Abstract
The aim of the present study is to examine the effects of dietary saturated fatty acids on liver adiponectin receptor 1 (AdipoR1) and adiponectin receptor 2 (AdipoR2) in ethanol-administered animals and in ethanol-exposed cultured hepatic cells, and to explore the underlying molecular mechanisms. The mRNA and protein levels of hepatic AdipoR2 were selectively increased by chronic ethanol feeding to mice consuming a diet high in saturated fat (HSF). Administration of an HSF diet blocked hyperacetylation of forkhead transcription factor 1 (FoxO1), a known target of sirtuin 1 (SIRT1), increased nuclear FoxO1 protein levels, and enhanced association of FoxO1 with the AdipoR2 promoter in the livers of ethanol-fed mice. Treatment of cultured hepatic cells with palmitic acid (a major saturated fatty acid in HSF diet) in the presence of ethanol robustly increased AdipoR2 mRNA expression and enhanced activity of a mouse AdipoR2 promoter. Knocking down SIRT1 or FoxO1 using the small silencing SIRT1 or FoxO1 plasmid blunted the palmitic acid effect. Taken together, these results reveal that dietary saturated fat selectively upregulates hepatic AdipoR2 through modulation of SIRT1-FoxO1 signaling in ethanol fed mice, and this effect may contribute to the protective effect of the HSF diet against alcoholic fatty liver. Antioxid. Redox Signal. 15, 425–435.
Introduction
Adiponectin is a hormone largely secreted by adipocytes, which circulates in the plasma in a mixture of quaternary structures (26). It predominantly exists as a low-molecular weight trimer (LMW), a middle-molecular weight hexamer (MMW), and a high-molecular weight multimer (HMW), each form conferring various biologic activities (35). These activities are thought to be mediated primarily through adiponectin receptor 1 (AdipoR1) and adiponectin receptor 2 (AdipoR2) (26, 35). AdipoR1 is expressed in various tissues with a relatively high level in skeletal muscle, whereas AdipoR2 is predominantly expressed in the liver.
Forkhead transcription factor O1 (FoxO1) is a member of the forkhead transcription factor class O family (9). FoxO1 has been established as a key player in the regulation of various signaling pathways involved in lipid metabolism and oxidative stress response. The regulation of FoxO1 is complex. FoxO1 is regulated by changes in subcellular localization coupled to post-translational modifications, including phosphorylation, ubiquitination, and acetylation (9).
Sirtuins 1 (SIRT1) is an NAD+-dependent protein deacetylase regulating gene expression through removing acetyl groups from modified lysine residues of histones and various transcriptional regulators (41). FoxO1 is known to be a major target of SIRT1. Studies have demonstrated that SIRT1 deacetylates lysine residues within the FoxO1 DNA binding domain, and promotes the nuclear retention of FoxO1 increasing its transcriptional activity (7, 8, 15, 22). Nevertheless, the effect of SIRT1-dependent deacetylation on FoxO1 function is somewhat controversial, with some studies suggesting that deacetylation inhibits-not increases-FoxO1-dependent transcriptional activity (21, 36).
While little is known about the regulation of adiponectin receptors, SIRT1-FoxO1 signaling has been shown to affect hepatic AdipoR1/R2 gene expression (1, 2, 6, 10, 11, 32). SIRT1 gain-of-function mice displayed reduced liver acetylated FoxO1 levels and significantly higher hepatic AdipoR2 mRNA levels compared to wild-type mice (2). Administration to animals of several known SIRT1 agonists, including resveratrol and SRT1720, significantly increased hepatic AdipoR1/R2 mRNA expression levels (1, 6).
Excess accumulation of fat in the liver represents an initial stage in the development of alcoholic liver disease. Alcoholic fatty liver can progress to more severe forms of liver injury such as fibrosis and cirrhosis in humans. Therefore, the development of therapeutic strategies such as nutritional modulation of alcoholic liver steatosis is highly important. Dietary fat content and composition have been known to contribute to the development of alcoholic liver injury (23). In several animal models, diets enriched in saturated fatty acids or medium-chain triglycerides prevent ethanol-induced liver injury, while diets containing polyunsaturated fatty acids promote liver injury. The molecular mechanisms by which saturated fat protects against alcoholic liver injury appear to be manifold. We have shown that a saturated fat diet partially exerts this effect by increasing circulating adiponectin levels in mice, resulting in enhanced hepatic adiponectin signaling (38). Increased adiponectin signaling stimulates a central lipid regulatory system, the SIRT1-AMPK axis, leading to a reduced rate of de novo lipogenesis and an increased rate of fatty acid oxidation, thereby preventing fat accumulation in the livers of ethanol-fed mice (1, 30, 38, 39).
In liver, adiponectin regulates lipid metabolism primarily through interacting with AdipoR1/R2 (40). However, the interactions of dietary saturated fat and ethanol on adiponectin receptors in the liver remain unclear. Therefore, in the present study, we examined the effect of dietary fatty acids on the hepatic expression of AdipoR1/R2 in ethanol-fed mice as well as in cultured hepatic cells exposed to ethanol, with a focus on the involvement of SIRT1-FoxO1 signaling pathway.
Materials and Methods
Plasmid constructs
Mouse adiponectin promoter-luciferase reporter plasmid was a kind gift of Dr. J. B. Kim (Seoul National University). The mouse AdipoR2 promoter-luciferase reporter plasmid was a kind gift of Dr. Y. Chen (Institute for Nutritional Sciences, Chinese Academy of Sciences). The small silencing SIRT1 plasmid (SIRT1siRNA) and small silencing FoxO1 plasmid (FoxO1siRNA) were purchased from Upstate Biotechnology (Lake Placid, NY).
Animal studies
The detailed animal feeding protocol and diet composition were described previously (38, 39). Liquid diets were based upon the modified Lieber–DeCarli formulation and provided 1 kcal/ml (prepared by Dyets, Inc., Bethlehem, PA). Protein content was constant at 18% of total calories and each diet had identical mineral and vitamin content and contained safflower oil (4% of calories) to provide essential fatty acids. Male C57BL/6J (6–8 weeks old) mice were divided into four dietary groups: (1) polyunsaturated fat pair-fed control diet (PUFA, 40% of calories from fat, primarily from corn oil); (2) ethanol-containing polyunsaturated fat diet [PUFA+E, identical to the control PUFA diet but with ethanol added to account for 27.5% of total calories and the caloric equivalent of carbohydrate (maltose-dextrin) removed]; (3) high saturated fat pair-fed control diet [HSF, 40% of calories from fat, primarily from cocoa butter]; (4) ethanol-containing high saturated fat diet [HSF+E, identical to the control HSF diet but with ethanol added to account for 27.5% of total calories]. The dietary and nutritional intake of control mice were matched to those of the ethanol-fed mice by pair-feeding the same volume of isocaloric liquid diet for 4 weeks. The protocols for these animal studies were approved by the Institutional Animal Care Use Committees of University of South Florida.
