Abstract
The BTB-Kelch protein Krp1 is highly and specifically expressed in skeletal muscle, where it is proposed to have a role in myofibril formation. We observed significant upregulation of Krp1 in C2 cells early in myoblast differentiation, well before myofibrillogenesis. Krp1 has a role in cytoskeletal organization and cell motility; since myoblast migration and elongation/alignment are important events in early myogenesis, we hypothesized that Krp1 is involved with earlier regulation of differentiation. Krp1 protein levels were detectable by 24 h after induction of differentiation in C2 cells and were significantly upregulated by 48 h, i.e., following the onset myogenin expression and preceding myosin heavy chain (MHC) upregulation. Upregulation of Krp1 required a myogenic stimulus as signaling derived from increased myoblast cell density was insufficient to activate Krp1 expression. Examination of putative Krp1 proximal promoter regions revealed consensus E box elements associated with myogenic basic helix-loop-helix binding. The activity of a luciferase promoter-reporter construct encompassing this 2,000-bp region increased in differentiating C2 myoblasts and in C2 cells transfected with myogenin and/or MyoD. Knockdown of Krp1 via short hairpin RNA resulted in increased C2 cell number and proliferation rate as assessed by bromodeoxyuridine incorporation, whereas overexpression of Krp1-myc had the opposite effect; apoptosis was unchanged. No effects of changed Krp1 protein levels on cell migration were observed, either by scratch wound assay or live cell imaging. Paradoxically, both knockdown and overexpression of Krp1 inhibited myoblast differentiation assessed by expression of myogenin, MEF2C, MHC, and cell fusion.
Keywords: Kelch-related protein 1, myoblast migration, myoblast proliferation
kelch-related protein 1 (Krp1), encoded by the murine Kbtbd10 gene, is a member of the Kelch domain containing family of proteins. The first identified Kelch protein, ORF1, was in the Drosophila egg chamber ring canal, where it was thought to have a role in cytoplasmic streaming (30). Since then, over 50 family members of this ancient family (4) have been proposed, although few have been functionally characterized. All possess four to seven Kelch motifs that encode distinct structures, each comprising one blade of a β-propeller-like structure that is thought to have important protein-protein interaction and scaffolding functions; the Kelch sequence identity is poor, suggesting that individual motifs may have the ability to interact with multiple partners (1). Krp1 has five Kelch repeats and was thus assumed to form a five-blade propeller; recently, however, sequence elements encoding sixth non-Kelch propeller were identified, raising the possibility that these blade structures do not exclusively require Kelch motifs (15). In addition to the distinct propeller structure, Kelch proteins may possess additional domains that are involved in further protein-protein interactions (1). Thus Krp1 also contains an NH2-terminal BTB (bric-a-brac-tramtrack-broad complex; also called Poxvirus and zinc finger) domain that is linked to the COOH-terminal propeller structure via a BACK domain.
The major site of Krp1 expression is skeletal muscle, where it was termed sarcosin, with some expression in cardiac tissue. Two transcripts have been identified, which have identical coding regions but different 3′-UTRs, the significance of which is not yet clear (28). Krp1 associates with the actin binding protein N-RAP and is proposed to have a role in myofibril assembly in both skeletal and cardiac muscle (16, 23). Small interfering (si)RNA-targeted Krp1 knockdown in primary cardiomyocytes resulted in decreased myofibrillar accumulation accompanied by “thin” fibrils, suggesting that Krp1 promoted lateral fusion (16). A further key function for Krp1 has been revealed in transformed cells, where it has been identified as an regulated activator protein-1 (26). Overexpression of Krp1 dramatically elongated pseudopodia in transformed rat fibroblasts, and a truncated Krp1 or siRNA targeted against Krp1 (27) reduced pseudopodial elongation, leading to the suggestion that Krp1 has a key role in the regulation of cell motility and invasion. Thus Krp1 is localized with F-actin and the actin binding protein Lasp-1 at the tips and membrane ruffles of extending pseudopodia in an activator protein-1-regulated manner in Fos- and Ras-transformed fibroblasts (25). A role for Krp1 in nontransformed cells is less clear, although the BTB-Poz Kelch protein Mayven interacts with actin and has an important role in lamellipodia and process formation in oligodendrocytes (29).
