Abstract
The underlying causes of nonalcoholic fatty liver disease (NAFLD) are unclear, although recent evidence has implicated the endoplasmic reticulum (ER) in both the development of steatosis and progression to nonalcoholic steatohepatitis. Disruption of ER homeostasis, often termed “ER stress,” has been observed in liver and adipose tissue of humans with NAFLD and/or obesity. Importantly, the signaling pathway activated by disruption of ER homeostasis, the unfolded protein response, has been linked to lipid biosynthesis, insulin action, inflammation, and apoptosis. Therefore, understanding the mechanisms that disrupt ER homeostasis in NAFLD and the role of ER-mediated signaling have become topics of intense investigation. The present review will examine the ER and the unfolded protein response in the context of NAFLD. Antioxid. Redox Signal. 15, 505–521.
Introduction
Nonalcoholic fatty liver disease (NAFLD) is an emerging obesity-related disorder characterized by fatty infiltration (steatosis) of the liver in the absence of chronic alcohol consumption. In some individuals, steatosis progresses to nonalcoholic steatohepatitis (NASH), which is characterized by steatosis, inflammation, apoptosis and fibrosis, and end-stage liver disease (39). NAFLD is now recognized as the most common cause of chronic liver enzyme elevations and cryptogenic cirrhosis (16). The prevalence of NAFLD has nearly doubled since 1980, and current estimates indicate that NAFLD may affect up to 25% of the general population and 80% of individuals with obesity (175). NAFLD has also emerged as a common pediatric problem, afflicting 3%–9% of all children in the United States and up to 50% of obese children (122).
The underlying causes of NAFLD are unclear, although recent evidence has implicated the endoplasmic reticulum (ER) in both the development of steatosis and progression to NASH. Disruption of ER homeostasis, often termed “ER stress,” has been observed in liver and adipose tissue of humans with NAFLD and/or obesity (9, 26, 45, 127, 146). Importantly, the signaling pathway activated by disruption of ER homeostasis, the unfolded protein response (UPR), has been linked to lipid biosynthesis, insulin action, inflammation, and apoptosis (44, 52, 65). Therefore, understanding the mechanisms that disrupt ER homeostasis in NAFLD and the role of ER-mediated signaling in NAFLD have become topics of intense investigation. The present review will examine the ER in the context of NAFLD.
The ER and the UPR
Endoplasmic reticulum
In general, the largest membrane in a eukaryotic cell encloses the ER, an array of tubules (cisternae) that forms a three-dimensional network (reticulum) stretching from the nuclear envelope to the cell surface. The smooth ER, which lacks ribosomes, produces structural phospholipids and cholesterol, as well as significant amounts of triacylglycerol and cholesterol esters that have nonstructural roles (167). The smooth ER is the main site of cholesterol synthesis, although much of this lipid is transported to other cellular organelles. Thus, the ER membrane is comprised of very low concentrations of cholesterol and complex sphingolipids (167). This loose packing of ER membrane lipids may provide an environment conducive to the insertion and transport of newly synthesized lipids and proteins (167). The requirement for such a specialized lipid environment within the ER has implications in diseases characterized by abnormal lipid accumulation, such as NAFLD.
All eukaryotic cells contain a significant amount of rough ER, the site for protein folding and maturation. Proteins destined for secretion or insertion into membranes require modification, such as glycosylation and disulfide bond formation, that cannot be achieved in the cytosol (88). The ER lumen provides a specialized environment for protein folding and maturation that is characterized by high concentrations of calcium, a low ratio of reduced glutathione to oxidized glutathione (1:1 to 3:1), and a unique complement of molecular chaperones and folding enzymes (141). The presence of ER-associated degradation (ERAD) machinery helps to ensure that improperly folded proteins are retrotranslocated to the cytoplasm and targeted for proteasomal degradation. The ability of the ER lumen to match folding and degradation to the rate of entry of newly synthesized proteins is monitored by the UPR, a highly conserved quality control system that functions to restore ER homeostasis following periods of stress.
Canonical UPR
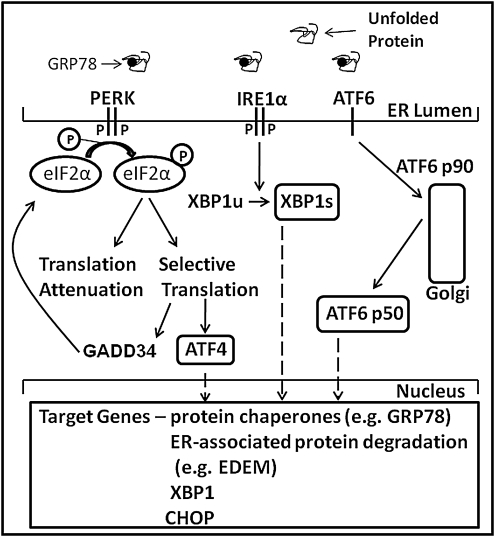
In mammalian cells, the UPR (Fig. 1) is initiated by three ER-localized proteins: inositol-requiring transmembrane kinase and endonuclease 1α (IRE1α), double-stranded RNA (dsRNA)-dependent protein kinase-like ER kinase (PERK), and activating transcription factor-6 (ATF6) (133). Each of these transmembrane proteins has an ER-luminal domain to sense unfolded proteins, a transmembrane domain for targeting to the ER membrane, and a cytosolic domain to transmit signals to the transcriptional and/or translational apparatus (182). It is currently thought that in un-stressed cells, all three proteins are maintained in an inactive state via their association with the ER protein chaperone glucose-regulated protein 78 (GRP78)/immunoglobulin-heavy-chain-binding protein. It has been postulated that upon ER stress, GRP78 is released and sequestered on unfolded proteins, thereby allowing activation of PERK, IRE1α, and ATF6 (194). PERK activation leads to phosphorylation of the α-subunit of the translation initiation factor eukaryotic initiation factor 2α (p-eIF2α) and subsequent attenuation of translation initiation. Paradoxically, p-eIF2α leads to selective translation of mRNAs containing open reading frames, such as activating transcription factor-4 (ATF4) (62, 142). Increased expression of growth arrest and DNA damage-inducible protein 34 (GADD34; which also contains open reading frames), a member of the growth arrest and DNA damage family of proteins, is involved in dephosphorylation of eIF2α and therefore reversal of translational attenuation (133). Activation of IRE1α promotes the splicing of X-box-binding protein-1 (XBP1s) mRNA and subsequent transcription of molecular chaperones (e.g., GRP78) and genes involved in ERAD (e.g., ER degradation-enhancing α-like protein [EDEM]) (142). ATF6 is a bZIP-domain containing transcription factor belonging to the cAMP-response element binding protein (CREB)/ATF family of transcription factors. Activation of ATF6 leads to its release from the ER membrane, processing in the Golgi, and entry into the nucleus. Transcriptional targets of ATF6 include protein chaperones and XBP1 (182). Thus, activation of the UPR initiates a spectrum of responses that transiently attenuate global protein synthesis and enhance the capacity for protein folding and degradation. These responses, in turn, attempt to ameliorate ER stress by restoring the balance between the protein load imposed on the ER lumen and the ability to fold and degrade entering proteins.
FIG. 1.
Overview of the mammalian unfolded protein response (UPR). The presence of unfolded proteins in the endoplasmic reticulum (ER) lumen leads to dimerization and autophosphorylation of protein kinase-like ER kinase (PERK) and (IRE1α), and the release and proteolytic cleavage of activating transcription factor 6 (ATF6) in the Golgi. PERK-mediated phosphorylation of eukaryotic initiation factor 2α (eIF2α) leads to transient attenuation of translation but selective translation of mRNAs containing upstream open reading frames, such as activating transcription factor 4 (ATF4). Increased transcription and translation of GADD34 subsequently leads to dephosphorylation of eIF2α and resumption of translation. Activation of IRE1α leads to the splicing of XBP1. Spliced X-box binding protein 1 (XBP1s), ATF4, and the cleaved form of ATF6 lead to transcriptional activation of a number of gene targets related to protein folding and ER-associated degradation (see text).
An expanded view of the UPR
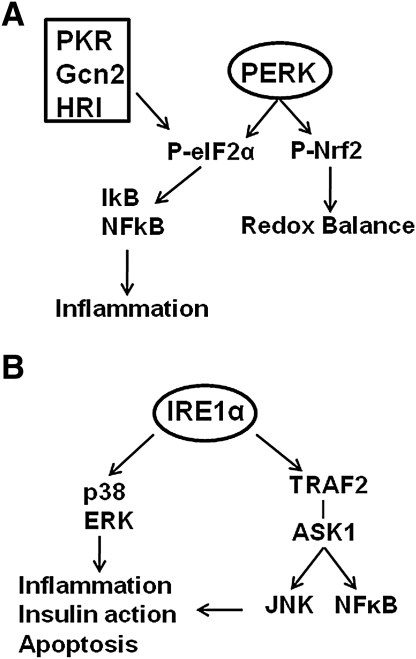
PERK is one of four protein kinases that can phosphorylate eIF2α (Fig. 2A); the other three are dsRNA-activated protein kinase (PKR), which is activated in response to viral infection, general control nonderepressible 2 kinase (GCN2), which is activated in response to amino acid deprivation, and heme-regulated inhibitor kinase (HRI), which is primarily expressed in reticulocytes and appears to coordinate globin polypeptide synthesis with heme availability (63). Protein kinase-mediated phosphorylation of eIF2α regulates not only translation but also the activation of nuclear factor kappa-β (NFκB), via reduction in the abundance of the NFκβ inhibitor (Iκβ) (121, 142, 183). Further, PERK can phosphorylate nuclear erythroid 2 p45-related factor 2 (Nrf2) triggering the dissociation of Nrf2/Keap1 complexes and subsequent nuclear import of Nrf2 (22). Thus, activation of this branch of the UPR links disruption of ER homeostasis to both inflammation, via NFκβ and redox balance, via Nrf2.
FIG. 2.
Protein kinase-mediated phosphorylation of eIF2α and additional downstream targets of p-eIF2α and IRE1α. (A) Phosphorylation of eIF2α can be mediated by four protein kinases: double-stranded RNA-activated protein kinase (PKR), general control nonderepressible 2 kinase (Gcn2), heme-regulated inhibitor kinase (HRI), and PERK. Phosphorylation of eIF2α is linked to the inflammatory response via activation of nuclear factor kappa-β (NFκβ). PERK-mediated phosphorylation of nuclear erythroid 2 p45-related factor 2 (Nrf2) links PERK activation to the regulation of redox balance (see text). (B) Activation of IRE1α can lead to activation of stress kinases, including p38 mitogen-activated protein kinase (MAPK), extracellular-regulated kinase (ERK), c-Jun-NH2-terminal kinase (JNK), and NFκβ (see text).
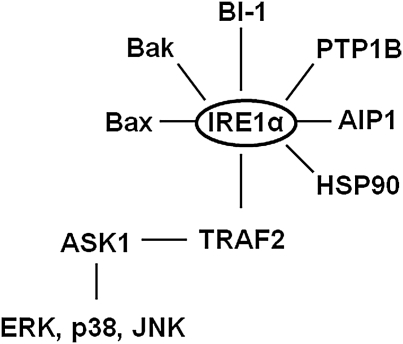
IRE1α, in addition to catalyzing XBP1 splicing, has additional functions related to cellular signaling (Fig. 2B). Activated IRE1α can interact with the adaptor protein TNFR-associated factor 2 (TRAF2) and lead to activation of c-Jun-NH2-terminal kinase (JNK) and NFκβ via apoptosis signaling-regulating kinase 1 (ASK1) (166). IRE1α activation has also been linked to the activation of p38 mitogen-activated protein kinase (p38 MAPK) and extracellular-regulated kinase (ERK) (50, 54, 104). These interactions suggest that the IRE1α branch of the UPR regulates not only adaptation to ER stress and cell survival via XBP1 splicing but also activation of signaling pathways involved in inflammation, insulin action, and apoptosis.
Several proteins that share sequence homology with ATF6α, including Tisp40 (CREB4 and CREBSL4), BBF2H7 (CREB3L2), Luman (CREB3), old astrocyte specifically induced substance (CREB3L1), and CREBH (CREB3L3), are similarly anchored to the ER membrane and activated via regulated intramembrane proteolysis (RIP) in response to ER stress (7, 98, 111). Although each of these proteins appears to be mobilized in response to ER stress their unique tissue distributions suggest that ER stress-mediated RIP may be a mechanism to achieve tissue/cell-specific outcomes. Thus, it appears likely that future studies related to ER stress-mediated RIP will expand the role of the UPR in cellular signaling.
It is important to emphasize that much of what we know about the UPR has been derived from studies that utilize pharmacologic agents (tunicamycin and thapsigargin) to induce severe, protracted ER stress and cell death. Much less is known about the UPR in the context of physiologic stressors that provoke ER stress. In vivo, the diversity of ER stress-mediated UPR signaling likely yields outcomes that are specific to the stress imposed and the needs of the involved cell but may be broadly grouped into three potential outputs: adaptation (ER stress→UPR activation→re-establishment of ER homeostasis), alarm (ER stress→UPR activation→activation of signaling pathways involved in inflammation, anti-oxidant defense, and/or insulin action→re-establishment of ER homeostasis or mild, chronic ER stress), and apoptosis (ER stress→UPR activation→failure to resolve severe ER stress→cell death) (65). In light of this complexity we will first review studies that link ER stress and/or the UPR to the pathogenesis of NAFLD and then discuss the signals that might provoke activation of the UPR in NAFLD, novel roles for the UPR in this disease and the physiologic implications of these studies.
The UPR and the Development of Hepatic Steatosis
NAFLD is characterized by lipid accumulation in the liver (≥10% of liver weight) in the absence of chronic alcohol consumption or other liver disease (39). Sources of hepatic lipids in NAFLD include dietary chylomicron remnants, free fatty acids released from adipose tissue triglycerides, and de novo lipogenesis. Recent work has emphasized the latter two mechanisms as the principle contributors to hepatic fat accumulation in humans with NAFLD (31, 32, 67). Several recent studies have linked the UPR to the regulation of lipogenesis and hepatic steatosis.
