Abstract
Myostatin deficiency causes dramatically increased skeletal muscle mass and reduced fat mass. Previously, myostatin-deficient mice were reported to have unexpectedly low total energy expenditure (EE) after normalizing to body mass, and thus, a metabolic cause for low fat mass was discounted. To clarify how myostatin deficiency affects the control of body fat mass and energy balance, we compared rates of oxygen consumption, body composition, and food intake in young myostatin-deficient mice relative to wild-type (WT) and heterozygous (HET) controls. We report that after adjusting for total body mass using regression analysis, young myostatin-deficient mice display significantly increased EE relative to both WT (+0.81 ± 0.28 kcal/day, P = 0.004) and HET controls (+0.92 ± 0.31 kcal/day, P = 0.005). Since food intake was not different between groups, increased EE likely accounts for the reduced body fat mass (KO: 8.8 ± 1.1% vs. WT: 14.5 ± 1.3%, P = 0.003) and circulating leptin levels (KO: 0.7 ± 0.2 ng/ml vs. WT: 1.9 ± 0.3 ng/ml, P = 0.008). Interestingly, the observed increase in adjusted EE in myostatin-deficient mice occurred despite dramatically reduced ambulatory activity levels (−50% vs. WT, P < 0.05). The absence of hyperphagia together with increased EE in myostatin-deficient mice suggests that increased leptin sensitivity may contribute to their lean phenotype. Indeed, leptin-induced anorexia (KO: −17 ± 1.2% vs. WT: −5 ± 0.3%) and weight loss (KO: −2.2 ± 0.2 g vs. WT: −1.6 ± 0.1, P < 0.05) were increased in myostatin-deficient mice compared with WT controls. We conclude that increased EE, together with increased leptin sensitivity, contributes to low fat mass in mice lacking myostatin.
Keywords: energy balance, locomotor activity, body fat, insulin sensitivity
myostatin, a member of the transforming growth factor-β family, is a paracrine factor that regulates skeletal muscle size and growth by favoring muscle atrophy and inhibiting anabolic signaling (12, 15). In mice (22) and humans (30), homozygous loss-of-function mutation of the myostatin gene causes a phenotype characterized by dramatic, whole body skeletal muscle hypertrophy and hyperplasia. Initial characterization of the energy homeostasis phenotype of myostatin-null mice revealed unexpected findings, suggesting a role for myostatin in the control of energy balance beyond its effect on skeletal muscle. Mature myostatin-null mice were found to have reduced body fat accumulation with age (23). Because energy expenditure (EE; normalized to body weight or lean body mass) in these animals was decreased relative to wild-type (WT) controls, suggestive of increased metabolic efficiency, the low-body fat phenotype remained unexplained (11, 23). A subsequent, extensive analysis of fat metabolism in these animals failed to identify a cause of low fat mass beyond increased skeletal muscle accrual (11).
Yet these studies, among others, also established that myostatin deficiency protects against high-fat diet-induced weight gain (11, 36) and associated comorbidity, including tissue inflammation (36), insulin resistance (11), and atherogenesis (33). Accordingly, several questions related to energy homeostasis remain unanswered in mice lacking myostatin, including 1) how does reduced body fat mass coexist with low metabolic rate, and 2) what mechanisms beyond increased lean body mass underlie the maintenance of reduced body fat mass? In the current work, we sought to answer these questions. We demonstrate that increased total EE without a corresponding increase of food intake likely accounts for the reduced body fat phenotype in myostatin-deficient mice and may be explained by increased leptin sensitivity. In addition, regression analysis to adjust EE for differences in body mass (16) demonstrates that myostatin-deficient mice are hypermetabolic, in contrast to what has been reported previously using traditional ratio normalization to adjust EE (11, 23).
MATERIALS AND METHODS
Animals.
Studies were conducted using both male and female adult myostatin-deficient mice from a colony maintained on a C57Bl/6 background and their littermate WT and heterozygous (HET) control mice (8) across a variety of ages, as detailed in results. Myostatin-deficient mice were developed and kindly provided by Dr. S. -J. Lee (Johns Hopkins University) (22). Both heterozygote-heterozygote and homozygote (male)-heterozygote (female) breeding strategies were used to generate animals for the studies described. Additional age-matched, nonlittermate, male and female C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME) were included in the calorimetry study to adjust total EE (TEE), as described below. Animals were housed individually in a temperature-controlled room (23 ± 2°C), maintained on a 12:12-h light-dark cycle, and provided ad libitum access to water and pelleted rodent chow (Test Diet 5015; LabDiet, Richmond, IN). For each study, mice were matched for sex difference between genotypes. All study protocols were approved by the Institutional Animal Care and Use Committee of the University of Washington and were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Indirect calorimetry.
Age- and sex-matched control and myostatin-deficient mice were individually housed after weaning and acclimated to metabolic cages for 3 days prior to data collection.
Total oxygen consumption (V̇o2) and carbon dioxide production (V̇co2) were measured continuously by indirect calorimetry using an eight-chamber Oxymax Lab Animal Monitoring System (Comprehensive Laboratory Animal Monitoring System; Columbus Instruments, Columbus, OH). V̇o2 was converted to individual TEE in kilocalories per hour by Columbus software, which uses the standard Lusk formula [TEE in kcal/h = (3.815 + 1.232 × RQ) × V̇o2 in l/h, where RQ is the ratio of V̇co2 to V̇o2] (20). A value of EE for each individual cage was recorded every 27 min.
Ambulatory activity.