During myogenesis, undifferentiated myoblasts must migrate from the somite to sites of muscle formation, elongate, and ultimately fuse to form multinucleated myotubes (5). Similarly, adult muscle satellite cells have the ability to move to sites of muscle injury for subsequent repair. In microarray studies in Lbx1 mutant mice, which have a severe myoblast migration defect, the expression of the Kelch domain only containing protein Klbdc2 was significantly decreased (24). Overexpression of Klbdc2 in C2C12 myoblasts abolished the ability of the cells to respond to the chemoattractant properties of hepatocyte growth factor, known to be important in myoblast migration (10). In our preliminary studies, we observed upregulation of Krp1 between 24 and 48 h following induction of myoblast differentiation in C2 myoblasts, considerably before myofibril formation and in agreement with previous observations made in differentiating primary human myoblasts (17, 22). We therefore decided to investigate the hypothesis that Krp1 might have important functions at earlier stages of myoblast differentiation, such as cell migration and elongation. This was pursued by examining the pattern of its expression in more detail and the consequences of its knockdown [via short hairpin (sh)RNA] and overexpression in the established C2 mouse myoblast cell line.
MATERIALS AND METHODS
Reagents.
Commercially available antibodies were obtained as follows: rabbit anti-MEF2C and rabbit anti-myogenin from Santa Cruz; mouse anti-myogenin and mouse anti-myosin heavy chain from Developmental Studies Hybridoma Banks, University of Iowa; rabbit anti-pp38 from Promega; rabbit anti-myc from Cell Signaling; rabbit anti-β-actin from Abcam; goat anti-mouse-HRP and goat anti-rabbit-HRP from Bio-Rad; and goat anti-rabbit Alexa Fluor 488 and goat anti-mouse Alexa Fluor 568 from Molecular Probes. Rabbit polyclonal anti-Krp1 antibodies (raised against the Kelch repeat region of Krp1) were raised in-house [Spence et al. (26)].
The full-length coding region of rat Krp1 with a carboxy terminal myc tag in pcDNA3.1 was generated as described by Spence et al. (26).
Generation of C2 cell lines stably overexpressing Krp1-myc.
Krp1-myc was subcloned from pcDNA3.1 into the pBabe puromycin retroviral vector via EcoR1 restriction sites using PCR and the following primers: 5′: CTAAGGAATTCATGGATTCCCAGCGG, and 3′: GTAGTCGAATTCTTAAACTCAATGATG. Plat-E helper cells were transfected using the Calphos mammalian transfection kit (Calbiochem) according to the manufacturer's instructions. Media were changed after 18 h and harvested after 24 h, filtered through a 0.45-μm filter, and made up to a final concentration of 4 μg/ml polybrene and 10% FBS and 10% new born calf serum. C2 myoblasts were infected with viral titres and seeded as 2 × 105 cells in a 1-ml viral suspension. Control cell lines were generated via the same procedures using empty vector. All cells were cultured in growth medium and selected for the presence of pBABE vector with 2 μg/ml puromycin for 2 wk, when all control cells were dead.
Generation of C2 cell lines expressing shRNA targeted against mouse Krp1.
