Abstract
Obesity and metabolic syndrome are associated with an increased risk for several diabetic complications, including diabetic nephropathy and chronic kidney diseases. Oxidative stress and mitochondrial dysfunction are often proposed mechanisms in various organs in obesity models, but limited data are available on the kidney. Here, we fed a lard-based high-fat diet to mice to investigate structural changes, cellular and subcellular oxidative stress and redox status, and mitochondrial biogenesis and function in the kidney. The diet induced characteristic changes, including glomerular hypertrophy, fibrosis, and interstitial scarring, which were accompanied by a proinflammatory transition. We demonstrate evidence for oxidative stress in the kidney through 3-nitrotyrosine and protein radical formation on high-fat diet with a contribution from iNOS and NOX-4 as well as increased generation of mitochondrial oxidants on carbohydrate- and lipid-based substrates. The increased H2O2 emission in the mitochondria suggests altered redox balance and mitochondrial ROS generation, contributing to the overall oxidative stress. No major derailments were observed in respiratory function or biogenesis, indicating preserved and initially improved bioenergetic parameters and energy production. We suggest that, regardless of the oxidative stress events, the kidney developed an adaptation to maintain normal respiratory function as a possible response to an increased lipid overload. These findings provide new insights into the complex role of oxidative stress and mitochondrial redox status in the pathogenesis of the kidney in obesity and indicate that early oxidative stress-related changes, but not mitochondrial bioenergetic dysfunction, may contribute to the pathogenesis and development of obesity-linked chronic kidney diseases.
Keywords: reactive oxygen species, chronic kidney diseases, obesity, mitochondria
obesity is a global health problem with numerous risks and consequences, including cardiovascular problems and other complications. Metabolic syndrome and obesity are associated with an increased risk for diabetic complications later, including diabetic nephropathy and chronic kidney diseases (CKDs) (2, 11). Novel data suggest that CKD can develop in obese individuals without diabetes (5, 12, 26). The proposed pathological mechanisms and alterations in the kidney include lipotoxicity and renal lipid accumulation (1, 8, 20, 21, 27, 36), proinflammatory changes and cytokines (45), hyperfiltration and hypertension (15), and reactive oxygen species (ROS) (16, 34). Others suggest a role for glucose intolerance and insulin resistance as well, although the kidney is not traditionally considered an insulin-sensitive organ (10, 33, 44). The characteristic obesity-linked changes always include glomerular hypertrophy, fibrosis, and interstitial scarring (18). Oxidative stress, and/or mitochondrial dysfunction are often proposed mechanisms in various organs affected by obesity including the kidney but are incompletely understood. Numerous studies have focused on diabetic nephropathy, but sparse data are available on the metabolic syndrome stage when there are no overt signs of severe kidney dysfunction (23). Subcellular components such as mitochondria and the mitochondrial redox status have received broad attention lately because, even in physiological circumstances, mitochondrial electron transport and ROS production play key roles in redox homeostasis. In the case of an imbalance in lipid and carbohydrate metabolism, such as metabolic syndrome-related diseases, the overload on the tricarboxylic acid (TCA) cycle can result in the accumulation of irregular metabolites (24). These in turn will affect the ubiquinone (CoQ) pool and translate into excessive mitochondrial superoxide production (29) and can lead to derailments of mitochondria-driven redox signaling and altered cellular milieu in the disease process.
High-fat diet is an excellent model for excess calorie intake, which will contribute to the development of obesity and metabolic syndrome. Feeding a high-fat diet has also been shown to induce renal injury and morphological changes in the kidney (18, 43). Therefore, to achieve diet-induced obesity, we fed a 45% kcal lard-based high-fat diet to C57BL mice for 12 and 16 wk to investigate both functional and structural changes in the kidney at the cellular and subcellular levels. Here, we demonstrate that oxidative stress is evident in the kidney, as shown by 3-nitrotyrosine and protein radical adduct formation in the glomeruli on high-fat diet, possibly mediated by the parallel increase in inducible NO synthase (iNOS) and NOX-4 expression. Oxidative stress was accompanied by morphological changes and a proinflammatory transition in both the glomeruli and tubuli. The kidney mitochondria increased its H2O2-releasing capacity on various substrates without derangements of its bioenergetics or biogenesis, which suggests altered redox status and mitochondrial ROS production but preserved energy production. More importantly, the kidney initially developed an adaptation-like response in respiratory function in response to a high-fat diet and increased free fatty acid overload despite the evident cellular and subcellular oxidative stress.
MATERIALS AND METHODS
Materials.
All chemicals were from Sigma (St. Louis, MO) and of purest grade available unless stated otherwise. Antibodies were from various sources indicated in each section specifically. DMPO was from Axxora (San Diego, CA). Amplex Red, horseradish peroxidase (HRP), and Western blotting materials were from Invitrogen (Carlsbad, CA). Masson's TriChrome staining kit was purchased from Poly Scientific (Bay Shore, NY).
Animals.
Male C57BL mice weighing 18–20 g (Jackson Laboratory) were used in all experiments. Mice were housed in a room with air conditioning and a 12:12-h light-dark cycle, were fed with diets detailed below, and had access to water ad libitum. Experiments were started after the quarantine period was over. Mice were randomly divided into the following groups: control diet (10% kcal fat from lard, D12450B) and high-fat diet for 12 or 16 wk (45% kcal fat from lard, D12451). Diets were purchased from Research Diets (New Brunswick, NJ). Body weight gain was monitored weekly in every group throughout the experiments. Blood glucose levels were measured at the end of the feeding experiments. At the end of the feeding, all cohorts were divided into two subgroups. One subgroup received the spin trap DMPO (1 g/kg ip twice, 1 and 2 h before being euthanized); the other subgroup was euthanized without spin traps. After CO2 euthanasia, blood was drawn by heart puncture, kidneys were excised, and one from each animal was used to prepare fresh mitochondria or kept in −80°C until further experiments. The other kidney was halved. One half was kept in 10% buffered formalin for fixation; the other half was embedded in OCT medium for stainings and confocal studies. Kidneys from DMPO-treated animals were used only to study the sites of protein radical formation. All studies were approved by the Institutional Review Board at the Pennington Biomedical Research Center (PBRC) and adhered to NIH guidelines for the care and handling of experimental animals.
Clinical chemistry.
For blood sample collection, mice were fasted overnight with free access to water and then euthanized the next morning. Blood was collected via heart puncture before the dissection. Blood parameters such as plasma glucose, insulin, triglyceride, creatinine, and blood urea nitrogen (BUN) levels were measured by the Clinical Chemistry core facility at the PBRC.
Histology and immunohistochemistry.
