Abstract
Endothelial progenitor cells (EPCs) play an essential role in angiogenesis but are functionally impaired in diabetes. We recently reported that decreased expression of manganese superoxide dismutase (MnSOD) critically contributes to diabetic EPC dysfunction. AMP-activated protein kinase (AMPK) activation has been shown to induce MnSOD and suppress hyperglycemia-induced mitochondrial ROS production in endothelial cells. However, whether AMPK protects EPCs from oxidative stress in diabetes is unknown. We tested the hypothesis that AMPK activation rescues impaired EPC functions through MnSOD induction in type 1 diabetes. Bone marrow-derived EPCs from adult male streptozotocin-induced diabetic mice and normal controls were used. AMPK activity was decreased in diabetic EPCs, indicated by reduced AMPK and acetyl-CoA carboxylase phosphorylation. AMPK activation by treating diabetic EPCs with its selective agonist AICAR rescued their in vitro functions, including Matrigel tube formation, adhesion, and migration. Furthermore, AICAR restored the decreased MnSOD protein and enzymatic activity and suppressed the mitochondrial superoxide level in diabetic EPCs, indicated by MitoSOX flow cytometry. These beneficial effects of AICAR on MnSOD and EPC functions were significantly attenuated by silencing MnSOD or AMPK antagonist compound C pretreatment. Finally, the expression of protein phosphatase 2A, a key enzyme for AMPK dephosphorylation and inactivation, was increased in diabetic EPCs, and its inhibition by siRNA or okadaic acid reversed the deficient AMPK activation and MnSOD level in diabetic EPCs. These findings demonstrate for the first time that AMPK activation rescues impaired EPC functions and suppresses mitochondrial superoxide by inducing MnSOD in type 1 diabetes.
Keywords: protein phosphatase 2A, angiogenesis, oxidase stress
bone marrow-derived endothelial progenitor cells (EPCs) express stem cell surface antigens and endothelial markers (32) and are able to migrate to the peripheral circulation (15). After homing to the site of ischemic injury, EPCs function to maintain vascular homeostasis and promote postnatal neovascularization (6, 15). In patients with type 1 or type 2 diabetes, EPCs are impaired in both their number and function (8, 9, 21), which contributes to the pathogenesis of vascular complications in diabetes, as manifested by impaired wound healing and reduced collateral formation in ischemia. However, the mechanisms underlying EPC dysfunction in diabetes are incompletely understood.
Normal EPCs are resistant to oxidative stress, because of their high intrinsic expression of antioxidant enzyme manganese superoxide dismutase (MnSOD) (5, 14). In diabetes, excessive oxidative stress impairs EPC functions (3, 36). We recently reported that decreased expression of MnSOD critically contributes to impaired EPC functions in type 2 diabetes (24). AMP-activated protein kinase (AMPK) is a serine/threonine protein kinase involved in the regulation of cellular and organismal metabolism. AMPK acts as a new regulator of angiogenesis that is specifically required for endothelial cell migration and differentiation under conditions such as hypoxia (27). Activating AMPK signaling was found to stimulate EPC angiogenesis in response to ischemic stress in vivo (6). More direct evidences demonstrate that activated AMPK promoted EPC differentiation, and angiogenesis in vitro and in vivo (20). However, little information exists regarding the role of AMPK on EPC angiogenic functions in diabetes.
Interestingly, AMPK activation was reported to reduce hyperglycemia-induced mitochondrial reactive oxygen species (ROS) production by induction of the MnSOD pathway in endothelial cells (19). These findings support the notion that AMPK exerts a beneficial effect on endothelial function by suppressing oxidative stress in endothelial cells. In our study, EPCs from streptozotocin (STZ)-induced type 1 diabetic mice demonstrated deficient AMPK activation and decreased expression of MnSOD. Based on these findings, the present study tested the hypothesis that AMPK activation suppresses EPC mitochondrial superoxide level and rescues their angiogenic dysfunction through MnSOD induction in type 1 diabetes.
MATERIALS AND METHODS
Animals and Induction of Type 1 Diabetes
All study procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Pittsburgh. Male C57BL/6 mice (Charles River), aged 8 to 9 wk, were injected intraperitoneally with or citrate buffer (control) or STZ (Sigma) dissolved in sterile citrate buffer (0.05 mol/l sodium citrate, pH 4.5, 60 mg/kg; Sigma), for 5 consecutive days during the first week of the study (22, 31, 38). Whole blood glucose levels were measured with a OneTouch meter (LifeScan). Mice with a blood glucose level > 280 mg/dl were considered diabetic (22) and used for EPC isolation following 4–6 wk of hyperglycemia.
EPC Culture and Characterization
Bone-marrow derived EPCs were isolated and cultured according to the latest published methods, including ours (10, 24, 40, 42). Briefly, mononuclear cells were obtained by flushing the femurs and tibias of C57BL/6 mice. Immediately following isolation, cells were plated onto rat plasma vitronectin (Sigma)-precoated six-well plates at a density of 1 × 106 cells/cm2 and cultured in endothelial cell growth medium 2 (EGM-2, Lonza) supplemented with 5% fetal bovine serum (FBS, Hyclone) in 37°C 5% CO2. After 4 days in culture, nonadherent cells were washed away, and fresh medium was added. EPCs after 7 days in culture were used for all experiments.
To characterize the endothelial phenotype of the in vitro-cultured EPCs using previously established methods (32), adherent cells after 7 days in culture were stained for DiI-acLDL (1 μg/ml, Invitrogen) and FITC-conjugated isolectin (1 μg/ml, Sigma). After nuclei staining by Hoechst 33258 (5 μg/ml, Sigma), the cells were observed with an inverted fluorescent microscope (Nikon). In addition, expression of Sca-1, Flk-1, CD34, VE-cadherin (CD144), and CD11b were analyzed by flow cytometry (FACScan) and compared with freshly isolated mononuclear cells according to published procedures (10, 42). The cells were detached and incubated with rat anti-mouse Sca-1-FITC antibody (1 μg/1 × 106 cells, BD) and rat anti-mouse Flk-1-PE antibody (0.5 μg/1 × 106 cells, BD), rat anti-mouse CD34-FITC antibody (1 μg/1 × 106 cells, BD), or rat anti-mouse CD11b-PE antibody (0.5 μg/1 × 106 cells, BD). The FITC or PE- conjugated isotype antibody was used as a control. To detect CD144, cells were incubated with goat anti-mouse CD144 antibody (2.5 μg/1 × 106 cells, R&D Systems) and then labeled with rabbit anti-goat Alexa 488 (0.625 μg/1 × 106 cells, Invitrogen). As a staining control, 0.5% goat serum was used together with the identical secondary antibodies. For flow cytometry analysis, 1 × 104 cells were acquired, and data were processed using CellQuest software (BD).
