Abstract
Female reproductive tissues such as mammary glands, ovaries, uterus, and placenta are phenotypically dynamic, requiring tight integration of bioenergetic and apoptotic mechanisms. Mitochondrial zinc (Zn) pools have emerged as a central player in regulating bioenergetics and apoptosis. Zn must first be imported into mitochondria to modulate mitochondrion-specific functions; however, mitochondrial Zn import mechanisms have not been identified. Here we documented that the Zn transporter ZnT2 is associated with the inner mitochondrial membrane and acts as an auxiliary Zn importer into mitochondria in mammary cells. We found that attenuation of ZnT2 expression significantly reduced mitochondrial Zn uptake and total mitochondrial Zn pools. Moreover, expression of a ZnT2-hemagglutinin (HA) fusion protein was localized to mitochondria and significantly increased Zn uptake and mitochondrial Zn pools, directly implicating ZnT2 in Zn import into mitochondria. Confocal microscopy of truncated and point mutants of ZnT2-green fluorescent protein (GFP) fusion proteins revealed a histidine-rich motif (51HHXH54) in the NH2 terminus that is important for mitochondrial targeting of ZnT2. More importantly, the expansion of mitochondrial Zn pools by ZnT2 overexpression significantly reduced ATP biogenesis and mitochondrial oxidation concurrent with increased apoptosis, suggesting a functional role for ZnT2-mediated Zn import into mitochondria. These results identify the first Zn transporter directly associated with mitochondria and suggest that unique secretory tissues such as the mammary gland require novel mechanisms to modulate mitochondrion-specific functions.
Keywords: zinc transporter, mitochondrial targeting, adenosine 5′-triphosphate biogenesis, apoptosis
female reproductive tissues such as mammary glands, ovaries, uterus, and placenta are phenotypically dynamic. As such, mammary glands differentiate from a nonsecreting, proliferative tissue to a highly differentiated secretory tissue during lactation. After weaning, this tissue involutes back to a nonsecreting, proliferative tissue. These changes require dramatic alterations in both bioenergetic and apoptotic mechanisms. During lactation, mammary glands are highly metabolically active and energetically demanding because of expanding tissue mass and nutrient production and secretion. After lactation ceases, weaning results in extensive apoptosis of mammary epithelial cells (MECs), reorganizing the tissue back to the prepregnant phenotypic state. Thus both bioenergetic and apoptotic events must be coordinated in a regulated manner to modulate these phenotypic dynamics.
Mitochondrial Zn pools play a central role in regulating bioenergetics and apoptosis. Zn treatment of hepatocytes depletes ATP (20). In addition to the effects of Zn on ATP generation, Zn accumulation in prostate cells (9) and neurons (27) activates apoptosis through the mitochondrial release of cytochrome c followed by caspase activation. Furthermore, Zn accumulation in mitochondria is also associated with mitochondrial depolarization, dysfunction, and cell death following neuronal ischemia (15). Thus the regulation of mitochondrial Zn expansion may assist in coordinating phenotype dynamics in the mammary gland. To regulate mitochondrial ATP production and apoptosis, Zn must first be imported into mitochondria. However, mitochondrial Zn import mechanisms have not been identified.
Zn transporters are expressed with cell and species specificity (22). Abundant expression of the Zn transporter ZnT2 is detectable in unique secretory and reproductive tissues such as the pancreas, prostate gland, placenta, ovary, and mammary glands (16). We previously determined that ZnT2 plays a major role in mammary gland Zn secretion into milk during lactation following the identification of a missense mutation in the gene that encodes human ZnT2 (SLC30A2) that substitutes an arginine for a highly conserved histidine residue (H54R) in the NH2-terminal domain. This substitution reduced Zn secretion into milk in afflicted women by ∼75% (3). Our recent studies indicate that ZnT2 is transcriptionally regulated by the lactogenic hormone prolactin (31) to facilitate Zn accumulation into exocytotic vesicles in secreting MECs (24). This clearly reflects the key role ZnT2 plays in Zn metabolism in the lactating mammary gland.
In addition to playing a major role in Zn secretion during lactation, ZnT2 expression is robustly detected in nonlactating mammary gland and nonsecreting MECs (31). This strongly suggests that ZnT2 plays a role in Zn metabolism of nonsecreting mammary cells independent of its role in Zn secretion. Here we report that ZnT2 is specifically localized to the inner mitochondrial membrane of MECs and facilitates Zn import into the matrix. Confocal microscopy of truncated and point mutants of NH2-terminal ZnT2-green fluorescent protein (GFP) fusion proteins provides evidence that an NH2-terminal histidine-rich motif is important in mitochondrial targeting of ZnT2. Critically, the expansion of mitochondrial Zn pools by ZnT2 overexpression significantly reduced ATP biogenesis and activated apoptosis, suggesting a functional role for ZnT2-mediated Zn import into mitochondria. These results identify the first Zn transporter directly associated with mitochondria and suggest that female reproductive tissues such as the mammary gland require novel mechanisms to modulate mitochondrion-specific functions such as ATP production and apoptosis.
MATERIALS AND METHODS
Cell culture.
Mouse MECs (HC11) were a gift from Dr. Jeffery Rosen (Baylor College of Medicine, Houston, TX) and used with permission of Dr. Bernd Groner (Institute for Biomedical Research, Frankfurt, Germany). Cells were maintained in “growth medium” [RPMI 1640 supplemented with 10% fetal bovine serum, insulin (5 μg/ml), epidermal growth factor (EGF; 10 ng/ml), and gentamycin].
Subcellular localization of ZnT2.
To identify the subcellular localization of endogenous ZnT2 in nonsecreting MECs, cells were seeded onto glass coverslips and cultured overnight until ∼80% confluence. Cells were fixed in phosphate-buffered paraformaldehyde (4%), pH 7.4, for 10 min, washed in PBS, and briefly permeabilized with Triton X-100 (0.2% in PBS). Nonspecific binding was blocked with 5% goat serum-1% bovine serum albumin in PBS for 20 min, followed by incubation with affinity-purified ZnT2 antibody (1 μg/ml) for 60 min at room temperature. ZnT2 antibody was affinity purified as previously described (24). ZnT2 antibody was detected with Alexa 488-conjugated anti-rabbit IgG (1 μg/ml; Invitrogen) for 45 min at room temperature and shielded from light. Mitochondria were visualized by indirect immunofluorescence using antibody directed against cytochrome-c oxidase subunit IV (COX IV; 1 μg/ml) and detected with Alexa 568-conjugated anti-mouse IgG (1 μg/ml; Invitrogen) or stained with MitoTracker (Invitrogen) diluted in Opti-MEM for 45 min according to the manufacturer's instructions. Immunofluorescent imaging was performed with an Olympus BX50WI with UPlanApo ×100 oil lens NA 1.35 (LaserSharp2000, version 4.1; Bio-Rad). Localization of hemagglutinin (HA)-tagged ZnT2 in mitochondria was verified by immunofluorescent detection of HA-tagged ZnT2 with rabbit anti-HA antibody (1 μg/ml; Invitrogen) and visualization with Alexa 568-conjugated IgG as described above.
Isolation and purification of mitochondria and mitoplasts.
To verify mitochondria localization of ZnT2, cells were scraped into ice-cold MB buffer (in mM: 10 HEPES, pH 7.5, 210 mannitol, 70 sucrose, 1 EDTA) and then Dounce homogenized with a glass homogenizer. The cell extract was centrifuged at 2,000 g for 5 min at 4°C, and the crude mitochondrial fraction was pelleted by centrifugation at 12,000 g for 12 min at 4°C. The crude mitochondrial fraction was resuspended in MB buffer, gently homogenized on ice, and then purified on a discontinuous Nycodenz gradient (22.5% and 9.5% diluted into MB buffer) by centrifugation at 141,000 g for 1 h at 4°C. Purified mitochondria were washed twice in MB buffer by centrifugation at 12,000 g for 12 min. To generate mitochondria devoid of the outer mitochondrial membrane (mitoplasts), purified mitochondria were hypotonically lysed in water on ice for 20 min and pelleted by centrifugation at 15,000 g for 15 min. To eliminate contaminating subcellular membranes, intact mitochondria or mitoplasts (10 μg) were rigorously digested with an equal amount of proteinase K (10 μg) on ice for 20 min. Enzymatic activity was inactivated with PMSF (20 mM) for 20 min on ice, and mitochondria or mitoplasts were repelleted by centrifugation.