For various stainings, OCT or paraffin-embedded kidneys from low-fat-fed and high-fat-fed mice were cut into 5-μm cross sections on a cryostat machine at the Pennington Imaging Core Facility. Sections were immediately mounted on charged SuperFrost slides (Fisher Scientific, Pittsburgh, PA), and the frozen sections were fixed with acetone and air-dried overnight. Sections were stained with 1) hematoxylin-eosin to evaluate general histology of the kidney, glomerular size, and hypertrophy; 2) TriChrome staining for fibrosis, scarring, and collagen; and 3) immunofluorescent stainings to detect fibroblast-specific protein-1 (FSP-1), 3-nitrotyrosine, protein radical adducts, and iNOS expression. 3-Nitrotyrosine (3-NT) staining was made with a monoclonal 3-NT antibody (Abcam, Cambridge, MA) according to the manufacturer's dilution instructions. FSP-1 staining was made using a rabbit FSP-1 antibody (Abcam). A monoclonal iNOS antibody was used for iNOS staining (Sigma). After washes, Alexa fluor 568 or 488 fluorescent rabbit or mouse secondary antibodies were applied for 1 h at room temperature (1:500 or 1:750, Invitrogen, Carlsbad, CA). After stainings, histology slides were scanned and analyzed with a NanoZoomer Digital Pathology Virtual Slide Viewer. Each slide was coded by the core facility, and low-fat- or high-fat-fed groups were revealed only after evaluation. Six mice in each group, two sections from each mouse, and 10–15 glomeruli on each section were analyzed. For fluorescent stainings, sections were covered with coverslips and observed with an LSM510 confocal laser microscope (Zeiss). In case of the glomeruli staining of 3-NT and iNOS, at least 15 glomeruli were recorded in each group, and grayscale intensities were compared using Image J software. For the FSP-1 staining, the area of positively stained pixels was compared and normalized as percentage of total pixels, comparing at least 15 pictures in each group.
Confocal microscopy of protein radicals.
To determine the amount and location of protein radical formation upon high-fat diet exposure, kidneys from DMPO-injected mice were fixed in 4% buffered formaldehyde for 24 h and then placed into 30% sucrose for 24 h. Tissues were then embedded into OCT medium, sliced on a cryocut microtome into 30-μm sections, and then immediately mounted on SuperFrost charged glass slides. Samples were permeabilized with 0.1% Surfact-Amps-X-100 for 30 min. After blocking with 1% goat serum for 30 min, stainings were performed on the slides. Kidney sections were isolated with a Pap Pen to prevent antibody loss, and a polyclonal chicken anti-DMPO antibody (1:1,500) was used to detect DMPO protein radical adducts in the kidneys. The noncommerical polyclonal chicken antibody was a generous gift from Dr. Ronald Mason's laboratory (National Institute of Environmental Health Sciences, Research Triangle Park, NC). After four washes with PBS, an Alexa fluor 488 donkey anti-chicken secondary antibody was used to visualize samples (1:500). Secondary controls were made to determine background and nonspecific fluorescence by omitting the primary antibody but applying the secondary antibody, as well as using a section from a non-DMPO-injected animal and applying both antibodies. After four rinses with PBS, sections were covered with coverslips and observed with an LSM510 confocal laser microscope (Zeiss). In case of glomeruli DMPO staining, at least 30 glomeruli were recorded and observed in each group, and grayscale intensities were compared using Image J software (http://rsb.info.nih.gov/ij/).
Mitochondria preparation.
Freshly excised kidneys were collected into a small amount of mitochondria isolation buffer (50 ml 0.1 M Tris-MOPS, 5 ml 0.1 M EGTA-Tris, 100 ml 1 M sucrose in 500 ml nanopure water, pH 7.4), chopped into small pieces with a scissors, and homogenized with a small Dounce homogenizer in isolation buffer. The homogenate was centrifuged first at 600 g, 4C for 10 min to remove debris, then the supernatant was centrifuged at 7,000 g, 4°C for 10 min twice to obtain a mitochondrial pellet. The preparation was resuspended in a small amount of isolation buffer, mitochondrial protein content was measured with a BCA protein assay kit (Pierce, Rockford, IL), and the preparation was used immediately for mitochondrial bioenergetic measurements in intact mitochondria or was stored at −80°C until further analysis.
Mitochondrial bioenergetics.
To assess mitochondrial function and bioenergetics in kidney mitochondria, we utilized the SeaHorse XF24 Extracellular Flux Analyzer (SeaHorse Bioscience, Billerica, MA) technology available at the PBRC. This technology allows us to determine a number of mitochondrial bioenergetic parameters in a single experiment, with a number of replicates for each mouse kidney used. In preliminary experiments, the optimal mitochondrial protein concentration was titrated to ensure an optimal and measurable OCR (oxygen consumption rate); 10, 5, and 2.5 μg/well mitochondria was loaded onto SeaHorse 24-well plates, and proportional responses were measured accordingly (data not shown). To ensure proper baseline readings recommended by the manufacturer, 2.5 μg/well kidney mitochondria was selected to load in all experiments. Accordingly, ADP, oligomycin, FCCP, and antimycin A concentrations were also optimized to obtain maximal effects and readings. We used sequential injections of these compounds to determine basal OCR, ATP-linked OCR, proton leak, nonmitochondrial OCR, and respiratory control ratio (RCR). Freshly prepared mitochondria were diluted to a concentration of 2.5 μg protein/well in MAS-1 buffer (70 mM sucrose, 220 mM mannitol, 10 mM KH2PO4, 5 mM MgCl2, 2 mM HEPES, 1 mM EGTA, and 0.2% BSA in nanopure water, pH 7.2, adjusted using KOH), loaded onto the 24-well SeaHorse plates, centrifuged, and incubated according to the manufacturer's protocol with rotenone-succinate as substrate. OCRs were measured loading both low-fat and high-fat diet-fed mouse kidney mitochondria on the same plate.
Western blotting of Cu/ZnSOD, NOX-4, MnSOD, mitochondrial complexes, biogenesis and cytochrome c.
For Western blot studies, equal amounts of kidney tissue or mitochondrial protein preparations were homogenized in lysis buffer containing a mixture of protease and phosphatase inhibitors, 2% Triton X-100, 300 mM NaCl, 20 mM Tris, 2 mM EDTA, and 1% NP-40. Samples were centrifuged at 10,000 rpm for 5 min at 4°C and were subject to 40 μl of SDS sample buffer including 2% β-mercaptoethanol as reducing agent and boiled for 10 min, and 20 μg of protein per well (equal loading was ensured by measuring protein content with a BCA reagent kit) was separated on reducing NuPAGE 4–12% Bis-Tris gel (Invitrogen) and transferred to a nitrocellulose membrane. In the mitochondrial complex studies, boiling was omitted according to recommendations of the antibody cocktail manufacturer (MitoSciences, Eugene, OR). After blocking (5% fish gelatin in 0.1 M PBS, pH 7.4), the membrane was probed with a mitochondrial complex antibody cocktail for complex Vα, COX I (complex IV), Complex III, Complex II, and Complex I expression. For cellular and mitochondrial oxidative stress enzymes, the membrane was probed with a goat polyclonal MnSOD antibody, goat polyclonal Cu/ZnSOD antibody (Santa Cruz Biotechnology, San Diego, CA), or rabbit polyclonal NOX-4 antibody (Novusbio, Littleton, CO). For biogenesis, PPARγ coactivator (PGC)-1α, PGC-1β, AMPK, and phospho-AMPK antibodies were used. For cytochrome c, mitochondrial and cytosolic fractions were separated as above, and the membranes were probed with a rabbit cytochrome c antibody (Cell Signaling, Danvers, MA). After washes, this was followed by the appropriate HRP-conjugated secondary antibody and ECL chemiluminescent substrate (Pierce, Rockford, IL). Western blot band intensities were quantified using the Image J program (download available at http://rsb.info.nih.gov/ij/).