In vitro EPC functions
Tube formation assay.
In vitro angiogenic activity of EPCs was determined by Matrigel tube formation assay, as described previously (12, 42), with minor modifications. EPCs were plated at a density of 5 × 104 cells/well in 48-well plates precoated with 150 μl/well growth factor-reduced Matrigel (BD). After a 24-h incubation in EGM-2 plus 5% FBS, images of tube morphology were taken by an inverted microscope (Nikon), and tube lengths were measured at five random fields at magnification of ×40 per sample.
Adhesion assay.
For adhesion assays (12), 2.0 × 104 EPCs were plated in 48-well plates coated with 1.0 μg/ml vitronectin in triplicate as we described (12, 42). After a 1-h incubation in EGM-2 plus 5% FBS, nonadherent cells were washed away, and adherent cells were fixed with 2% paraformaldehyde (PFA, Sigma). Nuclei were stained with Hoechst 33258 and were counted at five random fields in each well, at magnification ×100 for each sample.
Migration assay.
EPC migration was measured using Boyden chambers (Costar) with 8-μm pore size filters (34). EPCs were plated at a density of 5 × 104 per well in the upper chamber with 200 μl of EBM-2 (Lonza)/5% FBS, while 500 μl of EBM-2 with 5% FBS and recombinant human vascular endothelial growth factor (VEGF; 50 ng/ml, BD) was placed in the lower chamber. EPCs were allowed to migrate in a humidified incubator at 37°C for 24 h. Nonmigrated cells were removed by gently wiping the membrane's upper surface with a cotton swab. The cells on the membrane's lower side were fixed with 2% PFA and stained with Hoechst 33258. The number of EPCs was counted at magnification of ×200, and the mean value of five random fields was determined for each sample.
Western Blot Analysis
Western blotting was performed as we described previously (7, 22). Proteins were extracted from cultured cells using CelLytic MT Cell Lysis Reagent (Sigma) and separated on SDS-polyacrylamide gels. Proteins were probed with the following antibodies: against MnSOD (1:1,000, BD), CuZnSOD (1:50,000, Abcam), heme oxygenase-1 (HO-1; 1:1,000, StressGen), catalase (1:1,000, BD), glutathione peroxidase-1 (Gpx1; 1:200, Santa Cruz Biotechnology), AMPK (1:1,000, Cell signaling), Thr172-phospho-AMPK (1:1,000, Cell Signaling), Ser79-phospho-acetyl-CoA-Carboxylase (ACC; 1: 1,000, Cell Signaling), and PP2A (1:1,000, Cell Signaling). β-Actin (1:10,000, Sigma) was used as an internal control. Secondary antibodies (Rockland) were IR Dye 700-conjugated anti-rabbit (1:4,000) and IR Dye 800-conjugated anti-mouse (1:5,000). The blots were scanned with an Odyssey imager (LI-COR Biosciences), and band intensity was determined with Quantity One System (Bio-Rad).
Adenoviral Vector Transfection
EPCs after 7 days in culture were transfected by a dominant-negative AMPKα2 mutant-expressing adenovirus (Ad-AMPK-DN) that is known to inhibit basal and stimulated AMPK activity (25). Cells were transfected by Ad-AMPK-DN and internal control β-galactosidase-expressing adenovirus (Ad-β-gal) with a titer of 50 multiplicity of infection (MOI) as previously described (33). Adenovirus transfection was performed in EGM-2 supplemented with 2% FBS for 24 h followed by change of EGM-2 with 5% FBS. After a further 48 h of cultivation, transfected EPCs were subjected to other experiments.
Small Interfering RNA Transfection
EPCs after 7 days in culture were replated at a density of 2.5–3.0 × 104/cm2 and cultured overnight. For small interfering RNA (siRNA)-mediated gene knockdown, 100 nmol/l MnSOD or PP2A siRNA SMARTpool, synthesized by Dharmacon, was transfected into cells. The nonrelated scramble oligonucleotides were used as negative controls. All siRNAs were transfected into EPCs with DharmaFECT Transfection Reagent 1 (Dharmacon) according to the manufacturer's protocol and our published methods (42). After 72 h of transfection, the cells were harvested for the other experiments.
Detection of Mitochondrial Superoxide in Epcs
Measurements of mitochondrial superoxide anion formation in EPCs were performed using flow cytometry analysis and MitoSOX as previously described (26). Briefly, cultured EPCs were incubated with 5 μM MitoSOX (Invitrogen) for 20 min at 37°C. Cells were detached using EDTA (GIBCO) and washed three times with HBSS with Ca/Mg (GIBCO). Mean fluorescent intensity was measured by flow cytometry (FACScan, BD).
MnSOD Activity Assay
MnSOD activity in cultured EPCs was measured as we described (22, 24) with minor modification. Briefly, cells were lysed in cold buffer (20 mmol/l HEPES, 1 mmol/l EGTA, 210 mmol/l mannitol, and 70 mmol/l sucrose), and lysates were centrifuged at 1,500 g for 5 min at 4°C. To separate mitochondrial MnSOD from cytosolic SOD, the 1,500-g supernatant was centrifuged at 10,000 g for 15 min at 4°C. The pellet was homogenized in cold buffer (mentioned above) and subjected to a commercial SOD Assay Kit (Cayman Chemical).
Statistical Analysis
Data are expressed as means ± SE. Experimental means were subjected to either Student's t-test for two groups, or one-way ANOVA with Newman-Keuls multiple comparison test for more than two groups (7, 22). A probability value of P < 0.05 was considered statistically significant. The above statistical analyses were performed using GraphPad Prism 5.0.
RESULTS
Induction of Diabetes in Mice
A 5-day low-dose STZ injection regimen was used to ensure sustained hyperglycemia. Under such a regimen, STZ-induced type 1 diabetic mice were characterized by significantly elevated blood glucose levels (456.0 ± 24.46 vs. 165.3 ± 7.35 mg/dl; n = 36, P < 0.001) for 4–6 wk compared with control mice.
Characterization of EPCs
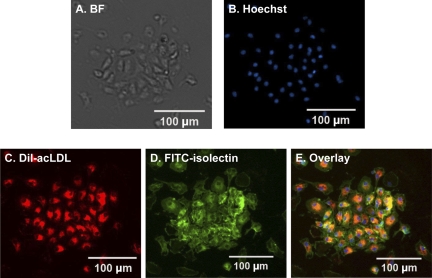
After 7 days of cultivation, the cells were visualized by inverted microscopy and characterized by fluorescence staining. In vitro expanded EPCs derived from the bone marrow of C57BL/6 mice exhibited endothelial cell-like morphology and formed endothelial colonies (Fig. 1A). EPCs were qualified as adherent cells, stained double positive for DiI-acLDL and FITC-isolectin (Fig. 1, B–E).
