Abstract
Peroxiredoxin 6 (Prdx6), a bifunctional protein with GSH peroxidase and lysosomal-type phospholipase A2 activities, has been localized to both cytosolic and acidic compartments (lamellar bodies and lysosomes) in lung alveolar epithelium. We postulate that Prdx6 subcellular localization affects the balance between the two activities. Immunostaining localized Prdx6 to lysosome-related organelles in the MLE12 and A549 alveolar epithelial cell lines. Inhibition of trafficking by brefeldin A indicated processing of the protein through the vesicular pathway. Trafficking of Prdx6 was decreased by inhibitors of PKC, ERK, and p38 MAPK. Immunocytochemistry, immunoprecipitation, and an in situ proximity ligation assay (Duolink) showed that binding of the lysosomal targeting sequence of Prdx6 (amino acids 31–40) to 14-3-3ε was dependent on activity of PKC, ERK, and p38 MAPK. Knockdown of 14-3-3ε with siRNA inhibited the lysosomal targeting of Prdx6. In vitro study with recombinant proteins by pull-down assay and surface plasmon resonance confirmed the interaction of Prdx6 and 14-3-3ε. These findings suggest that ERK and p38 MAPK regulate subcellular localization of Prdx6 by activation of 14-3-3ε as a chaperone protein, resulting in its translocation to acidic organelles.
Keywords: 1-cys peroxiredoxin, phospholipase A2, lung lamellar bodies, cell signaling, protein kinase C, protein chaperone
peroxiredoxin 6 (Prdx6) is a unique member of the peroxiredoxin family of ubiquitous antioxidant enzymes. Prdx6 has both glutathione peroxidase and Ca2+-independent phospholipase A2 (aiPLA2) activities and is highly expressed in respiratory epithelium (11, 14). Through its peroxidase activity, Prdx6 is capable of reducing a broad spectrum of peroxides and provides protection against oxidative stress (14). Through its aiPLA2 activity, Prdx6 participates in lung surfactant phospholipid turnover. Inactivation of Prdx6 in a mouse model markedly diminished lung surfactant phospholipid degradation and its remodeling by the reacylation pathway, whereas overexpression of the enzyme had the opposite effect (6, 7).
In vitro studies have demonstrated that the pH optimum for the PLA2 activity of the enzyme is in the acidic range (∼pH 4), whereas the peroxidase activity is expressed at cytosolic pH. Analysis by immunocytochemistry and subcellular fractionation has localized Prdx6 to lamellar bodies (lysosome-related secretory organelles) and lysosomes in lung alveolar epithelium (1, 11, 34). This localization of Prdx6 to acidic organelles is consistent with the low pH requirement for its PLA2 activity (11). Thus we have postulated that Prdx6 targeting to its specific subcellular compartment in part determines the balance between the low-pH-dependent PLA2 and the neutral-pH-dependent peroxidase activities of the protein. Prdx6 expression in lamellar bodies was confirmed in isolated rat lung alveolar type 2 cells and macrophages using both immunoblot and immunofluorescence analysis (34).
We have recently discovered that Prdx6 compartmentalization in acidic organelles and its consequent PLA2 activity rely on a 10-amino-acid sequence located not far from the NH2-terminal part of the protein (26). This sequence, comprising Prdx6 31–40 amino acids, can direct green fluorescent protein (GFP)-tagged Prdx6 peptides to lysosome-related organelles in MLE12 and A549 cells, cell lines derived from mouse and human lung epithelium, respectively. However, neither the mechanism for Prdx6 subcellular sorting nor the possible signaling pathways that direct its lysosomal compartmentalization have been defined.
The present results indicate that Prdx6 localization to lysosomal-like organelles in lung epithelial cells requires the activity of ERK1/2 and p38 MAPK, as well as PKC, a kinase upstream of MAPK. We determined that the role of both ERK and p38 MAPK in lysosomal compartmentalization of the protein does not involve Prdx6 serine/threonine phosphorylation but rather requires its interaction with a member of the 14-3-3 family of chaperone proteins. Thus our study suggests that Prdx6 utilizes a unique signaling pathway to determine its subcellular localization.
MATERIALS AND METHODS
Materials.
12-O-Tetradecanoyl-phorbol-13-acetate, brefeldin A (BFA), MAPK inhibitors (PD98059, SP600125, SB202190) and H7, a PKC inhibitor, were purchased from Calbiochem (EMD Biosciences, San Diego, CA). Nile Red was obtained from Sigma-Aldridge (St. Louis, MO). Specific 14-3-3ε siRNA, nontargeted control siRNA and the siRNA transfection reagent system were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Recombinant glutathione S-transferase (GST): 14-3-3ε fusion protein was a gift from Dr. Mitchell Weiss (Children's Hospital of Philadelphia, PA); the GST is the sequence for expression of the Shistosoma japonicum protein (35).
Cell culture, transfections, and inhibitors.
Lung epithelial cell lines MLE12 and A549 were obtained from the American Type Culture Collection (ATCC, Manassas, VA). The mouse lung epithelial cell line MLE12 (CRL-2110, ATCC) (32) was cultured in Ham's F12 (50:50 mix) medium with 0.005 mg/ml insulin, 0.01 mg/ml transferrin, 30 nM sodium selenite, 10 nM hydrocortisone, 10 nM β-estradiol, 10 mM HEPES buffer, 2 mM l-glutamine supplemented with 2% fetal bovine serum and antibiotics. These cells contain lysosomal-like organelles with some of the ultrastructural characteristics of lamellar bodies and secrete phospholipids (32). MLE12 cells (3 × 106) were transiently transfected with 3 μg pGFP-Prdx6 or pGFP-Prdx6 (amino acids 31–40) lysosomal targeting peptide using the Amaxa Nucleofactor program W-001 and the Normal Human Bronchial Epithelial Nucleofactor kit (Lonza, Walkersville, MD) according to the manufacturer's protocol. The mammalian expression plasmids encoding NH2-terminal GFP-tagged full-length Prdx6 and GFP-tagged amino acid 31–40 peptide were described previously (26). Following electroporation, cells in growth medium were plated on coverslips in the six-well plates and cultured for 48 h before experimental treatments.
A549 cells (CCL-185, ATCC), a human lung carcinoma cell line (13), were grown in DMEM (GIBCO Laboratories, Grand Island, NY) supplemented with 10% fetal bovine serum and antibiotics. Cells were maintained in 5% CO2 at 37°C. For transient knockdown of 14-3-3ε in A549 cells, cell layers at 70% confluence in six-well plates were transfected with 60 pmol of either specific 14-3-3ε siRNA or nontargeted control siRNA using the siRNA transfection reagent system (Santa Cruz Biotechnology) according to the manufacturer's protocol. Cells were subjected to experimental treatments 48 h after transfection.
To evaluate the effect of brefeldin A, MLE12 cells were incubated with a solution containing the agent at 10 μg/ml for 4 h and then fixed. To test the effect of PKC and/or MAPK signaling, MLE12 and A549 cells were subcultured as described above and treated for 1.5 h before fixation with the specific PKC or MAPK inhibitors. To inhibit PKC, cells were treated with 50 μM H7. For inhibition of MAPK, cells were treated with ERK1/2 inhibitor PD98059 (25 μM), p38 inhibitor SB202190 (50 μM), or JNK inhibitor SP600125 (50 μM).