Mitochondrial H2O2 release.
Mitochondrial H2O2 emission was measured by monitoring Amplex Red oxidation using a Molecular Devices M5 spectrofluorimeter with temperature control (excitation 560 nm, emission 590 nm). We prepared mitochondrial membranes by freeze-thawing them in liquid N2 three times as well as fresh mitochondria. The working solution contained 5 mM succinate or 75 μM palmitoyl-l-carnitine, 100 μM Amplex Red, 0.2 U/ml HRP and 5 mM ADP or 1 μg/ml oligomycin or 2 μM rotenone or the combination of these or 2 μM antimycin A. To initiate the assay, 50 μl of working solution was added to 50 μg of mitochondrial suspension (in MAS-1 buffer, equalized to the same volume) in 96-well microtiter plates. Measurements were run for 20–30 min with at least four samples in each group.
Statistical analysis.
Data are expressed as means ± SE. Statistical significance between groups was determined by ANOVA and Student's t-test as appropriate. P < 0.05 was considered the minimum level of statistical significance.
RESULTS
Body weights and clinical parameters.
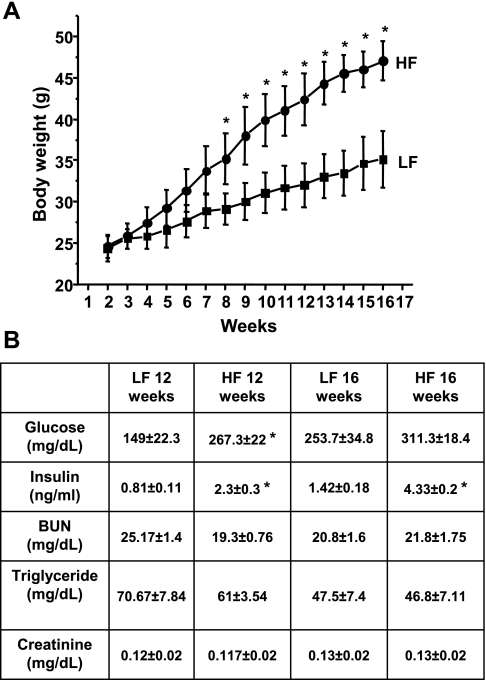
Feeding a 45% kcal lard high-fat diet to C57BL mice resulted in a significant increase in body weight from the 8th wk of the feeding period (Fig. 1A), accompanied by only minor changes in plasma glucose levels after 12 and 16 wk (Fig. 1B) but with pronounced hyperinsulinemia as expected. No significant changes were observed in creatinine or BUN levels at that time (Fig. 1B), indicating an early stage in the obese mice without severe functional kidney damage yet.
Fig. 1.
Body weight changes and clinical/kidney metabolic parameters in low- (LFD) and high-fat diet (HFD)-fed mice. A: representative graph of body weight changes in C57BL mice on 10% kcal LFD or 45% kcal HFD. B: typical clinical chemistry parameters of C57BL mice after 12 and 16 wk of LFD or HFD. Values are means ± SE; n = 12. *P < 0.05 vs. LFD fed.
High-fat diet induces glomerular hypertrophy, fibrosis and scarring in the kidney.
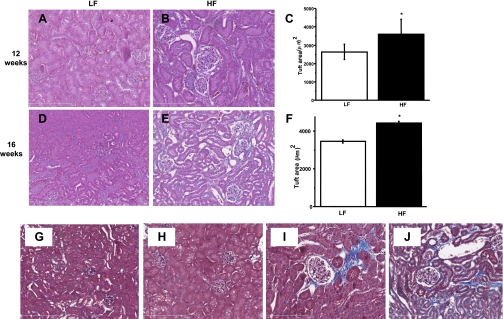
Representative histology pictures from kidneys of low-fat-fed and high-fat-fed mice are shown in Fig. 2, where hematoxylin-eosin staining was performed on renal sections after 12 and 16 wk of feeding. Kidneys taken from mice fed a normal low-fat diet had normal glomeruli with a constant tuft size at 12 or 16 wk, whereas in the high-fat-fed groups, glomerular size was already significantly increased at 12 wk and worsened by 16 wk (Fig. 2, A–F). TriChrome staining indicated several changes typical of progressive kidney pathology in high-fat diet-fed mice. Mesangial space expansion in the glomeruli, collagen deposits, fibrosis, interstitial scarring, and thickening basal membranes were abundant compared with the low-fat-fed cohorts (Fig. 2, G–J).
Fig. 2.
Glomerular hypertrophy and fibrosis, interstitial scarring, fibrosis, and mesangial space expansion in the HFD kidney. A–F: LFD-fed mice kidneys showed normal glomeruli structure at 12 and 16 wk (A and D), whereas kidneys in HFD-fed group showed gradually progressing increase in glomerular tuft size and Bowman's space expansion after 12 (B) and 16 wk (E). At least 30 glomeruli were measured in each group, data represent means ± SE; n = 30, *P < 0.05 vs. LFD. G: representative section of LFD-fed mouse kidney at ×100 with TriChrome staining, with normal glomeruli and mesangial cells at 12 wk. H: typical section of a 16-wk LFD-fed mouse kidney. I: representative of 12-wk HFD glomeruli with mesangial space expansion, fibrotic tissue, thickened membranes, and expanded Bowman's space stained in blue (×200). J: fibrosis, collagen, and interstitial scarring in a 16-wk HFD-fed mouse kidney (×200).
Indication of proinflammatory transformation and evidence of oxidative stress in the kidney: FSP-1, 3-NT, and protein radical formation on high-fat diet.
To determine whether proinflammatory changes and reactive oxygen and nitrogen species production accompany the development of changes in the kidney, we used confocal microscopy to localize FSP-1 positive staining and oxidative stress markers. FSP-1 is considered a hallmark of, and a good indication for, profibrotic renal changes and a possible epithelial-mesenchymal transition. FSP-1 staining was used as a marker of fibrosis related to transformation of tubular cells (32). We focused specifically on protein damage and protein nitration/oxidation because these changes can affect important enzymes and their function in the kidney cells and mitochondria. Protein nitration was determined using 3-NT staining, and protein radical formation was detected using a novel immunospin trapping technique. Here, the spin trap DMPO is given to animals and specifically reacts with radicals in vivo, forming stable adducts. These adducts can then undergo modifications that will make it impossible to detect them with conventional EPR spectroscopy. However, the anti-DMPO antiserum recognizes the protein radical-DMPO adducts in their EPR-silent form (6, 14, 38). This novel tool allows us to image protein radical formation for the first time in the kidney in both space and time.