Fig. 1.
Phenotype characterizations of mouse bone marrow-derived endothelial progenitor cells (EPCs) after 7 days in culture. A: in bright field (BF), attached cells exhibit endothelial cell-like morphology and show their capacity to form endothelial colonies. B: Hoechst staining to detect the cell nucleus. C: cells show Dil-acLDL uptake. D: cells wee stained by FITC-isolectin. E: overlay of the 3 stains.
To further characterize bone marrow-derived EPCs, the stem cell markers (Sca-1, CD34), endothelial cell markers (Flk-1, CD144), and a monocyte marker (CD11b) were examined by flow cytometry. Compared with freshly isolated mononuclear cells, the percentages of cell population-bearing stem cell markers in 7-day cultured EPCs were increased for Sca-1 from 16.61 ± 5.59 to 67.40 ± 5.67% and for CD34 from 21.35 ± 2.95 to 46.40 ± 9.82%. The percentages of endothelial cell markers were increased for Flk-1 from 3.18 ± 1.50 to 18.59 ± 5.21% and for CD144 from 1.97 ± 0.53 to 7.77 ± 3.69%. The percentage of monocyte marker CD11b was decreased from 68.14 ± 5.36 to 54.51 ± 5.93%. The percentage of Sca-1/Flk-1 double-positive cells was elevated from 4.32 ± 2.35% in mononuclear cells to 16.77 ± 2.57% in 7-day cultured cells.
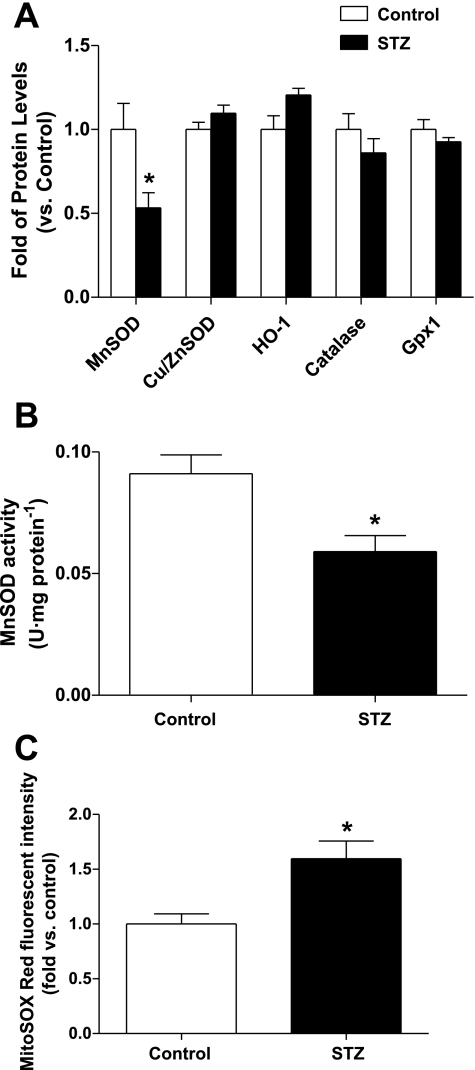
MnSOD Expression Is Deficient in Diabetic EPCs, Accompanied by Increased Mitochondrial Superoxide Level
Increased oxidative stress represents one critical mechanism underlying the impairment of in vivo and in vitro EPC functions in diabetes (36). Higher expressions of antioxidant enzymes play a critical role in equipping EPCs with more resistance to oxidative stress compared with mature endothelial cells (5, 14). To investigate the potential mechanism accounting for the excessive oxidative stress in diabetic EPCs, we first compared the protein levels of antioxidant enzymes between diabetic and control EPCs, including MnSOD, CuZnSOD, HO-1, catalase, and Gpx-1. As shown in Fig. 2A, MnSOD protein was significantly decreased in diabetic EPCs compared with controls (P < 0.05), whereas there was no significant difference in protein levels of CuZnSOD, HO-1, catalase, or Gpx-1. In parallel, MnSOD activity was also reduced in diabetic EPCs (P < 0.05 vs. controls; Fig. 2B). Subsequently, diabetic EPCs displayed increased mitochondrial superoxide level compared with EPCs from control mice, indicated by higher MitoSOX Red fluorescent intensity (P < 0.05; Fig. 2C).
Fig. 2.
Manganese superoxide dismutase (MnSOD) expression and activity are decreased, accompanied by increased mitochondrial superoxide production in diabetic EPCs. A: protein level of MnSOD was decreased in STZ-induced type 1 diabetic EPCs, whereas those of CuZnSOD, catalase, heme oxygenase-1 (HO-1), and glutathione peroxidase-1 (Gpx1) were not significantly changed; n = 4–6. *P < 0.05 vs. control. B: MnSOD activity was also decreased in diabetic EPCs compared with control; n = 5. *P < 0.05. C: mitochondrial superoxide level was elevated in diabetic EPCs; n = 4–5. *P < 0.05 vs. control.
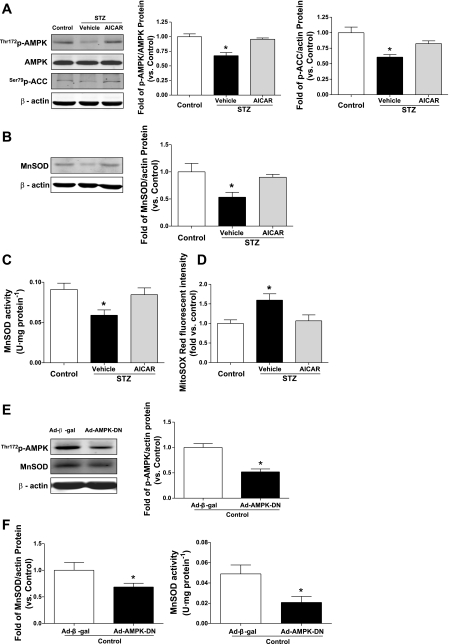
AMPK Activation Augments MnSOD and Reduces Mitochondrial Superoxide in Diabetic EPCs
Since AMPK activation was reported to induce MnSOD and reduce hyperglycemia-induced mitochondrial ROS production in endothelial cells (19), we further examined whether AMPK activation contributes to MnSOD upregulation in diabetic EPCs. AMPK activity in EPCs was detected using Western blot, demonstrated by the phosphorylation of AMPK-Thr172 and ACC-Ser79. Data showed that both the phosphorylation of AMPK-Thr172 and ACC-Ser79 were significantly decreased in diabetic EPCs compared with control EPCs, which were restored by incubation of diabetic EPCs with the AMPK agonist AICAR (aminoimidazole carboxamide ribonucleotide, 1 mM for 24 h; Sigma) (20, 41) (P < 0.05; Fig. 3A). Moreover, AMPK activation by exposure of diabetic EPCs to its selective agonist AICAR restored the deficient MnSOD protein level in diabetic EPCs (P < 0.05 vs. vehicle treatment; Fig. 3B). Consistently, the decreased MnSOD activity was also restored by AMPK activation (P < 0.05; Fig. 3C). Furthermore, AICAR inhibited the elevated mitochondrial superoxide level in diabetic EPCs (P < 0.05; Fig. 3D). In addition, compound C pretreatment abolished the increased MnSOD protein and activity caused by AICAR in diabetic EPCs [Supplemental Fig. S1, A and B (supplementary materials are found online at the Journal website)], suggesting that AICAR restores MnSOD through AMPK activation. To further confirm the role of AMPK activation in regulating MnSOD, the dominant-negative AMPKα2 mutant-expressing adenovirus Ad-AMPK-DN was transfected into normal EPCs to downregulate AMPK activation. Western blot data showed that the p-AMPK level was significantly reduced by Ad-AMPK-DN transfection (P < 0.05; Fig. 3E), and Ad-AMPK-DN transfection inhibited the protein level of MnSOD and its activity in normal EPCs compared with that with Ad-β-gal transfection (P < 0.05; Fig. 3F).
