR was performed to measure PDE-3B mRNA expression in adipose tissue, using Takara Ex Taq Polymerase (Takara, Japan). Primer sequences used were as follows: PDE-3B, F 5′-GAG GAA GGA CGA GCG CGA GC-3′, R 5′-CTC AGC AGC GTC CGA AGC CC-3′; GAPDH F 5′-AAT GCA TCC TGC ACC ACC AAC TGC-3′, R 5′-GGA GGC CAT GTA GGC CAT GAG GTC-3′. Thermal cycling was first carried out with a denaturation phase of 5 min at 95°C followed by 30 cycles of 30 s at 95°C, 30 s at 52°C, and 45 s at 72°C, with a final extension cycle of 5 min at 72°C. Amplification was carried out in a Veriti 96-well Thermal Cycler (Applied Biosystems). The final products were resolved on 1% agarose gel stained with ethidium bromide.
Other Assays
Plasma glucose was analyzed using the glucose oxidase method on a Beckman Glucose Analyzer II (Beckman, Fullerton, CA). Plasma insulin was measured using a radioimmunoassay kit from Linco Research (St. Charles, MO). Plasma FFA was measured using an acyl-CoA oxidase-based colorimetric kit (Wako Chemicals, Richmond, VA) and plasma glycerol using Glycerol Reagent from Sigma-Aldrich (St. Louis, MO). Plasma corticosterone was analyzed using an AssayMax ELISA kit from AssayPro (St. Charles, MO). Plasma epinephrine and norepinephrine were measured using a single-isotope derivative radioenzymatic assay (31).
Statistical Analysis
All data are expressed as means ± SE. The significance of differences in the mean values between groups was assessed by Student's t-test. A P value of <0.05 was considered to be statistically significant.
RESULTS
Effects of Continuous NA Infusion on Plasma FFA Levels
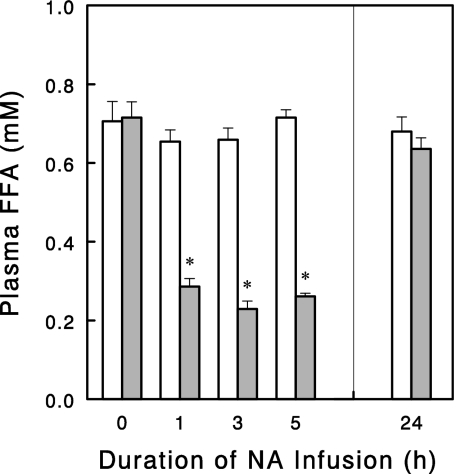
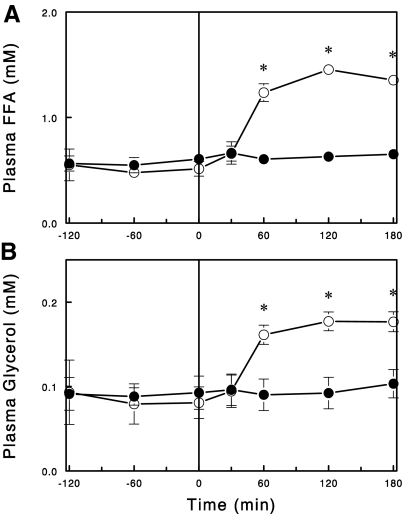
NA infusion rapidly (<1 h) lowered plasma FFA levels, and this effect (>60%, P < 0.01) was maintained for at least 5 h (Fig. 1). However, when this infusion was extended to 24 h, plasma FFA levels rebounded to the levels of saline-infused control rats. Thus, plasma FFA levels were no longer lowered by the NA infusion. We next tested whether the apparent lack of NA effect on plasma FFA was due to a downregulation of NA action or a compensatory increase in lipolysis after the 24-h NA infusion. To address this issue, the NA infusion was stopped after 24 h, and changes in plasma FFA and glycerol levels were observed during the following 3-h period. Plasma FFA levels rapidly increased more than twofold upon cessation of the NA infusion (Fig. 2). Plasma glycerol levels also increased in parallel to the increases in plasma FFA levels. These data suggest that after the 24-h infusion NA was still able to suppress lipolysis and thus plasma FFA levels, but this effect was masked by an increase in basal lipolysis.
Fig. 1.
Plasma FFA levels during saline (open bars) or nicotinic acid (NA) (30 μmol/h; gray bars) infusion in overnight-fasted rats. Effects of short (≤5 h) vs. 24-h infusion were determined in different rats such that plasma FFA levels were measured in similar fasting (18–24 h) states. Data are means ± SE (n = 5 for short and n = 7 or 9 for 24-h infusions). *P < 0.05 vs. saline control.
Fig. 2.
Plasma FFA (A) and glycerol (B) levels during a saline (○) or NA (●) infusion (0–180 min) following 24-h NA infusion. Data are means ± SE (n = 5). *P < 0.05 vs. control.
Effects of 24-h NA Infusion on Gene (mRNA) Expression in Adipose Tissue
Microarray analysis was conducted to determine whether the FFA rebound that occurred after the 24-h NA infusion was associated with changes in mRNA expression for proteins involved in lipolysis or its regulation. Lipolysis-TG degradation to FFA and glycerol involves three lipases: adipose triglyceride lipase (ATGL or PNPLA2), HSL (or LIPE), and monoglyceride lipase (MGLL). HSL expression decreased by 24% (P = 0.043), whereas ATGL and MGLL expressions were not significantly altered (Table 1). In addition, the expression of adiponutrin (PNPLA3), another lipase with a function that has not been well defined, decreased by 54% (P = 0.023). These changes in lipase expression cannot explain the increase in lipolysis (or FFA rebound) that occurred after 24 h of NA infusion. Interestingly, the expression of the lipid droplet-associated protein perilipin (PLIN1) decreased by 39% (P = 0.006), which can potentially explain some of the increase in lipolysis (see discussion). Another lipid droplet-associated protein, TIP47 (perilipin 3 or M6PRBP1), showed no significant change in gene expression.