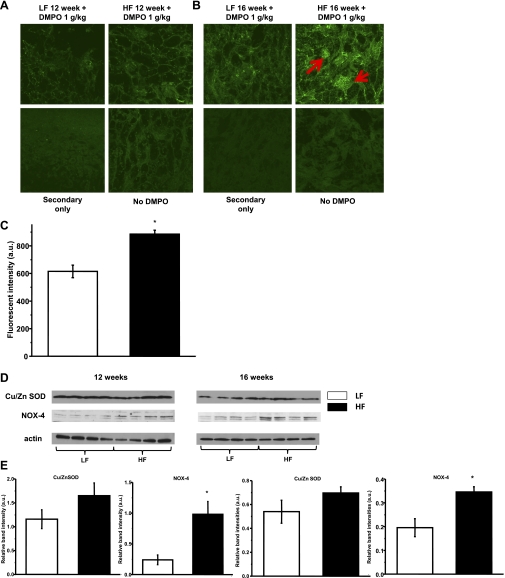
FSP-1 staining increased with time on the high-fat diet, localizing in the interstitial space, indicating progressive proinflammatory transition (Fig. 3, A and B). Significantly increased 3-NT staining was observed at both 12 and 16 wk in the high-fat diet-fed groups (Fig. 3, C and D); this was accompanied by iNOS expression, which peaked at 12 wk (Fig. 3, E and F). Only a small amount of DMPO staining was detected at 12 wk, but significant fluorescence indicating increased protein-DMPO adduct accumulation was observed at 16 wk. Similar to 3-NT, DMPO staining was located mainly in the glomeruli (Fig. 4, A–C). These results indicate that high-fat diet induces nitration and protein damage partially through protein radical formation in the kidney over time. We found increased iNOS and NOX-4 expression at 12 and 16 wk (Fig. 4. D and E) as possible sources further contributing to these cellular oxidative stress alterations. The expression of Cu/ZnSOD was not changed at these timepoints (Fig. 4, D and E).
Fig. 3.
Proinflammatory signs in the kidney from HFD mice. A: fibroblast-specific protein-1 (FSP-1) expression and accumulation over time in mouse kidneys after 12 and 16 wk of HFD feeding (green fluorescent interstitial staining). B: staining was evaluated as the positively green-stained percentage of total pixels in each group. Values are means ± SE; n = 12 pictures evaluated in each group. *P < 0.05 vs. LFD group. C: positive and progressing 3-nitrotyrosine (3-NT) staining in kidney glomeruli (red fluorescence mainly in glomeruli). D: evaluation of staining was measured as fluorescent intensity in the glomeruli. Values are means ± SE; n = 15–20 glomeruli per each group. *P < 0.05 vs. LFD group. E: representative iNOS staining in LFD and HFD kidney sections at both time points showing iNOS in the glomeruli. F: evaluation of staining was measured as fluorescent intensity in the glomeruli. Values are means ± SE; n = 15–20 glomeruli per each group, *P < 0.05 vs. LFD group.
Fig. 4.
Cellular oxidative stress and protein radical formation progress over time on HFD feeding in the kidney. Animals were injected with the spin trap DMPO (1 g/kg, twice) before being euthanized, and then a DMPO-protein radical adduct-specific anti-DMPO antibody was applied for staining. A: minimal green staining can be observed in kidney sections after 12 wk of HFD feeding. B: protein radical formation was observed as positive green staining mainly in glomeruli after 16 wk of HFD feeding (red arrows). Kidney sections from non-DMPO-treated animals and omitting primary antibody on sections served as negative controls. C: evaluation of staining was measured as fluorescent intensity in glomeruli of the 16-wk group. Data show means ± SE; n = 15–20 glomeruli per each group. *P < 0.05 vs. LFD group. D: representative Western blots of Cu/ZnSOD and NOX-4 protein levels in LFD and HFD groups showing no change in Cu/ZnSOD levels, but a robust increase in NOX-4 levels on HFD. E: Western blot band intensities were evaluated in each group (n = 4). Data are expressed as means ± SE. *P < 0.05 vs. LFD group.
High-fat diet induces defense mechanisms and increased H2O2 emission in kidney mitochondria.
To determine whether the high-fat diet compromised mitochondrial biogenesis and whether there was any indication of mitochondrial oxidative stress or dysfunction, we next investigated the expression of mitochondrial complexes, the level of the protective mitochondrial superoxide dismutase (MnSOD), and the degree of H2O2 release as an indication of mitochondrial ROS production. We speculated that, since we observed protein radical formation and nitration, mitochondria could be a significant source of oxidative stress, which is common in obesity-related syndromes.
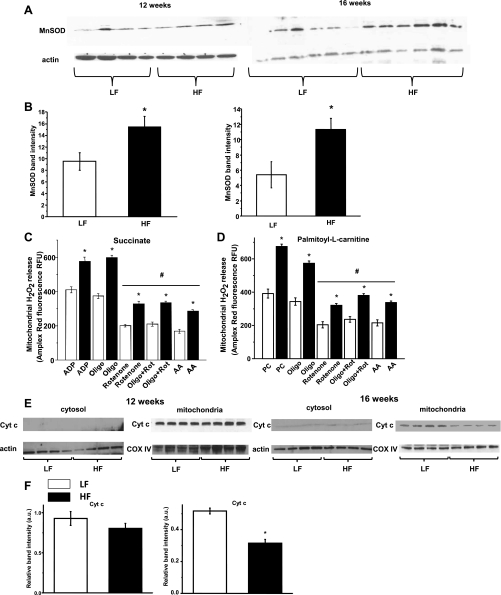
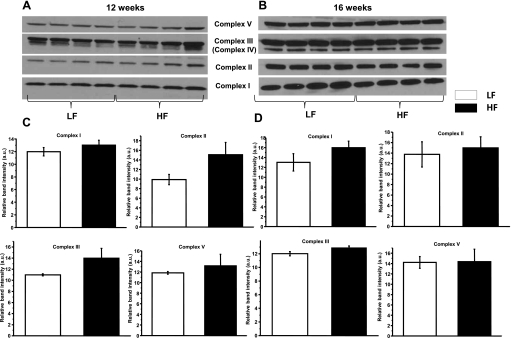
MnSOD expression levels were increased on the high-fat diet. This increase was modest but significant at 12 wk and further increased at 16 wk. Elevated MnSOD expression is possibly a compensatory response to increased ROS leaking and could protect the respiratory chain complexes in the kidney (Fig. 5, A and B). In in vitro studies, overexpression of MnSOD was shown to be protective in renal proximal tubular cells exposed to hyperglycemia (30). Consistent with the increased MnSOD expression, mitochondria from the high-fat diet groups were emitting significantly higher amounts of H2O2. The production of H2O2 was measured and compared using both carbohydrate- and lipid-based substrates (supported by succinate or palmitoyl-l-carnitine, respectively) (4, 37). This H2O2 production was significantly decreased by inhibiting Complex I with rotenone or applying antimycin A on both substrates. The result suggests a reverse electron flow to Complex I, which is generally considered the main site of superoxide leakage (Fig. 5, C and D). Decreased mitochondrial cytochrome c was also observed at 16 wk, but leaking to the cytosol fraction was either insignificant or inconsistent to detect at these time points. Mitochondrial biogenesis and the expression of the respiratory complexes were intact, indicating no overall reduction in levels of mitochondrial protein content (Fig. 6, A–D).