Stable shRNA cell lines were generated to knock down Krp1 using the pSiren-RetroQ-ZsGreen vector (Clontech). The Babraham Institute Inhibitory design tool (Babraham Bioinformatics) was used to predict putative target siRNA sequences against mouse Krp1 and against Escherichia coli maltose binding protein (MBP) as a control. Four sets of sequences were tested but only two consistently knocked down Krp1. Successful sequences are as follows: shK1 (target sequence: CGTGGATCCTGCTATACTTTT): sense: 5′-gatccGCGTGGATCCTGCTATACTTTTTTCAAGAGAAAAAGTATAGCAGGATCCACGTTTTTTACGCGTg-3′, and antisense: 5′-aattcACGCGTAAAAAACGTGGATCCTGCTATACTTTTTCTCTTGAAAAAAGTATAGCAGGATCCACGCg-3′; shK2 (target sequence: ATGAGTGCTACCTTACTGCTT): sense: 5′-gatccATGAGTGCTACCTTACTGCTTTTCAAGAGAAAGCAGTAAGGTAGCACTCATTTTTTTACGCGTg-3′, and antisense: 5′-aattcACGCGTAAAAAAATGAGTGCTACCTTACTGCTTTCTCTTGAAAAGCAGTAAGGTAGCACTCATg-3′; and MBP (target sequence: GCAGTACCGTTACACCATA): sense: 5′-gatccGCAGTACCGTTACACCATATTCAAGAGATATGGTGTAACGGTACTGCTTTTTTACGCGTg-3′, and antisense: 5′-aattcACGCGTAAAAAAGCAGTACCGTTACACCATATCTCTTGAATATGGTGTAACGGTACTGCg-3′. Plat-E helper cells were transfected with the generated sh constructs, and C2 cells were transfected as described above. FACS analysis was used to select ZsGreen-expressing cells; these populations were expanded, rechecked for ZsGreen expression (via FACS), and frozen at −80 C.
C2 myoblast cell culture.
C2 myoblasts (31), a gift from Claire Stewart (Manchester Metropolitan University, Manchester, UK) were seeded (1 × 105 cells) onto 2% gelatin-coated 60-mm plates and cultured in growth medium (GM: DMEM supplemented with 10% FBS and 10% new born calf serum). Differentiation was induced 48 h after seeding by replacing GM with differentiation medium (DM: DMEM with 2% horse serum).
Whole cell extraction.
C2 cells were washed in PBS and harvested in 250 μl of lysis buffer (20 mM Tris·HCl pH 7.5, 137 mM NaCl, 1 mM EGTA pH 8, 1% Triton X-100, 10% glycerol, and 1.5 mM MgCl2) containing protease and phosphatase inhibitors (10 mM NaF, 1 mM PMSF, 1 mM Na metavanadate, 5 μg/ml aprotinin, and 10 μg/ml leupeptin). Total protein was determined by Bradford assay (Bio-Rad).
Western blot analysis.
Thirty-five micrograms of protein from cell lysates or 35 μl of conditioned media were resolved by SDS-PAGE (10% acrylamide) and transferred to an Immobilon-P membrane (Millipore) by electroblotting. The membranes were blocked with 0.2% I-block (Applied Biosystems), 0.1% Tween 20 in PBS and probed with corresponding primary and secondary antibodies. Antigen-antibody complexes were visualized using ECL reagents (Amersham, Little Chalfont, UK). Scanned autoradiographs were semiquantified using AIDA two-dimensional densitometry software and normalized as a function of the loading control/total expression of the various proteins.
Immunofluorescence.
Myoblasts were seeded at 5.4 × 104 cells onto a 25-mm diameter Thermanox coverslip (Nunc, Roskilde, Denmark) coated in 2% gelatin. Cells were fixed in 4% paraformaldehyde and permeabilized with 0.2% Triton X-100, blocked, and incubated with appropriate primary antibody at 4°C overnight, followed by appropriate secondary antibody. Coverslips were mounted in VECTASHIELD HardSet Mounting Medium with DAPI (Vector Laboratories, Peterborough, UK). With the use of a ×40/1.4 NA objective, ∼70 fields of view were examined on each coverslip. Confocal images were captured on an Olympus FV 1000 Zeiss confocal microscope. Fluorescence excitations/emissions were as follows (in nm): 405/440–480 (DAPI), 488/505–550 (Alexa Fluor 488), and 561/604–668 (Alexa Fluor 568).
Isolation of Krp1 promoter and luciferase promoter reporter assays.