Table 1.
Effects of 24-h NA infusion on gene expression in adipose tissue detected by microarray analysis
| Hybridization/Intensity |
|||||
|---|---|---|---|---|---|
| Symbol | Description | Saline | NA | %Δ | P Value |
| Lipases and lipid droplet-associated proteins | |||||
| PNPLA2 | adipose triglyceride lipase (desnutrin) | 6,335 ± 191 | 6,540 ± 124 | ↑3% | 0.419 |
| LIPE | hormone sensitive lipase | 3,121 ± 120 | 2,374 ± 225 | ↓24% | 0.043 |
| MGLL | monoglyceride lipase | 1,672 ± 195 | 1,701 ± 34 | ↑2% | 0.890 |
| PNPLA3 | adiponutrin | 121 ± 18 | 55 ± 5 | ↓54% | 0.023 |
| PLIN1 | perilipin 1 | 1,950 ± 76 | 1,181 ± 119 | ↓39% | 0.006 |
| M6PRBP1 | TIP47, perilipin 3 | 85 ± 9 | 82 ± 4 | ↓3% | 0.769 |
| LPL | lipoprotein lipase | 6,273 ± 181 | 5,491 ± 112 | ↓12% | 0.021 |
| Enzymes of triglyceride synthesis | |||||
| AGPAT2 | 1-acylglycerol-3-phosphate O-acyltransferase-2 | 2,750 ± 248 | 1,655 ± 147 | ↓40% | 0.019 |
| AGPAT3 | 1-acylglycerol-3-phosphate O -acyltransferase-3 | 1,244 ± 102 | 606 ± 30 | ↓51% | 0.004 |
| AGPAT9 | 1-acylglycerol-3-phosphate O -acyltransferase-9 | 580 ± 39 | 256 ± 35 | ↓56% | 0.004 |
| DGAT1 | diacylglycerol O -acyltransferase-1 | 1,003 ± 119 | 582 ± 37 | ↓42% | 0.028 |
| DGAT2 | diacylglycerol O -acyltransferase-2 | 1,057 ± 102 | 535 ± 73 | ↓49% | 0.014 |
| GPAM | glycerol-3-phosphate acyltransferase | 1,458 ± 105 | 1,082 ± 168 | ↓26% | 0.131 |
| GPD1 | glycerol-3-phosphate dehydrogenase-1 (soluble) | 5,401 ± 323 | 2,799 ± 347 | ↓48% | 0.005 |
| PCK1 | phosphoenolpyruvate carboxykinase 1 | 6,229 ± 500 | 2,894 ± 91 | ↓54% | 0.003 |
| cAMP regulators | |||||
| ADCY6 | adenylate cyclase 6 | 630 ± 52 | 540 ± 34 | ↓14% | 0.222 |
| ADCY4 | adenylate cyclase 4 | 151 ± 8 | 192 ± 14 | ↑27% | 0.070 |
| PDE-3B | phosphodiesterase 3B | 1,627 ± 175 | 707 ± 77 | ↓57% | 0.009 |
| PRKAR2B | protein kinase, cAMP-dependent, regulatory, type IIβ | 1,845 ± 136 | 1,095 ± 32 | ↓41% | 0.006 |
| NIACR1 | nicotinic acid receptor, | 1,149 ± 93 | 428 ± 103 | ↓63% | 0.007 |
| PLA2G16 | AdPLA, phospholipase A2, group XVI | 1,604 ± 62 | 1,268 ± 75 | ↓21% | 0.026 |
| Other major proteins of adipocytes | |||||
| ADIPOQ | adiponectin | 6,616 ± 95 | 6,200 ± 316 | ↓6% | 0.276 |
| LEP | leptin | 453 ± 61 | 592 ± 46 | ↑31% | 0.141 |
| FABP4 | fatty acid binding protein-4 | 4,749 ± 155 | 4,305 ± 122 | ↓9% | 0.087 |
| FASN | fatty acid synthase | 954 ± 395 | 715 ± 128 | ↓25% | 0.596 |
| GADPH | glyceraldehyde-3-phosphate dehydrogenase | 5,219 ± 705 | 4,947 ± 67 | ↓5% | 0.720 |
| ACTB | β-actin | 4,890 ± 285 | 5,421 ± 126 | ↑11% | 0.164 |
| Transcription factors | |||||
| PPARG | PPARγ | 775 ± 30 | 562 ± 73 | ↓27% | 0.055 |
| CEBPA | C/EBPα | 786 ± 60 | 601 ± 63 | ↓24% | 0.101 |
| CEBPB | C/EBPβ | 97 ± 3 | 81 ± 2 | ↓17% | 0.008 |
| CEBPG | C/EBPγ | 108 ± 10 | 315 ± 25 | ↓23% | 0.026 |
NA, nicotinic acid.
Enzymes directly involved in TG synthesis include 1-acylglycerol-3-phosphate O-acyltransferase (AGPAT), diacylglycerol O-acyltransferase (DGAT), and glycerol-3-phosphate acyltransferase (GPAM). In addition, glycerol-3-phosphate dehydrogenase (GPD1) and phosphoenolpyruvate carboxykinase-1 (PCK1 or PEPCK1) contribute to TG synthesis by providing glycerol 3-phosphate, which is required for TG synthesis (15). With the exception of GPAM, all of these genes showed substantially (40–56%) decreased expression after 24 h of NA infusion (P < 0.05). These changes occurred without significant changes in the expression of major genes of adipocytes, such as adiponectin, leptin, fatty acid-binding protein-4, fatty acid synthase, glyceraldehyde-3-phosphate dehydrogenase, and β-actin (P > 0.05 for all).
cAMP is an important regulator of lipolysis in adipocytes, and its level is determined by the balance of the activities of adenylate cyclase, which synthesizes cAMP, and PDE, which degrades cAMP. Adenylate cyclase expression was not significantly altered. In contrast, PDE-3B (but not PDE-3A or PDE-4) expression decreased by 57% (P = 0.009). Moreover, cAMP-dependent protein kinase (PRKAR2B) expression decreased by 41% (P = 0.006). Interestingly, nicotinic acid receptor (NIACR1) expression decreased by 63% (P = 0.007), and adipose-specific phospholipase A2 (AdPLA or PLA2G16) expression decreased by 21% (P = 0.026) (see discussion). Finally, all of the transcription factors involved in adipocyte differentiation, such as C/EBPα, C/EBPβγ, C/EBPγ, and PPARγ (43), showed decreased expression, although only the changes in C/EBPβ and C/EBPγ gained statistical significance.