Small interfering RNA-mediated gene suppression.
Cells were plated in antibiotic-free Opti-MEM medium in 10-cm dishes (8 × 105 cells/dish) and cultured overnight until ∼50% confluent. Cells were transfected with SLC30A2-specific small interfering RNA (siRNA) (sense: 5′-CUGCAUAUCUGGGCGCUGA-3′, antisense: 5′-UCAGCGCCCAGAUAUGCAG-3′; Sigma-Aldrich) or mismatched control siRNA (sense: 5′-CCGCGUCCUUCCUUAUGUAGGAAUU-3′, antisense: 5′-AAUUCCUACAUAAGGAAGGACGCGG-3′; Invitrogen) in antibiotic-free Opti-MEM medium with Lipofectamine 2000 (Invitrogen) to attenuate ZnT2 expression. Transfection medium was replaced with serum-containing, antibiotic-free medium after 6 h, and cells were cultured for another 16 h before experiments. Cells were collected and mitochondria were isolated as described above for determination of mitochondrial ZnT2 abundance, 65Zn uptake, and mitochondrial Zn pools with the mitochondrial Zn-specific fluorophore RhodZin-3 AM (see below).
ZnT2 overexpression.
To verify the expression of ZnT2 in mitochondria, a COOH-terminal tandem HA-tagged ZnT2 plasmid was generated as previously described (3). HC11 cells were seeded in antibiotic-free growth medium in six-well plates for total crude membrane isolation or in 10-cm2 dishes for mitochondria isolation and cultured overnight until ∼95% confluent. The cells were transfected with 8 μg (6-well plates) or 48 μg (10 cm2 dishes) of HA-tagged ZnT2 plasmid in serum- and antibiotic-free growth medium with Lipofectamine 2000 at a DNA-to-transfection reagent ratio of 1:2.5 according to the manufacturer's specifications. Transfection medium was replaced 4–6 h later with antibiotic-free growth medium, and cells were cultured for an additional 24 h before experiments.
Truncated and mutated ZnT2 expression plasmid construction.
The NH2 terminus deletion mutant of ZnT2 (Δ2–50 ZnT2) was generated by PCR amplification of ZnT2 cDNA using primers 5′-CCACCATGCTGTATGTAGCC-3′ (containing an ATG codon and upstream Kozak consensus sequence for translation initiation) and 5′-CATCAGGCGTAGTCGGGGAC-3′ (containing the epitope tag and a terminal stop codon) and cloned into the pcDNA3.1/V5-His TOPO vector (Invitrogen). Mutant variants (3Mut-HA) were constructed with the QuickChange site-directed mutagenesis kit (Stratagene, La Jolla, CA) according to the manufacturer's protocol. To test whether the NH2-terminal region of ZnT2 contained a mitochondrial targeting sequence, we constructed fusion proteins containing the NH2-terminal region of ZnT2 [(1–73)ZnT2-GFP] or 3Mut [(1–73)3Mut-GFP] fused to GFP with PCR amplification. To generate the truncated NH2-terminal fragments, the primers for each fragment were designed to have a HindIII or SacII site at the 5′- and 3′-ends, respectively. Each DNA fragment was amplified and digested with HindIII and SacII restriction enzymes, followed by ligation in pEGFP-N1 vector (Clontech) cut with the same enzyme. The constructs were introduced into the pEGFP-N1 vector and fused to GFP. The sequences of all constructs were confirmed by directed sequencing (Pennsylvania State University Nucleic Acid Facility).
Immunoblotting.
Crude membrane proteins from mouse mammary gland or HC11 cells were isolated and ZnT2 was detected by immunoblotting as previously described (17, 18, 24). Membranes were stripped and reprobed for calnexin [endoplasmic reticulum (ER) marker, Abcam], lysosome-associated membrane protein 1 (LAMP1, lysosomal marker; Abcam), early endosomal antigen 1 (EEA1, early endosomal marker; Abcam), mannose 6-phosphate receptor (M6PR, late endosomal marker; Abcam), COX IV (mitochondrial inner membrane marker, Invitrogen), and voltage-dependent anion channel (VDAC)/porin (mitochondrial outer membrane marker; Abcam). Primary antibody was detected with horseradish peroxidase-conjugated IgG, and proteins were visualized by enhanced chemiluminescence (SuperSignal Femto, Pierce) after exposure to autoradiography film.
Zn uptake in mitoplasts.
To verify the role of ZnT2 in mitochondrial Zn transport in vitro, isolated mitoplasts from ZnT2-attenuated, ZnT2-overexpressing, or mock-transfected (control) HC11 cells were resuspended in serum-free growth medium (0.25–0.5 ml). Zn uptake into mitoplasts was assessed after addition of serum-free culture medium containing 0.1–1 μM ZnSO4 and 0.1 μCi 65Zn (50 mCi/mg; Oak Ridge National Laboratory, Oak Ridge, TN) and incubation at 37°C for up to 60 min. Mitoplasts were pelleted by centrifugation at 15,000 g for 15 min and washed two times with ice-cold PBS-EDTA (1 mM). Radioactivity in the mitoplast pellet was quantified in a gamma scintillation counter (Packard Minaxi-γ, Meriden, CT).
Assessment of changes in Zn pools in mitochondria from live cells.
RhodZin-3 AM is a high-affinity (∼65 nM), Zn-specific fluorophore that reacts with labile Zn specifically in mitochondria (35). RhodZin-3 fluorescence was used to quantify changes in mitochondrial Zn pools in vivo in ZnT2-attenuated and ZnT2-overexpressing HC11 cells. Cells were rinsed twice with PBS and then loaded with RhodZin-3 AM (1 μM in DMSO containing Pluronic acid 127 to a final concentration of 0.02%; Invitrogen) according to the manufacturer's instructions in Opti-MEM for 1 h at 37°C. Cells were briefly rinsed twice with PBS and washed with PBS for 30 min at 25°C with constant shaking. RhodZin-3 (emission 550 nm, excitation 575 nm) fluorescence was measured at 25°C with a FLUOstar OPTIMA plate reader (BMG Labtech, Budapest, Hungary) spectrofluorimeter. Protein concentration was measured with the Bradford assay, and fluorescence was normalized to total cellular protein.
Visualization of (1–73)ZnT2-GFP and (1–73)3Mut-GFP localization by confocal microscopy.
Cells were transfected as described above and then incubated with MitoTracker (Invitrogen) diluted in Opti-MEM for 45 min. The GFP images were captured by excitation at 488 nm and emission at 510–540 nm. The MitoTracker images were captured by excitation at 568 nm and emission at 600–640 nm. Images were collected as described above.
Intracellular ATP level.
The Enliten ATP Assay System (Promega, Madison, WI) was used for ATP measurement. Cells were transfected for 24 h as described above and transferred to microfuge tubes. Protein was precipitated with 50 μl of TCA (2.5%); samples were neutralized to pH 7.4 with 10 μl of 4 M Tris and diluted 10-fold with ATP-free water. The luciferase reagent was added 1 s before a 5-s measurement in the luminometer (Promega), as suggested by the supplier. Light photons were measured by a luminometer and were compared against a standard curve to calculate ATP concentration. Protein content in the supernatant was determined by the Bradford assay. The ATP level was normalized relative to total protein content. ATP content is expressed as picomoles per milligram of protein.
MTT mitochondrial oxidation assay.