Ambulatory activity was determined simultaneously with the collection of indirect calorimetry data using an Opto-Varimetrix-3 sensor system (Columbus Instruments). Consecutive adjacent infrared beam breaks in either the x- or y-axes were scored as an activity count, and a tally was recorded every 15 min.
Food intake.
Food intake was recorded continuously over a 24-h period during which standard chow was available ad libitum. Food and water intake were measured with the Feed-Scale System (Columbus Instruments). Measurements of feeding behavior (i.e., the amount of food/water consumed) were also quantified using feed scale (mass) measurements. For animals not housed in metabolic cages, food intake was measured daily (2 h into the light cycle) during experimental protocols.
Body composition analysis.
Body composition was evaluated in live, conscious animals in triplicate by quantitative nuclear magnetic resonance spectroscopy (EchoMRI 3-in-1 Animal Tissue Composition Analyzer; Echo Medical Systems, Houston, TX) (30a, 31a). A system test is performed routinely at the beginning of each measurement day, and the equipment is calibrated by scanning a calibration holder containing a known amount of fat to test the validity of measurement.
Measurements of insulin tolerance.
Insulin sensitivity was measured using an intraperitoneal (ip) insulin tolerance test performed in nonfasted animals 8 h into the light cycle. Following an ip injection (0.5 U/kg) of insulin (Humulin R; Eli Lilly, Indianapolis, IN), blood glucose levels were measured in blood obtained from the tail vein at t = 0, 30, 60, and 90 min using a hand-held glucometer (Accu-Chek; Roche, Indianapolis, IN).
Measurements of leptin sensitivity.
The effects of exogenous recombinant murine leptin (Dr. A. F. Parlow, National Hormone and Peptide Program) on food intake and body weight were evaluated in individually housed mice injected with leptin (0.375 mg/kg ip) at 12-h intervals for a total of four consecutive doses. Food intake and body weight were measured throughout the 48-h period and compared with the preceding 48-h period during which animals received twice daily ip injections of vehicle. Leptin-induced hypothalamic pSTAT3 content was determined in 4-h-fasted animals that were euthanized either 1 or 4 h following leptin administration (1.0 mg/kg ip).
Blood collection and tissue processing.
Brains were removed and immediately frozen under crushed dry ice. Mediobasal hypothalamus was dissected and stored at −80°C prior to protein extraction, as described previously (37). Trunk blood was collected in chilled, heparinized tubes, and plasma was collected and frozen at −80°C. Plasma leptin values were determined by mouse-specific leptin ELISA (Crystal Chem, Downers Grove, IL).
Protein extraction and Western blotting.
Total protein was extracted from hypothalamic tissue using T-PCR reagent (Thermo Scientific, Rockford, IL) and quantified by BCA protein assay kit (Thermo Scientific). Forty micrograms of protein was loaded on 4–20% gradient polyacrylamide gel. Western blotting was performed using a rabbit anti-pSTAT3 antibody (Cell Signaling Technology, Beverly, MA) and a rabbit anti-STAT3 antibody (Cell Signaling Technology) at 1:1,000 dilution. Hypothalamic pSTAT3 content was normalized to total STAT3 levels for each time point.
Statistical methods.
Because key outcome variables were not altered when data were analyzed separately by sex difference, data pooled from both sexes are presented for all studies. All results are expressed as means ± SE. For analyses that did not require adjustment for variation in body size, we used a one-way ANOVA for omnibus group-wide testing followed by a post hoc least significant difference between-subjects Student t-test for pairwise comparisons. To control for the influence of body size variation on TEE (16), group comparisons involving this outcome were adjusted for total body mass and for lean body mass in separate analyses using analysis of covariance (ANCOVA) (4), in keeping with recent work from our group (16). ANOVA was performed using Statistica (version 7.1; StatSoft), and ANCOVA was performed with the univariate general linear model module in PASW statistics (version 17; IBM, Chicago, IL). Significance was established at P < 0.05 (two-tailed t-test).
RESULTS
Body weight, body composition, and EE in myostatin-deficient mice and WT littermate controls.
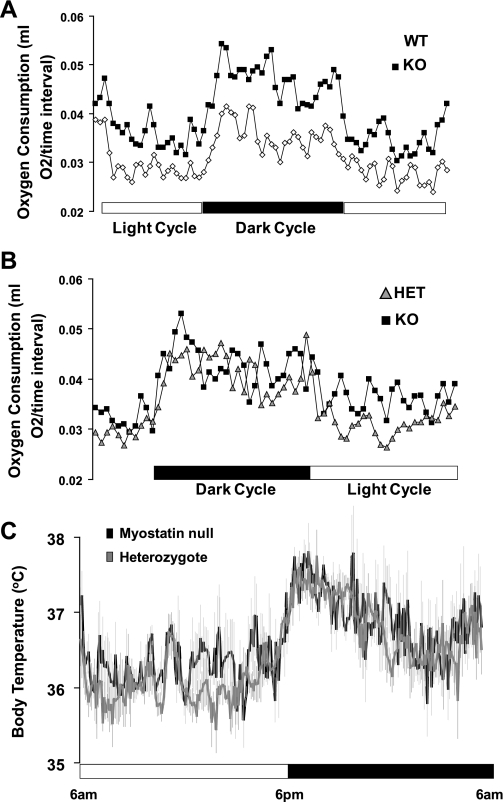
At age 6–8 wk, myostatin-deficient mice (KO) display increases in both body weight (KO: 23.6 ± 1.2 g vs. WT: 18.0 ± 1.4 g; n = 4 for each group, P = 0.01) and lean mass (Table 1) relative to age- and sex-matched WT controls. Even at this young age, myostatin-deficient mice also exhibited significant decreases in total body fat mass (Table 1), percent fat mass (8.8 ± 1.1 vs. 14.5 ± 1.3%, P = 0.003), and plasma leptin concentrations (0.7 ± 0.2 vs. 1.9 ± 0.3 ng/ml, P = 0.008). Despite low plasma leptin levels, mean daily food (Table 1) and water intake (3.1 ± 0.5 vs. 3.3 ± 0.4 ml, P = 0.4) were not significantly different between genotypes. Using continuous measurements obtained by indirect calorimetry, we evaluated oxygen consumption in myostatin-deficient and WT control mice (n = 4/group; Fig. 1A). Unadjusted EE during both the dark and light cycle was higher in myostatin-deficient mice than WT controls (Table 1) despite the fact that ambulatory activity was significantly lower in myostatin-deficient animals (Table 1). Thus, myostatin-deficient mice are larger and have higher total EE but equal food intake compared with WT controls.