A 2,000-bp region upstream of the Krp1 start site was identified using the Transcription Element Search System (TESS; Jonathan Schug and G. Christian Overton, 1997, University of Pennsylvania, www.cbil.upenn.edu/tess, in conjunction with Babraham Bioinformatics). To extract genomic DNA, the testes of a 4-mo-old wild-type C57 B6/J mouse were incubated overnight at 55°C in 10 mM Tris/1 mM EDTA buffer containing 0.1 M NaCl, 1% SDS, and 0.5 mg/ml proteinase K. DNA was isolated by addition of 0.5 ml of phenol and vigorous shaking for 30 s followed by centrifugation at 13,000 rpm. The aqueous phase containing genomic DNA was removed, and DNA was precipitated by addition 1 ml 100% ethanol and 40 μl 3 M sodium acetate and incubation at −20°C overnight. DNA was reextracted and washed in 70% ethanol; the final DNA pellet was resuspended in 30 μl Tris-EDTA buffer.
The 2,000-bp putative Krp1 promoter region was amplified using Velocity DNA polymerase (Bioline) to generate a blunt ended product that was cloned into the pGL3 luciferase reporter vector (Promega). Krp1 promoter-reporter activities were determined using the dual-luciferase reporter assay system (Promega), as described in the manufacturer's instructions. C2 myoblasts were additionally transiently transfected (using Lipofectimine 2000; Invitrogen; according to the manufacturer's instructions) as appropriate and lysates were harvested after 48 h in DM, as previously described (14).
Cell number and bromodeoxyuridine incorporation.
Cell number was determined using a hemocytometer. Cell proliferation was indicated by bromodeoxyuridine (BrdU) incorporation using a proliferation assay kit (Calbiochem), according to the manufacturer's instructions.
Caspase-3 assay.
Caspase-3 activity was determined by cleavage of the fluorigenic Ac-DEVD-AMC caspase-3 substrate (Calbiochem) according to the manufacturer's instructions. Cells (detached and adherent, total 50 μg protein per assay) were washed with ice cold PBS, harvested into caspase lysis buffer (50 mM Tris·HCl pH 7.5, 150 mM NaCl, 0.5 mM EDTA, and 0.5% Nonidet P-40), and incubated on ice for 20 min.
Fusion index.
Fusion index was calculated as the proportion of cells (myotubes) containing two or more nuclei (8). For each individual experiment, five microscope fields were counted, and each experiment was performed at least three times for each cell line.
Statistical analyses.
Data were analyzed by ANOVA, using treatment (Krp1 overexpression or knockdown vs. control) and time as main effects and employing a Bonferroni correction where multiple comparisons were made; they are presented as means ± SE. All observations are derived from at least three independent experiments.
RESULTS
Expression of Krp1 in differentiating C2 myoblasts.
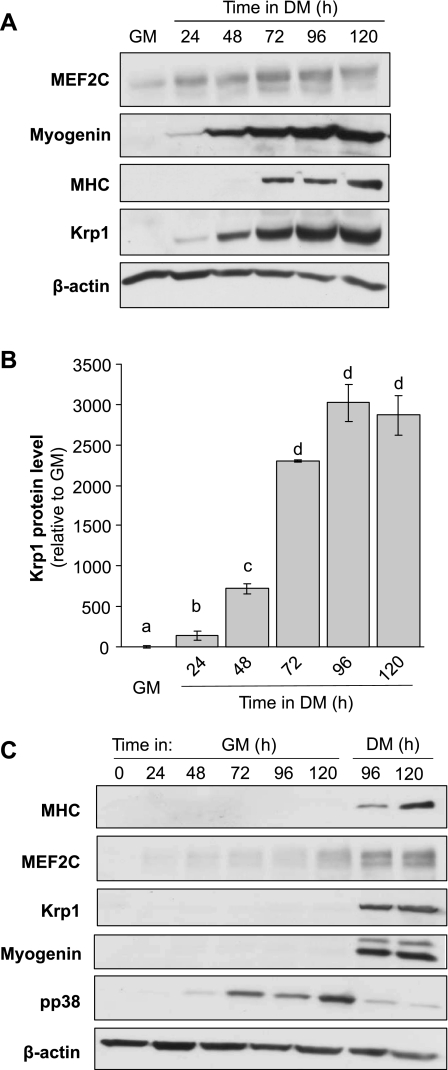
The protein levels of Krp1 were compared with those for the key myogenic markers, myogenin, myosin heavy chain (MHC), and MEF2C, following induction of differentiation by transfer of cells from high serum GM to low serum DM, as described in the materials and methods (Fig. 1). Withdrawal of serum to initiate differentiation is well established and induces upregulation of the strongly promyogenic growth factor IGF-II (13). As previously observed (7, 21), expression of the transcription factor myogenin is observed 24–48 h after transfer to DM and the later structural protein MHC at 72 h. The abundance of MEF2C gradually increased during differentiation and its apparent mobility decreased due to the increased phosphorylation of MEF2C that occurs as differentiation progresses. Krp1 protein was detectable as early as 24 h after transfer to DM, suggesting that it might have a role in the earlier phases of myogenesis. Its levels then increased massively and significantly to peak at 96 h (Fig. 1, A and B).