Fig. 3.
AMPK activation restores MnSOD protein and enzymatic activity, resulting in suppressed mitochondrial superoxide level in diabetic EPCs. A: level of phosphorylated AMPK-Thr172 and ACC-Ser79, indicating AMPK activity, was deficient in diabetic EPCs, which was restored by AICAR treatment (1 mM for 24 h). Blot is representative of 5 blots from 5 independent experiments; n = 5. *P < 0.05 vs. control and STZ + AICAR. B: AICAR increased MnSOD protein level in diabetic EPCs. Blot is representative of 5 blots from 5 independent experiments; n = 5–6. *P < 0.05 vs. control and STZ + AICAR. C: AICAR restored diminished MnSOD enzymatic activity in diabetic EPCs; n = 5. *P < 0.05 vs. control and STZ + AICAR. D: mitochondrial superoxide level was reduced by AICAR treatment in diabetic EPCs; n = 4–5. *P < 0.05 vs. control and STZ + AICAR. E: Ad-β-gal or Ad-AMPK-DN was transfected into normal EPCs. Western blot data showed that p-AMPK was decreased after Ad-AMPK-DN transfection. Blot is representative of 4 blots from 4 independent experiments; n = 4. *P < 0.05 vs. Control EPCs + Ad-β-gal. F: Ad-AMPK-DN transfection reduces MnSOD protein levels; n = 4. *P < 0.05; enzymatic activity, n = 5–6, *P < 0.05 in control EPCs vs. Ad-β-gal transfection.
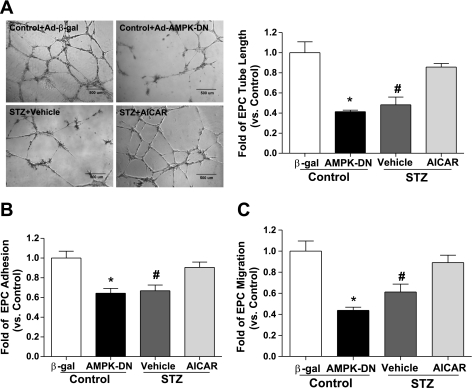
AMPK Activation Improves Diabetic EPC Functions
To determine whether AMPK activation exerted beneficial effects in protecting EPC functions in type 1 diabetes, we further performed in vitro function assays in EPCs. Results showed that the ability of in vitro tube formation on Matrigel was significantly impaired in diabetic EPCs, which was rescued by AMPK activation following AICAR treatment (1 mM for 24 h, P < 0.05; Fig. 4A). Moreover, the decreased cell number of adhesion to vitronectin was improved by AICAR treatment in diabetic EPCs (P < 0.05 vs. vehicle treatment; Fig. 4B). AICAR also rescued the impaired migratory capacity of diabetic EPCs (P < 0.05; Fig. 4C). In addition, AICAR-induced improvement of tube formation, adhesion, and migration was abolished by AMPK antagonist compound C pretreatment in diabetic EPCs (Supplemental Fig. S2, A–C). In normal EPCs, inhibiting AMPK activation by Ad-AMPK-DN transfection significantly impaired their normal abilities of tube formation, adhesion and migration (P < 0.05 vs. Ad-β-gal transfection; Fig. 4, A–C).
Fig. 4.
AMPK activation rescues impaired EPC angiogenic functions in type 1 diabetes. A: AICAR treatment rescued diabetic EPC tube formation on Matrigel, whereas inhibiting AMPK activation by Ad-AMPK-DN transfection impaired control EPC tube formation. Photomicrographs are representative of 4 independent experiments; n = 4–5. *P < 0.05 vs. control + Ad-β-gal, #P < 0.05 vs. control and STZ + AICAR. B: adhesion of diabetic EPCs was improved by AICAR treatment, whereas that of normal EPCs was impaired by Ad-AMPK-DN; n = 4–5. *P < 0.05 vs. control + Ad-β-gal; #P < 0.05 vs. control and STZ + AICAR. C: impaired migration in diabetic EPCs was rescued by AICAR treatment, and Ad-AMPK-DN transfection blunted migration of control EPCs; n = 4–5. *P < 0.05 vs. control + Ad-β-gal; #P < 0.05 vs. control and STZ + AICAR.
Silencing MnSOD Abolishes Improved Diabetic EPC Functions Induced by AMPK Activation
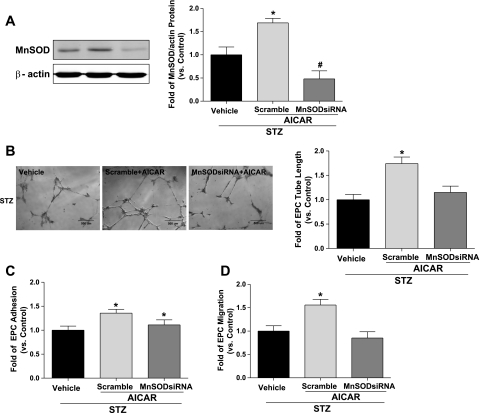
To further investigate whether MnSOD plays a causal role in AMPK-induced improvement of EPC functions, MnSOD siRNA was used to knock down MnSOD in diabetic EPCs before AICAR treatment. Western blot analysis confirmed that MnSOD siRNA decreased MnSOD level by 60% compared with scramble control transfection in diabetic EPCs (P < 0.05; Fig. 5A). The results of in vitro function assays showed that AICAR-induced improvement of EPC tube formation on Matrigel was abolished after MnSOD silencing (P < 0.05 vs. scramble control; Fig. 5B). Likewise, the improved capacity of EPC migration in diabetes was also inhibited by MnSOD downregulation (P < 0.05 vs. scramble control; Fig. 5D). However, silencing MnSOD did not alter the increased adhesion ability of EPCs following AICAR treatment (Fig. 5C).
