Abstract
BACKGROUND
Lymphangioleiomyomatosis (LAM) is a progressive, cystic lung disease in women; it is associated with inappropriate activation of mammalian target of rapamycin (mTOR) signaling, which regulates cellular growth and lymphangiogenesis. Sirolimus (also called rapamycin) inhibits mTOR and has shown promise in phase 1–2 trials involving patients with LAM.
METHODS
We conducted a two-stage trial of sirolimus involving 89 patients with LAM who had moderate lung impairment — a 12-month randomized, double-blind comparison of sirolimus with placebo, followed by a 12-month observation period. The primary end point was the difference between the groups in the rate of change (slope) in forced expiratory volume in 1 second (FEV1).
RESULTS
During the treatment period, the FEV1 slope was −12±2 ml per month in the placebo group (43 patients) and 1±2 ml per month in the sirolimus group (46 patients) (P<0.001). The absolute between-group difference in the mean change in FEV1 during the treatment period was 153 ml, or approximately 11% of the mean FEV1 at enrollment. As compared with the placebo group, the sirolimus group had improvement from baseline to 12 months in measures of forced vital capacity, functional residual capacity, serum vascular endothelial growth factor D (VEGF-D), and quality of life and functional performance. There was no significant between-group difference in this interval in the change in 6-minute walk distance or diffusing capacity of the lung for carbon monoxide. After discontinuation of sirolimus, the decline in lung function resumed in the sirolimus group and paralleled that in the placebo group. Adverse events were more common with sirolimus, but the frequency of serious adverse events did not differ significantly between the groups.
CONCLUSIONS
In patients with LAM, sirolimus stabilized lung function, reduced serum VEGF-D levels, and was associated with a reduction in symptoms and improvement in quality of life. Therapy with sirolimus may be useful in selected patients with LAM. (Funded by the National Institutes of Health and others; MILES ClinicalTrials.gov number, NCT00414648.)
Lymphangioleiomyomatosis (LAM) is an uncommon systemic disease that is associated with cystic destruction of the lung, chylous pleural effusions, and abdominal tumors such as renal angiomyolipomas.1,2 LAM affects women almost exclusively and occurs sporadically, developing in about 5 persons per 1 million; it also affects 30 to 40% of women with tuberous sclerosis complex (TSC). Lung function, measured as the forced expiratory volume in 1 second (FEV1), declines at the rate of 75 to 118 ml per year3–5; clinically important respiratory impairment, recurrent pneumothoraxes, and hypoxemia develop in most patients within a decade after the onset of symptoms.6
Smooth-muscle cells that infiltrate the lung in patients with LAM appear to be benign histologically,7 arise from an unknown source, circulate in the blood,8 and harbor biallelic, inactivating TSC gene mutations.9 Loss of TSC gene function constitutively activates the mammalian target of rapamycin (mTOR) signaling pathway, which regulates multiple cellular functions, including growth, motility, and survival.10 LAM cells also express two lymphangiogenic growth factors, vascular endothelial growth factor C (VEGF-C) and vascular endothelial growth factor D (VEGF-D), and spread through lymphatic channels.11,12 Current evidence, together with reports of recurrence of LAM after lung transplantation,13,14 suggests that LAM is a low-grade, metastatic neoplasm that selectively targets the lung (see video).
Sirolimus (also called rapamycin) blocks mTOR activation of downstream kinases and restores homeostasis in cells with defective TSC gene function.10 The cells that make up LAM lesions in the lung exhibit activation of the mTOR pathway and ex vivo sensitivity to the antimitogenic effects of sirolimus.15 Administration of sirolimus in rodent models of TSC has been shown to cause regression of neoplastic growths in the liver and kidney.16,17 Recent phase 1–2 trials18,19 of sirolimus in patients with TSC or LAM showed that there was a reduction in the size of angiomyolipomas and, in some cases, improvement in lung function; however, the relative risks and benefits of sirolimus in patients with LAM remain unclear.20 We conducted an international, multi-center, randomized, placebo-controlled study to test the hypothesis that treatment with sirolimus for 1 year would improve lung function in patients with LAM.
METHODS
STUDY PATIENTS
Patients were eligible for inclusion in the study if they were women 18 years of age or older, had an FEV1 after bronchodilation of 70% of the predicted value or less, and had received a diagnosis of LAM on the basis of findings of compatible cystic change on high-resolution computed tomography plus at least one of the following criteria: confirmation of LAM by means of a biopsy, a serum VEGF-D level of 800 pg per milliliter or higher,21 or clinically consistent findings (an existing diagnosis of TSC, a prior chylous pleural effusion, or a history of renal angiomyolipoma). Exclusion criteria were a current or planned pregnancy, large chylous fluid collections, and prior lung transplantation. All patients provided written informed consent on documents approved by the local committee charged with oversight of human subjects research. Further details of the inclusion and exclusion criteria are provided in the Supplementary Appendix, available with the full text of this article at NEJM.org.
STUDY DESIGN AND END POINTS
The study was designed by the investigators, approved by the data and safety monitoring board at the National Center for Research Resources and the institutional review board at each participating site, and conducted within the National Institutes of Health Rare Lung Diseases Consortium. The LAM Foundation assisted with recruitment of patients and with study logistics. The data, collected with the use of Internet-based electronic case-report forms, were reported to the data management and coordinating center, where they were securely held and analyzed. All the authors participated in the writing of the first and subsequent drafts of the manuscript and in the decision to submit the manuscript for publication and vouch for the completeness and veracity of the data and data analyses. Pfizer provided the drug and the money for the costs of study visits but had no role in the design or conduct of the study or the analysis or reporting of the data. The protocol, including the statistical analysis plan, is available at NEJM.org.
