Abstract
Parkinson's disease is one of the major neurodegenerative disorders. Neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) can cause Parkinson's disease–like symptoms and biochemical changes in humans and animals. Hydrogen sulfide (H2S) has been shown to protect neurons. The goal of this study was to examine the effects of inhaled H2S in a mouse model of Parkinson's disease induced by MPTP. Male C57BL/6J mice received MPTP at 80 mg/kg and breathed air with or without 40 ppm H2S for 8 h/day for 7 days. Administration of MPTP induced movement disorder and decreased tyrosine hydroxylase (TH)-containing neurons in the substantia nigra and striatum in mice that breathed air. Inhalation of H2S prevented the MPTP-induced movement disorder and the degeneration of TH-containing neurons. Inhaled H2S also prevented apoptosis of the TH-containing neurons and gliosis in nigrostriatal region after administration of MPTP. The neuroprotective effect of inhaled H2S after MPTP administration was associated with upregulation of genes encoding antioxidant proteins, including heme oxygenase-1 and glutamate-cysteine ligase. These observations suggest that inhaled H2S prevents neurodegeneration in a mouse model of Parkinson's disease induced by MPTP, potentially via upregulation of antioxidant defense mechanisms and inhibition of inflammation and apoptosis in the brain. Antioxid. Redox Signal. 15, 343–352.
Introduction
Parkinson's disease (PD) is one of the most common neurodegenerative diseases (29). It is characterized by a slow and progressive degeneration of dopaminergic neurons in the substantia nigra (10). Although the etiology of PD is not fully understood, several mechanisms responsible for the neurodegeneration in PD have been suggested, including abnormal protein handling, oxidative stress, mitochondrial dysfunction, excitotoxicity, neuroinflammation, and apoptosis (11).
A number of animal models of PD, both toxin-induced and genetically engineered, have been created. Although the toxin-induced PD models have provided considerable therapeutic insight into basal ganglia physiology and response to drug therapy, genetic models provide a powerful new set of molecular tools to study the etiology of PD (22). None of the animal models accurately recapitulates the pathophysiologic features of PD, but 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) currently represents the most important and most frequently used parkinsonian toxin applied in animal models (1, 26, 27). MPTP is the only known dopaminergic neurotoxin capable of causing a clinical picture indistinguishable from idiopathic PD in humans (18). Moreover, MPTP produces a reliable and reproducible lesion of the nigrostriatal dopaminergic pathway after its systemic administration, which is often not the case for other neurotoxins (3).
Hydrogen sulfide (H2S) is an important gaseous signaling molecule. In the brain, H2S is synthesized by cystathionine β-synthase (CBS) and 3-mercaptopyruvate sulfurtransferase (3MST) (28), whereas the ability of 3MST to produce H2S directly has been recently questioned (15). Although cystathionine γ-lyase (CSE) may also produce H2S in cerebral vasculature, its contribution to the brain levels of H2S appears to be limited (13).
Hydrogen sulfide appears to confer cytoprotection via multiple mechanisms including antioxidant and antiapoptotic effects. For example, Kimura and colleagues (17) demonstrated by using a model of glutamate-induced oxidative stress that H2S protects neurons from cell death by increasing the levels of the antioxidant, glutathione. A recent study revealed that NaHS, an H2S donor, protects PC12 cells from cytotoxicity and apoptosis induced by 1-methy-4-phenylpyridinium ion (MPP+), the active metabolite of MPTP (33). Although molecular mechanisms responsible for the cytoprotective effects of H2S are incompletely defined, Calvert and colleagues (4) suggested that H2S may upregulate endogenous antioxidants through a nuclear-factor-E2–related factor-2 (Nrf2)-dependent signaling pathway. Nrf2 regulates gene expression of a number of antioxidant proteins [for example, heme oxygenase-1 (HO-1)] and phase II detoxification enzymes [(for example, glutathione S-transferase (GST)] (19). Whether H2S protects neurons by upregulating Nrf2-dependent antioxidant mechanisms remains to be determined.
In the current study, we sought to examine effects of H2S inhalation in the clinically relevant MPTP-induced PD model in mice. We also examined whether inhaled H2S upregulates antioxidant and detoxification enzymes in the brain. Here, we report that H2S inhalation prevents MPTP-induced degeneration of dopaminergic neurons and movement disorder in mice.
Materials and Methods
Animals
All experiments were carried out in accordance with institutional guidelines and approved by the Massachusetts General Hospital Subcommittee on Research Animal Care. We used 8- to 10-weeks-old age- and weight-matched male C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME). Mice had free access to food and water, and were maintained in a 12-h light/dark cycle (lights on 7:00–19:00).
MPTP-induced Parkinson's disease model in mice
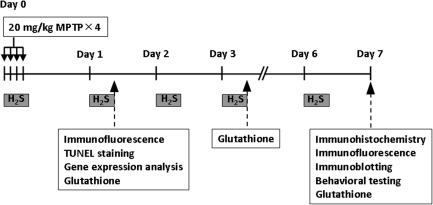
MPTP (Sigma-Aldrich, St. Louis, MO) was prepared in saline solution just before use. Male C57BL/6J wild-type mice received four intraperitoneal injections of MPTP (20 mg/kg; n = 62) or saline (n = 17) every 2 h for a total of four doses of MPTP (total, 80 mg/kg) or equivalent amount of saline on Day 0, according to a previously described protocol (14). Mice breathed air alone (n = 31) or air mixed with H2S (40 ppm; n = 31) for 8 h each day in custom-made plastic chambers, starting immediately after the first injection of MPTP on Day 0 (Fig. 1). We chose to examine the effects of 40 ppm H2S in these experiments, based on our previous study (30) in which breathing H2S at 80 or 120 ppm, but not at 40 ppm, reduced carbon dioxide production in mice. To minimize the potentially confounding influence of H2S-induced hypometabolism/hypothermia on the neuroprotective effects of H2S breathing, we chose 40 ppm H2S in the current study. The H2S breathing session was performed from 9 AM until 5 PM on each of 7 consecutive days from Day 0 through Day 6. Rectal temperature was measured in subgroups of mice treated with MPTP that breathed air (n = 3) and mice treated with MPTP that breathed H2S (n = 5) at 9 AM and 5 PM every day from Day 0 to Day 6 and before killing for tissue harvesting on Day 7.