Mitochondrial metabolic function was assessed by the conversion of the dye 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) to formazan. This assay is based on the ability of the mitochondrial enzyme succinate dehydrogenase to metabolize MTT into formazan. The cells were incubated for 4 h with MTT (5 mg/ml), and then MTT solvent (isopropyl alcohol containing 0.1 N HCl) was added to dissolve the formazan formed. The resulting formazan was quantified spectrophotometrically at 570 nm, and the background was subtracted at 690 nm with a plate reader (BioTek). Cellular protein concentration was determined by the Bradford assay, and optical density was normalized to total protein concentration.
In situ localization of apoptotic cells.
Staining of apoptotic cells was done with annexin V-FITC (Trevigen, Gaithersburg, MD) according to the manufacturer's instructions. Cells were transfected as described above and then stained with annexin V-FITC. Glass coverslips were mounted onto glass slides with ProLong Gold (Invitrogen), visualized at ×10 magnification with an Epi-Fluorescence microscope with a FITC Filter Cube (Olympus BX61 Epi-Fluorescence Microscope, Center Valley, PA), and imaged with SlideBook 4.1 software.
Assessment of ZnT2 topology.
To assess the topology of mitochondrial ZnT2, isolated mitochondria were preincubated with affinity-purified ZnT2 antibody (1–20 μg/ml; directed against the putative COOH-terminal domain) or goat anti-rabbit IgG (1–20 μg/ml; negative control) for 30 min at room temperature before measurement of 65Zn uptake. To confirm the topology of ZnT2 within the inner mitochondrial membrane, the Fluorescence Biotin Quantitation kit (Pierce) was used to detect the presence of biotinylated antibody associated with mitochondria. Briefly, mitochondria and mitoplasts were purified from cells ectopically expressing ZnT2-HA and incubated with either biotinylated mouse anti-HA antibody (10 μg/ml) or mouse IgG (control antibody; 10 μg/ml) for 30 min at room temperature. Unbound antibody was removed by washing twice with PBS, and mitochondria and mitoplasts were pelleted by centrifugation at 15,000 g for 15 min. Biotin was detected with the Fluorescence Biotin Quantitation Kit as instructed by the manufacturer. Fluorescence (emission 494 nm, excitation 520 nm) was measured at 25°C with a FLUOstar OPTIMA plate reader (BMG Labtech) spectrofluorimeter and compared against a biocytin standard curve to calculate the amount of biotin associated with each fraction. Protein concentration was determined with the Bradford assay, and fluorescence measurements were normalized to mitochondria/mitoplast protein concentration. Biotin content is expressed as picomoles per microgram of protein.
Statistical analysis.
Results are presented as means ± SD. A minimum of at least two independent experiments were conducted with sample sizes as indicated. Statistical comparisons were performed with Student's t-test or one-way ANOVA where appropriate (Prism Graph Pad, Berkeley, CA), and a significant difference was demonstrated at P < 0.05. Estimation of colocalization between subcellular markers and ZnT2 was calculated with Pearson's coefficient where indicated. The value of Pearson's coefficient can range from +1 to −1, with +1 illustrating a positive correlation, −1 illustrating a negative correlation, and 0 illustrating no correlation.
RESULTS
ZnT2 is associated with inner mitochondrial membrane in MECs.
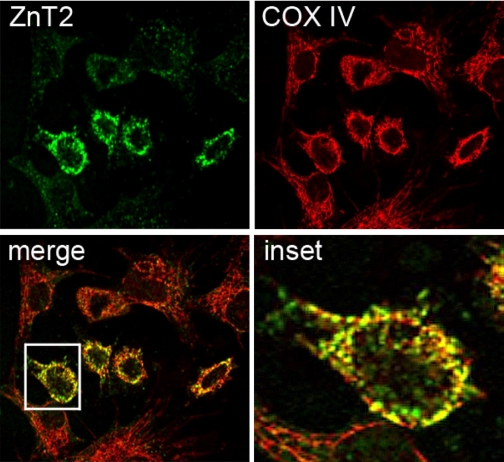
We previously identified two discrete ZnT2 isoforms that transport Zn either directly across the cell membrane or into exocytotic secretory vesicles in secreting MECs (24). However, ZnT2 is also abundantly expressed in nonsecreting MECs (31) and nonlactating (nonsecreting) mammary gland (Fig. 1). Confocal microscopy revealed that ZnT2 often displayed a distinct, reticular staining pattern in nonsecreting mammary cells (Fig. 2). This staining pattern was reminiscent of mitochondrial morphology. Partial colocalization of ZnT2 with COX IV strongly suggested that ZnT2 was associated with mitochondria. It is important to note that exclusive localization of ZnT2 to mitochondria was never observed.
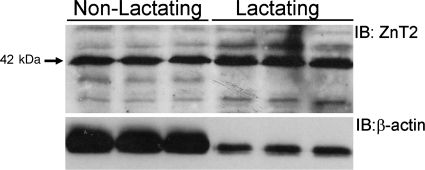
Fig. 1.
ZnT2 is abundantly expressed in the nonlactating mammary gland: representative immunoblot of ZnT2 detected in total crude membrane proteins (50 μg) isolated from nonlactating and lactating mouse mammary gland. Membranes were stripped and immunoblotted (IB) with antibody against β-actin.
Fig. 2.
Distinct localization of endogenous ZnT2 in mitochondria in nonsecreting mammary epithelial cells. Representative laser confocal micrographs suggesting the partial colocalization (merge; yellow) of ZnT2 (green) and the mitochondrial marker cytochrome-c oxidase subunit IV (COX IV; red) in nonsecreting mammary cells (Pearson's coefficient = 0.8). Inset illustrates distinct overlap (yellow) between ZnT2 and COX IV.
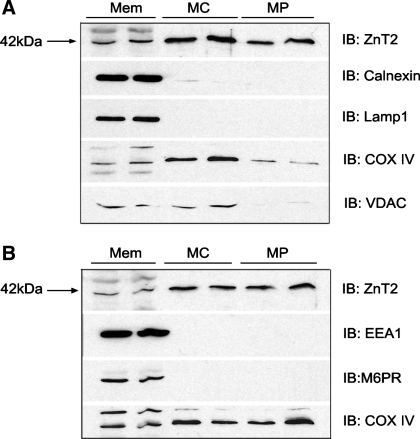
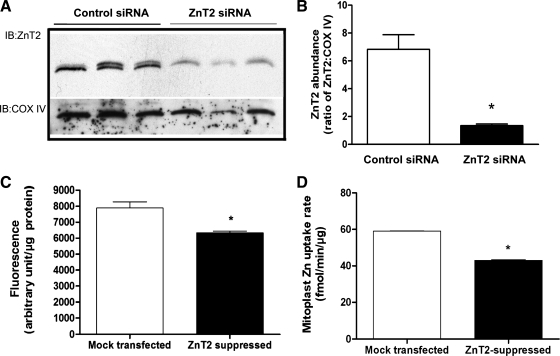
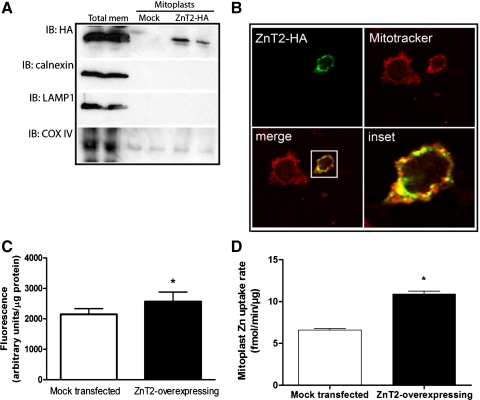
To verify mitochondrial localization of ZnT2, intact mitochondria and mitoplasts (mitochondria devoid of the outer mitochondrial membrane) were isolated and digested with proteinase K and mitochondrial proteins were immunoblotted for ZnT2. We first confirmed the lack of contamination in the mitochondrial fraction of ER, lysosomes, early and late endosomal membranes, and outer mitochondrial membrane as our purified mitochondria and mitoplast fractions showed no presence of calnexin, LAMP1, EEA1, M6PR, or VDAC/porin, respectively (Fig. 3). Importantly, robust detection of ZnT2 was observed in highly purified mitochondria and mitoplasts (Fig. 3). To confirm that the protein we detected in inner mitochondrial membrane was a specific product of the gene that encodes ZnT2 (SLC30A2), we transfected cells with SLC30A2 siRNA. ZnT2 protein abundance in isolated, purified mitoplasts was significantly attenuated by ∼70% (Fig. 4, A and B).