Immunofluorescence and confocal microscopy.
Cells cultured on glass coverslips were rinsed with PBS and either fixed with cold ethanol-acetone mixture (1:1 in volume) for 5 min on ice or with 3% paraformaldehyde for 10 min at room temperature followed by 10-min permeabilization with 1% Triton X-100 solution in PBS. Both methods gave similar results. Following permeabilization, cells on coverslips were immunolabeled with primary antibodies [1:200 dilution in 0.2% Triton X-100 solution in PBS (T-PBS)] for 1 h at room temperature. The monoclonal antibody to Prdx6 was purchased from Chemicon (Millipore, Billerica, MA). Polyclonal (rabbit) anti-lysosomal-associated membrane protein 1 (LAMP1) antibody (Cell Signaling Technology, Danvers, MA) was used as a marker for lysosomal organelles, and anti-calnexin antibody (Stressgen, Victoria, Canada) was used as a marker of endoplasmic reticulum (ER). After being washed with T-PBS (5 times for 5 min each), cells were incubated for 1 h at room temperature with secondary Alexa Fluor-594-conjugated goat anti-mouse (red) and Alexa Fluor-488-conjugated goat anti-rabbit (green) IgG antibodies (Molecular Probes, Eugene, OR) at 1:1,000 dilution in T-PBS. After a final washing (5 times for 5 min each with T-PBS and twice for 5 min each with PBS), the cells were mounted with Vectashield mounting medium (Vector Laboratories, Burlingame, CA) and subcellular distribution of Prdx6, and/or its targeting peptide in cells, was observed under a confocal microscope (Radiance 2000; Bio-Rad, Hercules, CA) at ×60 magnification.
Nile red and GFP staining.
Nile Red, a lipid stain, was used to stain lamellar body-like structures in MLE12 cells fixed in 3% paraformaldehyde (3). These organelles have been shown to represent modified lysosomes similar to the acidic (pH ∼5.5) lamellar bodies of alveolar type 2 cells (4). A saturated solution of Nile Red (0.1 mg/ml) (Sigma-Aldrich) was prepared in acetone and stored protected from light at −20°C. Nile Red stock solution (0.5 μl) was added to 1 ml of a 75:25 glycerol-water mixture to prepare a working solution of the dye. Fixed MLE12 cells transfected with constructs expressing GFP-tagged full-length Prdx6 or its 31–40 amino acid lysosomal-targeting peptide (26) were subjected to a 5-min incubation at room temperature with 25 μl of the Nile Red working solution. The subcellular distribution of GFP-tagged protein or peptide was observed by confocal microscopy at ×60 magnification.
Subcellular fractionation of MLE12 cells in isotonic sucrose.
Lysosomal organelles from MLE12 cells were isolated in isotonic sucrose as described previously (26). Briefly, 0.5 g of pelleted cells were washed once with PBS, resuspended in 5 ml of buffer containing 0.25 M sucrose in 10 mM Tris·HCl, pH 7.4, and homogenized with 10–15 strokes in a Teflon Dounce homogenizer. The cell debris was centrifuged at 12,000 g for 10 min at 4°C. CaCl2 was added to the supernatant to a final concentration of 8 mM, and the sample was recentrifuged at 25,000 g for 15 min at 4°C. The supernatant was analyzed as the cytosolic fraction. The pellet containing lysosomal organelles was washed in 4 ml of 150 mM KCl in 10 mM Tris·HCl buffer, pH 7.4, and organelles were resedimented by a final centrifugation at 25,000 g for 15 min at 4°C. Prdx6 localization was analyzed by Western blot.
Immunoprecipitation assay.
The immunoprecipitation assay was performed as described previously (33). Briefly, cells transfected with GFP-tagged Prdx6 31–40 amino acid wild-type or S32A-mutant peptide were lysed in 1× cell lysis buffer (Cell Signaling Technology) supplemented with protease inhibitor cocktail (Sigma-Aldrich) and phosphatase inhibitors (NaF, 2-glycerophosphate, Na3VO4, and EDTA). Equal amounts of total protein (1.5 mg) were mixed with anti-GFP monoclonal antibody (Clontech, Mountain View, CA) followed by incubation with Protein G-conjugated agarose (Invitrogen Life Technologies, Carlsbad, CA), which was added to bind the immune complexes formed in the solution. After centrifugation, the pellet was extensively washed with cell lysis buffer, and the components of the precipitated immune complexes were extracted with SDS-PAGE sample buffer and analyzed by Western blotting.
DuoLink in situ proximity ligation assay.
To detect the interaction between Prdx6 and 14-3-3ε, we utilized the DuoLink in situ Proximity Ligation Assay (PLA) (Olink Bioscience, Uppsala, Sweden) according to the manufacturer's protocol. A549 cells, cultured on glass coverslips, were rinsed with PBS and fixed with cold ethanol-acetone mixture (1:1 in volume) for 5 min on ice, followed by 10-min incubation with 1% Triton X-100 solution in PBS and further 40-min blocking in a 3% solution of bovine serum albumin in PBS containing 0.2% T-PBS. Cells were immunolabeled with primary antibodies (1:100 dilution in 0.2% Triton X-100 solution in T-PBS) for 1 h at room temperature. The monoclonal antibody to Prdx6 and polyclonal antibody (T-16, Santa Cruz Biotechnology) to 14-3-3ε were used to detect protein-to-protein interaction as observed by confocal microscopy at ×60 magnification. The secondary antibodies with attached PLA probes are supplied in the Duolink kit. A fluorescence signal indicates that two proteins within cells are separated by <40 nm (8) (www.olink.com).
GST pull-down assay.
To identify Prdx6 interaction with 14-3-3ε in vitro, a GST pull-down assay was performed according to a previously described protocol (5). Briefly, purified recombinant GST:14-3-3ε fusion and untagged Prdx6 proteins were incubated in the presence of glutathione-Sepharose beads with end-over-end mixing at 4°C for 2 h (Amersham Biosciences, Uppsala, Sweden). The beads were washed five times with PBS (2.7 mM KCl, 1.5 mM KH2PO4, 137 mM NaCl, and 8.1 mM Na2HPO4, pH 7.4) and resuspended in SDS sample buffer. The presence of GST:14-3-3ε and Prdx6 proteins indicating complex formation of the two proteins with glutathione attached to the beads was analyzed by SDS-PAGE.
Surface plasmon resonance assay.
