Abstract
In perifused immortalized GnRH neurons (GT1–7), simultaneous measurements of GnRH and cAMP revealed that the secretory profiles for both GnRH and cAMP are pulsatile. An analysis of GnRH and cAMP pulses in 16 independent experiments revealed that 25% of pulses coincide. Inversion of the peak and nadir levels was found in 33% and random relationship between GnRH and cAMP found in 42% of analyzed pulses. The random relation between GnRH and cAMP pulse resets to synchronous after an inverse relation between pulses occurred during the major GnRH release, indicating that GnRH acts as a switching mechanism to synchronize cAMP and GnRH release in perifused GT1–7 neurons. Activation of GnRH receptors with increasing agonist concentrations caused a biphasic change in cAMP levels. Low nanomolar concentrations increased cAMP production, but at high concentrations the initial increase was followed by a rapid decline to below the basal level. Blockade of the GnRH receptors by peptide and nonpeptide antagonists generated monotonic nonpulsatile increases in both GnRH and cAMP production. These findings indicate that cAMP positively regulates GnRH secretion but does not participate in the mechanism of pulsatile GnRH release.
Keywords: gonadotropin-releasing hormone; adenosine 3′,5′-cyclic monophosphate
the neuroendocrine control of reproductive function is expressed through the episodic secretion of gonadotropic hormones from the anterior pituitary gland in response to pulsatile stimulation by gonadotropin-releasing hormone (GnRH) produced by a network of peptidergic neurons in the hypothalamus (8, 12, 34). The characteristic pulsatile secretion of GnRH from hypothalamic neurons is dependent on an autocrine interaction between GnRH and its receptors expressed in GnRH-producing neurons. The amplitude of GnRH pulses is increased by GnRH agonist stimulation and diminished by GnRH antagonist treatment. These findings demonstrated that expression of GnRH receptors, GnRH-dependent activation of Ca2+ signaling, and autocrine regulation of GnRH release are evident in early fetal GnRH neurons and could provide a mechanism for gene expression and regulated GnRH secretion during embryonic migration (17, 31).
The endogenous GnRH receptor (GnRH-R) expressed in native and immortalized GnRH neurons activates at least three G proteins, as indicated by the agonist-induced release of their specific α-subunits from the plasma membrane (13). Such coupling to a diverse array of G proteins provides GnRH neurons with several potential signaling pathways to transduce incoming signals. Decrease in membrane-associated Gαs at low nanomolar GnRH concentrations and of Gq and Gαi3 at higher concentrations suggested that an agonist concentration-dependent switch in coupling of the GnRH-R between specific G proteins modulates neuronal Ca2+ signaling via Gs-cAMP stimulatory and Gi-cAMP inhibitory mechanisms. This autocrine mechanism appears to serve as a timer to determine the frequency of pulsatile GnRH release by regulating Ca2+- and cAMP-dependent signaling and GnRH neuronal firing (10, 11).
The presence of several types of receptors in GT1–7 neuronal cell lines has indicated the broad range of receptor-activated signaling and transduction pathways that may participate in the regulation of GnRH secretion into the portal circulation (1, 16, 19, 24, 25). Activation of protein kinase A (PKA) and protein kinase C (PKC) mediates GnRH gene transcription and GnRH secretion (7, 9, 21, 22, 27, 28, 32). This suggests that both Ca2+-inositol 1,4,5-trisphosphate (InsP3) and adenosine 3′,5′-cyclic monophosphate (cAMP) pathways are involved in the transduction of plasma membrane signals in GnRH systems.
In the present study, the relationships between pulsatile GnRH secretion and cAMP production were examined in perifused GT1–7 neurons.
MATERIALS AND METHODS
Cell culture.
Immortalized GnRH neurons (GT1–7) were obtained from Dr. P. L. Mellon (University of California at San Diego) (18). The culture medium for GT1–7 neurons was Dulbecco's modified Eagle's medium and F-12 (1:1; DMEM-F-12) mixture (Sigma Chemical, St. Louis, MO) supplemented with 100 ml of 10% heat-inactivated fetal bovine serum (Biofluids, Rockville, MD), 2.5 gm/l sodium bicarbonate (Sigma Chemical), and 100 mg/l gentamicin reagent solution (Sigma Chemical). The cell cultures were maintained at 37°C in a water-saturated atmosphere of 95% air and 5% CO2. After reaching confluence, the cells were dissociated by trypsin (Sigma Chemical) treatment and plated for either static or perfusion studies.
Perifusion studies.
For perifusion studies, 1.5 × 107 GT1–7 cells were allowed to attach to preswollen Cytodex-2 beads (Pharmacia Biotech, Piscataway, NJ) in tissue culture dishes (Falcon Labware; Becton-Dickson, Franklin Lakes, NJ); the cell-coated cytodex beads were then incubated in culture medium for 24 h. The culture medium was removed and replaced with serum-free culture medium, and the cells were incubated for another 24 h. The cell-coated cytodex beads were collected by sedimentation and placed into a temperature-controlled perifusion chamber (Acusyst-S Cellex Biosciences, Minneapolis, MN).
The chambers were then perifused with HEPES-buffered DMEM-F-12 (1:1) containing 0.1% bovine serum albumin and 0.03% bacitracin (Sigma Chemical) at a flow rate of 15 ml/h. Prior to sample collection, the chambers were perifused for 60 min to allow the cells to equilibrate, and samples were collected at 5-min intervals for a total of 5 h. The collected samples were stored at −20°C until radioimmunoassays for cAMP and GnRH were performed. All perifusion studies were repeated a minimum of four times.
Perifused GT1–7 cells were treated with the phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine (IBMX; Sigma Chemical) and 8-bromoadenosine (8-BR) cAMP (Sigma Chemical) to evaluate GnRH responses to elevated cAMP levels. Two specific PKA inhibitors, Rp-cyclic adenosine 3′,5′-phosphorothioate and H-89 dihydrocholide (Calbiochem-Novabiochem, La Jolla, CA), were used to examine the effects of PKA inhibition on GnRH. For perifusion treatments, each PKA inhibitor was used at a concentration of 10 μM.