Fig. 3.
Detection of ZnT2 in purified mitochondria and mitoplasts from nonsecreting MECs. A: representative immunoblot of ZnT2 detected in total crude membranes (Mem, 20 μg), purified mitochondria (MC; 20 μg protein), and purified mitoplasts (MP; 20 μg protein). Membranes were stripped and immunoblotted with antibodies against calnexin [endoplasmic reticulum (ER) membrane marker], lysosome-associated membrane protein 1 (Lamp1; lysosome membrane marker), COX IV (mitochondria inner membrane marker), and voltage-dependent anion channel (VDAC; mitochondrial outer membrane marker). B: representative immunoblot of ZnT2 detected in total crude membranes (20 μg), purified mitochondria (20 μg protein), and purified mitoplasts (20 μg protein). Membranes were stripped and immunoblotted with antibodies against early endosomal antigen 1 (EEA1; early endosomal membrane marker), mannose 6-phosphate receptor (M6PR; late endosomal membrane marker), and COX IV (mitochondria marker).
Fig. 4.
Mitochondrial Zn pools are reduced in ZnT2-suppressed cells. A: representative immunoblot of ZnT2 in isolated mitoplasts from cells transiently transfected with scrambled control small interfering RNA (siRNA) (control siRNA, n = 3) or SLC30A2 siRNA (ZnT2 siRNA, n = 3). Membranes were stripped and reprobed for COX IV as a loading control. B: densitometry quantification of immunoblot. Data represent mean ± SD ratio of ZnT2 to COX IV (arbitrary densitometry units); n = 3 samples/treatment. *Significant effect of ZnT2 suppression on ZnT2 abundance, P < 0.0001. C: cells were transiently transfected with SLC30A2 siRNA to suppress ZnT2 expression (ZnT2 suppressed), and effects on labile Zn pools in mitochondria were quantified with RhodZin-3 AM by fluorometry. Results were compared with mock-transfected cells. Data represent mean ± SD fluorescence (arbitrary units/μg protein); n = 8 samples/treatment. *Significant effect of ZnT2 suppression on RhodZin-3 fluorescence, P < 0.05. D: Zn uptake was measured with 65Zn in isolated mitoplasts from cells transiently transfected with SLC30A2 siRNA (ZnT2 suppressed) or scrambled control siRNA (mock transfected). Data represent mean ± SD Zn uptake rate (fmol·min−1·μg protein−1); n = 3 samples/treatment. *Significant effect of ZnT2 gene attenuation on Zn uptake, P < 0.001.
ZnT2 transports Zn into mitochondria.
To determine whether ZnT2 mediates Zn transport into mitochondria, we generated cells to suppress or overexpress ZnT2 and measured mitochondrial Zn import, using RhodZin-3 fluorometry and 65Zn uptake. RhodZin-3 AM is a mitochondrion-specific Zn fluorophore (34, 35) that we used to quantify changes in mitochondrial Zn pools in live cells (25). RhodZin-3 fluorometry documented that ZnT2-attenuated cells had significantly lower (20%, P < 0.05) mitochondrial Zn pools (Fig. 4C) compared with mock-transfected cells. To verify that mitochondrial Zn uptake was directly affected by ZnT2 suppression, mitoplasts were generated and Zn uptake was directly measured in vitro with 65Zn. Our data indicated that the initial rate of Zn uptake into purified mitoplasts was routinely reduced by ∼20–30% (P < 0.05) in mitoplasts isolated from ZnT2-suppressed cells relative to mock-transfected cells, clearly documenting a role for ZnT2 in mitochondrial Zn import (Fig. 4D).
We next overexpressed HA-tagged ZnT2 in MECs to confirm that we could modulate mitochondrial Zn levels. Mitoplasts were isolated, and Zn accumulation in live cells and Zn uptake in vitro were measured (Fig. 5). These data indicated that HA-tagged ZnT2 could be directly detected in mitochondria (Fig. 5, A and B). Cells overexpressing ZnT2 had ∼23% (P < 0.05) greater RhodZin-3 fluorescence than mock-transfected cells (Fig. 5C). Moreover, ZnT2 overexpression increased the initial rate of Zn uptake in vitro by ∼38% (P < 0.05) compared with mock-transfected cells, providing further evidence that ZnT2 plays a role in the expansion of mitochondrial Zn pools (Fig. 5D).
Fig. 5.
Zn pools in mitochondria are increased in ZnT2-overexpressing cells. A: representative immunoblot of hemagglutinin (HA)-tagged ZnT2 detected in total crude membranes from cells transfected with HA-tagged ZnT2 (Total mem), mitoplasts isolated from mock-transfected cells (Mock) or mitoplasts isolated from cells transfected with HA-tagged ZnT2 (ZnT2-HA). Membranes were immunoblotted with HA antibody and then stripped and reprobed for calnexin, Lamp1, and COX IV. B: confocal micrograph illustrates colocalization (merge, yellow) of HA-tagged ZnT2 (green) and MitoTracker (red), indicating that ZnT2 is partially localized to mitochondria (Pearson's coefficient = 0.86). C: to verify changes in mitochondrial Zn pools, labile Zn pools in mitochondria were detected with RhodZin-3 AM. Cells were transfected with HA-tagged ZnT2, and RhodZin-3 fluorescence was compared with mock-transfected cells. Data represent mean ± SD fluorescence (arbitrary units/μg protein); n = 8 samples/treatment. *Significant effect of ZnT2 overexpression on fluorescence, P < 0.01. D: Zn uptake was measured with 65Zn in isolated mitoplasts from cells transiently overexpressing ZnT2-HA. Data represent mean ± SD Zn uptake (fmol·min−1·μg protein−1); n = 3 samples/treatment. *Significant effect of ZnT2 overexpression on Zn uptake, P < 0.001.
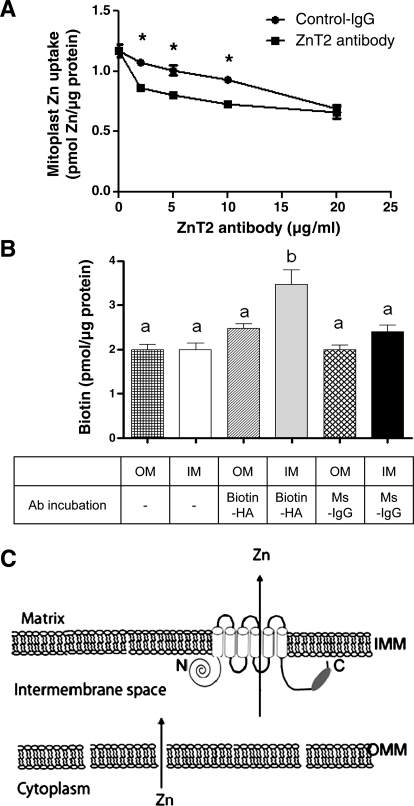
To further document that ZnT2 mediates Zn import into mitoplasts, we aimed to block Zn uptake into isolated mitoplasts with ZnT2 antibody. We reasoned that because our antibody was generated against the COOH terminus of ZnT2, if pretreatment with ZnT2 antibody decreased Zn import into mitoplasts, the COOH terminus of ZnT2 was accessible to antibody binding in the intermembrane space. Purified mitoplasts were preincubated with affinity-purified ZnT2 antibody or rabbit IgG (control) before the addition of 65Zn in serum-free medium, and 65Zn uptake into mitoplasts was quantified after 15 min. As illustrated in Fig. 6A, preincubation with ZnT2 antibody significantly reduced Zn uptake in a dose-dependent manner. To verify the topology of ZnT2 within the inner mitochondrial membrane, we developed a biotin detection assay. Since our ZnT2 construct contained a COOH-terminal HA tag, we postulated that treatment with a biotin-conjugated HA antibody would allow us to detect the COOH terminus of ZnT2 if it was within the intermembrane space. As shown in Fig. 6B, a significant amount of biotin was only detected when the outer membrane of mitochondria was removed (∼30% greater, relative to nonspecific binding; P < 0.05). Our ability to inhibit Zn uptake and to capture the COOH terminus of ZnT2 in intact mitoplasts suggests that, based on topology prediction, both the NH2- and COOH-terminal domains of ZnT2 reside in the cytoplasm-contiguous intermembrane space (Fig. 6C).