FIG. 1.
Experimental protocol. Mice breathed air with or without H2S (40 ppm) for 8 h (9 AM to 5 PM) on each day in custom-made plastic chambers.
Immunohistochemical detection of tyrosine hydroxylase (TH), TH immunoblots, and behavioral testing were conducted on Day 7 after all H2S breathing sessions were completed. Glutathione assay was performed on brain tissue samples obtained on Days 1, 3, and 7 to examine time-dependent changes of glutathione levels in the brain. Detection of DNA fragmentation and gene-expression analysis were conducted on Day 1 based on the results of pilot studies that showed that the degree of DNA fragmentation and the changes of gene expression of antioxidant proteins were greater on Day 1 than on Day 7.
Behavioral test
All behavioral studies were performed and scored by an investigator blinded to the treatment that mice received. All behavioral tests were performed on Day 7 in saline-treated mice (control; n = 9), mice treated with MPTP and breathed air (n = 8), and mice treated with MPTP and breathed H2S (n = 7).
Open-field test
The open-field test was performed according to the method described previously, with some modifications (20, 32). In brief, mice were placed individually on a gray plastic rectangular box (50 × 30 × 15 cm) with 10-cm interval black grids and allowed to move freely for 3 min to habituate to the experimental environment. Grid crosses by the mouse were manually counted as a measure of total distance moved. Furthermore, the rearing times (number of exploratory activities) was manually counted while a mouse was in the open field.
Rotarod test
Rotarod tests were performed according to the method described previously, with some modifications (21). In brief, after five training sessions, mice were placed individually on the rotating rod, and the speed of rod rotation was increased from 0 to 50 rpm over a period of 3 min. The length of time mice remained on the rod (fall latency) was recorded and used as a measure of motor function.
Tail-suspension test
Tail-suspension tests were performed according to the method described previously (24). In brief, mice were suspended by the tails. The duration of immobility was measured for a period of 5 min. When mice climbed up their tails or dropped from the attachment during the test session, the data were omitted. The experiments were carried out in a quiet soundproof room.
Histologic studies
Detection of tyrosine hydroxylase
After inactivation of endogenous peroxidase activity by 3% hydrogen peroxide, the sections were incubated for 1 h in blocking solution (10% normal goat serum in PBS) and were incubated overnight at 4°C with the primary rabbit anti-tyrosine hydroxylase (TH) antibody (1:1,000; Millipore, Billerica, MA). The sections were washed in PBS and incubated with the biotinylated secondary goat anti-rabbit IgG antibody (1:200; Vector Laboratories, Burlingame, CA) and visualized by using the avidin-biotin-peroxidase complex method with diaminobenzidine tetrahydrochloride (DAB) as the chromogen, according to the protocol recommended by the manufacturer (2).
Detection of glial activation
Sections were incubated for 1 h in blocking solution and incubated overnight at 4°C with primary antibodies against markers of activated astrocytes (glial fibrillary acidic protein, GFAP, 1:500; Dako, Carpinteria, CA) or activated microglia (ionized calcium-binding adaptor molecule 1, Iba-1, 1:500; Wako Chemicals USA, Richmond, VA). After rinsing, sections were incubated with secondary antibodies for 1 h: Rhodamine RedTX-conjugated goat anti-rabbit antibody (for GFAP, 1:200; Jackson ImmunoReasearch, West Grove, PA) or Alexa Fluor 488 goat anti-rabbit antibody (for Iba-1, 1:200; Invitrogen, Carlsbad, CA). The fluorescence images were captured by using appropriate filters with a fluorescence microscope (Nikon ECLIPSE TE-2000-S).
Detection of DNA fragmentation
To identify cells undergoing DNA fragmentation, we performed the terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assay (DeadEnd Fluorometric TUNEL System; Promega, Madison, WI) and multicolor immunohistochemical detection for TH, GFAP, or Iba-1 on brain sections obtained on Day 1. Subsequent to visualization of the fragmented DNA by using the fluorescent TUNEL method, sections were incubated in 10% normal goat serum and 0.40% Triton X-100 in PBS for 1 h. The primary antibodies for TH, GFAP, or Iba-1 (1:100 for all three) were applied and incubated overnight at 4°C. After rinsing, sections were incubated with the biotinylated goat anti-rabbit IgG antibody for 1 h followed by Texas red-Avidin D (Vector Laboratories, Burlingame, CA) for 1 h. Sections were mounted with Vectashield mounting medium containing 4′,6-diamidino-2-phenylindole (DAPI) (Vector Laboratories) and observed by using a fluorescence microscope with appropriate filters.
Immunoblotting
To determine TH protein levels, substantia nigra and striatum were dissected from mice treated with saline (n = 5), mice treated with MPTP and breathed air (n = 5), and mice treated with MPTP and breathed H2S (n = 4) and frozen on Day 7 and homogenized in lysis buffer [50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1% Triton X-100, 1 mM EDTA, 2 mM sodium vanadate, 1 mM DTT, 5 μM Trichostatin A, 10 mM nicotinamide and Protease Inhibitor Cocktail (Sigma-Aldrich, St. Louis, MO)]. Protein samples (15 μg/lane) were subjected to a standard tris-glycine SDS-polyacrylamide gel electrophoresis (10%) with a Mini-Protean System (Bio-Rad, Hercules, CA) and were electrophoretically transferred to a polyvinylidene difluoride membrane. The membranes were soaked in blocking buffer (1% nonfat dry milk in TBS) for 1 h and incubated overnight at 4°C with the primary rabbit anti-TH antibody (1:50,000; Millipore), and then incubated with horseradish peroxidase–labeled anti-rabbit IgG antibody (1:75,000; GE Healthcare, Waukesha, WI) for 1 h at room temperature. Immunoreactive bands were detected with Amersham ECL Advanced Western Blotting Detection Kit (GE Healthcare). GAPDH was visualized with the specific antibody (1: 75,000; Trevigen, Gaithersburg, MD) on the identical membrane after stripping of anti-TH antibody. Densitometric analysis of the results was carried out with NIH Image software (version 1.62).