IRE1α-XBP1 pathway
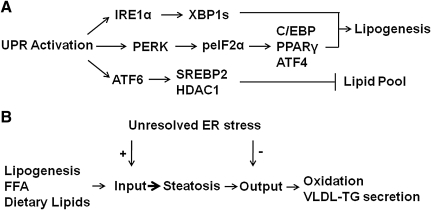
The downstream targets of IRE1α activation include splicing of homologous to ATF/CREB1 (HAC1) mRNA in yeast and XBP1 mRNA in mammalian cells (63). IRE1α-mediated splicing of HAC1 mRNA produces Hac1p, whereas IRE1α-mediated splicing of XBP1 produces XBP1s, both of which are critical to membrane lipid synthesis in yeast and mammalian cells, respectively (18, 153). In fact, the enforced expression of XBP1s in NIH-3T3 fibroblasts was sufficient to induce the synthesis of phosphatidylcholine, the primary phospholipid in the ER membrane (153). To investigate the role of XBP1 in the postnatal liver, Lee et al. utilized mice bearing an inducible, conditional disruption of the Xbp1 gene (71). The phenotype of mice with conditional disruption of Xbp1 in the liver included reduced plasma levels of triglyceride, cholesterol, and free fatty acids. Primary hepatocytes isolated from these mice were characterized by reduced incorporation of [14C] acetate into fatty acids and sterols, suggesting that XBP1 was required for de novo lipogenesis. Chromatin immunoprecipitation assays, using liver extracts from mice fed a high fructose diet, demonstrated that XBP1 was able to bind to promoter regions of several lipogenic genes. The IRE1α-XBP1 pathway has also been linked to adipogenesis in mouse embryonic fibroblasts and 3T3-L1 cells (145). Thus, XBP1 splicing may, under the appropriate conditions, promote hepatic lipogenesis (Fig. 3A) and perhaps contribute to the development of hepatic steatosis.
FIG. 3.
The UPR is linked to regulation of lipogenesis and hepatic lipid stores. (A) The IRE1α-XBP1 and the PERK-peIF2α pathways can upregulate the lipogenic gene program. In contrast, interactions among ATF6, sterol regulatory element binding protein 2 (SREBP2), and histone deacytelase-1 (HDAC1) can limit lipogenesis. (B) Inability to resolve ER stress may promote hepatic steatosis via upregulation of pathways that contribute to lipid input and downregulation of lipid output pathways.
PERK-eIF2α pathway
PERK and phosphorylation of eIF2α also appear to regulate lipogenesis and hepatic steatosis. Targeted deletion of PERK in mammary epithelium reduced free fatty acids in mouse milk and led to growth retardation in suckling pups (8). Further analysis revealed that PERK deletion resulted in reduced expression of several lipogenic genes, including sterol regulatory element binding protein 1 (SREBP1). This study led to the hypothesis that PERK-mediated phosphorylation of eIF2α promotes SREBP1 activation during mid-lactation via depletion of insulin-induced gene 1 (INSIG1) protein, an ER-localized protein that anchors the SREBP-SREBP cleavage-activating protein (SCAP) complex in the ER membrane (72). Whether PERK-mediated regulation of lipogenesis occurs in hepatocytes is presently unknown. GADD34 (PPP1R15a) encodes a regulatory subunit of a phosphatase that selectively dephosphorylates eIF2α (phosphoserine 51). Enforced expression of an active C-terminal fragment of GADD34 from a liver-specific albumin promoter was used to study the role of p-eIF2α-mediated signaling in the mouse liver (114). The presence of the transgene resulted in a number of metabolic adaptations, including reduced hepatic steatosis in mice fed a high-fat diet. The reduction in hepatic steatosis was associated with lower expression of the adipogenic nuclear receptor peroxisome proliferator-activated receptor-γ (PPARγ) and upstream regulators of PPARγ, CCAAT/enhancer-binding protein-α and -β (C/EBPα and C/EBPβ). Protein kinase-mediated phosphorylation of eIF2α increases the translation of a subset of genes that include ATF4. The phenotype of ATF4 heterozygous mice includes protection from age-related and diet-induced obesity and diet-induced hepatic steatosis (144). Thus, when these studies are considered together it would appear that protein kinase-mediated eIF2α phosphorylation can regulate the lipogenic transcriptional program and perhaps contribute to the development of hepatic steatosis (Fig. 3A).
ATF6
ATF6 and SREBPs are both ER-membrane-bound transcription factors that are activated by proteolytic cleavage. At least one study has demonstrated that the nuclear form of ATF6 inhibits the transcriptional activity of SREBP2 by forming a complex with SREBP2 that recruits histone deacytelase-1 (HDAC1) (192). The functional consequence of this interaction was to reduce Oil-Red-O staining in liver cells (Fig. 3A). Thus, all three proximal UPR sensors, PERK, IRE1α, and ATF6α, can regulate lipid stores in the liver. The degree to which the UPR contributes to hepatic steatosis may depend on the relative activation of the three proximal UPR sensors, PERK, IRE1α, and ATF6, coupled with appropriate downstream protein–protein and/or protein–DNA interactions.
Homeostatic model
A fundamental function of the UPR is to restore ER homeostasis in response to the accumulation of unfolded proteins by reducing the protein load entering the ER lumen and increasing the capacity of the ER to fold and degrade proteins. The presence of ER stress and activation of the UPR in chronic diseases such as obesity and NAFLD implies that the ability to resolve ER stress has been compromised. A recent study has examined the role of the UPR in hepatic steatosis from this perspective (134). Genetic ablation of eIF2α, IRE1α, or ATF6α resulted in hepatic steatosis in response to chemical induction of ER stress. Steatosis, in this model, appeared to result from impairments in the capacity to oxidize fatty acids and was potentially augmented by impaired lipoprotein secretion. Thus, the UPR may promote lipid homeostasis (i.e., prevent hepatic steatosis) via its ability to maintain or rapidly re-establish ER homeostasis after ER stress. Perhaps development and/or exacerbation of hepatic steatosis involves selective impairments to the UPR that reduce the ability of the UPR to resolve ER stress (Fig. 3B). Further work is necessary to investigate this hypothesis using physiologic models of hepatic steatosis.
Recent studies using GRP78+/− mice and adenoviral-mediated overexpression of GRP78 in vivo support the homeostatic model concept (61, 189). GRP78+/− mice were resistant to high-fat-diet-induced insulin resistance, hepatic steatosis, white adipose tissue inflammation, and hyperglycemia (189). It was postulated that GRP78 heterozygosity triggered the adaptive UPR via upregulation of other ER chaperones (e.g., GRP94) and components of the ERAD machinery, improvement of ER homeostasis, and attenuation of the deleterious consequences of a high-fat diet. The results of this study predict that selective upregulation of protein chaperones in the liver should improve insulin action and hepatic steatosis. Indeed, a recent study demonstrated that selective overexpression of GRP78 in the liver improved ER homeostasis, hepatic steatosis, and insulin action in ob/ob mice (61).
The UPR and Disease Progression in NAFLD
Liver pathology in NASH can include macrovesicular steatosis, inflammation, fibrosis, apoptosis, and necrosis (5, 37, 48, 112, 120, 156). Multiple factors, including insulin action, oxidative stress, cytokine-mediated signaling, inflammation, bacterial endotoxin, and excess fatty acids likely function in concert to provoke disease progression in NAFLD. In this section we will consider potential ways in which the UPR, via interactions with some of these factors, may contribute to the development of NASH and progressive liver disease.
JNK: a common link to insulin action, apoptosis, and inflammation
Obesity and insulin resistance are thought to play an important role in the pathogenesis of NAFLD (12, 23, 87, 120, 152). An elegant study identified ER stress as a molecular link between obesity and deterioration of insulin action in liver and adipose tissue (117). In this study, obesity-induced ER stress reduced insulin signaling via an IRE1α-dependent activation of JNK, a protein kinase that can interfere with insulin signaling via serine phosphorylation of insulin-receptor substrate-1 (51, 53, 166). Notably, activation of JNK can also lead to liver damage and hepatocyte apoptosis (17, 24, 68, 105), the latter of which is a characteristic feature of NASH and correlates with disease severity (38, 176). In addition, the expression of proinflammatory cytokines was reduced in mice lacking JNK (52, 164), leading to the inference that IRE1α-mediated activation of JNK may also lead to a pro-inflammatory state. Global deletion of JNK1, but not JNK2, reduced hepatic triglyceride accumulation, inflammation, liver injury, and apoptosis in methionine-choline diet-fed mice (140). In contrast, specific ablation of JNK1 in hepatocytes produced a phenotype that included glucose intolerance, insulin resistance, and hepatic steatosis (135), suggesting that JNK isoforms may have tissue-specific actions that dictate their roles in NAFLD. Thus, more work is needed to elucidate the role of JNK in NAFLD, how and under what conditions IRE1α regulates JNK, the cell types responsible for IRE1α-JNK-mediated outcomes (25), and the molecular mechanisms that distinguish ER lumen-mediated regulation of JNK from that which occurs from signals originating in the cytosol or plasma membrane. Clearly, JNK represents an important nexus for control of insulin action, inflammation, and cell death. The ability of IRE1α to regulate JNK activity suggests that ER stress and the UPR play an important role in disease progression in NAFLD.
Inflammation
The liver plays a critical role in the production of pro-inflammatory mediators, such as C-reactive protein (CRP), amyloid P-component, fibrinogen, and interleukin-6 (IL-6). Low-level activation of NFκβ in the liver was sufficient to increase the hepatic production of these pro-inflammatory mediators to an extent that was equivalent to what occurred in wild type mice fed a high-fat diet (14). Thus, it is now well appreciated that the NFκβ pathway is an important determinant of the inflammatory response and subsequent changes in insulin action (149). UPR-mediated signaling can lead to activation of NFκβ via the PERK, IRE1α, and/or ATF6 pathways (52, 142, 186). However, the importance of UPR-mediated signaling to the activation of NFκβ in the context of obesity and NAFLD are presently unknown. If the formation of the IRE1α-TRAF2 complex is crucial to activation of both JNK and NFκβ in NAFLD (54, 166), it will be important to understand how and under what physiologic conditions these two proteins interact with the IRE1α-TRAF2 complex. Alternatively, it will be important to consider whether ER stress-mediated activation of JNK and NFκβ is shared among the three proximal UPR sensors, and if so, to identify the mechanism by which this is accomplished. This latter possibility may be particularly relevant given that NFκβ can protect hepatocytes from oxidative stress and TNFα-induced cell death (42, 80), as well as steatohepatitis and hepatocellular carcinoma (83). Thus, the ultimate outcome of NFκβ activation likely depends on such factors as the duration and magnitude of the stimulus, and the interaction of NFκβ with other signaling networks.
PKR is an interferon-induced serine/threonine protein kinase that is activated by dsRNA and, as previously described (Fig. 2A), is one of four protein kinases that can phosphorylate eIF2α in response to ER stress (33). PKR appears to be required for NFκβ activation in response to dsRNA, and therefore PKR has been linked to immune and inflammatory responses (34). PKR activity is increased in adipose tissue and liver of murine models of obesity and inhibits insulin signaling directly and indirectly, the latter via activation of JNK (101). The ability of PKR to respond to pathogens, nutrients, and organelle stress and to regulate inflammatory and insulin signaling pathways suggest that PKR may be a core component of an inflammatory complex (52, 101, 188). PKR exemplifies the complexity of UPR signaling and its downstream outcomes. For example, dsRNA-activated PKR can use catalysis-dependent and -independent activities to function both as a pro- and anti-apoptotic factor, via regulation of NFκβ and phosphorylation of eIF2α, respectively (34). Thus, it has been proposed that “PKR may serve as a molecular clock to time the sequential events of survival and death following virus infection” (34). It is feasible that other multifunctional, UPR-linked protein kinases employ similar strategies to elicit cell- or stress-selective outcomes.
RIP, the release and transport of ER-resident proteins from the ER membrane to the Golgi for processing, may represent an important link between the ER and inflammation (97, 130). The three-proximal UPR sensor, ATF6α, and the lipogenic transcription factor, SREBP, both undergo RIP before their entry into the nucleus (194); thus, RIP is required for the activation of one arm of the UPR and for transcriptional regulation of the lipogenic program in the liver. CREBH, a transcription factor belonging to the CREB/ATF family of transcription factors, is an RIP-regulated, liver-enriched protein that appears to be required for the hepatic synthesis of amyloid P-component and CRP (82, 195). In addition, pro-inflammatory cytokines and lipopolysaccharide (LPS) induce the cleavage of CREBH in the liver in vivo (195). Thus, ER stress in the liver may be linked to systemic inflammation via RIP-mediated mobilization of CREBH.
Oxidative stress
Oxidative stress is thought to be an important pathogenic event in NAFLD. The ER provides a unique oxidizing environment for protein folding and disulfide bond formation. Each disulfide bond formed during oxidative protein folding produces a single reactive oxygen species. It has been estimated that secretory cells produce 3–6 million disulfide bonds per minute; thus, protein folding in the ER is intimately linked to the generation of reaction oxygen species and potentially oxidative stress (138, 148). Conversely, cellular oxidative stress can disrupt ER homeostasis and induce ER stress (47, 184, 190). Therefore, it is not surprising that the UPR can activate an antioxidant program via the transcription factor Nrf2 (21). Nrf2 belongs to the Cap ’n’ Collar family of basic leucine zipper transcription factors and regulates the inducible expression of antioxidant response element-containing genes (21). Nrf2 is highly expressed in the liver and kidney and is a substrate of the proximal UPR sensor PERK (96). Importantly, Nrf2 deletion results in rapid onset, and progression of steatohepatitis in mice provided a methionine-choline-deficient diet (155). In addition, Nrf2-deficient mice were characterized by increased mortality in response to endotoxin-induced and cecal ligation and puncture-induced septic shock (162). These studies have led to the proposal that Nrf2 participates in the regulation of the innate immune response. As noted, PERK-mediated phosphorylation of eIF2α also leads to the upregulation of ATF4. Along with Nrf2, this transcription factor has been linked to the maintenance of cellular glutathione (22). Thus, the PERK arm of the UPR appears to play a critical role in the defense against oxidative stress and the downstream substrate Nrf2 has been directly linked to steatohepatitis. In addition to the PERK arm of the UPR, recent evidence has also linked the IRE1α-XBP1 branch of the UPR to the regulation of antioxidant defenses (81). In this study, hydrogen peroxide-mediated cell death occurred more extensively in mouse embryonic fibroblast cells deficient in XBP1. XBP1 deficiency resulted in reduced catalase expression, and overexpression of XBP1 restored catalase expression in XBP1-deficient cells. Thus, XBP1 may provide protection from oxidative stress; however, whether this regulation occurs in hepatocytes is presently unknown.