Fig. 1.
Expression of Kelch-related protein 1 (Krp1) in differentiating myoblasts. A: Western blot to reveal protein levels of MEF2C, myogenin, myosin heavy chain (MHC), Krp1, and β-actin (loading control) in cell lysates derived from C2 cells cultured either in growth medium (GM) or differentiation medium (DM) for the time indicated; representative of n = 4 independent experiments. B: fold increase in Krp1 protein levels from individual blots relative to levels in GM. Data are means ± SE; n = 4. Bars with different letters have values that are significantly different as assessed by ANOVA (P < 0.05). C: Western blot for MHC, MEF2C, Krp1, myogenin, phospho-p38 (pp38), and β-actin (loading control) in cell lysates derived from C2 cells cultured either in GM or DM for the time points indicated; representative image from n = 3 independent experiments.
As myoblasts continue to proliferate during the early stages of myogenesis and cell density consequently increases, the stimulus for Krp1 expression could be derived either from cell-cell contact mechanisms or by differentiation-specific cues. We therefore cultured cells in either GM or DM and determined Krp1 protein levels. As a positive control for signaling stimuli derived from cell-cell contact, phospho-p38 levels were determined (21) and as expected these increased in the cells maintained in GM, which stimulated increased cell density (Fig. 1C); however, cells in GM did not differentiate, as indicated by the lack of upregulation of myogenin, MHC, or MEF2C. In contrast, these were observed in myoblasts transferred to DM (Fig. 1C, right two lanes vs. left six lanes). Significantly, Krp1 protein was only observed in the cells cultured in DM, indicating that a myogenic stimulus was required to upregulate its expression.
Krp1 is upregulated following myogenin expression.
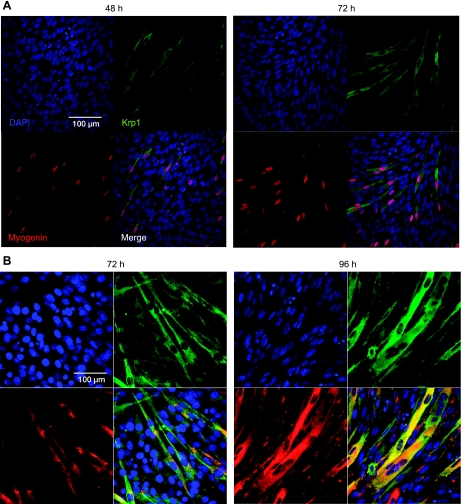
In the Western blot analysis in Fig. 1A, it appeared that Krp1 protein was upregulated at either about the same time or just after myogenin but before MHC, and we decided to investigate this temporal relationship further. Figure 2A shows immunofluorescence to display myogenin (red) and Krp1 in differentiating C2 cells. At 48 h (Fig. 2, A and B, left), myogenin can be detected in the nuclei of a minority of cells, as they begin to enter terminal differentiation. Krp1 was observed in a proportion of myogenin-positive cells these cells are elongated and undergoing migration and alignment (see merged images, Fig. 2, A and B, bottom right). However, Krp1 was never observed in the absence of myogenin, confirming that myogenin is upregulated before Krp1, and also suggesting that it could have a role in the regulation of Krp1 transcription. In agreement with previous studies (26), Krp1 localization was cytoplasmic but we did not observe Krp1 concentrated at putative pseudopodial structures, as has been observed in transformed fibroblasts (26). Observations at 72 h (Fig. 2, A and B, right) supported these findings. The localization of Krp1 and MHC was next investigated at 72 and 96 h after the induction of differentiation (Fig. 2B). Again, Krp1 localization was cytoplasmic, and at 96 h, it was observed in cells that were beginning to fuse. MHC was only observed in cells that were Krp1 positive at both 72 and 96 h, suggesting that MHC is upregulated after Krp1. Thus we have established a hierarchy for Krp1 expression during myoblast differentiation, with myogenin preceding and MHC following Krp1 upregulation.