Measurements of gene expression
Total RNA was extracted from substantia nigra and striatum of mice at baseline (n = 9), mice treated with MPTP and breathed air (n = 7), and mice treated with MPTP and breathed H2S (n = 7) on Day 1 by using the Illustra RNA spin Mini kit (GE Healthcare), and cDNA was synthesized by using MMLV-RT (Promega). Glutathione S-transferase A4 (GST A4), glutathione S-transferase Mu1 (GST Mu1), NAD(P)H quinone oxidoreductase-1 (NQO1), heme oxygenase-1 (HO-1), glutamate-cysteine ligase catalytic subunit (GCLC), and 18S ribosomal RNA transcript levels were measured with real-time PCR by using a Realplex 2 system (Eppendorf, Westbury, NY). Primers used were as follows: GST A4 (5′-TGATTGCCGTGGCTCCATTTA-3′, 5′-CAACGAGAAAAGCCTCTCCGT-3′), GST Mu1 (5′-GTCAGTCCTGCTGAAGCCAG-3′, 5′-TGGCTTCTGTCAAAGTCGGG-3′), NQO1 (5′-AGGATGGGAGGTACTCGAATC-3′, 5′-AGGCGTCCTTCCTTATATGCTA-3′), HO-1 (5′-AAGCCGAGAATGCTGAGTTCA-3′, 5′-GCCGTGTAGATATGGTACAAGGA-3′), GCLC (5′-GGACAAACCCCAACCATCC-3′, 5′-GTTGAACTCAGACATCGTTCCTC-3′), and 18S rRNA (5′-CGGCTACCACATCCAAGGAA-3′, 5′-GCTGGAATTACCGCGGCT-3′). Changes in the relative gene expression normalized to levels of 18S rRNA were determined by using the relative CT method. The mean value of samples from control mice was set as 1.
Glutathione assay
Tissue levels of reduced glutathione (GSH) and oxidized glutathione (GSSG) were measured in the substantia nigra and striatum of mice at baseline (control; n = 4) and 1, 3, and 7 days after MPTP administration in mice treated with MPTP and breathed air (n = 5, 4, and 7, respectively), and in mice treated with MPTP and breathed H2S (n = 5, 5, and 7, respectively) by using a commercially available kit (Cayman Chemical) according to the manufacturer's recommendation.
Statistical analysis
All data are expressed as mean ± SEM. Normally distributed data were analyzed with the one-way analysis of variance (ANOVA) with Bonferroni or Newman-Keuls post hoc test. Percentages of TUNEL-positive cells were compared with the Mann-Whitney U test because the values are not normally distributed. StatView software (Abacus Concepts, Berkeley, CA) was used for statistical analyses.
Results
Effects of administration of MPTP and H2S inhalation on body temperature
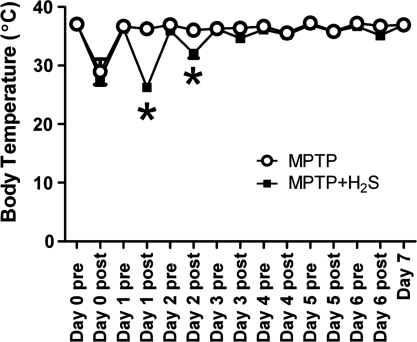
Administration of MPTP markedly decreased body temperature in all mice without or with H2S breathing to a similar extent on Day 0 (Fig. 2). Although H2S inhalation decreased body temperature on Day 1 and 2, a hypothermic response to H2S breathing was not observed after Day 3 to Day 6.
FIG. 2.
Change of body temperature after MPTP administration. Open circle, MPTP, mice that breathed air, treated with MPTP (n = 3). Solid square, MPTP + H2S, mice treated with MPTP that breathed H2S (n = 5). Day 0 is the day when MPTP was administered to mice. Pre, body temperature of mice before breathing H2S or air in the experimental chamber; post, body temperature of mice immediately after breathing H2S or air in the chamber. *p < 0.05 vs. MPTP.
Inhaled H2S prevented movement disorder induced by MPTP
Open-field test
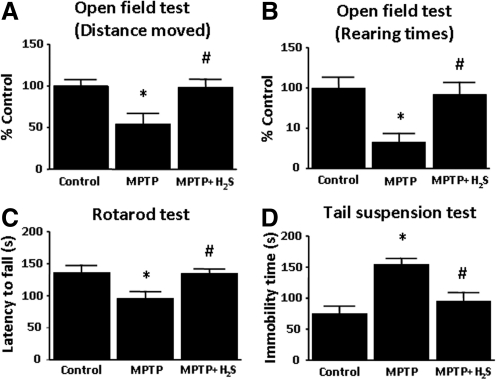
MPTP treatment decreased the distance moved and the number of rearing activity compared with the saline-treated controls (Fig. 3A and B). Inhaled H2S prevented the reduction of locomotion and rearing activity on Day 7 (p < 0.05 vs. MPTP for both).
FIG. 3.
Results of behavioral tests performed at 7 days after administration of MPTP or saline. Results of Distance moved (A) and Rearing times (B) in the Open-field test are shown as a percentage of the values in saline-treated control mice. (C) Latency to fall in the Rotorod test is shown in seconds. (D) Immobility times in the Tail-suspension test are shown in seconds. Control indicates saline-treated mice; MPTP, mice treated with MPTP; MPTP + H2S, mice treated with MPTP and inhaled H2S. n = 7 to 9 in each group in all tests. *p < 0.05 vs. control. #p < 0.05 vs. MPTP.
Rotarod test
Administration of MPTP reduced the duration of time that mice were able to stay on the rotarod compared with the saline controls (96 ± 11 sec vs. 136 ± 12 sec; p < 0.05). Mice that breathed H2S exhibited no motor impairment assessed by the rotarod on Day 7(p < 0.05 vs. MPTP; Fig. 3C).