The ER and cell death
Hepatocyte apoptosis is increased in patients with NASH and correlates with disease severity; therefore, apoptosis has been proposed as a component of disease progression in NAFLD (38, 176). Failure of the UPR to ameliorate ER stress can lead to cell death via several mechanisms. C/EBP homologous protein (Chop) is among the best characterized of the UPR-regulated pro-apoptotic proteins (116). Chop expression is regulated by ATF4 and perhaps ATF6, and deletion of Chop provides some protection from ER stress-induced cell death in both cells and animals (84, 109, 116, 132, 151). Chop deficiency delayed the development of ER stress-mediated diabetes in Akita mice and attenuated cholestasis-induced liver fibrosis (115, 157). However, the role of Chop in NAFLD is unclear as recent evidence demonstrated that methionine-choline-deficient diet-induced liver injury was not reduced in Chop knockout mice (125). The Chop protein is unstable when compared to protein chaperones, such as GRP78. Therefore, the role of Chop as a pro-apoptotic protein may be determined by the level of Chop expression, the presence of factors that increase its stability and/or protein–protein interactions that direct cell-specific effects (89, 132, 180).
It is possible that ER-mediated calcium release links the ER to alterations in mitochondrial function and oxidative stress in NAFLD. For example, the release of ER calcium and subsequent calcium influx into mitochondria can lead to mitochondrial membrane permeabilization and activation of the intrinsic apoptotic pathway (30). A number of studies have demonstrated that ER stress, ER-localized proteins, and B-cell leukemia/lymphoma 2 protein (Bcl-2) protein family members interacting with ER localized proteins can regulate ER calcium flux (30, 74, 107, 143). Although the mechanisms involved in the regulation of ER calcium are not fully understood available data implicate the inositol triphosphate (IP3) receptor and truncated variants of the sarcoendoplasmic reticulum calcium-ATPase (74, 107). It also appears that the ER and mitochondria physically and functionally interact, in part, via tethers that link both smooth and rough ER to mitochondria (20, 131). A reduction in the length of these tethers has been observed in response to apoptotic agents (20). Although it is unclear whether ER calcium influences disease progression and apoptosis in NAFLD, it is possible that the hepatic milieu in NAFLD modifies ER calcium flux perhaps via alterations in IP3 receptor function and or ER-mitochondrial interactions.
Murine caspase-12 and human caspase-4 are members of the IL-1β-converting enzyme subfamily of caspases that are 48% identical at the amino acid level and have predicted structures that are consistent with initiator caspases (69, 108). Both of these caspases appear to be ER-localized and cleaved in response to ER stress. Caspase-12 knockout mice and human cell lines in which caspase-4 was knocked down were protected from ER stress-induced apoptosis (99, 100). Whether these caspases play a functional role in ER stress-mediated apoptosis is controversial, in part due to the fact that caspase-4 belongs to a group of caspases that are best known for proteolytic activation of cytokines and not apoptosis (108, 147).
As noted above, IRE1α, via interaction with TRAF2, can mediate activation of the pro-inflammatory and pro-apoptotic protein, JNK (166). In addition, IRE1α appears to be a platform for the interaction of several Bcl-2 proteins, including the pro-apoptotic proteins Bcl-2-associated X protein (Bax) and Bcl-2 antagonist/killer (Bak) (49, 65, 107, 143). Although there are clearly a number of potential mechanisms linking the ER to the apoptotic program, the extent to which these mechanisms play a role in NAFLD remains unclear. It is also important to emphasize that cell death linked to persistent ER dysfunction can proceed independently of caspase activity, and that cell death in response to ER stress can involve caspase-dependent apoptosis and caspase-independent cell necrosis (65).
Autophagy, in principle, is a cellular degradation process for long-lived proteins and unnecessary or damaged organelles (66, 93, 94). At least three forms of autophagy have been identified, chaperone-mediated autophagy, microautophagy, and macroautophagy, that have distinct physiologic functions and modes of cargo delivery to the lysosome (73). Recent evidence has demonstrated that inhibition of macroautophagy in cultured hepatocytes and mouse liver increased triglyceride storage in lipid droplets (150). ER stress can trigger macroautophagy via mechanisms that may require calcium-mediated activation of protein kinase Cθ (137, 191). In addition, autophagy is a necessary pathway for the maintenance of structure, mass, and function in pancreatic β-cells (60). One can envision that ER stress-mediated activation of autophagy may be part of the protective, adaptive component of the UPR. The extent to which autophagy contributes to the development of hepatic steatosis or disease progression in NAFLD requires further study.
Summary
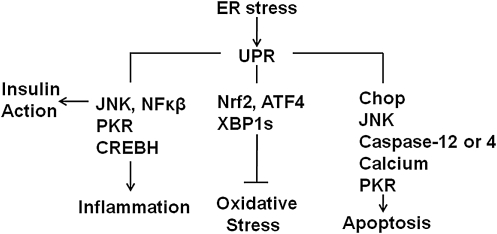
There are a number of viable candidate mechanisms that link unresolved ER stress and the UPR to insulin resistance, inflammation, and apoptosis (Fig. 4). In addition, the process of protein folding in the ER lumen can generate reactive oxygen species and the UPR has the ability to mount a protective response against the development of oxidative stress (Fig. 4). Thus, ER-mediated signals are linked to a number of downstream pathways that contribute to the pathogenesis of NAFLD. However, whether ER stress and the UPR contribute to disease progression in NAFLD will ultimately depend on the ability of the UPR to alleviate the insult that led to disruption of ER homeostasis. The scenario most conducive to ER stress-mediated disease progression likely involves chronic insults that provoke continuous ER stress coupled to signals that reduce or impair the UPR's ability to alleviate those insults. Next we will consider potential factors that elicit activation of the UPR and are relevant to NAFLD.
FIG. 4.
The UPR is linked to insulin action, inflammation, oxidative stress, and apoptosis. The UPR is linked to insulin action via interactions with JNK, to inflammation via PKR, regulated intramembrane proteolysis of CREBH, and interactions with JNK, to protection from oxidative stress via phosphorylation of Nrf2, splicing of XBP1 and selective translation of ATF4, to apoptosis via interactions with JNK, upregulation of C/EBP homologous protein (Chop), activation of caspase-12, and extrusion of luminal calcium (see text).
Determinants of UPR Activation in NAFLD: Steatosis
The upstream signals that mediate the putative link between the UPR and lipogenesis are presently unclear. Recent studies have demonstrated that the postprandial environment can elicit IRE1α activation, XBP1 splicing, and phosphorylation of eIF2α (71, 114, 126). It is possible that the postprandial environment provokes ER stress by transiently increasing protein synthesis above the capacity for protein folding and degradation. Alternatively, it is possible that the postprandial environment activates selective proximal UPR sensors that allow for the regulation of lipogenesis independent of ER stress per se. In support of this latter concept, hepatic XBP1s was induced in mice fed a 60% fructose diet in the absence of changes in GRP78 or Chop (71). Previous studies have also identified novel links between PERK and the expression of growth factors (75), and between PKR and phosphoinositide-3 kinase signaling (64), that may be independent of unfolded protein accumulation. Moreover, the basal expression of at least some ER chaperones appears to be dependent on a mitogenic pathway that is distinct from the ER stress-induced UPR (11). The critical role of the UPR in ER membrane biogenesis may require such independent regulation (106).
It is certainly possible that individual UPR sensors or downstream signaling components may be regulated by signals that do not necessarily involve ER stress (179). Although PERK and IRE1α share functionally similar ER-luminal sensing domains and both appear to be simultaneously activated by chemically induced ER stress, they may be selectively engaged in vivo (50). In particular, recent studies have identified several proteins that directly interact with and/or regulate the activity of IRE1α (46, 49, 78). This has led to the postulate that IRE1α activity in mammalian cells is determined by the formation of a complex protein platform, discussed above, that is assembled at the ER membrane (Fig. 5). Thus, the activity of IRE1α and its ability to interact with and regulate downstream pathways may be dependent on the composition of the IRE1α protein platform. In addition, the transcriptional response to increased splicing of XBP1 may be modulated by protein interactions with XBP1s that are dependent on the cellular environment (123, 178, 185). Indeed, recent work has demonstrated that the nuclear localization of XBP1s requires the interaction of XBP1s with the p85 subunit of phosphoinositide 3-kinase (123, 178). Ultimately, what may be a critical determinant of UPR-mediated hepatic steatosis is the ability to turn off postprandial activation of IRE1α or related UPR components. In this regard, mammalian target of rapamycin (mTOR) has been linked to the postprandial activation of the IRE1α-XBP1 pathway in the liver (126). In addition, chronic activation of mTOR can provoke UPR-mediated inhibition of insulin signaling and susceptibility to apoptosis (118). Thus, the ability to regulate mTOR may play an important role in UPR-mediated regulation of lipogenesis and subsequent development of hepatic steatosis. Recent findings have also linked the IRE1α-XBP1 pathway to the regulation of hepatic lipid metabolism by the circadian clock (19). This disruption of the circadian clock may represent another important link between the UPR and hepatic steatosis.
FIG. 5.
IRE1α can interact with a diverse set of proteins. The activity of IRE1α can be modified by a number of interacting proteins, including B-cell leukemia/lymphoma 2 protein (Bcl-2)-associated X protein (Bax), Bcl-2 antagonist/killer (Bak), Bax inhibitor (BI)-1, protein tyrosine phosphatase-1B (PTP1B), apoptosis signaling-regulating kinase 1 (ASK1)-interacting protein 1 (AIP1), and HSP90. In turn, IRE1α, via interactions with TNFR-associated factor 2 (TRAF2), can modify the activity of ERK, p38, JNK, and NFκβ (see text).
Determinants of UPR Activation in NAFLD: Disease Progression
Fatty acid composition
A large portion of the elevated hepatic triglyceride stores in NAFLD appear to arise from re-esterification of circulating free fatty acids (32). Elevated circulating free fatty acids are a characteristic feature of NAFLD and are positively correlated with liver disease severity (103). A growing body of evidence has demonstrated that elevated free fatty acids, in particular, long chain saturated fatty acids, induce ER stress and activation of the UPR in liver cells (113, 173). In addition, in vivo studies have demonstrated that a reduction in the ability to desaturate fatty acids or a dietary-induced increase in the amount of saturated fatty acids in the liver provoke ER stress, apoptosis, and/or liver injury (76, 170). Saturated fatty acids disrupt ER homeostasis and induce apoptosis in liver cells via mechanisms that do not appear to involve ceramide accumulation (173). It is possible that the link between circulating and intrahepatic fatty acids and ER stress is mediated by changes in intracellular phospholipid pools (161, 181). Several studies suggest that saturated fatty acids disrupt ER homeostasis via selective, structural effects to the ER (10, 13, 95, 165). The highly unsaturated, fluid environment of the ER membrane, and ER membrane-bound UPR sensor proteins may be particularly susceptible to even minor increases in the amount of saturated fatty acids (167). Another mechanism by which saturated fatty acids may disrupt ER homeostasis is through reduced ER calcium stores in liver cells, perhaps via compromised calcium-dependent ER protein chaperone function (124, 172). Finally, recent data have demonstrated that saturated fatty acids increase the expression of phosphotidylinositol 3-kinase interacting protein 1, and SiRNA-mediated knockdown of this protein protected HepG2 cells from palmitate-mediated ER stress (27). However, it is presently unclear whether fatty acid composition plays a significant role in disease progression in human NAFLD (3, 29, 35, 43, 102).
Apolipoprotein B100
Apolipoprotein B100 (ApoB100) is an important client protein in the liver. The biogenesis of ApoB100 requires co- and post-translational modification and studies have identified interactions between newly synthesized ApoB100 polypeptides and ER chaperone proteins, such as GRP78, glucose-regulated protein-94 (GRP94), endoplasmic reticulum protein-72 (ERp72), calreticulin, and calnexin (15, 129, 193). Prolonged and/or severe ER stress can reduce ApoB100 secretion; however, it has also been postulated that ApoB100 may serve as a molecular link between fatty acid-induced ER stress and insulin resistance in hepatocytes (113, 154). These data suggest a scenario in which fatty acids stimulate ApoB100 synthesis to an extent that exceeds the ability of the ER lumen to modify this protein, leading to ER stress and activation of stress signaling proteins (e.g., JNK and glycogen synthase kinase-α) that can influence insulin action, inflammation, and/or apoptosis.
Hexosamine biosynthetic pathway
Glycosylation is an essential ER luminal modification for proper stability, folding, translocation, and function of many proteins (63). The hexosamine biosynthetic pathway plays a key role in glycosylation and increased flux through this pathway, which can occur under conditions of hyperglycemia and/or hyperlipidemia, has been linked to PERK-dependent ER stress and attenuation of ApoB100 synthesis (128, 136). Overexpression of glutamine:fructose-6-phosphate amidotransferase (GFAT), the rate-limiting enzyme of the hexosamine biosynthetic pathway induced ER stress, lipid accumulation, and upregulation of genes associated with inflammatory pathways in HepG2 cells (136). Treatment of cells with a GFAT antagonist blocked these responses to GFAT overexpression. Thus, one can envision a scenario in which increased flux of fatty acids and glucose may compromise the functional capacity of the ER lumen via direct effects on ApoB100 processing and maturation.
Oxidative stress
Changes in nutrient flux in NAFLD may also influence the functional capacity of the ER via affects on redox balance. Oxidative stress has been implicated in many diseases associated with protein misfolding, such as Prion disease, Alzheimer disease, and Parkinson disease (86). Oxidative stress has also been linked to the development and progression of a number of chronic diseases, including diabetes, obesity, and NAFLD (40, 169). Although the oxidation of cysteine residues during disulfide bond formation in the ER may be a significant source of reactive oxygen species and lead to the development of oxidative stress (86), extraluminal sources of prooxidants can also induce ER stress and promote the formation of inclusion bodies in liver cells (47, 85). The ability of free fatty acids, as well as TNFα, to provoke ER stress and UPR activation may be linked, at least in part, to the generation of reactive oxygen species outside of the ER lumen (6, 79, 138, 184).