Native GnRH and GnRH agonist analog des-Gly10[d-Ala6]-GnRH ethylamide ([d-Ala6]Ag; Peninsula Laboratories, San Carlos, CA) were used in perifusion studies to evaluate the effect of exogenous GnRH on cAMP secretion. We have shown previously that [d-Ala6]Ag does not cross-react with GnRH in RIAs (12). All perifusions were performed using a 3-h basal perifusion, followed by 1 h of agonist treatment and 2 h of washout. The agonist doses used were 1 nM, 10 nM, 100 nM, and 1 μM.
The GnRH antagonist d-Phe(pCl)2,d-Pal (3)3,d-Cit6,d-Ala10 (SB-75) was a gift from Dr. A. V. Shally (Veterans Affairs Hospital, New Orleans, LA), and the nonpeptide GnRH antagonist [1H]quinoline was provided by Dr. James Schaeffer (Merck Research Laboratories, Rahway, NJ). There was no detectable cross-reactivity of the GnRH agonist or antagonist analogs used in the GnRH RIA (12). For antagonist treatment, the GT1–7 cells were perifused for 2 h with perifusion medium, followed by 4 h of antagonist perifusion and 1 h of basal perifusion. The antagonist doses used were 1 nM, 10 nM, 100 nM, and 1 μM.
GnRH radioimmunoassay.
GnRH levels in medium from perifusion and static cultures were determined by RIA as described previously using 125I-GnRH (Amersham Pharmacia Biotech, Arlington Heights, IL), GnRH standards (Peninsula Laboratories, Belmont, CA), and primary antibody donated by Dr. V. D. Ramirez (University of Illinois, Urbana, IL) (15). All samples from an experiment were analyzed in the same assay in duplicate. The intra- and interassay coefficients of variation at 80% binding in standard samples (15 pg/ml) were 12 and 14%, respectively.
cAMP radioimmunoassay.
Medium levels of cAMP from perifusion and static cultures were determined by RIA as described previously using 125I-cAMP (Covance Laboratories, Vienna, VA), cAMP standards (Sigma Chemical), and primary cAMP antiserum (5). Using a specific cAMP antiserum at a titer of 1:5,000, this assay demonstrated no cross-reaction with 3′,5′-cyclic monophosphate, 2′,3′-cAMP, ADP, GDP, CTP, or IBMX. The intra- and interassay coefficients of variation at 80% binding in standard samples (0.015 pmol/ml) were 8 and 10%, respectively.
Data analyses.
All data values are reported as means ± SE. GnRH and cAMP pulses were identified, and their parameters were determined by algorithm cluster analysis. Individual point standard deviations were calculated using a power function variance model from experimental duplicates. A 2 × 2 cluster configuration and a t-statistic of 2 for the upstroke and downstroke were used to maintain false-positive and false-negative error rates <10% (29). An unpaired t-test was used to assess differences in the mean between controls and treatments. One-way analysis of variance was used to assess means among a set of treatments. Bonferroni's multiple comparison test was used for post hoc analysis. An α-error of 0.05 was considered significant for all calculations.
RESULTS
GnRH and cAMP secretory profiles in perifused GT1–7 cells.
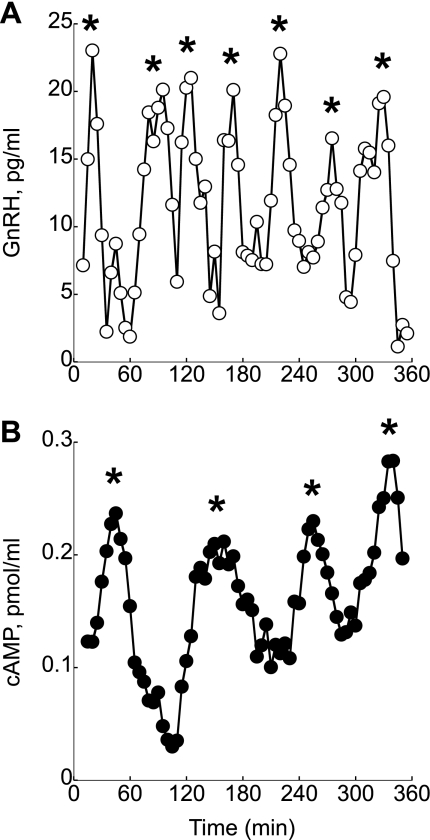
Multiple experiments (n = 16) were performed to analyze GnRH and cAMP secretion from perifused GT1–7 neurons. Cluster analysis of the obtained perifusion data revealed that both GnRH and cAMP were secreted in a pulsatile manner, with pulse frequencies of 40–60 min for GnRH and 54–80 min for cAMP (Fig. 1, A and B). In total, 112 GnRH and cAMP pulses were analyzed. GnRH and cAMP pulses coincided in 25% of the analyzed data. In 33% of the analyzed data, peak of GnRH pulses coincided with nadir cAMP levels. The GnRH peak levels coincided with random levels of cAMP 42% of the time.
Fig. 1.
Representative traces of the simultaneous measurements of gonadotropin-releasing hormone (GnRH; A) and cAMP secretion (B) in perfuse GT1–7 neurons. Both secretory profiles are pulsatile. *Pulses. Shown are representative traces from 16 independent experiments.
