Abstract
Sarcopenia is the age-associated loss of skeletal muscle mass and strength. Recent evidence suggests that an age-associated loss of muscle precursor cell (MPC) functionality contributes to sarcopenia. The objectives of the present study were to examine the influence of activated T cells on MPCs and determine whether an age-related defect in this signaling occurs. MPCs were collected from the gastrocnemius and plantaris of 3-mo-old (young) and 32-mo-old (old) animals. Splenic T cells were harvested using anti-CD3 Dynabead isolation. T cells were activated for 48 h with costimulation of 100 IU/ml interleukin-2 (IL-2) and 5 μg/ml of anti-CD28. Costimulation increased 5-bromo-2′-deoxyuridine incorporation of T cells from 13.4 ± 4.6% in control to 64.8 ± 6.0% in costimulated cells. Additionally, T cell cytokines increased proliferation on MPCs isolated from young muscle by 24.0 ± 5.7%, whereas there was no effect on MPCs isolated from aged muscle. T cell cytokines were also found to be a chemoattractant. T cells were able to promote migration of MPCs isolated from young muscle; however, MPCs isolated from aged muscle did not respond to the T cell-released chemokines. Conversely, whereas T cell-released cytokines did not affect myogenesis of MPCs isolated from young animals, there was a decrease in MPCs isolated from old animals. These data suggest that T cells may play a critical role in mediating MPC function. Furthermore, aging may alter T cell-induced MPC function. These findings have implications for developing strategies aimed at increasing MPC migration and proliferation leading to an improved regenerative capacity of aged skeletal muscle.
Keywords: satellite cell, proliferation, migration, immune cell, regeneration
aging is associated with decreased strength and skeletal muscle mass, a condition known as sarcopenia. One contributing factor to sarcopenia is the diminished capacity for aged skeletal muscle to regenerate (14), hypertrophy (5), and regrow after a bout of atrophy (15). Moreover, in aging there is a marked increase in skeletal muscle fibrosis in concert with decreased muscle tissue resulting in decreased muscle quality (23, 31). After illness, the reduced skeletal muscle quality is strongly associated with increased age (32). Diminished muscle mass, strength, and quality lead to impaired skeletal muscle function in terms of mobility, independence, and quality of life.
In skeletal muscle, the resident stem cells responsible for tissue repair are termed satellite cells (originally named for their anatomical location). The progeny of activated satellite cells are referred to as muscle precursor cells (MPCs). Recent evidence suggests that an age-associated loss of MPC functionality is the primary factor responsible for the loss of regenerative potential and increased atrophy and fibrosis of aged skeletal muscle (19). MPC function is predominately dictated by the surrounding environment or the local milieu. However, it is also known that MPCs from aged skeletal muscle respond to the environment differently compared with MPCs isolated from young muscle (6, 17, 18, 36, 38, 41, 45, 46, 49).
One of the main contributing factors to the local milieu of MPCs in injured skeletal muscle is infiltrating immune cells. T cell infiltration into damaged skeletal muscle occurs rapidly following injury (16). A recent report demonstrated T cell infiltration begins at about 3 days following injury and remains until at least day 10 of regeneration (16). However, very little is known about the specific role of T cells in skeletal muscle repair.
Current evidence suggests that T cells may play an integral role in skeletal muscle repair and fibrosis. Dermatomyositis and polymyositis are disabling rheumatic diseases characterized by an appreciable number of T cells infiltrating muscle tissue (27). Moreover, in muscular dystrophy, there is a chronic infiltration of macrophages and T cells due to repeated cycles of degeneration and regeneration (68). The infiltrating T cells have been implicated in the pathology of muscular dystrophy (68). Recently, the use of immunodeficient/dystrophic mouse models demonstrated that T cell depletion resulted in both reduced tranforming growth factor-β1 (TGF-β1) and skeletal muscle fibrosis in dystrophic skeletal muscle (26, 55). These immunodeficient/dystrophic mice demonstrated improved skeletal muscle regeneration and decreased fibrosis (26, 55). Thus one possible interpretation is that the chronic infiltration of T cells in the lesions of dystrophic skeletal muscle promotes the fibrotic aspects of muscular dystrophy. However, the role of T cells in skeletal muscle repair of nondystrophic skeletal muscle is not known. Transient T cell infiltration after an acute bout of injury in nondystrophic muscle may provoke a different outcome on the regenerative process compared with the chronic infiltration observed in dystrophic skeletal muscle. Moreover, it is known that nondystrophic animals lacking T cells (nude mice) exhibit impaired muscle growth (56), indicating a possible role for T cells in myogenesis.
T cell infiltration begins at the early stages of regeneration during MPC migration, proliferation, and differentiation suggesting that T cells may play an integral role in skeletal muscle repair. Despite the simultaneous accumulation of T cells and MPC action, very little is known about the potential regulatory function of T cells on MPCs. However, in terms of sarcopenia, delineating mechanisms of T cell-mediated MPC function in aging may facilitate the development of treatment strategies aimed at improving the skeletal muscle repair process leading to increased mass and strength in our aging and physically frail populations.
The purpose of the present study was twofold. The first was to establish whether activated T cells regulate MPC proliferation, migration, and differentiation. To do this, MPCs isolated from young rats were treated with conditioned media collected from activated T cells. The second was to establish whether an age-related difference exists in the responsiveness of MPCs to activated T cells. To do this, MPCs were isolated from skeletal muscle of aged/sarcopenic rats, and T cell-mediated proliferation, migration, and differentiation were determined.
MATERIALS AND METHODS
Animals.
All procedures were approved by the Institutional Animal Care and Use Committee at Colorado State University. Fisher 344x Brown Norway F1 hybrid male rats, 3- and 32-mo-old, were obtained from the National Institute on Aging. Animals were housed at 21°C on a 12-h light/12-h dark cycle and allowed free access to food and water. For tissue collection, animals were given an intraperitoneal injection of ketamine (80 mg/kg), xylazine (10 mg/kg), and acepromazine (4 mg/kg), the animals were killed by removing the heart, and the tissue was excised.
MPC isolation and culture.
MPC isolation was modified from Allen et al. (2) as described previously (38, 41). Briefly, cells were isolated from the gastrocnemius and plantaris muscles by pronase digestion and preplated for 24 h on tissue-culture treated 150-mm plates. MPCs were cultured on Matrigel (BD Biosciences, San Jose, CA)-coated plates (0.1 mg/ml Matrigel, 60 min at 37°C) and passaged only one time [growth media (GM), 20% FBS in Ham's F-10; 6% O2-5% CO2-89% N2 at 37°C].