Bacterial endotoxin
Bacterial translocation through the intestinal wall and small intestinal bacterial overgrowth may be involved in the pathogenesis of NASH (177, 187). Recent studies have confirmed the presence of increased plasma endotoxin and intestinal permeability in humans with NAFLD (91, 163). It is perhaps relevant that LPS, contained in the cell wall of gram-negative bacteria, induces ER stress and activation of the UPR in the normal liver and more sustained activation of the UPR in the liver of cirrhotic rats (160, 195). It is also interesting that LPS, as well as several cytokines (IL-6, IL-1β), also induce the cleavage of the proinflammatory CREBH protein from the ER membrane (195). Thus, an environment characterized by hyperlipidemia, elevated cytokines, and endotoxin can promote ER stress in the liver and also potentially provoke systemic inflammation via mobilization of CREBH.
Novel Roles for the UPR in NAFLD
Although hepatic iron accumulation in NAFLD may be mild, some studies have found an association between iron overload and disease progression (1, 92). In addition, hepatic iron overload can induce oxidative stress. Two recent studies have demonstrated that the UPR can regulate hepatic iron metabolism (110, 168). Hepcidin, a defensin-like peptide, is a critical humoral regulator of innate immunity and host defense, and regulates iron stores in the liver via the iron export protein, ferroportin. It appears that hepcidin expression is regulated by the UPR in a biphasic manner, reduced initially and then increased if ER stress is sustained, via both CREBH and Chop. These data lend further support to the notion that the role of the UPR extends beyond protein quality control and now link ER stress to the regulation of hepatic iron metabolism.
Free fatty acids derived from adipose tissue are thought to play an important role in the development of hepatic steatosis (32). A recent study provided strong evidence that adipocyte apoptosis may be a key early event for macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in mice and humans (2). ER stress is present in adipose tissue of humans with obesity and NAFLD (9, 146). It is of interest, therefore, that a recent study in C. elegans suggested that IRE1 and HSP-4, the nematode IRE1 and GRP78 homologs, respectively, regulate the expression of the fasting-induced lipases, FIL-1 and −2 (59). These lipases were both necessary and sufficient for fasting-induced fat granule hydrolysis. It is presently unclear whether the UPR can regulate lipolysis in mammalian adipose tissue; however, one can envision a role for this pathway in adipose tissue lipolysis and apoptosis in the context of obesity and NAFLD.
ER Stress, the UPR, and Human NAFLD
There is currently only one study that has compared markers of ER stress in humans with or without NAFLD (127). In this study, liver samples were obtained from subjects with metabolic syndrome and normal liver histology (controls, n=17), subjects with metabolic syndrome and hepatic steatosis (NAFL, n=21), and subjects with metabolic syndrome and NASH (NASH, n=21). Although livers from NAFL and NASH were characterized by increased phosphorylation of eIF2α, several other markers of ER stress were not increased, including ATF4 mRNA and protein, Chop mRNA, GADD34 mRNA, unspliced XBP1 mRNA, and EDEM mRNA. Livers from NASH subjects were additionally characterized by a reduction in the amount of XBP1s and increased phosphorylation of JNK.
Two studies have examined ER stress markers in adipose tissue of obese, insulin-resistant subjects (9, 146). In one of these studies, subcutaneous fat biopsies were obtained from the upper thigh in lean (body mass index [BMI] 24±1.2 kg/m2, n=6) and obese (BMI 33.5±1.6 kg/m2, n=6) healthy subjects (9). Adipose tissue from obese subjects was characterized by increased protein levels of calnexin, calreticulin, and protein disulfide isomerase, as well as increased XBP1s mRNA and phosphorylation of JNK. In the second study, adipose tissue was obtained from 78 healthy, nondiabetic subjects over a spectrum of BMI (146). Several gene markers associated with the UPR, including GRP78, ATF6α, PERK, XBP1s, EDEM1, calreticulin, and oxygen-regulated protein 150, were significantly correlated to BMI. Correlations with BMI remained significant after controlling for contributions made by macrophages using CD68 gene expression.
One study examined both liver and adipose tissue in morbidly obese subjects (BMI 51.3±3 kg/m2, n=11) before and 1 year after gastric bypass surgery (45). Subjects lost ∼40% of body weight at the 1 year follow-up at which time significant reductions were observed in adipose tissue GRP78 mRNA, XBP1s mRNA, phosphorylation of eIF2α, and phosphorylation of JNK. Liver samples were characterized by reduced staining for GRP78 and phosphorylated eIF2α. Thus, weight loss appears to modulate the levels of several gene and protein markers of ER stress in liver and adipose tissue.
A recent study analyzed hepatic gene networks in morbidly obese patients with (BMI 49.6±7.4 kg/m2, n=24, 89% female) or without (BMI 48.8±5.9 kg/m2, n=25, 96% female) NAFLD (41). Three genes associated with the fibrosis pathway (COL1A1, IL-10, and IGFBP3) were upregulated and one gene associated with the UPR (HSPA5, also known as GRP78) was downregulated in patients with NAFLD compared to patients without NAFLD.
These results emphasize several limitations in our current understanding of ER stress in the context of human obesity and NAFLD. These include the lack of lean individuals with normal liver histology as a comparison group, limited tissue access, heavy reliance on gene expression analysis for determination of ER stress and UPR activation, and limited analysis of proteins associated with the UPR. Future in vivo studies in both animal models of chronic disease and humans with obesity and NAFLD need to carefully and comprehensively examine the UPR in liver and adipose tissue at multiple time points and under both fasted and fed conditions. Such data will allow us to better define ER stress and understand the UPR within a physiologic context.
Chemical Chaperones and NAFLD
Several studies have demonstrated that chemical chaperones alleviate ER stress in model systems used to study lysosomal storage disease, hereditary hemochromatosis, and cholangiocarcinoma (4, 28, 171). Oral administration of 4-phenyl butyric acid (PBA) or taurine-conjugated ursodeoxycholic acid (TUDCA) to ob/ob mice normalized blood glucose levels improved insulin sensitivity, reduced hepatic steatosis, normalized liver enzymes, and reduced biochemical markers of ER stress in liver and adipose tissue (119). PBA and TUDCA have also proven effective in reducing fatty acid-induced ER stress in liver cells (113, 125). The role and effectiveness of chemical chaperones in human NAFLD is presently unclear. Three trials, one that studied 24 patients over a 12-month period, one that studied 15 patients over a 3-month period, and one that studied 29 patients for 6 months, suggested that UDCA treatment (10–15 mg/kg/day) produced beneficial effects by reducing liver enzymes (36, 70, 139). In contrast, two trials, one that studied 14 women for a 1.5-month period and one that studied 80 patients for a 24-month period, suggested that UDCA (13–15 mg/kg/day) did not have a significant effect on biochemical or imaging markers of liver disease compared to weight reduction or placebo, respectively (77, 90). Future trials using chemical chaperones that can enhance protein-folding capacity should assess ER stress markers, taking into considerations the limitations noted in the previous section.
Concluding Remarks
Nutrient excess is a critical mediator of obesity and local organ/tissue lipid accumulation. Accumulating evidence suggests that the ER plays an important role in nutrient sensing and can interact with multiple metabolic pathways, in part, via the UPR. These features, coupled with the apparent activation of components of the UPR in obesity and NAFLD, suggest that disturbances in ER homeostasis likely contribute to the development and/or progression of NAFLD. Elegant studies employing selective genetic manipulation and cell-based systems have clearly demonstrated that both proximal sensors/initiators of the UPR and downstream responses can participate in a diverse array of cellular functions, including differentiation, ER and mitochondrial biogenesis, insulin action, glucose and lipid metabolism, inflammation, and apoptosis. However, much less is known about the role and regulation of ER homeostasis and the UPR in the setting of chronic metabolic diseases (50, 52, 55, 133) or multiple liver diseases (56–58, 158, 159, 174) in vivo. For example, what are the primary signals that provoke ER stress in vivo, and are these signals tissue/organ specific? Can activation of the UPR in vivo occur via signal-mediated activation of a proximal UPR sensor independently of the accumulation of unfolded proteins? How is the diverse set of outputs controlled by the UPR regulated in vivo and in response to chronic stress? Finally, since ER stress is defined by the activation of the UPR, and not all components of the UPR appear to be activated or increased in human NAFLD, should our focus be on potential impairments in the UPR and how these are linked to disease progression?
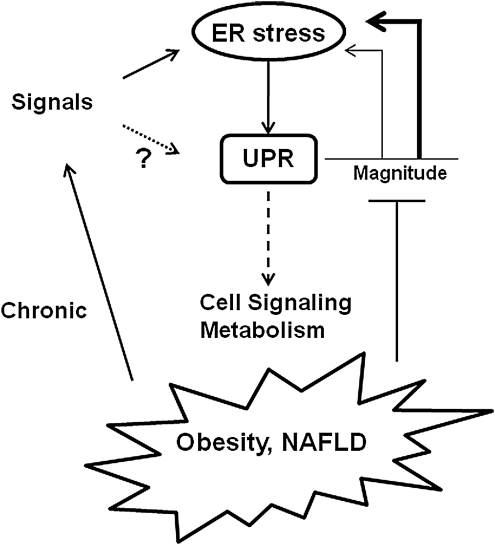
In conclusion, the UPR is a robust, highly efficient pathway that functions to remove unfolded proteins from the ER lumen but also interacts with multiple obligatory cellular signaling pathways (Fig. 6). The ability to resolve ER stress is closely linked to the magnitude and duration of the UPR. It is therefore hypothesized that in the setting of chronic diseases the inability to mitigate signals that induce ER stress and/or activate the UPR coupled with signals that impair the magnitude of the UPR will provide an environment that will promote disease progression.
FIG. 6.
Hypothetical model for the UPR in chronic diseases. Signals that lead to ER stress activate the UPR. The ability of the UPR to alleviate ER stress is linked to the magnitude of the UPR (thin line represents a lower magnitude response; thicker line represents higher magnitude response). The UPR is also linked to multiple cell signaling and metabolic pathways. In chronic diseases like obesity and nonalcoholic fatty liver disease (NAFLD) chronic signals induce ER stress, and perhaps activation of the UPR independently of ER stress. In addition, obesity and/or NAFLD may be characterized by signals that impair the UPR, thus dampening the magnitude of the response and the ability to alleviate ER stress (see text).
Abbreviations Used
- AIP1
ASK1-interacting protein 1
- ApoB100
apolipoprotein B100
- ASK1
apoptosis signaling-regulating kinase 1
- ATF4
activating transcription factor 4
- ATF6
activating transcription factor 6
- Bak
Bcl-2 antagonist/killer
- Bax
Bcl-2-associated X protein
- BBF2H7
BBF human homolog on chromosome 7
- Bcl-2
B-cell leukemia/lymphoma 2 protein
- BI
Bax inhibitor
- BMI
body mass index
- C/EBP
CCAAT/enhancer-binding protein
- Chop
C/EBP homologous protein
- CREB
cAMP-response element binding protein
- CRP
C-reactive protein
- dsRNA
double-stranded RNA
- EDEM
ER degradation-enhancing α-like protein
- eIF2α
eukaryotic initiation factor 2α
- ER
endoplasmic reticulum
- ERAD
ER-associated degradation
- ERK
extracellular-regulated kinase
- ERp72
endoplasmic reticulum protein-72
- GADD34
growth arrest and DNA damage-inducible protein 34
- Gcn2
general control non-derepressible 2 kinase
- GFAT
glutamine:fructose-6-phosphate amidotransferase
- GRP78
glucose-regulated protein 78
- GRP94
glucose-regulated protein 94
- HAC1
homologous to ATF/CREB1
- HDAC1
histone deacytelase-1
- HRI
heme-regulated inhibitor kinase
- Iκβ
NFκβ inhibitor
- IL-6
interleukin-6
- INSIG1
insulin-induced gene 1
- IP3
inositol triphosphate
- IRE1α
inositol-requiring transmembrane kinase and endonuclease 1α
- JNK
c-Jun-NH2-terminal kinase
- LPS
lipopolysaccharide
- MAPK
mitogen-activated protein kinase
- mTOR
mammalian target of rapamycin
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- NFκβ
nuclear factor kappa-β
- Nrf2
nuclear erythroid 2 p45-related factor 2
- PBA
4-phenyl butyric acid
- PERK
protein kinase-like ER kinase
- PKR
double-stranded RNA-activated protein kinase
- PPAR
peroxisome proliferator-activated receptor
- PTP1B
protein tyrosine phosphatase-1B
- RIP
regulated intramembrane proteolysis
- SCAP
SREBP cleavage-activating protein
- SREBP
sterol regulatory element binding protein
- Tisp40
transcript induced in spermiogenesis-40
- TRAF2
TNFR-associated factor 2
- TUDCA
taurine-conjugated ursodeoxycholic acid
- UPR
unfolded protein response
- XBP1s
spliced X-box binding protein 1
Acknowledgments
The authors acknowledge research support from NIH and the Lillian Fountain Smith Endowment.