Tail-suspension test
Administration of MPTP markedly increased the immobility time during a 5-min period compared with saline controls (155 ± 10 sec vs. 75 ± 12 sec; p < 0.05). Inhalation of H2S prevented the increments of immobility time on Day 7 (p < 0.05 vs. MPTP; Fig. 3D).
Inhaled H2S protected dopaminergic neurons from MPTP toxicity
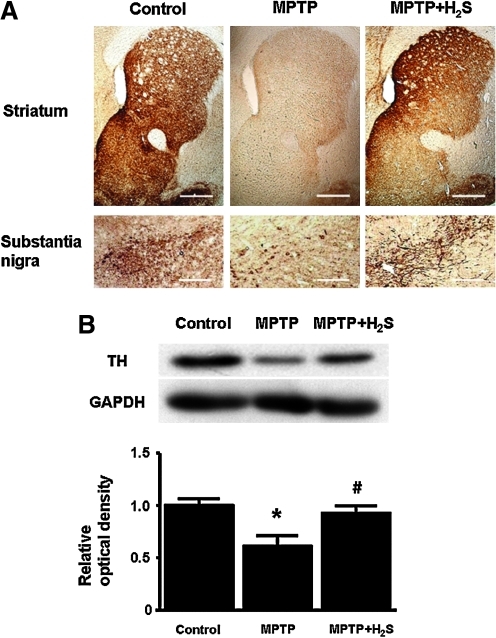
Immunohistochemistry revealed a marked loss of TH immunoreactivity in striatum and substantia nigra 7 days after administration of MPTP in mice that breathed air. In contrast, H2S inhalation for 7 days prevented the loss of TH in striatum and substantia nigra (Fig. 4A). These observations were confirmed with immunoblot analysis that demonstrated loss of TH in striatum and substantia nigra of mice that breathed air, but not in mice that breathed H2S, 7 days after MPTP administration (Fig. 4B).
FIG. 4.
Representative staining and immunoblots. (A) Representative immunohistochemical staining of TH-positive neurons in mice obtained 7 days after administration of MPTP or saline in striatum and substantia nigra. (B) Representative immunoblots showing expression of TH in the striatum and substantia nigra of mice 7 days after administration of MPTP or saline. Relative TH levels were quantitated by dividing the TH immunoreactivity by GAPDH immunoreactivity and normalized to values of control mice; n = 4–5 in each group. *p < 0.05 vs. control. #p < 0.05 vs. MPTP. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
Inhaled H2S protected nigrostriatal neurons from apoptosis induced by MPTP
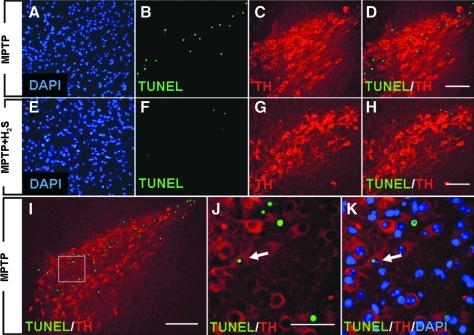
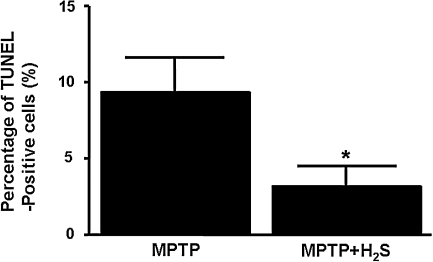
Administration of MPTP increased the number of apoptotic cells in substantia nigra 1 day after MPTP administration, as indicated by the presence of TUNEL-positive nuclei (Fig. 5A–D). In contrast, H2S inhalation prevented the MPTP-induced apoptosis (Fig. 5E–H). Immunofluorescence double labeling demonstrated that the TUNEL-positive nuclei were found exclusively in substantia nigra, especially in the area where TH-positive neurons reside (Fig. 5D, H, and I). Although the majority of the TUNEL-positive nuclei did not overlie with TH-positive neurons (Fig. 5D, H–J), triple labeling with TUNEL, TH, and DAPI showed that a small number of TH-positive neurons contained a TUNEL-positive nucleus (Fig. 5J and K). These observations suggest that at least a part of TH-positive neurons died through apoptosis. Inhaled H2S markedly attenuated the MPTP-induced increase of TUNEL-positive cells, as indicated by the changes in the ratio between TUNEL-positive (green fluorescent) nuclei and DAPI-positive (blue) nuclei (9 ± 2 vs. 3 ± 1%; p < 0.05; Fig. 6).
FIG. 5.
Representative photomicrographs of brain sections containing substantia nigra. These were obtained from mice 1 day after administration of MPTP without H2S (MPTP, A–D, I–K) or MPTP with H2S breathing (MPTP + H2S, E–H). Sections were subjected to the TUNEL assay (green) and stained with DAPI (blue) and anti-TH (red) antibody. Size bar = 100 μm for A–H, 200 μm for I, and 5 μm for J and K. White arrows in J and K, a TH-positive neuron containing TUNEL-positive nuclei. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
FIG. 6.
The percentages of TUNEL-positive cells in all cells (DAPI-positive) in substantia nigra. The percentages of TUNEL-positive cells were counted in four brain sections obtained from three mice in each experimental group. *p < 0.05 vs. MPTP.
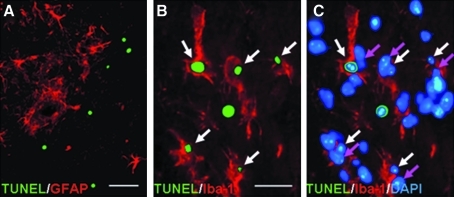
Further to identify the cell types undergoing apoptosis in substantia nigra after MPTP administration, we stained brain sections with antibodies against GFAP (activated astrocytes) or Iba-1 (activated microglia) after TUNEL assay. Double labeling with TUNEL and GFAP revealed that GFAP-positive activated astrocytes did not overlie with any TUNEL-positive nuclei (Fig. 7A). Conversely, double labeling with TUNEL and Iba-1 revealed that many TUNEL-positive nuclei were enveloped by Iba-1–positive activated microglia (Fig. 7B). In fact, triple labeling with TUNEL, Iba-1, and DAPI showed that majority of TUNEL-positive nuclei were distinct from nuclei of microglia (Fig. 7C), suggesting phagocytosis of apoptotic neurons by activated microglia. Taken together, these observations suggest that MPTP induced apoptosis of TH-positive neurons in substantia nigra, and apoptotic neurons were phagocytosed by activated microglia.
