Abstract
Fatty liver disease (FLD), associated with chronic alcohol consumption or obesity, is a serious medical problem. Strong evidence indicates that oxidative stress and dysregulation of redox-sensitive signaling pathways are central to the pathobiology of FLD. Herein, this Forum summarizes current knowledge regarding mechanisms of FLD from both clinical and experimental studies. Special emphasis is given to the role of redox biology disturbances in the initiation and progression of FLD from both chronic alcohol consumption and obesity. Focus areas in this Forum include discussions on the (i) multi-hit hypothesis; (ii) interaction of adipokines and redox signaling pathways; (iii) role of sub-cellular organelle systems (i.e., endoplasmic reticulum and mitochondria); and (iv) contribution of the innate immune system, in FLD. A state-of-the-art discussion is also included highlighting key lessons learned from experimental studies using rodent models of FLD. Antioxid. Redox Signal. 15, 421–424.
Fatty liver disease (FLD), a pathology of chronic alcohol consumption or obesity with or without type 2 diabetes mellitus (T2DM), is a serious medical and public health problem for many countries. Briefly, alcoholic and nonalcoholic fatty liver disease (AFLD and NAFLD) are a spectrum of liver pathologies encompassed by the initial early stage of steatosis (hepatocyte triglyceride accumulation) to the more serious conditions of steatohepatitis and fibrosis. Notably, both alcoholic and nonalcoholic steatohepatitis (ASH and NASH) are recognized as potential precursors to cirrhosis and hepatocellular carcinoma. Heavy alcohol consumption is ranked as the third leading cause of preventable death in the United States, with AFLD continuing to be a significant cause of morbidity and mortality. It is estimated that ∼12,000 deaths occur each year from alcohol-related chronic liver disease and cirrhosis (18). It has been a long-standing belief that AFLD is largely dependent on both the total dose and duration of alcohol intake, with decades of heavy alcohol drinking required to cause serious end-stage liver diseases like ASH and cirrhosis. However, this simple mechanism of AFLD causation has recently been expanded to include the possibility of a complex interplay of genetic, epigenetic, metabolic, viral, immune, and/or environmental factors with chronic alcohol to facilitate the progression from steatosis to cirrhosis and cancer (5). Importantly, metabolic derangements linked to the cardiometabolic syndrome like obesity, hyperlipidemia, and insulin resistance may even exacerbate liver injury in the chronic alcohol consumer (2, 17, 25). Therefore, future studies (clinical and basic) should be directed at investigating the impact of cardiometabolic disease risk factors like obesity and hyperlipidemia to increase the hepatotoxicity of alcohol.
Unlike AFLD, NAFLD is a more recently recognized pathology emerging onto the clinical scene in only the past 20 years to become the most common cause of chronic liver disease for both adults and children (12, 20). It is currently estimated that the prevalence of NAFLD is 20% in the general U.S. population (24). Ludwig and colleagues (13) first classified NASH as a sub-category of FLD that is histologically identical to ASH but occurs in overweight and/or T2DM patients who do not drink alcohol to excess; for example, alcohol intake no greater than 40 g/day for men and 20 g/day for women (20). NASH patients typically present with moderate to severe steatosis with lobular inflammation and fibrosis present in the majority of patients (3). As with AFLD, the pathobiology of NAFLD is linked to multiple factors and stressors including obesity, central adiposity, hyperlipidemia, and T2DM, with insulin resistance postulated as an essential causative factor (7). Recent data also support an involvement of insulin resistance in AFLD (23); thus, highlighting the overlapping molecular mechanisms and targets involved in liver disease development from two dissimilar impacts: chronic alcohol intake and obesity/T2DM.