References
- 1.Aigner E. Theurl I. Theurl M. Lederer D. Haufe H. Dietze O. Strasser M. Datz C. Weiss G. Pathways underlying iron accumulation in human nonalcoholic fatty liver disease. Am J Clin Nutr. 2008;87:1374–1383. doi: 10.1093/ajcn/87.5.1374. [DOI] [PubMed] [Google Scholar]
- 2.Alkhouri N. Gornicka A. Berk MP. Thapaliya S. Dixon LJ. Kashyap S. Schauer PR. Feldstein AE. Adipocyte apoptosis, a link between obesity, insulin resistance, and hepatic steatosis. J Biol Chem. 2010;285:3428–3438. doi: 10.1074/jbc.M109.074252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allard JP. Aghdassi E. Mohammed S. Raman M. Avand G. Arendt BM. Jalali P. Kandasamy T. Prayitno N. Sherman M. Guindi M. Ma DWL. Heathcote JE. Nutritional assessment and hepatic fatty acid composition in non-alcoholic fatty liver disease (NAFLD): a cross-sectional study. J Hepatol. 2008;48:300–307. doi: 10.1016/j.jhep.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 4.Alpini G. Kanno N. Phinizy JL. Glaser S. Francis H. Taffetani S. LeSage G. Tauroursodeoxycholate inhibits human cholangiocarcinoma growth via calcium-, PKC-, and MAPK-dependent pathways. Am J Physiol Gastrointest Liver Physiol. 2004;286:G973–G982. doi: 10.1152/ajpgi.00270.2003. [DOI] [PubMed] [Google Scholar]
- 5.Argo CK. Caldwell SH. Epidemiology and natural history of non-alcoholic steatohepatitis. Clin Liver Dis. 2009;13:511–531. doi: 10.1016/j.cld.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 6.Aronis A. Madar Z. Tirosh O. Mechanism underlying oxidative stress-mediated lipotoxicity: exposure of J774.2 macrophages to triacylglycerols facilitates mitochondrial reactive oxygen species production and cellular necrosis. Free Radic Biol Med. 2005;38:1221–1230. doi: 10.1016/j.freeradbiomed.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 7.Bailey D. O'Hare P. Transmembrane bZIP transcription factors in ER stress signaling and the unfolded protein response. Antioxid Redox Signal. 2007;9:2305–2321. doi: 10.1089/ars.2007.1796. [DOI] [PubMed] [Google Scholar]
- 8.Bobrovnikova-Marjon E. Hatzivassiliou G. Grigoriadou C. Romero M. Cavener DR. Thompson CB. Diehl JA. PERK-dependent regulation of lipogenesis during mouse mammary gland development and adipocyte differentiation. Proc Natl Acad Sci U S A. 2008;105:16314–16319. doi: 10.1073/pnas.0808517105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boden G. Duan X. Homko C. Molina EJ. Song W. Perez O. Cheung P. Merali S. Increase in endoplasmic reticulum stress-related proteins and genes in adipose tissue of obese, insulin-resistant individuals. Diabetes. 2008;57:2438–2444. doi: 10.2337/db08-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borradaile NM. Han X. Harp JD. Gale SE. Ory DS. Schaffer JE. Disruption of endoplasmic reticulum structure and integrity in lipotoxic cell death. J Lipid Res. 2006;47:2726–2737. doi: 10.1194/jlr.M600299-JLR200. [DOI] [PubMed] [Google Scholar]
- 11.Brewer JW. Cleveland JL. Hendershot LM. A pathway distinct from the mammalian unfolded protein response regulates expression of endoplasmic reticulum chaperones in non-stressed cells. EMBO J. 1997;16:7207–7216. doi: 10.1093/emboj/16.23.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bugianesi E. Moscatiello S. Ciaravella MF. Marchesini G. Insulin resistance in nonalcoholic fatty liver disease. Curr Pharm Des. 2010;16:1841–1951. doi: 10.2174/138161210791208875. [DOI] [PubMed] [Google Scholar]
- 13.Busch AK. Gurisik E. Cordery DV. Sudlow M. Denyer GS. Laybutt DR. Hughes WE. Biden TJ. Increased fatty acid desaturation and enhanced expression of stearoyl coenzyme A desaturase protects pancreatic B-cells from lipoapoptosis. Diabetes. 2005;54:2917–2924. doi: 10.2337/diabetes.54.10.2917. [DOI] [PubMed] [Google Scholar]
- 14.Cai D. Yuan M. Frantz DF. Melendez PA. Hansen L. Lee J. Shoelson SE. Local and systemic insulin resistance resulting from hepatic activation of IKK-β and NF-κB. Nat Med. 2005;11:183–190. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Y. Le Caherec F. Chuck SL. Calnexin and other factors that alter translocation affect the rapid binding of ubiquitin to apoB in the Sec61 complex. J Biol Chem. 1998;273:11887–11894. doi: 10.1074/jbc.273.19.11887. [DOI] [PubMed] [Google Scholar]
- 16.Clark JM. Diehl A. Nonalcoholic fatty liver disease: an underrecognized cause of cryptogenic cirrhosis. JAMA. 2003;289:3000–3004. doi: 10.1001/jama.289.22.3000. [DOI] [PubMed] [Google Scholar]
- 17.Corazza N. Jakob S. Schaer C. Frese S. Keogh A. Stroka D. Kassahn D. Torgler R. Mueller C. Schneider P. Brunner T. TRAIL receptor-mediated JNK activation and Bim phosphorylation critically regulate Fas-mediated liver damage and lethality. J Clin Invest. 2006;116:2493–2499. doi: 10.1172/JCI27726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cox JS. Chapman RE. Walter P. The unfolded protein response coordinates the production of endoplasmic reticulum protein and endoplasmic reticulum membrane. Mol Biol Cell. 1997;8:1805–1814. doi: 10.1091/mbc.8.9.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cretenet G. Le Clech M. Gachon F. Circadian clock-coordinated 12 Hr period rhythmic activation of the IRE1alpha pathway controls lipid metabolism in mouse liver. Cell Metab. 2010;11:47–57. doi: 10.1016/j.cmet.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 20.Csordas G. Renken C. Varnai P. Walter L. Weaver D. Buttle KF. Balla T. Mannella CA. Hajnoczky G. Structural and functional features and significance of the physical linkage between ER and mitochondria. J Cell Biol. 2006;174:915–921. doi: 10.1083/jcb.200604016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cullinan SB. Diehl JA. Coordination of ER and oxidative stress signaling: the PERK/Nrf2 signaling pathway. Int J Biochem Cell Biol. 2006;38:317–332. doi: 10.1016/j.biocel.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 22.Cullinan SB. Zhang D. Hannink M. Arvisais E. Kaufman RJ. Diehl JA. Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Mol Cell Biol. 2003;23:7198–7209. doi: 10.1128/MCB.23.20.7198-7209.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cusi K. Role of insulin resistance and lipotoxicity in non-alcoholic steatohepatitis. Clin Liver Dis. 2009;13:545–563. doi: 10.1016/j.cld.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 24.Czaja MJ. The future of GI and liver research: editorial perspectives III. JNK/AP-1 regulation of hepatocyte death. Am J Physiol Gastrointest Liver Physiol. 2002;284:G875–G879. doi: 10.1152/ajpgi.00549.2002. [DOI] [PubMed] [Google Scholar]
- 25.Das M. Sabio G. Jiang F. Rincon M. Flavell RA. Davis RJ. Induction of hepatitis by JNK-mediated expression of TNF-alpha. Cell. 2009;136:249–260. doi: 10.1016/j.cell.2008.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Das SK. Chu WS. Mondal AK. Sharma NK. Kern PA. Rasouli N. Elbein SC. Effect of pioglitazone treatment on endoplasmic reticulum stress response in human adipose and palmitate-induced stress in human liver and adipose cell lines. Am J Physiol Endocrinol Metab. 2008;295:E393–E400. doi: 10.1152/ajpendo.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Das SK. Mondal AK. Elbein SC. Distinct gene expression profiles characterize cellular responses to palmitate and oleate. J Lipid Res. 2010;51:2121–2131. doi: 10.1194/jlr.M004275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Almeida SF. Picarote G. Fleming JV. Carom-Fonseca M. Azevedo JE. de Sousa M. Chemical chaperones reduce endoplasmic reticulum stress and prevent mutant HFE aggregate formation. J Biol Chem. 2007;282:27905–27912. doi: 10.1074/jbc.M702672200. [DOI] [PubMed] [Google Scholar]
- 29.de Piano A. Prado WL. Caranti DA. Siqueira KO. Stella SG. Lofrano M. Tock L. Cristofalo DM. Lederman H. Tufik S. de Mello MT. Damaso AR. Metabolic and nutritional profile of obese adolescents with nonalcoholic fatty liver disease. J Pediatr Gastroenterol Nutr. 2007;44:446–452. doi: 10.1097/MPG.0b013e31803815d9. [DOI] [PubMed] [Google Scholar]
- 30.Deniaud A. Sharaf el dein O. Maillier E. Poncet D. Kroemer G. Lemaire C. Brenner C. Endoplasmic reticulum stress induces calcium-dependent permeability transition, mitochondrial outer membrane permeabilization and apoptosis. Oncogene. 2008;27:285–299. doi: 10.1038/sj.onc.1210638. [DOI] [PubMed] [Google Scholar]
- 31.Diraison F. Moulin P. Beylot M. Contribution of hepatic de novo lipogenesis and reesterification of plasma non esterified fatty acids to plasma triglyceride synthesis during non-alcoholic fatty liver disease. Diabetes Metab. 2003;29:478–485. doi: 10.1016/s1262-3636(07)70061-7. [DOI] [PubMed] [Google Scholar]
- 32.Donnelly KL. Smith CI. Schwarzenberg SJ. Jessurun J. Boldt MD. Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115:1343–1351. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Donze O. Abbas-Terki T. Picard D. The Hsp90 chaperone complex is both a facilitator and a repressor of the dsRNA-dependent kinase PKR. EMBO J. 2001;20:3771–3780. doi: 10.1093/emboj/20.14.3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Donze O. Deng J. Curran J. Sladek R. Picard D. Sonenberg N. The protein kinase PKR: a molecular clock that sequentially activates survival and death programs. EMBO J. 2004;23:564–571. doi: 10.1038/sj.emboj.7600078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elizondo A. Araya J. Rodrigo R. Signorini C. Sgherri C. Comporti M. Poniachik J. Videla LA. Effects of weight loss on liver and erythrocyte polyunsaturated fatty acid pattern and oxidative stress status in obese patients with non-alcoholic fatty liver disease. Biol Res. 2008;41:59–68. [PubMed] [Google Scholar]
- 36.Ersoz G. Gunsar F. Karasu Z. Akay S. Batur Y. Akarca US. Management of fatty liver disease with vitamin E and C compared to ursodeoxycholic acid treatment. Turk J Gastroenterol. 2005;16:124–128. [PubMed] [Google Scholar]
- 37.Fassio E. Alvarez E. Dominguez N. Landeira G. Longo C. Natural history of nonalcoholic steatohepatitis: a longitudinal study of repeat liver biopsies. Hepatology. 2004;40:820–826. doi: 10.1002/hep.20410. [DOI] [PubMed] [Google Scholar]
- 38.Feldstein AE. Canbay A. Angulo P. Taniai M. Burgart LJ. Lindor KD. Gores GJ. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology. 2003;125:437–443. doi: 10.1016/s0016-5085(03)00907-7. [DOI] [PubMed] [Google Scholar]
- 39.Festi D. Colecchia A. Sacco T. Bondi M. Roda E. Marchesini G. Hepatic steatosis in obese patients: clinical aspects and prognostic significance. Obes Rev. 2004;5:27–42. doi: 10.1111/j.1467-789x.2004.00126.x. [DOI] [PubMed] [Google Scholar]
- 40.Furukawa S. Fujita T. Shimabukuro M. Iwaki M. Yamada Y. Nakajima Y. Nakayama O. Makishima M. Matsuda M. Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gawrieh S. Baye TM. Carless M. Wallace J. Komorowski R. Kleiner DE. Andris D. Makladi B. Cole R. Charlton M. Curran J. Dyer TD. Charlesworth J. Wilke R. Blangero J. Kissebah AH. Olivier M. Hepatic gene networks in morbidly obese patients with nonalcoholic fatty liver disease. Obes Surg. 2010;20:1698–1709. doi: 10.1007/s11695-010-0171-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Geisler F. Algul H. Paxian S. Schmid RM. Genetic inactivation of RelA/p65 sensitizes adult mouse hepatocytes to TNF-induced apoptosis in vivo and in vitro. Gastroenterology. 2007;132:2489–2503. doi: 10.1053/j.gastro.2007.03.033. [DOI] [PubMed] [Google Scholar]
- 43.Gill HK. Wu GY. Non-alcoholic fatty liver disease and the metabolic syndrome: effects of weight loss and a review of popular diets. Are low carbohydrate diets the answer? World J Gastroenterol. 2006;12:345–353. doi: 10.3748/wjg.v12.i3.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Glimcher LH. Lee AH. From sugar to fat: how the transcription factor XBP1 regulates hepatic lipogenesis. Ann N Y Acad Sci. 2009;1173(Suppl 1):E2–E9. doi: 10.1111/j.1749-6632.2009.04956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gregor MF. Yang L. Fabbrini E. Mohammed BS. Eagon JC. Hotamisligil GS. Klein S. Endoplasmic reticulum stress is reduced in tissues of obese subjects after weight loss. Diabetes. 2009;58:693–700. doi: 10.2337/db08-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gu F. Nguyen DT. Stuible M. Dube N. Tremblay ML. Chevet E. Protein-tyrosine phosphatase 1B potentiates IRE1 signaling during endoplasmic reticulum stress. J Biol Chem. 2004;279:49689–49693. doi: 10.1074/jbc.C400261200. [DOI] [PubMed] [Google Scholar]
- 47.Hanada S. Harada M. Kumemura H. Omary MB. Koga H. Kawaguchi T. Taniguchi E. Yoshida T. Hisamoto T. Yanagimoto C. Maeyama M. Ueno T. Sata M. Oxidative stress induces the endoplasmic reticulum stress and facilitates inclusion formation in cultured cells. J Hepatol. 2007;47:93–102. doi: 10.1016/j.jhep.2007.01.039. [DOI] [PubMed] [Google Scholar]
- 48.Harrison SA. Kadakia S. Lang KA. Schenker S. Nonalcoholic steatohepatitis: what we know in the new millennium. Am J Gastroenterol. 2002;97:2714–2724. doi: 10.1111/j.1572-0241.2002.07069.x. [DOI] [PubMed] [Google Scholar]