T cell isolation and culture.
Splenocytes collected from the spleen were incubated with the mouse monoclonal antibody to rat CD3 (CALTAG Laboratories, Invitrogen, Carlsbad, CA) followed by incubation with anti-mouse IgM Dynabeads (Dynal, Invitrogen, Oslo, Norway). CD3+ cells were then separated using the Dynabead magnet and resuspended in 10 ml OpTmizer T-cell Expansion media (GIBCO, Invitrogen). T cell stimulation was induced with treatment of 100 IU/ml of IL-2 (PreproTech, Rocky Hill, NJ) and 5 μg/ml of anti-CD28 (BD, San Diego, CA). After 2 days, cells were either collected to assess activation/proliferation, or the medium was changed to a basal media (BM) for cytokine collection. Cells used for activation/proliferation assessment were pulsed with 5-bromo-2′-deoxyuridine-5′-monophosphate (BrdU) and fixed for analysis via flow cytometry. For cytokine collection, following the 2-day T cell stimulation, the medium was changed to BM (2% FBS in DMEM), and cells were cultured for an additional 24 h. During this time, the T cells released cytokines into the cell culture media, a process known as conditioning media.
MPC migration.
After the first passage, MPC migration was measured using the FluoroBlok cell migration plates (8 μm pore size; BD Biosciences, San Jose, CA) with inserts coated with Matrigel. MPCs (75,000) were loaded into the upper chamber of the migration plate in BM containing calcein-AM (5 μg/ml). The lower chamber contained either BM, or conditioned media (CM) obtained from T cells. To detect the rate of migration, the plates were read with bottom reading fluorescence (Ex485/Em530) every 30 min up to 120 min (Soft Max Pro Software; Spectra Max M5, Molecular Devices, Silicone Valley, CA).
Proliferation.
To analyze cell proliferation of both T cells and MPCs, BrdU incorporation was determined using flow cytometry (39). As mentioned above, T cells were pulsed with BrdU for 60 min beginning 47 h following isolation in either the presence or absence of IL-2 (100 IU/ml) and anti-CD28 antibody (5 μg/ml). For MPC proliferation, cells were plated in 10% FBS in Ham's F-10 and cultured for 24 h. After 24 h, the medium was replaced with a 1:1 mixture containing one part 20% FBS in Ham's F-10 and either BM or CM. MPCs were then pulsed with BrdU for 60 min beginning 23 h following treatment with either BM or CM. Briefly, after the 60-min pulse, the cells were washed twice with PBS, removed from the plates, centrifuged at 500 g for 5 min, and fixed with ice-cold 70% ethanol. DNA was acid denatured (2 N HCl, 30 min), and incorporated BrdU was detected using a fluorescein-conjugated monoclonal antibody raised against BrdU (5 μg/ml, Roche Applied Sciences, Indianapolis, IN) in PBS with 0.1% bovine serum albumin (BSA). Cells (20,000) were analyzed using a Epics XL-MCL Coulter flow cytometer (Beckman Coulter, Brea, CA) and FCS Express (De Novo Software, Los Angeles, CA) (41).
MPC differentiation.
To determine the myogenic capacity of MPCs, myosin heavy chain (MyHC) was determined using previously described methods (40). Briefly. 75,000 MPCs were plated onto each well of a six-well plate in GM and allowed to adhere overnight. To induce MPC differentiation, the medium was changed to a low mitogen-serum mixture. MPCs were either exposed to medium containing CM (1:1 mixture of 2% FBS in DMEM and CM) or BM (1:1 mixture of 2% FBS in DMEM and BM). The medium was changed every 24 h with the treatments remaining the same. Equal amounts of protein were loaded and separated using SDS-PAGE and transferred onto nitrocellulose membranes. The MyHC antibody (MF 20) was purchased from the Developmental Studies Hybridoma Bank. Immunocomplexes were visualized using Immu-Blot AP kit (Bio-Rad, Hercules, CA). The signal bands were scanned and quantified using ImageJ software.
Statistics.
Data are presented as means ± SE. Sample sizes are indicated for each measurement in the figure legends, where n represents independent isolations from separate animals. Comparisons between groups were done using the two-way repeated measures ANOVA (SigmaStat software, Systat, Chicago, IL). Significance was accepted at P ≤ 0.05.
RESULTS
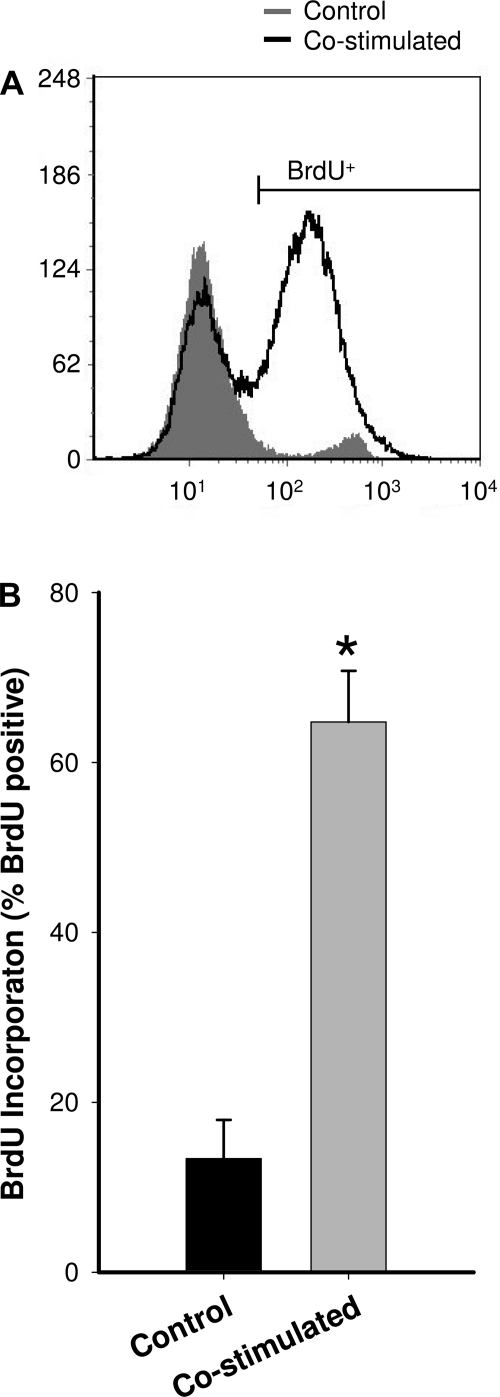
To determine whether T cells may contribute to MPC function, naïve splenic T cells were isolated and activated in vitro. After the isolation of CD3+ T cells from the spleen, T cells were activated via costimulation with IL-2 (100 IU/ml) and anti-CD28 antibody (5 μg/ml). To verify successful activation of the T cells via costimulation, proliferation was determined using BrdU incorporation. Based on the flow cytometry analysis (Fig. 1), the combined IL-2 and anti-CD28 stimulation significantly increased T cell proliferation compared with isolated CD3+ T cells that did not receive the combined IL-2 and anti-CD28 treatment. T cell stimulation increased T cell proliferation by approximately fivefold. T cells that were costimulated were 64.8% BrdU positive, whereas only 13.4% of T cells that did not receive combined IL-2 and anti-CD28 treatment were BrdU positive.