Fig. 5.
Mitochondrial redox status changes and oxidant production upon HFD feeding in obese mouse kidneys. A: representative Western blots show slightly increased MnSOD expression in the 12-wk feeding group, which was further increased at 16 wk. B: Western blot band intensities were evaluated in each group (n = 4–5), and experiments were repeated 3 times. Data are expressed as means ± SE. *P < 0.05 vs. LFD group. C: HFD increased kidney mitochondrial H2O2 emission at 12 wk time point on ADP or in state IV respiration vs. LFD group, using succinate or palmitoyl-l-carnitine (D) as substrates. Inhibition of complex I significantly reduced H2O2 emitting capacity. Rot, rotenone; PC, palmitoyl-l-carnitine; AA, antimycin A. Data represent means ± SE; n = 5 in duplicated experiments. *P < 0.05 vs. LFD group; #P < 0.05 vs. corresponding state IV respiration groups. E: representative Western blot analyses of cytochrome c levels in cytosolic and mitochondrial fractions from LFD and HFD kidney samples. F: band intensities were evaluated in the mitochondrial fraction as above (n = 4). Data are expressed as means ± SE. *P < 0.05 vs. LFD group.
Fig. 6.
Mitochondrial biogenesis and protein expression levels of respiratory chain complexes. A and B: representative Western blots of respiratory chain complex proteins after 12 and 16 wk of feeding. C and D: evaluation of Western blot band intensities revealed intact biogenesis of the electron transport chain (n = 4, triplicate experiments). Data are shown as means ± SE. *P < 0.05 vs. LFD group.
High-fat diet induces an initial adaptation in mitochondrial bioenergetics in the kidney with preserved biogenesis despite oxidative stress.
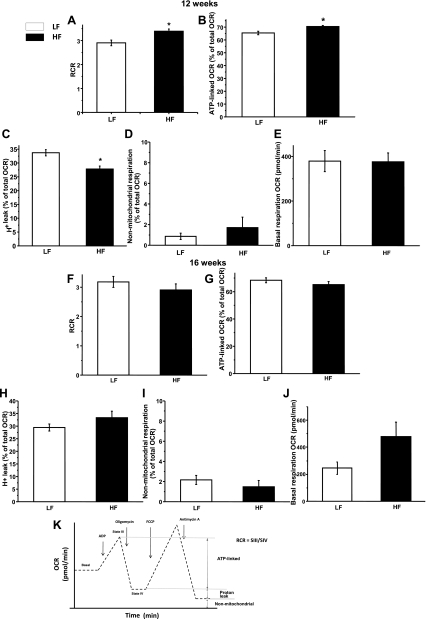
To further study and assess the impact of lard-based high-fat diet feeding on kidney mitochondria function and bioenergetics, we used a SeaHorse analyzer to measure several parameters of mitochondrial respiration. This approach allowed us to simultaneously compare kidney mitochondria from low- and high-fat diet-fed mice, minimizing variation from isolation or preparation. Interestingly, rather than showing a decay in mitochondrial bioenergetics, kidney mitochondria of the 12-wk group repeatedly showed a steadily maintained bioenergetics and, moreover, a modest but significant adaptation to the increased lipid load (Fig. 7). Both RCR, which is a commonly used parameter of mitochondrial function, and ATP-linked OCR were significantly higher in the high-fat diet-fed group mitochondria, suggesting an adaptive-type response in the kidney (Fig. 7, A and B). Note that kidney is one of the organs with high ATP needs; therefore, this response may be important to maintain its ATP homeostasis. Furthermore, the 12-wk high-fat kidney mitochondria demonstrated significantly reduced proton leak percentages (Fig. 7C), which, together with the increased ATP-linked respiration, translates to improved efficiency. No significant changes were observed in succinate-based basal respiration or after antimycin A addition in nonoxidative phosphorylation-linked respiration, which includes any other nonmitochondrial oxygen consumption (Fig. 7, D and E). In the 16-wk feeding group, the adaptation was no longer apparent, but the high-fat group kidney mitochondria maintained normal respiratory parameters compared with the low-fat-fed group in RCR and ATP production (Fig. 7, F and G). We observed a slightly elevated but not statistically significant proton leak and higher basal respiration. These parameters are indicative of some degree of uncoupling (Fig. 7, H and J). There was no change in the nonmitochondrial respiration rates (Fig. 7I). These findings suggest that the kidney develops an initial increase in mitochondrial bioenergetics as a response to HFD feeding, which slowly diminished with the progession of obesity but was still maintained at normal levels at 16 wk.
Fig. 7.
Mitochondrial bioenergetic adaptation to HFD feeding in isolated kidney mitochondria. Experiments were run on a SeaHorse XF24 extracellular flux analyzer to obtain bioenergetic profiles from LFD- and HFD-fed groups at 12 and 16 wk. OCR, oxygen consumption rate. A–E: typical bioenergetic parameters in the 12-wk feeding group. Some parameters showed improvement as an adaptation. A: respiratory control ratio (RCR). B: ATP-linked respiration. C: proton leak. D: nonmitochondrial respiration. E: basal respiration rates. F–J: same as A–E in the 16-wk feeding group. K: philosophy of the SeaHorse extracellular flux analyzer machine and interpretation of the various parameters. Data represent means ± SE for 2 independent cohorts (n = 8 per group). *P < 0.05 vs. LFD group.
DISCUSSION
High-fat diet and obesity can lead to chronic kidney disease with or without developing diabetes (5, 11). There is accumulating evidence showing the role of altered lipogenesis and lipotoxicity in this process (1, 8, 27) and how the kidney changes morphologically as well as functionally (18, 43). Although several studies have focused on diabetic nephropathy as a complication, sparse data are available on precise oxidative stress markers and, moreover, on the possible role and relation of mitochondrial derailments (mitochondrial oxidative stress, biogenesis, and bioenergetics) to the early stage of the kidney on high dietary fat intake.
Here, we demonstrate evidence for oxidative stress through mitochondrial ROS production and protein radical formation/nitration, which develops in parallel with fibrotic and proinflammatory changes in the kidney on high-fat diet while the kidney maintains normal mitochondrial biogenesis. Furthermore, we suggest the implication of a novel paradigm similar to that of skeletal muscle to address how the kidney develops an initial increase in bioenergetics despite the early signs of oxidative stress.