Effects of 24-h NA Infusion on Protein Expression and Phosphorylation in Adipose Tissue
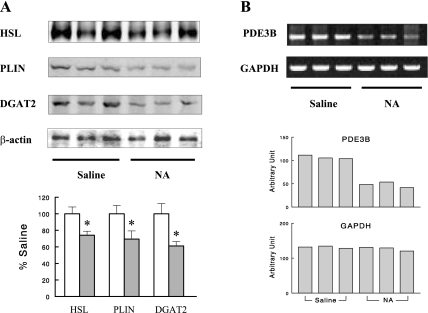
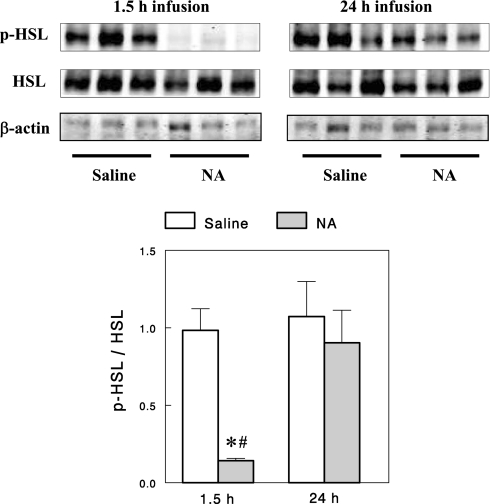
We also examined changes in protein expression in adipose tissue after the 24-h NA infusion. HSL, perilipin, and DGAT2 protein contents decreased by 26, 31, and 39%, respectively (Fig. 3A), similar to the corresponding changes in mRNA expression, supporting the reliability of the microarray analysis. NA is known to decrease lipolysis by inhibiting adenylate cyclase and lowering cAMP. The finding of 57% decreased PDE-3B mRNA expression after 24-h NA infusion suggests that the initial decrease in cAMP by NA (due to reduced cAMP synthesis) may be reversed by reduced cAMP degradation arising from decreased PDE-3B activity, explaining the FFA rebound that occurred after 24-h of NA infusion. To test this idea, we measured the phosphorylation status of HSL (Ser563), which reflects cAMP-dependent protein kinase [i.e., protein kinase A (PKA)] activity and thus the cAMP level. As expected, phospho-HSL normalized to total HSL was profoundly decreased by a short (1.5-h) NA infusion, but after a 24-h NA infusion it rebounded to a level not different from controls (Fig. 4). These data suggest that decreases in PDE-3B gene expression may be a major mechanism responsible for FFA rebound after 24-h NA infusion. The effect of NA infusion on PDE-3B expression was not tested at the protein level because of technical problems with antibodies. However, using semiquantitative RT-PCR with gene-specific primers, we confirmed that PDE-3B mRNA levels indeed decreased after the 24-h NA infusion (Fig. 3B). The decrease was quantified to be 55% (P < 0.01), very similar to the 57% decrease detected by the microarray analysis.
Fig. 3.
Representative Western blots and quantitative data showing hormone-sensitive lipase (HSL), perilipin (PLIN1), and diacylglycerol acyltransferase-2 (DGAT2) protein contents (A) and semiquantitative RT-PCR showing phosphodiesterase (PDE)-3B mRNA levels (B) in adipose tissue in 24-h saline- or NA-infused rats (open and gray bars, respectively, in A). Data are means ± SE (n = 7). *P < 0.05 vs. saline control.
Fig. 4.
Representative Western blots for total and phosphorylated HSL and quantitation of the ratio of phosphorylated to total HSL. Data are means ± SE (n = 5 for 1.5-h and 7 for 24-h infusions). *P < 0.05 vs. saline control; #P < 0.05 vs. 24-h infusion.