FIG. 7.
Representative photomicrographs of brain sections 1 day after MPTP administration without H2S breathing. (A) Double labeling with TUNEL (green) and GFAP (astrocytes, red). Size bar = 5 μm. (B) Double labeling with TUNEL (green) and Iba-1 (microglia, red). (C) Triple labeling with TUNEL (green), Iba-1 (red), and DAPI (blue). Size bar = 2.5 μm for B and C. White arrows, TUNEL-positive nuclei, and pink arrows, nuclei of microglia distinct from the TUNEL-positive nuclei. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
Inhaled H2S prevented glial cell activation induced by MPTP
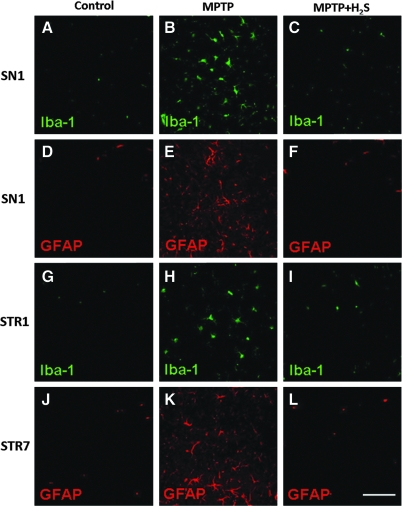
Immunofluorescence staining demonstrated few activated microglia and astrocytes in substantia nigra and striatum of saline-treated mice (Fig. 8A, D, G, and J). In contrast, MPTP increased the number of activated microglia and astrocytes in substantia nigra on Day 1 (Fig. 8B and E). In striatum, the number of activated microglia increased on Day 1 (Fig. 8H), and the number of activated astrocytes increased on Day 7 after MPTP injection (Fig. 8K). Inhaled H2S prevented the activation of both microglia and astrocytes in substantia nigra and striatum after MPTP administration (Fig. 8C, F, I, and L).
FIG. 8.
Representative immunofluorescence staining. The staining was for Iba-1 (microglia, green) and GFAP (astrocytes, red) in substantia nigra (SN) and striatum (STR) of saline-treated control mice (Control, A, D, G, and J), mice treated with MPTP that breathed air (MPTP, B, E, H, and K), and mice treated with MPTP that breathed H2S (MPTP + H2S, C, F, I, and L). These sections were obtained from mice 1 day (SN1 and STR1) or 7 days (STR7) after administration of MPTP or saline. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
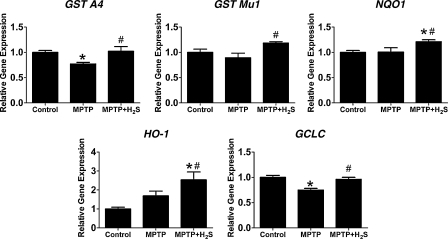
Inhaled H2S upregulated detoxifying enzymes and antioxidant proteins in nigrostriatal region of the brain
To begin to explore the molecular mechanisms responsible for the neuroprotective effects of inhaled H2S, we measured expression levels of genes encoding antioxidant proteins and phase II detoxification enzymes that are regulated by Nrf2 (20). Administration of MPTP did not affect expression of GST Mu1, NQO1, and HO-1 but decreased expression of GST A4 and GCLC (Fig. 9). Inhalation of H2S increased gene expression of all five genes compared with mice that received MPTP without H2S breathing on Day 1 (p < 0.05 vs. MPTP for all five genes). These results suggest that H2S inhalation conferred neuroprotection against MPTP toxicity via upregulation of detoxification enzymes and antioxidant proteins.
FIG. 9.
Relative gene-expression levels in substantia nigra and striatum from mice 1 day after MPTP administration. Control, saline-treated mice; MPTP, mice treated with MPTP; MPTP + H2S, mice treated with MPTP and inhaled H2S. Gene expression was normalized to 18S rRNA expression level, and the mean values for control mice were set to 1. GST A4, glutathione S-transferase A4; GST Mu1, glutathione S-transferase Mu1; NQO1, NAD(P)H quinone oxidoreductase-1; HO-1, heme oxygenase-1; GCLC, glutamate-cysteine ligase catalytic subunit. n = 7–9 in each group. *p < 0.05 vs. control; #p < 0.05 vs. MPTP.
Reduced glutathione levels in the brain were not modified by MPTP or H2S inhalation or both
Administration of MPTP with or without H2S inhalation did not significantly affect levels of reduced glutathione (GSH) and the ratio of reduced and oxidized glutathione ratio (GSH/GSSG) in striatum and substantia nigra on Days 1, 3, and 7 (Table 1).
Table 1.
Glutathione Levels in Brain
| Group | GSH (% of control) | GSH/GSSG ratio (% of control) |
|---|---|---|
| Control | 100 ± 3 | 100 ± 6 |
| Day 1 MPTP | 95 ± 2 | 96 ± 10 |
| Day 1 MPTP + H2S | 91 ± 3 | 93 ± 9 |
| Day 3 MPTP | 93 ± 6 | 91 ± 6 |
| Day 3 MPTP + H2S | 90 ± 4 | 84 ± 5 |
| Day 7 MPTP | 100 ± 3 | 89 ± 7 |
| Day 7 MPTP + H2S | 94 ± 3 | 82 ± 7 |
Values are expressed as mean ± SEM.
Control indicates saline-treated mice; MPTP, mice treated with MPTP; MPTP + H2S, mice treated with MPTP and inhaled H2S; Day 1, 1 day after MPTP treatment; Day 3, 3 days after MPTP treatment; Day 7, 7 days after MPTP treatment; GSH, reduced glutathione; and GSSG, oxidized glutathione. GSH and GSH/GSSG levels were normalized to values of control mice (%). No significant difference in GSH and GSH/GSSG ratio was found between time points and treatments; n = 4–7 in each group.