In addition to insulin resistance and disrupted lipid metabolism, mitochondrial dysfunction, oxidative stress, and dysregulated cytokine networks are proposed to be critical factors or “hits” responsible for the progression from simple steatosis to either ASH or NASH (15). Currently, the chief mechanism for AFLD and NAFLD is the “multi-hit” hypothesis, with the “first hit” being steatosis, which when accompanied by additional “hits” triggers the progression to the more serious pathologies of ASH and NASH (Fig. 1). Numerous examples of hits have been proposed and include external factors like diet and environmental toxicants, and internal factors like reactive oxygen species (ROS) and reactive nitrogen species, inflammatory mediators, and hepatocyte death (necrosis and/or apoptosis), just to name a few. Moreover, what many of these hits have in common is that they induce oxidative and/or nitrative stress and disrupt redox signaling in liver. A more precise understanding of the molecular processes involved in dysregulated redox biology will be critical in implementing efficacious therapies for treating AFLD and NAFLD. In this Forum, we summarize current knowledge about FLD pathogenesis (Fig. 1), together with a comprehensive overview of new work highlighting the instrumental role of oxidative stress and the ensuing disruption of redox signaling in development of AFLD and NAFLD.
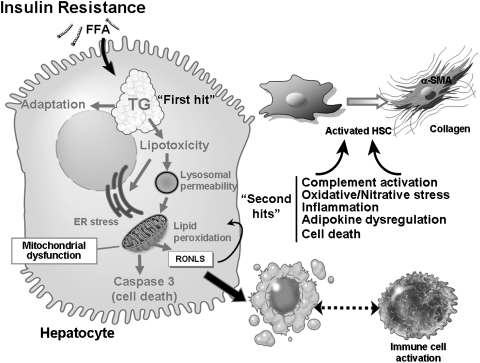
FIG. 1.
Current understanding of the pathophysiology of alcoholic steatohepatitis and nonalcoholic steatohepatitis. Recent advances point to the following molecular events being critical for fatty liver disease (FLD) pathology. Insulin resistance from both chronic alcohol consumption and obesity is now recognized as a critical event for development of steatosis: the first-hit of FLD. Early in the disease process the liver adapts to excess free fatty acid (FFA) to prevent triglyceride (TG) accumulation; however, over time these adaptive mechanisms fail resulting to TG accumulation, steatosis, and lipotoxicity. Lipotoxicity can trigger ER stress, lysosomal permeability, and mitochondrial dysfunction. As a consequence, there is increased production of a wide variety of reactive oxygen, nitrogen, and lipid species (RONLS), which dysregulate multiple redox-sensitive signaling pathways leading to increased TG accumulation. The ensuing disruption of mitochondrial function triggers apoptotic and necrotic cell death: key second-hits in FLD pathobiology. In conjunction, release of reactive species from hepatocytes can trigger activation of immune cells and convert the normally quiescent stellate cells to a fibrosis-inducing cell. It is this combination of these events and others presented within this Forum that are involved in the progression from steatosis to steatohepatitis to fibrosis/cirrhosis.
Part of this Forum is comprised of five review articles. In one review Maher (14) gives an inclusive perspective on multiple rodent models of NAFLD and NASH. This review focuses on many of the key lessons learned about NAFLD and NASH pathogenesis from studies using rodents with a genetic predisposition to overeat to rodents (wild-type or transgenic) that are fed high-energy (fat or carbohydrate) diets. Emphasized in this review is that many, if not all, genetic or dietary rodent models of FLD fail to precisely replicate the disease found in human patients. Therefore, the continuing challenge for FLD researchers is to find the correct combination of genetic background, diet, and environment (e.g., exercise and housing) that best reproduces the human disease. While not discussed in this review, the same concerns exist for models of AFLD and ASH. Indeed, proper characterization and understanding of the functional and pathological differences among experimental models is required to elucidate the role of oxidative stress in FLD.
Findings from numerous experimental and clinical studies of FLD have demonstrated a clear role for the disruption of adipokine (adipose tissue cytokines) networks in FLD pathogenesis. Importantly, adipose tissue inflammation alters the pattern of adipokine secretion from adipose tissue with the negative consequence of contributing, in part, to FLD from both alcohol and obesity. On this topic, Parola and Marra (21) provide a new understanding of the potential role of ROS in affecting adipokine action in the development of NAFLD, hepatic insulin resistance, inflammation, and fibrosis. Emphasis is given to understanding the interplay between adiponectin, leptin, and resistin and ROS, and downstream effects on key redox-sensitive signaling pathways.