Fig. 1.
In vitro stimulation of isolated CD3+ T cells. A: representative histogram from flow cytometric analysis of T cells labeled for bromo-2′-deoxyuridine (BrdU) incorporation. The large increase in proliferation due to costimulation with interleukin (IL)-2 and anti-CD28 is observed by the large number of cells in the BrdU+ population (the peak on the right). B: group mean data for BrdU incorporation for Control and Costimulated T cells (n = 5). *Significantly different from control (P ≤ 0.05).
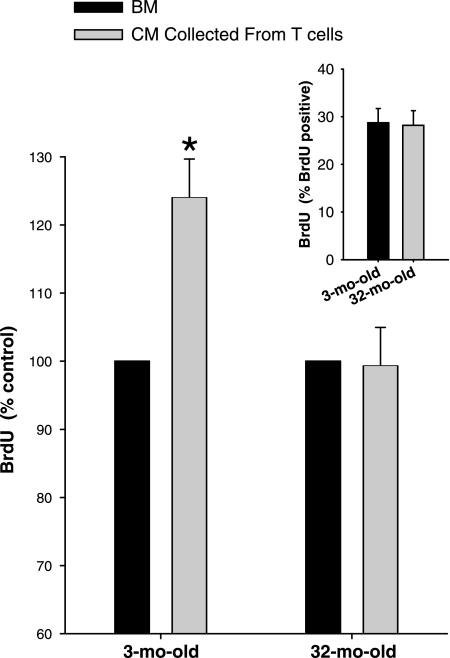
To determine the potential effect of T cell-released cytokines on MPC function, activated T cell cultures were changed to BM and allowed to condition the media for 24 h. CM was then collected and used to treat MPCs before pulsing with BrdU. First, proliferation of MPCs isolated from 3- and 32-mo-old animals was determined in medium containing the BM (1:1 mixture of 20% FBS in Ham's F-10 and BM). Under these conditions, there was no difference in the proliferation of MPCs isolated from either age (Fig. 2, inset).
Fig. 2.
Proliferation of muscle precursor cells (MPCs) in conditioned media (CM). CM collected from activated CD3+ T cells for 24 h following the costimulation with IL-2 and anti-CD28 (n = 4). Proliferation in response to CM is expressed as percent control (proliferation when treated with basal media, BM). Inset: proliferation of muscle precursor cells (MPCs) in BM. Group mean data for BrdU incorporation for MPCs isolated from 3- and 32-mo-old animals in BM (n = 4). *Significantly different from BM (P ≤ 0.05).
However, when MPCs were treated with CM, age-related differences emerged. CM from activated T cells caused an increase in proliferation of MPCs isolated from 3-mo-old animals (Fig. 2). T cell-released cytokines increased proliferation of MPCs isolated from young muscle by 24.0 ± 5.7%. However, CM had no effect on the proliferation of MPCs isolated from 32-mo-old animals (Fig. 2). These data demonstrate that while MPCs isolated from young skeletal muscle are responsive to the mitogenic factors released from activated T cell, MPCs isolated from old skeletal muscle are not responsive to these factors.
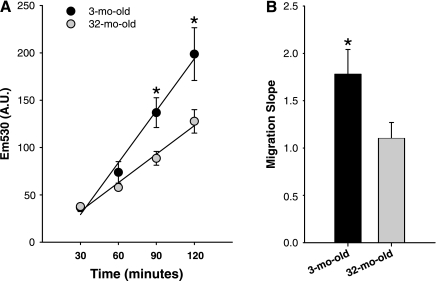
After skeletal muscle injury, MPCs migrate to the site of injury. The responsiveness to chemotactic factors that attract MPCs to the site of injury is a critical step in the regenerative process. To determine whether age-associated differences exist in the MPC response to chemotactic agents, a migration assay was performed. The migration of MPCs isolated from 3- and 32-mo-old were first tested in BM. Migration toward BM was measured every 30 min for 2 h (Fig. 3). A higher number of MPCs isolated from the 3-mo-old animals had migrated at the 90- and 120-min time points compared with MPCs isolated from the 32-mo-old rats (Fig. 3A). The slope calculated from the regression line plotted for the cell migration indicates the relative rate of migration of MPCs. From the slopes it was determined that the MPCs isolated from young animals exhibited a higher rate of migration compared with MPCs isolated from aged animals (Fig. 3B). These data indicate that there may be an intrinsic deficiency in MPC migration with age.
Fig. 3.
MPC migration toward BM. A: migration of MPCs isolated from 3- and 32-mo-old animals was assessed in BM every 30 min for 2 h (n = 4). B: rate of migration is represented as the calculated slopes of the migration plots. *Significantly different from 32-mo-old (P ≤ 0.05).
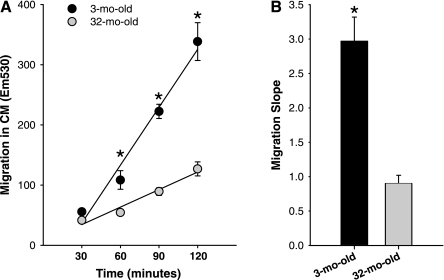
Activated T cells are known to release chemokines. To determine whether activated T cells promote MPC chemotaxis, cell migration toward CM was performed. CM-induced migration of MPCs was examined at 30-min intervals for 2 h (Fig. 4). A higher number of MPCs isolated from the 3-mo-old animals had migrated at the 60-, 90-, and 120-min time points compared with MPCs isolated from the 32-mo-old rats (Fig. 4A). Moreover, it was determined that the MPCs isolated from young animals exhibited an approximately threefold higher rate of migration toward CM compared with MPCs isolated from aged animals (Fig. 4B).