Effects of Continuous NA Infusion on Insulin Action
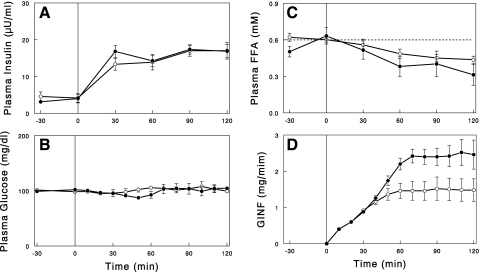
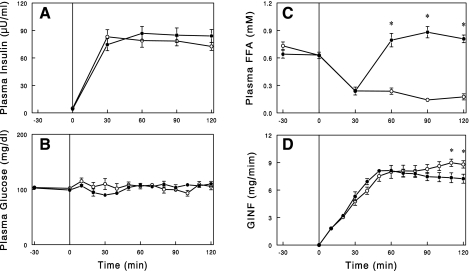
Hyperinsulinemic euglycemic clamps were conducted in 24-h saline or NA-infused rats to assess insulin's actions to suppress lipolysis and to stimulate glucose metabolism. First, the clamp was performed by infusing insulin at a rate of 1 mU·kg−1·min−1 while the saline or NA infusion was continued. During the insulin infusion, plasma insulin levels were raised to similar levels of ∼17 μU/ml in both groups (Fig. 5). Plasma FFA levels were lowered by insulin similarly in both groups. The GINF required to maintain plasma glucose, which reflects insulin's action to suppress hepatic glucose production and to increase peripheral glucose uptake, was significantly higher during the second hour of the clamp in the NA-infused rats compared with the saline-infused control rats (average GINF: 2.44 ± 0.25 vs. 1.48 ± 0.29 mg/min, P < 0.05). In separate experiments, we conducted a hyperinsulinemic clamp in which the NA infusion was stopped at the beginning of the clamp. In this experiment, plasma FFA rebounded above basal levels due to waning of the NA effect to suppress lipolysis (Fig. 2). To suppress the FFA rebound/overshoot, insulin was infused at a higher rate of 5 mU·kg−1·min−1, which raised plasma insulin to similar levels of ∼80 μU/ml in both groups (Fig. 6). Even with the increased insulin infusion, plasma FFA levels were not fully suppressed in NA-infused rats; plasma FFA levels were similarly decreased by insulin at 30 min in both groups, but while this effect was maintained throughout the clamp in control rats, plasma FFA levels rebounded after 30 min in the 24-h NA-infused rats. GINF during the first hour of the clamp was similar or slightly greater in the 24-h NA-infused rats than in the control rats. GINF showed a tendency for further increases during the second hour in control rats. In contrast, in NA-infused rats, GINF showed a tendency for decreases, presumably due to the rebound of plasma FFA (see discussion), resulting in slightly but significantly lower GINF toward the end of the clamps (P < 0.05).
Fig. 5.
Plasma insulin (A), glucose (B), and FFA (C) levels and glucose infusion rate (GINF; D) during hyperinsulinemic euglycemic clamp (insulin infusion = 1 mU·kg−1·min−1) following 24-h saline (○) or NA (●) infusion. In the NA group, NA infusion was continued during the clamp. Data are means ± SE (n = 5).
Fig. 6.
Plasma insulin (A), glucose (B), and FFA (C) levels and GINF (D) during hyperinsulinemic euglycemic clamp (insulin infusion=5 mU·kg−1·min−1) following 24-h saline (○) or NA (●) infusion. During the clamps, NA infusion was stopped. Data are means ± SE (n = 7 or 9). *P < 0.05, saline vs. NA.
Effects of 24-h NA Infusion on Plasma Corticosterone and Catecholamine Levels
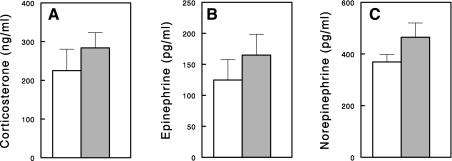
Previous human studies have shown that NA raised plasma epinephrine levels at resting states (28, 40). In addition, NA infusion in rats significantly increased plasma corticosterone levels (29). These hormones are known to increase lipolysis in adipocytes and are potentially responsible for FFA rebound. We measured plasma levels of corticosterone and catecholamines in the 24-h saline- or NA-infused rats. There were tendencies for these hormones to increase after the 24-h NA infusion, but the changes were not statistically significant (P > 0.05 for all; Fig. 7).
Fig. 7.
Plasma corticosterone (A), epinephrine (B), and norepinephrine (C) levels in 24-h saline- (open bars) or NA (gray bars)-infused rats. Data are means ± SE (n = 6 or 8).
DISCUSSION
In the present study, we found that a 24-h continuous NA infusion in rats resulted in FFA rebound; while NA infusion lowered plasma FFA levels for the initial 5 h, plasma FFA levels rebounded to preinfusion levels when the NA infusion was extended to 24 h. This was not due to a downregulation of NA action, because when the NA infusion was stopped, plasma FFA levels rapidly increased more than twofold, demonstrating that NA was still able to suppress lipolysis. Microarray analysis revealed that the FFA rebound was accompanied by many changes in gene expression in adipocytes that would increase lipolysis. In particular, PDE-3B gene expression decreased significantly, which would increase cAMP levels and thus lipolysis. In addition, the expression of several key enzymes of triglyceride synthesis was markedly decreased, which would also contribute to the FFA rebound (see below). Thus, the present study provides an animal model and potential molecular mechanisms for the FFA rebound observed in clinical studies with chronic NA treatment.
Previous studies in humans and rodents had demonstrated a characteristic pattern of change in plasma FFA levels following acute administration of NA, a rapid decrease followed by a rebound and an overshoot above pretreatment levels that occurs within a few hours (29, 39). The rebound may largely reflect waning of the initial NA effect following an injection or oral administration of NA; NA has a short half-life in blood [e.g., 1 h in rats (30)], and its effect may last for only a few hours. On the other hand, the overshoot above basal levels may be due to increases in lipolytic hormones such as epinephrine, glucocorticoids, and/or growth hormone induced by acute NA administration (28, 29, 40). Previous studies in rats had demonstrated that the overshoot could be completely prevented by the combination of adrenalectomy and hypophysectomy, but not by either treatment alone, suggesting that both the adrenal gland and pituitary hormones are involved. However, when NA (or long-acting derivatives) is administered in high and frequent doses, the plasma FFA level can be maintained for an extended period without a rebound or an overshoot (40, 42). In this case, the inhibitory effect of NA on lipolysis may dominate acute influences of lipolytic hormones. In our studies, continuous NA infusion in rats maintained its ability to lower plasma FFA for at least 5 h (present study) or 7 h (12). However, when this NA infusion was extended to 24 h, plasma FFA rebounded to control levels, suggesting that there was a slowly developing process that caused the FFA rebound. This was not due to a downregulation of NA action, because when the NA infusion was stopped after 24 h the plasma FFA level rapidly increased. The ratio of the average plasma FFA level with NA to that without NA was 0.46 during the 3-h follow-up period after 24-h NA infusion (Fig. 2), which was similar to the value of 0.36 observed during the initial hours of NA infusion (Fig. 1), indicating that the ability of NA to suppress lipolysis was not greatly affected. Thus, our data suggest that the FFA rebound occurred not because of a downregulation of NA action but because of an increase in the basal rate of lipolysis.