Studies by Sansom et al. determined a time course on high-fat diet when the kidney glomeruli start to show morphological changes (43). On the basis of those studies, we started looking at morphological alterations, fibrosis, and oxidative stress as well as proinflammatory markers at 12 and 16 wk of high-fat diet feeding. At this stage, C57BL mice do not develop diabetes but are significantly more obese, in a state with no signs of severe kidney function damage (Fig. 1). Glomerular hypertrophy was apparent, as well as various obesity-related morphological changes, with pronounced interstitial scarring and fibrosis, which are common signs of progressing renal disease. Scarring and fibrosis are linked to numerous factors such as growth factors, cytokines, proinflammation, and oxidative stress. Since obesity is often associated with a proinflammatory stage in tissues, it is important to link this fibrosis to a marker of low-grade inflammation in the kidney. FSP-1 expression is an indication for such alteration, where tubular epithelial cells migrate to the interstitial space and change their phenotype, also expressing FSP-1 (19, 22, 32). In agreement with the presence of fibrotic/collagen tissue, FSP-1 staining was prominent in the interstitial space, confirming a possible link between high-fat diet and proinflammation in the kidney that was worsening in time.
Oxidative stress as a rather broad and vague definition is also often mentioned and linked to pathological kidney alterations. We looked for specific markers of protein damage in these studies to achieve a more precise picture and elucidate whether the accumulation of these oxidized and nitrated proteins is induced by high-fat diet and correlates with the scarring and glomeruli damage. The novel immunospin-trapping method allowed us to identify and localize protein radical formation in the kidney, which was preceded by nitrotyrosine formation starting at 12 wk (Figs. 3 and 4). The time course accumulation of FSP-1 and nitrotyrosine together with subsequent protein radical formation is a novel observation that indicates a transformation in the high-fat diet-fed kidney, which involves epithelial-mesenchymal transition as well as accumulation of oxidized and damaged proteins, which may have an impact on the function of the kidney over time. Our data also indicate that iNOS and NOX-4 can be important cellular enzymatic sources of radicals upon high-fat diet feeding, allowing the possibility for superoxide anion and NO to form simultaneously. This pathway very likely contributes to the nitration process, as shown in Fig. 3C, through the formation of peroxynitrite or other similar radical mechanisms. It is important to note that, although nitrotyrosine and protein adduct accumulation were observed mainly in glomerular structures, the FSP-1 staining pattern refers to the tubular epithelium and the interstitial space where those FSP-1-positive epithelial cells may migrate on transformation. The oxidative and profibrotic damage happens in parallel in these structures, influencing both glomerular function and tubular integrity at the same time with the progression of the disease.
To further dissect a contribution from mitochondrial oxidative stress in these processes, as often happens in diet-induced obesity, we looked for mitochondrial defense mechanisms as well as H2O2 release as an indication of mitochondrial ROS production. In the H2O2 emission experiments, we applied mitochondrial membrane preparations to overcome any possible interference from mitochondrial defense mechanisms (42). Also, applying Amplex Red has some considerations. For example, GSH or NADH can interfere with the assay; this way the risk of potential false readings was minimized. In some experiments, as a comparison, we also used fresh mitochondria to confirm these results, where we found no difference from those obtained from freeze-thawed preparations. Note that succinate is not the most physiologically accurate substrate, but mitochondrial membrane preparations will not consume glutamate or malate. Succinate represents a carbohydrate-based substrate; therefore, to compare this approach with a fatty acid substrate-supported scenario, we also used palmitoyl-l-carnitine. Compared with succinate, using palmitoyl-l-carnitine as a substrate resulted in higher H2O2 production when applied alone but not in state IV respiration or in the inhibition experiments. While high-fat diet-induced MnSOD overexpression and H2O2 release from the mitochondria, which was inhibitable at the level of Complex I (Fig. 5, C and D), suggesting some redox disturbances, interestingly, biogenesis and the amount of mitochondrial complex proteins were intact. Cytochrome c was only leaking to the cytosol in a negligible amount at 16 wk or was repeatedly inconsistent to detect (possibly due to large variations in the high-fat diet-fed mice after those feeding time points), but the protein was decreased in the mitochondria. As cytochrome c is important in passing electrons from Complex III to Complex IV, decreased levels can further contribute to a reverse electron flow and enhanced H2O2 release. Consistently, previous data on much earlier timepoints in high-fat diet-fed mice indicates an initial leakage of cytochrome c to the cytosol in the kidney (23), while downregulation of important genes related to mitochondrial function including cytochrome c was observed by others in skeletal muscle as well (35). When we looked at factors controlling biogenesis and oxidative phosphorylation such as PGC-1α, PGC-1β, and AMPK/phospho-AMPK, their expression was also unchanged (data not shown).
More importantly, on high-fat diet the kidney initially developed an adaptation-like response in our experiments, increasing its bioenergetic capacity and its H2O2 emission from mitochondria on both carbohydrate- and fatty acid-based substrates, shifting its redox state, which is probably also an initial adaptive response to the free fatty acid overload exceeding metabolic demand. This behavior seemed to follow a cycle in the kidney, as we could only observe this repeatedly with different cohorts in the 12 wk group, and by the 16th week this initial response was not detectable anymore. Regardless, the kidney had no derailments in its bioenergetic balance (Fig. 7). There is mounting evidence indicating that, in case of a metabolic disturbance (out-of-balance metabolic state) like high-fat diet feeding, substrate oversupply to the TCA can have a large impact on function. Novel data suggest also an adaptive “shift” in skeletal muscle (3) as well as an initial increase in bioenergetics or biogenesis (7, 17, 40) as a response to this oversupply, but a similar concept has never been proposed in the kidney. When, due to this free fatty acid overburdening, various new metabolites appear, they can significantly alter the rate of superoxide/H2O2 production through changing the redox state of Complex I and CoQ pool (29). This is often referred to as derailed handling of lipids in the mitochondria (25, 31). The increasing MnSOD expression over time was certainly the first indication that it might be a response to increased ROS production in the mitochondria, dismutating superoxide to H2O2. In vitro evidence in renal cell cultures supports that overexpressing MnSOD is protective in hyperglycemia as well as supressing collagen deposition (13, 30). This is intriguing, as herein we show that in an in vivo high-fat diet feeding model the kidney has elevated levels of MnSOD. This will presumably reduce mitochondrial superoxide levels, but on the other hand may lead to H2O2 accumulation, depending on other cellular defense mechanisms such as catalase or peroxidases. Indeed, kidney mitochondria from high-fat diet-fed mice had increased H2O2 release. This was significantly attenuated by rotenone, which acts on Complex I, inhibiting the production of radicals derived from a reverse electron flow from complex II, or antimycin A, which can also decrease H2O2 release from reverse flow by decreasing membrane potential (39). Complex I is a well-known site of ROS production in the mitochondria.