- 49.Hetz C. Bernasconi P. Fisher J. Lee AH. Bassik MC. Antonsson B. Brandt GS. Iwakoshi NN. Schinzel A. Glimcher LH. Korsmeyer SJ. Proapoptotic BAX and BAK modulate the unfolded protein response by a direct interaction with IRE1alpha. Science. 2006;312:572–576. [Google Scholar]
- 50.Hetz C. Glimcher LH. Fine-tuning of the unfolded protein response: assembling the IRE1alpha interactome. Mol Cell. 2009;35:551–561. doi: 10.1016/j.molcel.2009.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hirosumi J. Tuncman G. Chang L. Gorgun CZ. Uysal KT. Maeda K. Karin M. Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 52.Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140:900–917. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hotamisligil GS. Inflammatory pathways and insulin action. Int J Obes Relat Metab Disord. 2003;27(Suppl 3):S53–S55. doi: 10.1038/sj.ijo.0802502. [DOI] [PubMed] [Google Scholar]
- 54.Hu P. Han Z. Couvillon AD. Kaufman RJ. Exton JH. Autocrine tumor necrosis factor alpha links endoplasmic reticulum stress to the membrane death receptor pathway through IRE1alpha-mediated NF-kB activation and down-regulation of TRAF2 expression. Mol Cell Biol. 2006;26:3071–3084. doi: 10.1128/MCB.26.8.3071-3084.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ji C. Dissection of endoplasmic reticulum stress signaling in alcoholic and non-alcoholic liver injury. J Gastroenterol Hepatol. 2008;23(Suppl 1):S16–S24. doi: 10.1111/j.1440-1746.2007.05276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ji C. Kaplowitz N. Betaine decreases hyperhomocysteinemia, endoplasmic reticulum stress, and liver injury in alcohol-fed mice. Gastroenterology. 2003;124:1488–1499. doi: 10.1016/s0016-5085(03)00276-2. [DOI] [PubMed] [Google Scholar]
- 57.Ji C. Kaplowitz N. ER stress: can the liver cope? J Hepatol. 2006;45:321–333. doi: 10.1016/j.jhep.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 58.Ji C. Mehrian-Shai R. Chan C. Hsu YH. Kaplowitz N. Role of CHOP in hepatic apoptosis in the murine model of intragastric ethanol feeding. Alcohol Clin Exp Res. 2005;29:1496–1503. doi: 10.1097/01.alc.0000174691.03751.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jo H. Shim J. Lee JH. Lee J. Kim JB. IRE-1 and HSP-4 contribute to energy homeostasis via fasting-induced lipases in C. elegans. Cell Metab. 2009;9:440–448. doi: 10.1016/j.cmet.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 60.Jung HS. Chung KW. Won Kim J. Kim J. Komatsu M. Tanaka K. Nguyen YH. Kang TM. Yoon KH. Kim JW. Jeong YT. Han MS. Lee MK. Kim KW. Shin J. Lee MS. Loss of autophagy diminishes pancreatic beta cell mass and function with resultant hyperglycemia. Cell Metab. 2008;8:318–324. doi: 10.1016/j.cmet.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 61.Kammoun HL. Chabanon H. Hainault I. Luquet S. Magnan C. Koike T. Ferre P. Foufelle F. GRP78 expression inhibits insulin and ER stress-induced SREBP-1c activation and reduces hepatic steatosis in mice. J Clin Invest. 2009;119:1201–1215. doi: 10.1172/JCI37007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kaufman RJ. Orchestrating the unfolded protein response in health and disease. J Clin Invest. 2002;110:1389–1398. doi: 10.1172/JCI16886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kaufman RJ. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev. 1999;13:1211–1233. doi: 10.1101/gad.13.10.1211. [DOI] [PubMed] [Google Scholar]
- 64.Kazemi S. Mounir Z. Baltzis D. Raven JF. Wang S. Krishnamoorthy JL. Pluquet O. Pelletier J. Koromilas AE. A novel function of eIF2alpha kinases as inducers of the phosphoinositide-3 kinase signaling pathway. Mol Biol Cell. 2007;18:3635–3644. doi: 10.1091/mbc.E07-01-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim I. Xu W. Reed JC. Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat Rev Drug Discov. 2008;7:1013–1030. doi: 10.1038/nrd2755. [DOI] [PubMed] [Google Scholar]
- 66.Klionsky DJ. Autophagy: from phenomenology to molecular understanding in less than a decade. Nat Rev Mol Cell Biol. 2007;8:931–937. doi: 10.1038/nrm2245. [DOI] [PubMed] [Google Scholar]
- 67.Korenblat KM. Fabbrini E. Mohammed BS. Klein S. Liver, muscle, and adipose tissue insulin action is directly related to intrahepatic triglyceride content in obese subjects. Gastroenterology. 2008;134:1369–1375. doi: 10.1053/j.gastro.2008.01.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kurinna SM. Tsao CC. Nica AF. Jiffar T. Ruvolo PP. Ceramide promotes apoptosis in lung cancer-derived A549 cells by a mechanism involving c-Jun NH2-terminal kinase. Cancer Res. 2004;64:7852–7856. doi: 10.1158/0008-5472.CAN-04-1552. [DOI] [PubMed] [Google Scholar]
- 69.Lamkanfi M. Kalai M. Vandenabeele P. Caspase-12: an overview. Cell Death Differ. 2004;11:365–368. doi: 10.1038/sj.cdd.4401364. [DOI] [PubMed] [Google Scholar]
- 70.Laurin J. Lindor KD. Crippin JS. Gossard A. Gores GJ. Ludwig J. Rakela J. McGill DB. Ursodeoxycholic acid or clofibrate in the treatment of non-alcohol-induced steatohepatitis: a pilot study. Hepatology. 1996;23:1464–1467. doi: 10.1002/hep.510230624. [DOI] [PubMed] [Google Scholar]
- 71.Lee A-H. Scapa EF. Cohen DE. Glimcher LH. Regulation of hepatic lipogenesis by the transcription factor XBP1. Science. 2008;320:1492–1496. doi: 10.1126/science.1158042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee JN. Ye J. Proteolytic activation of sterol regulatory element-binding protein induced by cellular stress through depletion of Insig-1. J Biol Chem. 2004;279:45257–45265. doi: 10.1074/jbc.M408235200. [DOI] [PubMed] [Google Scholar]
- 73.Levine B. Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li G. Mongillo M. Chin KT. Harding H. Ron D. Marks AR. Tabas I. Role of ERO1-alpha-mediated stimulation of inositol 1,4,5-triphosphate receptor activity in endoplasmic reticulum stress-induced apoptosis. J Cell Biol. 2009;186:783–792. doi: 10.1083/jcb.200904060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li Y. Iida K. O'Neil J. Zhang P. Li S. Frank A. Gabai A. Zambito F. Liang SH. Rosen CJ. Cavener DR. PERK eIF2alpha kinase regulates neonatal growth by controlling the expression of circulating insulin-like growth factor-I derived from the liver. Endocrinology. 2003;144:3505–3513. doi: 10.1210/en.2003-0236. [DOI] [PubMed] [Google Scholar]
- 76.Li ZZ. Berk M. McIntyre TM. Feldstein AE. Hepatic lipid partitioning and liver damage in nonalcoholic fatty liver disease: role of stearoyl-CoA desaturase. J Biol Chem. 2009;284:5637–5644. doi: 10.1074/jbc.M807616200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lindor KD. Kowdley KV. Heathcote EJ. Harrison ME. Jorgensen R. Angulo P. Lymp JF. Burgart L. Colin P. Ursodeoxycholic acid for treatment of nonalcoholic steatohepatitis: results of a randomized trial. Hepatology. 2004;39:770–778. doi: 10.1002/hep.20092. [DOI] [PubMed] [Google Scholar]
- 78.Lisbona F. Rojas-Rivera D. Thielen P. Zamorano S. Todd D. Martinon F. Glavic A. Kress C. Lin JH. Walter P. Reed JC. Glimcher LH. Hetz C. BAX inhibitor-1 is a negative regulator of the ER stress sensor IRE1alpha. Mol Cell. 2009;33:679–691. doi: 10.1016/j.molcel.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Listenberger LL. Ory DS. Schaffer JE. Palmitate-induced apoptosis can occur through a ceramide-independent pathway. J Biol Chem. 2001;276:14890–14895. doi: 10.1074/jbc.M010286200. [DOI] [PubMed] [Google Scholar]
- 80.Liu H. Lo CR. Czaja MJ. NF-kappaB inhibition sensitizes hepatocytes to TNF-induced apoptosis through a sustained activation of JNK and c-Jun. Hepatology. 2002;35:772–778. doi: 10.1053/jhep.2002.32534. [DOI] [PubMed] [Google Scholar]
- 81.Liu Y. Adachi M. Zhao S. Hareyama M. Koong AC. Luo D. Rando TA. Imai K. Shinomura Y. Preventing oxidative stress: a new role for XBP1. Cell Death Differ. 2009;16:847–857. doi: 10.1038/cdd.2009.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Luebke-Wheeler J. Zhang K. Battle M. Si-Tayeb K. Garrison W. Chhinder S. Li J. Kaufman RJ. Duncan SA. Hepatocyte nuclear factor 4alpha is implicated in endoplasmic reticulum stress-induced acute phase response by regulating expression of cyclic adenosine monophosphate responsive element binding protein H. Hepatology. 2008;48:1242–1250. doi: 10.1002/hep.22439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Luedde T. Beraza N. Kotsikoris V. van Loo G. Nenci A. De Vos R. Roskams T. Trautwein C. Pasparakis M. Deletion of NEMO/IKKψ in liver parenchymal cells causes steatohepatitis and hepatocellular carcinoma. Cancer Cell. 2007;11:119–132. doi: 10.1016/j.ccr.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 84.Ma Y. Brewer JW. Diehl JA. Hendershot LM. Two distinct stress signaling pathways converge upon the CHOP promoter during the mammalian unfolded protein response. J Mol Biol. 2002;318:1351–1365. doi: 10.1016/s0022-2836(02)00234-6. [DOI] [PubMed] [Google Scholar]
- 85.Malhotra JD. Kaufman RJ. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid Redox Signal. 2007;9:2277–2293. doi: 10.1089/ars.2007.1782. [DOI] [PubMed] [Google Scholar]
- 86.Malhotra JD. Miao H. Zhang K. Wolfson A. Pennathur S. Pipe SW. Kaufman RJ. Antioxidants reduce endoplasmic reticulum stress and improve protein secretion. Proc Natl Acad Sci U S A. 2008;105:18525–18530. doi: 10.1073/pnas.0809677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Marchesini G. Brizi M. Morselli-Labate AM. Bianci G. Bugianesi E. McCullough AJ. Forlani G. Melchionda N. Association of nonalcoholic fatty liver disease with insulin resistance. Am J Med. 1999;107:450–455. doi: 10.1016/s0002-9343(99)00271-5. [DOI] [PubMed] [Google Scholar]
- 88.Marciniak S. Ron D. Endoplasmic reticulum stress signaling in disease. Physiol Rev. 2006;86:1133–1149. doi: 10.1152/physrev.00015.2006. [DOI] [PubMed] [Google Scholar]
- 89.Masciarelli S. Fra AM. Pengo N. Bertolotti M. Cenci S. Fagioli C. Ron D. Hendershot LM. Sitia R. CHOP-independent apoptosis and pathway-selective induction of the UPR in developing plasma cells. Mol Immunol. 2010;47:1356–1365. doi: 10.1016/j.molimm.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mendez-Sanchez N. Gonzalez V. Chavez-Tapia N. Ramos MH. Uribe M. Weight reduction and ursodeoxycholic acid in subjects with nonalcoholic fatty liver disease. A double-blind, placebo-controlled trial. Ann Hepatol. 2004;3:108–112. [PubMed] [Google Scholar]
- 91.Miele L. Valenza V. La Torre G. Montalto M. Cammarota G. Ricci R. Masciana R. Forgione A. Gabrieli ML. Perotti G. Vecchio FM. Rapaccini G. Gasbarrini G. Day CP. Grieco A. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology. 2009;49:1877–1887. doi: 10.1002/hep.22848. [DOI] [PubMed] [Google Scholar]
- 92.Mitsuyoshi H. Yasui K. Harano Y. Endo M. Tsuji K. Minami M. Itoh Y. Okanoue T. Yoshikawa T. Analysis of hepatic genes involved in the metabolism of fatty acids and iron in nonalcoholic fatty liver disease. Hepatol Res. 2009;39:366–373. doi: 10.1111/j.1872-034X.2008.00464.x. [DOI] [PubMed] [Google Scholar]
- 93.Mizushima N. Physiological functions of autophagy. Curr Top Microbiol Immunol. 2009;335:71–84. doi: 10.1007/978-3-642-00302-8_3. [DOI] [PubMed] [Google Scholar]
- 94.Mizushima N. The pleiotropic role of autophagy: from protein metabolism to bactericide. Cell Death Differ. 2005;12(Suppl 2):1535–1541. doi: 10.1038/sj.cdd.4401728. [DOI] [PubMed] [Google Scholar]
- 95.Moffitt JH. Fielding BA. Evershed R. Berstan R. Currie JM. Clark A. Adverse physicochemical properties of tripalmitin in beta cells lead to morphological changes and lipotoxicity in vitro. Diabetologia. 2005;48:1819–1829. doi: 10.1007/s00125-005-1861-9. [DOI] [PubMed] [Google Scholar]
- 96.Moi P. Chan K. Asunis I. Cao A. Kan YW. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proc Natl Acad Sci U S A. 1994;91:9926–9930. doi: 10.1073/pnas.91.21.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mori K. Frame switch splicing and regulated intramembrane proteolysis: key words to understand the unfolded protein response. Traffic. 2003;4:519–528. doi: 10.1034/j.1600-0854.2003.00112.x. [DOI] [PubMed] [Google Scholar]
- 98.Murakami T. Kondo S. Ogata M. Kanemoto S. Saito A. Wanaka A. Imaizumi K. Cleavage of the membrane-bound transcription factor OASIS in response to endoplasmic reticulum stress. J Neurochem. 2006;96:1090–1100. doi: 10.1111/j.1471-4159.2005.03596.x. [DOI] [PubMed] [Google Scholar]
- 99.Nakagawa T. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 2000;403:98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- 100.Nakagawa T. Yuan J. Cross-talk between two cysteine protease families: activation of caspase-12 by calpain in apoptosis. J Cell Biol. 2000;150:887–894. doi: 10.1083/jcb.150.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nakamura T. Furuhashi M. Li P. Cao H. Tuncman G. Sonenberg N. Gorgun CZ. Hotamisligil GS. Double-stranded RNA-dependent protein kinase links pathogen sensing with stress and metabolic homeostasis. Cell. 2010;140:338–348. doi: 10.1016/j.cell.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nakamuta M. Kohjima M. Higuchi N. Kato M. Kotoh K. Yoshimoto T. Yada M. Yada R. Takemoto R. Fukuizumi K. Harada N. Taketomi A. Maehara Y. Nakashima M. Enjoji M. The significance of differences in fatty acid metabolism between obese and non-obese patients with non-alcoholic fatty liver disease. Int J Mol Med. 2008;22:663–667. [PubMed] [Google Scholar]