Table 1.
Energy balance in KO and WT mice
| WT | KO | |
|---|---|---|
| Unadjusted energy expenditure, ml O2/h | ||
| TEE (dark) | 82 ± 7 | 98 ± 2* |
| TEE (light) | 59 ± 6 | 73 ± 2* |
| Ambulatory activity (beam breaks × 103) | ||
| Dark cycle | 32 ± 4 | 16 ± 2* |
| Light cycle | 8.1 ± 0.4 | 5.2 ± 1.0* |
| Lean mass, g | 14.6 ± 1.4 | 20.5 ± 1.2* |
| Fat mass, g | 2.6 ± 0.1 | 2.1 ± 0.3* |
| Food intake, g | 4.6 ± 0.2 | 4.6 ± 0.6 |
Data are means ± SE; n = 4.
KO, myostatin-deficient mice; WT, wild-type mice; TEE, total energy expenditure.
Oxygen consumption (ml O2/h), TEE during the dark and the light cycles, ambulatory activity during the dark and light cycles, lean and fat mass (by quantitative magnetic resonance), and 24-h food intake data are presented.
P < 0.05 between groups by 2-tailed Student t-test.
Fig. 1.
Total energy expenditure in myostatin-deficient mice (KO) and controls [heterozygous (HET) and wild type (WT)]. Total oxygen consumption (ml O2/27-min time interval) is shown during 2 light cycles and 1 dark cycle in wild-type littermates (◊) and myostatin-deficient mice (■) (A) (n = 4/group) and heterozygote littermates (gray triangle) and myostatin-deficient mice (■) (B) (n = 6–8/group). C: body temperature in KO mice and HET littermate controls. Core body temperature was measured continuously throughout the day via implanted (ip) temperature transducers in 2 groups of age- and weight-matched mice (n = 8/group): 1) HET littermates (gray line) and 2) KO mice (black line). Light and dark cycles indicated by the open and and black bars, respectively. Data presented are means ± SE.
Body weight, body composition, and EE in myostatin-deficient mice and heterozygous littermate controls.
Previous studies have shown that heterozygous mice with only one functioning myostatin allele have an intermediate body weight phenotype (23). Because evaluation of energy homeostasis is influenced strongly by differences in body weight and lean mass, we evaluated a separate cohort of young, weight-matched, myostatin-deficient (24.5 ± 0.8 g), and heterozygote control mice (25.6 ± 0.7 g, P = 0.2) aged 6–10 wk (n = 6/group). Again, we observed significantly reduced fat mass (Table 2) and percent body fat (8.9 ± 0.8 vs. 11.5 ± 0.7%, P = 0.01) in myostatin-deficient relative to heterozygous mice. Importantly, differences in body adiposity between myostatin-deficient and heterozygous control mice were detected despite no difference in lean body mass between groups (Table 2). As before, we found no differences in either food (Table 2) or water intake (3.2 ± 0.3 vs. 3.0 ± 0.2 ml, P = 0.3) between groups. Continuous evaluation of oxygen consumption revealed increased EE in myostatin-deficient animals, especially during the light cycle (Fig. 1B), and unadjusted EE was higher in myostatin-deficient mice than in heterozygote controls during both the dark and light cycles (Table 2), whereas ambulatory activity was significantly lower in myostatin-deficient animals only during the dark cycle (Table 2). Increased EE could not be explained by altered body temperature since there was no difference in body temperature between myostatin-deficient and heterozygous mice during either the dark or light cycle (Fig. 1C).
Table 2.
Energy balance in KO and HET control mice
| HET | KO | |
|---|---|---|
| Unadjusted energy expenditure, ml O2/h | ||
| TEE (dark) | 98 ± 4 | 107 ± 4* |
| TEE (light) | 66 ± 2 | 76 ± 2* |
| Ambulatory activity (beam breaks ×103) | ||
| Dark cycle | 28 ± 2 | 19.6 ± 2* |
| Light cycle | 5.0 ± 0.4 | 5.6 ± 0.7 |
| Lean mass, g | 21.0 ± 0.5 | 21.4 ± 1.2 |
| Fat mass, g | 2.9 ± 0.2 | 2.2 ± 0.2* |
| Food intake, g | 4.2 ± 0.2 | 4.3 ± 0.4 |
Data are means ± SE; n = 6. HET, heterozygous mice. Oxygen consumption (ml O2/h), TEE during the dark and the light cycles, ambulatory activity during the dark and light cycles, lean and fat mass (by quantitative magnetic resonance), and 24-h food intake data are presented.
P < 0.05 between groups by 2-tailed Student t-test.