Fig. 4.
MPC migration toward CM. A: migration of MPCs isolated from 3- and 32-mo-old animals was assessed in CM every 30 min for 2 h (n = 4). B: rate of migration is represented as the calculated slopes of the migration plots. *Significantly different from 32-mo-old (P ≤ 0.05).
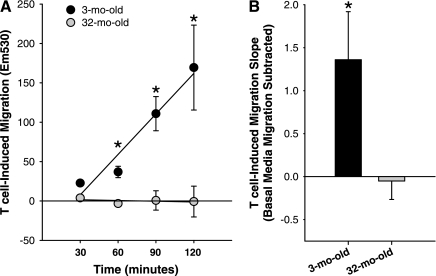
However, since migration occurs in BM alone, to determine the cell migration induced by the chemokines in CM, migration in BM was subtracted to give T cell-induced migration (Fig. 5). In response to T cell-released chemokines, more MPCs isolated from the 3-mo-old animals had migrated at the 60-, 90-, and 120-min time points compared with MPCs isolated from the 32-mo-old rats (Fig. 5A). Importantly, T cell-released factors induced a significant increase in the rate of migration of MPCs isolated from 3-mo-old animals, while there was no difference in rate of migration of 32-mo-old animals in toward CM or BM (Fig. 5B). These data indicate that while MPCs isolated from young skeletal muscle are responsive to the chemotactic factors released from activated T cell, MPCs isolated from old skeletal muscle are not responsive to these factors.
Fig. 5.
T cell-induced MPC migration. A: to determine migration due to T cell-released factors, migration of MPCs isolated from 3- and 32-mo-old animals was assessed in CM every 30 min for 2 h, and the migration toward BM was subtracted (n = 4). B: T cell-induced rate of migration is represented as the calculated slopes of the migration plots. *Significantly different from 32-mo-old (P ≤ 0.05).
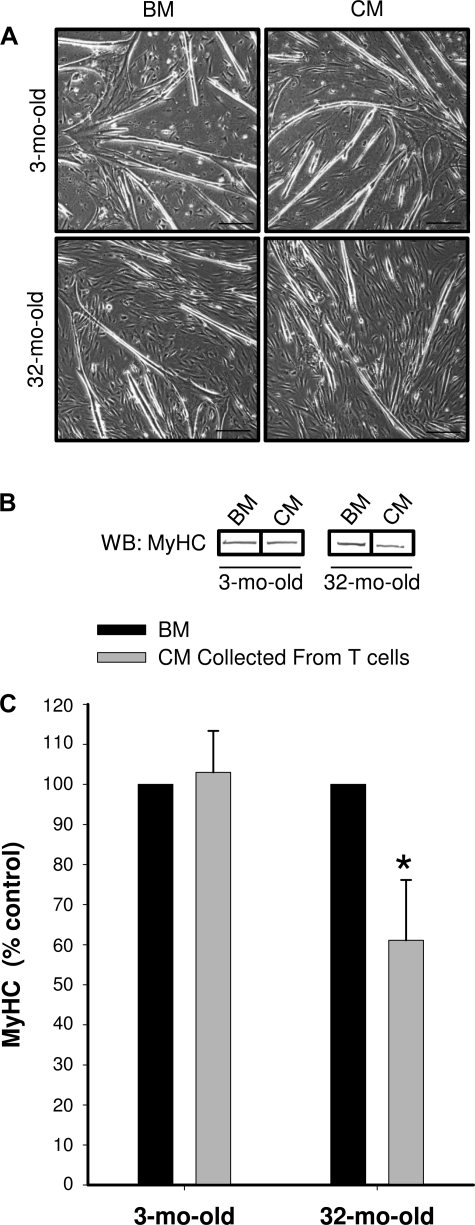
To determine the effect of T cell-released cytokines on myogenesis, MPCs were induced to differentiate in media containing either the BM (1:1 mixture of 2% FBS in DMEM and BM) or media containing CM (1:1 mixture of 2% FBS in DMEM and CM). MPCs were allowed to differentiate for 48 h. T cell-released factors in CM had no influence on MyHC expression in MPCs isolated from 3-mo-old animals (Fig. 6). However, in contrast to the MPCs isolated from young animals, MPCs isolated from aged animals were sensitive to the CM. MyHC expression was decreased in MPCs isolated from the 32-mo-old animals when exposed to the CM compared with the BM (Fig. 6).
Fig. 6.
Differentiation of MPCs in CM. CM collected from activated CD3+ T cells for 24 h following the costimulation with IL-2 and anti-CD28 (n = 4). A: representative images of MPCs induced to differentiate for 48 h in either the presence of CM or BM. Scale bar represents 250 nm. B: representative bands from the Western blot (WB) probed with anti-sarcomeric myosin heavy chain (MyHC) antibody. The representative bands were not necessarily from adjacent lanes on the Western blot. C: group mean data for MyHC WB of differentiated MPCs collected from 3-mo-old (n = 4) and 32-mo-old CM are expressed as percent control (differentiation when treated with BM). *Significantly different from BM (P ≤ 0.05).
DISCUSSION
Aging is associated with diminished MPC function, leading to impaired skeletal muscle regeneration (15, 19). Infiltrating immune cells are a main contributor to the local milieu of MPCs in injured skeletal muscle. Despite the known importance of the infiltrating immune cell on the regenerative process (70), very little is known about a potential regulatory role of T cells influencing MPC function. To our knowledge, we are the first to demonstrate that cytokines released from activated T cells possess both mitogenic and chemotattractant functions on MPCs isolated from young skeletal muscle. Moreover, this is the first report demonstrating that MPCs isolated from aged skeletal muscle are not responsive to the mitogens and chemokines released from activated T cells. Taken together, our findings support the notion that intrinsic differences in the response to environmental cues exist between MPCs isolated from skeletal muscle of young and old animals. The inability of aged MPCs to respond to environment cues of infiltrating immune cells may contribute to age-associated impaired skeletal muscle regeneration.
A link exists between inflammatory cell function and the skeletal muscle repair. When nonsteroidal anti-inflammatory drugs (NSAID) are administered, there is impaired muscle regeneration (4, 53) that leads to weaker musculotendinosus units compared with untreated animals (7, 48). It has also been established that NSAID treatment and specific inhibition of cyclooxygenase enzymes causes diminished MPC function (47, 51). More recently, macrophage depletion led to increased fibrosis in regenerating skeletal muscle postinjury (21).