Cell culture
Rat hepatoma McA-RH7777 cell line was purchased from from the American Type Culture Collection (Manassas, VA). The cells were maintained at 37°C, 5% CO2 in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum (Gemini Bio-Products, Woodland, CA). Various dietary fatty acids were dissolved in 3% (wt/vol) fatty acid-free bovine serum albumin (BSA). At 80%–90% confluence, the cells were incubated for 16 h in serum-free DMEM. The cells were then treated with serum-free DMEM containing fatty acid:BSA complex and ethanol. The rat primary hepatocytes were isolated and cultured as described (37, 38).
Transfections, luciferase assay, and small interfering RNA transfection
Transfection procedures were performed using the Lipofectamine and Plus reagents, according to the manufacturer's instructions (Invitrogen, Frederick, MD). Luciferase assays were performed according to the manufacturer's instructions (Promega, Fitchburg, WI) (38). β-Galactosidase was used as an internal control. The small silencing RNA for SIRT1 (SIRT1 siRNA) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The small silencing RNA for FoxO1 (FoxO1siRNA) was purchased from Cell Signaling Technology (Danvers, MA). The Small Interfering RNA transfection procedures were carried out as described (30).
Total RNA isolation and real-time quantitative reverse transcription
Liver or cell total RNA was prepared as described. RT of total RNA (2.5 μg/μl) to cDNA was performed using the StrataScript QPCR cDNA Synthesis kit (Stratagene, La Jolla, CA) (30, 38). Quantitative reverse transcription–polymerase chain reaction (qRT-PCR) amplification was performed in an iCycler thermal cycler (BioRad, Hercules, CA) using a SYBR Green QPCR Master Mix (SABiosciences, Frederick, MD). Primer sets optimized for the tested targets for SYBR Green based qRT-PCR, including SIRT1, AdipoR1, AdipoR2, SIRT1, FoxO1, or glyceraldehyde 3-phosphate dehydrogenase (GAPDH), were purchased from SABiosciences Corporation. The relative amount of target mRNA was calculated using the comparative threshold (Ct) method by normalizing target mRNA Ct to those for GAPDH (ΔCt).
Western blot analysis
Western Blot analyses were performed using 30–50 μg whole liver extracts, nuclear extracts or cell extracts separated by electrophoresis in a 10%, 8%, or 5% SDS-polyacrylamide gel and transferred to nitrocellulose filters. SIRT1 and AdipoR2 were visualized using antibodies from Santa Cruz Biotechnology and AlphaDiagnostic (San Antonio, TX), respectively. Polyclonal rabbit anti-β-actin antibody (Sigma Aldrich, St. Louis, MO) or Lamin A/C antibody (Santa Cruz Biotechnology, Inc.) was used to normalize the signal obtained for total or nuclear liver or cellular protein extracts. FoxO1 protein was immunoprecipitated from liver protein extracts by use of an anti-FoxO1 antibody (Santa Cruz Biotechnology, Inc.). Equal amounts of immunoprecipitates were then subjected to Western blot analysis, using specific antibodies for FoxO1, and acetyl-lysine (Cell Signaling). Immunoblots were imaged and quantified with a ChemiDoc XRS imaging system (BioRad).
Chromatin immunoprecipitation assay and real-time qRT-PCR
The chromatin immunoprecipitation (ChIP) assay was performed with the ChIP assay kit (Upstate Biotechnology), according to the protocols provided by the manufacturer (39). Briefly, liver samples were fixed with 1% formaldehyde and sonicated in the SDS-lysis buffer (39). Fragmented soluble chromatin was immunoprecipitated with an antibody against FoxO1. The precipitates were reversely cross-linked for DNA isolation. The samples were subjected to real-time qRT-PCR analysis using the following primers that amplify a region of the mouse AdipoR2 promoter containing the predicted FoxO binding sites (5’-ATGTGCCCACGAATTTGACC-3’) (5’-TCTCCTCCCTCCCTCCTTT-3’). The primer set (5'-GGCTCACAACCATCTATAATCAGGT-3') (5'-ACAGCTTTCTGCTTGCATGTATG-3') for GAPDH was used as a control. The results were normalized to control IgG, GAPDH, and input DNA.
Nuclear extracts of liver or cells
Nuclear proteins from portions of fresh liver or cells were extracted using a nuclear extraction kit (Active Motif, Carlsbad, CA) according to manufacturer's protocol.
Immunofluorescence microscopy
The immortalized mouse AML-12 hepatocytes (American Type Culture Collection) were grown in Chamber BD FalconTM Cultureslides (BD Biosciences, Bedford, MA). Following treatment, AML-12 cells were fixed for 10 min with 4% paraformaldehyde. Fixed cells were permeabilized for 10 min in 0.3% Triton X-100 in PBS, and blocked for 1 h using 4% BSA in PBS at room temperature. Coverslips were incubated with a mouse-specific SIRT1 antibody (Cell Signaling) overnight at 4°C. Samples were then incubated with the secondary anti-rabbit Cy5 antibody (Jackson ImmunoResearch Laboratories; West Grove, PA) for 1 h at room temperature. After washing with PBS, cells were mounted with DAPI (ProLong Gold, Invitrogen). Samples without primary antibodies were used as negative controls. Images were obtained with a Leica DM4000 upright microscope and with Leica DFC350X camera. Images were processed with Leica LAS software.
Evaluation of the formation of reactive oxygen species in mouse livers and in hepatic cells
The lipid peroxidation product in mouse liver samples was determined using a kit (ZeptoMetrix, Buffalo, NY) based on the formation of the thiobarbituric reactive substances (TBARS) and expressed as the extent of malondialdehyde (MDA) production as described (1). H2O2 levels in hepatic cells were quantified using a H2O2 Assay Kit (Cayman Chemical, Ann Arbor, MI) (4). The procedure is based on the oxidation of ferrous ions to ferric ions by H2O2 under acidic conditions. The ferric ions bind with the dye xylenol orange to form a stable colored complex which is measured at 595 nm. Catalase, which decreases H2O2 to an undetectable level, serves as a control.
Statistical analysis
Data are presented as means±SD. Multiple comparisons were evaluated by two-way ANOVA, followed by Tukey's multiple-comparison procedure with p<0.05 being considered significant.
Results
A high saturated fat diet selectively increased AdipoR2 mRNA and protein expression levels in the livers of ethanol-fed mice
Previously, we reported that feeding mice a liquid diet containing high levels of polyunsaturated fat plus ethanol (PUFA+E) for 4 weeks led to development of liver steatosis (38, 39). At the same time, we showed that fatty liver was prevented in mice that received a liquid diet containing high levels of saturated fat plus ethanol (HSF+E) (38, 39). In those reports we provided evidence for the involvement of SIRT1 deacetylase activity in the observed effects. In order to explore the molecular basis of these effects more comprehensively, cryopreserved liver samples from those treatment groups were used to generate new additional data for the present study.