Kidney and skeletal muscle hold some common features in that they are both organs with high metabolic needs and their function relies heavily on ATP availability. We believe that, similarly to skeletal muscle, an adaptation process may occur in the kidney on high-fat diet to maintain bioenergetic needs regardless of the indications of early oxidative stress, including mitochondrial ROS production, protein radical formation, and activation of iNOS and NOX-4 and their contribution to modulating nitration mechanisms. Our data suggest that these oxidative changes and protein damage develop in the kidney in time, partially through nitration and protein radical formation, as was demonstrated by a novel immunospin trapping tool. This was accompanied by morphological changes and transformation of tubular epithelial cells, indicating proinflammation, while the kidney maintained normal mitochondrial bioenergetics and, moreover, developed an initial adaptation to the increased lipid load by increasing H2O2 emission and overexpressing MnSOD. Therefore, these initial changes, but not mitochondrial bioenergetic dysfunction, may be contributing to the early pathological changes and altered cellular milieu in the kidney. The bioenergetic adaptation is particularly puzzling and warrants further investigation, as it may reveal important details about how cellular metabolism is altered in the kidney on nutrition overload to maintain ATP needs. This adaptation results from feeding a high-fat diet but may not be linked to adiposity per se, as the degree of obesity between 12- and 16-wk feeding is not a drastic change. One alternative could be an initial increase in mitochondrial biogenesis; however, at this time we have not observed an increase in either AMPK phosphorylation or PGC-1α or -β levels. Therefore, we suggest that, to some extent, the data indicate that, as obesity progresses, cellular and subcellular oxidative stress will precede mitochondrial energy imbalance, which may decline later in time. Lower levels of cytochrome c could also contribute to increased reverse electron flow and mitochondrial oxidant emission. Clearly, the interesting phenomenon of adaptation requires further studies. Taken together, our findings herein provide novel important insights on the effects of a high-fat dietary intake in the kidney.
One question that arises from our data is the possible application of mitochondrial targeted small peptide antioxidants or other targeted approaches to modulate either H2O2 emission or enzymes in the TCA cycle. This approach would further explore the adaptation mechanism and the possible links between oxidative stress and morphological changes outside the mitochondria. These interventions lately showed very promising results in skeletal muscle (3) as well as type 1 diabetic nephropathy (9). If a targeted approach prevents increased ROS production in the mitochondrial electron transport chain in high-fat diet and subsequently decreases proinflammatory changes and oxidative stress in the kidney, it will provide more precise details to understand an obesity-induced condition in this organ. Similarly, modulating enzymes related to β-oxidation and the TCA cycle could explore novel pathways linking TCA disturbances and consequent adaptation mechanisms in the kidney (28, 41). This will certainly translate into clinical relevancy and better design of therapeutic approaches specifically targeting the kidney, to prevent the progression of metabolic syndrome-related damage and chronic kidney disease.
GRANTS
Research in this laboratory is supported partially by NIH (DK-083615) to K. Stadler and the Pennington Foundation and by a NORC P & F grant (2P30DK072476). The work utilized the facilities of the Cell Biology and Bioimaging Core, which are supported in part by COBRE (NIH P20 RR-021945) and CNRU (NIH 1P30 DK-072476) center grants from the NIH.
DISCLOSURES
No conflicts of interest are reported by the authors.
ACKNOWLEDGMENTS
We thank Drs. Rea-Annunciado Koza, David Burk, Barry Robert, and Vishwa Deep Dixit for helpful discussions and Katie Bailey for careful proofreading of the manuscript.
REFERENCES
- 1. Abrass CK. Cellular lipid metabolism and the role of lipids in progressive renal disease. Am J Nephrol 24: 46–53, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Abrass CK. Overview: obesity: what does it have to do with kidney disease? J Am Soc Nephrol 15: 2768–2772, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Anderson EJ, Lustig ME, Boyle KE, Woodlief TL, Kane DA, Lin CT, Price JW, 3rd, Kang L, Rabinovitch PS, Szeto HH, Houmard JA, Cortright RN, Wasserman DH, Neufer PD. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Anderson EJ, Yamazaki H, Neufer PD. Induction of endogenous uncoupling protein 3 suppresses mitochondrial oxidant emission during fatty acid-supported respiration. J Biol Chem 282: 31257–31266, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Bagby SP. Obesity-initiated metabolic syndrome and the kidney: a recipe for chronic kidney disease? J Am Soc Nephrol 15: 2775–2791, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Bonini MG, Siraki AG, Atanassov BS, Mason RP. Immunolocalization of hypochlorite-induced, catalase-bound free radical formation in mouse hepatocytes. Free Radic Biol Med 42: 530–540, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bonnard C, Durand A, Peyrol S, Chanseaume E, Chauvin MA, Morio B, Vidal H, Rieusset J. Mitochondrial dysfunction results from oxidative stress in the skeletal muscle of diet-induced insulin-resistant mice. J Clin Invest 118: 789–800, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bonnet F, Cooper ME. Potential influence of lipids in diabetic nephropathy: insights from experimental data and clinical studies. Diabetes Metab 26: 254–264, 2000 [PubMed] [Google Scholar]
- 9. Chacko B, Reily C, Srivastava A, Johnson MS, Ulasova E, Agarwal A, Zinn K, Murphy MP, Kalyanaraman B, Darley-Usmar V. Prevention of diabetic nephropathy in Ins2+/−AkitaJ mice by the mitochondria-targeted therapy Mito Q. Biochem J 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen J, Muntner P, Hamm LL, Fonseca V, Batuman V, Whelton PK, He J. Insulin resistance and risk of chronic kidney disease in nondiabetic US adults. J Am Soc Nephrol 14: 469–477, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Chen J, Muntner P, Hamm LL, Jones DW, Batuman V, Fonseca V, Whelton PK, He J. The metabolic syndrome and chronic kidney disease in U. S adults Ann Intern Med 140: 167–174, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS. Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Craven PA, Phillips SL, Melhem MF, Liachenko J, DeRubertis FR. Overexpression of manganese superoxide dismutase suppresses increases in collagen accumulation induced by culture of mesangial cells in high-media glucose. Metabolism 50: 1043–1048, 2001 [DOI] [PubMed] [Google Scholar]
- 14. Detweiler CD, Deterding LJ, Tomer KB, Chignell CF, Germolec D, Mason RP. Immunological identification of the heart myoglobin radical formed by hydrogen peroxide. Free Radic Biol Med 33: 364–369, 2002 [DOI] [PubMed] [Google Scholar]