Two articles in this Forum summarize advances in our understanding of the role of key sub-cellular organelles, the endoplasmic reticulum (ER) and the mitochondrion, in FLD. Recent studies have reported that disruption in ER homeostasis, termed “ER stress,” occurs in liver of obese and/or NAFLD patients (10). Important to these observations is that a key consequence of ER stress; the unfolded protein response (UPR) is linked to lipid metabolism, insulin signaling, and inflammation; pathways similarly altered under conditions of oxidative stress. Gentile et al. (8) discuss the interaction of UPR and redox signaling pathways in the context of NAFLD. Importantly, several UPR-dependent pathways may be critical in activating cellular defenses against oxidative stress in NAFLD; however, when these protective systems fail, ER stress-mediated disease progression may occur leading to severe pathology in patients.
As a site critical for fat metabolism and ROS production, the mitochondrion has been at the forefront of AFLD and NAFLD research for many years. Indeed, mitochondrial ROS production was proposed to be the key second hit responsible for disease progression from simple steatosis to steatohepatitis from alcohol or nonalcohol sources (16, 22). As presented, by Morris et al. (19) the role of mitochondria in liver disease pathogenesis continues to expand. Studies are presented in this review showing the direct impact of mitochondrial ROS on regulation of redox-sensitive signaling pathways like AMPK and the use of antioxidants (coenzyme Q10) in the treatment of FLD. Nonmitochondrial sources of ROS and reactive nitrogen species are also chief contributors to oxidative stress in AFLD and NAFLD especially from nonparenchymal liver cells. Cohen et al. (4) discuss the critical role of cells of the innate immune system in the pathobiology of AFLD. Studies are highlighted which indicate that chronic alcohol consumption (1) enhances TLR-4-mediated cytokine expression presumably through dysregulation of key redox signaling pathways and (2) decreases a key antioxidant thioredoxin, in liver. Moreover, emerging evidence implicates alcohol-mediated ROS production in activation of the complement system, which worsens AFLD through induction of various pro-inflammatory cytokine networks. Taken together, these reviews showcase the complexity of AFLD and NAFLD pathogenesis, which involves multiple cellular and sub-cellular networks dynamically interacting to exacerbate liver injury through dysregulation of redox-sensitive signaling pathways and targets.
To complete this Forum, three original research contributions are presented highlighting the role of redox disturbances in both AFLD and NAFLD. Liang et al. (11) show an interaction between saturated fat and chronic alcohol to upregulate hepatic adiponectin receptor 2 (AdipoR2) through a sirtuin 1 (SIRT1) and forkhead transcription O1-dependent mechanism. These findings are significant as downregulation of AdipoR2 occurs in response to chronic alcohol consumption and contributes to AFLD (1). Importantly, the work presented in this Forum shows for the first time that the alcohol-dependent movement of SIRT1 from the nucleus to the cytoplasm is redox dependent. Thus, Liang et al. (11) provide evidence to support the concept that nutritional and/or pharmacological interventions targeting the SIRT1-forkhead transcription O1-AdipoR2 axis may be novel therapies for AFLD and NAFLD. Further evidence in support of redox dysregulation in FLD comes from the work of Gornicka et al. (9), showing the impact of a NASH-inducing diet (methionine-choline deficient diet) on antioxidant gene expression profiles. Specifically, this work shows increases in flavin-containing mono-oxygenase 2, glutathione peroxidase, and peroxiredoxins, whereas stearoyl-CoA desaturase-1 (SCD1) was dramatically decreased in livers of mice with NASH compared to controls. SCD1 facilitates the movement of free fatty acids into triglycerides, phospholipids, and cholesterol esters. The decrease in SCD1 is important as this could have the effect of enhancing the delivery of free fatty acids to mitochondria, resulting in increased mitochondrial ROS production, which was indirectly verified through increased markers of oxidative tissue damage (i.e., increased 4-hydroxynonenal adducts).