Surface plasmon resonance (SPR) experiments were performed on a dual channel Reichert SPR Refractometer (SR7000DC; Reichert, Depew, NY) using Reichert 500-kDa carboxymethyl dextran SPR7000 gold sensor chips. Buffers were filtered and thoroughly degassed before use. The running buffer was PBS (2.7 mM KCl, 1.5 mM KH2PO4, 137 mM NaCl, and 8.1 mM Na2HPO4, pH 7.4) with 0.01% Tween 20 (PBST) that was injected at a flow rate of 25 μl/min. Carboxylic acids were activated with a 15-min injection of 1-ethyl-3-(3-dimethylaminopropyl) carbodiamide and N-hydroxysuccinimide at a molar ratio of 4:1 in distilled water. In the experimental channel, 14-3-3ε fused to GST was allowed to couple for 12 min at a concentration of 100 μg/ml in 20 mM sodium acetate buffer, pH 5.2, which resulted in the immobilization of 433 refractive response units of protein; the reference channel received no 14-3-3ε. The remaining activated esters in both channels were quenched with 1 M ethanolamine, pH 8.5, injected over 10 min. The surface was then treated for 3 min with 20 mM HCl to remove nonbound 14-3-3ε, and the signal baseline was established with injection of PBST. Prdx6 concentrations from 6.16 μM to 385 nM were injected over immobilized 14-3-3ε. Prdx6 was injected for a period of 7 min (association), and the resulting Prdx6/14-3-3ε complex was subjected to 4 min of buffer flow only (dissociation). The surface was then regenerated by injection of 10 mM glycine, pH 2.5, over 3 min. This PBST-Prdx6 (association/dissociation)-glycine sequence was repeated in triplicate for all Prdx6 concentrations analyzed.
Individual association/dissociation curves for each Prdx6 concentration were analyzed with Scrubber 2.0 of Biologic Software (Reichert) where all traces were zeroed, cropped, aligned, and referenced (curve-for-curve subtraction of the reference channel from the experimental channel). The traces were then analyzed for concentration-dependent changes in the steady-state signal, and Prdx6/14-3-3ε association/dissociation constants were calculated.
Western blot analysis.
Gels subjected to PAGE were stained by SimplyBlue Safe Stain according to manufacturer's protocol (Invitrogen). Western blot analysis was performed using the two-color Odyssey LI-COR (Lincoln, NE) technique as previously described (18). A polyclonal antibody (16) was used to detect Prdx6. Polyclonal antibody T-16 was used to detect 14-3-3ε. Anti-MAPK antibodies were monoclonal anti-p38 (T180/Y182) for phosphorylated p38 and anti-p38 α/SAPK 2A for total p38 (BD Transduction Laboratories, San Diego, CA) and monoclonal E-4, C-14, G-7, and E-5 (Santa Cruz Biotechnology) for phosphorylated ERK, total ERK, phosphorylated JNK, and total JNK, respectively. Anti-GFP monoclonal and LAMP1 polyclonal antibodies are described above. The secondary antibody was IrDye 800 goat anti-rabbit (Rockland, Gilberstville, PA) for imaging on the green 800-nm channel.
Statistical analysis.
Data are expressed as means ± SE. Statistical significance was assessed with SigmaStat software (Jandel Scientific, San Jose, CA). Group differences were evaluated by one-way ANOVA. Differences between mean values were considered statistically significant at P < 0.05.
RESULTS
Prdx6 targeting to lysosomal organelles involves the secretory protein transport pathway.
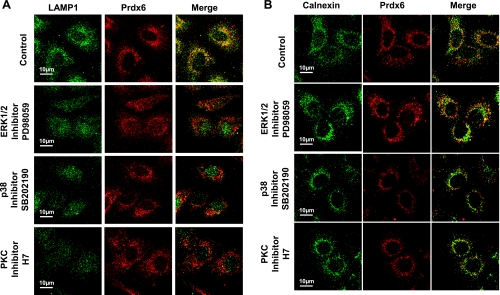
Our previous studies demonstrated Prdx6 expression in lysosomes and lamellar bodies of lung alveolar type 2 cells (1, 7, 34). Moreover, Prdx6 was predominantly localized to the vesicular lumen (26). To further investigate the possible molecular mechanisms of Prdx6 targeting to lysosome-related organelles, we studied the expression of endogenous Prdx6 in MLE12 cells, a cell line derived from mouse lung epithelium (Fig. 1). We first tested the effect of BFA, an inhibitor of the classical pathway for secretory protein transport, on targeting of endogenous Prdx6 to lysosomal organelles as identified by staining with LAMP1, a marker of lysosome-related compartments (9). Treatment with BFA markedly decreased Prdx6 colocalization with LAMP1 (Fig. 1A) with perinuclear accumulation of the protein in ER as shown by colocalization with calnexin, an ER marker (Fig. 1B). These data suggest that Prdx6 targeting to lysosomal compartments in MLE12 cells occurs through the classical secretory/exocytotic protein transport pathway, compatible with its presence in the extracellular bronchoalveolar lavage fluid, as reported in a previous study (22).
Fig. 1.
The secretory protein transport pathway, MAPKs, and PKC are involved in lysosomal localization of endogenous peroxiredoxin 6 (Prdx6) in MLE12 mouse lung epithelial cells. Fixed, permeabilized cells were stained for lysosomal-associated membrane protein 1 (LAMP1) or calnexin (green) and Prdx6 (red). Cells were incubated with brefeldin A (BFA) for 4 h and with other inhibitors for 1.5 h before fixation. Inhibitors used were BFA (10 μg/ml), PD98059 (25 μM), SB202190 (50 μM), SP600125 (50 μM), or H7 (50 μM). Shown in green (left) are lysosomal organelles stained with LAMP1 (A) or the endoplasmic reticulum (ER) stained with calnexin (B). Prdx6 expression in A and B is shown in red (middle), and colocalization is detected by yellow color in the merged image (right).
ERK1/2 and p38 MAPK regulate Prdx6 localization in lysosomal organelles in MLE12 cells.
Our recent studies have shown that both ERK and p38 MAPK are able to phosphorylate both Prdx6 in intact cells and recombinant Prdx6 in vitro (33). Moreover, the inhibition of either ERK, p38 MAPK, or PKC abolished phorbol ester-stimulated Prdx6 PLA2 activity in lung epithelial cells (33). PKC is upstream of and can directly activate MAPK (23). These previous results led us to study the role of MAPKs in regulation of Prdx6 targeting to lysosomal organelles although the demonstrated site of phosphorylation of Prdx6 lies outside of the 10-amino-acid-targeting peptide (33). MLE12 cells were treated with ERK inhibitor (PD98059), p38 MAPK inhibitor (SB202190), JNK inhibitor (SP600125), or PKC inhibitor (H7) for 1.5 h before fixation. Inhibition of either ERK or p38 MAPK markedly depressed Prdx6 lysosomal compartmentalization as reflected in Prdx6 colocalization with LAMP1, whereas inhibition of JNK kinase had no effect (Fig. 1A). Inhibition of PKC also abolished Prdx6 lysosomal localization (Fig. 1A). However, treatment of MLE12 cells with MAPK and PKC inhibitors had no effect on Prdx6 accumulation in the perinuclear region of the ER as shown by its colocalization with calnexin (Fig. 1B). Inhibition of targeting to lysosomes by inhibition of MAPK (ERK, p38) and PKC also was shown for A549 cells (Fig. 2, A and B). Thus it appears that MAPK activity is required for Prdx6 to exit the ER and enter the vesicular/secretory pathway.
Fig. 2.
Inhibition of PKC, ERK1/2, or p38 MAPK but not JNK affects lysosomal localization of endogenous Prdx6 in A549 cells. The protocol for cell staining and use of inhibitors is the same, except for the different cell type, as shown in Fig. 1.