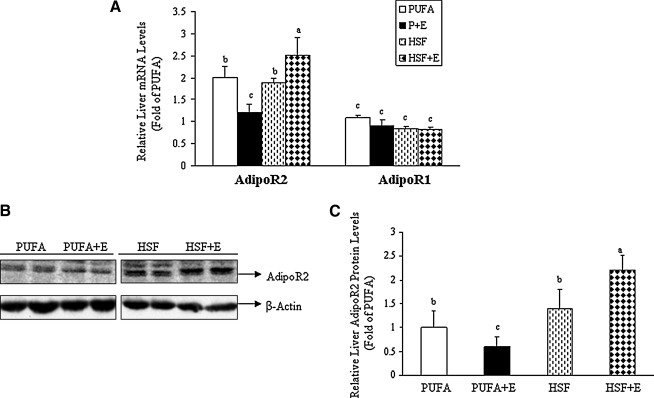
Levels of hepatic adiponectin receptor mRNA and protein were determined using real-time qRT-PCR and Western blot analysis. Concerning the differential expression of specific subtypes of adiponectin receptor in the livers of control group animals, real-time qRT-PCR analysis revealed that AdipoR2 mRNA levels are roughly double those of AdipoR1 (Fig. 1A). Addition of ethanol to these diets did not significantly change the levels of hepatic AdipoR1 mRNA and protein (Fig.1A and data not shown). However, with respect to hepatic AdipoR2, the interaction of dietary fats and ethanol induced marked changes in mRNA and protein levels. As shown in Figure1, addition of ethanol to the PUFA diet resulted in decreased mRNA and protein levels of AdipoR2: by approximately 40% and 25%, respectively. Alternatively, addition of ethanol to the HSF diet resulted in increases of AdipoR2 in the range of 40%–50% for both mRNA and protein. Collectively, these data demonstrate that hepatic AdipoR2 was selectively upregulated by an ethanol-containing HSF diet in mice.
FIG. 1.
A high saturated fat diet selectively increased AdipoR2 mRNA and protein expression levels in the livers of ethanol-fed mice. Mice were fed a high polyunsaturated fat diet (PUFA), or a high saturated fat diet (HSF) with (+E) or without ethanol. (A) Relative mRNA levels of AdipoR2 and AdipoR1. (B) Representative Western blots of AdipoR2. (C) Relative quantification of AdipoR2 normalized to β-Actin. All data are expressed as means±SD; n=6–8 animals. Means without a common letter differ, p<0.05.
A high saturated fat diet blocked hepatic FoxO1 hyperacetylation and enhanced nuclear FoxO1 protein levels in the livers of ethanol-fed mice
Liver SIRT1 was upregulated by ethanol administration in mice fed the HSF diet (39). Hence, we examined the effect of dietary fat on FoxO1, a known target of SIRT1, by measuring the FoxO1 mRNA and protein expression and its acetylation levels in various diet groups.
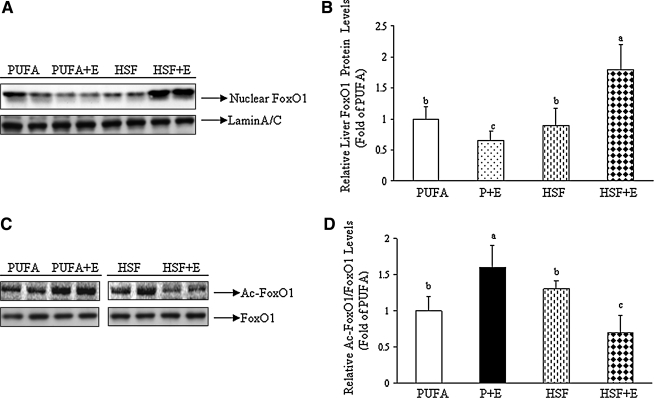
Western blot analysis of liver nuclear and cytosolic fractions was performed with an anti-FoxO1 antibody. As shown in Figures 2A and 2B, the amount of nuclear FoxO1 protein was reduced by about 35% in the livers of PUFA+E fed mice as compared with control mice receiving only the PUFA diet. However, the addition of ethanol to the HSF diet robustly increased FoxO1 protein in the nucleus. The FoxO1 mRNA levels and protein levels in the cytoplasm were not altered in any of the diet groups (data not shown).
FIG. 2.
A high saturated fat diet blocked hepatic FoxO1 hyperacetylation and enhanced nuclear FoxO1 protein levels, in the livers of ethanol-fed mice. Mice were fed diets as described in Figure 1. (A) Representative Western blots of nuclear FoxO1 protein. (B) Relative quantification of nuclear FoxO1 protein levels normalized to Lamin A/C. (C) Representative Western blots of acetylated FoxO1 and total FoxO1. FoxO1 was immunoprecipitated from liver nuclear extracts and then immunoblotted with either an anti-acetylated lysine antibody to determine the extent of FoxO1 acetylation or with a FoxO1 antibody to determine the FoxO1 amount as an internal control. (D) Relative quantification of acetylated FoxO1 normalized to total FoxO1. All data are expressed as means±SD; n=6–8 animals. Means without a common letter differ, p<0.05.
FoxO1 acetylation status was analyzed by Western blot analysis from liver extracts using an anti-acetylated lysine antibody, and was normalized to the level of total FoxO1. As shown in Figures 2C and 2D, in combination with a PUFA diet, ethanol feeding led to a nearly 50% increase in the acetylation of FoxO1. Conversely, when ethanol was combined with an HSF diet, acetylation of FoxO1 was cut nearly in half (Figs. 2C and 2D).
Taken together, these findings suggest that an HSF diet stimulated liver SIRT1-FoxO1 signaling in ethanol-fed mice.
A high saturated fat diet increased the association of FoxO1 with AdipoR2 promoter in the livers of ethanol-fed mice
ChIP assays were utilized to examine the effect of dietary fats on the association of SIRT1 or FoxO1 with the AdipoR2 promoter in the livers of ethanol-fed mice and pair-fed controls.
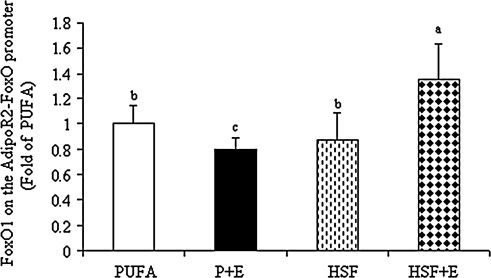
ChIP assays were performed on liver samples from various diet groups using anti-FoxO1 antibody and IgG as a negative control. Immunoprecipitated DNA was amplified by real time PCR with primers for the AdipoR2 promoter region containing a FoxO binding sequence. As shown in Figure 3, HSF+E significantly increased the interaction of FoxO1 with the AdipoR2-FoxO1 promoter by ∼30%, whereas PUFA+E significantly reduced it by about 20%.
FIG. 3.
A high saturated fat diet increased the association of FoxO1 with AdipoR2 promoter in the livers of ethanol-fed mice. ChIP assays were performed as described in the “Experimental Procedures” section. Immunoprecipitations (IP) were carried out using antibody directed against FoxO1 from liver samples of mice fed diets as described in Figure 1. Bound and input DNA was analyzed with primers for the AdipoR2-FoxO promoter by qRT-PCR. Values are given as means±SD (n=8 animals). Means without a common letter differ, p<0.05.