In the remaining article of this Forum, Eccleston et al. (6) present novel findings demonstrating that chronic exposure to a high-fat diet induces steatosis, modifies the liver mitochondrial proteome, and decreases nitric oxide (NO) bioavailability in mice. Twenty-plus proteins were altered in abundance in response to a high-fat diet, including proteins involved in energy metabolism, chaperone function, beta-oxidation, and sulfur amino acid metabolism. These results reveal novel targets in mitochondria that might be suitable for forthcoming NAFLD/NASH therapeutic studies. Moreover, a chronic high-fat diet decreased NO concentrations in liver. This is predicted to disrupt mitochondrial function as NO functions through a redox-dependent mechanism to stimulate mitochondrial biogenesis. Thus, low NO might be considered as a second hit in the disease progression of NAFLD.
In summary, this Forum explores the complex mechanisms through which chronic alcohol consumption and obesity amplify oxidative stress and disrupt signaling pathways that are under redox control in liver. Aggressive research efforts are warranted to further elucidate the pathogenesis of AFLD and NAFLD with the goal of developing accurate disease biomarker tests for both diseases and novel agents and regimens for treatment. We would like to thank the authors who took time out of their busy schedules to make this Forum possible, as well as to all the reviewers who provided outstanding feedback on the articles. We hope that the articles presented in this Forum will increase understanding of the molecular mechanisms involved in ALFD and NAFLD and stimulate new research investigations focused on redox biology and signaling in the context of these important diseases.
Abbreviations Used
- adipoR2
adiponectin receptor 2
- AFLD
alcoholic fatty liver disease
- ASH
alcoholic steatohepatitis
- ER stress
endoplasmic reticulum stress
- FFA
free fatty acid
- FLD
fatty liver disease
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- NO
nitric oxide
- RONLS
reactive oxygen, nitrogen, and lipid species
- ROS
reactive oxygen species
- SCD1
stearoyl-CoA desaturase-1
- SIRT1
sirtuin 1
- T2DM
type 2 diabetes mellitus
- TG
triglyceride
- UPR
unfolded protein response
References
- 1.Ajmo JM. Liang X. Rogers CQ. Pennock B. You M. Resveratrol alleviates alcoholic fatty liver in mice. Am J Physiol Gastrointest Liver Physiol. 2008;295:G833–G842. doi: 10.1152/ajpgi.90358.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bailey SM. Mantena SK. Millender-Swain T. Cakir Y. Jhala NC. Chhieng D. Pinkerton KE. Ballinger SW. Ethanol and tobacco smoke increase hepatic steatosis and hypoxia in the hypercholesterolemic apoE-/- mouse: implications for a “multi-hit” hypothesis of fatty liver disease. Free Radic Biol Med. 2009;46:928–938. doi: 10.1016/j.freeradbiomed.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brunt EM. Pathology of nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2010;7:195–203. doi: 10.1038/nrgastro.2010.21. [DOI] [PubMed] [Google Scholar]
- 4.Cohen JI. Chen X. Nagy LE. Redox signaling and the innate immune system in alcoholic liver disease. Antioxid Redox Signal. 2011;15:523–534. doi: 10.1089/ars.2010.3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Day CP. Genes or environment to determine alcoholic liver disease and non-alcoholic fatty liver disease. Liver Int. 2006;26:1021–1028. doi: 10.1111/j.1478-3231.2006.01323.x. [DOI] [PubMed] [Google Scholar]
- 6.Eccleston HB. Andringa KK. Betancourt AM. King AL. Mantena SK. Swain TM. Tinsley HN. Nolte RN. Nagy TR. Abrams GA. Bailey SM. Chronic exposure to a high-fat diet induces hepatic steatosis, impairs nitric oxide bioavailability, and modifies the mitochondrial proteome in mice. Antioxid Redox Signal. 2011;15:447–459. doi: 10.1089/ars.2010.3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feldstein AE. Novel insights into the pathophysiology of nonalcoholic fatty liver disease. Semin Liver Dis. 2010;30:391–401. doi: 10.1055/s-0030-1267539. [DOI] [PubMed] [Google Scholar]