The study design included a screening visit and a 12-month, double-blind, placebo-controlled treatment period, followed by a 12-month observation period during which no patients received a study drug and all patients remained unaware of their treatment assignment. Patients who met the eligibility criteria were randomly assigned by the data management and coordinating center, in a 1:1 ratio, to receive oral sirolimus, at an initial dose of 2 mg per day, or matched placebo. Sirolimus levels were measured at each follow-up visit; the results of these measurements were revealed only to an independent medical monitor, who made dosing recommendations to maintain sirolimus trough levels between 5 and 15 ng per milliliter, as well as corresponding sham dose adjustments in the placebo group.
The primary outcome measure was the FEV1 response, which was assessed as the rate of change in FEV1 (FEV1 slope) in milliliters per month. Secondary outcome measures included responses in forced vital capacity (FVC), measured as changes from baseline to 12 months; lung volumes (residual volume, functional residual capacity, and total lung capacity); the distance covered on a 6-minute walk test; diffusing capacity of the lung for carbon monoxide; serum VEGF-D levels; and scores on the St. George’s Respiratory Questionnaire, the Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36), the Functional Performance Inventory, the General Well-Being Questionnaire, and the EuroQOL visual-analogue scales assessing fatigue, dyspnea, and quality of life. Study visits occurred at baseline, at 3 weeks, and at 3, 6, 9, 12, 18, and 24 months. Primary and secondary end points were measured at baseline and at every visit after the 3-week visit, as described in the study calendar in the Supplementary Appendix.
Adverse events were assessed according to the Common Terminology Criteria for Adverse Events (version 3.0). Laboratory testing to assess safety included hematologic, serum chemical, and urine chemical tests.
STATISTICAL ANALYSIS
A planned interim analysis was conducted with the use of the O’Brien–Fleming stopping boundary when 40 patients had completed the 12-month visit. A significance level of 0.002 was chosen to preserve a nominal significance level of 0.049 for efficacy at the end of the study. The interim analysis did not occur until late in the study, owing to regulatory and contracting hindrances that delayed the opening of some sites and prolonged the enrollment period. Although the interim stopping rule met the threshold for early termination, the data and safety monitoring board recommended that the trial be continued until all the patients had completed the 12-month visit. The investigators later learned that this action was taken to ensure that a full complement of efficacy and safety data would be available for the primary analysis in the event that the effect size was small. The data and safety monitoring board also endorsed an investigator-initiated proposal to truncate the observation phase of the study, owing to the impending termination of the funding period. The treatment assignments and the deliberations of the data and safety monitoring board remained concealed until the release of the final analysis.
The analyses were performed according to the intention-to-treat principle. The primary outcome, the FEV1 response measured in milliliters per month over the course of 1 year (termed the FEV1 slope), was analyzed as the difference in the FEV1 slope between the placebo group and the sirolimus group. This was calculated with the use of spirometric data obtained at baseline and at 3, 6, 9, and 12 months during the treatment phase. A linear mixed-effects model was used to evaluate the between-group and within-group differences in the FEV1 slope. The model included the time since enrollment, the treatment assignment, and the interaction between time and treatment. The PROC MIXED procedure with the Kenward–Roger correction (SAS Institute) was used to fit the model, without imputation of missing data. A general linear model was used to compare the difference between the two groups in the mean change from baseline to 12 months, after adjustment for baseline values. A Wilcoxon signed-rank test was used to assess the difference from baseline to 12 months within each group. For categorical outcomes, the data were compared with the use of Fisher’s exact test or the chi-square test, as appropriate. For continuous variables, the medians were compared with the use of the Wilcoxon rank-sum test. P values of less than 0.05 were considered to indicate statistical significance. All reported P values are two-sided, and have not been adjusted for multiple testing. All analyses were performed with the use of SAS software, version 9.2.
RESULTS
ENROLLMENT AND BASELINE CHARACTERISTICS OF THE PATIENTS
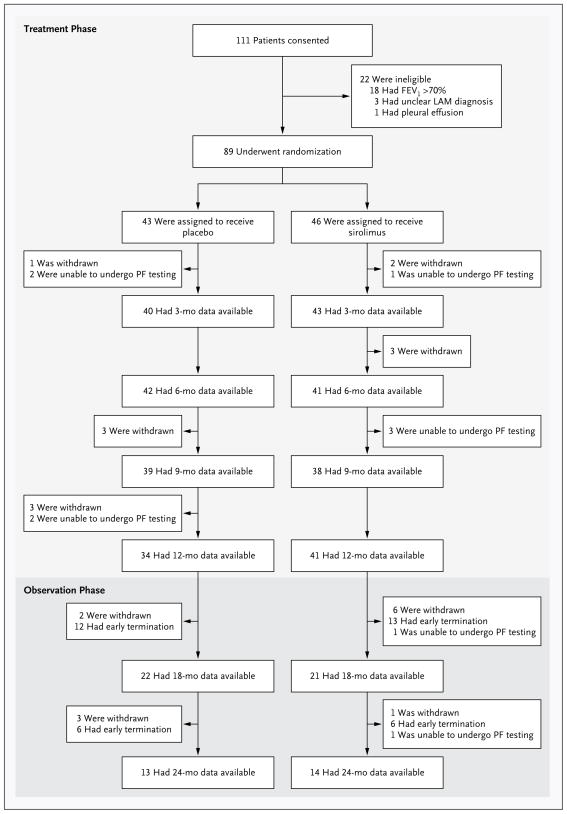
From December 2006 through September 2010, a total of 111 patients consented to participate in the study (Fig. 1). Of these, 89 patients were eligible for the study and underwent randomization — 43 to the placebo group and 46 to the sirolimus group. The baseline characteristics of the patients were similar in the two groups (Table 1, and Table A in the Supplementary Appendix). The patients who were enrolled in the study had moderately severe lung disease; the mean (±SD) FEV1 was 47.7±14.4% of the predicted value in the placebo group and 49.3±13.3% of the predicted value in the sirolimus group (P = 0.77). There was also evidence of airflow obstruction, gas trapping, and impaired gas exchange. For additional details regarding the baseline characteristics of the patients and the results of the trial see the Supplementary Appendix.