Collectively, our results suggest that the HSF diet increases SIRT1–FoxO1 activity resulting in enhanced association of FoxO1 with the promoter of AdipoR2, and subsequently causes upregulation of AdipoR2 gene expression.
Palmitic acid, a dietary saturated fatty acid, selectively increased AdipoR2 mRNA expression levels in hepatoma cells exposed to ethanol
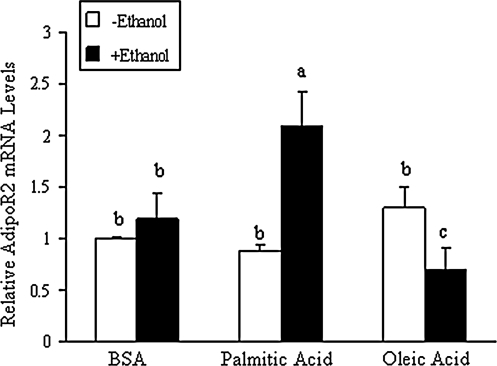
We examined the effects of dietary fatty acids and ethanol on AdipoRs expression in vitro, using cultured hepatocytes. Palmitic acid is the most abundant saturated fatty acid in the HSF diet (from cocoa butter) whereas oleic acid is the principle unsaturated fatty acid in the PUFA diet (from corn oil) (38). Rat primary hepatocytes were incubated with palmitic acid or oleic acid (0.2 mM complexed with 3% BSA with or without ethanol (50 mM) for 18 h. Ethanol treatment alone slightly increased AdipoR2 mRNA levels. However, when palmitic acid and ethanol were co-administered, a robust increase in AdipoR2 mRNA was observed (Fig. 4).On the contrary, addition of oleic acid to the ethanol diet significantly reduced the AdipoR2 mRNA levels (Fig. 4). Consistent with in vivo findings, no significant changes were seen with various fatty acids and ethanol treatments on AdipoR1 mRNA levels (data not shown).
FIG. 4.
Palmitic acid, a dietary saturated fatty acid, selectively increased AdipoR2 mRNA expression levels in hepatoma cells exposed to ethanol. Rat primary hepatocytes were incubated for 4 h in serum-free medium and then treated with various fatty acid:BSA complexes in the absence or presence of ethanol (50 mM) for 16 h. Levels of mRNA were determined using qRT-PCR. Values are given as means±SD from at least three experiments performed in duplicate. Means without a common letter differ, p<0.05.
Palmitic acid stimulated AdipoR2 promoter activity through activation of SIRT1-FoxO1 signaling in hepatoma cells exposed to ethanol
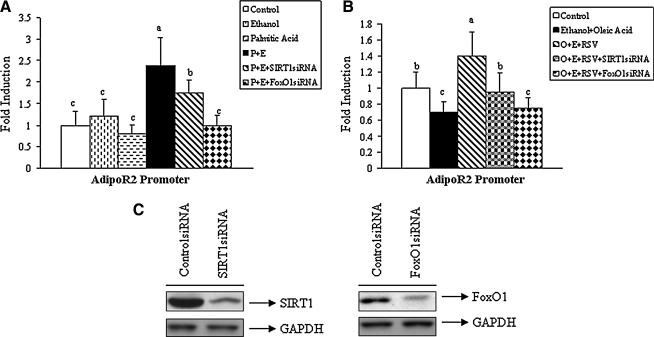
To determine the role of SIRT1-FoxO1 signaling in the dietary fatty acids-dependent regulation of AdipoR2 gene expression, a luciferase reporter assay was employed. Rat McA-RH7777 cells were co-transfected with a mouse AdipoR2 promoter reporter or a vector control, and expression plasmids for either SIRT1 siRNA or FoxO1 siRNA.
As shown in Figure 5A, neither ethanol (50 mM) nor palmitic acid (0.2 mM) treatment alone had an effect on AdipoR2 luciferase activity. However, when cells were treated with a combination of ethanol and palmitate there was a substantial increase (∼2-fold) in AdipoR2 promoter activity (Fig. 5A). This increase was partially blocked by the expression of SIRT1 siRNA and was completely blocked by expression of FoxO1 siRNA (Fig. 5A), suggesting that SIRT1–FoxO1 signaling is involved in the elevation of AdipoR2 promoter activity in hepatoma cells exposed to ethanol and palmitic acid. Knockdown of SIRT1 or FoxO1 in the absence of palmitic acid or ethanol slightly reduced AdipoR2 reporter activity (data not shown).
FIG. 5.
Palmitic acid stimulated AdipoR2 promoter activity through activation of SIRT1–FoxO1 signaling in hepatoma cells exposed to ethanol. Hepatoma McA-7777 cells were transfected with a mouse AdipoR2 promoter-luciferase reporter and expression plasmids for SIRT1 siRNA or FoxO1 siRNA. Following transfection, cells were treated with various fatty acid:BSA complexes in the absence or presence of ethanol. (A) Promoter activity following treatment of palmitic acid group. (B) Promoter activity following treatment of oleic acid group. (C) Representative Western blots of homogenates from cells treated with control siRNA, SIRT1siRNA, or FoxO1siRNA, and probed for SIRT1, FoxO1, or glyceraldehyde phosphate dehydrogenase (GAPDH.) Abbreviations used: E, ethanol; O, Oleic acid; P, palmitic acid; RSV, resveratrol. Normalized luciferase activities are shown as mean±SD from at least three experiments performed in duplicate. Means without a common letter differ, p<0.05.
Conversely, incubation with oleic acid (0.2 mM) plus ethanol (50 mM) significantly reduced AdipoR2 promoter activity. Pretreatment of cells with 30 μM resveratrol (RSV) (a SIRT1 activator) completely reversed the inhibition of AdipoR2 promoter mediated by oleic acid plus ethanol (Fig. 5B). Further, knockdown of either SIRT1 or FoxO1 nullified effects of resveratrol, indicating that both proteins are required for induction of the AdipoR2 promoter activity. Note that effective suppression of both SIRT1 and FoxO1 proteins was achieved by use of siRNA vectors (Fig. 5C).
Taken together, these data suggest that elevation of AdipoR2 expression by the combination of palmitic acid and ethanol are mediated, at least in part, through activation of SIRT1-FoxO1 signaling.