- 103.Nehra V. Angulo P. Buchman AL. Lindor KD. Nutritional and metabolic considerations in the etiology of nonalcoholic steatohepatitis. Dig Dis Sci. 2001;46:2347–2352. doi: 10.1023/a:1012338828418. [DOI] [PubMed] [Google Scholar]
- 104.Nguyen DT. Kebache S. Fazel A. Wong HN. Jenna S. Emadali A. Lee EH. Bergeron JJ. Kaufman RJ. Larose L. Chevet E. Nck-dependent activation of extracellular signal-regulated kinase-1 and regulation of cell survival during endoplasmic reticulum stress. Mol Biol Cell. 2004;15:4248–4260. doi: 10.1091/mbc.E03-11-0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ni HM. Chen X. Shi YH. Liao Y. Beg AA. Fan J. Yin XM. Genetic delineation of the pathways mediated by bid and JNK in tumor necrosis factor-alpha-induced liver injury in adult and embryonic mice. J Biol Chem. 2009;284:4373–4382. doi: 10.1074/jbc.M807259200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nunnari J. Walter P. Regulation of organelle biogenesis. Cell. 1996;84:389–394. doi: 10.1016/s0092-8674(00)81283-0. [DOI] [PubMed] [Google Scholar]
- 107.Oakes SA. Scorrano L. Opferman JT. Bassik MC. Nishino M. Pozzan T. Korsmeyer SJ. Proapoptotic BAX and BAK regulate the type 1 inositol trisphosphate receptor and calcium leak from the endoplasmic reticulum. Proc Natl Acad Sci U S A. 2005;102:105–110. doi: 10.1073/pnas.0408352102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Obeng EA. Boise LH. Caspase-12 and caspase-4 are not required for caspase-dependent endoplasmic reticulum stress-induced apoptosis. J Biol Chem. 2005;280:29578–29587. doi: 10.1074/jbc.M502685200. [DOI] [PubMed] [Google Scholar]
- 109.Okada T. Yoshida H. Akazawa R. Negishi M. Mori K. Distinct roles of activating transcription factor 6 (ATF6) and double-stranded RNA-activated protein kinase-like endoplasmic reticulum kinase (PERK) in transcription during the mammalian unfolded protein response. Biochem J. 2002;366:585–594. doi: 10.1042/BJ20020391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Oliveira SJ. Pinto JP. Picarote G. Costa VM. Carvalho F. Rangel M. de Sousa M. de Almeida SF. ER stress-inducible factor CHOP affects the expression of hepcidin by modulating C/EBPalpha activity. PLoS One. 2009;4:e6618. doi: 10.1371/journal.pone.0006618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Omori Y. Imai J-I. Watanabe M. Komatsu T. Suzuki Y. Kataoka K. Watanabe S. Tanigami A. Sugano S. CREB-H: a novel mammalian transcription factor belonging to the CREB/ATF family and functioning via the box-B element with a liver-specific expression. Nucleic Acids Res. 2001;29:2154–2162. doi: 10.1093/nar/29.10.2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ong JP. Elariny H. Collantes R. Younoszai A. Chandhoke V. Reines HD. Goodman Z. Younossi ZM. Predictors of nonalcoholic steatohepatitis and advanced fibrosis in morbidly obese patients. Obes Surg. 2005;15:310–315. doi: 10.1381/0960892053576820. [DOI] [PubMed] [Google Scholar]
- 113.Ota T. Gayet C. Ginsberg HN. Inhibition of apolipoprotein B100 secretion by lipid-induced hepatic endoplasmic reticulum stress in rodents. J Clin Invest. 2008;118:316–322. doi: 10.1172/JCI32752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Oyadomari S. Harding H. Zhang Y. Oyadomari M. Ron D. Dephosphorylation of translation initiation factor 2α enhances glucose tolerance and attenuates hepatosteatosis in mice. Cell Metab. 2008;7:520–532. doi: 10.1016/j.cmet.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Oyadomari S. Koizumi A. Takeda K. Gotoh T. Akira S. Araki E. Mori M. Targeted disruption of the Chop gene delays endoplasmic reticulum-stress mediated diabetes. J Clin Invest. 2002;109:525–532. doi: 10.1172/JCI14550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Oyadomari S. Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11:381–389. doi: 10.1038/sj.cdd.4401373. [DOI] [PubMed] [Google Scholar]
- 117.Ozcan U. Cao Q. Yilmaz E. Lee A-H. Iwakoshi NN. Ozdelen E. Tuncman G. Gorgun CZ. Glimcher LH. Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action and type 2 diabetes. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 118.Ozcan U. Ozcan L. Yilmaz E. Duvel K. Sahin M. Manning BD. Hotamisligil GS. Loss of the tuberous sclerosis complex tumor suppressors triggers the unfolded protein response to regulate insulin signaling and apoptosis. Mol Cell. 2008;29:541–551. doi: 10.1016/j.molcel.2007.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ozcan U. Yilmaz E. Ozcan L. Furuhashi M. Vaillancourt E. Smith RO. Gorgun CZ. Hotamisligil GS. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–1140. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pagadala M. Zein CO. McCullough AJ. Predictors of steatohepatitis and advanced fibrosis in non-alcoholic fatty liver disease. Clin Liver Dis. 2009;13:591–606. doi: 10.1016/j.cld.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 121.Pahl HL. Baeuerle PA. A novel signal transduction pathway from the endoplasmic reticulum to the nucleus is mediated by transcription factor NF-kappa B. EMBO J. 1995;14:2580–2588. doi: 10.1002/j.1460-2075.1995.tb07256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Papandreou D. Rousso I. Mavromichalis I. Update on non-alcoholic fatty liver disease in children. Clin Nutr. 2007;26:409–415. doi: 10.1016/j.clnu.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 123.Park SW. Zhou Y. Lee J. Lu A. Sun C. Chung J. Ueki K. Ozcan U. The regulatory subunits of PI3K, p85alpha and p85beta, interact with XBP-1 and increase its nuclear translocation. Nat Med. 2010;16:429–437. doi: 10.1038/nm.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pena F. Jansens A. van Zadelhoff G. Braakman I. Calcium as a crucial cofactor for low density lipoprotein receptor folding in the endoplasmic reticulum. J Biol Chem. 2010;285:8656–8664. doi: 10.1074/jbc.M110.105718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pfaffenbach KT. Gentile CL. Nivala AM. Wang D. Wei Y. Pagliassotti MJ. Linking endoplasmic reticulum stress to cell death in hepatocytes: roles of C/EBP homologous protein and chemical chaperones in palmitate-mediated cell death. Am J Physiol Endocrinol Metab. 2010;298:E1026–E1035. doi: 10.1152/ajpendo.00642.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pfaffenbach KT. Nivala AM. Reese L. Ellis F. Wang D. Wei Y. Pagliassotti MJ. Rapamycin inhibits postprandial-mediated X-box-binding protein-1 splicing in rat liver. J Nutr. 2010;140:879–884. doi: 10.3945/jn.109.119883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Puri P. Mirshahi F. Cheung O. Natarajan R. Maher JW. Kellum JM. Sanyal AJ. Activation and dysregulation of the unfolded protein response in nonalcoholic fatty liver disease. Gastroenterology. 2008;134:568–576. doi: 10.1053/j.gastro.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 128.Qiu W. Su Q. Rutledge AC. Zhang J. Adeli K. Glucosamine-induced endoplasmic reticulum stress attenuates apolipoprotein B100 synthesis via PERK signaling. J Lipid Res. 2009;50:1814–1823. doi: 10.1194/jlr.M800343-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rashid KA. Hevi S. Chen Y. Le Caherec F. Chuck SL. A proteomic approach identifies proteins in hepatocytes that bind nascent apolipoprotein B. J Biol Chem. 2002;277:22010–22017. doi: 10.1074/jbc.M112448200. [DOI] [PubMed] [Google Scholar]
- 130.Rawson RB. Regulated intramembrane proteolysis: from the endoplasmic reticulum to the nucleus. Essays Biochem. 2002;38:155–168. doi: 10.1042/bse0380155. [DOI] [PubMed] [Google Scholar]
- 131.Rizzuto R. Pinton P. Carrington W. Fay FS. Fogarty KE. Lifshitz LM. Tuft RA. Pozzan T. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science. 1998;280:1763–1766. doi: 10.1126/science.280.5370.1763. [DOI] [PubMed] [Google Scholar]
- 132.Rutkowski DT. Arnold SM. Miller CN. Wu J. Li J. Gunnison KM. Mori K. Akha AAS. Raden D. Kaufman RJ. Adaptation to ER stress is mediated by differential stabilities of pro-survival and pro-apoptotic mRNAs and proteins. PLoS Biol. 2006;4:2024–2041. doi: 10.1371/journal.pbio.0040374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rutkowski DT. Kaufman RJ. A trip to the ER: coping with stress. Trends Cell Biol. 2004;14:20–28. doi: 10.1016/j.tcb.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 134.Rutkowski DT. Wu J. Back SH. Callaghan MU. Ferris SP. Iqbal J. Clark R. Miao H. Hassler JR. Fornek J. Katze MG. Hussain MM. Song B. Swathirajan J. Wang J. Yau GD. Kaufman RJ. UPR pathways combine to prevent hepatic steatosis caused by ER stress-mediated suppression of transcriptional master regulators. Dev Cell. 2008;15:829–840. doi: 10.1016/j.devcel.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sabio G. Cavanagh-Kyros J. Ko HJ. Jung DY. Gray S. Jun JY. Barrett T. Mora A. Kim JK. Davis RJ. Prevention of steatosis by hepatic JNK1. Cell Metab. 2009;10:491–498. doi: 10.1016/j.cmet.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sage AT. Walter LA. Shi Y. Khan MI. Kaneto H. Capretta A. Werstuck GH. Hexosamine biosynthesis pathway flux promotes endoplasmic reticulum stress, lipid accumulation, and inflammatory gene expression in hepatic cells. Am J Physiol Endocrinol Metab. 2010;298:E499–E511. doi: 10.1152/ajpendo.00507.2009. [DOI] [PubMed] [Google Scholar]
- 137.Sakaki K. Kaufman RJ. Regulation of ER stress-induced macroautophagy by protein kinase C. Autophagy. 2008;4:841–843. doi: 10.4161/auto.6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Santos CX. Tanaka LY. Wosniak J. Laurindo FR. Mechanisms and implications of reactive oxygen species generation during the unfolded protein response: roles of endoplasmic reticulum oxidoreductases, mitochondrial electron transport, and NADPH oxidase. Antioxid Redox Signal. 2009;11:2409–2427. doi: 10.1089/ars.2009.2625. [DOI] [PubMed] [Google Scholar]
- 139.Santos VN. Lanzoni VP. Szejnfeld J. Shigueoka D. Parise ER. A randomized double-blind study of the short-time treatment of obese patients with nonalcoholic fatty liver disease with ursodeoxycholic acid. Braz J Med Biol Res. 2003;36:723–729. doi: 10.1590/s0100-879x2003000600007. [DOI] [PubMed] [Google Scholar]
- 140.Schattenberg JM. Singh R. Wang Y. Lefkowitch JH. Rigoli RM. Scherer PE. Czaja MJ. JNK1 but not JNK2 promotes the development of steatohepatitis in mice. Hepatology. 2006;43:163–172. doi: 10.1002/hep.20999. [DOI] [PubMed] [Google Scholar]
- 141.Schroder M. Kaufman RJ. ER stress and the unfolded protein response. Mutat Res. 2005;569:29–63. doi: 10.1016/j.mrfmmm.2004.06.056. [DOI] [PubMed] [Google Scholar]
- 142.Schroder M. Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739–789. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- 143.Scorrano L.S.A.O. Opferman JT. Cheng EH. Sorcinelli MD. Pozzan T. Korsmeyer SJ. Bax and Bak regulation of endoplasmic reticulum calcium: a control point for apoptosis. Science. 2003;300:135–139. doi: 10.1126/science.1081208. [DOI] [PubMed] [Google Scholar]
- 144.Seo J. Fortuno ES., 3rd Suh JM. Stenesen D. Tang W. Parks EJ. Adams CM. Townes T. Graff JM. Atf4 regulates obesity, glucose homeostasis, and energy expenditure. Diabetes. 2009;58:2565–2573. doi: 10.2337/db09-0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sha H. He Y. Chen H. Wang C. Zenno A. Shi H. Yang X. Zhang X. Qi L. The IRE1alpha-XBP1 pathway of the unfolded protein response is required for adipogenesis. Cell Metab. 2009;9:556–564. doi: 10.1016/j.cmet.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sharma NK. Das SK. Mondal AK. Hackney OG. Chu WS. Kern PA. Rasouli N. Spencer HJ. Yao-Borengasser A. Elbein SC. Endoplasmic reticulum stress markers are associated with obesity in nondiabetic subjects. J Clin Endocrinol Metab. 2008;93:4532–4541. doi: 10.1210/jc.2008-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sharma R. Gow A. Minimal role for caspase 12 in the unfolded protein response in oligodendrocytes in vivo. J Neurochem. 2007;101:889–897. doi: 10.1111/j.1471-4159.2007.04541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Shimizu Y. Hendershot LM. Oxidative folding: cellular strategies for dealing with the resultant equimolar production of reactive oxygen species. Antioxid Redox Signal. 2009;9:2317–2331. doi: 10.1089/ars.2009.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Shoelson SE. Obesity, inflammation, and insulin resistance. Gastroenterology. 2007;132:2169–2180. doi: 10.1053/j.gastro.2007.03.059. [DOI] [PubMed] [Google Scholar]