Adjusted EE in myostatin-deficient mice and WT and heterozygous controls.
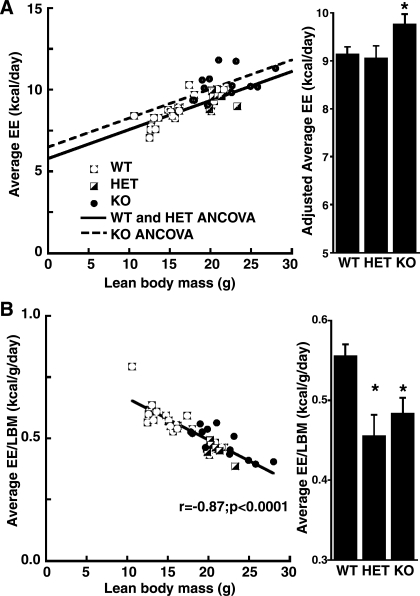
Previous studies have concluded that, despite their large lean mass, myostatin-deficient mice are hypometabolic; i.e., they have lower than expected EE after normalizing to their increased lean body mass (11, 23). Importantly, these studies expressed EE as a ratio (e.g., divided by) of total body mass or lean body mass, which can yield confounded outcomes when these variables differ significantly between groups. Based on these concerns, we used regression analysis to compare EE after adjusting for differences of either total body mass or lean body mass (16). After adjustment for total body mass using ANCOVA, myostatin-deficient mice exhibited significantly higher EE than either HET (by 0.92 ± 0.31 kcal/day, P = 0.005) or WT mice (by 0.81 ± 0.28 kcal/day, P = 0.004), whereas the average daily EE of HET mice did not differ significantly from that of WT mice (difference = 0.08 ± 0.33 kcal/day, P = 0.81). Using ANCOVA to adjust for lean body mass yielded similar group differences, with EE being greater in myostatin-deficient mice than in either HET (+0.71 ± 0.29 kcal/day, P = 0.02) or WT controls (+0.63 ± 0.28 kcal/day, P = 0.03) (Fig. 2A). Thus, controlling for the influence of total or lean body mass on EE using appropriate normalization methodology (16) reveals myostatin-deficient mice to be hypermetabolic compared with WT or HET animals. Notably, the hypermetabolic phenotype of the KO mice occurs despite the fact that they exhibit the lowest mean activity level of the three groups (P = 0.006 relative to WT mice and P = 0.052 relative to HET mice). For comparison purposes, we also performed the analysis using a traditional ratio normalization method whereby EE is divided by lean body mass. Using this method, myostatin-deficient mice appear to be hypometabolic relative to WT mice (by −0.072 ± 0.023 kcal·g lean body mass−1·day−1, P = 0.004) but no different than HET mice (P = 0.39) (Fig. 2B). This incorrect conclusion is an inevitable mathematical consequence of the confounding influence of a positive y-intercept (5.63 + 0.48 kcal/day, P = 0.00001) in the regression of total EE on lean body mass as shown in Fig. 2A, as described recently (16).
Fig. 2.
Adjusted energy expenditure (EE) in relation to lean body mass (LBM) in KO mice and controls (HET and WT). A: the scatterplot shows lines of best fit for EE as a function of group and LBM using analysis of covariance (ANCOVA). The EE fit line for KO mice (dashed line) is higher than that for WT and HET controls (solid line). Note the significant, positive y-intercept (5.63 ± 0.48 kcal/day, P = 0.00001) in the regression of EE on LBM. The bar graph shows group comparisons adjusted for LBM. *P ≤ 0.03 compared with WT or HET. B: traditional ratio normalization (division of EE by LBM) yields a confounded measurement, as demonstrated by the remaining strong negative correlation between the normalized construct and LBM. The bar graph demonstrates that KO mice incorrectly appear hypometabolic using this method. *P ≤ 0.004 compared with WT.
Leptin sensitivity and insulin sensitivity in myostatin-deficient mice and WT littermate controls.
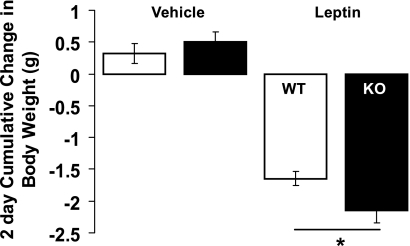
The combination of high EE without compensatory increased energy intake in myostatin-deficient mice led us to ask whether leptin sensitivity is increased in these animals. To address this question, we compared behavioral responses to injection of leptin (0. 375 mg/kg total ip twice daily for 2 days) between myostatin-deficient and WT littermate control mice (age 14–24 wk; n = 11–13/group). Body weights were similar between groups (KO: 33.9 ± 1.7 g vs. WT: 33.5 ± 2.3 g, P = 0.4) despite higher lean mass (87 ± 0.7 vs. 79 ± 2.4%, P < 0.01), lower fat mass (7.8 ± 0.3 vs. 15.4 ± 2.7%, P < 0.05), and reduced plasma leptin concentrations (1.8 ± 0.5 vs. 5.1 ± 0.9 ng/ml, P < 0.005) in mutant animals, consistent with our previous results. As shown in Fig. 3, myostatin-deficient mice were clearly more sensitive than controls to the effect of leptin to reduce body weight. The reduction in cumulative food intake over the 2-day period relative to food intake during the control injection period was also greater in the myostatin-deficient than WT mice (KO: −1.2 ± 0.4 g vs. WT: −0.2 ± 0.3, P < 0.05). Thus, myostatin-deficient mice are more sensitive to leptin-induced anorexia and weight loss than weight-matched littermate controls.