Fig. 5.
Silencing MnSOD abolishes improved diabetic EPC functions induced by AMPK activation. A: MnSOD siRNA transfection before AICAR treatment reduced MnSOD protein level by 60% vs. scramble control transfection in diabetic EPCs; n = 5, *P < 0.05 vs. STZ and STZ + MnSOD siRNA + AICAR. B: silencing MnSOD abolished improved tube formation caused by AICAR treatment (1 mM for 24 h) in diabetic EPCs. Photomicrographs are representative of 5 independent experiments; n = 5. *P < 0.05 vs. STZ and STZ + MnSODsiRNA + AICAR. C: increased adhesion by AICAR was not significantly affected by silencing MnSOD; n = 4–5. *P < 0.05 vs. STZ. D: improved migration of diabetic EPCs was reversed by MnSOD siRNA pretreatment; n = 4–5. *P < 0.05 vs. STZ and STZ + MnSOD siRNA + AICAR.
Increased PP2A Contributes to AMPK Deficiency in Diabetic EPCs
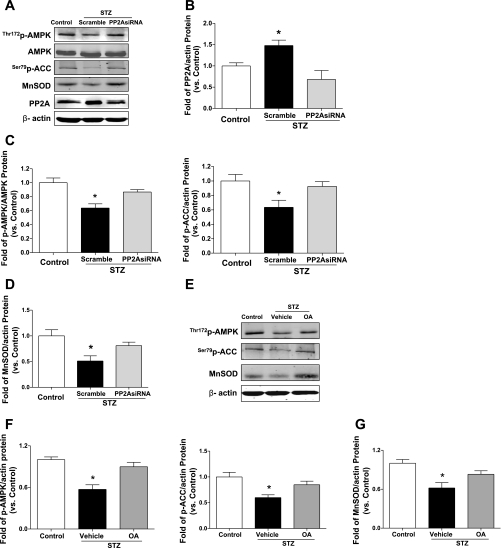
PP2A inactivates the phosphorylated form of AMPK in endothelial cells (39). We examined a potential mechanism underlying AMPK deficiency in diabetic EPCs. Diabetic EPCs showed a significantly higher level of PP2A protein (P < 0.05 vs. control EPCs; Fig. 6, A and B). Next, we performed PP2A silencing in diabetic EPCs to examine the role of PP2A in regulating AMPK activation. As shown in Fig. 6B, PP2A protein level in diabetic EPCs was decreased by 50% (P < 0.05). Consequently, the decreased AMPK-Thr172 and ACC-Ser79 phosphorylations and MnSOD protein levels were restored due to PP2A inhibition in diabetic EPCs (P < 0.05 vs. scramble control; Fig. 6, C and D). We also treated diabetic EPCs with okadaic acid [OA; 20 nM for 2 h (39)], a selective PP2A inhibitor, to obverse the changes of AMPK activity and MnSOD levels. The Western blot data showed that OA restored the deficient AMPK activation (phosphorylation of AMPK-Thr172 and ACC-Ser79) and consequently reversed MnSOD protein level in diabetic EPCs (*P <0.05; Fig. 6, E–G). These results are consistent with those after PP2A siRNA transfection.
Fig. 6.
Protein phosphatase 2A (PP2A) inhibition results in AMPK activation and MnSOD upregulation in diabetic EPCs. A: representative blots for effect of silencing PP2A on AMPK activity and MnSOD level in diabetic EPCs. Blots are representative of 4 blots from 4 independent experiments. B: PP2A protein was elevated in diabetic EPCs and was reduced by 50% following PP2A siRNA transfection; n = 4. *P < 0.05 vs. control and STZ + PP2A siRNA. C: silencing PP2A in diabetic EPCs leads to AMPK activation, demonstrated by higher levels of AMPK-Thr172 phosphorylation and ACC-Ser79 phosphorylation; n = 4. *P < 0.05 vs. control and STZ + PP2A siRNA. D: MnSOD expression was upregulated following PP2A inhibition in diabetic EPCs; n = 4. *P < 0.05 vs. control and STZ + PP2A siRNA. E–G: diabetic EPCs were treated with okadaic acid (OA, 20 nM) for 2 h. OA treatment restored AMPK-Thr172 and ACC-Ser79 phosphorylation and increased MnSOD protein level in diabetic EPCs. Blot is representative of 4 blots from 4 independent experiments; n = 4–5. * P <0.05 vs. control and STZ + OA.
DISCUSSION
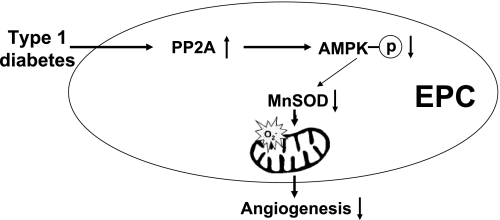
The major new findings of this study unveiled several new mechanisms of AMPK protection on diabetic EPC angiogenesis. First, EPCs from SZT-induced type 1 diabetic mice displayed deficient AMPK activity, which is normalized by AICAR in vitro treatment. Second, AMPK activation restored the deficiency of both MnSOD protein and activity and suppressed mitochondrial superoxide level in diabetic EPCs. Third, AMPK activation-induced improvements of diabetic EPC tube formation and migration were dependent on MnSOD induction in type 1 diabetes. Finally, PP2A inhibition restored the deficient AMPK activation and MnSOD protein level in diabetic EPCs. A schematic illustration is presented in Fig. 7.
Fig. 7.
Schematic illustration of possible signals through which AMPK activation improves EPC angiogenic functions in type 1 diabetes. AMPK activation reduces mitochondrial superoxide level in diabetic EPCs by promoting MnSOD induction, resulting in improvement of EPC functions in type 1 diabetes.
Recently, EPC therapy has become an attractive alternative strategy to current pharmacological, interventional, or surgical treatments for vascular complications characterized by impaired angiogenesis in diabetes, such as refractory wounds (1). However, several clinical trials of EPC therapy for ischemia revealed that autologous cell therapy for diabetic patients did not exert the same clinical efficacy as in nondiabetic patients (18, 28). A major reason could be that EPCs from diabetic patients have extensive dysfunction in repairing tissue, consequently leading to inefficient therapy in the clinic. Consistent with EPC studies in patients with diabetes (21, 37), our study herein demonstrated that bone marrow-derived EPCs from STZ-induced type 1 diabetic mice exhibited distinctly poor angiogenic properties. Therefore, EPCs biologically modified to augment their angiogenic functions may be a potent therapeutic tool. Therefore, it is imperative to first understand the potential mechanisms underlying EPC dysfunction in diabetes.