Figure 1. Screening, Randomization, and Follow-up.
The treatment period was 12 months in duration and was followed by a 12-month observation period during which no patients received a study drug and all patients remained unaware of their treatment assignment. Spirometry was performed every 3 months during the treatment year and every 6 months during the observation year. Patients who were unable to undergo pulmonary-function (PF) testing at one visit could undergo testing at a subsequent visit. The reasons for withdrawal of patients from the study included a decision to use sirolimus outside the study (3 patients in the placebo group and 5 in the sirolimus group), pneumothorax (2 patients in the placebo group), infection (3 patients in each group), placement on a list for transplantation (2 patients in the placebo group), non-adherence to visits or testing (2 patients in the sirolimus group), rash (1 patient in the sirolimus group), anxiety (1 patient in the sirolimus group), and death (2 deaths in the placebo group, 1 due to stroke and 1 in a house fire). FEV1 denotes forced expiratory volume in 1 second and LAM lymphangioleiomyomatosis.
Table 1.
Baseline Demographic and Clinical Characteristics of the Patients.*
| Characteristic | All Patients (N = 89) | Placebo Group (N = 43) | Sirolimus Group (N = 46) | P Value |
|---|---|---|---|---|
| Age — yr | 45.4±10.6 | 45.9±10.3 | 45.0±10.9 | 0.74 |
| Race — no. (%)† | ||||
| White | 59 (66) | 30 (70) | 29 (63) | 0.58‡ |
| Asian | 27 (30) | 12 (28) | 15 (33) | |
| Other | 3 (3) | 1 (2) | 2 (4) | |
| Clinical features — no. (%) | ||||
| Tuberous sclerosis complex | 8 (9) | 4 (9) | 4 (9) | 1.00§ |
| Postmenopause | 30 (34) | 16 (37) | 14 (30) | 0.50‡ |
| History of angiomyolipoma | 44 (49) | 22 (51) | 22 (48) | 0.75‡ |
| History of pneumothorax | 53 (60) | 29 (67) | 24 (52) | 0.14‡ |
| Oxygen-therapy requirement | ||||
| Continuous use | 28 (31) | 14 (33) | 14 (30) | 0.83‡ |
| Intermittent use | 52 (58) | 23 (53) | 29 (63) | 0.36‡ |
| Pulmonary-function testing | ||||
| FEV1 | ||||
| Volume — ml | 1367±420 | 1378±446 | 1357±400 | 0.69¶ |
| % of predicted value | 48.54±13.77 | 47.73±14.37 | 49.29±13.31 | 0.77¶ |
| FVC | ||||
| Volume — ml | 2791±692 | 2909±749 | 2682±622 | 0.14¶ |
| % of predicted value | 79.71±16.60 | 80.77±17.62 | 78.73±15.70 | 0.55¶ |
| Ratio of FEV1 to FVC | 0.50±0.15 | 0.48±0.15 | 0.52±0.16 | 0.35¶ |
| Total lung capacity — % of predicted value | 105.21±25.63 | 106.70±29.45 | 103.83±21.71 | 0.61¶ |
| Functional residual capacity | ||||
| Volume — ml | 3000±905 | 3175±1059 | 2838±710 | 0.20¶ |
| % of predicted value | 112.49±31.32 | 116.61±38.29 | 108.67±22.97 | 0.43 |
| Residual volume — % of predicted value | 141.42±59.22 | 147.48±69.25 | 135.78±48.15 | 0.80¶ |
| DLCO | ||||
| Diffusing capacity — ml/mm Hg/min | 10.23±4.61 | 10.42±4.82 | 10.05±4.47 | 0.52¶ |
| % of predicted value | 43.43±18.97 | 43.77±20.56 | 43.12±17.66 | 0.70¶ |
| 6-Minute walk distance — m | 403±105 | 399±115 | 407±96 | 0.78¶ |
| Health-related symptom scores | ||||
| EuroQOL visual-analogue scale for quality of life|| | 67.82±19.25 | 67.09±20.11 | 68.5±18.61 | 0.83¶ |
| Functional Performance Inventory** | 2.29±0.50 | 2.35±0.49 | 2.25±0.51 | 0.35¶ |
| Serum VEGF-D concentration — pg/ml | 2029±2343 | 2223±2997 | 1848±1514 | 0.57¶ |
Plus–minus values are means ±SD. DLCO denotes diffusing capacity for carbon monoxide, FEV1 forced expiratory volume in 1 second, FVC forced vital capacity, and VEGF-D vascular endothelial growth factor D.
Race was self-reported.
P value was calculated with the use of the chi-square test.
P value was calculated with the use of Fisher’s exact test.
P value was calculated with the use of the Wilcoxon rank-sum test.
The EuroQOL visual-analogue scale measures self-reported ratings of health status. Scores range from 0 to 100, with lower scores indicating worse functioning.
Scores on the Functional Performance Inventory range from 1 to 4, with lower scores indicating lower health status.
SERUM LEVELS OF SIROLIMUS AND ADHERENCE TO MEDICATION
The serum levels of sirolimus in the placebo group were below the detection limit throughout the study. Other than brief out-of-range excursions, the sirolimus levels in the active-treatment group were maintained between 5 and 15 ng per milliliter, except in the case of four patients in whom levels were intentionally kept below therapeutic levels for 3 months or more in order to control side effects.