Microarray analysis revealed that the FFA rebound after 24-h NA infusion was associated with many changes in gene expression in adipocytes. Among these changes, the decrease in PDE-3B gene expression is of particular interest. This change was very specific, as genes encoding other isoforms of PDE (i.e., PDE-3A, PDE-4A–D) were not affected (data not shown). PDE-3B degrades cAMP, a major regulator of lipolysis in adipocytes, and decreased PDE-3B helps increase the cAMP level and thus lipolysis (27). The mechanism by which NA inhibits lipolysis in adipocytes is well established (9, 10). NA binds to and activates the NA receptor, a G protein (Gi)-coupled receptor, to inhibit adenylate cyclase and lower the cAMP level. Low cAMP levels in turn decrease PKA activity and the phosphorylation (and activity) of proteins involved in lipolysis (e.g., HSL and perilipin). Thus, cAMP levels are expected to be reduced (to suppress lipolysis) during the initial hours of NA infusion, but this change may be reversed after 24-h NA infusion due to decreased PDE-3B that will, in turn, decrease cAMP degradation. To test this idea, we first measured cAMP concentrations in adipose tissue and found that it may not well reflect changes in adipocytes. For example, a short (i.e., 1.5-h) NA infusion decreased tissue cAMP concentration, but this effect was small (14%) and statistically insignificant (P > 0.05). This may be due to the fact that adipose tissues contain cells other than adipocytes, which may contribute more than adipocytes to the tissue cAMP pool. Therefore, we chose to measure the phosphorylation levels of HSL (Ser563), which reflect PKA activity and cAMP levels. As expected, phosphorylation levels of HSL were profoundly decreased after 1.5-h NA infusion. It is important to note that most of the decrease was reversed after 24-h NA infusion, supporting the concept that the short-term effects of NA to decrease cAMP and lipolysis were reversed by the effects of decreased PDE-3B to increase cAMP after 24 h of exposure to NA. These data suggest that decreased PDE-3B expression may be a major mechanism underlying the FFA rebound after a prolonged exposure to NA.
When plasma FFA levels rise after cessation of NA infusion (Fig. 2), cAMP levels are expected to rise in adipocytes, and this would increase phosphorylation of HSL. Interestingly, we did not see any significant increase in phospho-HSL (Ser563) (p-HSL) above the levels seen after 24-h NA infusion (data not shown). These data may suggest that cAMP levels did not rise after cessation of NA infusion, contrary to our expectation. However, this is unlikely, based on the rapid increase of lipolysis after cessation of NA infusion. Alternatively, it is possible that cAMP levels rose but p-HSL did not increase. Previous studies have demonstrated that a rapid increase in lipolysis in adipocytes leads to an activation of AMP-dependent protein kinase (AMPK) (16), and increased AMPK activity inhibits phosphorylation of HSL (Ser563) (3). Therefore, it may be conceivable that the rapid increase in lipolysis after cessation of NA infusion, arising from increased cAMP levels, activates AMPK activity to inhibit phosphorylation of HSL (Ser563), resulting in no significant increase in p-HSL with an increase in cAMP level, which can increase lipolysis by acting on other molecules, such as adipose triglyceride lipase or perilipin.
Other changes in gene expression that might also contribute to the FFA rebound after a 24-h NA infusion were the 40–60% decreases in the expression of key enzymes of TG synthesis, including AGPAT, DGAT, GPD1, and PEPCK1. The fatty acyltransferases AGPAT and DGAT are directly involved in TG synthesis (or FFA reesterification). In addition, GPD1 and PEPCK1 contribute to TG synthesis by providing glycerol 3-phosphate required for FFA reesterification (15). FFA release from the adipose tissue is determined by the balance between lipolysis and FFA reesterification, and decreased FFA reesterification helps increase the net rate of TG degradation into FFA and glycerol. For example, decreased DGAT2 activity decreases diacylglycerol conversion to TG, thereby increasing the availability of diacylglycerol for lipolysis to monoacylglycerol and to FFA and glycerol. Previous studies established a role of PEPCK1, the key enzyme involved in glyceroneogenesis in adipocytes (15), in the regulation of FFA reesterification. Glucocorticoids are known to decrease the expression of PEPCK1 in adipocytes, and this may contributes to the action of the hormone to promote FFA release from adipocytes (15). Also, INFγ was shown to promote FFA release from adipose tissue by decreasing PEPCK expression without affecting lipolysis (23). Thus, our novel finding of the coordinated decreases in gene expression of the enzymes of TG synthesis suggests that the FFA rebound after a 24-h NA infusion may result, at least in part, from decreased TG synthesis or FFA reesterification in adipocytes.
There were other changes in gene expression that might also have contributed to the increase in lipolysis after a 24-h NA infusion. First, perilipin mRNA and protein expression decreased by 39 and 31%, respectively. Perilipin coats lipid droplets in adipocytes and suppresses lipolysis by reducing lipase accessibility to the surface of lipid droplets (36). Perilipin knockout mice were lean, had elevated basal lipolysis, and were resistant to diet-induced obesity (1, 26). Therefore, the 31% decrease in perilipin protein content after 24 h of NA likely contributed to the increase in lipolysis. Second, there was a 21% decrease in adipose-specific phospholipase A2 (AdPLA or PLA2G16) gene expression. AdPLA is a recently identified, membrane-associated, calcium-dependent phospholipase that is expressed exclusively in adipocytes and is the major phospholipase in adipocytes. AdPLA suppresses lipolysis by producing prostaglandin E2, which decreases cAMP via EP receptors (19). AdPLA-null mice showed elevated lipolysis in adipocytes and were resistant to diet-induced obesity (35). These data suggest that the small (21%) but significant decrease in AdPLA gene expression might have contributed to the increase in lipolysis that occurred after 24-h NA infusion (assuming that a similar change in protein level occurred). Finally, NA receptor gene expression decreased by 63% after 24-h exposure to NA. However, as discussed above, the NA effect to suppress lipolysis was not significantly affected, suggesting that signaling through the NA receptor was still strong enough to suppress lipolysis under our experimental conditions, possibly due to spare receptors and/or high plasma NA levels. It is conceivable, however, that such a change in nicotinic acid receptor expression, which would decrease NA signaling and action, may account for some of FFA rebound in clinical situations when plasma NA levels are not too high.