Fig. 6.
Inhibition of Zn uptake by ZnT2 antibody predicts the topology of ZnT2 within the inner mitochondrial membrane. A: Zn uptake was measured with 65Zn in isolated mitoplasts pretreated with either control rabbit IgG or anti-ZnT2 IgG in a dose-dependent manner. Data represent mean ± SD uptake; n = 3 samples/treatment. *Significant effect of ZnT2 antibody pretreatment on Zn uptake, P < 0.05. B: biotin was detected in mitochondria in the absence of the outer membrane. Mitochondria with (OM) or without (IM) the outer mitochondrial membrane were isolated from cells expressing ZnT2-HA. To detect ZnT2-HA, mitochondria with (hatched bar) or without (gray bar) the outer membrane were treated with biotinylated anti-HA antibody. Nonspecific fluorescence was assessed in mitochondria with (checkered bar) or without (open bar) the outer membrane not treated with antibody or in mitochondria with (cross-hatched bar) or without (filled bar) the outer membrane treated with anti-mouse-IgG mitochondria as a control antibody. Biotin level was assessed with the DyLight Reporter fluorescence assay. Data represent mean ± SD picomoles of biotin (pmol/μg protein); n = 4 samples/treatment. Means with different letters are significantly different as analyzed by 1-way analysis of variance followed by Tukey post-test (P < 0.05). C: schematic model illustrating the putative orientation of ZnT2 in mitochondria postulated as a result of the accessibility of the ZnT2 antibody epitope (dark oval) to ZnT2 antibody in isolated mitoplasts. Zn likely traverses the outer mitochondrial membrane (OMM) through porin channels, as the intermembrane space is contiguous with the cytoplasm. Topology prediction suggests that Zn2+ is transported from the intermembrane space (arrow) within which the NH2 and COOH termini reside. IMM, inner mitochondrial membrane.
NH2-terminal domain of ZnT2 facilitates targeting to mitochondria.
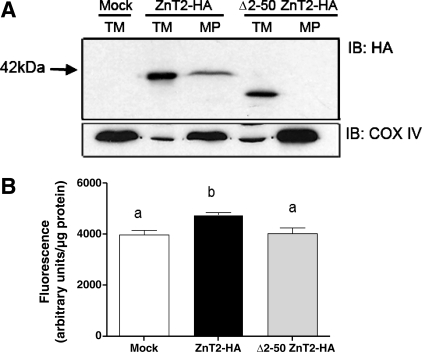
We next aimed to identify key motifs involved in mitochondrial targeting. We first determined that deletion of the first 50 amino acids (Δ2–50 ZnT2-HA) was efficiently translated with predicted molecular mass. Importantly, the Δ2–50 ZnT2-HA truncation mutant was not detected in mitochondria (Fig. 7A). Moreover, expression of the Δ2–50 ZnT2-HA deletion mutant abrogated Zn accumulation in mitochondria, which resulted from overexpression of wild-type ZnT2 (Fig. 7B). Taken together, our data suggest that a domain(s) within or proximal to the first 50 amino acids of ZnT2 is important for insertion of ZnT2 into the mitochondrial membrane.
Fig. 7.
Truncation of the first 50 amino acids of the NH2 terminus of ZnT2 eliminated detection in mitochondria and reduced Zn uptake. A: representative immunoblot of total crude membranes (TM) and purified mitoplasts (MP) from cells transfected with HA-tagged ZnT2 (ZnT2-HA) and the NH2 terminus truncation mutant Δ2–50 ZnT2-HA with anti-HA antibody. ZnT2-HA was detected in purified mitoplasts isolated from cells transfected with wild-type ZnT2-HA (ZnT2-HA MP), but truncation of the first 50 amino acids of NH2 terminus of ZnT2 (Δ2–50 ZnT2-HA MP) eliminated its detection in mitoplasts. B: expression of Δ2–50 ZnT2-HA abrogated Zn accumulation into mitochondria as monitored with the mitochondrion-specific Zn fluorophore RhodZin-3. Data represent mean ± SD fluorescence; n = 6 samples/treatment. Means with different letters are significantly different, P < 0.05.
NH2-terminal histidine-rich motif of ZnT2 plays critical role in its mitochondrial targeting.
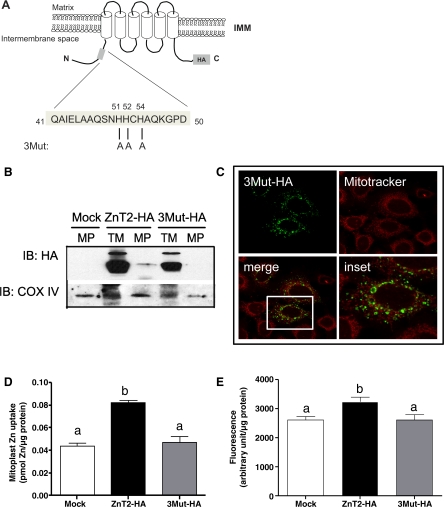
Sequence analysis detected a unique 51HHCH54 domain in this NH2-terminal region. We examined the effect on mitochondrial localization of replacing all three histidine residues in this domain with alanine by site-directed mutagenesis (Fig. 8A). Our data indicated that mutation of all three histidine residues to alanines (3Mut-HA) completely abolished mitochondrial localization of ZnT2 (Fig. 8, B and C). Moreover, expression of the 3Mut-HA mutant abolished 65Zn uptake into mitochondria (Fig. 8D) in vitro and Zn accumulation in mitochondria (Fig. 8E) in live cells, which resulted from overexpression of wild-type ZnT2. Collectively, these findings suggest that this histidine-rich motif in the NH2 terminus of ZnT2 is essential for mitochondrial targeting of ZnT2.
Fig. 8.
Mitochondrial localization of ZnT2 requires the NH2-terminal histidine-rich motif. A: schematic illustration of the 3Mut-HA protein with highlighted histidine-rich sequence in the NH2 terminus. Mutations introduced within this sequence are shown. B: representative immunoblot of total crude membranes (TM) and purified mitoplasts (MP) from cells transfected with HA-tagged ZnT2 (ZnT2-HA) and the 3Mut-HA with anti-HA antibody. ZnT2-HA was detected in purified mitoplasts isolated from cells transfected with wild-type ZnT2-HA (ZnT2-HA MP), but the 3Mut-HA eliminated its detection in mitoplasts. C: confocal micrograph illustrates that HA-tagged 3Mut (green) did not colocalize with MitoTracker (red), indicating that 3Mut is not localized to mitochondria. D: expression of 3Mut-HA abrogated Zn uptake into mitochondria. Data represent mean ± SD Zn uptake (pmol Zn/μg protein); n = 3 samples/treatment. E: to verify changes in mitochondrial Zn pools, labile Zn pools in mitochondria were detected with RhodZin-3 AM. Data represent mean ± SD fluorescence (arbitrary units/μg protein); n = 8 samples/treatment. *Means with different letters are significantly different: D, P < 0.001; E, P < 0.01.