PRIMARY ANALYSIS
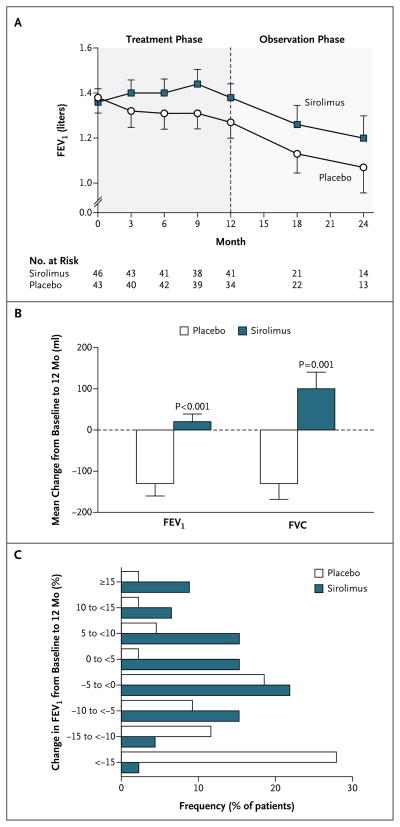
In the placebo group, the FEV1 slope from baseline to 12 months was −12±2 ml per month; the slope was significantly less than zero (P<0.001), a finding that was consistent with declining lung function (Table 2). The FEV1 slope in the sirolimus group was 1±2 ml per month, which was not significantly different from zero; this was indicative of the stabilization of lung function during treatment (Fig. 2A). There was a significant difference between the two groups in the FEV1 slope (P<0.001). The absolute difference in the mean change in FEV1 during the treatment period, calculated as the difference between the mean change in the placebo group (−134±182 ml) and the mean change in the sirolimus group (19±124 ml), was 153 ml (P<0.001 for the between-group difference) (Fig. 2B). A total of 12% of the patients in the placebo group, as compared with 46% of the patients in the sirolimus group, had FEV1 values at or above baseline values at the 12-month visit (P<0.001) (Fig. 2C).
Table 2.
Effects of Sirolimus on Primary and Selected Secondary Outcome Variables during the Treatment Period.*
| Variable | Value at 12 Months | Change from Baseline | Rate of Change per Month | |||||
|---|---|---|---|---|---|---|---|---|
| Placebo (N = 34) | Sirolimus (N = 41) | Placebo (N = 34) | Sirolimus (N = 41) | P Value† | Placebo (N = 43) | Sirolimus (N = 46) | P Value‡ | |
| Pulmonary function
| ||||||||
| FEV1 (ml) | 1272±414 | 1383±394 | −134±182§ | 19±124 | <0.001 | −12±2¶ | 1±2 | <0.001 |
|
| ||||||||
| FVC (ml) | 2843±668 | 2780±735 | −129±233§ | 97±260 | 0.001 | −11±3¶ | 8±3¶ | <0.001 |
|
| ||||||||
| Total lung capacity (ml) | 5464±1217 | 4944±982 | −7±650 | 94±504 | 0.65 | −2±7 | 8±7 | 0.34 |
|
| ||||||||
| Residual volume (ml) | 2502±969 | 2112±617 | −16 ±514 | 38±538 | 0.61 | −3±7 | 4±7 | 0.46 |
|
| ||||||||
| Functional residual capacity (ml) | 3260±968 | 2912±660 | −123±521 | 53±335 | 0.43 | −11±6 | 6±6 | 0.049 |
|
| ||||||||
| DLCO (ml/mm Hg/min) | 9.61±4.06 | 9.62±3.92 | −0.62±2.89§ | −0.06±1.50 | 0.38 | −0.06±0.03¶ | −0.01±0.02 | 0.17 |
|
| ||||||||
| 6-Minute walk distance (m) | 418±107 | 431±104 | 26±51§ | 24±59§ | 0.99 | 1.47±0.87 | 1.65±0.81¶ | 0.88 |
|
| ||||||||
| Score on EuroQOL visual-analogue scale for quality of life|| | 65.60±18.47** | 73.71±18.03 | −2.34±15.77 | 6.10±16.96 | 0.02 | −0.21±0.20 | 0.39±0.19¶ | 0.03 |
|
| ||||||||
| Total score on Functional Performance Inventory†† | 2.33±0.47 | 2.35±0.49 | −0.05±0.24 | 0.10±0.38 | 0.08 | −0.009±0.004¶ | 0.005±0.004 | 0.03 |
|
| ||||||||
| Serum VEGF-D (pg/ml) | 2444±3862** | 862±540 | −14.81±1113 | −1032±1301§ | 0.001 | −2.42±17.23 | −88.01±16.61¶ | 0.001 |
Plus–minus values are means ±SD. DLCO denotes diffusing capacity for carbon monoxide, FEV1 forced expiratory volume in 1 second, FVC forced vital capacity, and VEGF-D vascular endothelial growth factor D.
Two-sided P values for the comparison between the placebo and sirolimus groups of the mean change from baseline were calculated with the use of a general linear model, with adjustment for baseline levels of variables.
Two-sided P values for the comparison between the placebo and sirolimus groups of the rate of change per month were calculated with the use of a linear mixed-effects model.
The two-sided P value for the change from baseline within the placebo or sirolimus group, as calculated with the use of the Wilcoxon signed-rank test, was less than 0.05 for FVC and 6-minute walk distance, less than 0.01 for DLCO, and less than 0.001 for FEV1 and VEGF-D level.