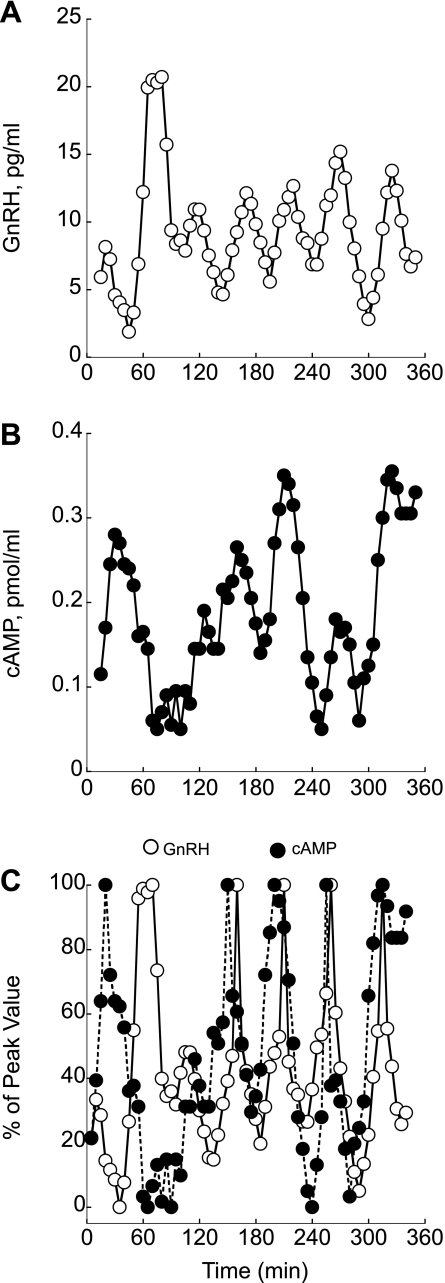
In perifused GT1–7 neurons, we observed occasional GnRH pulses in which the amplitude was significantly higher than other pulses (Fig. 2A). Measurement of cAMP in samples where major GnRH peaks were found revealed the lowest cAMP release (Fig. 2B). The synchronous relation between GnRH and cAMP pulse resets after inverse relation between pulses, suggesting that GnRH acts as a switching mechanism that synchronizes cAMP and GnRH release in perifused GT1–7 neurons (Fig. 2C).
Fig. 2.
The degree of coincidence between pulses of GnRH (A) and cAMP (B) under basal conditions. C: relation between GnRH and cAMP production normalized as the percentage of the maximum peak value for GnRH and cAMP. In this perifusion, inverse relation between the major GnRH pulse at 60 min coincided with the lowest cAMP level and pulses synchronized thereafter. Shown are representative traces from 16 independent experiments.
Fig. 3.
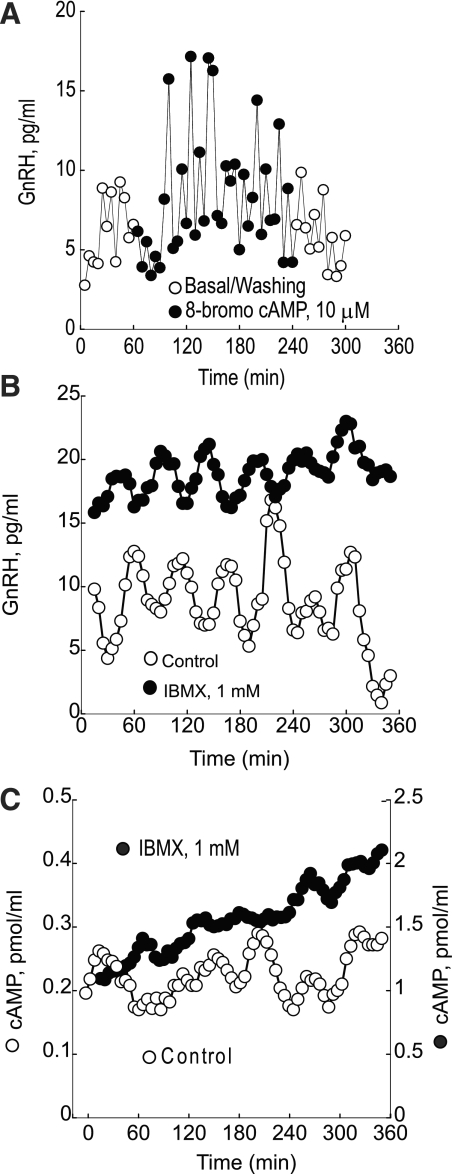
A: continuous treatment of perifused GT1–7 neurons with 8-bromoadenosine (8-bromo) cAMP increases pulse amplitude but not the frequency of GnRH pulses. B: GnRH release was increased significantly, but the pulsatility remained unchanged compared with untreated cells during application of 1 mM 3-isobutyl-1-methylxanthine (IBMX). C: treatment of perifused GT1–7 neurons with 1 mM IBMX terminates pulsatile cAMP release and causes an ≤10-fold monotonic increase in cAMP production. Shown are representative traces from 4 independent experiments.
Modulation of GnRH release and cAMP production in perifused GT1–7 neurons by cAMP analogs and inhibitors of phosphodiesterase and protein kinase A.
Treatment of perifused GT1–7 neurons with 8-BR cAMP caused a significant increase in basal GnRH release, which increased significantly from 24.7 ± 0.6 pg/ml in controls to 39.7 ± 0.9 pg/ml (P < 0.01) in 8-BR cAMP-treated cells. Despite the increase in baseline GnRH release, the frequency of GnRH pulses remained unchanged (Fig. 3A).
During IBMX treatment, GnRH release increased significantly, but the pulsatility remained unchanged compared with untreated cells. GnRH baseline levels were increased by 40% from a mean of 5.6 ± 0.2 to 9.4 ± 0.3 pg/ml (P < 0.01) and remained at these levels throughout the perifusion. GnRH mean peak height increased from 9.3 ± 0.6 to 15.8 ± 1.1 pg/ml (P < 0.05). The mean interval between the peaks for GnRH was not statistically changed (Fig. 3B).
Application of 1 mM of a nonselective phosphodiesterase inhibitor (IBMX) caused loss of cAMP pulsatility and an ≤10-fold monotonic increase in cAMP production. IBMX increased baseline levels of cAMP by 762% from a mean of 0.21 ± 0.01 to 1.6 ± 0.03 pmol/ml (P < 0.01), and there was a continual increase in cAMP as the perifusion progressed, which was associated with a marked decrease in cAMP pulsatility (Fig. 3C).