- 150.Singh R. Kaushik S. Wang Y. Xiang Y. Novak I. Komatsu M. Tanaka K. Cuervo AM. Czaja MJ. Autophagy regulates lipid metabolism. Nature. 2009;458:1131–1135. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Song B. Scheuner D. Ron D. Pennathur S. Kaufman RJ. Chop deletion reduces oxidative stress, improves beta cell function, and promotes cell survival in multiple mouse models of diabetes. J Clin Invest. 2008;118:3378–3389. doi: 10.1172/JCI34587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sorrentino P. Terracciano L. D'Angelo S. Ferbo U. Bracigliano A. Vecchione R. Predicting fibrosis worsening in obese patients with NASH through parenchymal fibronectin, HOMA-IR, and hypertension. Am J Gastroenterol. 2010;105:336–344. doi: 10.1038/ajg.2009.587. [DOI] [PubMed] [Google Scholar]
- 153.Sriburi R. Jackowski S. Mori K. Brewer JW. XBP1: a link between the unfolded protein response, lipid biosynthesis, and biogenesis of the endoplasmic reticulum. J Cell Biol. 2004;167:35–41. doi: 10.1083/jcb.200406136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Su Q. Tsai J. Xu E. Qiu W. Bereczki E. Santha M. Adeli K. Apolipoprotein B100 acts as a molecular link between lipid-induced endoplasmic reticulum stress and hepatic insulin resistance. Hepatology. 2009;50:77–84. doi: 10.1002/hep.22960. [DOI] [PubMed] [Google Scholar]
- 155.Sugimoto H. Okada K. Shoda J. Warabi E. Ishige K. Ueda T. Taguchi K. Yanagawa T. Nakahara A. Hyodo I. Ishii T. Yamamoto M. Deletion of nuclear factor-E2-related factor-2 leads to rapid onset and progression of nutritional steatohepatitis in mice. Am J Physiol Gastrointest Liver Physiol. 2010;298:G283–G294. doi: 10.1152/ajpgi.00296.2009. [DOI] [PubMed] [Google Scholar]
- 156.Syn WK. Choi SS. Diehl AM. Apoptosis and cytokines in non-alcoholic steatohepatitis. Clin Liver Dis. 2009;13:565–580. doi: 10.1016/j.cld.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tamaki N. Hatano E. Taura K. Tada M. Kodama Y. Nitta T. Iwaisako K. Seo S. Nakajima A. Ikai I. Uemoto S. CHOP deficiency attenuates cholestasis-induced liver fibrosis by reduction of hepatocyte injury. Am J Physiol Gastrointest Liver Physiol. 2008;294:G498–G505. doi: 10.1152/ajpgi.00482.2007. [DOI] [PubMed] [Google Scholar]
- 158.Tardif K. Mori K. Kaufman RJ. Siddiqui A. Hepatitis C virus suppresses the IRE1-XBP1 pathway of the unfolded protein response. J Biol Chem. 2004;279:17158–17164. doi: 10.1074/jbc.M312144200. [DOI] [PubMed] [Google Scholar]
- 159.Tardif KD. Mori K. Siddiqui A. Hepatitis C virus subgenomic replicons induce endoplasmic reticulum stress activating an intracellular signaling pathway. J Virol. 2002;76:7453–7459. doi: 10.1128/JVI.76.15.7453-7459.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Tazi KA. Bieche I. Paradis V. Guichard C. Laurendeau I. Dargere D. Legrand A. Fay M. Pedruzzi E. Robin MA. Cazals-Hatem D. Tellier Z. Bernuau D. Feldmann G. Vidaud M. Lebrec D. Ogier-Denis E. Moreau R. In vivo altered unfolded protein response and apoptosis in livers from lipopolysaccharide-challenged cirrhotic rats. J Hepatol. 2007;46:1075–1088. doi: 10.1016/j.jhep.2007.01.034. [DOI] [PubMed] [Google Scholar]
- 161.Testerink N. van der Sanden MH. Houweling M. Helms JB. Vaandrager AB. Depletion of phosphatidylcholine affects endoplasmic reticulum morphology and protein traffic at the Golgi complex. J Lipid Res. 2009;50:2182–2192. doi: 10.1194/jlr.M800660-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Thimmulappa RK. Lee H. Rangasamy T. Reddy SP. Yamamoto M. Kensler TW. Biswal S. Nrf2 is a critical regulator of the innate immune response and survival during experimental sepsis. J Clin Invest. 2006;116:984–995. doi: 10.1172/JCI25790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Thuy S. Ladurner R. Volynets V. Wagner S. Strahl S. Konigsrainer A. Maier KP. Bischoff SC. Bergheim I. Nonalcoholic fatty liver disease in humans is associated with increased plasma endotoxin and plasminogen activator inhibitor 1 concentrations and with fructose intake. J Nutr. 2008;138:1452–1455. doi: 10.1093/jn/138.8.1452. [DOI] [PubMed] [Google Scholar]
- 164.Tuncman G. Hirosumi J. Solinas G. Chang L. Karin M. Hotamisligil GS. Functional in vivo interactions between JNK1 and JNK2 isoforms in obesity and insulin resistance. Proc Natl Acad Sci U S A. 2006;103:10741–10746. doi: 10.1073/pnas.0603509103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Turner MD. Fatty acyl CoA-mediated inhibition of endoplasmic reticulum assembly. Biochim Biophys Acta. 2004;1693:1–4. doi: 10.1016/j.bbamcr.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 166.Urano F. Wang X. Bertolotti A. Zhang Y. Chung P. Harding HP. Ron D. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664–666. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- 167.van Meer G. Voelker DR. Feigenson GW. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol. 2008;9:112–124. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Vecchi C. Montosi G. Zhang K. Lamberti I. Duncan SA. Kaufman RJ. Pietrangelo A. ER stress controls iron metabolism through induction of hepcidin. Science. 2009;325:877–880. doi: 10.1126/science.1176639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Videla LA. Rodrigo R. Orellana M. Fernandez V. Tapia G. Quinones L. Varela N. Contreras J. Lazarte R. Csendes A. Rojas J. Maluenda F. Burdiles P. Diaz JC. Smok G. Thielemann L. Poniachik J. Oxidative stress-related parameters in the liver of non-alcoholic fatty liver disease patients. Clin Sci (Lond) 2004;106:261–268. doi: 10.1042/CS20030285. [DOI] [PubMed] [Google Scholar]
- 170.Wang D. Wei Y. Pagliassotti MJ. Saturated fatty acids promote endoplasmic reticulum stress and liver injury in rats with hepatic steatosis. Endocrinology. 2006;147:943–951. doi: 10.1210/en.2005-0570. [DOI] [PubMed] [Google Scholar]
- 171.Wei H. Kim SJ. Zhang Z. Tsai PC. Wisniewski KE. Mukherjee AB. ER and oxidative stresses are common mediators of apoptosis in both neurodegenerative and non-neurodegenerative lysosomal storage disorders and are alleviated by chemical chaperones. Hum Mol Genet. 2008;17:469–477. doi: 10.1093/hmg/ddm324. [DOI] [PubMed] [Google Scholar]
- 172.Wei Y. Wang D. Gentile CL. Pagliassotti MJ. Reduced endoplasmic reticulum luminal calcium links saturated fatty acid-mediated endoplasmic reticulum stress and cell death in liver cells. Mol Cell Biochem. 2009;331:31–40. doi: 10.1007/s11010-009-0142-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wei Y. Wang D. Topczewski F. Pagliassotti MJ. Saturated fatty acids induce endoplasmic reticulum stress and apoptosis independently of ceramide in liver cells. Am J Physiol Endocrinol Metab. 2006;291:E275–E281. doi: 10.1152/ajpendo.00644.2005. [DOI] [PubMed] [Google Scholar]
- 174.Werstuck GH. Lentz SR. Dayal S. Hossain GS. Sood SK. Shi YY. Zhou J. Maeda N. Krisans SK. Malinow MR. Austin RC. Homocysteine-induced endoplasmic reticulum stress causes dysregulation of the cholesterol and triglyceride biosynthetic pathways. J Clin Invest. 2001;107:1263–1273. doi: 10.1172/JCI11596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wieckowska A. McCullough AJ. Feldstein AE. Noninvasive diagnosis and monitoring of nonalcoholic steatohepatitis: present and future. Hepatology. 2007;46:582–589. doi: 10.1002/hep.21768. [DOI] [PubMed] [Google Scholar]
- 176.Wieckowska A. Zein NN. Yerian LM. Lopez AR. McCullough AJ. Feldstein AE. In vivo assessment of liver cell apoptosis as a novel biomarker of disease severity in nonalcoholic fatty liver disease. Hepatology. 2006;44:27–33. doi: 10.1002/hep.21223. [DOI] [PubMed] [Google Scholar]
- 177.Wigg AJ. Roberts-Thomson IC. Dymock RB. McCarthy PJ. Grose RH. Cummins AG. The role of small intestinal bacterial overgrowth, intestinal permeability, endotoxaemia, and tumour necrosis factor alpha in the pathogenesis of non-alcoholic steatohepatitis. Gut. 2001;48:206–211. doi: 10.1136/gut.48.2.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Winnay JN. Boucher J. Mori MA. Ueki K. Kahn CR. A regulatory subunit of phosphoinositide 3-kinase increases the nuclear accumulation of X-box-binding protein-1 to modulate the unfolded protein response. Nat Med. 2010;16:438–445. doi: 10.1038/nm.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wiseman RL. Zhang Y. Lee KP. Harding HP. Haynes CM. Price J. Sicheri F. Ron D. Flavonol activation defines an unanticipated ligand-binding site in the kinase-RNase domain of IRE1. Mol Cell. 2010;38:291–304. doi: 10.1016/j.molcel.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Woo CW. Cui D. Arellano J. Dorweiler B. Harding H. Fitzgerald KA. Ron D. Tabas I. Adaptive suppression of the ATF4-CHOP branch of the unfolded protein response by toll-like receptor signalling. Nat Cell Biol. 2009;11:1473–1480. doi: 10.1038/ncb1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wouters K. van Bilsen M. van Gorp PJ. Bieghs V. Lutjohann D. Kerksiek A. Staels B. Hofker MH. Shiri-Sverdlov R. Intrahepatic cholesterol influences progression, inhibition and reversal of non-alcoholic steatohepatitis in hyperlipidemic mice. FEBS Lett. 2010;584:1001–1005. doi: 10.1016/j.febslet.2010.01.046. [DOI] [PubMed] [Google Scholar]
- 182.Wu J. Kaufman RJ. From acute ER stress to physiological roles of the unfolded protein response. Cell Death Differ. 2006;13:374–384. doi: 10.1038/sj.cdd.4401840. [DOI] [PubMed] [Google Scholar]
- 183.Wu S. Tan M. Hu Y. Wang JL. Scheuner D. Kaufman RJ. Ultraviolet light activates NFkappaB through translational inhibition of IkappaBalpha synthesis. J Biol Chem. 2004;279:34898–34902. doi: 10.1074/jbc.M405616200. [DOI] [PubMed] [Google Scholar]
- 184.Xue X. Piao JH. Nakajima A. Sakon-Komazawa S. Kojima Y. Mori K. Yagita H. Okumura K. Harding H. Nakano H. Tumor necrosis factor alpha (TNFalpha) induces the unfolded protein response (UPR) in a reactive oxygen species (ROS)-dependent fashion, and the UPR counteracts ROS accumulation by TNFalpha. J Biol Chem. 2005;280:33917–33925. doi: 10.1074/jbc.M505818200. [DOI] [PubMed] [Google Scholar]
- 185.Yamamoto K. Sato T. Matsui T. Sato M. Okada T. Yoshida H. Harada A. Mori K. Transcriptional induction of mammalian ER quality control proteins is mediated by single or combined action of ATF6alpha and XBP1. Dev Cell. 2007;13:365–376. doi: 10.1016/j.devcel.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 186.Yamazaki H. Hiramatsu N. Hayakawa K. Tagawa Y. Okamura M. Ogata R. Huang T. Nakajima S. Yao J. Paton AW. Paton JC. Kitamura M. Activation of the Akt-NF-kappaB pathway by subtilase cytotoxin through the ATF6 branch of the unfolded protein response. J Immunol. 2009;183:1480–1487. doi: 10.4049/jimmunol.0900017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Yang SQ. Lin HZ. Lane MD. Clemens M. Diehl AM. Obesity increases sensitivity to endotoxin liver injury: implications for the pathogenesis of steatohepatitis. Proc Natl Acad Sci U S A. 1997;94:2557–2562. doi: 10.1073/pnas.94.6.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Yang X. Chan C. Repression of PKR mediates palmitate-induced apoptosis in HepG2 cells through regulation of Bcl-2. Cell Res. 2009;19:469–486. doi: 10.1038/cr.2009.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Ye R. Jung DY. Jun JY. Li J. Luo S. Ko HJ. Kim JK. Lee AS. Grp78 heterozygosity promotes adaptive unfolded protein response and attenuates diet-induced obesity and insulin resistance. Diabetes. 2010;59:6–16. doi: 10.2337/db09-0755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Yokouchi M. Hiramatsu N. Hayakawa K. Okamura M. Du S. Kasai A. Takano Y. Shitamura A. Shimada T. Yao J. Kitamura M. Involvement of selective reactive oxygen species upstream of proapoptotic branches of unfolded protein response. J Biol Chem. 2008;283:4252–4260. doi: 10.1074/jbc.M705951200. [DOI] [PubMed] [Google Scholar]
- 191.Yorimitsu T. Nair U. Yang Z. Klionsky DJ. Endoplasmic reticulum stress triggers autophagy. J Biol Chem. 2006;281:30299–30304. doi: 10.1074/jbc.M607007200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Zeng L. Lu M. Mori K. Luo S. Lee AS. Zhu Y. Shyy JY. ATF6 modulates SREBP2-mediated lipogenesis. EMBO J. 2004;23:950–958. doi: 10.1038/sj.emboj.7600106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zhang J. Herscovitz H. Nascent lipidated apolipoprotein B is transported to the Golgi as an incompletely folded intermediate as probed by its association with network of endoplasmic reticulum molecular chaperones, GRP94, ERp72, BiP, calreticulin, and cyclophilin B. J Biol Chem. 2003;278:7459–7468. doi: 10.1074/jbc.M207976200. [DOI] [PubMed] [Google Scholar]
- 194.Zhang K. Kaufman RJ. From endoplasmic reticulum stress to the inflammatory response. Nature. 2008;454:455–462. doi: 10.1038/nature07203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Zhang K. Shen X. Wu J. Sakaki K. Saunders T. Rutkowski DT. Back SH. Kaufman RJ. Endoplasmic reticulum stress activates cleavage of CREBH to induce a systemic inflammatory response. Cell. 2006;124:587–599. doi: 10.1016/j.cell.2005.11.040. [DOI] [PubMed] [Google Scholar]