The two-sided P value for the rate of change per month within the placebo or sirolimus group, as calculated with the use of a linear mixed-effects model, was less than 0.05 for the total score of the Functional Performance Inventory, the DLCO, and the EuroQOL visual-analogue scale, less than 0.01 for the FVC, and less than 0.001 for VEGF-D level and FEV1.
The EuroQOL visual-analogue scale measures self-reported ratings of health status. Scores range from 0 to 100, with lower scores indicating worse functioning.
The two-sided P value for the difference between the placebo and sirolimus groups at 12 months was less than 0.05, as calculated with the use of the Wilcoxon rank-sum test.
Scores on the Functional Performance Inventory range from 1 to 4, with lower scores indicating lower health status.
Figure 2. Change in Lung Function during the Treatmentand Observation Phases of the Trial.
Panel A shows the mean forced expiratory volume in 1 second (FEV1) at baseline and at each follow-up visit in the placebo and sirolimus groups. The number of patients for whom FEV1 data were available at each time point is also shown. Panel B shows the mean changes from baseline to 12 months in FEV1 and forced vital capacity (FVC) in the 34 patients in the placebo group and the 41 patients in the sirolimus group for whom 12-month data were available. Panel C shows the frequency of FEV1 changes, in increments or decrements of 5% of the baseline value, from baseline to 12 months. The percentage of patients who had any improvement in FEV1 was significantly greater in the sirolimus group than in the placebo group (46% vs. 12%, P<0.001). Conversely, a significantly greater percentage of patients in the placebo group than in the sirolimus group had some worsening of FEV1 (67% vs. 44%). In Panels A and B, T bars indicate standard errors.
SECONDARY ANALYSES
The FVC slope during the treatment phase was −11±3 ml per month in the placebo group, as compared with 8±3 ml per month in the sirolimus group (P<0.001) (Table 2). The FVC slope was significantly less than zero in the placebo group (P = 0.001), which was consistent with a decline in lung function, and the slope was significantly greater than zero in the sirolimus group (P = 0.009), which was consistent with an improvement in lung function during treatment. The absolute difference in the mean change in FVC during the treatment phase, calculated as the difference between the mean change in the placebo group (–129±233 ml) and the mean change in the sirolimus group (97±260 ml), was 226 ml (P = 0.001 for the between-group difference) (Fig. 2B). A total of 23% of the patients in the placebo group, as compared with 54% of patients in the sirolimus group, had FVC values that were at or above baseline values at the 12-month visit (P<0.001). The between-group difference in the slope for functional residual capacity during the treatment phase was also significant (P = 0.049), but the differences in the slopes for total lung capacity, residual volume, diffusing capacity for carbon monoxide, and distance covered on a 6-minute walk test were not significant (Table 2).
There were significant differences favoring sirolimus in the change from baseline to 12 months in the score on the EuroQOL visual-analogue scale for quality of life and in the total score on the Functional Performance Inventory. The changes in other measures of health-related symptoms did not differ significantly between the two groups (Table 2). Mean VEGF-D levels were similar in the two groups at baseline but were significantly lower in the sirolimus group than in the placebo group at 6 and 12 months (Tables 1 and 2).
ANALYSES OF DATA FROM THE OBSERVATION YEAR
FEV1 declined in both groups during the observation year (a decline of 8±2 ml per month in the placebo group and of 14±3 ml per month in the sirolimus group) (Fig. 2A). Although these slopes were both less than zero (P = 0.005 and P<0.001, respectively), the difference between them did not reach significance (P = 0.08). The mean change in FEV1 from baseline to 24 months did not differ significantly between the two groups (−180±100 ml in the placebo group and −150±170 ml in the sirolimus group). Similarly, with respect to FVC, there were no significant between-group differences in the observation-year slopes or the mean changes from baseline to 24 months. The mean serum VEGF-D levels at 24 months remained elevated in the placebo group (2107±2146 pg per milliliter in the 13 patients for whom data were available at 24 months) and depressed in the sirolimus group (930±461 pg per milliliter in the 14 patients for whom data were available at 24 months). There were no significant differences in the slopes from 12 to 24 months or in the mean change from baseline to 24 months in any other variables measured, including lung volumes, diffusing capacity for carbon monoxide, distance covered on a 6-minute walk test, and symptoms.
ADVERSE EVENTS
The most common adverse events during the treatment period were mucositis, diarrhea, nausea, hypercholesterolemia, acneiform rash, and swelling in the lower extremities (Table 3). The excess adverse events in the sirolimus group occurred mainly in eight categories: blood or bone marrow events, gastrointestinal events, dermatologic problems (1.9 events per patient in the placebo group vs. 3.0 events per patient in the sirolimus group, P<0.001), metabolic disturbances or abnormal laboratory results (2.2 events per patient in the placebo group vs. 2.8 events per patient in the sirolimus group, P = 0.04), musculo-skeletal or soft-tissue events, pain, neurologic events, and ocular or visual problems. Serious adverse cardiac events occurred only in the sirolimus group and included pericarditis, atrial arrhythmia (2 events in 1 patient), and tachycardia and fluid overload after embolization of an angiomyolipoma (2 events in 1 patient). Serious adverse pulmonary or upper respiratory events occurred only during the treatment period and were reported more frequently among patients receiving placebo than among those receiving sirolimus (P<0.001 by Fisher’s exact test). During the observation year, considerably fewer adverse events (both overall and per patient) occurred in both groups, but serious adverse events occurred more frequently in the placebo group than in the sirolimus group.
Table 3.