It is known that infiltrating cells of the immune system into damaged skeletal muscle are part of a well-choreographed set of events during skeletal muscle repair. After skeletal muscle injury, white blood cells infiltrate into the skeletal muscle compartment occurs (10, 28, 34, 54, 57, 62). Neutrophils are the first to migrate and arrive within the injury site immediately following injury peaking between 1 and 4 h postinjury and may remain within the skeletal muscle for up to 4 days (29, 52, 65). Neutrophils serve to phagocytose necrotic myofibers and cellular damage. Neutrophils also release proinflammatory cytokines such as IL-6, transforming growth factor-β (TGF-β), and tumor necrosis factor-α (TNF-α) that may augment the inflammatory process (1, 9, 13, 52, 59, 61, 65). Additionally, neutrophils release IL-1 and IL-8, which are chemoattractant cytokines for macrophages (28).
Within 24 h, the concentration of neutrophils within the damaged muscle decreases (66) and monocyte infiltration begins. Once within the skeletal muscle the monocytes mature and differentiate into tissue macrophages. Macrophages can remain within the skeletal muscle for 7–14 days. The ED1+ macrophages typically occur in greater concentration within the first 4 days postinjury and release proinflammatory cytokines such as IL-1, IL-2, IFN-γ, and TNF. These macrophages help remove damaged myofibrillar debris (8, 22, 25, 28, 42, 65). The appearance of ED2+ macrophages occurs around day 4. ED2+ macrophages are nonphagocytic but instead release growth factors and cytokines linked to regulating muscle precursor cell function. ED2+ macrophages release anti-inflammatory cytokines including IL-4, IL-5, IL-6, IL-10, and IL-13, and are thought to promote tissue repair (3, 28, 50, 58, 69).
While less studied than macrophages, T cell infiltration also occurs following skeletal muscle injury. A recent report examining postinjury immune cell infiltration of T cells was similar to macrophages at day 3 postinjury and were significantly elevated at days 5 and 10 of regeneration (16). Importantly, the time course of T cell infiltration overlaps with the regenerative phase following skeletal muscle injury. The current findings support a possible role for T cells in mediating MPC function leading to tissue repair. Since MPC migration begins within the first 24 h (64) and proliferation is first detected after the first day following injury (60), early mitogenic/chemoattractant cues are likely to come from alternate sources (e.g., neutrophils). However, T cell-released chemokines may serve to retain MPCs at the site of injury/repair, and the promitogenic function of T cell-released cytokines may play a role in continued proliferation of MPCs at the site of injury. While the data collected in the present study support the notion that T cells are able to stimulate MPC function during skeletal muscle regeneration, a better understanding of the specific role of various T cell subpopulations is needed.
Currently, very little is known about the specific subpopulations of T cells present in injured muscle and the influence they might have on the repair process. It is known that both CD8+ and CD4+ T cells are found in dystrophic skeletal muscle (67). It was found that the chronic infiltration of both the CD8+ and CD4+ T cells had an influence on the pathology of dystrophic muscle (67). A more recent report characterized the distribution of the Vβ T-cell receptors (TCR) found in the mdx model of muscular dystrophy. While several subtypes were found, the predominant subpopulation was Vβ8.1/8.2+ TCR (72). However, it is thought that the infiltrating immune cell profile and resulting cytokines differs somewhat in response to an acute injury versus chronic inflammation present in muscular dystrophy (71). Consequently, a better characterization of the T cell infiltration after an acute injury is needed to determine the role of T cells in the regenerative process.
Aging is associated with decreased strength and skeletal muscle mass, a condition known as sarcopenia. Sarcopenia leads to decreased quality of life and increased mortality in our aging population. One contributing factor to sarcopenia is the diminished capacity for aged skeletal muscle to regenerate (5), hypertrophy (12), and regrow after a bout of atrophy (15). In addition, there is a marked increase in skeletal muscle fibrosis in concert with decreased muscle tissue with aging. Recent evidence suggests that an age-associated loss of MPC functionality is the primary factor responsible for the loss of regenerative potential and increased atrophy and fibrosis of aged skeletal muscle (19). While MPCs have a clearly defined role in facilitating skeletal muscle regeneration, MPCs isolated from aged skeletal muscle have been shown to exhibit a transition from a myogenic to a fibrogenic lineage (12, 35). Also, previous reports demonstrate that aged MPCs respond differently to environmental cues compared with MPCs isolated from young muscle (6, 17, 36, 38, 41, 45, 46, 49). In the present study we demonstrate that MPCs isolated from aged skeletal muscle fail to respond to the mitogenic agents released from activated T cells. In addition, MPCs isolated from aged skeletal muscle exhibited diminished migration (∼40% slower rate of migration toward BM). More importantly, whereas chemokines from activated T cells increased the rate of migration MPCs isolated from young skeletal muscle, MPCs isolated from aged skeletal muscle did not respond to these chemotactic agents. Interestingly, during MPC differentiation, T cell-released factors did not seem to affect myofusion and MyHC expression in MPCs isolated from young animals. However, MPCs isolated from aged animals exhibited a decrease in MyHC expression when exposed to the CM collected from activated T cells. Therefore, the presence of T cells may have a negative influence on myogenesis during the regenerative process in aged skeletal muscle.
Based on previous reports it is possible to speculate on potential candidates for growth factors and cytokines that might be responsible for the observed communication between T cells and MPCs. Activated T cells are known to release a number of different growth factors and cytokines including fibroblast growth factor-2 (FGF-2) (11), interferon-γ (IFN-γ) (24, 37), TGF-β (43, 44), TNF-α (24, 63, 73), and interleukin-4 (IL-4) (24, 30). Interestingly, all of these growth factors and cytokines have been shown to influence MPC function. For example, both TGF-β1 and FGF-2 (20, 33, 36) have been shown to have mitogenic effects on MPCs. Interestingly, we recently reported an age-related decrease in the mitogenic response to FGF-2 (36). MPCs isolated from aged skeletal muscle exhibited a diminished proliferative response to FGF-2 treatment. While no age-related differences were observed in receptor expression at the mRNA level for FGF receptors 1–4, in the presence of FGF-2, an age-related differential expression level of FGF receptors 1 and 2 was detected (36). Future studies will need to be aimed at delineating the specific T cell-released factors involved in modulating MPC function. Additionally, determining the level at which age-related impairments may exist needs to be accomplished. Any number of growth factors and cytokines, either alone or in combination, may be responsible. Moreover, age-related differences may exist at several different points in the signaling cascade possibly involving receptor expression, ligand binding, and receptor activation and intracellular signaling events.