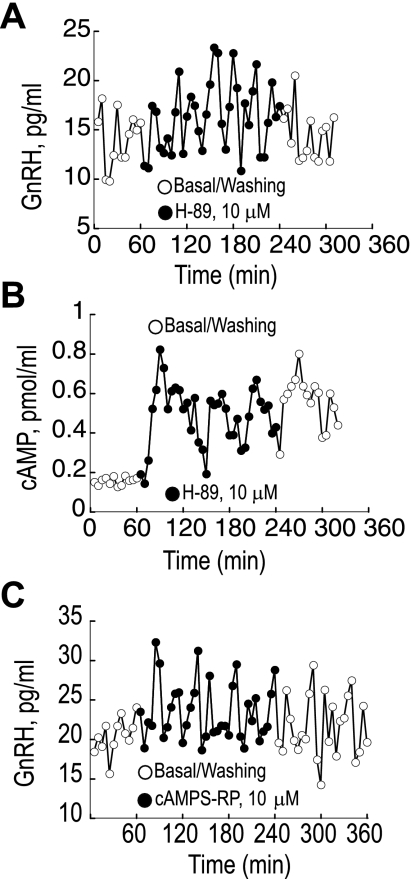
Treatment with the PKA inhibitors cAMP 8Br-Rp-isomer and H-89 significantly increased GnRH episodic secretion levels by 111% from 7.2 ± 0.3 to 15.22 ± 0.6 pg/ml (P < 0.01) and by 81% from 7.5 ± 0.5 to 13.59 ± 0.7 pg/ml (Fig. 4, A and B), respectively. During the washout period, the GnRH levels decreased but remained higher than the initial baseline values. GnRH pulsatility was unchanged by the cAMP 8Br-Rp. cAMP release remained pulsatile but was elevated during the treatment of perifused GT1–7 neurons with H-89 (Fig. 4C).
Fig. 4.
A: pulsatile GnRH release increases significantly during treatment with the nonselective PKA inhibitor H-89. B: treatment with H-89 also markedly increases pulsatile cAMP secretion. C: treatment of perifused GT1–7 neurons with selective cAMP-dependent PKA inhibitor cAMPS-R also increases pulsatile GnRH release. Shown are representative traces from 4 independent experiments.
Roles of GnRH receptor agonist and antagonist analogs in pulsatile GnRH and cAMP release.
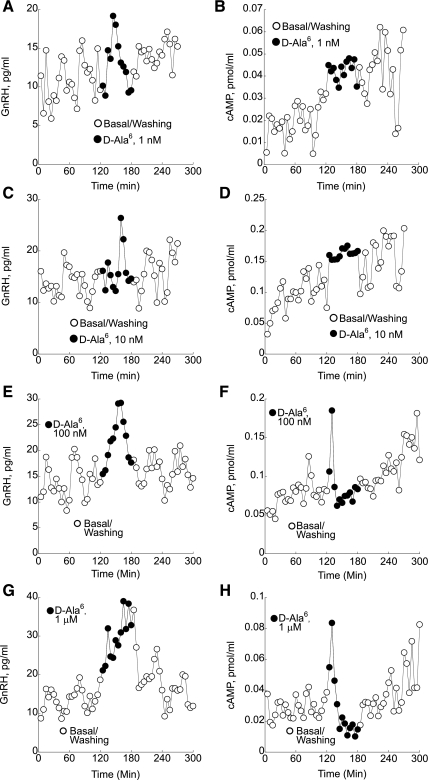
Treatment of perifused GT1–7 neurons with 1 nM [d-Ala6]Ag concentrations caused initial but not significant changes in GnRH pulse amplitude and frequency (Fig. 5A). In contrast, a significant 28% increase in cAMP production from 0.07 ± 0.004 to 0.09 ± 0.005 pmol/ml (P < 0.01) was observed during treatment with 1 nM [d-Ala6]Ag (Fig. 5B). Treatment with 10 nM [d-Ala6]Ag increased GnRH levels by 21.4% from 14.1 ± 0.37 to 17.12 ± 0.76 pg/ml (P < 0.01; Fig. 5C) as well as cAMP levels by 44% from 0.07 ± 0.003 to 0.11 ± 0.007 pmol/ml (P < 0.01; Fig. 5D). Increased concentrations (100 nM) of [d-Ala6]Ag caused a significant transient increase in endogenous GnRH release (Fig. 5E). The initial increase in cAMP production after treatment with 100 nM [d-Ala6]Ag was followed by a decrease from 0.06 ± 0.002 to 0.04 ± 0.003 pmol/ml (P < 0.01; Fig. 5F). [d-Ala6]Ag at 1-μM levels increased GnRH by 123% from 17.4 ± 0.9 to 38.7 ± 2.4 pg/ml (P < 0.01; Fig. 5G). The initial increase in cAMP production after treatment with 1 μM [d-Ala6]Ag was followed by a decrease from 0.08 ± 0.003 to 0.03 ± 0.002 pmol/ml (P < 0.01; Fig. 5H).
Fig. 5.
GnRH and cAMP responses to perifusion with various doses of the GnRH agonist des-Gly10[d-Ala6]-6nRH ethylamide ([d-Ala6]Ag; ●). A: treatment of perifused GT1–7 neurons with low 1 nM [d-Ala6]Ag concentrations caused initial but not significant changes in GnRH pulse amplitude and frequency. B: in contrast, a significant 28% increase in cAMP production was observed during treatment with 1 nM [d-Ala6]Ag. C and D: treatment with 10 nM [d-Ala6]Ag significantly increased both GnRH and cAMP levels. E: increased concentrations (100 nM) of [d-Ala6]Ag caused a significant transient increase in GnRH release. F: the initial increase in cAMP production after treatment with 100 nM [d-Ala6]Ag was followed by a decrease. G: treatment of perifused GT1–7 neurons with 1 μM [d-Ala6]Ag also caused a significant increase in GnRH secretion. H: the initial increase in cAMP production after treatment with 1μM [d-Ala6]Ag was followed by a significant decrease. Shown are representative traces from 5 independent experiments.