Total Adverse Events and Serious Adverse Events in the Two Study Groups.*
| Category | Total Adverse Events | Serious Adverse Events | ||||||
|---|---|---|---|---|---|---|---|---|
| Treatment Period | Observation Period | Treatment Period | Observation Period | |||||
| Placebo | Sirolimus | Placebo | Sirolimus | Placebo | Sirolimus | Placebo | Sirolimus | |
| number of events | ||||||||
| Allergy or immunologic event | 13 | 11 | 1 | 1 | ||||
|
| ||||||||
| Auditory or ear-related event | 2 | 4 | 0 | 1 | ||||
|
| ||||||||
| Blood or bone-marrow event | 4 | 12 | 1 | 0 | 0 | 1 | 0 | 0 |
|
| ||||||||
| Cardiac arrhythmia | 0 | 2 | 0 | 2 | ||||
|
| ||||||||
| Cardiac event, general | 12 | 15 | 3 | 2 | 0 | 5 | 0 | 0 |
|
| ||||||||
| Constitutional symptom | 35 | 46 | 7 | 7 | ||||
|
| ||||||||
| Death not related to an event | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
|
| ||||||||
| Dermatologic event | 41 | 106 | 8 | 11 | ||||
|
| ||||||||
| Endocrinologic event | 3 | 2 | 2 | 0 | 0 | 0 | 1 | 0 |
|
| ||||||||
| Gastrointestinal event | 181 | 275 | 12 | 20 | 1 | 3 | 0 | 0 |
|
| ||||||||
| Hemorrhage or bleeding event | 14 | 17 | 3 | 1 | 0 | 0 | 1† | 0 |
|
| ||||||||
| Hepatobiliary or pancreatic event | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
|
| ||||||||
| Infection | 74 | 78 | 20 | 24 | 3 | 2 | 1 | 0 |
|
| ||||||||
| Lymphatic event | 8 | 15 | 6 | 0 | ||||
|
| ||||||||
| Metabolic event or abnormal laboratory result | 26 | 56 | 1 | 6 | 0 | 1 | 0 | 0 |
|
| ||||||||
| Musculoskeletal or soft-tissue event | 21 | 35 | 2 | 4 | 0 | 1 | 0 | 1 |
|
| ||||||||
| Neurologic event | 27 | 33 | 4 | 7 | ||||
|
| ||||||||
| Ocular or visual problem | 3 | 8 | 1 | 2 | ||||
|
| ||||||||
| Pain | 115 | 130 | 9 | 13 | 1 | 7 | 0 | 0 |
|
| ||||||||
| Pulmonary or upper respiratory event | 121 | 97 | 17 | 32 | 13 | 2 | 0 | 0 |
|
| ||||||||
| Renal or genitourinary event | 8 | 11 | 0 | 2 | ||||
|
| ||||||||
| Sexual or reproductive problem | 8 | 5 | 0 | 0 | ||||
|
| ||||||||
| Vascular event | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 |
|
| ||||||||
| Total adverse events | 718 | 959 | 99 | 135 | ||||
|
| ||||||||
| Total serious adverse events | 18 | 23 | 5 | 1 | ||||
|
| ||||||||
| Definitely not related to study drug | 1 | 11 | 2 | 1 | ||||
|
| ||||||||
| Probably not related to study drug | 8 | 7 | 0 | 0 | ||||
|
| ||||||||
| Possibly related to study drug | 5 | 3 | 3 | 0 | ||||
|
| ||||||||
| Probably related to study drug | 4 | 2 | 0 | 0 | ||||
Adverse events were assessed with the use of the Common Terminology Criteria for Adverse Events (version 3.0). Details of the statistical analysis plan for adverse events are provided in the Supplementary Appendix.
A level 5 serious adverse event due to intracranial hemorrhage resulted in the death of one patient in the 18th month after the beginning of the study (the 6th month of the observation period).
DISCUSSION
Our study shows that treatment with sirolimus for 1 year has beneficial effects in patients with LAM, including the stabilization of FEV1 and improvement in FVC, quality of life, and some functional performance measures. No effects were observed on the diffusing capacity of the lung for carbon monoxide or on exercise tolerance, and the positive effects on airflow waned after sirolimus was discontinued. Sirolimus was associated with an increased frequency of adverse events, as compared with placebo, though the rates of serious adverse events were similar in the two study groups.
Although the minimum clinically important differences for measures of lung function have not been established in patients with LAM, the minimum clinically important difference in FEV1 in patients with chronic obstructive lung disease has been estimated to be 100 to 140 ml,22 which is a change that patients can perceive, that is a typical response after use of a bronchodilator, and that predicts a relapse after an exacerbation.22 The absolute mean between-group change of 153 ml in the FEV1 over the course of 1 year in our study compares favorably with this estimate. In the placebo group, the observed annual mean decline of 134 ml per year in the FEV1 from the baseline level of 1.38 liters indicates that lung function is lost at the rate of almost 10% per year in patients with moderately severe LAM. Therapies that stabilize lung function could potentially delay the need for lung transplantation, with its associated risks. The importance of the observed changes in lung function is further supported by the positive correlation with the scores on the Functional Performance Inventory and the EuroQOL visual-analogue scale for quality of life, both of which have been validated in patients with other lung diseases.23,24
Sirolimus therapy positively affected lung function only during the treatment period. After discontinuation of sirolimus, the FEV1 in patients in the sirolimus group declined in parallel with the decline in the placebo group, and the mean change from baseline to 24 months did not differ significantly between the two groups. Thus, sirolimus therapy for 1 year did not appear to accelerate the subsequent rate of decline in lung function — a theoretical concern raised by the apparent rebound increase in growth rates of angiomyolipomas after withdrawal of sirolimus.18 These data should be interpreted with caution, given the high withdrawal rate in the observation period and the early termination of the second trial year for some patients. Other study limitations include the possibility that the treatment assignments may have been inadvertently revealed owing to cholesterol elevations and the development of mouth ulcers and rashes in some patients in the sirolimus group.