A better understanding of the potential role immune cells may exert on MPCs is vital to delineating mechanisms responsible for skeletal muscle regeneration. Just as important, the age-associated differences in the response to environmental cues driving MPC function need to be identified. To our knowledge, this study was the first to specifically examine influence of T cells on MPC function. In this report we have established that activated T cells have the capacity to promote both proliferation and migration of MPCs isolated from young skeletal muscle. However, MPCs isolated from aged skeletal muscle are not responsive to the mitogenic factors and did not respond to chemokines released from activated T cells. Moreover, while the myogenic response was not affected in MPCs isolated from young muscle, there was a decreased myogenic response in aged MPCs. Taken together, these data suggest that T cells may mediate MPC function during skeletal muscle regeneration and aging may alter T cell-induced MPC function. While future studies are needed to further elucidate the specific mechanisms mediating the influences of T cells on MPC function in aging, these findings have implications for the development of potential treatment interventions aimed at improving the skeletal muscle repair process as we age.
DISCLOSURES
No conflicts of interest, financial, or otherwise are declared by the author(s).
ACKNOWLEDGEMENTS
This study was supported by the NASA Colorado Space Grant (S. J. Lees), the Colorado State University Center on Aging Pilot Grant (S. J. Lees), and National Institutes of Health RO1 (AG-18780 P.I., Frank W. Booth).
REFERENCES
- 1. Adams GR, Caiozzo VJ, Haddad F, Baldwin KM. Cellular and molecular responses to increased skeletal muscle loading after irradiation. Am J Physiol Cell Physiol 283: C1182–C1195, 2002 [DOI] [PubMed] [Google Scholar]
- 2. Allen RE, Boxhorn LK. Regulation of skeletal muscle satellite cell proliferation and differentiation by transforming growth factor-beta, insulin-like growth factor I, and fibroblast growth factor. J Cell Physiol 138: 311–315, 1989 [DOI] [PubMed] [Google Scholar]
- 3. Allen RE, Sheehan SM, Taylor RG, Kendall TL, Rice GM. Hepatocyte growth factor activates quiescent skeletal muscle satellite cells in vitro. J Cell Physiol 165: 307–312, 1995 [DOI] [PubMed] [Google Scholar]
- 4. Almekinders LC, Gilbert JA. Healing of experimental muscle strains and the effects of nonsteroidal antiinflammatory medication. Am J Sports Med 14: 303–308, 1986 [DOI] [PubMed] [Google Scholar]
- 5. Alway SE, Degens H, Krishnamurthy G, Smith CA. Potential role for Id myogenic repressors in apoptosis and attenuation of hypertrophy in muscles of aged rats. Am J Physiol Cell Physiol 283: C66–C76, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Barani AE, Durieux AC, Sabido O, Freyssenet D. Age-related changes in the mitotic and metabolic characteristics of muscle-derived cells. J Appl Physiol 95: 2089–2098, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Barlow Y, Willoughby J. Pathophysiology of soft tissue repair. Br Med Bull 48: 698–711, 1992 [DOI] [PubMed] [Google Scholar]
- 8. Bischoff R. Proliferation of muscle satellite cells on intact myofibers in culture. Dev Biol 115: 129–139, 1986 [DOI] [PubMed] [Google Scholar]
- 9. Bischoff R. A satellite cell mitogen from crushed adult muscle. Dev Biol 115: 140–147, 1986 [DOI] [PubMed] [Google Scholar]
- 10. Bischoff R, Heintz C. Enhancement of skeletal muscle regeneration. Dev Dyn 201: 41–54, 1994 [DOI] [PubMed] [Google Scholar]
- 11. Blotnick S, Peoples GE, Freeman MR, Eberlein TJ, Klagsbrun M. T lymphocytes synthesize and export heparin-binding epidermal growth factor-like growth factor and basic fibroblast growth factor, mitogens for vascular cells and fibroblasts: differential production and release by CD4+ and CD8+ T cells. Proc Natl Acad Sci USA 91: 2890–2894, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, Rando TA. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science 317: 807–810, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Braun T, Buschhausen-Denker G, Bober E, Tannich E, Arnold HH. A novel human muscle factor related to but distinct from MyoD1 induces myogenic conversion in 10T1/2 fibroblasts. EMBO J 8: 701–709, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brooks SV, Faulkner JA. Contraction-induced injury: recovery of skeletal muscles in young and old mice. Am J Physiol Cell Physiol 258: C436–C442, 1990 [DOI] [PubMed] [Google Scholar]
- 15. Chakravarthy MV, Davis BS, Booth FW. IGF-I restores satellite cell proliferative potential in immobilized old skeletal muscle. J Appl Physiol 89: 1365–1379, 2000 [DOI] [PubMed] [Google Scholar]
- 16. Cheng M, Nguyen MH, Fantuzzi G, Koh TJ. Endogenous interferon-gamma is required for efficient skeletal muscle regeneration. Am J Physiol Cell Physiol 294: C1183–C1191, 2008 [DOI] [PubMed] [Google Scholar]
- 17. Conboy IM, Conboy MJ, Smythe GM, Rando TA. Notch-mediated restoration of regenerative potential to aged muscle. Science 302: 1575–1577, 2003 [DOI] [PubMed] [Google Scholar]
- 18. Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 433: 760–764, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Conboy IM, Rando TA. Aging, stem cells and tissue regeneration: lessons from muscle. Cell Cycle 4: 407–410, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Cook DR, Doumit ME, Merkel RA. Transforming growth factor-beta, basic fibroblast growth factor, and platelet-derived growth factor-BB interact to affect proliferation of clonally derived porcine satellite cells. J Cell Physiol 157: 307–312, 1993 [DOI] [PubMed] [Google Scholar]