Fig. 3.
Leptin-induced weight loss in KO mice and WT littermate controls. Body weight was measured before and after bid administration of leptin (0.375 mg/kg ip) × 4 doses or vehicle in 2 groups of mice (n = 11–13/group): 1) WT littermates (open bars) and 2) KO mice (black bars). Data presented are means ± SE. *P < 0.05 by standard 2-tailed Student t-test.
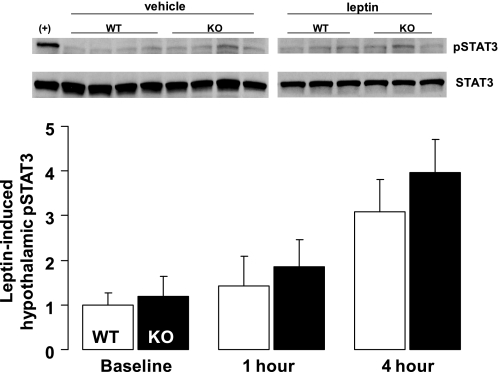
In a separate study, we tested the ability of leptin administration (1 mg/kg ip) to increase hypothalamic content of pSTAT3, a key signal transduction molecule downstream of the leptin receptor (n = 3–4 per group per time point). As expected, systemic leptin administration induced a time-dependent increase in hypothalamic pSTAT3 content (relative to total STAT3) in both groups compared with ip vehicle treatment (P = 0.007). Despite significantly lower plasma leptin levels in myostatin-deficient mice, baseline hypothalamic pSTAT3 content was equivalent to WT controls, suggesting greater basal leptin sensitivity; however, the effect of pharmacological leptin administration to increase hypothalamic pSTAT3 (at 1 and 4 h) in mutant mice was not significantly different from control mice (Fig. 4).
Fig. 4.
Hypothalamic pSTAT3 in KO and WT littermate controls. Representative Western blots showing pSTAT3 and total STAT3 protein within a medio-basal wedge of hypothalamic tissue 4 h following ip leptin administration (1.0 mg/kg) or vehicle injection. B: averaged leptin-induced hypothalamic Western blot pSTAT3/STAT3 data normalized to vehicle-injected animals 1 or 4 h following ip leptin administration in 2 groups of mice (n = 3–5/group): 1) WT littermates (open bars) and 2) KO mice (black bars). Data presented are means ± SE.
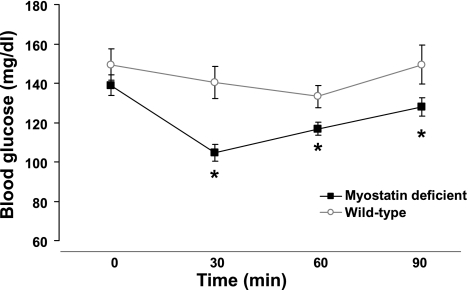
To confirm that myostatin-deficient animals demonstrate increased insulin sensitivity, as has been shown previously (11), we also performed an insulin tolerance test in myostatin-deficient mice (age 10–16 wk; mean body weight 30.7 ± 1.2 g, lean mass 86 ± 0.6%, fat mass 10.2 ± 0.5%) and WT controls (age 10–16 wk; weight 23.7 ± 0.6 g, lean mass 80 ± 0.5%, fat mass 14.2 ± 0.7%) (n = 10/group). Myostatin-deficient mice were significantly more insulin sensitive than littermate controls (Fig. 5) at all time points following ip insulin administration (0.5 μg/kg). Thus, myostatin-deficient mice demonstrate increased insulin sensitivity, consistent with previous findings (11).
Fig. 5.
Blood glucose response to an ip insulin tolerance test in KO mice and WT littermate controls. Insulin sensitivity was measured by ip insulin tolerance testing in nonfasted animals 8 h into the light cycle. Following an ip injection of insulin, blood glucose levels were measured in blood obtained from the tail vein at t = 0, 30, 60, and 90 min in 2 groups of mice (n = 10/group): 1) WT littermates (○) and 2) KO mice (■). Data presented are means ± SE. *P < 0.05 by standard 2-tailed Student t-test.
DISCUSSION
Deficient myostatin signaling has been shown to confer a dramatic phenotype of increased skeletal muscle mass (22) that is accompanied by reduced fat mass accumulation with increasing age (11, 23). The current studies were undertaken to clarify the impact of myostatin deficiency on key determinants of energy balance. We report that young myostatin-deficient mice have increased energy expenditure relative to WT and heterozygote controls based on both unadjusted analyses and on normalization methodology that appropriately controls for the influence of body size variation on energy expenditure (16). Importantly, increased energy expenditure in combination with equivalent food intake provides a feasible explanation for the reduced fat mass characteristic of mice lacking myostatin. Our finding of increased behavioral leptin sensitivity provides a plausible mechanism that may contribute to the defense of reduced body adiposity in these animals without compensatory hyperphagia.
Taken together, our findings suggest that, in addition to increasing the growth of skeletal muscle, myostatin deficiency alters energy homeostasis regulation so as to defend a reduced level of body fat mass that in turn is associated with improved glucose metabolism despite reduced voluntary physical activity. These observations raise the interesting possibility that a muscle-related factor(s) may lower body fat mass and improve metabolic function as well as regulate spontaneous physical activity, and these hypotheses warrant additional investigation.