Sirolimus was associated with an improvement in FVC; the absolute mean change in FVC (230 ml) was similar in magnitude to the minimum clinically important difference of 250 ml proposed for scleroderma-related lung disease.25 The increase in FVC with sirolimus was accompanied by an increase in the slope for functional residual capacity and trends toward increases in the slopes for total lung capacity and residual volume, suggesting that a reduction in restrictive impairment is a potential mechanism for higher airflow. The reduction in residual volume noted in a previous open-label trial, which suggested a reduction in gas trapping, was not seen in our trial.18
Serious adverse respiratory events occurred less frequently in the sirolimus group than in the placebo group during the treatment period, a finding that is consistent with a potential beneficial effect of the drug on other manifestations of the disease. The between-group difference of 8.44 from baseline to 12 months in the quality of life scores on the EuroQOL visual-analogue scale (on which scores range from 0 to 100, with lower scores indicating worse functioning) was greater than half the standard deviation, an accepted threshold for clinical significance.26 The lack of a significant between-group difference in the distance covered on a 6-minute walk test suggests that improvement in lung function may not be accompanied by an increase in exercise capacity, though a treatment effect might have been obscured by the relatively high baseline exercise tolerance of the patients or limitations in the performance characteristics of the test.
Levels of serum VEGF-D, a lymphangiogenic growth factor implicated in the pathophysiology of LAM,11,12 were reduced in response to sirolimus. The persistent depression in mean VEGF-D levels after discontinuation of the drug may be consistent with a durable treatment effect in some patients, but this finding is difficult to interpret, given the attrition that occurred during the observation year. Further study is needed to determine whether it is possible to predict which patients will benefit from sirolimus treatment and whether the VEGF-D level can serve as a biomarker of disease severity, disease progression, or treatment response.
These results indicate that sirolimus may be useful in treating patients with moderately severe LAM-related lung disease. LAM typically progresses slowly, and given the risks of sirolimus therapy, treatment decisions should be made on an individual basis. The mean FEV1 for patients in this trial (48% of the predicted value) was lower than the population-based mean among patients in the LAM Registry of the NIH27 (70% of the predicted value), making it difficult to generalize the results from the patients in this study to patients with milder or more severe lung disease due to LAM. Since the stabilization of lung function appears to require continuous exposure to sirolimus, additional trials are needed to determine which patients will benefit from treatment and the optimal dose and duration of treatment. Given the side-effect profile of the drug during a 1-year period, future studies must carefully evaluate the long-term safety of sirolimus.
Supplementary Material
Acknowledgments
Supported by grants from the NIH Office of Rare Disease Research, administered by the National Center for Research Resources (RR019498, to Drs. Trapnell and McCormack; and RR019259, to Drs. Lee and Krischer), the Food and Drug Administration (FD003362, to Dr. McCormack), Canadian Institutes of Health Research (to Drs. Downey, Cohen, and Singer), Pfizer (formerly Wyeth) Pharmaceuticals (to Dr. McCormack), the Japanese Ministry of Health, Labor, and Welfare (H 19 Rinshoshiken 008, to Drs. Nakata and Inoue), the LAM Foundation (to Dr. McCormack), the Tuberous Sclerosis Alliance (Rothberg Courage Award, to Dr. McCormack), Cincinnati Children’s Hospital Medical Center (Institutional Clinical and Translational Science Award 1UL1RR026314 01, to Drs. Young and McCormack, and Translational Research Initiative Award, to Drs. McCormack and Trapnell), Vi and John Adler, and the Adler Foundation. Drs. Moss and Taveira-DaSilva were supported by the Division of Intramural Research, National Heart, Lung, and Blood Institute. Pfizer provided the study drug and money for study visit costs.
We thank John Bissler, M.D., for his assistance in providing the Cine MRI of a woman with tuberous sclerosis and lymphangioleiomyomatosis.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Taveira-DaSilva AM, Steagall WK, Moss J. Lymphangioleiomyomatosis. Cancer Control. 2006;13:276–85. doi: 10.1177/107327480601300405. [DOI] [PubMed] [Google Scholar]
- 2.McCormack FX. Lymphangioleiomyomatosis: a clinical update. Chest. 2008;133:507–16. doi: 10.1378/chest.07-0898. [DOI] [PubMed] [Google Scholar]
- 3.Urban T, Lazor R, Lacronique J, et al. Pulmonary lymphangioleiomyomatosis: a study of 69 patients. Medicine (Baltimore) 1999;78:321–37. doi: 10.1097/00005792-199909000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Johnson SR, Tattersfield AE. Decline in lung function in lymphangioleiomyomatosis: relation to menopause and progesterone treatment. Am J Respir Crit Care Med. 1999;160:628–33. doi: 10.1164/ajrccm.160.2.9901027. [DOI] [PubMed] [Google Scholar]
- 5.Taveira-DaSilva AM, Stylianou MP, Hedin CJ, Hathaway O, Moss J. Decline in lung function in patients with lymphangioleiomyomatosis treated with or without progesterone. Chest. 2004;126:1867–74. doi: 10.1378/chest.126.6.1867. [DOI] [PubMed] [Google Scholar]