- 21. Cornelison DD. Context matters: in vivo and in vitro influences on muscle satellite cell activity. J Cell Biochem 105: 663–669, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cornelison DD, Wold BJ. Single-cell analysis of regulatory gene expression in quiescent and activated mouse skeletal muscle satellite cells. Dev Biol 191: 270–283, 1997 [DOI] [PubMed] [Google Scholar]
- 23. Corsetti G, Pasini E, D'Antona G, Nisoli E, Flati V, Assanelli D, Dioguardi FS, Bianchi R. Morphometric changes induced by amino acid supplementation in skeletal and cardiac muscles of old mice. Am J Cardiol 101: 26E–34E, 2008 [DOI] [PubMed] [Google Scholar]
- 24. De Rosa SC, Lu FX, Yu J, Perfetto SP, Falloon J, Moser S, Evans TG, Koup R, Miller CJ, Roederer M. Vaccination in humans generates broad T cell cytokine responses. J Immunol 173: 5372–5380, 2004 [DOI] [PubMed] [Google Scholar]
- 25. DiMario J, Buffinger N, Yamada S, Strohman RC. Fibroblast growth factor in the extracellular matrix of dystrophic (mdx) mouse muscle. Science 244: 688–690, 1989 [DOI] [PubMed] [Google Scholar]
- 26. Farini A, Meregalli M, Belicchi M, Battistelli M, Parolini D, D'Antona G, Gavina M, Ottoboni L, Constantin G, Bottinelli R, Torrente Y. T and B lymphocyte depletion has a marked effect on the fibrosis of dystrophic skeletal muscles in the scid/mdx mouse. J Pathol 213: 229–238, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Fasth AE, Dastmalchi M, Rahbar A, Salomonsson S, Pandya JM, Lindroos E, Nennesmo I, Malmberg KJ, Soderberg-Naucler C, Trollmo C, Lundberg IE, Malmstrom V. T cell infiltrates in the muscles of patients with dermatomyositis and polymyositis are dominated by CD28null T cells. J Immunol 183: 4792–4799, 2009 [DOI] [PubMed] [Google Scholar]
- 28. Fava RA, Olsen NJ, Postlethwaite AE, Broadley KN, Davidson JM, Nanney LB, Lucas C, Townes AS. Transforming growth factor beta 1 (TGF-beta 1) induced neutrophil recruitment to synovial tissues: implications for TGF-beta-driven synovial inflammation and hyperplasia. J Exp Med 173: 1121–1132, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Florini JR, Ewton DZ, Magri KA. Hormones, growth factors, and myogenic differentiation. Annu Rev Physiol 53: 201–216, 1991 [DOI] [PubMed] [Google Scholar]
- 30. Fritsch RD, Shen X, Sims GP, Hathcock KS, Hodes RJ, Lipsky PE. Stepwise differentiation of CD4 memory T cells defined by expression of CCR7 and CD27. J Immunol 175: 6489–6497, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Goldspink G, Fernandes K, Williams PE, Wells DJ. Age-related changes in collagen gene expression in the muscles of mdx dystrophic and normal mice. Neuromuscul Disord 4: 183–191, 1994 [DOI] [PubMed] [Google Scholar]
- 32. Griffiths RD. Muscle mass, survival, and the elderly ICU patient. Nutrition 12: 456–458, 1996 [DOI] [PubMed] [Google Scholar]
- 33. Hathaway MR, Pampusch MS, Hembree JR, Dayton WR. Transforming growth factor beta-1 facilitates establishing clonal populations of ovine muscle satellite cells. J Anim Sci 72: 2001–2007, 1994 [DOI] [PubMed] [Google Scholar]
- 34. Hawke TJ, Garry DJ. Myogenic satellite cells: physiology to molecular biology. J Appl Physiol 91: 534–551, 2001 [DOI] [PubMed] [Google Scholar]
- 35. Hidestrand M, Richards-Malcolm S, Gurley CM, Nolen G, Grimes B, Waterstrat A, Zant GV, Peterson CA. Sca-1-expressing nonmyogenic cells contribute to fibrosis in aged skeletal muscle. J Gerontol A Biol Sci Med Sci 63: 566–579, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jump SS, Childs TE, Zwetsloot KA, Booth FW, Lees SJ. Fibroblast growth factor 2-stimulated proliferation is lower in muscle precursor cells from old rats. Exp Physiol 94: 739–748, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Krug N, Madden J, Redington AE, Lackie P, Djukanovic R, Schauer U, Holgate ST, Frew AJ, Howarth PH. T-cell cytokine profile evaluated at the single cell level in BAL and blood in allergic asthma. Am J Respir Cell Mol Biol 14: 319–326, 1996 [DOI] [PubMed] [Google Scholar]
- 38. Lees SJ, Childs TE, Booth FW. Age-dependent FOXO regulation of p27Kip1 expression via a conserved binding motif in rat muscle precursor cells. Am J Physiol Cell Physiol 295: C1238–C1246, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lees SJ, Childs TE, Booth FW. p21(Cip1) expression is increased in ambient oxygen, compared to estimated physiological (5%) levels in rat muscle precursor cell culture. Cell Prolif 41: 193–207, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lees SJ, Rathbone CR, Booth FW. Age-associated decrease in muscle precursor cell differentiation. Am J Physiol Cell Physiol 290: C609–C615, 2006 [DOI] [PubMed] [Google Scholar]
- 41. Lees SJ, Zwetsloot KA, Booth FW. Muscle precursor cells isolated from aged rats exhibit an increased tumor necrosis factor- alpha response. Aging Cell 8: 26–35, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lefaucheur JP, Sebille A. Basic fibroblast growth factor promotes in vivo muscle regeneration in murine muscular dystrophy. Neurosci Lett 202: 121–124, 1995 [DOI] [PubMed] [Google Scholar]
- 43. Levings MK, Bacchetta R, Schulz U, Roncarolo MG. The role of IL-10 and TGF-beta in the differentiation and effector function of T regulatory cells. Int Arch Allergy Immunol 129: 263–276, 2002 [DOI] [PubMed] [Google Scholar]
- 44. Levings MK, Sangregorio R, Sartirana C, Moschin AL, Battaglia M, Orban PC, Roncarolo MG. Human CD25+CD4+ T suppressor cell clones produce transforming growth factor beta, but not interleukin 10, and are distinct from type 1 T regulatory cells. J Exp Med 196: 1335–1346, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lorenzon P, Bandi E, de Guarrini F, Pietrangelo T, Schafer R, Zweyer M, Wernig A, Ruzzier F. Ageing affects the differentiation potential of human myoblasts. Exp Gerontol 39: 1545–1554, 2004 [DOI] [PubMed] [Google Scholar]