- 15. El-Atat FA, Stas SN, McFarlane SI, Sowers JR. The relationship between hyperinsulinemia, hypertension and progressive renal disease. J Am Soc Nephrol 15: 2816–2827, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Forbes JM, Coughlan MT, Cooper ME. Oxidative stress as a major culprit in kidney disease in diabetes. Diabetes 57: 1446–1454, 2008 [DOI] [PubMed] [Google Scholar]
- 17. Hancock CR, Han DH, Chen M, Terada S, Yasuda T, Wright DC, Holloszy JO. High-fat diets cause insulin resistance despite an increase in muscle mitochondria. Proc Natl Acad Sci USA 105: 7815–7820, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Henegar JR, Bigler SA, Henegar LK, Tyagi SC, Hall JE. Functional and structural changes in the kidney in the early stages of obesity. J Am Soc Nephrol 12: 1211–1217, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest 110: 341–350, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jiang T, Liebman SE, Lucia MS, Li J, Levi M. Role of altered renal lipid metabolism and the sterol regulatory element binding proteins in the pathogenesis of age-related renal disease. Kidney Int 68: 2608–2620, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Jiang T, Wang Z, Proctor G, Moskowitz S, Liebman SE, Rogers T, Lucia MS, Li J, Levi M. Diet-induced obesity in C57BL/6J mice causes increased renal lipid accumulation and glomerulosclerosis via a sterol regulatory element-binding protein-1c-dependent pathway. J Biol Chem 280: 32317–32325, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest 112: 1776–1784, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kartha GK, Moshal KS, Sen U, Joshua IG, Tyagi N, Steed MM, Tyagi SC. Renal mitochondrial damage and protein modification in type-2 diabetes. Acta Diabetol 45: 75–81, 2008 [DOI] [PubMed] [Google Scholar]
- 24. Koves TR, Li P, An J, Akimoto T, Slentz D, Ilkayeva O, Dohm GL, Yan Z, Newgard CB, Muoio DM. Peroxisome proliferator-activated receptor-gamma co-activator 1alpha-mediated metabolic remodeling of skeletal myocytes mimics exercise training and reverses lipid-induced mitochondrial inefficiency. J Biol Chem 280: 33588–33598, 2005 [DOI] [PubMed] [Google Scholar]
- 25. Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, Bain J, Stevens R, Dyck JR, Newgard CB, Lopaschuk GD, Muoio DM. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab 7: 45–56, 2008 [DOI] [PubMed] [Google Scholar]
- 26. Kramer H, Luke A, Bidani A, Cao G, Cooper R, McGee D. Obesity and prevalent and incident CKD: the Hypertension Detection and Follow-Up Program. Am J Kidney Dis 46: 587–594, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Kume S, Uzu T, Araki S, Sugimoto T, Isshiki K, Chin-Kanasaki M, Sakaguchi M, Kubota N, Terauchi Y, Kadowaki T, Haneda M, Kashiwagi A, Koya D. Role of altered renal lipid metabolism in the development of renal injury induced by a high-fat diet. J Am Soc Nephrol 18: 2715–2723, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Li LO, Ellis JM, Paich HA, Wang S, Gong N, Altshuller G, Thresher RJ, Koves TR, Watkins SM, Muoio DM, Cline GW, Shulman GI, Coleman RA. Liver-specific loss of long chain acyl-CoA synthetase-1 decreases triacylglycerol synthesis and beta-oxidation and alters phospholipid fatty acid composition. J Biol Chem 284: 27816–27826, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu SS. Cooperation of a “reactive oxygen cycle” with the Q cycle and the proton cycle in the respiratory chain—superoxide generating and cycling mechanisms in mitochondria. J Bioenerg Biomembr 31: 367–376, 1999 [DOI] [PubMed] [Google Scholar]
- 30. Munusamy S, MacMillan-Crow LA. Mitochondrial superoxide plays a crucial role in the development of mitochondrial dysfunction during high glucose exposure in rat renal proximal tubular cells. Free Radic Biol Med 46: 1149–1157, 2009 [DOI] [PubMed] [Google Scholar]
- 31. Muoio DM, Newgard CB. Obesity-related derangements in metabolic regulation. Annu Rev Biochem 75: 367–401, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Rossini M, Cheunsuchon B, Donnert E, Ma LJ, Thomas JW, Neilson EG, Fogo AB. Immunolocalization of fibroblast growth factor-1 (FGF-1), its receptor (FGFR-1), and fibroblast-specific protein-1 (FSP-1) in inflammatory renal disease. Kidney Int 68: 2621–2628, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Sarafidis PA, Ruilope LM. Insulin resistance, hyperinsulinemia, and renal injury: mechanisms and implications. Am J Nephrol 26: 232–244, 2006 [DOI] [PubMed] [Google Scholar]
- 34. Shah SV, Baliga R, Rajapurkar M, Fonseca VA. Oxidants in chronic kidney disease. J Am Soc Nephrol 18: 16–28, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Sparks LM, Xie H, Koza RA, Mynatt R, Hulver MW, Bray GA, Smith SR. A high-fat diet coordinately downregulates genes required for mitochondrial oxidative phosphorylation in skeletal muscle. Diabetes 54: 1926–1933, 2005 [DOI] [PubMed] [Google Scholar]
- 36. Spencer MW, Muhlfeld AS, Segerer S, Hudkins KL, Kirk E, LeBoeuf RC, Alpers CE. Hyperglycemia and hyperlipidemia act synergistically to induce renal disease in LDL receptor-deficient BALB mice. Am J Nephrol 24: 20–31, 2004 [DOI] [PubMed] [Google Scholar]
- 37. St-Pierre J, Buckingham JA, Roebuck SJ, Brand MD. Topology of superoxide production from different sites in the mitochondrial electron transport chain. J Biol Chem 277: 44784–44790, 2002 [DOI] [PubMed] [Google Scholar]
- 38. Stadler K, Bonini MG, Dallas S, Duma D, Mason RP, Kadiiska MB. Direct evidence of iNOS-mediated in vivo free radical production and protein oxidation in acetone-induced ketosis. Am J Physiol Endocrinol Metab 295: E456–E462, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tahara EB, Navarete FD, Kowaltowski AJ. Tissue-, substrate-, and site-specific characteristics of mitochondrial reactive oxygen species generation. Free Radic Biol Med 46: 1283–1297, 2009 [DOI] [PubMed] [Google Scholar]
- 40. Turner N, Bruce CR, Beale SM, Hoehn KL, So T, Rolph MS, Cooney GJ. Excess lipid availability increases mitochondrial fatty acid oxidative capacity in muscle: evidence against a role for reduced fatty acid oxidation in lipid-induced insulin resistance in rodents. Diabetes 56: 2085–2092, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Ussher JR, Koves TR, Jaswal JS, Zhang L, Ilkayeva O, Dyck JR, Muoio DM, Lopaschuk GD. Insulin-stimulated cardiac glucose oxidation is increased in high-fat diet-induced obese mice lacking malonyl CoA decarboxylase. Diabetes 58: 1766–1775, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. von Bergen NH, Koppenhafer SL, Spitz DR, Volk KA, Patel SS, Roghair RD, Lamb FS, Segar JL, Scholz TD. Fetal programming alters reactive oxygen species production in sheep cardiac mitochondria. Clin Sci (Lond) 116: 659–668, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wei P, Lane PH, Lane JT, Padanilam BJ, Sansom SC. Glomerular structural and functional changes in a high-fat diet mouse model of early-stage Type 2 diabetes. Diabetologia 47: 1541–1549, 2004 [DOI] [PubMed] [Google Scholar]
- 44. Whaley-Connell A, Pavey BS, Afroze A, Bakris GL. Obesity and insulin resistance as risk factors for chronic kidney disease. J Cardiometab Syndr 1: 209–216, 2006 [DOI] [PubMed] [Google Scholar]
- 45. Wisse BE. The inflammatory syndrome: the role of adipose tissue cytokines in metabolic disorders linked to obesity. J Am Soc Nephrol 15: 2792–2800, 2004 [DOI] [PubMed] [Google Scholar]