- 6.Johnson SR, Whale CI, Hubbard RB, Lewis SA, Tattersfield AE. Survival and disease progression in UK patients with lymphangioleiomyomatosis. Thorax. 2004;59:800–3. doi: 10.1136/thx.2004.023283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henske EP. Metastasis of benign tumor cells in tuberous sclerosis complex. Genes Chromosomes Cancer. 2003;38:376–81. doi: 10.1002/gcc.10252. [DOI] [PubMed] [Google Scholar]
- 8.Crooks DM, Pacheco-Rodriguez G, DeCastro RM, et al. Molecular and genetic analysis of disseminated neoplastic cells in lymphangioleiomyomatosis. Proc Natl Acad Sci U S A. 2004;101:17462–7. doi: 10.1073/pnas.0407971101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carsillo T, Astrinidis A, Henske EP. Mutations in the tuberous sclerosis complex gene TSC2 are a cause of sporadic pulmonary lymphangioleiomyomatosis. Proc Natl Acad Sci U S A. 2000;97:6085–90. doi: 10.1073/pnas.97.11.6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sengupta S, Peterson TR, Sabatini DM. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol Cell. 2010;40:310–22. doi: 10.1016/j.molcel.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumasaka T, Seyama K, Mitani K, et al. Lymphangiogenesis-mediated shedding of LAM cell clusters as a mechanism for dissemination in lymphangioleiomyomatosis. Am J Surg Pathol. 2005;29:1356–66. doi: 10.1097/01.pas.0000172192.25295.45. [DOI] [PubMed] [Google Scholar]
- 12.Kumasaka T, Seyama K, Mitani K, et al. Lymphangiogenesis in lymphangioleiomyomatosis: its implication in the progression of lymphangioleiomyomatosis. Am J Surg Pathol. 2004;28:1007–16. doi: 10.1097/01.pas.0000126859.70814.6d. [DOI] [PubMed] [Google Scholar]
- 13.Karbowniczek M, Astrinidis A, Balsara BR, et al. Recurrent lymphangiomyomatosis after transplantation: genetic analyses reveal a metastatic mechanism. Am J Respir Crit Care Med. 2003;167:976–82. doi: 10.1164/rccm.200208-969OC. [DOI] [PubMed] [Google Scholar]
- 14.Bittmann I, Rolf B, Amann G, Lohrs U. Recurrence of lymphangioleiomyomatosis after single lung transplantation: new insights into pathogenesis. Hum Pathol. 2003;34:95–8. doi: 10.1053/hupa.2003.50. [DOI] [PubMed] [Google Scholar]
- 15.Goncharova EA, Goncharov DA, Eszterhas A, et al. Tuberin regulates p70 S6 kinase activation and ribosomal protein S6 phosphorylation: a role for the TSC2 tumor suppressor gene in pulmonary lymphangioleiomyomatosis (LAM) J Biol Chem. 2002;277:30958–67. doi: 10.1074/jbc.M202678200. [DOI] [PubMed] [Google Scholar]
- 16.Kwiatkowski DJ, Zhang H, Bandura JL, et al. A mouse model of TSC1 reveals sex-dependent lethality from liver hemangiomas, and up-regulation of p70S6 kinase activity in Tsc1 null cells. Hum Mol Genet. 2002;11:525–34. doi: 10.1093/hmg/11.5.525. [DOI] [PubMed] [Google Scholar]
- 17.Lee L, Sudentas P, Donohue B, et al. Efficacy of a rapamycin analog (CCI-779) and IFN-gamma in tuberous sclerosis mouse models. Genes Chromosomes Cancer. 2005;42:213–27. doi: 10.1002/gcc.20118. [DOI] [PubMed] [Google Scholar]
- 18.Bissler JJ, McCormack FX, Young LR, et al. Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N Engl J Med. 2008;358:140–51. doi: 10.1056/NEJMoa063564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davies DM, Johnson SR, Tattersfield AE, et al. Sirolimus therapy in tuberous sclerosis or sporadic lymphangioleiomyomatosis. N Engl J Med. 2008;358:200–3. doi: 10.1056/NEJMc072500. [DOI] [PubMed] [Google Scholar]
- 20.Paul E, Thiele E. Efficacy of sirolimus in treating tuberous sclerosis and lymphangioleiomyomatosis. N Engl J Med. 2008;358:190–2. doi: 10.1056/NEJMe0707153. [DOI] [PubMed] [Google Scholar]
- 21.Young LR, Vandyke R, Gulleman PM, et al. Serum vascular endothelial growth factor-D prospectively distinguishes lymphangioleiomyomatosis from other diseases. Chest. 2010;138:674–81. doi: 10.1378/chest.10-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Donohue JF. Minimal clinically important differences in COPD lung function. COPD. 2005;2:111–24. doi: 10.1081/copd-200053377. [DOI] [PubMed] [Google Scholar]
- 23.Rutten-van Mölken MP, Oostenbrink JB, Tashkin DP, Burkhart D, Monz BU. Does quality of life of COPD patients as measured by the generic EuroQol five-dimension questionnaire differentiate between COPD severity stages? Chest. 2006;130:1117–28. doi: 10.1378/chest.130.4.1117. [DOI] [PubMed] [Google Scholar]
- 24.Larson JL, Kapella MC, Wirtz S, Covey MK, Berry J. Reliability and validity of the Functional Performance Inventory in patients with moderate to severe chronic obstructive pulmonary disease. J Nurs Meas. 1998;6:55–73. [PubMed] [Google Scholar]
- 25.Khanna D, Brown KK, Clements PJ, et al. Systemic sclerosis-associated interstitial lung disease — proposed recommendations for future randomized clinical trials. Clin Exp Rheumatol. 2010;28:S55–62. [PubMed] [Google Scholar]
- 26.Norman GR, Sloan JA, Wyrwich KW. The truly remarkable universality of half a standard deviation: confirmation through another look. Expert Rev Pharmacoecon Outcomes Res. 2004;4:581–5. doi: 10.1586/14737167.4.5.581. [DOI] [PubMed] [Google Scholar]
- 27.Ryu JH, Moss J, Beck GJ, et al. The NHLBI lymphangioleiomyomatosis registry: characteristics of 230 patients at enrollment. Am J Respir Crit Care Med. 2006;173:105–11. doi: 10.1164/rccm.200409-1298OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.