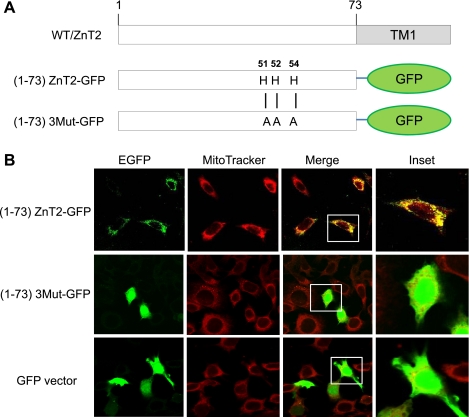
To confirm that the 51HHCH54 motif could direct ZnT2 to mitochondria, we generated chimeric proteins consisting of the first 73 amino acids of wild-type ZnT2 fused to GFP [(1–73)ZnT2-GFP] or of the first 73 amino acids of ZnT2 in which 51HHCH54 had been replaced with 51AACA54 [(1–73)3Mut-GFP] (Fig. 9A). As illustrated in Fig. 9B, expression of (1–73)ZnT2-GFP colocalized with MitoTracker, illustrating mitochondrion-specific distribution, whereas (1–73)3Mut-GFP did not colocalize with MitoTracker but instead showed diffuse localization in the cytoplasm similar to that observed for cells expressing the GFP vector alone. These data confirmed that the NH2-terminal histidine-rich motif is critical to the mitochondrial localization of ZnT2.
Fig. 9.
The NH2-terminal histidine-rich motif of ZnT2 is responsible for mitochondrial targeting. A: the NH2-terminally truncated mutants of (1–73)ZnT2-GFP and (1–73)3Mut-GFP were constructed and expressed in HC11 cells. Numbers refer to amino acid positions starting from the NH2 terminal. B: (1–73)ZnT2-GFP (green) and Mitotracker (red) were colocalized (yellow), but (1–73)3Mut-GFP and GFP vector alone showed a diffuse staining pattern within the cells. EGFP, enhanced GFP.
Mitochondrial function is affected in ZnT2-overexpressing cells.
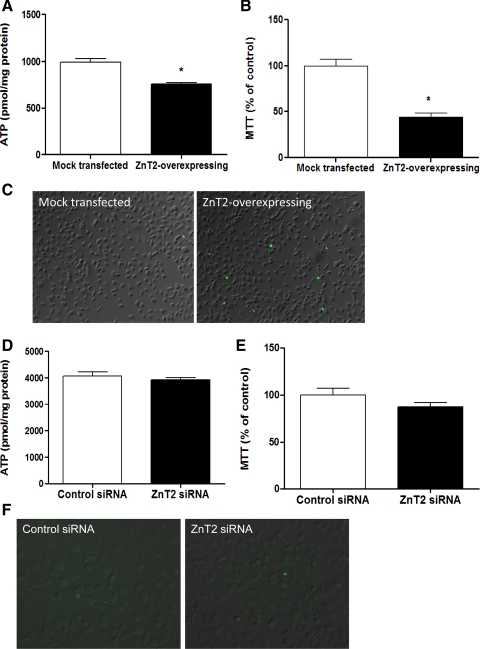
We next hypothesized that changes in mitochondrial Zn pools mediated by ZnT2 expression would regulate mitochondrial function. Our data indicated that ZnT2 overexpression reduced both ATP concentration by 25% (P < 0.05; Fig. 10A) and mitochondrial oxidation by ∼56% (P < 0.05; Fig. 10B) compared with mock-transfected cells. Moreover, ZnT2 overexpression showed greater abundance of annexin V positive-staining cells compared with mock-transfected cells (Fig. 10C), indicating that ZnT2 overexpression resulted in increased apoptosis. In addition to effects of ZnT2 overexpression, we tested the effects of ZnT2 attenuation on mitochondrial function. Our data indicated that ZnT2 suppression had no effect on either ATP levels (Fig. 10D) or mitochondrial oxidation (Fig. 10E) compared with mock-transfected cells. Furthermore, ZnT2 suppression showed no difference in the abundance of annexin V positive-staining cells compared with mock-transfected cells (Fig. 10F). Although effects of ZnT2 on mitochondrial function were not reciprocal, these results indicate that the expansion of mitochondrial Zn pools by ZnT2 overexpression modulates mitochondrion-specific functions, suggesting a functional role for ZnT2-mediated Zn import into mitochondria.
Fig. 10.
ZnT2 overexpression in the mitochondria inhibited mitochondrial function. A: cells were transiently transfected with ZnT2-HA to overexpress ZnT2 (ZnT2 overexpressing), and effects on ATP levels were measured with bioluminescence. Results were compared with mock-transfected cells. Data represent mean ± SD ATP level (pmol/mg protein); n = 3 samples/treatment. *Significant effect of ZnT2 overexpression on ATP level, P < 0.05. B: effects of ZnT2 overexpression on metabolic function in the mitochondria were measured with the 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay. Data represent mean ± SD % of values observed in the mock-transfected cells (% of control); n = 8 samples/treatment. *Significant effect of ZnT2 overexpression on metabolic function, P < 0.05. C: effects of ZnT2 overexpression on apoptosis were detected with annexin V-FITC. The number of apoptotic cells generated to overexpress ZnT2 was increased (green, FITC-conjugated annexin V) compared with mock-transfected cells. Scale bar, 10 μm. To demonstrate the reciprocal effect of ZnT2 on mitochondrial function, cells were transfected with SLC30A2-specific small interfering RNA (siRNA) or mismatched control siRNA to attenuate ZnT2 expression. D–F: effects of ZnT2 suppression on ATP levels (D), metabolic function in the mitochondria (E), and apoptosis (F) were measured. Results were compared with mismatched control siRNA-transfected cells as described above.
DISCUSSION
In addition to playing a major role in Zn secretion during lactation, ZnT2 is detected in nonsecreting mammary cells (31) and nonlactating mammary gland, suggesting that ZnT2 has diverse roles in mammary gland Zn metabolism. A key finding is our characterization of ZnT2-mediated Zn expansion of mitochondria, which may serve a regulatory role. Our data indicate that ZnT2 is a specific component of the inner mitochondrial membrane. An alternative interpretation is that the ZnT2 protein we detected in isolated mitoplast preparations may actually reside in the ER membrane associated with focal contact microdomains between the ER and mitochondria (30) that remained intact despite our rigorous digestion with proteinase K. While this cannot be conclusively eliminated, we believe that the effectiveness of our protease treatment makes this interpretation unlikely, as we confirmed that classical markers of vesicular membranes such as ER, lysosomes, and endosomes (8, 13) were absent.
It has been postulated that Zn-transporting proteins likely reside in the mitochondrial membrane (27). Previous studies by Costello and colleagues described the kinetics of mitochondrial Zn uptake in prostate cells (11) and further reported that the Zn-binding protein metallothionein may function as a chaperone in this process (5). These key observations provide evidence that mitochondrial Zn uptake systems exist. However, specific Zn transporters that play a direct role in the process of mitochondrial Zn uptake have not yet been identified. Recently Zip8 has been detected at the plasma membrane and in mitochondria in lung epithelium (1, 12). While this has not been conclusively documented, topological analysis predicts that Zip8 would export Zn from mitochondria into the cytoplasm. To our knowledge, no Zn importers have been identified in mitochondria to date. It is important to note that all ZnT proteins thus far [with the exception of a ZnT5 isoform (14)] are responsible for Zn export from the cytoplasm either into subcellular organelles/vesicles or into the extracellular space. Results from both our ZnT2-attenuation and -overexpression studies support an ancillary role for ZnT2 in Zn import into the mitochondria from the cytoplasm. Studies conducted in purified mitoplasts in vitro documented a robust effect of ZnT2 attenuation on Zn import. Interestingly, studies in live cells using the mitochondrion-specific Zn fluorophore RhodZin-3 illustrated a significant yet more modest effect of ZnT2 attenuation on mitochondrial Zn uptake. This may reflect the potential lack of a linear relationship between RhodZin-3 fluorescence and Zn content or the previously documented role of ZnT2 in other subcellular, vesicular compartments (25).