- 46. Machida S, Booth FW. Increased nuclear proteins in muscle satellite cells in aged animals as compared to young growing animals. Exp Gerontol 39: 1521–1525, 2004 [DOI] [PubMed] [Google Scholar]
- 47. Mendias CL, Tatsumi R, Allen RE. Role of cyclooxygenase-1 and -2 in satellite cell proliferation, differentiation, and fusion. Muscle Nerve 30: 497–500, 2004 [DOI] [PubMed] [Google Scholar]
- 48. Merrick MA. Secondary injury after musculoskeletal trauma: a review and update. J Athl Train 37: 209–217, 2002 [PMC free article] [PubMed] [Google Scholar]
- 49. Mezzogiorno A, Coletta M, Zani BM, Cossu G, Molinaro M. Paracrine stimulation of senescent satellite cell proliferation by factors released by muscle or myotubes from young mice. Mech Ageing Dev 70: 35–44, 1993 [DOI] [PubMed] [Google Scholar]
- 50. Michalopoulos GK, Zarnegav R. Hepatocyte growth factor. Hepatology 15: 149–155, 1992 [DOI] [PubMed] [Google Scholar]
- 51. Mikkelsen UR, Langberg H, Helmark IC, Skovgaard D, Andersen LL, Kjaer M, Mackey AL. Local NSAID infusion inhibits satellite cell proliferation in human skeletal muscle after eccentric exercise. J Appl Physiol 107: 1600–1611, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Miner JH, Wold B. Herculin, a fourth member of the MyoD family of myogenic regulatory genes. Proc Natl Acad Sci USA 87: 1089–1093, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mishra DK, Friden J, Schmitz MC, Lieber RL. Anti-inflammatory medication after muscle injury. A treatment resulting in short-term improvement but subsequent loss of muscle function. J Bone Joint Surg Am 77: 1510–1519, 1995 [DOI] [PubMed] [Google Scholar]
- 54. Morgan JE, Partridge TA. Muscle satellite cells. Int J Biochem Cell Biol 35: 1151–1156, 2003 [DOI] [PubMed] [Google Scholar]
- 55. Morrison J, Lu QL, Pastoret C, Partridge T, Bou-Gharios G. T-cell-dependent fibrosis in the mdx dystrophic mouse. Lab Invest 80: 881–891, 2000 [DOI] [PubMed] [Google Scholar]
- 56. Morrison J, Partridge T, Bou-Gharios G. Nude mutation influences limb skeletal muscle development. Matrix Biol 23: 535–542, 2005 [DOI] [PubMed] [Google Scholar]
- 57. Muir AR, Kanji AH, Allbrook D. The structure of the satellite cells in skeletal muscle. J Anat 99: 435–444, 1965 [PMC free article] [PubMed] [Google Scholar]
- 58. Muraguchi A, Hirano T, Tang B, Matsuda T, Horii Y, Nakajima K, Kishimoto T. The essential role of B cell stimulatory factor 2 (BSF-2/IL-6) for the terminal differentiation of B cells. J Exp Med 167: 332–344, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Pinney DF, Pearson-White SH, Konieczny SF, Latham KE, Emerson CP., Jr Myogenic lineage determination and differentiation: evidence for a regulatory gene pathway. Cell 53: 781–793, 1988 [DOI] [PubMed] [Google Scholar]
- 60. Rantanen J, Hurme T, Lukka R, Heino J, Kalimo H. Satellite cell proliferation and the expression of myogenin and desmin in regenerating skeletal muscle: evidence for two different populations of satellite cells. Lab Invest 72: 341–347, 1995 [PubMed] [Google Scholar]
- 61. Rhodes SJ, Konieczny SF. Identification of MRF4: a new member of the muscle regulatory factor gene family. Genes Dev 3: 2050–2061, 1989 [DOI] [PubMed] [Google Scholar]
- 62. Rudnicki MA, Le Grand F, McKinnell I, Kuang S. The molecular regulation of muscle stem cell function. Cold Spring Harb Symp Quant Biol 73: 323–331, 2008 [DOI] [PubMed] [Google Scholar]
- 63. Schaerli P, Britschgi M, Keller M, Steiner UC, Steinmann LS, Moser B, Pichler WJ. Characterization of human T cells that regulate neutrophilic skin inflammation. J Immunol 173: 2151–2158, 2004 [DOI] [PubMed] [Google Scholar]
- 64. Schultz E, Jaryszak DL, Valliere CR. Response of satellite cells to focal skeletal muscle injury. Muscle Nerve 8: 217–222, 1985 [DOI] [PubMed] [Google Scholar]
- 65. Sheehan SM, Tatsumi R, Temm-Grove CJ, Allen RE. HGF is an autocrine growth factor for skeletal muscle satellite cells in vitro. Muscle Nerve 23: 239–245, 2000 [DOI] [PubMed] [Google Scholar]
- 66. Smith C, Kruger MJ, Smith RM, Myburgh KH. The inflammatory response to skeletal muscle injury: illuminating complexities. Sports Med 38: 947–969, 2008 [DOI] [PubMed] [Google Scholar]
- 67. Spencer MJ, Montecino-Rodriguez E, Dorshkind K, Tidball JG. Helper (CD4(+)) and cytotoxic (CD8(+)) T cells promote the pathology of dystrophin-deficient muscle. Clin Immunol 98: 235–243, 2001 [DOI] [PubMed] [Google Scholar]
- 68. Spencer MJ, Tidball JG. Do immune cells promote the pathology of dystrophin-deficient myopathies? Neuromuscul Disord 11: 556–564, 2001 [DOI] [PubMed] [Google Scholar]
- 69. Tatsumi R, Liu X, Pulido A, Morales M, Sakata T, Dial S, Hattori A, Ikeuchi Y, Allen RE. Satellite cell activation in stretched skeletal muscle and the role of nitric oxide and hepatocyte growth factor. Am J Physiol Cell Physiol 290: C1487–C1494, 2006 [DOI] [PubMed] [Google Scholar]
- 70. Tidball JG. Inflammatory processes in muscle injury and repair. Am J Physiol Regul Integr Comp Physiol 288: R345–R353, 2005 [DOI] [PubMed] [Google Scholar]
- 71. Tidball JG, Villalta SA. Regulatory interactions between muscle and the immune system during muscle regeneration. Am J Physiol Regul Integr Comp Physiol 298: R1173–R1187, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Vetrone SA, Montecino-Rodriguez E, Kudryashova E, Kramerova I, Hoffman EP, Liu SD, Miceli MC, Spencer MJ. Osteopontin promotes fibrosis in dystrophic mouse muscle by modulating immune cell subsets and intramuscular TGF-beta. J Clin Invest 119: 1583–1594, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Zanin-Zhorov A, Cahalon L, Tal G, Margalit R, Lider O, Cohen IR. Heat shock protein 60 enhances CD4+ CD25+ regulatory T cell function via innate TLR2 signaling. J Clin Invest 116: 2022–2032, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]