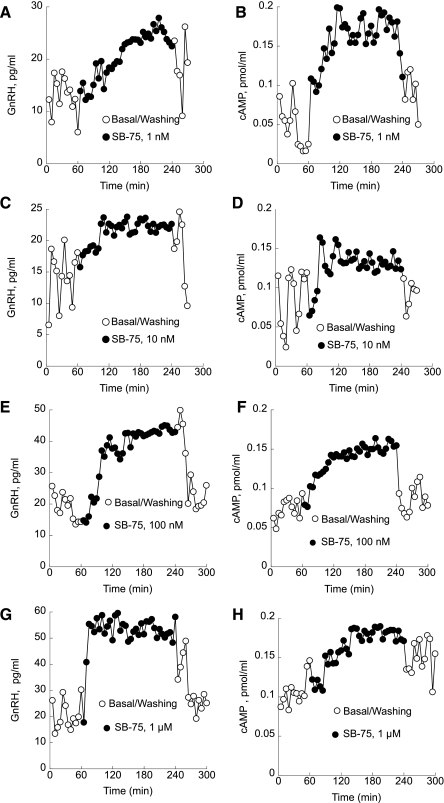
Inactivation of GnRH receptors with a GnRH antagonist (SB-75), which does not cross-react with the GnRH antibody, inhibited pulsatile GnRH and caused a slow monotonic increase in GnRH release. Treatment with 1 nM SB-75 abolished pulsatile GnRH release and caused a significant increase in GnRH release from 7.4 ± 0.5 to 20.9 ± 0.4 pg/ml (P < 0.01; Fig. 6A). Levels of cAMP also increased by 50% from 0.09 ± 0.01 to 0.18 ± 0.017 pmol/ml with 1-nM treatment (P < 0.01; Fig. 6B). Basal GnRH secretion increased from 7.1 ± 0.4 to 20.3 ± 0.6 pg/ml during treatment with 10 nM SB-75 (P < 0.01). During the washout, GnRH levels decreased to control levels (Fig. 6C). Levels of cAMP increased by 42.1% from 0.19 ± 0.01 to 0.28 ± 0.007 pmol/ml with 10 nM treatment (P < 0.01; Fig. 6D).
Fig. 6.
Perifusion of GT1–7 cells with the GnRH antagonist SB-75 (●). A: treatment with 1 nM SB-75 abolished pulsatile GnRH release and caused a significant increase in GnRH production. B: levels of cAMP also increased significantly during the 1 nM treatment. C: basal GnRH secretion also increased during treatment with 10 nM SB-75 and returned to control levels during washout. D: levels of cAMP were also increased significantly. E: basal GnRH levels rose significantly in GT1–7 cells treated with 100 nM SB-75. F: during the washout period, the GnRH pulsatility returned to the baseline levels. cAMP production also significantly increased during 100 nM SB-75 treatment and returned to the basal level during washout. G: treatment with 1 μM SB-75 abolished pulsatile GnRH release and caused a significant increase in GnRH secretion. H: levels of cAMP also monotonically increased during treatment with 1 μM SB-75. Shown are representative traces from 5 independent experiments.
Basal GnRH levels rose by 25% from 20.14 ± 0.05 to 25.17 ± 0.3 pg/ml when treated with 100 nM SB-75 (P < 0.01). During the washout period, GnRH pulsatility and the baseline levels returned to control levels (Fig. 6E). cAMP production also significantly increased during 100 nM SB-75 treatment from 0.07 ± 0.01 to 0.14 ± 0.02 pmol/ml (P < 0.01) and returned to the basal level during washout (Fig. 6F).
Treatment with 1 μM SB-75 abolished pulsatile GnRH release and caused a significant increase in GnRH secretion from 20.9 ± 0.65 pg/ml control to 53.2 ± 2.3 pg/ml during SB-75 treatment (Fig. 6G). The levels of cAMP also monotonically increased during treatment with 1 μM SB-75 from 0.11 ± 0.03 to 0.17 ± pmol/ml (P < 0.05) with reduced pulsatility (Fig. 6H).
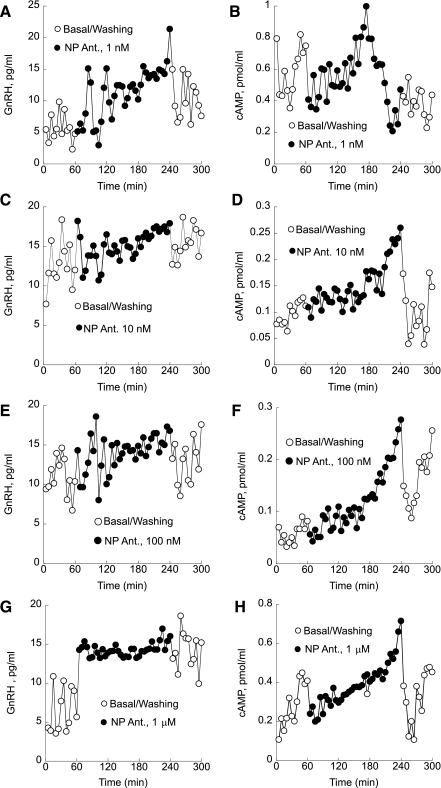
Similarly to treatment with the peptide GnRH-R antagonist SB-75, application of a nonpeptide GnRH-R antagonist increased both GnRH and cAMP secretion, causing monotonic increase and loss of pulsatility. After an initial delay, treatment with 1 nM nonpeptide antagonist decreased pulse amplitude and abolished both pulsatile GnRH (Fig. 7A) and cAMP secretion (Fig. 7B). The amounts of released GnRH and cAMP during treatment with 1 nM nonpeptide GnRH antagonist increased significantly from 6.3 ± 0.45 to 14.2 ± 1.3 pg/ml (P < 0.01) for GnRH (Fig. 7A) and from 0.4 ± 0.08 to 0.9 ± 0.05 pmol/ml for cAMP, respectively (Fig. 7B). Significant increases in both GnRH and cAMP production were also observed during treatment with 10 nM nonpeptide antagonist. Basal GnRH increased from 13.68 ± 0.6 to 16.29 ± 0.2 pg/ml (Fig. 7C), and cAMP rose from 0.11 ± 0.02 to 0.16 ± 0.1 pmol/ml (Fig. 7D) during the treatment with 10 nM nonpeptide GnRH antagonist (P < 0.01). Treatment with 100 nM nonpeptide GnRH antagonist inhibited pulsatile GnRH release and caused a significant monotonic increase in GnRH release from 11.2 ± 0.9 to 16.6 ± 1.1 pg/ml (P < 0.05; Fig. 7E). Production of cAMP also increased monotonically with loss of pulsatility during treatment with 100 nM nonpeptide GnRH antagonist (0.08 ± 0.009 pg/ml basal to 0.18 ± 0.1 pmol/ml antagonist treated, P < 0.05; Fig. 7F). Basal GnRH levels rose by 123% from 6.02 ± 0.5 to 13.41 ± 0.2 pg/ml with 1 μM treatment (P < 0.01; Fig. 7G). Analysis of variance revealed that treatment with 1 μM nonpeptide GnRH antagonist increased cAMP significantly by 58% from 0.3 ± 0.05 to 0.52 ± 0.09 (P < 0.05; Fig. 7H).