Characterization of ZnT2 as a functional Zn importer into mitochondria begs the question, How is ZnT2 targeted to mitochondria? It is important to note that there is no general consensus sequence(s) for mitochondrial targeting signals (MTS). Studies have found that many nonclassical MTS have hydrophobic amino acid residues or a proline-rich region surrounded by positively charged residues in the NH2 terminus (19, 28, 32). Bioinformatic analysis using MitoProt (http://ihg2.helmholtz-muenchen.de/ihg/mitoprot.html) and Target P (http://www.cbs.dtu.dk/services/TargetP/) failed to identify an MTS in the predicted ZnT2 amino acid sequence. Consistent with a weaker MTS score and a lower whole protein net charge (7) compared with proteins that are exclusively targeted to mitochondria, ZnT2 is dually targeted to exocytotic vesicles (24) and mitochondria. Mutational analysis determined that substitution of all three histidine residues in an NH2-terminal domain (51HHCH54) abrogated mitochondrial targeting, suggesting that this 51HHXH54 motif of ZnT2 in the NH2 terminus is critical for mitochondria targeting. Mutation of individual histidine residues at H52 and H54 abolished mitochondrial targeting of ZnT2, and mutation of H51 was not translated (Y. A. Seo, unpublished observations), suggesting that each histidine in the histidine-rich motif is indispensable for ZnT2 targeting to the mitochondria. Interestingly, H51 and H54 residues in ZnT2 are conserved in the mammalian ZnT2 homologs, suggesting that there may be a role of this motif in Zn-dependent function and/or subcellular localization of ZnT2. Although the role of this motif as a Zn binding domain has not been confirmed, the histidine-rich motif is reminiscent of Zn binding centers and is involved in the active sites of many Zn-binding proteins, presumably because of their nitrogen-containing imidazole. Thus it is conceivable that Zn may bind this histidine-rich motif and act as a signaling molecule to modulate the subcellular localization of ZnT2 in the mammary cell. The importance of the histidine-rich motif is consistent with our previous determination that a mutation that substitutes H54 for an arginine results in a significant defect in ZnT2 function (3). Moreover, it appears that this histidine-rich region may be important to several members of the ZnT family (ZnT2, ZnT3, and ZnT8). Further studies are needed to explore the role of this motif in other Zn transporters and systems.
Most mitochondrial proteins are nuclear encoded and have to be transported into the mitochondria, implying a highly organized protein import system. Two major classes of MTS have been elucidated, those contained within an NH2-terminal presequence and those contained within the protein (7). Our data suggest that ZnT2 protein belongs to the second class of proteins and that the histidine motif identified here appears to be the dominant MTS within the ZnT2 protein sequence. Tom 70 is often required for the import of this second class of proteins destined for the inner mitochondrial membrane (2). One hypothesis is that Tom 70 may associate with ZnT2 through the histidine-rich motif and then translocate through the general import pore of the outer membrane and interact with the Tim9-Tim10 complex in the intermembrane space to guide the ZnT2 protein across the intermembrane space to the TIM22 translocase. Alternatively, this region may be required by ZnT2 to form intermolecular complexes that target ZnT2 to the mitochondrial membrane. For example, heat shock protein (Hsp)70 is a ubiquitous protein that performs critical functions such as binding to unfolded segments of protein substrates to assist in protein folding, cellular transport, and translocation across intracellular membranes (36). Analysis of the yeast homolog mtHsp70 (Ssc1) implicates its essentiality in the translocation of cytosolic proteins across both mitochondrial membranes (37). Interestingly, expression of Hsp70 is reduced by the lactogenic hormone prolactin (6). One intriguing postulate is that in the absence of prolactin (nonsecreting cells) Hsp70 may associate with ZnT2 and facilitate ZnT2 targeting to mitochondria, while prolactin stimulation may abrogate this facilitation. While our data indicate that this region appears to directly assist in ZnT2 targeting, it is possible that lack of detection of the mutant protein in mitochondria may result from protein misfolding or denaturation. Studies are needed to determine specifically how ZnT2 is inserted into the mitochondrial membrane and whether molecular chaperones play a role in this process.
Understanding the functional relevance of ZnT2 in mitochondria in hormonally responsive secretory tissues may have important physiological considerations. It is important to stress that ZnT2 localization is never exclusively restricted to either mitochondria or vesicles. Thus changes in the relative distribution of ZnT2 between these compartments may regulate or reflect cellular function. Modulation of vesicular Zn pools would clearly assist in Zn secretion from the cell during lactation. Changes in mitochondrial Zn pools may regulate cellular functions such as bioenergetics and/or apoptosis (reviewed in Ref. 10). Several lines of evidence suggest that mitochondrial Zn accumulation results in ATP depletion (33) through inhibition of aconitase, α-ketoglutarate dehydrogenase, isocitrate dehydrogenase-NAD+ dependent, succinate dehydrogenase, or COX activities (20). In support of these observations, our data indicate that mitochondrial Zn expansion facilitated by ZnT2 overexpression restrained ATP biogenesis. Similar to our observations in MECs, extensive investigation by Costello and colleagues in prostate cells has revealed that high mitochondrial Zn levels (∼3- to 10-fold higher than in mitochondria from liver and kidney; Ref. 23) functionally inhibit m-aconitase activity, permitting citrate accumulation in seminal fluid (4) and restraining ATP levels. This suggests that a similar bioenergetic regulatory role for the regulated redistribution of Zn in mammary gland and prostate mitochondria may exist. Zn accumulation in mitochondria is also associated with mitochondrial depolarization, dysfunction, and cell death following neuronal ischemia (27). Interestingly, the exposure of prostate cells to Zn inhibited cell growth due, in part, to an increase in apoptosis (21). Further studies revealed that the apoptotic effect is due to Zn-induced mitochondrial apoptogenesis (9). This is consistent with our determination that expansion of mitochondrial Zn pools mediated by ZnT2 overexpression activated apoptosis. Regulation of these bioenergetic and apoptogenic mechanisms may be particularly important in modulating mammary gland transition between nonsecreting and secreting phenotypes (29). We speculate that reductions in ZnT2-mediated mitochondrial Zn pools associated with a secreting phenotype may inhibit apoptogenesis while permitting enhanced ATP production as required for tissue differentiation and secretory function. Studies are currently under way to explore the functional relevance of these observations.
In summary, this study indicates that ZnT2 expands mitochondrial Zn pools. To our knowledge this is the first direct evidence of a mitochondrial Zn transporter, and studies are currently under way to further characterize the function and regulation of ZnT2 in mitochondria. Importantly, our data clearly indicate that ZnT2 plays an ancillary role in Zn import into mitochondria, which suggests that MEC mitochondria may have a unique functional requirement to integrate ATP production and apoptosis.
GRANTS
This work was supported by National Institute of Child Health and Human Development Grant HD-058614 to S. L. Kelleher.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors gratefully acknowledge the excellent technical expertise of Jose Ignacio Gonsalez Zambra. Moreover, we thank Dr. Bo Lönnerdal for his mentorship and support, Dr. Dennis Winge for his insightful discussion, and members of the Kelleher Lab for their generous input and constructive comments.