Fig. 2.
Immunofluorescence to reveal Krp1 localization in differentiating C2 cells. C2 cells were cultured in DM for the time points indicated, and immunofluorescence was performed to display DAPI (blue), Krp1 (green), and myogenin (red) in A or DAPI (blue), Krp1 (green), and MHC (red) in B. Merged images are also displayed; representative images from 3 independent experiments.
The association of Krp1 with either F-actin (phalloidin staining) or the structural protein α-actinin was investigated; even through limited colocalization of Krp1 with these proteins was sometimes observed, there was no evidence for an enrichment of expression at myoblast membrane projections (data not shown).
Identification of E box elements within the Krp1 promoter.
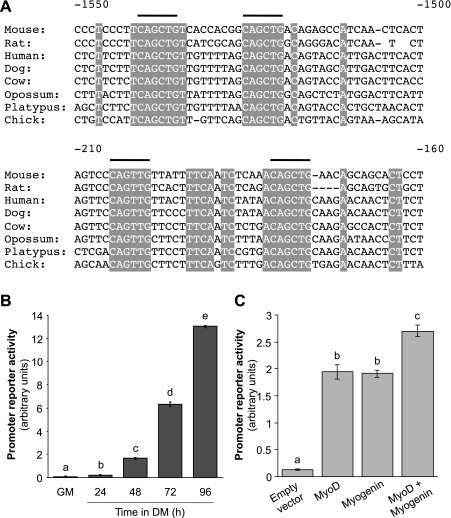
The transcription element search software identified eight clusters of putative myogenic E boxes (CAnnTG) in genomic DNA sequence 2,000-bp upstream from the Krp1 start site to which basic helix-loop-helix (bHLH) myogenic transcription factor family members bind strongly when in complex with E proteins (20). Two regions are conserved across mammals, opossum, platypus, and chicken, as shown in Fig. 3A, indicating their potential importance; one region is proximal to the Krp1 transcription start site (∼ −175 to −205) and the other is more distal (∼ −1,520 to −1,540). To test the activity of this combined region, a fragment of genomic DNA 2,000-bp upstream of the Krp1 start site was cloned into a luciferase reporter vector as described in the materials and methods. The activity of this promoter was very low in GM but increased significantly by almost 14-fold after C2 cells had been in DM for 96 h (Fig. 3B). As E boxes are consensus binding sites for all members of the myogenic bHLH proteins myogenic transcription factor family, we tested the ability of the early MyoD and terminal myogenin bHLH proteins (6) to activate the Krp1 promoter construct. C2 myoblasts were maintained in GM and transiently transfected with either MyoD or myogenin, or both. Both constructs significantly increased Krp1 promoter-reporter activity individually by twofold vs. empty vector transfected cells; in combination they further induced promoter-reporter activity to almost threefold (Fig. 3C). These increases are not as great as those observed following transfer of C2 cells from GM to DM but simple transfection with a bHLH protein in growth medium will not be such a strong myogenic stimulus. Overall, these findings suggest that Krp1 expression is regulated by bHLH proteins.
Fig. 3.