What are the mechanisms by which these changes in gene expression in adipose tissue were brought about by NA? NA lowers cAMP and thus PKA activity in adipocytes. Therefore, one possibility is that the cAMP/PKA-dependent pathways mediated some gene expression changes via regulation of the activity of transcription factors such as CREB and CREM (17), which bind to cAMP response elements of gene promoters. Alternatively, NA may regulate gene expression by decreasing the plasma levels of FFAs, which are endogenous ligands of the PPARs. Although plasma FFA levels rebounded after the 24-h NA infusion, some of the changes in gene expression might be a delayed response to earlier decreases in plasma FFA levels. On the other hand, as discussed above, NA has been shown to increase plasma epinephrine and glucocorticoid levels (28, 29, 27). We found that NA infusion significantly increased plasma levels of both epinephrine and corticosterone at 1 h (data not shown) although these effects were not statistically significant at 24 h (Fig. 7). Therefore, some gene expression changes may have arisen from increases in these hormones early during NA infusion. Interestingly, the 24-h NA infusion decreased the expression of transcription factors involved in adipocyte differentiation, such as C/EBPβ, C/EBPγ, and PPARγ (43), suggesting that NA may affect differentiation/dedifferentiation status. However, major adipocyte-specific genes such as adiponectin, leptin, fatty-acid synthase, and fatty-acid-binding protein showed no significant changes in gene expression, suggesting that adipocyte differentiation/dedifferentiation might not play a role. Thus, there could be several different mechanisms by which gene expression changes were induced. Future studies are warranted to elucidate the underlying mechanisms.
The present rat model of FFA rebound with 24-h NA infusion may not be directly relevant to clinical situations with chronic treatments with NA or its derivatives. This is because the mode, dose, and duration of NA administration used in the present study are different from those used in clinical studies. Moreover, we studied the phenomenon in normal metabolic states, whereas clinical studies involve patients with dyslipidemia. Nonetheless, our study provided potential molecular mechanisms for FFA rebound during prolonged exposure to NA, such as gene-expression changes in adipocytes, which can be tested in clinical studies using fat biopsy samples taken before and after chronic NA treatment. If similar changes occur in patients during chronic NA treatment, our 24-h NA infusion model would be an excellent model with which to further study molecular mechanisms underlying the changes.
The 24-h NA infusion improved insulin's action to stimulate glucose metabolism as assessed with continued NA infusion, presumably due to decreased plasma FFA levels that were present during a significant portion of the 24-h infusion period. Our additional data show that the effect of NA to lower plasma FFA level was well maintained after 12-h NA infusion (data not shown). Therefore, although plasma FFA rebounded to control levels after 24-h NA infusion, plasma FFA levels appeared to be lowered during most (>12 h) of the 24-h NA infusion period. Previous studies in humans have demonstrated that lowering plasma FFA using the NA analog acipimox for a short term (hours or days) increased insulin sensitivity (13, 34). Our preliminary data also show a 37% decrease in hepatic TG concentration (P < 0.05, n = 4) after 24-h NA infusion despite the FFA rebound, reflecting the effect of earlier decreases in plasma FFA levels, consistent with the effect on insulin sensitivity. However, when NA infusion was stopped, basal lipolysis increased, and insulin was not able to fully suppress lipolysis (or lower plasma FFA), resulting in impaired insulin action. It is well established that the plasma FFA level is a major determinant of insulin's action (4). Although the effect of FFA rebound on insulin action was small (i.e., 18%), this was likely due to a short exposure to elevated plasma FFA. Previous studies have shown that it takes time for elevated FFA levels to decrease insulin's action on glucose uptake. For example, in human studies (5, 7), it took 2–3 h to see any effect on insulin action (i.e., GINF) even with FFA levels elevated higher than those in the present study. In our own study in rats (25), insulin action was inhibited by elevated plasma FFA in a time-dependent manner, i.e., ∼10% at 1 h, ∼25% at 2 h, and >50% at 4 h. In the present study, NA-infused rats were exposed to elevated plasma FFA levels only during the final 1–1.5 h of the clamp, explaining the small effect (i.e., 18%) of FFA rebound on insulin sensitivity in NA-infused rats. Thus, our data support the previous suggestion that insulin resistance develops as a result of FFA rebound during NA treatment (2, 22, 32, 42).
In conclusion, a 24-h continuous NA infusion in rats resulted in FFA rebound, accompanied by many changes in gene expression and increased basal lipolysis in adipose tissue. In particular, PDE-3B gene expression decreased significantly, which might have led to increased cAMP levels and HSL activity to cause the FFA rebound. In addition, the expression of several key enzymes of triglyceride synthesis was markedly decreased, suggesting the possibility that decreased TG synthesis contributes to the FFA rebound. Thus, the present study provides an animal model and potential molecular mechanisms for the FFA rebound observed in clinical studies with chronic NA treatment. Future studies are warranted to determine whether similar changes occur in humans to account for FFA rebound during chronic NA treatment.
ACKNOWLEDGMENTS
This study was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-080233 (to J. H. Youn), the Basic Science Research Program through the National Research Foundation (NRF) of Korea (MEST, no. 2009-0073746 to I. Kang), the Korea Research Foundation Grant funded by the Korean Government (MOEHRD, KRF-2006-352-G00008 to K. S. Oh), and the Basic Science Research Program through the NRF of Korea (2010-0016256 to S. Choi).