REFERENCES
- 1. Besecker B, Bao S, Bohacova B, Papp A, Sadee W, Knoell DL. The human zinc transporter SLC39A8 (Zip8) is critical in zinc-mediated cytoprotection in lung epithelia. Am J Physiol Lung Cell Mol Physiol 294: L1127–L1136, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Chacinska A, Pfanner N, Meisinger C. How mitochondria import hydrophilic and hydrophobic proteins. Trends Cell Biol 12: 299–303, 2002 [DOI] [PubMed] [Google Scholar]
- 3. Chowanadisai W, Lonnerdal B, Kelleher SL. Identification of a mutation in SLC30A2 (ZnT-2) in women with low milk zinc concentration that results in transient neonatal zinc deficiency. J Biol Chem 281: 39699–39707, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Costello LC, Franklin RB, Liu Y, Kennedy MC. Zinc causes a shift toward citrate equilibrium of the m-aconitase reaction of prostate mitochondria. J Inorg Biol 78: 161–165, 2000 [DOI] [PubMed] [Google Scholar]
- 5. Costello LC, Guan Z, Franklin RB, Feng P. Metallothionein can function as a chaperone for zinc uptake transport into prostate and liver mitochondria. J Inorg Biochem 98: 664–666, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Deane EE, Kelly SP, Lo CK, Woo NY. Effects of GH, prolactin and cortisol on hepatic heat shock protein 70 expression in a marine teleost Sparus sarba. J Endocrinol 161: 413–421, 1999 [DOI] [PubMed] [Google Scholar]
- 7. Dinur-Mills M, Tal M, Pines O. Dual targeted mitochondrial proteins are characterized by lower MTS parameters and total net charge. PLoS One 3: e2161, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Falcon-Perez JM, Dell'Angelica EC. Zinc transporter 2 (SLC30A2) can suppress the vesicular zinc defect of adaptor protein 3-depleted fibroblasts by promoting zinc accumulation in lysosomes. Exp Cell Res 313: 1473–1483, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Feng P, Liang JY, Li TL, Guan ZX, Zou J, Franklin R, Costello LC. Zinc induces mitochondria apoptogenesis in prostate cells. Mol Urol 4: 31–36, 2000 [PubMed] [Google Scholar]
- 10. Franklin B, Costello L. The important role of the apoptotic effects of zinc in the development of cancers. J Cell Biochem 106: 750–757, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guan Z, Kukoyi B, Feng P, Kennedy MC, Franklin RB, Costello LC. Kinetic identification of a mitochondrial zinc uptake transport process in prostate cells. J Inorg Biochem 97: 199–206, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. He L, Girijashanker K, Dalton TP, Reed J, Li H, Soleimani M, Nebert DW. ZIP8, member of the solute-carrier-39 (SLC39) metal-transporter family: characterization of transporter properties. Mol Pharmacol 70: 171–180, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Iguchi K, Usui S, Inoue T, Sugimura Y, Tatematsu M, Hirano K. High-level expression of zinc transporter-2 in the rat lateral and dorsal prostate. J Androl 23: 819–824, 2002 [PubMed] [Google Scholar]
- 14. Jackson KA, Helston RM, McKay JA, O'Neill ED, Mathers JC, Ford D. Splice variants of the human zinc transporter ZnT5 (SLC30A5) are differentially localised and regulated by zinc through transcription and mRNA stability. J Biol Chem 282: 10423–10431, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Jiang D, Sullivan PG, Sensi SL, Steward O, Weiss JH. Zn2+ induces permeability transition pore opening and release of pro-apoptotic peptides from neuronal mitochondria. J Biol Chem 276: 47524–47529, 2001 [DOI] [PubMed] [Google Scholar]
- 16. Kambe T, Yamaguchi-Iwai Y, Sasaki R, Nagao M. Overview of mammalian zinc transporters. Cell Mol Life Sci 61: 49–68, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kelleher SL, Lonnerdal B. Zinc transporters in the mammary gland respond to marginal zinc and vitamin A intake during lactation in rats. J Nutr 132: 3280–3285, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Kelleher SL, Lonnerdal B. Zn transporter levels and localization change throughout lactation in rat mammary gland and are regulated by Zn in mammary cells. J Nutr 133: 3378–3385, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Lee EW, Lai Y, Zhang H, Unadkat JD. Identification of the mitochondrial targeting signal of the human equilibrative nucleoside transporter 1 (hENT1): implications for interspecies differences in mitochondrial toxicity of fialuridine. J Biol Chem 281: 16700–16706, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Lemire J, Mailloux R, Appanna V. Zinc toxicity alters mitochondrial metabolism and leads to decreased ATP production in hepatocytes. J Appl Toxicol 28: 175–182, 2008 [DOI] [PubMed] [Google Scholar]
- 21. Liang JY, Liu YY, Zou J, Franklin RB, Costello LC, Feng P. Inhibitory effect of zinc on human prostatic carcinoma cell growth. Prostate 40: 200–207, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lichten LA, Cousins RJ. Mammalian zinc transporters: nutritional and physiologic regulation. Annu Rev Nutr 29: 153–176, 2009 [DOI] [PubMed] [Google Scholar]
- 23. Liu Y, Franklin RB, Costello LC. Prolactin and testosterone regulation of mitochondrial zinc in prostate epithelial cells. Prostate 30: 26–32, 1997 [DOI] [PubMed] [Google Scholar]
- 24. Lopez V, Kelleher SL. Zinc transporter-2 (ZnT2) variants are localized to distinct sub-cellular compartments and functionally transport zinc. Biochem J 29: 43–52, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lopez V, Kelleher SL. Zip6-attenuation promotes epithelial-to-mesenchymal transition in ductal breast tumor (T47D) cells. Exp Cell Res 316: 366–375 [DOI] [PubMed] [Google Scholar]
- 26. Malaiyandi LM, Honick AS, Rintoul GL, Wang QJ, Reynolds IJ. Zn2+ inhibits mitochondrial movement in neurons by phosphatidylinositol 3-kinase activation. J Neurosci 25: 9507–9514, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Medvedeva YV, Lin B, Shuttleworth CW, Weiss JH. Intracellular Zn2+ accumulation contributes to synaptic failure, mitochondrial depolarization, and cell death in an acute slice oxygen-glucose deprivation model of ischemia. J Neurosci 29: 1105–1114, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Neve E, Ingelman-Sundberg M. Intracellular transport and localization of microsomal cytochrome P450. Anal Bioanal Chem 392: 1075–1084, 2008 [DOI] [PubMed] [Google Scholar]
- 29. Neville M, Mather I. Introduction: secretory activation: from the past to the future. J Mammary Gland Biol Neoplasia 12: 205–210, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Pizzo P, Pozzan T. Mitochondria-endoplasmic reticulum choreography: structure and signaling dynamics. Trends Cell Biol 17: 511–517, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Qian L, Lopez V, Seo Y, Kelleher S. Prolactin regulates ZNT2 expression through the JAK/STAT signaling pathway in mammary cells. Am J Physiol Cell Physiol 297: C369–C377, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Robin MA, Anandatheerthavarada HK, Biswas G, Sepuri NBV, Gordon DM, Pain D, Avadhani NG. Bimodal targeting of microsomal CYP2E1 to mitochondria through activation of an N-terminal chimeric signal by cAMP-mediated phosphorylation. J Biol Chem 277: 40583–40593, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rudolf E. Depletion of ATP and oxidative stress underlie zinc-induced cell injury. Acta Medica (Hradec Kralove) 50: 43–49, 2007 [PubMed] [Google Scholar]
- 34. Sensi SL, Ton-That D, Sullivan PG, Jonas EA, Gee KR, Kaczmarek LK, Weiss JH. Modulation of mitochondrial function by endogenous Zn2+ pools. Proc Natl Acad Sci USA 100: 6157–6162, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sensi SL, Ton-That D, Weiss JH, Rothe A, Gee KR. A new mitochondrial fluorescent zinc sensor. Cell Calcium 34: 281–284, 2003 [DOI] [PubMed] [Google Scholar]
- 36. Strub A, Zufall N, Voos W. The putative helical lid of the Hsp70 peptide-binding domain is required for efficient preprotein translocation into mitochondria. J Mol Biol 334: 1087–1099, 2003 [DOI] [PubMed] [Google Scholar]
- 37. Wadhwa R, Takano S, Kaur K, Aida S, Yaguchi T, Kaul Z, Hirano T, Taira K, Kaul SC. Identification and characterization of molecular interactions between mortalin/mtHsp70 and HSP60. Biochem J 391: 185–190, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]