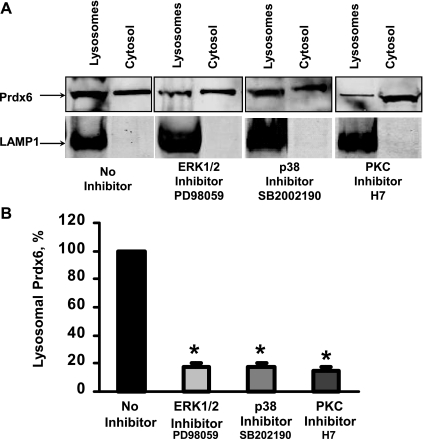
To further evaluate the effect of MAPK on Prdx6 subcellular localization, we isolated lysosomal-like organelles and the cytosolic fraction from MLE12 cells. Identity of the lysosome-related compartment was confirmed by staining with LAMP1. There was no LAMP1 staining in the cytosolic fraction indicating that it was free of contamination with lysosomal-like organelles. Consistent with previous reports (1), Prdx6 expression was demonstrated in both subcellular fractions by Western blot analysis (Fig. 3A). Quantification of the Western blots shows a large (∼75%) decrease in lysosomal Prdx6 in cells treated with an inhibitor of ERK, p38 MAPK, or PKC (Fig. 3B).
Fig. 3.
Inhibition of ERK1/2 and p38 MAPK affects localization of endogenous Prdx6 in lysosomes in MLE12 cells. A: Western blot analysis of Prdx6 expression in lysosomal organelles and cytosolic fraction isolated from MLE12 cells. Cells were treated for 1.5 h with PKC, ERK, or p38 MAPK inhibitors before assay. The whole isolated lysosomal fraction was loaded so that lysosomal protein per lane as indicated by the LAMP1 band was variable. B: quantification of Western blots shown in A. For each sample, Prdx6 expression was normalized for the expression of the lysosomal protein, LAMP1. Bars represent the percentage Prdx6 expression in lysosomal organelles compared with Prdx6 expression in lysosomal organelles isolated from nontreated MLE12 cells, shown as 100%. Values are means ± SE for n = 3 independent experiments, *P < 0.001 vs. nontreated.
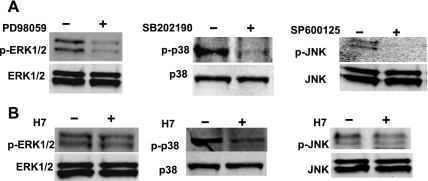
The effect of inhibitors on activity (phosphorylation) of ERK, p38, or JNK was evaluated by Western blot analysis. Treatment of MLE12 cells with PD98059, SB202190, and SP600125, respectively, resulted in a decrease in the phosphorylation of ERK by 61%, p38 by 58%, and JNK by >90% (Fig. 4A). The effect of PKC inhibition on ERK, p38, and JNK MAPK activation was also evaluated. Treatment of MLE12 cells with PKC inhibitor H7 resulted in the inhibition of ERK, p38, and JNK phosphorylation by 23, 62, and 18%, respectively (Fig. 4B).
Fig. 4.
Western blot analysis of the effect of inhibitors on MAPK activation in A549 cells. A549 cells were treated with PD98059 (25 μM), SB202190 (50 μM), or SP600125 (50 μM) to inhibit ERK, p38, or JNK MAPK, respectively (A). H7 (50 μM) was used to inhibit PKC (B). Cells were treated with inhibitors for 1.5 h and subjected to Western blot analysis for both phosphorylated (top) and total (bottom) protein.
ERK1/2 and p38 MAPK regulate the subcellular localization of both the Prdx6 full-length protein and the lysosomal targeting peptide in MLE12 cells.
We have recently reported that the Prdx6 31–40-amino-acid NH2-terminal region is necessary and sufficient for protein targeting to lysosomal compartments of MLE12 cells (26). To further study the signaling pathways that regulate Prdx6 targeting, we expressed the GFP-tagged Prdx6 31–40-amino-acid peptide in MLE12 cells and examined its subcellular localization in the presence of MAPK-specific inhibitors. It should be noted that most Prdx6 in lung epithelial cells is present in the cytoplasm and that only a small fraction is targeted to lamellar bodies. Thus the green fluorescence of the cells attributable to cytoplasmic localization of GFP-tagged peptide dominates the observed images (not shown). However, after extensive washing, the organellar localization of GFP could be discerned (Fig. 5). The GFP-tagged peptide showed good colocalization with Nile Red-stained organelles in MLE12 cells (Fig. 5A). Inhibition of ERK or p38 MAPK markedly affected the GFP-peptide lysosomal compartmentalization, whereas inhibition of JNK had no effect. Inhibition of PKC with H7 also abolished lysosomal localization of the GFP-Prdx6 (31–40 amino acid) peptide.
Fig. 5.
Effect of ERK1/2 and p38 inhibition on lysosomal targeting of GFP-tagged full-length Prdx6 and a peptide comprising Prdx6 amino acid (aa) 31–40 in MLE12 cells. GFP-tagged or Prdx6(31–40 aa) peptide (A) Prdx6 full-length (FL) (B) fusion construct expressed in the transfected cells is shown in green (left). Lysosomal-like organelles are stained with Nile Red (middle). Colocalization is detected by yellow color in the merged image (right). Inhibitors were applied, where indicated, 1.5 h before fixation of transfected MLE12 cells.
To extend these results, we expressed the full-length GFP-tagged Prdx6 in MLE12 cells. This construct also showed good colocalization with Nile Red-stained organelles (Fig. 5B). As with the peptide, inhibition of ERK, p38 MAPK, or PKC markedly affected the GFP-Prdx6 compartmentalization, whereas inhibition of JNK kinase had no effect (Fig. 5B). These data indicate that lysosomal localization of newly synthesized Prdx6 is mediated via PKC and MAP kinase signaling and relies on both ERK and p38 MAPK, but not on JNK.
ERK1/2 and p38 MAPK regulate the interaction of Prdx6 and 14-3-3ε in A549 cells.
It has been recently reported that 14-3-3ε interacts with Prdx6 as well as with ERK and p38 MAPK (12). The ϵ-isoform is a member of the 14-3-3 protein chaperone system, which is known to facilitate transport of signaling proteins in the early secretory pathway (12). We have now detected colocalization between 14-3-3ε and Prdx6 in intact A549 lung epithelial cells by immunofluorescence analysis (Fig. 6A) and by the DuoLink PLA (Fig. 6B). The 14-3-3ε/Prdx6 colocalization was especially strong in the perinuclear region of the cell. To evaluate the effect of PKC and MAPK signaling on 14-3-3ε/Prdx6 interaction, A549 cells were treated with inhibitors of MAPK and PKC for 1.5 h before fixation. Inhibition of either ERK, p38 MAPK, or PKC markedly diminished the 14-3-3ε/Prdx6 colocalization signal, whereas inhibition of JNK had no effect (Fig. 6B). These findings suggest that 14-3-3ε and Prdx6 interact in the intact lung cell, that the interaction is dependent on MAPK activity, and that 14-3-3ε modulates Prdx6 translocation along the exocytotic pathway.
Fig. 6.