Discussion
The current study revealed that inhalation of H2S at 40 ppm for 8 h/day for 7 days prevented MPTP-induced neurodegeneration and movement disorder in a mouse model of PD. The neuroprotective effects of H2S inhalation were associated with marked attenuation of the MPTP-induced loss of TH-containing neurons in substantia nigra and striatum. Breathing H2S prevented apoptosis and gliosis in substantia nigra 1 day after MPTP administration. The neuroprotective effects of H2S breathing were associated with upregulation of antioxidant proteins and phase II detoxification enzymes in the nigrostriatal region of the brain. Taken together, these observations suggest that inhaled H2S confers protection against the neurotoxicity of MPTP in mice. Our results suggest that the neuroprotection afforded by inhaled H2S is mediated at least in part by Nrf2-dependent upregulation of antioxidant defense mechanisms.
Neuroprotective effects of H2S donors (i.e., NaHS and Na2S) have been reported in vitro and in vivo in a variety of animal models (16, 17). In particular, a recent report by Hu and colleagues (12) demonstrated neuroprotective effects of NaHS in rat models of PD induced by 6-OHDA or rotenone. The current results support the neuroprotective effects of H2S and extend the findings of Hu and colleagues in several aspects. Neuroprotection by the inhalation of authentic H2S observed in our study lends support that the salutary effects observed after administration of NaHS in the Hu study were actually mediated by H2S. A solution of NaHS has been found to include polysulfides and elemental sulfur in addition to H2S (6). Unlike other toxins, MPTP induces symptoms virtually identical to those of idiopathic PD in humans and directly elicits a specific intoxication of dopaminergic neurons (18). Therefore, the observation that inhaled H2S prevents MPTP-induced neurodegeneration has important clinical implications. Conversely, toxin-induced acute models of PD were used in both of these studies. Parkinson's disease is characterized by the slowly progressive movement disorder. The therapeutic potential of H2S remains to be examined in more chronic and progressive animal models of PD induced by genetic modifications.
We previously reported that the neuroprotective effects of Na2S after cardiac arrest and CPR were associated with inhibition of caspase-3 activation in the brain (23). Similarly, in the current study, we observed that H2S inhalation markedly attenuated apoptosis of neurons in substantia nigra 1 day after MPTP administration. Our multicolor immunohistochemical studies revealed that at least a part of the TH-positive neurons die through apoptosis after MPTP administration. Although the number of TH-positive and TUNEL-positive neurons appears to be small compared with the TH-negative and TUNEL-positive cells in the substantia nigra 1 day after MPTP administration (see Fig. 5), it is possible that apoptotic dopaminergic neurons may be quickly phagocytosed, losing TH positivity (see Fig. 7B and C). Our observation that TUNEL-positive neurons were almost exclusively found in substantia nigra also suggests that the majority of TH-positive neurons underwent apoptosis after MPTP administration. Taken together, these observations suggest that inhaled H2S prevented MPTP-induced neurodegeneration, at least in part by inhibiting apoptosis of dopaminergic neurons.
Inhaled H2S also prevented the activation of microglia in substantia nigra and striatum 1 day after MPTP administration (see Figs. 7 and 8). Neuroinflammation has been suggested to play an important role in the pathogenesis of neurodegenerative diseases (9). It is conceivable that MPTP-induced apoptosis in a small number of vulnerable neurons in substantia nigra triggered glial activation by sending “danger” signals. Nonetheless, because the loss of TH-positive dopaminergic neurons did not become apparent in our histologic assessment until 7 days after administration of MPTP (see Fig. 4), it is likely that inhibition of gliosis by inhaled H2S contributed to the neuroprotective effects of H2S.
The protective impact of Nrf2-dependent signaling has been reported in mouse models of PD induced by MPTP administration (5). In the current study, we found that the neuroprotective effects of inhaled H2S are associated with Nrf2-dependent upregulation of antioxidant and detoxification proteins. These observations are in line with a recent article in which Calvert and colleagues showed that H2S confers cardioprotection against ischemia–reperfusion via upregulation of Nrf2-dependent signaling (4). Conversely, we did not observe significant changes of GSH levels in the tissue homogenates of striatum and substantia nigra 1, 3, and 7 days after administration of MPTP with or without H2S inhalation. Although these results do not support an important role for GSH in the neuroprotective effects of H2S inhalation in our model, it is possible that GSH levels change only in a subgroup of cells in substantia nigra or striatum that were not detectable with the current method. Further studies are needed to define the molecular mechanisms responsible for the neuroprotective effects of H2S breathing in PD.
Organ-protective effects of inhaled H2S have generally been attributed to its ability to induce hypothermia in rodents. For example, Florian and colleagues (7) reported that sustained (48 h) deep hypothermia induced by H2S breathing reduced cerebral-infarct volume in rats after focal ischemia (7). In the current study, administration of MPTP alone reduced the body temperature of mice on Day 0, and H2S breathing did not further decrease the body temperature. Although inhalation of H2S decreased the body temperature of mice for 8 h each on Day 1 and 2 compared with MPTP alone, mice rapidly developed tolerance by Day 3 and exhibited no hypothermic response to inhaled H2S thereafter. It is possible that this brief period of hypothermia contributed to the neuroprotective effects of H2S inhalation. However, it is likely that inhaled H2S conferred its ameliorating effects via other mechanisms unrelated to hypothermia, given the protracted time course of neurodegeneration induced by administration of MPTP.