Fig. 7.
Treatment with 1 nM of a nonpeptide antagonist abolished both pulsatile GnRH (A) and cAMP secretion after an initial delay (B). C and D: significant increases in both GnRH (C) and cAMP production (D) were also observed during treatment with 10 nM nonpeptide antagonist. E: treatment with 100 nM nonpeptide GnRH antagonist inhibited pulsatile GnRH release and caused a significant monotonic increase in GnRH release. F: production of cAMP also increased monotonically with loss of pulsatility during treatment with 100 nM nonpeptide GnRH antagonist. G and H: basal GnRH levels rose significantly during treatment with 1 μM nonpeptide GnRH antagonist (G) as well as cAMP production (H). Shown are representative traces from 5 independent experiments.
DISCUSSION
Calcium and cAMP are important factors in the mechanism of episodic signaling in hypothalamic GnRH neurons (10, 20, 30). The observation that cAMP production in GT1–7 neurons is stimulated by increased extracellular Ca2+ and the Ca2+ channel agonist BK-8644 and is diminished by low extracellular Ca2+ and treatment with dihydropyridine analogs is consistent with activation and/or inhibition of the calcium-dependent adenylyl cyclase type I (ACI) expressed in these cells (14). These findings indicate that cAMP production in GnRH neuronal cells is maintained by Ca2+ entry through voltage-sensitive calcium channels, leading to activation of ACI, and that Ca2+ influx-dependent activation of ACI acts in conjunction with AC-regulatory G proteins to determine basal and agonist-stimulated levels of cAMP production (14). Also, cAMP-induced activation of cyclic nucleotide-gated channels promotes Ca2+ entry and causes increase in GnRH secretion (2, 3).
In this study, we performed a detailed analysis of the patterns of cAMP and GnRH release from perifused GT1–7 cells. The degree of coincidence between pulses of GnRH and cAMP under basal conditions was determined by concomitant measurements of both responses in samples collected from perifused GnRH neurons. We found that major GnRH pulses in basal GnRH release from perifused GT1–7 neurons frequently corresponded with the lowest cAMP level, indicating an inhibitory action of increased GnRH concentrations on cAMP production.
Agonist activation of GnRH receptors leads to stimulation of phospholipase C, formation of inositol 1,4,5-trisphosphate, mobilization of Ca2+ from intracellular stores, GnRH release, and cAMP production (22, 27). Modulation of GnRH receptor activity in GnRH neurons by GnRH agonist and antagonist analogs and measurements of GnRH and cAMP production were used to investigate the regulatory mechanisms underlying GnRH and cAMP pulsatile release. The GnRH agonist [d-Ala6]Ag was used to evaluate cAMP's response to GnRH-R receptor activation. Continuous exposure of perifused GT1–7 neurons to high, 100 nM, and 1 μM GnRH receptor agonist analog [d-Ala6]Ag caused an increase in GnRH pulse amplitude and transiently stimulated cAMP production followed by prolonged inhibition. This is consistent with the findings that high GnRH concentrations caused coupling of GnRH-R to Gi and inhibited cAMP production (13). During the treatment of perifused GT1–7 neurons with low GnRH receptor agonist concentrations, 1 nM and 10 nM, the amplitude of GnRH pulses remained unchanged, with concomitant increase in cAMP pulses. These findings indicate that, at low GnRH concentrations, GnRH receptors couple to adenylyl cyclase stimulatory G proteins and cause an increase in cAMP production (13, 14). Thus, GnRH exerts a biphasic effect on cAMP production, where low GnRH concentrations stimulate cAMP production and at high concentrations GnRH inhibits cAMP production (10, 13).
Inactivation of GnRH receptors by both peptide and nonpeptide GnRH antagonist abolishes pulsatile GnRH release. The cessation of pulsatile GnRH release was dose dependent, and a switch to monotonic increase at high GnRH antagonist concentrations was observed. Cessation of pulsatile cAMP release was also evident during GnRH antagonist treatment, and cAMP production monotonically increased with rising GnRH antagonist concentrations, suggesting that GnRH/GnRH-R mediated inhibition is required to maintain both pulsatile GnRH and cAMP secretion. GnRH antagonist-induced inhibition of GnRH-R and increase in cAMP production may account for cAMP-mediated increase in GnRH secretion. These data suggest that pulsatile cAMP secretion in GT1–7 neurons is driven by a GnRH/GnRH-R autoregulatory system in which dose-dependent switching of GnRH-R coupling to adenylyl cyclase stimulatory (Gs) and inhibitory (Gi) G proteins mediates both pulsatile GnRH and cAMP secretion.