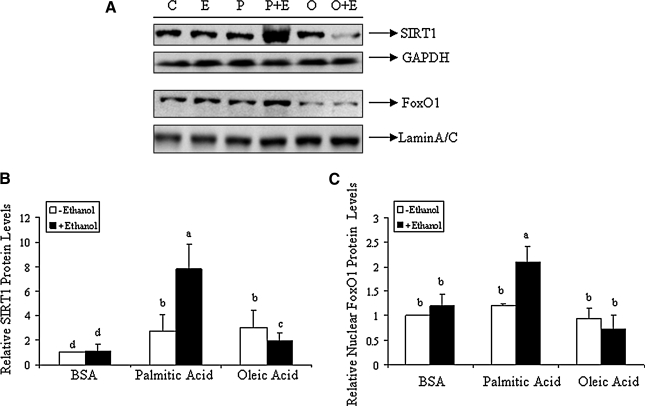
Upregulated SIRT1 and FoxO1 by palmitic acid in rat primary hepatocytes exposed to ethanol
We examined the interaction of dietary fatty acids with ethanol on SIRT1 and FoxO1 expression in rat primary hepatocytes. Western blot analysis of nuclear and cytosolic extracts of hepatocytes treated with dietary fatty acids (palmitic acid or oleic acid) in the presence or absence of ethanol for 18 h were performed. As shown in Figure 6, incubation of the primary hepatocytes with palmitic acid (0.2 mM) and ethanol (50 mM) significantly increased total SIRT1 and nuclear FoxO1 protein levels compared to controls. Moreover, there was a significant decrease in SIRT1 and FoxO1 protein levels observed in primary hepatocytes treated with ethanol in the presence of oleic acid (0.2 mM) (Fig. 6).
FIG. 6.
Upregulated SIRT1 and FoxO1 by palmitic acid in rat primary hepatocytes exposed to ethanol. Primary rat hepatocytes were treated with various fatty acid:BSA complexes in the presence or absence of ethanol, and levels of SIRT1 and FoxO1 were determined. (A) Representative Western blots of total SIRT1 protein and nuclear FoxO1 protein. (B) Relative quantification of total SIRT1 protein normalized to GAPDH. (C) Relative quantification of nuclear FoxO1 protein normalized to Lamin A/C. Values are given as means±SD from at least three experiments performed in duplicate. Means without a common letter differ, p<0.05.
Effects of dietary fatty acids on generation of oxidative stress in the livers of ethanol-fed mice or cultured hepatocytes exposed to ethanol
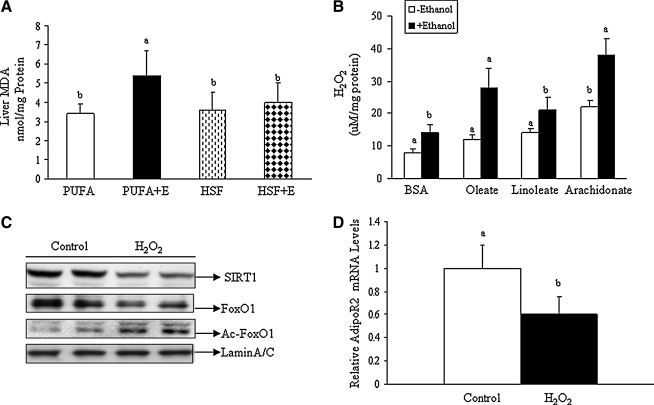
Both SIRT1 and FoxO1 can be regulated by oxidative stress. We investigated the role of reactive oxygen species (ROS) in mediating the effects of dietary fatty acids on SIRT1–FoxO1–AdipoR2 in ethanol-fed mice and in hepatic cells exposed to ethanol. In mice, while PUFA+E feeding significantly increased the liver malondialdehyde (MDA) levels by 1.8-fold, a diet of HSF+E did not affect MDA levels (Fig. 7A).
FIG. 7.
Effects of dietary fatty acids on generation of oxidative stress in the livers of ethanol-fed mice or cultured hepatocytes exposed to ethanol. (A) Malondialdehyde (MDA) equivalent units were determined and graphed for samples from livers of mice fed different diets as described in Figure 1. (B) Primary rat hepatocytes were treated with various fatty acid:BSA complexes in the presence or absence of ethanol, and cellular H2O2 levels were measured and graphed. (C) Representative Western blots of homogenates from hepatocytes treated with or without H2O2 (20 μM) and probed for SIRT1, FoxO1, Ac-FoxO1, or Lamin A/C. (D) Relative AdipoR2 mRNA levels of hepatocytes treated with or without H2O2 (20 μM). Values are given as means±SE from at least three experiments performed in duplicate. Means without a common letter differ, p<0.05.
Further, we determined the effect of various dietary fatty acids on ROS generation in rat primary hepatocytes exposed to ethanol. Incubation of hepatocytes with unsaturated fatty acids (oleic acid, linoleic acid, or arachidonic acid) (0.2 mM) for 18 h robustly raised the cellular H2O2 levels in hepatocytes exposed to ethanol (Fig. 7B). Western blot analysis indicated that treatment of hepatocytes with 20 μM hydrogen peroxide (H2O2) for 18 h significantly reduced levels of total SIRT1 and nuclear FoxO1 protein while increasing total FoxO1 acetylation (Fig. 7C).
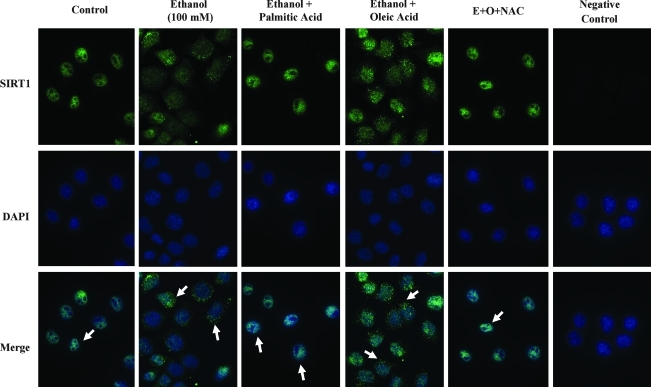
Oxidative stress regulates SIRT1 nucleocytoplasmic shuttling (3). Therefore, we further performed immunofluorescence to determine the effects of dietary fatty acids and ethanol on SIRT1 nucleocytoplasmic shuttling using mouse AML-12 hepatocytes. As shown in Figure 8, SIRT1 was exclusively present in the nucleus prior to ethanol exposure. However, SIRT1 was partially shuttled out of the nucleus in response to ethanol treatment. Interestingly, co-addition of palmitic acid blocked the ethanol-induced SIRT1 nucleocytoplasmic shuttling. Conversely, oleic acid treatment augmented the ability of ethanol to induce SIRT1 cytoplasmic localization. Pretreatment with an antioxidant, N-acetylcystine (NAC; 2 mM), largely prevented the ethanol-induced shuttling of SIRT1 to the cytoplasm. It is worthwhile to point out that treatment with H2O2 (50 μM) for 18 h also led to shuttling of SIRT1 out of the nucleus in AML-12 cells (data not shown). Similar to the depletion of nuclear SIRT1, AdipoR2 mRNA levels were also cut in half by treatment with H2O2 (Fig. 7D).
FIG. 8.
Effects of dietary fatty acids on nucleocytoplasmic shuttling of SIRT1 by ethanol. Mouse AML-12 hepatocytes were treated with ethanol (E; 100 mM) alone, or in combination with palmitic acid (P; 0.25 mM) or oleic acid (O; 0.25 mM) in the presence or absence of N-acetylcystine (NAC; 2 mM) for 18 h. The cells were fixed with 4% paraformaldehyde and used for immunostaining. SIRT1 was visualized using a primary mouse SIRT1 antibody. Nuclei were stained with DAPI. Samples without primary SIRT1 antibody were used as negative assay controls. Images represent at least 3 separate experiments.