Hyperglycemia is the metabolic hallmark of diabetes and leads to widespread cellular damage. An excess of glucose sets off a chain of metabolic events that culminate in superoxide anion overproduction from the mitochondrial electron transport chain (2). In diabetes, continuous exposure of endothelial cells (mature and EPC) to ROS makes them a prime target for oxidative stress (35). Protection against oxidative stress by ROS is accomplished by a complex defense system composed of several antioxidant enzymes that reduce the damaging effects of ROS. The organelles most vulnerable to oxidative stress are the mitochondria because of the permanent potential for the production of superoxide anions. Superoxide anions are converted to hydrogen peroxide by SODs, whereas hydrogen peroxide is detoxified by the enzymes catalase and glutathione peroxidase (GPx). Because of the localization of MnSOD and GPx-1 in the matrix of the mitochondria, in close proximity to the production of ROS by the electron transport chain, these two enzymes are believed to be the primary antioxidant defense systems in the mitochondria (11). In vitro studies demonstrated that EPCs express higher levels of MnSOD and Gpx1 (5, 14). Our recent study has demonstrated that decreased expression of MnSOD in EPCs contributes to delayed wound healing in type 2 diabetes (24). These findings support the notion that EPCs require antioxidant enzymes for their functional capacity, especially in diabetes. In the present study, we found that the protein level and enzymatic activity of MnSOD were significantly reduced in EPCs from STZ-induced type 1 diabetic mice, similarly with their changes in EPCs from type 2 diabetes recently reported by our group (24). They were accompanied by accumulating mitochondrial superoxide level and significant impairment of EPCs' in vitro functions. In contrast, other antioxidant enzymes, including CuZnSOD, HO-1, catalase, and Gpx-1, remained unchanged. These results suggest that MnSOD may act as one critical antioxidant enzyme to protect EPCs from oxidative stress in type 1 diabetes.
AMPK, a serine/threonine protein kinase, has recently come into focus because of its potential roles in regulating other signaling pathways in addition to its role in controlling energy metabolism, such as in regulating oxidative stress (43). Ido et al. (17) showed that the AMPK activator AICAR increased AMPK activity in human umbilical vein endothelial cells (HUVEC) and completely prevented the high-glucose-induced increase in HUVEC apoptosis induced by oxidative stress, suggesting that AMPK could play an important role in protecting the endothelial cells against the adverse effects of sustained hyperglycemia. Ouslimani et al. (30) reported that metformin, an AMPK activator, decreases intracellular ROS level in aortic endothelial cells from NADPH oxidase and the respiratory mitochondrial chain. These studies indicate that AMPK is beneficial to endothelial function via suppressing oxidative stress. In the present study, bone marrow-derived EPCs isolated from type 1 diabetic mice displayed decreased AMPK activity. By utilizing a selective agonist of AMPK (AICAR), we observed that AMPK activity was normalized in diabetic EPCs. AICAR also restored the deficient MnSOD protein level and its enzymatic activity, resulting in the reduction of mitochondrial superoxide level in diabetic EPCs. In addition, AMPK antagonist compound C was found to abolish AICAR-induced MnSOD upregulation in diabetic EPCs, suggesting that AICAR restores MnSOD levels through AMPK activation in diabetic EPCs. This is consistent with the previous finding in endothelial cells reported by Kukidome et al. (19) that AMPK activation reduces hyperglycemia-induced mitochondrial ROS production by induction of MnSOD in HUVEC. To further verify the role of AMPK activation in regulating MnSOD levels in EPCs, a dominant-negative AMPK mutant (Ad-AMPK-DN) was transfected into EPCs to downregulate AMPK activation. AMPK downregulation reduced the protein level MnSOD and also inhibited its activity in normal EPCs. Taken together, these findings demonstrate that AMPK activation alleviates oxidative stress by upregulating MnSOD in EPCs, a critical antioxidant enzyme that maintains their normal EPC functions.
AMPK has been shown to serve as a new regulator of angiogenesis under some conditions, such as hypoxia (27). Dominant-negative AMPK mutant was reported to suppress both endothelial cell migration to VEGF and in vitro differentiation into tube-like structures under hypoxic conditions (27). AMPK has also been shown to promote EPC differentiation, and in vitro and in vivo angiogenesis in human circulating EPCs (20). In the present study, we observed that bone marrow-derived EPCs isolated from type 1 diabetic mice had reduced AMPK activity. By utilizing the selective agonist of AMPK AICAR, we found that AMPK activity was normalized in diabetic EPCs, leading to their restored angiogenic functions including Matrigel tube formation, adhesion, and migration. In addition, AICAR-induced improvement of diabetic EPCs functions was abolished by AMPK antagonist compound C. These results suggest that insufficient AMPK activation may be one critical factor resulting in EPC dysfunctions in type 1 diabetes, and AICAR rescues diabetic EPC dysfunction through AMPK activation. AMPK activation upregulates MnSOD level, which plays an essential role in suppressing oxidative stress in diabetic EPCs, suggesting that upregulation of MnSOD may play a crucial role in mediating AMPK protective effects on diabetic EPC functions. This point was further confirmed by the evidence that MnSOD silencing blunted the improvement of EPC tube formation and migration caused by AMPK activation. But AMPK-induced EPC adhesion ability was not altered by silencing MnSOD. This may be elucidated by other angiogenesis-related mechanisms, which are supposed to be regulated by AMPK activation. Activated AMPK is reported to increase endothelial (e)NOS phosphorylation, causing EPC-EC differentiation, and in vitro and in vivo angiogenesis (20). And AMPK activation can increase VEGF production and promote angiogenesis in muscle in response to ischemic injury (29). Therefore, eNOS or VEGF may be involved in the improvement of EPC adhesion ability by AMPK activation. Certainly, their roles in mediating AMPK in protecting EPC functions under diabetic condition need to be further investigated.
Because AMPK activity was deficient in cultured EPCs from type 1 diabetes, we further delineated the upstream regulation accounting for this deficiency. The delicate AMPK phosphorylation is maintained by upstream protein kinase and serine/threonine phosphatase. Protein kinases responsible for AMPK activation include Peutz-Jeghers syndrome kinase LKB1 (LKB1) (13) and the Ca2+/calmodulin-dependent protein kinase kinase (CaMKK) (16). In contrast, PP2A and PP2C, two serine/threonine phosphatases, were identified to inactivate the active and phosphorylated form of AMPK in cell-free assays (4). In the present study, we examined the levels of LKB1, CaMKK, PP2A, and PP2C in diabetic EPCs and found that diabetic EPCs possessed an elevated PP2A level, whereas others were not altered (data not shown). Furthermore, silencing PP2A restored AMPK activity and MnSOD level in diabetic EPCs. These observations suggest that increased PP2A may contribute to the deficient AMPK activation in diabetic EPCs.