- 8.Gentile CL. Frye M. Pagliassotti MJ. Endoplasmic reticulum stress and the unfolded protein response in nonalcoholic fatty liver disease. Antioxid Redox Signal. 2011;15:505–521. doi: 10.1089/ars.2010.3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gornicka A. Morris-Stiff G. Thapaliya S. Papouchado BG. Berk M. Feldstein AE. Transcriptional profile of genes involved in oxidative stress and antioxidant defense in a dietary murine model of steatohepatitis. Antioxid Redox Signal. 2011;15:437–445. doi: 10.1089/ars.2010.3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kapoor A. Sanyal AJ. Endoplasmic reticulum stress and the unfolded protein response. Clin Liver Dis. 2009;13:581–590. doi: 10.1016/j.cld.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 11.Liang X. Hu M. Rogers CQ. Shen Z. You M. Role of SIRT1-FoxO1 signaling in dietary saturated fat-dependent upregulation of liver adiponectin receptor 2 in ethanol-administered mice. Antioxid Redox Signal. 2011;15:425–435. doi: 10.1089/ars.2010.3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loomba R. Sirlin CB. Schwimmer JB. Lavine JE. Advances in pediatric nonalcoholic fatty liver disease. Hepatology. 2009;50:1282–1293. doi: 10.1002/hep.23119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ludwig J. Viggiano TR. McGill DB. Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980;55:434–438. [PubMed] [Google Scholar]
- 14.Maher JJ. New insights from rodent models of fatty liver disease. Antioxid Redox Signal. 2011;15:535–550. doi: 10.1089/ars.2010.3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mantena SK. King AL. Andringa KK. Eccleston HB. Bailey SM. Mitochondrial dysfunction and oxidative stress in the pathogenesis of alcohol- and obesity-induced fatty liver diseases. Free Radic Biol Med. 2008;44:1259–1272. doi: 10.1016/j.freeradbiomed.2007.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mantena SK. King AL. Andringa KK. Landar A. Darley-Usmar V. Bailey SM. Novel interactions of mitochondria and reactive oxygen/nitrogen species in alcohol mediated liver diseases. World J Gastroenterol. 2007;13:4967–4973. doi: 10.3748/wjg.v13.i37.4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marrero JA. Fontana RJ. Fu S. Conjeevaram HS. Su GL. Lok AS. Alcohol, tobacco and obesity are synergistic risk factors for hepatocellular carcinoma. J Hepatol. 2005;42:218–224. doi: 10.1016/j.jhep.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Minino AM. Heron MP. Smith BL. Deaths: preliminary data for 2004. Natl Vital Stat Rep. 2006;54:1–49. [PubMed] [Google Scholar]
- 19.Morris EM. Rector RS. Thyfault JP. Ibdah JA. Mitochondria and redox signaling in steatohepatitis. Antioxid Redox Signal. 2011;15:485–504. doi: 10.1089/ars.2010.3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neuschwander-Tetri BA. Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology. 2003;37:1202–1219. doi: 10.1053/jhep.2003.50193. [DOI] [PubMed] [Google Scholar]
- 21.Parola M. Marra F. Adipokines and redox signaling: impact on fatty liver disease. Antioxid Redox Signal. 2011;15:461–483. doi: 10.1089/ars.2010.3848. [DOI] [PubMed] [Google Scholar]
- 22.Pessayre D. Role of mitochondria in non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2007;22(Suppl 1):S20–S27. doi: 10.1111/j.1440-1746.2006.04640.x. [DOI] [PubMed] [Google Scholar]
- 23.Ronis MJ. Wands JR. Badger TM. de la Monte SM. Lang CH. Calissendorff J. Alcohol-induced disruption of endocrine signaling. Alcohol Clin Exp Res. 2007;31:1269–1285. doi: 10.1111/j.1530-0277.2007.00436.x. [DOI] [PubMed] [Google Scholar]
- 24.Ruhl CE. Everhart JE. Epidemiology of nonalcoholic fatty liver. Clin Liver Dis. 2004;8:501–519. doi: 10.1016/j.cld.2004.04.008. , vii. [DOI] [PubMed] [Google Scholar]
- 25.Tsukamoto H. Machida K. Dynnyk A. Mkrtchyan H. “Second hit” models of alcoholic liver disease. Semin Liver Dis. 2009;29:178–187. doi: 10.1055/s-0029-1214373. [DOI] [PMC free article] [PubMed] [Google Scholar]