Taken together, these data suggest that unsaturated fatty acids-induced oxidative stress may be largely responsible for reduction of SIRT1 total protein and FoxO1 nuclear protein levels in hepatocytes exposed to ethanol.
Discussion
In the present study, we demonstrated that an HSF diet selectively increased hepatic AdipoR2 mRNA and protein expression levels in ethanol-fed mice. Concurrently, this diet led to increased nuclear FoxO1 protein levels, blocked hepatic FoxO1 hyperacetylation, and enhanced association of FoxO1 with the promoter of AdipoR2 in the livers of ethanol-fed mice. Treatment of hepatic cells with palmitic acid, the primary saturated fatty acid in the HSF diet, in the presence of ethanol selectively increased AdipoR2 promoter activity and AdipoR2 gene expression. Furthermore, we provided evidence that upregulation of liver AdipoR2 by dietary saturated fatty acids plus ethanol may be largely mediated by stimulation of SIRT1-FoxO1 signaling.
The present study agrees with our previous work in which we have consistently reported that liver AdipoR2 but not AdipoR1 is selectively downregulated by chronic ethanol feeding in mice (1, 30). AdipoR2 is predominantly expressed in the liver, where its importance in the development of hepatic steatosis has been demonstrated in both rodents and in humans (14, 17–19, 24, 33). Interestingly, it is reported that gene expression of AdipoR2, but not AdipoR1, is decreased in liver samples from nonalcoholic steatohepatitis (NASH) patients (17). Our current study suggests that in addition to altering adiponectin synthesis in adipose tissue, chronic ethanol exposure also selectively downregulates hepatic AdipoR2, thus impairing adiponectin receptor-mediated signaling pathways in the liver, reducing the otherwise protective effects of adiponectin and promoting development of hepatic steatosis.
Our current data show that the ethanol-dependent increases of hepatic AdipoR2 mRNA and protein levels found in mice consuming an HSF diet are modest. However, we have found that an HSF diet significantly increased circulating total adiponectin levels and enhanced the high molecular-weight (HMW) form of adiponectin in ethanol-fed mice (39 and X. Liang and M. You, unpublished observation). The HMW form represents the major form of adiponectin regulating lipid metabolism in the liver (26, 35). It is likely that the HSF diet reverses abnormalities in hepatic lipid metabolism in ethanol-fed mice through a stimulation of hepatic adiponectin signaling via combination of upregulating circulating total HMW adiponectin and hepatic AdipoR2 expression.
A previous study from our group has demonstrated that an HSF diet significantly increased SIRT1 activity in the livers of ethanol-fed mice (39). Conceivably, the HSF+E diet-mediated activation of SIRT1 causes FoxO1 deacetylation, promoting nuclear retention of FoxO1 and increasing FoxO1 transcriptional activity. The activation of SIRT1-FoxO1 signaling by HSF diet may directly contribute to the increased AdipoR2 gene expression through enhanced binding of FoxO1 to AdipoR2 promoter sites in livers of ethanol-fed mice.
The precise cellular events by which ethanol, in combination with specific dietary fatty acids, regulate hepatic AdipoR2 levels call for more investigation. However, it appears clear at this time that the SIRT1–FoxO1 axis is involved. Reactive oxygen species (ROS) have long been established as a major contributor in the pathogenesis of alcoholic liver disease (5). SIRT1 activity is known to be regulated by nucleocytoplasmic shuttling. Accumulating evidence has shown that a number of stimuli can lead to the translocation of SIRT1 from the nucleus, and the subsequent impairment of SIRT1 activity (3, 12, 34). Our present study, for the first time, demonstrated that ethanol with or without an unsaturated fatty acid induced transport of SIRT1 from the nucleus to the cytoplasm of AML-12 hepatocytes. More importantly, our data suggest that ethanol-induced shuttling of SIRT1 is redox dependent. The role of ethanol-induced SIRT1 subcellular localization and subsequent effects on FoxO1 activity, and AdipoR2 gene expression, will be of great interest to investigate in the future.
The SIRT1–FoxO1 pathway plays a key role in regulating ROS production (3, 29, 42). Our present data demonstrate that both SIRT1 and FoxO1 are inhibited by polyunsaturated fatty acids in the presence of ethanol, correlating with a significant increase in the production of ROS and a decrease in AdipoR2 expression, suggesting that ROS generated by ethanol is likely a major triggering molecular event leading to impairment of SIRT1–FoxO1–AdipoR2 signaling and excess hepatic fat accumulation in ethanol-fed mice. On the other hand, our data have also shown that administration of an HSF diet to ethanol-fed mice did not significantly reduce the ROS levels compared to pair-fed controls, suggesting that the ROS-independent mechanisms may also be involved.
SIRT1 protein expression levels are known to be upregulated by pyruvate in cultured hepatic cells and in animal livers (27). Ethanol metabolism in liver through alcohol dehydrogenase and aldehyde dehydrogenase causes decreased levels of pyruvate due to NAD+ depletion (20). It is possible that SIRT1 protein levels in mice fed the HSF+E diet might be mediated by preventing the ethanol metabolism-induced loss of NAD+ and reduced pyruvate. However, in comparison to pair-fed control mice, the HSF diet did not significantly increase the pyruvate levels in ethanol-fed mice (X. Liang and M. You, unpublished observation). Further studies are necessary to clarify the mechanisms involved in the increase of SIRT1–FoxO1 signaling by dietary saturated fatty acids with ethanol.
In addition to SIRT1-FoxO1 signaling, other mechanisms may play an important role in the upregulation of liver AdipoR2 expression by the HSF+E diet. Studies have suggested that saturated fatty acids-induced endoplasmic reticulum (ER) stress may cause downregulation of AdipoR2 in cultured hepatic cells (16, 25). The ER stress response has been associated with the pathogenesis of alcoholic liver disease (13). ER stress is also linked with induced reactive oxygen species production (28). Therefore, it is possible that the HSF+E diet may upregulate AdipoR2 expression by attenuating ER stress. However, several known key ER stress markers including CCAAT/enhancer binding protein beta (C/EBPβ), eukaryotic translation initiation factor 2α (eIF2α), and the pro-apoptotic transcription factor CHOP were not significantly altered in our ethanol-fed mice supplemented with either an HSF or a PUFA diet (X. Liang and M. You, unpublished observation), suggesting the involvement of ER-independent signaling pathways.
In summary, our present study is the first report to demonstrate that dietary saturated fat in the presence of ethanol selectively upregulates liver AdipoR2 through SIRT1 and FoxO1. Our study suggests that nutritional or pharmacological modulation of the SIRT1–FoxO1–AdipoR2 axis may be a potential therapeutic strategy for treating human alcoholic fatty liver disease.