Colocalization and protein-protein interaction between Prdx6 and 14-3-3ε in A549 cells. A: immunofluorescence assay. A549 cells were stained for 14-3-3ε shown in green (left) and for Prdx6 shown in red (middle). Colocalization was detected by yellow color in merged image (right). B: in situ proximity ligation assay (PLA). Shown are fluorescence images to indicate interaction between 2 proteins. Treatment with primary Prdx6 mouse monoclonal and 14-3-3ε rabbit polyclonal antibodies was followed by treatment with species-specific secondary antibodies with PLA probes. PLA signal (red dots) was detected in A549 cells using confocal microscopy (No inhibitor). As indicated, A549 cells were treated with PKC or MAPK inhibitors for 1.5 h before fixation. Reduction of the PLA signal indicates that inhibition of PKC, ERK, or p38 greatly attenuated the interaction of Prdx6 with 14-3-3ε, whereas JNK inhibitor had no effect. Normal mouse and rabbit IgG were used as an isotype control (Control).
14-3-3ε interacts with Prdx6 31–40-amino-acid peptide.
Because the targeting 31–40 amino-acid peptide can direct Prdx6 to lysosomes, we evaluated 14-3-3ε interaction with GFP-tagged wild-type or S32A mutant 31–40-amino-acid Prdx6 peptide expressed in A549 cells. Interaction of the wild-type peptide with 14-3-3ε was detected by the DuoLink in situ PLA (Fig. 7A). Binding of 14-3-3ε to the wild-type peptide also was detected using an anti-GFP pull-down assay (Fig. 7B). Mutation of serine 32 in the peptide to alanine (S32A), which was previously shown to abolish Prdx6 lysosomal targeting (26), resulted in a significant decrease in the interaction of 14-3-3ε with the Prdx6 31–40-amino-acid peptide (Fig. 7B). As quantitated by scanning of Western blots and subtracting the IgG control, relative binding of 14-3-3ε to the S32A mutant compared with the wild-type sequence was only 21.3 ± 5.4% (n = 3) (Fig. 7C). Thus these results provide additional evidence that 14-3-3ε plays a role in Prdx6 lysosomal targeting and that the Prdx6 31–40 amino acid NH2-terminal targeting sequence serves as a 14-3-3ε binding site.
Fig. 7.
Prdx6 31–40 aa peptide serves as a binding site for the 14-3-3ε. A: in situ PLA of A549 cells transiently transfected with green fluorescent protein (GFP)-tagged Prdx6 31–40 aa peptide. Staining with primary anti-GFP mouse monoclonal and anti-14-3-3ε rabbit polyclonal antibodies was followed by species-specific secondary antibodies with PLA probes. The PLA signal indicating interaction (shown in red) was detected in A549 cells using confocal microscopy. B: immunoprecipitation (IP) assay with immunoblot analysis of A549 cells transfected with GFP-tagged wild-type (WT) or S32A mutant Prdx6 31–40 aa peptides. Top: using anti-GFP monoclonal antibody for IP, both 14-3-3ε and GFP-tagged Prdx6 WT peptide (31–40 aa) were pulled down from cell lysates. IP with nonspecific IgG was used as a control. IgGHC and IgGLC indicate bands for the heavy and light chains of IgG, respectively. Bottom: expression of GFP-tagged Prdx6 WT or S32A mutant peptides and 14-3-3ε in whole cell lysates, representing the input for the top gel. C: quantification of Western blot analysis of 14-3-3ε bound to Prdx6 31–40 aa WT or S32A mutant peptides in A549 cells. For each sample, 14-3-3ε expression was normalized for amount of immunoprecipitated GFP-tagged peptide. Bars represent the percentage of 14-3-3ε bound to Prdx6 peptides. The amount of 14-3-3ε bound to the WT Prdx6 31–40 aa peptide is shown as 100%. Values are means ± SE for n = 3 independent experiments, *P < 0.001 vs. values for WT Prdx6 31–40 aa peptide.
Prdx6 directly interacts with 14-3-3ε in vitro.
Because studies with cells indicated that Prdx6/14-3-3ε interaction appears to play a role in Prdx6 targeting to lysosomal organelles, we evaluated interaction between purified recombinant Prdx6 and 14-3-3ε proteins in vitro. Binding of Prdx6 to 14-3-3ε was evaluated using GST pull down (Fig. 8A). In this assay, GST-tagged 14-3-3ε binds to GSH immobilized on Sepharose beads. The presence of (untagged) Prdx6 in the eluate from the beads indicates its binding to GST-tagged 14-3-3ε. SPR analysis demonstrated tight binding of Prdx6 with immobilized GST-14-3-3ε (Fig. 8B, left). GST-14-3-3ε coupled to the surface of the Reichert chip was subjected to a range of Prdx6 concentrations spanning 385 nM to 6.16 μM and showed an increasing response as a function of concentration. Mutation of serine 32 to alanine (S32A), which was previously shown to abolish Prdx6 lysosomal targeting (26), markedly decreased the magnitude of the interaction between the proteins (Fig. 8B, right). The difference between the wild-type and mutant peptides increased with increasing peptide concentration (Fig. 8C). The association rates (ka) were 210 and 170 M−1/s for 14-3-3ε interaction with the wild-type and S32A mutant Prdx6, respectively, as calculated using Scrubber 2.0 Biologic Software. Equilibrium dissociation (KD) constants were 0.6 mM and 1.05 mM for 14-3-3ε interaction with wild-type and S32 mutant Prdx6, respectively.
Fig. 8.
Analysis of the Prdx6 protein binding to 14-3-3ε in vitro. A: in vitro pull down of recombinant Prdx6 with glutathione S-transferase (GST)-tagged 14-3-3ε using glutathione Sepharose beads as described in materials and methods. 14-3-3ε/Prdx6 binding was analyzed with SDS-PAGE. Prdx6 only (lane 1) was used as a control for nonspecific binding. Incubation of glutathione-Sepharose beads with 14-3-3ε only (lane 2) indicates binding of the protein to the beads. WT Prdx6 is pulled down in the presence of 14-3-3ε (lane 3), whereas pull down of S32A mutated Prdx was markedly decreased (lane 4). Lanes 5–8 are recombinant proteins (1 μg): GST, GST-14-3-3ε, WT Prdx6, and S32A mutant Prdx6, respectively. B: analysis of Prdx6 protein binding to 14-3-3ε by surface plasmon resonance as described in materials and methods. GST fused to 14-3-3ε or bovine serum albumin was immobilized on a 500-kDa carboxymethyl dextran SPR7000 gold sensor slide, and surface plasmon resonance was utilized to measure the interaction with WT (left) or S32A mutant (right) Prdx6. Prdx6 in varying concentrations (a–e) was injected over immobilized 14-3-3ε. Prdx6 injection was at zero time; the switch to Prdx6-free buffer (dissociation) is indicated by the arrow. a, 6.16 μM; b, 3.08 μM; c, 1.5 μM; d, 770 nM; e, 385 nM. C: response units (RU) from experiments shown in B are plotted as a function of Prdx6 concentration (M). Values are mean for n = 3 replicates.
Inhibition of 14-3-3ε affects Prdx6 targeting to lysosomal organelles.