Previous efforts to characterize energy balance in myostatin-deficient mice yielded confusing results. Two studies demonstrated that total (23) and resting energy expenditure (11) are decreased in myostatin-deficient animals when the rate of oxygen consumption is “corrected” for total or lean body mass by dividing energy expenditure by either variable. Although this is a commonly used approach, it can yield confounded outcomes, and in this case it led the authors to conclude that these animals are hypometabolic (11, 23) and, by extension, that the low fat mass of these mice could not be explained by differences of metabolic rate. However, when the effect of energy balance on body composition is evaluated, the difference between absolute energy intake and energy expenditure is ultimately critical. We found that energy expenditure is increased in myostatin-deficient animals when they are measured at a young age, when the high muscle/low fat phenotype is still developing, and therefore less likely to confound results. This finding is in agreement with previously reported data both in myostatin-deficient mice (11, 23) and in studies using pharmacological inhibition of myostatin in wild-type mice (6, 18). Combined with our finding that energy intake is not increased in myostatin-deficient mice, low body fat mass, as documented by us and others (11, 23), is the expected phenotype in these mice and does not require further mechanistic explanation (11). These considerations raise two additional questions related to energy balance: 1) what mechanisms underlie reduced energy intake relative to energy expenditure, and 2) is metabolic rate truly low in these mice with markedly increased muscle mass?
To answer the first question, we evaluated leptin signaling in myostatin-deficient mice since this hormone is a key regulator of energy balance and circulating levels are proportional to fat mass (21). Consistent with previous studies in myostatin-deficient animals that documented decreased fat mass (11, 23) and reduced adipogenesis (19), we observed that moderate to marked reduction of whole body fat content is an early manifestation of myostatin deficiency and is evident compared with both WT and HET littermate controls. As expected for animals with reduced fat mass, we also observed a clear reduction of plasma leptin levels in mice lacking myostatin, as shown previously (23).
Since myostatin-deficient mice did not exhibit hyperphagia characteristic of WT animals with reduced leptin levels (9, 26), as well as some hypermetabolic mouse models (1, 9), we hypothesized that sensitivity to leptin in these animals is increased such that relatively low leptin levels provide a leptin signal to the brain of sufficient magnitude to maintain food intake at levels similar to that of normal animals with higher leptin levels. Our finding that the ability of exogenous leptin to reduce body weight and food intake is increased in mice lacking myostatin provides direct evidence in support of this hypothesis, although additional work is needed to determine the relative loss of muscle and fat mass induced by leptin. How myostatin deficiency might affect the response to leptin remains unknown. Insight into this question may be gained from several other mouse models characterized by a hypermetabolic phenotype without compensatory hyperphagia. Interestingly, these models including neuron-specific deletion of endogenous inhibitors of leptin action such as suppressor of cytokine signaling-3 (25) and protein tyrosine phosphatase 1B (5, 38), and administration of drugs such as resveratrol (17) are each marked by increased leptin sensitivity and reduced body fat mass, and our data add myostatin-deficient mice to this list. However, the lean phenotype of these mice may also involve mechanisms independent of increased leptin sensitivity, since myostatin deficiency partially corrects obesity in ob/ob mice that lack leptin (23), and thus we investigated the effect of myostatin deficiency on metabolic efficiency.
The question of how to analyze mouse metabolic efficiency has been controversial (7, 16). Traditional methods of normalizing energy expenditure involve division by lean or total body mass, which may be allometrically scaled (28, 34, 35). These methods are often confounded by a parent linear relationship between energy expenditure and lean or total body mass that is typically characterized by a positive y-intercept (energy expenditure) term (3, 16, 31). Consequently, dividing energy expenditure by body mass mathematically leads to heavier mice having a lower normalized energy expenditure than smaller mice (16, 31). An alternative normalization method that is widely used in clinical obesity studies involves multiple regression methodology to adjust energy expenditure for differences in body mass so as to eliminate the influence of body size variation per se (32). Recent evidence suggests that a similar regression-based approach that accounts for variation in total or lean body mass is the best method to adjust energy expenditure in mice (16). In the current study, regression analysis (whether adjusted for activity or not) provided evidence that myostatin-deficient mice have increased energy expenditure for a given lean body mass compared with wild-type and heterozygous controls. Therefore, this finding indicates that a hypermetabolic phenotype might contribute to the leanness of myostatin-deficient mice. Importantly, this study provides a critical example wherein analyzing data by traditional ratio normalization of energy expenditure to lean body mass erroneously classifies myostatin-deficient mice as hypometabolic rather than hypermetabolic. The mechanism of altered metabolic efficiency in myostatin-deficient mice remains to be determined. Because resting EE is the largest component of total EE and the most closely linked to variation in lean body mass, we favor the hypothesis that hypermetabolism in myostatin-deficient mice is due to physiological changes in skeletal muscle that increase resting EE; however, alterations in thermoregulation or activity-related muscle efficiency could also contribute to altered metabolic efficiency in the mutant animals.
Our study also demonstrates dramatically reduced ambulatory activity in young myostatin-deficient mice, in contrast to what has been demonstrated in older mice (11). The mechanism of reduced ambulatory activity in young myostatin-deficient mice remains unclear. Previous studies have examined skeletal muscle morphology and physiology, muscle strength, force generation and contractile properties, biomechanical integrity, mitochondrial number, and muscle fiber types, as well as gait and ground reaction forces in myostatin-deficient mice, cumulatively describing numerous abnormalities (2, 10, 13, 22, 24, 29). Whether changes in muscle structure and function related to lacking myostatin contribute to decreased ambulatory activity remains to be determined, but intriguingly, other genetic strategies to increase muscle mass also cause downward regulation of ambulatory activity and fat mass (14). Because voluntary physical activity is a regulated variable in the control of energy homeostasis, and the central pathways governing ambulatory activity are increasingly defined (27), it is equally plausible that a central nervous system-mediated behavioral mechanism contributes to reduced ambulatory activity in myostatin-deficient mice, and this hypothesis awaits further study. Importantly, because ambulation has a greater energy cost in heavier animals, the extent to which reduced ambulatory activity in myostatin-deficient mice reduces activity energy expenditure remains to be determined.