Abbreviations Used
- AdipoR
adiponectin receptor
- AMPK
AMP-activated protein kinase
- C/EBP
CCAAT/enhancer binding protein
- ChIP
chromatin immunoprecipitation
- Ct
comparative threshold
- eIF2α
eukaryotic translation initiation factor 2α
- ER
endoplasmic reticulum
- FoxO1
forkhead transcription factor O 1
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- H2O2
hydrogen peroxide
- HSF
high saturated fat
- MDA
malondialdehyde
- PUFA
polyunsaturated fat
- qRT-PCR
quantitative reverse transcription–polymerase
chain reaction
- SIRT1
sirtuin 1
- TBARS
thiobarbituric reactive substances.
Acknowledgment
This study was supported by National Institute on Alcoholism and Alcohol Abuse Grants AA-015951 and AA-013623 (to M. You).
Author Disclosure Statement
No competing finanacial interests exist.
References
- 1.Ajmo JM. Liang X. Rogers CQ. Pennock B. You M. Resveratrol alleviates alcoholic fatty liver in mice. Am J Physiol Gastrointest Liver Physiol. 2008;295:G833–842. doi: 10.1152/ajpgi.90358.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banks AS. Kon N. Knight C. Matsumoto M. Gutiérrez–Juárez R. Rossetti L. Gu W. Accili D. SirT1 gain of function increases energy efficiency and prevents diabetes in mice. Cell Metab. 2008;8:333–341. doi: 10.1016/j.cmet.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caito S. Rajendrasozhan S. Cook S. Chung S. Yao H. Friedman AE. Brookes PS. Rahman I. SIRT1 is a redox-sensitive deacetylase that is post-translationally modified by oxidants and carbonyl stress. FASEB J. 2010;24:3145–3159. doi: 10.1096/fj.09-151308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao Q. Mak KM. Lieber CS. Leptin represses matrix metalloproteinase-1 gene expression in LX2 human hepatic stellate cells. J Hepatol. 2007;46:124–133. doi: 10.1016/j.jhep.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 5.Cederbaum AI. Lu Y. Wu D. Role of oxidative stress in alcohol-induced liver injury. Arch Toxicol. 2009;83:519–548. doi: 10.1007/s00204-009-0432-0. [DOI] [PubMed] [Google Scholar]
- 6.Feige JN. Lagouge M. Canto C. Strehle A. Houten SM. Milne JC. Lambert PD. Mataki C. Elliott PJ. Auwerx J. Specific SIRT1 activation mimics low energy levels and protects against diet-induced metabolic disorders by enhancing fat oxidation. Cell Metab. 2008;8:347–358. doi: 10.1016/j.cmet.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 7.Frescas D. Valenti L. Accili D. Nuclear trapping of the forkhead transcription factor FoxO1 via Sirt-dependent deacetylation promotes expression of glucogenetic genes. J Biol Chem. 2005;280:20589–20595. doi: 10.1074/jbc.M412357200. [DOI] [PubMed] [Google Scholar]
- 8.Gan L. Han Y. Bastianetto S. Dumont Y. Unterman TG. Quirion R. FoxO-dependent and -independent mechanisms mediate SirT1 effects on IGFBP-1 gene expression. Biochem Biophys Res Commun. 2005;337:1092–1096. doi: 10.1016/j.bbrc.2005.09.169. [DOI] [PubMed] [Google Scholar]
- 9.Gross DN. Wan M. Birnbaum MJ. The role of FOXO in the regulation of metabolism. Curr Diab Rep. 2009;9:208–214. doi: 10.1007/s11892-009-0034-5. [DOI] [PubMed] [Google Scholar]
- 10.Hasseine LK. Hinault C. Lebrun P. Gautier N. Paul–Bellon R. Van Obberghen E. miR-139 impacts FoxO1 action by decreasing FoxO1 protein in mouse hepatocytes. Biochem Biophys Res Commun. 2009;390:1278–1282. doi: 10.1016/j.bbrc.2009.10.135. [DOI] [PubMed] [Google Scholar]
- 11.Huang H. Iida KT. Sone H. Ajisaka R. The regulation of adiponectin receptors expression by acute exercise in mice. Exp Clin Endocrinol Diabetes. 2007;115:417–422. doi: 10.1055/s-2007-981660. [DOI] [PubMed] [Google Scholar]
- 12.Jin Q. Yan T. Ge X. Sun C. Shi X. Zhai Q. Cytoplasm-localized SIRT1 enhances apoptosis. J Cell Physiol. 2007;213:88–97. doi: 10.1002/jcp.21091. [DOI] [PubMed] [Google Scholar]
- 13.Kaplowitz N. Ji C. Unfolding new mechanisms of alcoholic liver disease in the endoplasmic reticulum. J Gastroenterol Hepatol. 2006;21:S7–9. doi: 10.1111/j.1440-1746.2006.04581.x. [DOI] [PubMed] [Google Scholar]
- 14.Kaser S. Moschen A. Cayon A. Kaser J. Crespo F. Pons–Romero C. Ebenbichler F. Patsch JR. Tilg H. Adiponectin and its receptors in non-alcoholic steatohepatitis. Gut. 2005;54:117–121. doi: 10.1136/gut.2003.037010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kitamura YI. Kitamura T. Kruse JP. Raum JC. Stein R. Gu W. Accili D. FoxO1 protects against pancreatic beta cell failure through NeuroD and MafA induction. Cell Metab. 2005;2:153–163. doi: 10.1016/j.cmet.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 16.Koh IU. Lim JH. Joe MK. Kim WH. Jung MH. Yoon JB. Song J. AdipoR2 is transcriptionally regulated by ER stress-inducible ATF3 in HepG2 human hepatocyte cells. FEBS J. 2010;277:2304–2317. doi: 10.1111/j.1742-4658.2010.07646.x. [DOI] [PubMed] [Google Scholar]
- 17.Kotronen A. Yki–Järvinen H. Aminoff A. Bergholm R. Pietiläinen KH. Westerbacka J. Talmud PJ. Humphries SE. Hamsten A. Isomaa B. Groop L. Orho–Melander M. Ehrenborg E. Fisher RM. Genetic variation in the ADIPOR2 gene is associated with liver fat content and its surrogate markers in three independent cohorts. Eur J Endocrinol. 2009;160:593–602. doi: 10.1530/EJE-08-0900. [DOI] [PubMed] [Google Scholar]
- 18.López–Bermejo A. Botas–Cervero P. Ortega–Delgado F. Delgado E. García–Gil MM. Funahashi T. Ricart W. Fernández–Real JM. Association of ADIPOR2 with liver function tests in type 2 diabetic subjects. Obesity (Silver Spring) 2008;16:2308–2313. doi: 10.1038/oby.2008.344. [DOI] [PubMed] [Google Scholar]
- 19.Ma H. Gomez V. Lu L. Yang X. Wu X. Xiao SY. Expression of adiponectin and its receptors in livers of morbidly obese patients with non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2009;24:233–237. doi: 10.1111/j.1440-1746.2008.05548.x. [DOI] [PubMed] [Google Scholar]