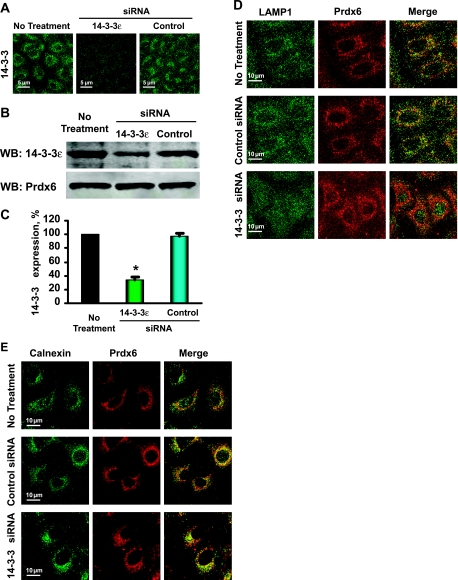
To further evaluate the role of 14-3-3ε in Prdx6 subcellular localization, we treated A549 cells with siRNA specific for 14-3-3ε. The effectiveness of knockdown was confirmed by both immunofluorescence (Fig. 9A) and Western blot (Fig. 9B) analysis. The latter demonstrated 66% knockdown compared with control (Fig. 9C). Treatment with the specific 14-3-3ε siRNA markedly diminished Prdx6 lysosomal localization, as detected by colocalization of endogenous Prdx6 with LAMP1 (Fig. 9D). This treatment had no effect on Prdx6 accumulation in the ER as shown by colocalization of Prdx6 with calnexin (Fig. 9E). These data further support the requirement for 14-3-3ε for Prdx6 lysosomal targeting.
Fig. 9.
Inhibition of 14-3-3ε expression abolished Prdx6 targeting to lysosomal organelles in A549 cells. Immunofluorescence (A) and Western blot (B) analysis of 14-3-3ε expression in untreated A549 cells or cells transfected with a specific 14-3-3ε or control (nontargeted) siRNA. C: quantitation of Western blots. Results are means ± SE for n = 3 experiments. *P < 0.05 vs. control. D and E: colocalization of Prdx6 in A549 cells with lysosomal and ER marker proteins by immunofluorescence. Shown in green (left) are lysosomal organelles stained with LAMP1 (D) and endoplasmic reticulum (ER) stained with calnexin (E). Prdx6 expression in cells transfected with the specific 14-3-3ε and control (nontargeted) siRNAs is shown in red (middle). Colocalization is detected by yellow color in the merged image (right).
DISCUSSION
The goal for this study was to evaluate signaling mechanisms that regulate the subcellular targeting of Prdx6 to lysosome-related organelles in lung epithelial cells. We studied the MLE12 and A549 cell lines derived from mouse and human lung epithelium, respectively, as well characterized models that express many characteristics of lung alveolar epithelial type 2 cells. Both cell lines are readily transfectable and, similar to primary type 2 lung epithelial cells, express endogenous Prdx6 in the luminal compartment of their lamellar body-like structures (26). Furthermore, a GFP-Prdx6 fusion full-length protein expressed in these cell lines is targeted to organelles that costain with markers for lysosomal-like structures. We have previously shown that amino acids 31–40 of Prdx6 are necessary and sufficient for protein localization to lysosome-related organelles and its consequent PLA2 activity in MLE12 and A549 cells (26).
In this study, we investigated the signaling pathway for lysosomal targeting of Prdx6. Our studies show some level of activation (phosphorylation) for all three MAPKs (ERK, p38, JNK) under baseline conditions although the signaling pathway responsible for activation was not otherwise determined. This activation was abolished by the use of specific inhibitors. Inhibition of either ERK1/2 or p38 MAPK markedly inhibited the presence of endogenous Prdx6 in lysosomes as well as targeting of GFP-tagged Prdx6 protein and peptide to lysosome-related compartments. Inhibition of JNK kinase had no effect. Thus active ERK and p38 MAPK in these lung epithelial cells is required for the translocation of newly synthesized Prdx6 to its specific subcellular location. Because inhibition of MAPK altered lysosomal targeting but did not affect ER localization, we postulate that MAPK activity is necessary for exit of Prdx6 from the ER and “packaging” into the vesicular lysosomal/exocytotic pathway.
We extended these observations with use of the inhibitor H7 to block the activation of MAPK by an upstream activator such as PKC. H7 inhibits PKC as well as several other protein kinases (2, 10), and PKC in turn can activate MAPK (23). In the present studies, the treatment of cells with H7 significantly inhibited p38 MAPK phosphorylation but had only a minor effect on ERK and JNK activation. H7 treatment of cells markedly diminished the localization of endogenous Prdx6 in lysosomal organelles. This effect of H7 could have reflected inhibition of p38 MAPK although an effect that was independent of the MAPK pathway cannot be excluded. A role for PKC in determining the composition of lysosomes has been suggested previously by several studies. Upon its activation by phorbol ester, PKC translocated to cellular membranes, including the limiting membrane of lysosomes (19), and it also associated with the outer surface of melanosomes (21), lysosome-related secretory granules similar to lung lamellar bodies. Furthermore, the formation of transport vesicles at the trans-Golgi network in human hepatoma Hep G2 cells was stimulated by phorbol ester and inhibited by calphostin C, a PKC-specific inhibitor (30). Finally, downregulation of PKC in phorbol ester-stimulated fibroblasts alters lysosomal granule formulation and reduces the activities of some lysosomal enzymes, i.e., serine protease cathepsin G and esterase (27). A possible mechanism for the PKC effect on protein lysosomal targeting has not been reported previously.
We considered several mechanisms for the effect of MAPK enzymes on Prdx6 targeting. One possibility is that the phosphorylation of Prdx6 functions as a signal for protein translocation. We have recently determined that activation of ERK1/2 and p38 MAPK leads to Prdx6 phosphorylation in alveolar epithelial type 2 cells stimulated with phorbol ester (33). However, T177, the only amino acid in Prdx6 that is phosphorylated by MAPK in vitro (33), is not in the targeting peptide. Furthermore, mass-spectroscopic study of the synthetic Prdx6 lysosomal-targeting peptide failed to detect its phosphorylation in vitro by either ERK1/2 or p38 MAPK (data not shown). Thus these studies do not support MAPK-mediated phosphorylation of Prdx6 as the determinant of protein targeting to its specific subcellular location. A possible alternative role for MAPK in Prdx6 targeting is the phosphorylation and activation of a chaperone molecule.
On the basis of a recent report that 14-3-3ε interacts with Prdx6 as well as with ERK and p38 MAPK (12), we focused on this protein as the possible binding partner for targeting. In support of the proposed role, mass-spectroscopic analysis has demonstrated expression of both 14-3-3ε and Prdx6 in exosomes released by keratinocyte-like cells (17). The 14-3-3 proteins are known chaperones that facilitate transport of signaling proteins in the early secretory pathway (20, 25, 36). Our study shows that Prdx6 and 14-3-3ε interact in vitro; it also demonstrates a MAPK-dependent interaction of both Prdx6 and the Prdx6 31–40-amino-acid peptide (DSWGILFSHP) with 14-3-3ε in lung epithelial cells. The 14-3-3 chaperones bind to target proteins that express one of the conserved arginine-based sequences: mode 1 (RSXpSXP), or mode 2 (RXXXpSXP), where pS represents phospho-serine (20, 25, 36). Although the Prdx6 31–40-amino-acid targeting peptide includes a terminal SXP sequence that resembles the consensus mode 2 14-3-3-binding motif, it does not contain the arginine residue, and neither the terminal serine (S38) nor S32 is phosphorylated. Thus the Prdx6 31–40-amino-acid lysosomal-targeting peptide may represent a novel 14-3-3ε binding sequence.