The lack of expression of cAMP receptors in mammalian cells as well as GT1–7 neurons indicates that, in contrast to invertebrates, where cAMP and its receptors provide for pulsatile cAMP release (4, 6), in mammalian cells the pulsatile cAMP is differently regulated. Activation of phosphodiesterase (PDE) by PKA has been reported to have a role in establishing cAMP oscillations, which might constitute a biological clock for GnRH pulsatile release (23, 26, 35).
Pharmacological blockade of PDE activity by IBMX, a nonselective PDE inhibitor, abolishes pulsatile cAMP secretion and causes a monotonic increase in cAMP production. In contrast, GnRH secretion remained pulsatile with unchanged pulse frequency and increased pulse amplitude. Likewise, continuous treatment with 8-BR cAMP significantly increased basal GnRH release and pulse amplitude without altering GnRH pulse frequency. These findings indicate that cAMP pulsatility does not drive GnRH pulsatile GnRH secretion in hypothalamic GnRH neurons. Also, the preserved fertility in mice lacking adenylyl cyclase 1 and 8 activity implies that cAMP is not essential in GnRH pulsatility (33).
Treatment of perifused GT1–7 neurons with PKA inhibitor H-89 increased the pulse amplitude but not pulse frequency for both GnRH and cAMP. These data suggest that under basal conditions cAMP inhibitory adenylyl cyclase V and VI are tonically activated by PKA and mediate inhibitory effects on both GnRH and cAMP secretion (30).
In summary, it is evident that pulsatile cAMP secretion in hypothalamic GnRH neurons is driven by a GnRH autoregulatory system in which changes in GnRH pulse amplitude cause an asynchronous relation between GnRH and cAMP pulses. The pulses synchronize after occurrence of the major GnRH pulse, providing a resetting mechanism by which GnRH and cAMP pulses may synchronize. Inhibition of pulsatile cAMP release by PDE inhibitors did not affect pulsatile GnRH release, suggesting that cAMP does not participate in the regulatory mechanism that is essential for GnRH pulsatility. Thus, it is evident that although cAMP is an active participant in GnRH secretion, it is not an essential component that drives pulsatile GnRH release, which is dependent on a GnRH autoregulatory system in hypothalamic GnRH neurons.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1. Al-Damluji S, Shen WB, White S, Barnard EA. alpha(1B) adrenergic receptors in gonadotrophin-releasing hormone neurones: relation to Transport-P. Br J Pharmacol 132: 336–344, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Charles A, Weiner R, Costantin J. cAMP modulates the excitability of immortalized H=hypothalamic (GT1) neurons via a cyclic nucleotide gated channel. Mol Endocrinol 15: 997–1009, 2001 [DOI] [PubMed] [Google Scholar]
- 3. El Majdoubi M, Weiner RI. Localization of olfactory cyclic nucleotide-gated channels in rat gonadotropin-releasing hormone neurons. Endocrinology 143: 2441–2444, 2002 [DOI] [PubMed] [Google Scholar]
- 4. Elzie CA, Colby J, Sammons MA, Janetopoulos C. Dynamic localization of G proteins in Dictyostelium discoideum. J Cell Sci 122: 2597–2603, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fujita K, Aguilera G, Catt KJ. The role of cyclic AMP in aldosterone production by isolated zona glomerulosa cell. J Biol Chem 254: 8567–8574, 1979 [PubMed] [Google Scholar]
- 6. Halloy J, Lauzeral J, Goldbeter A. Modeling oscillations and waves of cAMP in Dictyostelium discoideum cells. Biophys Chem 72: 9–19, 1998 [DOI] [PubMed] [Google Scholar]
- 7. Hawes BE, Waters SB, Janovick JA, Bleasdale JE, Conn PM. Gonadotropin-releasing hormone-stimulated intracellular Ca2+ fluctuations and luteinizing hormone release can be uncoupled from inositol phosphate production. Endocrinology 130: 3475–3483, 1992 [DOI] [PubMed] [Google Scholar]
- 8. Herbison AE, Pape JR, Simonian SX, Skynner MJ, Sim JA. Molecular and cellular properties of GnRH neurons revealed through transgenics in the mouse. Mol Cell Endocrinol 185: 185–194, 2001 [DOI] [PubMed] [Google Scholar]
- 9. Huckle WR, Conn PM. The relationship between gonadotropin-releasing hormone-stimulated luteinizing hormone release and inositol phosphate production: studies with calcium antagonists and protein kinase C activators. Endocrinology 120: 160–169, 1987 [DOI] [PubMed] [Google Scholar]
- 10. Krsmanovic LZ, Hu L, Leung PK, Feng H, Catt KJ. The hypothalamic GnRH pulse generator: multiple regulatory mechanisms. Trends Endocrinol Metab 20: 402–408, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Krsmanovic LZ, Hu L, Leung PK, Feng H, Catt KJ. Pulsatile GnRH secretion: roles of G protein-coupled receptors, second messengers and ion channels. Mol Cell Endocrinol 314: 158–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Krsmanovic LZ, Martinez-Fuentes AJ, Arora KK, Mores N, Navarro CE, Chen HC, Stojilkovic SS, Catt KJ. Autocrine regulation of gonadotropin-releasing hormone secretion in cultured hypothalamic neurons. Endocrinology 140: 1423–1431, 1999 [DOI] [PubMed] [Google Scholar]
- 13. Krsmanovic LZ, Mores N, Navarro CE, Arora KK, Catt KJ. An agonist-induced switch in G protein coupling of the gonadotropin-releasing hormone receptor regulates pulsatile neuropeptide secretion. Proc Natl Acad Sci USA 100: 2969–2974, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Krsmanovic LZ, Mores N, Navarro CE, Tomic M, Catt KJ. Regulation of Ca2+-sensitive adenylyl cyclase in gonadotropin-releasing hormone neurons. Mol Endocrinol 15: 429–440, 2001 [DOI] [PubMed] [Google Scholar]