DISCLOSURES
No conflicts of interest are reported by the authors.
REFERENCES
- 1. Ahamadian M, Sul HS. Lipolysis in adipocytes. Int J Biochem Cell Biol 42: 555–559, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alvarsson M, Grill V. Impact of nicotinic acid treatment on insulin secretion and insulin sensitivity in low and high insulin responders. Scand J Clin Lab Invest 56: 563–570, 1996 [DOI] [PubMed] [Google Scholar]
- 3. Anthony NM, Gaidhu MP, Ceddia RB. Regulation of visceral and subcutaneous adipocyte lipolysis by acute AICAR-induced AMPK activation. Obesity 17: 1312–1317, 2009 [DOI] [PubMed] [Google Scholar]
- 4. Bergman RN, Ader M. Free fatty acids and pathogenesis of type 2 diabetes mellitus. Trends Endocrinol Metab 11: 351–356, 2000 [DOI] [PubMed] [Google Scholar]
- 5. Boden G, Jadali F, White J, Liang Y, Mozzoli M, Chen X, Coleman E, Smith C. Effects of fat on insulin-stimulated carbohydrate metabolism in normal men. J Clin Invest 88: 960–966, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bodor ET, Offermanns S. Nicotinic acid: an old drug with a promising future. Br J Pharmacol 153: S68–S75, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bonadonna RC, Zych K, Boni C, Ferrannini E, DeFronzo RA. Time dependence of the interaction between lipid and glucose in humans. Am J Physiol Endocrinol Metab 257: E49–E56, 1989 [DOI] [PubMed] [Google Scholar]
- 8. Capurso A. Drugs affecting triglycerides. Cardiology 78: 218–225, 1991 [DOI] [PubMed] [Google Scholar]
- 9. Carlson LA. Nicotinic acid: the broad-spectrum lipid drug. A 50th anniversary review J Int Med 258: 94–114, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Carlson LA, Oro L. The effect of nicotinic acid on the plasma free fatty acid; demonstration of a metabolic type of sympathicolysis. Acta Med Scand 172: 641–645, 1962 [DOI] [PubMed] [Google Scholar]
- 11. Choi CS, Kim YB, Lee FL, Zabolotny JM, Kahn BB, Youn JH. Lactate induces insulin resistance in skeletal muscle by suppressing glycolysis and impairing insulin signaling. Am J Physiol Endocrinol Metab 283: E233–E240, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Choi S, Yoon H, Oh KS, Oh YT, Kim YI, Kang I, Youn JH. Widespread effects of nicotinic acid on gene expression in insulin-sensitive tissues: implications for unwanted effects of nicotinic acid treatment. Metabolism 60: 134–144, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cusi K, Kashyap S, Gastaldelli A, Bajaj M, Cersosimo E. Effects on insulin secretion and insulin action of a 48-h reduction of plasma free fatty acids with acipimox in nondiabetic subjects genetically predisposed to type 2 diabetes. Am J Physiol Endocrinol Metab 292: E1775–E1781, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Drood JM, Zimetbaum PJ, Frishman WH. Nicotinic acid for the treatment of hyperlipoproteinemia. J Clin Pharmacol 31: 641–650, 1991 [DOI] [PubMed] [Google Scholar]
- 15. Forest C, Tordjman J, Glorian M, Duplus E, Chauvet G, Quette J, Beale EG, Antoine B. Fatty acid recycling in adipocytes: a role for glyceroneogenesis and phosphoenolpyruvate carboxykinase. Biochem Soc Trans 31: 1125–1129, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Gauthier MS, Miyoshi H, Souza SC, Cacicedo JM, Saha AK, Greenberg AS, Ruderman NB. AMP-activated protein kinase is activated as a consequence of lipolysis in the adipocyte: potential mechanism and physiological relevance. J Biol Chem 283: 16514–16524, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gregoire FM, Smas CM, Sul HS. Understanding adipocyte differentiation. Physiol Reviews 78: 783–809, 1998 [DOI] [PubMed] [Google Scholar]
- 18. Guyton JR, Capuzzi DM. Treatment of hyperlipidemia with combined niacin-statin regimens. Am J Cardiol 82(12A): 82U–84U, 1998 [DOI] [PubMed] [Google Scholar]
- 19. Jaworski K, Ahmadian M, Duncan RE, Sarkadi-Nagy E, Varady KA, Hellerstein MK, Lee HY, Samuel VT, Shulman GI, Kim KH, de Val S, Kang C, Sul HS. AdPLA ablation increases lipolysis and prevents obesity induced by high-fat feeding or leptin deficiency. Nat Med 15: 159–168, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kamanna VS, Kashyap ML. Mechanism of action of niacin. Am J Cardiol 101: 20B–26B, 2008 [DOI] [PubMed] [Google Scholar]
- 21. Karpe F, Frayn KN. The nicotinic acid receptor—a new mechanism for an old drug. Lancet 363: 1892–1894, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Kelly JJ, Lawson JA, Campbell LV, Storlien LH, Jenkins AB, Whitworth JA, O'Sullivan AJ. Effects of nicotinic acid on insulin sensitivity and blood pressure in healthy subjects. J Hum Hypertens 14: 567–572, 2000 [DOI] [PubMed] [Google Scholar]
- 23. Khazen W, Distel E, Collinet M, Chaves VE, M'Bika JP, Chany C, Achour A, Benelli C, Forest C. Acute and selective inhibition of adipocyte glyceroneogenesis and cytosolic phosphoenolpyruvate carboxykinase by interferon gamma. Endocrinology 148: 4007–4014, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Kim JK, Wi JK, Youn JH. Metabolic impairment precedes insulin resistance in skeletal muscle during high fat feeding in rats. Diabetes 45: 651–658, 1996 [DOI] [PubMed] [Google Scholar]