In conclusion, we report that reduced ambulatory activity and increased leptin sensitivity are unexpected but integral aspects of the phenotype of mice lacking myostatin. We also report that low fat mass in these mice is readily explained by the combination of increased absolute or adjusted energy expenditure with no corresponding increase in energy intake. These findings suggest that myostatin signaling either directly or indirectly via increased skeletal muscle mass has far-reaching effects on energy homeostasis and raise the possibility that an unknown muscle-related factor(s) induced by myostatin deficiency may confer metabolic benefit.
ACKNOWLEDGMENTS
We are grateful to I. David, J. D. Fischer, Phil May, Miles Matsen, and A. Cubelo for their technical assistance and to the Seattle Metabolic Mouse Phenotyping Core (U24-DK-076126).
GRANTS
This work was supported by grants from the National Institutes of Health to B. E. Wisse (DK-061384 and DK-071784), M. W. Schwartz (DK-068384, DK-052989, and DK-083042), and Z. Yablonka-Reuveni (AG-021566, AG-035377, and AR-057794) and the Clinical Nutrition and Research Unit (P30-DK-035816; B. E. Wisse) and the Diabetes Endocrinology Research Center (P30-DK-17047, B. E. Wisse) at the University of Washington. Z. Yablonka-Reuveni acknowledges additional support from the Muscular Dystrophy Association (135908).
DISCLOSURES
The authors have nothing to disclose.
REFERENCES
- 1. Abu-Elheiga L, Matzuk MM, Abo-Hashema KA, Wakil SJ. Continuous fatty acid oxidation and reduced fat storage in mice lacking acetyl-CoA carboxylase 2. Science 291: 2613–2616, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Amthor H, Macharia R, Navarrete R, Schuelke M, Brown SC, Otto A, Voit T, Muntoni F, Vrbova G, Partridge T, Zammit P, Bunger L, Patel K. Lack of myostatin results in excessive muscle growth but impaired force generation. Proc Natl Acad Sci USA 104: 1835–1840, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arch JR, Hislop D, Wang SJ, Speakman JR. Some mathematical and technical issues in the measurement and interpretation of open-circuit indirect calorimetry in small animals. Int J Obes (Lond) 30: 1322–1331, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Armitage PB, Matthews JN. Statistical Methods in Medical Research. New York: Blackwell Science, 2002 [Google Scholar]
- 5. Bence KK, Delibegovic M, Xue B, Gorgun CZ, Hotamisligil GS, Neel BG, Kahn BB. Neuronal PTP1B regulates body weight, adiposity and leptin action. Nat Med 12: 917–924, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Bernardo BL, Wachtmann TS, Cosgrove PG, Kuhn M, Opsahl AC, Judkins KM, Freeman TB, Hadcock JR, LeBrasseur NK. Postnatal PPARdelta activation and myostatin inhibition exert distinct yet complimentary effects on the metabolic profile of obese insulin-resistant mice. PLoS One 5: e11307, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Butler AA, Kozak LP. A recurring problem with the analysis of energy expenditure in genetic models expressing lean and obese phenotypes. Diabetes 59: 323–329, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Day K, Shefer G, Shearer A, Yablonka-Reuveni Z. The depletion of skeletal muscle satellite cells with age is concomitant with reduced capacity of single progenitors to produce reserve progeny. Dev Biol 340: 330–343, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gelling RW, Yan W, Al-Noori S, Pardini A, Morton GJ, Ogimoto K, Schwartz MW, Dempsey PJ. Deficiency of TNFalpha converting enzyme (TACE/ADAM17) causes a lean, hypermetabolic phenotype in mice. Endocrinology 149: 6053–6064, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Girgenrath S, Song K, Whittemore LA. Loss of myostatin expression alters fiber-type distribution and expression of myosin heavy chain isoforms in slow- and fast-type skeletal muscle. Muscle Nerve 31: 34–40, 2005 [DOI] [PubMed] [Google Scholar]
- 11. Guo T, Jou W, Chanturiya T, Portas J, Gavrilova O, McPherron AC. Myostatin inhibition in muscle, but not adipose tissue, decreases fat mass and improves insulin sensitivity. PLoS One 4: e4937, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Guttridge DC. Signaling pathways weigh in on decisions to make or break skeletal muscle. Curr Opin Clin Nutr Metab Care 7: 443–450, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Hennebry A, Berry C, Siriett V, O'Callaghan P, Chau L, Watson T, Sharma M, Kambadur R. Myostatin regulates fiber-type composition of skeletal muscle by regulating MEF2 and MyoD gene expression. Am J Physiol Cell Physiol 296: C525–C534, 2009 [DOI] [PubMed] [Google Scholar]
- 14. Izumiya Y, Hopkins T, Morris C, Sato K, Zeng L, Viereck J, Hamilton JA, Ouchi N, LeBrasseur NK, Walsh K. Fast/Glycolytic muscle fiber growth reduces fat mass and improves metabolic parameters in obese mice. Cell Metab 7: 159–172, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Joulia-Ekaza D, Cabello G. The myostatin gene: physiology and pharmacological relevance. Curr Opin Pharmacol 7: 310–315, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Kaiyala KJ, Morton GJ, Leroux BG, Ogimoto K, Wisse B, Schwartz MW. Identification of body fat mass as a major determinant of metabolic rate in mice. Diabetes 59: 1657–1666, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 127: 1109–1122, 2006 [DOI] [PubMed] [Google Scholar]