Limitations
Although we attempted to measure total sulfide levels in plasma and brain tissue homogenates of mice that breathed H2S for 8 h by using the “methylene blue formation method,” we were not able to detect any changes in total sulfide levels in a reproducible manner. Whereas a number of studies used the “methylene blue formation method” and reported “H2S levels” in tissue and plasma [reviewed in (25)], the accuracy of such measurements has been questioned (8, 15, 31). Because we did not have access to more-accurate methods to measure H2S at this point (e.g., gas chromatography), we did not further pursue measurements of tissue H2S levels in the current study. Inhaled H2S may be transported to peripheral organs (e.g., brain) and directly confer protection against a variety of stresses (e.g., inflammation or apoptosis). Alternatively, inhaled H2S may modify blood-borne cells (e.g., leukocytes) or humoral factors (e.g., cytokines) as they pass through the pulmonary circulation, thereby indirectly attenuating the impact of stressors on peripheral tissues. To elucidate how exogenous H2S affects the integrity of peripheral tissues under stress, it is mandatory to determine accurately the impact of exogenously administered H2S on plasma and tissue levels of H2S in vivo. The lack of such data is a major limitation of the current study.
In conclusion, the current study demonstrates that inhalation of H2S prevents MPTP-induced neurodegeneration and movement disorder in mice. We also observed that the neuroprotective effects of inhaled H2S are associated with upregulation of Nrf2-dependent antioxidant and detoxification proteins in the brain. Potential therapeutic effects of H2S inhalation remain to be examined in genetically induced chronic models of PD in future studies.
Abbreviations Used
- 3MST
3-mercaptopyruvate sulfurtransferase
- 6-OHDA
6-hydroxydopamine
- CBS
cystathionine β-synthase
- CPR
cardiopulmonary resuscitation
- CSE
cystathionine γ-lyase
- DAB
diaminobenzidine tetrahydrochloride
- DAPI
4′,6-diamidino-2-phenylindole
- DTT
dithiothreitol
- EDTA
ethylenediaminetetraacetic acid
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GCL
glutamate-cysteine ligase
- GCLC
glutamate-cysteine ligase catalytic subunit
- GFAP
glial fibrillary acidic protein
- GSH
glutathione
- GSSG
oxidized glutathione, glutathione disulfide
- GST
glutathione S-transferase
- H2S
hydrogen sulfide
- HO-1
heme oxygenase-1
- Iba-1
ionized calcium-binding adaptor molecule 1
- MPP +
1-methy-4-phenylpyridinium ion
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- Na2S
sodium sulfide
- NaHS
sodium hydrosulfide
- NQO1
NAD(P)H quinone oxidoreductase-1
- Nrf2
nuclear-factor-E2–related factor-2
- PBS
phosphate-buffered saline
- PD
Parkinson's disease
- SDS
sodium dodecyl sulfate
- SN
substantia nigra
- STR
striatum
- TBS
tris-buffered saline
- TH
tyrosine hydroxylase
- TUNEL
terminal deoxynucleotidyl transferase dUTP nick-end labeling
Acknowledgments
This work was supported by grants from NIH DK05827 to Dr. Kaneki and HL101930 to Dr. Ichinose.
Author Disclosure Statement
The authors verify that no competing financial interests exist.
References
- 1.Beal MF. Experimental models of Parkinson's disease. Nat Rev Neurosci. 2001;2:325–334. doi: 10.1038/35072550. [DOI] [PubMed] [Google Scholar]
- 2.Boenisch T. Handbook of Immunohistochemical Staining Methods. Carpinteria, CA: Dako Cytomation; 2003. [Google Scholar]
- 3.Bove J. Zhou C. Jackson-Lewis V. Taylor J. Chu Y. Rideout HJ. Wu DC. Kordower JH. Petrucelli L. Przedborski S. Proteasome inhibition and Parkinson's disease modeling. Ann Neurol. 2006;60:260–264. doi: 10.1002/ana.20937. [DOI] [PubMed] [Google Scholar]
- 4.Calvert JW. Jha S. Gundewar S. Elrod JW. Ramachandran A. Pattillo CB. Kevil CG. Lefer DJ. Hydrogen sulfide mediates cardioprotection through Nrf2 signaling. Circ Res. 2009;105:365–374. doi: 10.1161/CIRCRESAHA.109.199919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen PC. Vargas MR. Pani AK. Smeyne RJ. Johnson DA. Kan YW. Johnson JA. Nrf2-mediated neuroprotection in the MPTP mouse model of Parkinson's disease: critical role for the astrocyte. Proc Natl Acad Sci U S A. 2009;106:2933–2938. doi: 10.1073/pnas.0813361106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doeller JE. Isbell TS. Benavides G. Koenitzer J. Patel H. Patel RP. Lancaster JR., Jr Darley-Usmar VM. Kraus DW. Polarographic measurement of hydrogen sulfide production and consumption by mammalian tissues. Anal Biochem. 2005;341:40–51. doi: 10.1016/j.ab.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 7.Florian B. Vintilescu R. Balseanu AT. Buga AM. Grisk O. Walker LC. Kessler C. Popa-Wagner A. Long-term hypothermia reduces infarct volume in aged rats after focal ischemia. Neurosci Lett. 2008;438:180–185. doi: 10.1016/j.neulet.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 8.Furne J. Saeed A. Levitt MD. Whole tissue hydrogen sulfide concentrations are orders of magnitude lower than presently accepted values. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1479–R1485. doi: 10.1152/ajpregu.90566.2008. [DOI] [PubMed] [Google Scholar]
- 9.Glass CK. Saijo K. Winner B. Marchetto MC. Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirsch E. Graybiel AM. Agid YA. Melanized dopaminergic neurons are differentially susceptible to degeneration in Parkinson's disease. Nature. 1988;334:345–348. doi: 10.1038/334345a0. [DOI] [PubMed] [Google Scholar]
- 11.Hirsch EC. Hunot S. Neuroinflammation in Parkinson's disease: a target for neuroprotection? Lancet Neurol. 2009;8:382–397. doi: 10.1016/S1474-4422(09)70062-6. [DOI] [PubMed] [Google Scholar]