- 25. Lee FN, Zhang L, Zheng D, Choi WS, Youn JH. Insulin suppresses PDK-4 expression in skeletal muscle independently of plasma FFA. Am J Physiol Endocrinol Metab 287: E69–E74, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Martinez-Botas J, Anderson JB, Tessier D, Lapillonne A, Chang BH, Quast MJ, Gorenstein D, Chen KH, Chan L. Absence of perilipin results in leanness and reverses obesity in Lepr(db/db) mice. Nat Genet 26: 474–479, 2000 [DOI] [PubMed] [Google Scholar]
- 27. Mei J, Holst LS, Landström TR, Holm C, Brindley D, Manganiello V, Degerman E. C(2)-ceramide influences the expression and insulin-mediated regulation of cyclic nucleotide phosphodiesterase 3B and lipolysis in 3T3-L1 adipocytes. Diabetes 51: 631–637, 2002 [DOI] [PubMed] [Google Scholar]
- 28. O'Neill M, Watt MJ, Heigenhauser GJ, Spriet LL. Effects of reduced free fatty acid availability on hormone-sensitive lipase activity in human skeletal muscle during aerobic exercise. J Appl Physiol 97: 1938–1945, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Pereira JN. The plasma free fatty acid rebound induced by nicotinic acid. J Lipid Res 8: 239–244, 1967 [PubMed] [Google Scholar]
- 30. Petrack B, Greengard P, Kalinsky H. On the relative efficacy of nicotinamide and nicotinic acid as precursors of nicotinamide adenine dinucleotide. J Biol Chem 241: 2367–2372, 1966 [PubMed] [Google Scholar]
- 31. Peuler JD, Johnson GA. Simultaneous single isotope radioenzymatic assay of plasma norepinephrine, epinephrine and dopamine. Life Sci 21: 625–636, 1977 [DOI] [PubMed] [Google Scholar]
- 32. Poynten AM, Gan SK, Kriketos AD, O'Sullivan A, Kelly JJ, Ellis BA, Chisholm DJ, Campbell LV. Nicotinic acid-induced insulin resistance is related to increased circulating fatty acids and fat oxidation but not muscle lipid content. Metabolism 52: 699–704, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Rasouli N, Hale T, Kahn SE, Spencer HJ, Elbein SC. Effects of short-term experimental insulin resistance and family history of diabetes on pancreatic b-cell function in nondiabetic individuals. J Clin Endocr Metab 90: 5825–5833, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Santomauro AT, Boden G, Silva ME, Rocha DM, Santos RF, Ursich MJ, Strassmann PG, Wajchenberg BL. Overnight lowering of free fatty acids with Acipimox improves insulin resistance and glucose tolerance in obese diabetic and nondiabetic subjects. Diabetes 48: 1836–1841, 1999 [DOI] [PubMed] [Google Scholar]
- 35. Sassone-Corsi P. Coupling gene expression to cAMP signalling: role of CREB and CREM. Int J Biochem Cell Biol 30: 27–38, 1998 [DOI] [PubMed] [Google Scholar]
- 36. Tansey JT, Sztalryd C, Gruia-Gray J, Roush DL, Zee JV, Gavrilova O, Reitman ML, Deng CX, Li C, Kimmel AR, Londos C. Perilipin ablation results in a lean mouse with aberrant adipocyte lipolysis, enhanced leptin production, and resistance to diet-induced obesity. Proc Natl Acad Sci USA 98: 6494–6499, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tunaru S, Kero J, Schaub A, Wufka C, Blaukat A, Pfeffer K, Offermanns S. PUMA-G and HM74 are receptors for nicotinic acid and mediate its anti-lipolytic effect. Nat Med 9: 352–355, 2003 [DOI] [PubMed] [Google Scholar]
- 38. Walldius G, Wahlberg G. Effects of nicotinic acid and its derivatives on lipid metabolism and other metabolic factors related to atherosclerosis. Adv Exp Med Biol 183: 281–293, 1985 [DOI] [PubMed] [Google Scholar]
- 39. Wang W, Basinger A, Neese RA, Christiansen M, Hellerstein MK. Effects of nicotinic acid on fatty acid kinetics, fuel selection, and pathways of glucose production in women. Am J Physiol Endocrinol Metab 279: E50–E59, 2000 [DOI] [PubMed] [Google Scholar]
- 40. Watt MJ, Holmes AG, Steinberg GR, Mesa JL, Kemp BE, Febbraio MA. Reduced plasma FFA availability increases net triacylglycerol degradation, but not GPAT or HSL activity, in human skeletal muscle. Am J Physiol Endocrinol Metab 287: E120–E127, 2004 [DOI] [PubMed] [Google Scholar]
- 41. Wise A, Foord SM, Fraser NJ, Barnes AA, Elshourbagy N, Eilert M, Ignar DM, Murdock PR, Steplewski K, Green A, Brown AJ, Dowell SJ, Szekeres PG, Hassall DG, Marshall FH, Wilson S, Pike NB. Molecular identification of high and low affinity receptors for nicotinic acid. J Biol Chem 278: 9869–9874, 2003 [DOI] [PubMed] [Google Scholar]
- 42. Worm D, Henriksen JE, Vaag A, Thye-Rønn P, Melander A, Beck-Nielsen H. Pronounced blood glucose-lowering effect of the antilipolytic drug acipimox in noninsulin-dependent diabetes mellitus patients during a 3-day intensified treatment period. J Clin Endocrinol Metab 78: 717–721, 1994 [DOI] [PubMed] [Google Scholar]
- 43. Zimmermann R, Lass A, Haemmerle G, Zechner R. Fate of fat: the role of adipose triglyceride lipase in lipolysis. Biochim Biophys Acta 1791: 494–500, 2009 [DOI] [PubMed] [Google Scholar]