- 20.Mezey E. Dietary fat and alcoholic liver disease. Hepatology. 1998;28:901–905. doi: 10.1002/hep.510280401. [DOI] [PubMed] [Google Scholar]
- 21.Motta MC. Divecha N. Lemieux M. Kamel C. Chen D. Gu W. Bultsma Y. McBurney M. Guarente L. Mammalian SIRT1 represses forkhead transcription factors. Cell. 2004;116:551–563. doi: 10.1016/s0092-8674(04)00126-6. [DOI] [PubMed] [Google Scholar]
- 22.Nakae J. Cao Y. Daitoku H. Fukamizu A. Ogawa W. Yano Y. Hayashi Y. The LXXLL motif of murine forkhead transcription factor FoxO1 mediates Sirt1-dependent transcriptional activity. J Clin Invest. 2006;116:2473–2483. doi: 10.1172/JCI25518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nanji AA. Role of different dietary fatty acids in the pathogenesis of experimental alcoholic liver disease. Alcohol. 2004;34:21–25. doi: 10.1016/j.alcohol.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 24.Peng Y. Rideout D. Rakita S. Sajan M. Farese R. You M. Murr MM. Downregulation of adiponectin/AdipoR2 is associated with steatohepatitis in obese mice. J Gastrointest Surg. 2009;13:2043–2049. doi: 10.1007/s11605-009-1032-2. [DOI] [PubMed] [Google Scholar]
- 25.Rahman SM. Qadri I. Janssen RC. Friedman JE. Fenofibrate and PBA prevent fatty acid-induced loss of adiponectin receptor and pAMPK in human hepatoma cells and in hepatitis C virus-induced steatosis. J Lipid Res. 2009;50:2193–2202. doi: 10.1194/jlr.M800633-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rogers CQ. Ajmo JM. You M. Adiponectin and alcoholic fatty liver disease. IUBMB Life. 2008;60:790–797. doi: 10.1002/iub.124. [DOI] [PubMed] [Google Scholar]
- 27.Rodgers JT. Lerin C. Haas W. Gygi SP. Spiegelman BM. Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 28.Santos CX. Tanaka LY. Wosniak J. Laurindo FR. Mechanisms and implications of reactive oxygen species generation during the unfolded protein response: Roles of endoplasmic reticulum oxidoreductases, mitochondrial electron transport, and NADPH oxidase. Antioxid Redox Signal. 2009;11:2409–2427. doi: 10.1089/ars.2009.2625. [DOI] [PubMed] [Google Scholar]
- 29.Sedding DG. FoxO transcription factors in oxidative stress response and ageing, A new fork on the way to longevity? Biol Chem. 2008;389:279–283. doi: 10.1515/BC.2008.033. [DOI] [PubMed] [Google Scholar]
- 30.Shen Z. Liang X. Rogers CQ. Rideout D. You M. Involvement of adiponectin-SIRT1-AMPK signaling in the protective action of rosiglitazone against alcoholic fatty liver in mice. Am J Physiol Gastrointest Liver Physiol. 2010;298:G364–374. doi: 10.1152/ajpgi.00456.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shimizu A. Takamura T. Matsuzawa N. Nakamura S. Nabemoto S. Takeshita Y. Misu H. Kurita S. Sakurai M. Yokoyama M. Zen Y. Sasaki M. Nakanuma Y. Kaneko S. Regulation of adiponectin receptor expression in human liver and a hepatocyte cell line. Metabolism. 2007;56:1478–1485. doi: 10.1016/j.metabol.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 32.Tsuchida A. Yamauchi T. Ito Y. Hada Y. Maki T. Takekawa S. Kamon J. Kobayashi M. Suzuki R. Hara K. Kubota N. Terauchi Y. Froguel P. Nakae J. Kasuga M. Accili D. Tobe K. Ueki K. Nagai R. Kadowaki T. Insulin/Foxo1 pathway regulates expression levels of adiponectin receptors and adiponectin sensitivity. J Biol Chem. 2004;279:30817–30822. doi: 10.1074/jbc.M402367200. [DOI] [PubMed] [Google Scholar]
- 33.Tomita K. Oike Y. Teratani T. Taguchi T. Noguchi M. Suzuki T. Mizutani A. Yokoyama H. Irie R. Sumimoto H. Takayanagi A. Miyashita K. Akao M. Tabata M. Tamiya G. Ohkura T. Hibi T. Hepatic AdipoR2 signaling plays a protective role against progression of nonalcoholic steatohepatitis in mice. Hepatology. 2008;48:458–73. doi: 10.1002/hep.22365. [DOI] [PubMed] [Google Scholar]
- 34.Tanno M. Sakamoto J. Miura T. Shimamoto K. Horio Y. Nucleocytoplasmic shuttling of the NAD+-dependent histone deacetylase SIRT1. J Biol Chem. 2007;282:6823–6832. doi: 10.1074/jbc.M609554200. [DOI] [PubMed] [Google Scholar]
- 35.Wang Y. Lam KS. Yau MH. Xu A. Post-translational modifications of adiponectin: mechanisms and functional implications. Biochem J. 2008;409:623–633. doi: 10.1042/BJ20071492. [DOI] [PubMed] [Google Scholar]
- 36.Yang Y. Hou H. Haller EM. Nicosia SV. Bai W. Suppression of FOXO1 activity by FHL2 through SIRT1-mediated deacetylation. EMBO J. 2005;24:1021–1032. doi: 10.1038/sj.emboj.7600570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.You M. Matsumoto M. Pacold CM. Cho WK. Crabb DW. The role of AMP-activated protein kinase in the action of ethanol in the liver. Gastroenterology. 2004;127:1798–1808. doi: 10.1053/j.gastro.2004.09.049. [DOI] [PubMed] [Google Scholar]
- 38.You M. Considine RV. Leone TC. Kelly DP. Crabb DW. Role of adiponectin in the protective action of dietary saturated fat against alcoholic fatty liver in mice. Hepatology. 2005;42:568–577. doi: 10.1002/hep.20821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.You M. Cao Q. Liang X. Ajmo JM. Ness GC. Mammalian sirtuin 1 is involved in the protective action of dietary saturated fat against alcoholic fatty liver in mice. J Nutr. 2008;138:497–501. doi: 10.1093/jn/138.3.497. [DOI] [PubMed] [Google Scholar]
- 40.You M. Rogers CQ. Adiponectin: A key adipokine in alcoholic fatty liver. Exp Biol Med (Maywood) 2009;234:850–859. doi: 10.3181/0902-MR-61. [DOI] [PubMed] [Google Scholar]
- 41.Yu J. Auwerx J. The role of sirtuins in the control of metabolic homeostasis. Ann NY Acad Sci. 2009;1173:E10–19. doi: 10.1111/j.1749-6632.2009.04952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zee RS. Yoo CB. Pimentel DR. Perlman DH. Burgoyne JR. Hou X. McComb ME. Costello CE. Cohen RA. Bachschmid M. Redox regulation of sirtuin-1 is mediated by S-glutathiolation. Antioxid Redox Signal. 2010;13:1023–1032. doi: 10.1089/ars.2010.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]