Because our studies showed lack of phosphorylation of the Prdx6 31–40-amino-acid peptide by ERK or p38 MAPK in vitro and the nonphosphorylated peptide bound to 14-3-3ε, it appears that phosphorylation of Prdx6 is not necessary for its binding to 14-3-3ε and subsequent targeting to lysosomal organelles. On the other hand, S32A mutation did diminish the interaction of Prdx6 with 14-3-3ε on the basis of SPR, GST pull down, and coimmunoprecipitation although the kinetics of interaction showed only modest differences between the wild-type and mutant protein. The results suggest that the binding site for interaction with 14-3-3ε is more accessible in the wild-type than in the mutant protein, possibly related to a conformational change in S32A mutant Prdx6 as previously described (15).
Lamellar bodies are members of a subclass of lysosome-related organelles referred to as secretory lysosomes (29). However, unlike lysosomes, lamellar bodies are specialized for storage and secretion rather than degradation of their contents. The relatively rapid (1.5 h) decrease in the endogenous Prdx6 in lysosomal organelles of MLE12 cells treated with inhibitors (BFA, PD98059, SB202190, H7) is unlikely to reflect secretion, but rather suggests retrograde trafficking. Retrograde trafficking from lysosomes to ER has been described for cholesterol (28) and from Golgi to ER for Shiga toxin (31). However, the retrograde trafficking pathway of luminal lysosomal proteins to the ER is poorly understood. Transfer from the lysosomal compartment to recycling organelles may be involved, but further study is necessary to sort out this pathway. This study also has not evaluated mechanisms for the cytosolic localization, the site for its glutathione peroxidase activity, of the major fraction of Prdx6 protein (24). We propose that newly synthesized Prdx6 is primarily cytosolic, whereas some fraction gets recruited to the ER for subsequent transport to secretory organelles. 14-3-3ε does not seem to be involved in this cytosol to ER step because its knockdown did not alter the presence of Prdx6 in the ER. At this time, the mechanisms for regulation of Prdx6 distribution between cytoplasm and ER and the signals for activation of PKC/MAPK pathways that determine lysosomal targeting remain to be determined.
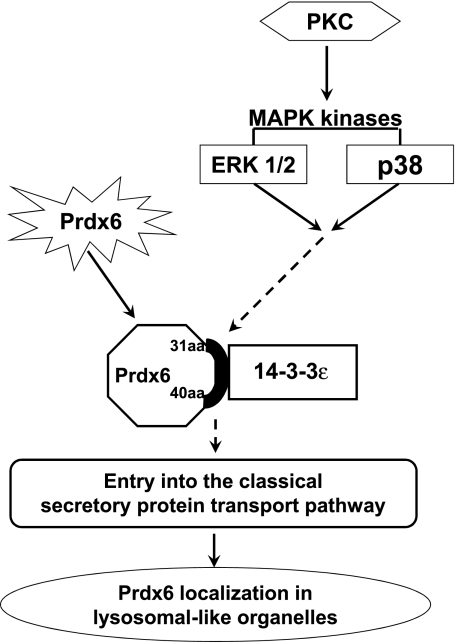
In conclusion, this study has demonstrated a signaling pathway that is required for Prdx6 targeting to its specific subcellular location in lysosome-related organelles of lung epithelial cells. The proposed scheme (Fig. 10) requires MAPK-dependent activation of 14-3-3ε as a chaperone protein that binds to the 31–40-amino-acid sequence of Prdx6, resulting in Prdx6 entry into the vesicular pathway and its further targeting to lysosomal compartments.
Fig. 10.
A proposed mechanism of ERK1/2 and p38 effect on Prdx6 targeting to lysosomal organelles. Prdx6 in ER interacts through its targeting sequence with 14-3-3ε, which acts as a chaperone for entry of Prdx6 into the secretory pathway with subsequent targeting to lysosomal organelles.
GRANTS
This study was supported by Grant HL-075587 from National Heart, Lung, and Blood Institute and presented in part at the FASEB annual meetings in San Diego, CA, April 2008, New Orleans, LA, April 2009, and Anaheim, CA, April 2010.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We thank Dr. David Speicher (Wistar Institute) for mass-spectroscopic analysis, Drs. Mitchell Weiss and Guowei Zhao (Children's Hospital of Philadelphia) for purified recombinant 14-3-3ε, Drs. Madesh Muniswamy and Kevin Yu for technical assistance with confocal microscopy, Drs. Sandra Bates and Altaf Kazi for valuable comments, and Victoria Brown for secretarial support.
REFERENCES
- 1. Akiba S, Dodia C, Chen X, Fisher AB. Characterization of acidic Ca(2+)-independent phospholipase A2 of bovine lung. Comp Biochem Physiol B Biochem Mol Biol 120: 393–404, 1998 [DOI] [PubMed] [Google Scholar]
- 2. Boulis NM, Davis M. Blockade of the spinal excitatory effect of cAMP on the startle reflex by intrathecal administration of the isoquinoline sulfonamide H-8: comparison to the protein kinase C inhibitor H-7. Brain Res 525: 198–204, 1990 [DOI] [PubMed] [Google Scholar]
- 3. Brown WJ, Sullivan TR, Greenspan P. Nile red staining of lysosomal phospholipid inclusions. Histochemistry 97: 349–354, 1992 [DOI] [PubMed] [Google Scholar]
- 4. Chander A, Johnson RG, Reicherter J, Fisher AB. Lung lamellar bodies maintain an acidic internal pH. J Biol Chem 261: 6126–6131, 1986 [PubMed] [Google Scholar]
- 5. Einarson M, Pugacheva E, Orlinick J. Identification of Protein-Protein Interactions with Glutathione-S-Transferase (GST) Fusion Proteins. CSH Protoc 2007: pdbtop11, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Fisher AB, Dodia C, Feinstein SI, Ho YS. Altered lung phospholipid metabolism in mice with targeted deletion of lysosomal-type phospholipase A2. J Lipid Res 46: 1248–1256, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Fisher AB, Dodia C, Yu K, Manevich Y, Feinstein SI. Lung phospholipid metabolism in transgenic mice overexpressing peroxiredoxin 6. Biochim Biophys Acta 1761: 785–792, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Fredriksson S, Gullberg M, Jarvius J, Olsson C, Pietras K, Gustafsdottir SM, Ostman A, Landegren U. Protein detection using proximity-dependent DNA ligation assays. Nat Biotechnol 20: 473–477, 2002 [DOI] [PubMed] [Google Scholar]
- 9. Huynh KK, Eskelinen EL, Scott CC, Malevanets A, Saftig P, Grinstein S. LAMP proteins are required for fusion of lysosomes with phagosomes. EMBO J 26: 313–324, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jarvis WD, Turner AJ, Povirk LF, Traylor RS, Grant S. Induction of apoptotic DNA fragmentation and cell death in HL-60 human promyelocytic leukemia cells by pharmacological inhibitors of protein kinase C. Cancer Res 54: 1707–1714, 1994 [PubMed] [Google Scholar]
- 11. Kim TS, Dodia C, Chen X, Hennigan BB, Jain M, Feinstein SI, Fisher AB. Cloning and expression of rat lung acidic Ca2+-independent PLA2 and its organ distribution. Am J Physiol Lung Cell Mol Physiol 274: L750–L761, 1998 [DOI] [PubMed] [Google Scholar]