- 12.Hu LF. Lu M. Tiong CX. Dawe GS. Hu G. Bian JS. Neuroprotective effects of hydrogen sulfide on Parkinson's disease rat models. Aging Cell. 2010;9:135–146. doi: 10.1111/j.1474-9726.2009.00543.x. [DOI] [PubMed] [Google Scholar]
- 13.Ishii I. Akahoshi N. Yu XN. Kobayashi Y. Namekata K. Komaki G. Kimura H. Murine cystathionine gamma-lyase: complete cDNA and genomic sequences, promoter activity, tissue distribution and developmental expression. Biochem J. 2004;381:113–123. doi: 10.1042/BJ20040243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jackson-Lewis V. Przedborski S. Protocol for the MPTP mouse model of Parkinson's disease. Nat Protoc. 2007;2:141–151. doi: 10.1038/nprot.2006.342. [DOI] [PubMed] [Google Scholar]
- 15.Kabil O. Banerjee R. Redox biochemistry of hydrogen sulfide. J Biol Chem. 2010;285:21903–21907. doi: 10.1074/jbc.R110.128363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kimura Y. Goto Y. Kimura H. Hydrogen sulfide increases glutathione production and suppresses oxidative stress in mitochondria. Antioxid Redox Signal. 2010;12:1–13. doi: 10.1089/ars.2008.2282. [DOI] [PubMed] [Google Scholar]
- 17.Kimura Y. Kimura H. Hydrogen sulfide protects neurons from oxidative stress. FASEB J. 2004;18:1165–1167. doi: 10.1096/fj.04-1815fje. [DOI] [PubMed] [Google Scholar]
- 18.Langston JW. Ballard P. Tetrud JW. Irwin I. Chronic parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983;219:979–980. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- 19.Lee JM. Calkins MJ. Chan K. Kan YW. Johnson JA. Identification of the NF-E2-related factor-2-dependent genes conferring protection against oxidative stress in primary cortical astrocytes using oligonucleotide microarray analysis. J Biol Chem. 2003;278:12029–12038. doi: 10.1074/jbc.M211558200. [DOI] [PubMed] [Google Scholar]
- 20.Luchtman DW. Shao D. Song C. Behavior, neurotransmitters and inflammation in three regimens of the MPTP mouse model of Parkinson's disease. Physiol Behav. 2009;98:130–138. doi: 10.1016/j.physbeh.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 21.Masliah E. Rockenstein E. Veinbergs I. Mallory M. Hashimoto M. Takeda A. Sagara Y. Sisk A. Mucke L. Dopaminergic loss and inclusion body formation in alpha-synuclein mice: implications for neurodegenerative disorders. Science. 2000;287:1265–1269. doi: 10.1126/science.287.5456.1265. [DOI] [PubMed] [Google Scholar]
- 22.Melrose HL. Lincoln SJ. Tyndall GM. Farrer MJ. Parkinson's disease: a rethink of rodent models. Exp Brain Res. 2006;173:196–204. doi: 10.1007/s00221-006-0461-3. [DOI] [PubMed] [Google Scholar]
- 23.Minamishima S. Bougaki M. Sips PY. De Yu J. Minamishima YA. Elrod JW. Lefer DJ. Bloch KD. Ichinose F. Hydrogen sulfide improves survival after cardiac arrest and cardiopulmonary resuscitation via a nitric oxide synthase 3-dependent mechanism in mice. Circulation. 2009;120:888–896. doi: 10.1161/CIRCULATIONAHA.108.833491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mori A. Ohashi S. Nakai M. Moriizumi T. Mitsumoto Y. Neural mechanisms underlying motor dysfunction as detected by the tail suspension test in MPTP-treated C57BL/6 mice. Neurosci Res. 2005;51:265–274. doi: 10.1016/j.neures.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 25.Olson KR. Is hydrogen sulfide a circulating “gasotransmitter” in vertebrate blood? Biochim Biophys Acta. 2009;1787:856–863. doi: 10.1016/j.bbabio.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 26.Przedborski S. Jackson-Lewis V. Naini AB. Jakowec M. Petzinger G. Miller R. Akram M. The parkinsonian toxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP): a technical review of its utility and safety. J Neurochem. 2001;76:1265–1274. doi: 10.1046/j.1471-4159.2001.00183.x. [DOI] [PubMed] [Google Scholar]
- 27.Schober A. Classic toxin-induced animal models of Parkinson's disease: 6-OHDA and MPTP. Cell Tissue Res. 2004;318:215–224. doi: 10.1007/s00441-004-0938-y. [DOI] [PubMed] [Google Scholar]
- 28.Shibuya N. Tanaka M. Yoshida M. Ogasawara Y. Togawa T. Ishii K. Kimura H. 3-Mercaptopyruvate sulfurtransferase produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxid Redox Signal. 2009;11:703–714. doi: 10.1089/ars.2008.2253. [DOI] [PubMed] [Google Scholar]
- 29.Twelves D. Perkins KS. Counsell C. Systematic review of incidence studies of Parkinson's disease. Mov Disord. 2003;18:19–31. doi: 10.1002/mds.10305. [DOI] [PubMed] [Google Scholar]
- 30.Volpato GP. Searles R. Yu B. Scherrer-Crosbie M. Bloch KD. Ichinose F. Zapol WM. Inhaled hydrogen sulfide: a rapidly reversible inhibitor of cardiac and metabolic function in the mouse. Anesthesiology. 2008;108:659–668. doi: 10.1097/ALN.0b013e318167af0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whitfield NL. Kreimier EL. Verdial FC. Skovgaard N. Olson KR. Reappraisal of H2S/sulfide concentration in vertebrate blood and its potential significance in ischemic preconditioning and vascular signaling. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1930–R1937. doi: 10.1152/ajpregu.00025.2008. [DOI] [PubMed] [Google Scholar]
- 32.Yamada M. Chiba T. Sasabe J. Terashita K. Aiso S. Matsuoka M. Nasal Colivelin treatment ameliorates memory impairment related to Alzheimer's disease. Neuropsychopharmacology. 2008;33:2020–2032. doi: 10.1038/sj.npp.1301591. [DOI] [PubMed] [Google Scholar]
- 33.Yin WL. He JQ. Hu B. Jiang ZS. Tang XQ. Hydrogen sulfide inhibits MPP(+)-induced apoptosis in PC12 cells. Life Sci. 2009;85:269–275. doi: 10.1016/j.lfs.2009.05.023. [DOI] [PubMed] [Google Scholar]