- 18. LeBrasseur NK, Schelhorn TM, Bernardo BL, Cosgrove PG, Loria PM, Brown TA. Myostatin inhibition enhances the effects of exercise on performance and metabolic outcomes in aged mice. J Gerontol A Biol Sci Med Sci 64: 940–948, 2009 [DOI] [PubMed] [Google Scholar]
- 19. Lin J, Arnold HB, Della-Fera MA, Azain MJ, Hartzell DL, Baile CA. Myostatin knockout in mice increases myogenesis and decreases adipogenesis. Biochem Biophys Res Commun 291: 701–706, 2002 [DOI] [PubMed] [Google Scholar]
- 20. McLean JT. Animal and Human Calorimetry. New York: Cambridge University, 1987. [Google Scholar]
- 21. McMinn JE, Baskin DG, Schwartz MW. Neuroendocrine mechanisms regulating food intake and body weight. Obes Rev 1: 37–46, 2000 [DOI] [PubMed] [Google Scholar]
- 22. McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature 387: 83–90, 1997 [DOI] [PubMed] [Google Scholar]
- 23. McPherron AC, Lee SJ. Suppression of body fat accumulation in myostatin-deficient mice. J Clin Invest 109: 595–601, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mendias CL, Marcin JE, Calerdon DR, Faulkner JA. Contractile properties of EDL and soleus muscles of myostatin-deficient mice. J Appl Physiol 101: 898–905, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mori H, Hanada R, Hanada T, Aki D, Mashima R, Nishinakamura H, Torisu T, Chien KR, Yasukawa H, Yoshimura A. Socs3 deficiency in the brain elevates leptin sensitivity and confers resistance to diet-induced obesity. Nat Med 10: 739–743, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature 443: 289–295, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Novak CM, Levine JA. Central neural and endocrine mechanisms of non-exercise activity thermogenesis and their potential impact on obesity. J Neuroendocrinol 19: 923–940, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Packard GC, Birchard GF. Traditional allometric analysis fails to provide a valid predictive model for mammalian metabolic rates. J Exp Biol 211: 3581–3587, 2008 [DOI] [PubMed] [Google Scholar]
- 29. Schmitt D, Zumwalt AC, Hamrick MW. The relationship between bone mechanical properties and ground reaction forces in normal and hypermuscular mice. J Exp Zool A Ecol Genet Physiol 313: 339–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schuelke M, Wagner KR, Stolz LE, Hubner C, Riebel T, Komen W, Braun T, Tobin JF, Lee SJ. Myostatin mutation associated with gross muscle hypertrophy in a child. N Engl J Med 350: 2682–2688, 2004 [DOI] [PubMed] [Google Scholar]
- 30a. Taicher GZ, Tinsley FC, Reiderman A, Heiman ML. Quantitative magnetic resonance (QMR) method for bone and whole-body-composition analysis. Anal Bioanal Chem 377: 990–1002, 2003 [DOI] [PubMed] [Google Scholar]
- 31. Tanner JM. Fallacy of per-weight and per-surface area standards, and their relation to spurious correlation. J Appl Physiol 2: 1–15, 1949 [DOI] [PubMed] [Google Scholar]
- 31a. Tinsley FC, Taicher GZ, Heiman ML. Evaluation of a quantitative magnetic resonance method for mouse whole body composition analysis. Obes Res 12: 150–160, 2004 [DOI] [PubMed] [Google Scholar]
- 32. Toth MJ. Comparing energy expenditure data among individuals differing in body size and composition: statistical and physiological considerations. Curr Opin Clin Nutr Metab Care 4: 391–397, 2001 [DOI] [PubMed] [Google Scholar]
- 33. Tu P, Bhasin S, Hruz PW, Herbst KL, Castellani LW, Hua N, Hamilton JA, Guo W. Genetic disruption of myostatin reduces the development of proatherogenic dyslipidemia and atherogenic lesions in Ldlr null mice. Diabetes 58: 1739–1748, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. White CR, Cassey P, Blackburn TM. Allometric exponents do not support a universal metabolic allometry. Ecology 88: 315–323, 2007 [DOI] [PubMed] [Google Scholar]
- 35. White CR, Seymour RS. Allometric scaling of mammalian metabolism. J Exp Biol 208: 1611–1619, 2005 [DOI] [PubMed] [Google Scholar]
- 36. Wilkes JJ, Lloyd DJ, Gekakis N. Loss-of-function mutation in myostatin reduces tumor necrosis factor alpha production and protects liver against obesity-induced insulin resistance. Diabetes 58: 1133–1143, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wisse BE, Ogimoto K, Schwartz MW. Role of hypothalamic interleukin-1beta (IL-1beta) in regulation of energy homeostasis by melanocortins. Peptides 27: 265–273, 2006 [DOI] [PubMed] [Google Scholar]
- 38. Zabolotny JM, Bence-Hanulec KK, Stricker-Krongrad A, Haj F, Wang Y, Minokoshi Y, Kim YB, Elmquist JK, Tartaglia LA, Kahn BB, Neel BG. PTP1B regulates leptin signal transduction in vivo. Dev Cell 2: 489–495, 2002 [DOI] [PubMed] [Google Scholar]