- 12. Liang S, Yu Y, Yang P, Gu S, Xue Y, Chen X. Analysis of the protein complex associated with 14-3-3 epsilon by a deuterated-leucine labeling quantitative proteomics strategy. J Chromatogr B Analyt Technol Biomed Life Sci 877: 627–634, 2009 [DOI] [PubMed] [Google Scholar]
- 13. Lieber M, Smith B, Szakal A, Nelson-Rees W, Todaro G. A continuous tumor-cell line from a human lung carcinoma with properties of type II alveolar epithelial cells. Int J Cancer 17: 62–70, 1976 [DOI] [PubMed] [Google Scholar]
- 14. Manevich Y, Fisher AB. Peroxiredoxin 6, a 1-Cys peroxiredoxin, functions in antioxidant defense and lung phospholipid metabolism. Free Radic Biol Med 38: 1422–1432, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Manevich Y, Reddy KS, Shuvaeva T, Feinstein SI, Fisher AB. Structure and phospholipase function of peroxiredoxin 6: identification of the catalytic triad and its role in phospholipid substrate binding. J Lipid Res 48: 2306–2318, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Manevich Y, Sweitzer T, Pak JH, Feinstein SI, Muzykantov V, Fisher AB. 1-Cys peroxiredoxin overexpression protects cells against phospholipid peroxidation-mediated membrane damage. Proc Natl Acad Sci USA 99: 11599–11604, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Medina A, Ghahary A. Transdifferentiated circulating monocytes release exosomes containing 14-3-3 proteins with matrix metalloproteinase-1 stimulating effect for dermal fibroblasts. Wound Repair Regen 18: 245–253, 2010 [DOI] [PubMed] [Google Scholar]
- 18. Milovanova T, Chatterjee S, Manevich Y, Kotelnikova I, Debolt K, Madesh M, Moore JS, Fisher AB. Lung endothelial cell proliferation with decreased shear stress is mediated by reactive oxygen species. Am J Physiol Cell Physiol 290: C66–C76, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science 258: 607–614, 1992 [DOI] [PubMed] [Google Scholar]
- 20. Nufer O, Hauri HP. ER export: call 14-3-3. Curr Biol 13: R391–R393, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Park HY, Perez JM, Laursen R, Hara M, Gilchrest BA. Protein kinase C-beta activates tyrosinase by phosphorylating serine residues in its cytoplasmic domain. J Biol Chem 274: 16470–16478, 1999 [DOI] [PubMed] [Google Scholar]
- 22. Power JH, Nicholas TE. Immunohistochemical localization and characterization of a rat Clara cell 26-kDa protein (CC26) with similarities to glutathione peroxidase and phospholipase A2. Exp Lung Res 25: 379–392, 1999 [DOI] [PubMed] [Google Scholar]
- 23. Roberson ED, English JD, Adams JP, Selcher JC, Kondratick C, Sweatt JD. The mitogen-activated protein kinase cascade couples PKA and PKC to cAMP response element binding protein phosphorylation in area CA1 of hippocampus. J Neurosci 19: 4337–4348, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schremmer B, Manevich Y, Feinstein SI, Fisher AB. Peroxiredoxins in the lung with emphasis on peroxiredoxin VI. Subcell Biochem 44: 317–344, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Shikano S, Coblitz B, Wu M, Li M. 14-3-3 proteins: regulation of endoplasmic reticulum localization and surface expression of membrane proteins. Trends Cell Biol 16: 370–375, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Sorokina EM, Feinstein SI, Milovanova TN, Fisher AB. Identification of the amino acid sequence that targets peroxiredoxin 6 to lysosome-like structures of lung epithelial cells. Am J Physiol Lung Cell Mol Physiol 297: L871–L880, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tanabe F, Cui SH, Ito M. Abnormal down-regulation of PKC is responsible for giant granule formation in fibroblasts from CHS (beige) mice—a thiol proteinase inhibitor, E-64-d, prevents giant granule formation in beige fibroblasts. J Leukoc Biol 67: 749–755, 2000 [DOI] [PubMed] [Google Scholar]
- 28. Underwood KW, Jacobs NL, Howley A, Liscum L. Evidence for a cholesterol transport pathway from lysosomes to endoplasmic reticulum that is independent of the plasma membrane. J Biol Chem 273: 4266–4274, 1998 [DOI] [PubMed] [Google Scholar]
- 29. Weaver TE, Na CL, Stahlman M. Biogenesis of lamellar bodies, lysosome-related organelles involved in storage and secretion of pulmonary surfactant. Semin Cell Dev Biol 13: 263–270, 2002 [DOI] [PubMed] [Google Scholar]
- 30. Westermann P, Knoblich M, Maier O, Lindschau C, Haller H. Protein kinase C bound to the Golgi apparatus supports the formation of constitutive transport vesicles. Biochem J 320: 651–658, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. White J, Johannes L, Mallard F, Girod A, Grill S, Reinsch S, Keller P, Tzschaschel B, Echard A, Goud B, Stelzer EH. Rab6 coordinates a novel Golgi to ER retrograde transport pathway in live cells. J Cell Biol 147: 743–760, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wikenheiser KA, Vorbroker DK, Rice WR, Clark JC, Bachurski CJ, Oie HK, Whitsett JA. Production of immortalized distal respiratory epithelial cell lines from surfactant protein C/simian virus 40 large tumor antigen transgenic mice. Proc Natl Acad Sci USA 90: 11029–11033, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wu Y, Feinstein SI, Manevich Y, Chowdhury I, Pak JH, Kazi A, Dodia C, Speicher DW, Fisher AB. Mitogen-activated protein kinase-mediated phosphorylation of peroxiredoxin 6 regulates its phospholipase A(2) activity. Biochem J 419: 669–679, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wu YZ, Manevich Y, Baldwin JL, Dodia C, Yu K, Feinstein SI, Fisher AB. Interaction of surfactant protein A with peroxiredoxin 6 regulates phospholipase A2 activity. J Biol Chem 281: 7515–7525, 2006 [DOI] [PubMed] [Google Scholar]
- 35. Yu D, dos Santos CO, Zhao G, Jiang J, Amigo JD, Khandros E, Dore LC, Yao Y, D'Souza J, Zhang Z, Ghaffari S, Choi J, Friend S, Tong W, Orange JS, Paw BH, Weiss MJ. miR-451 protects against erythroid oxidant stress by repressing 14-3-3zeta. Genes Dev 24: 1620–1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yuan H, Michelsen K, Schwappach B. 14-3-3 dimers probe the assembly status of multimeric membrane proteins. Curr Biol 13: 638–646, 2003 [DOI] [PubMed] [Google Scholar]