- 15. Krsmanovic LZ, Stojilkovic SS, Merelli F, Dufour SM, Virmani MA, Catt KJ. Calcium signaling and episodic secretion of gonadotropin-releasing hormone in hypothalamic neurons. Proc Natl Acad Sci USA 89: 8462–8466, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Marks DL, Smith MS, Vrontakis M, Clifto DK, Steiner RA. Regulation of galanin gene expression in gonadotropin-releasing neurons during estrous cycle of the rat. Endocrinology 132: 1836–1844, 1993 [DOI] [PubMed] [Google Scholar]
- 17. Martinez-Fuentes AJ, Hu L, Krsmanovic LZ, Catt KJ. Gonadotropin-releasing hormone (GnRH) receptor expression and membrane signaling in early embryonic GnRH neurons: role in pulsatile neurosecretion. Mol Endocrinol 18: 1808–1817, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Mellon PL, Windle JJ, Goldsmith PC, Padula CA, Roberts JL, Weiner RI. Immortalization of hypothalamic GnRH neurons by genetically targeted tumorigenesis. Neuron 5: 1–10, 1990 [DOI] [PubMed] [Google Scholar]
- 19. Mitchell V, Prevot V, Jennes L, Aubert JP, Croix D, Beauvillain JC. Presence of mu and kappa opioid receptor mRNAs in galanin but not in GnRH neurons in the female rat. Neuroreport 8: 3167–3172, 1997 [DOI] [PubMed] [Google Scholar]
- 20. Naor Z. Signaling by G-protein-coupled receptor (GPCR): studies on the GnRH receptor. Front Neuroendocrinol 30: 10–29, 2009 [DOI] [PubMed] [Google Scholar]
- 21. Naor Z, Leifer AM, Catt KJ. Calcium-dependent actions of gonadotropin-releasing hormone on pituitary guanosine 3′,5′-monophosphate production and gonadotropin release. Endocrinology 107: 1438–1445, 1980 [DOI] [PubMed] [Google Scholar]
- 22. Naor Z, Shacham S, Harris D, Seger R, Reiss N. Signal transduction of the gonadotropin releasing hormone (GnRH) receptor: cross-talk of calcium, protein kinase C (PKC), and arachidonic acid. Cell Mol Neurobiol 15: 527–544, 1995 [DOI] [PubMed] [Google Scholar]
- 23. Paruthiyil S, eL Majdoubi M, Conti M, Weiner RI. Phosphodiesterase expression targeted to gonadotropin-releasing hormone neurons inhibits luteinizing hormone pulses in transgenic rats. Proc Natl Acad Sci USA 99: 17191–17196, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pompolo S, Pereira A, Estrada KM, Clarke IJ. Colocalization of kisspeptin and gonadotropin-releasing hormone in the ovine brain. Endocrinology 147: 804–810, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Quaynor S, Hu L, Leung PK, Feng H, Mores N, Krsmanovic LZ, Catt KJ. Expression of a functional g protein-coupled receptor 54-kisspeptin autoregulatory system in hypothalamic gonadotropin-releasing hormone neurons. Mol Endocrinol 21: 3062–3070, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Sakakibara H, Conti M, Weiner RI. Role of phosphodiesterases in the regulation of gonadotropin- releasing hormone secretion in GT1 cells. Neuroendocrinology 68: 365–373, 1998 [DOI] [PubMed] [Google Scholar]
- 27. Stojilkovic SS, Catt KJ. Novel aspects of GnRH-induced intracellular signaling and secretion in pituitary gonadotrophs. J Neuroendocrinol 7: 739–757, 1995 [DOI] [PubMed] [Google Scholar]
- 28. Stojilkovic SS, Chang JP, Ngo D, Tasaka K, Izumi S, Catt KJ. Mechanism of action of GnRH: the participation of calcium mobilization and activation of protein kinase C in gonadotropin secretion. J Steroid Biochem 33: 693–703, 1989 [DOI] [PubMed] [Google Scholar]
- 29. Urban RJ, Johnson ML, Veldhuis JD. In vivo biological validation and biophysical modeling of the sensitivity and positive accuracy of endocrine peak detection. I. The LH pulse signal. Endocrinology 124: 2541–2547, 1989 [DOI] [PubMed] [Google Scholar]
- 30. Vitalis EA, Costantin JL, Tsai PS, Sakakibara H, Paruthiyil S, Iiri T, Martini JF, Taga M, Choi AL, Charles AC, Weiner RI. Role of the cAMP signaling pathway in the regulation of gonadotropin-releasing hormone secretion in GT1 cells. Proc Natl Acad Sci USA 97: 1861–1866, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wada K, Hu L, Mores N, Navarro CE, Fuda H, Krsmanovic LZ, Catt KJ. Serotonin (5-HT) receptor subtypes mediate specific modes of 5-HT-induced signaling and regulation of neurosecretion in gonadotropin-releasing hormone neurons. Mol Endocrinol 20: 125–135, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Wetsel WC, Eraly SA, Whyte DB, Mellon PL. Regulation of gonadotropin-releasing hormone by protein kinase-A and -C in immortalized hypothalamic neurons. Endocrinology 132: 2360–2370, 1993 [DOI] [PubMed] [Google Scholar]
- 33. Wong ST, Athos J, Figueroa XA, Pineda VV, Schaefer ML, Chavkin CC, Muglia LJ, Storm DR. Calcium-stimulated adenylyl cyclase activity is critical for hippocampus-dependent long-term memory and late phase LTP. Neuron 23: 787–798, 1999 [DOI] [PubMed] [Google Scholar]
- 34. Wray S. Development of gonadotropin-releasing hormone-1 neurons. Front Neuroendocrinol 23: 292–316, 2002 [DOI] [PubMed] [Google Scholar]
- 35. Yoshida H, Beltran-Parrazal L, Butler P, Conti M, Charles AC, Weiner RI. Lowering cyclic adenosine-3′,5′-monophosphate (cAMP) levels by expression of a cAMP-specific phosphodiesterase decreases intrinsic pulsatile gonadotropin-releasing hormone secretion from GT1 cells. Mol Endocrinol 17: 1982–1990, 2003 [DOI] [PubMed] [Google Scholar]