In summary, our current study demonstrates, for the first time, that AMPK activation suppresses mitochondrial superoxide level and exerts a proangiogenic effect by inducing MnSOD in EPCs from type 1 diabetes. These findings may provide a new mechanistic basis for promoting therapeutic angiogenesis by activating AMPK to rescue deficient MnSOD in EPCs under cardiovascular disease conditions including diabetes.
GRANTS
This work was funded by Grant R01 GM-077352 from the National Institute of General Medical Science; Grant 7-08-RA-23 from the American Diabetes Association (to A. F. Chen). X. R. Wang is the recipient of an American Heart Association postdoctoral fellowship award (09POST2370060).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
REFERENCES
- 1. Barcelos LS, Duplaa C, Krankel N, Graiani G, Invernici G, Katare R, Siragusa M, Meloni M, Campesi I, Monica M, Simm A, Campagnolo P, Mangialardi G, Stevanato L, Alessandri G, Emanueli C, Madeddu P. Human CD133 + progenitor cells promote the healing of diabetic ischemic ulcers by paracrine stimulation of angiogenesis and activation of Wnt signaling. Circ Res 104: 1095–1102, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature 414: 813–820, 2001 [DOI] [PubMed] [Google Scholar]
- 3. Callaghan MJ, Ceradini DJ, Gurtner GC. Hyperglycemia-induced reactive oxygen species and impaired endothelial progenitor cell function. Antioxid Redox Signal 7: 1476–1482, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Davies SP, Helps NR, Cohen PT, Hardie DG. 5′-AMP inhibits dephosphorylation, as well as promoting phosphorylation, of the AMP-activated protein kinase. Studies using bacterially expressed human protein phosphatase-2C alpha and native bovine protein phosphatase-2AC. FEBS Lett 377: 421–425, 1995 [DOI] [PubMed] [Google Scholar]
- 5. Dernbach E, Urbich C, Brandes RP, Hofmann WK, Zeiher AM, Dimmeler S. Antioxidative stress-associated genes in circulating progenitor cells: evidence for enhanced resistance against oxidative stress. Blood 104: 3591–3597, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Diller GP, van Eijl S, Okonko DO, Howard LS, Ali O, Thum T, Wort SJ, Bedard E, Gibbs JS, Bauersachs J, Hobbs AJ, Wilkins MR, Gatzoulis MA, Wharton J. Circulating endothelial progenitor cells in patients with Eisenmenger syndrome and idiopathic pulmonary arterial hypertension. Circulation 117: 3020–3030, 2008 [DOI] [PubMed] [Google Scholar]
- 7. Du YH, Guan YY, Alp NJ, Channon KM, Chen AF. Endothelium-specific GTP cyclohydrolase I overexpression attenuates blood pressure progression in salt-sensitive low-renin hypertension. Circulation 117: 1045–1054, 2008 [DOI] [PubMed] [Google Scholar]
- 8. Fadini GP, Miorin M, Facco M, Bonamico S, Baesso I, Grego F, Menegolo M, de Kreutzenberg SV, Tiengo A, Agostini C, Avogaro A. Circulating endothelial progenitor cells are reduced in peripheral vascular complications of type 2 diabetes mellitus. J Am Coll Cardiol 45: 1449–1457, 2005 [DOI] [PubMed] [Google Scholar]
- 9. Fadini GP, Sartore S, Albiero M, Baesso I, Murphy E, Menegolo M, Grego F, Vigili de Kreutzenberg S, Tiengo A, Agostini C, Avogaro A. Number and function of endothelial progenitor cells as a marker of severity for diabetic vasculopathy. Arterioscler Thromb Vasc Biol 26: 2140–2146, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Feng Y, van Eck M, Van Craeyveld E, Jacobs F, Carlier V, Van Linthout S, Erdel M, Tjwa M, De Geest B. Critical role of scavenger receptor-BI-expressing bone marrow-derived endothelial progenitor cells in the attenuation of allograft vasculopathy after human apo A-I transfer. Blood 113: 755–764, 2009 [DOI] [PubMed] [Google Scholar]
- 11. Haendeler J, Dimmeler S. Inseparably tied: functional and antioxidative capacity of endothelial progenitor cells. Circ Res 98: 157–158, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Hamada H, Kim MK, Iwakura A, Ii M, Thorne T, Qin G, Asai J, Tsutsumi Y, Sekiguchi H, Silver M, Wecker A, Bord E, Zhu Y, Kishore R, Losordo DW. Estrogen receptors alpha and beta mediate contribution of bone marrow-derived endothelial progenitor cells to functional recovery after myocardial infarction. Circulation 114: 2261–2270, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Hawley SA, Boudeau J, Reid JL, Mustard KJ, Udd L, Makela TP, Alessi DR, Hardie DG. Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J Biol 2: 28, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. He T, Peterson TE, Holmuhamedov EL, Terzic A, Caplice NM, Oberley LW, Katusic ZS. Human endothelial progenitor cells tolerate oxidative stress due to intrinsically high expression of manganese superoxide dismutase. Arterioscler Thromb Vasc Biol 24: 2021–2027, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Hristov M, Erl W, Weber PC. Endothelial progenitor cells: mobilization, differentiation, and homing. Arterioscler Thromb Vasc Biol 23: 1185–1189, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Hurley RL, Anderson KA, Franzone JM, Kemp BE, Means AR, Witters LA. The Ca2+/calmodulin-dependent protein kinase kinases are AMP-activated protein kinase kinases. J Biol Chem 280: 29060–29066, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Ido Y, Carling D, Ruderman N. Hyperglycemia-induced apoptosis in human umbilical vein endothelial cells: inhibition by the AMP-activated protein kinase activation. Diabetes 51: 159–167, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Jimenez-Quevedo P, Silva GV, Sanz-Ruiz R, Oliveira EM, Fernandes MR, Angeli F, Willerson JT, Dohmann HF, Perin EC. [Diabetic and nondiabetic patients respond differently to transendocardial injection of bone marrow mononuclear cells: findings from prospective clinical trials in “no-option” patients]. Rev Esp Cardiol 61: 635–639, 2008 [PubMed] [Google Scholar]
- 19. Kukidome D, Nishikawa T, Sonoda K, Imoto K, Fujisawa K, Yano M, Motoshima H, Taguchi T, Matsumura T, Araki E. Activation of AMP-activated protein kinase reduces hyperglycemia-induced mitochondrial reactive oxygen species production and promotes mitochondrial biogenesis in human umbilical vein endothelial cells. Diabetes 55: 120–127, 2006 [PubMed] [Google Scholar]
- 20. Li X, Han Y, Pang W, Li C, Xie X, Shyy JY, Zhu Y. AMP-activated protein kinase promotes the differentiation of endothelial progenitor cells. Arterioscler Thromb Vasc Biol 28: 1789–1795, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Loomans CJ, de Koning EJ, Staal FJ, Rookmaaker MB, Verseyden C, de Boer HC, Verhaar MC, Braam B, Rabelink TJ, van Zonneveld AJ. Endothelial progenitor cell dysfunction: a novel concept in the pathogenesis of vascular complications of type 1 diabetes. Diabetes 53: 195–199, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Luo JD, Wang YY, Fu WL, Wu J, Chen AF. Gene therapy of endothelial nitric oxide synthase and manganese superoxide dismutase restores delayed wound healing in type 1 diabetic mice. Circulation 110: 2484–2493, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Marrotte EJ, Chen DD, Hakim JS, Chen AF. Manganese superoxide dismutase expression in endothelial progenitor cells accelerates wound healing in diabetic mice. J Clin Invest 120: 4207–4219, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mu J, Brozinick JT, Jr, Valladares O, Bucan M, Birnbaum MJ. A role for AMP-activated protein kinase in contraction- and hypoxia-regulated glucose transport in skeletal muscle. Mol Cell 7: 1085–1094, 2001 [DOI] [PubMed] [Google Scholar]
- 26. Mukhopadhyay P, Rajesh M, Yoshihiro K, Hasko G, Pacher P. Simple quantitative detection of mitochondrial superoxide production in live cells. Biochem Biophys Res Commun 358: 203–208, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nagata D, Mogi M, Walsh K. AMP-activated protein kinase (AMPK) signaling in endothelial cells is essential for angiogenesis in response to hypoxic stress. J Biol Chem 278: 31000–31006, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Nyolczas N, Gyongyosi M, Beran G, Dettke M, Graf S, Sochor H, Christ G, Edes I, Balogh L, Krause KT, Jaquet K, Kuck KH, Benedek I, Hintea T, Kiss R, Preda I, Kotevski V, Pejkov H, Dudek D, Heba G, Sylven C, Charwat S, Jacob R, Maurer G, Lang I, Glogar D. Design and rationale for the Myocardial Stem Cell Administration After Acute Myocardial Infarction (MYSTAR) Study: a multicenter, prospective, randomized, single-blind trial comparing early and late intracoronary or combined (percutaneous intramyocardial and intracoronary) administration of nonselected autologous bone marrow cells to patients after acute myocardial infarction. Am Heart J 153: 212 e211–e217, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Ouchi N, Shibata R, Walsh K. AMP-activated protein kinase signaling stimulates VEGF expression and angiogenesis in skeletal muscle. Circ Res 96: 838–846, 2005 [DOI] [PubMed] [Google Scholar]
- 30. Ouslimani N, Peynet J, Bonnefont-Rousselot D, Therond P, Legrand A, Beaudeux JL. Metformin decreases intracellular production of reactive oxygen species in aortic endothelial cells. Metabolism 54: 829–834, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Schatteman GC, Hanlon HD, Jiao C, Dodds SG, Christy BA. Blood-derived angioblasts accelerate blood-flow restoration in diabetic mice. J Clin Invest 106: 571–578, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schroder K, Kohnen A, Aicher A, Liehn EA, Buchse T, Stein S, Weber C, Dimmeler S, Brandes RP. NADPH oxidase Nox2 is required for hypoxia-induced mobilization of endothelial progenitor cells. Circ Res 105: 537–544, 2009 [DOI] [PubMed] [Google Scholar]
- 33. Schulz E, Anter E, Zou MH, Keaney JF., Jr Estradiol-mediated endothelial nitric oxide synthase association with heat shock protein 90 requires adenosine monophosphate-dependent protein kinase. Circulation 111: 3473–3480, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Smadja DM, Bieche I, Uzan G, Bompais H, Muller L, Boisson-Vidal C, Vidaud M, Aiach M, Gaussem P. PAR-1 activation on human late endothelial progenitor cells enhances angiogenesis in vitro with upregulation of the SDF-1/CXCR4 system. Arterioscler Thromb Vasc Biol 25: 2321–2327, 2005 [DOI] [PubMed] [Google Scholar]
- 35. Son SM, Whalin MK, Harrison DG, Taylor WR, Griendling KK. Oxidative stress and diabetic vascular complications. Curr Diab Rep 4: 247–252, 2004 [DOI] [PubMed] [Google Scholar]
- 36. Sorrentino SA, Bahlmann FH, Besler C, Muller M, Schulz S, Kirchhoff N, Doerries C, Horvath T, Limbourg A, Limbourg F, Fliser D, Haller H, Drexler H, Landmesser U. Oxidant stress impairs in vivo reendothelialization capacity of endothelial progenitor cells from patients with type 2 diabetes mellitus: restoration by the peroxisome proliferator-activated receptor-gamma agonist rosiglitazone. Circulation 116: 163–173, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Tepper OM, Galiano RD, Capla JM, Kalka C, Gagne PJ, Jacobowitz GR, Levine JP, Gurtner GC. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation 106: 2781–2786, 2002 [DOI] [PubMed] [Google Scholar]
- 38. Tie L, Li XJ, Wang X, Channon KM, Chen AF. Endothelium-specific GTP cyclohydrolase I overexpression accelerates refractory wound healing by suppressing oxidative stress in diabetes. Am J Physiol Endocrinol Metab 296: E1423–E1429, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wu Y, Song P, Xu J, Zhang M, Zou MH. Activation of protein phosphatase 2A by palmitate inhibits AMP-activated protein kinase. J Biol Chem 282: 9777–9788, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Xie HH, Zhou S, Chen DD, Channon KM, Su DF, Chen AF. GTP cyclohydrolase I/BH4 pathway protects EPCs via suppressing oxidative stress and thrombospondin-1 in salt-sensitive hypertension. Hypertension 56: 1137–1144, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Xie Z, Zhang J, Wu J, Viollet B, Zou MH. Upregulation of mitochondrial uncoupling protein-2 by the AMP-activated protein kinase in endothelial cells attenuates oxidative stress in diabetes. Diabetes 57: 3222–3230, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42. Zhao T, Li J, Chen AF. MicroRNA-34a induces endothelial progenitor cell senescence and impedes its angiogenesis via suppressing silent information regulator 1. Am J Physiol Endocrinol Metab 299: E110–E116, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zou MH, Wu Y. AMP-activated protein kinase activation as a strategy for protecting vascular endothelial function. Clin Exp Pharmacol Physiol 35: 535–545, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.