Putative Krp1 promoter regions have consensus E box elements. A: alignment of Krp1 sequence from different species upstream of the start site to display 4 conserved E boxes. B: promoter-reporter activity of the putative Krp1 reporter sequence encompassing sequence −2,000 bp from the Krp1 start site derived from C2 cells cultured in GM or DM for the time points indicated. C: promoter-reported activity in C2 cells transfected with either empty vector, MyoD, myogenin, or both MyoD and myogenin. Data are means ± SE; n = 3. Bars with different letters are significantly different as assessed by ANOVA (P < 0.05).
shRNA knockdown of Krp1 protein levels.
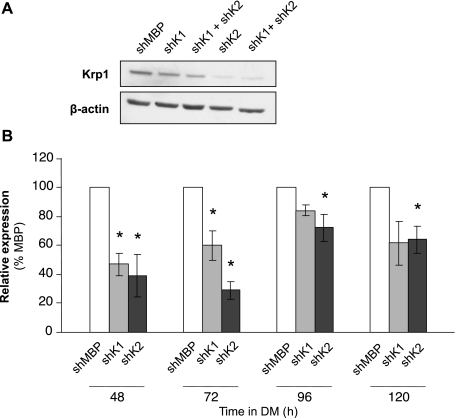
Since we demonstrated that Krp1 protein levels increased in response to myogenic stimuli and that its reporter contained consensus E box elements, we next determined the effects of Krp1 knockdown. This was achieved by generation of C2 myoblasts that stably expressed shRNAs targeted to regions within the coding sequence, as described in materials and methods. Four targets were assessed; all of these reduced Krp1 protein levels in early differentiating C2 myoblasts (data not shown), but the knockdown was only sustained for two of these, termed shK1 and shK2. Figure 4A shows Krp1 protein levels in C2 myoblasts stably expressing the shRNA targeted to MBP (lane 1) and to shK1 and -2 alone (lanes 2 and 4), 72 h after the initiation of differentiation. shK2 was consistently more effective than shK1 in Krp1 knockdown, as demonstrated by quantification of three independent differentiation time courses (48 to 120 h; Fig. 4B). The ability of a combination of shK1 and shK2 to further reduce Krp1 protein levels was assessed by transient transfection of the individual stable C2 cell lines with the reciprocal shRNA; this did not result in exacerbated knockdown (Fig. 4A, lanes 3 and 5), and therefore the stable cell lines expressing either shK1 and -2, together with the control shMBP line, were used for further studies.
Fig. 4.
Short hairpin (sh)RNA-mediated knockdown of Krp1 protein levels. A: Western blots for Krp1 and β-actin (loading control) in lysates of cells stably transfected with shRNA targeted to MBP (shMBP) or Krp1 (shK1 and shK2) and harvested after 72 h in DM. B: quantification of Krp1 protein levels assessed by Western blot as in A at the time points indicated in DM, and expressed relative to levels in the shMBP line. Data are means ± SE. *P < 0.05, significantly different from shMBP cells as assessed by t-test; n = 3 independent experiments.
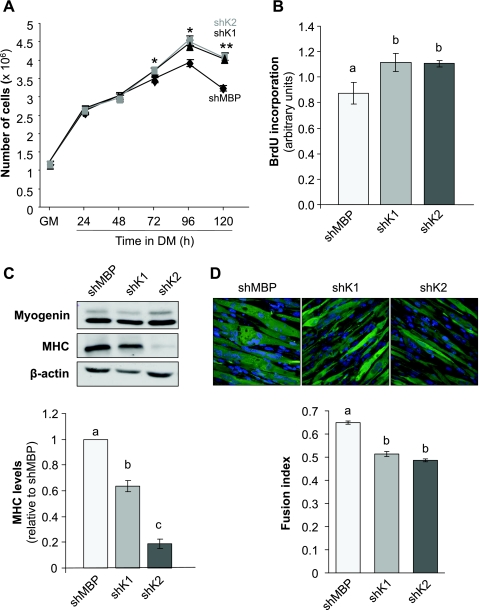
The phenotype of Krp1 knockdown and control C2 cells was assessed in terms of ability to proliferate, to resist apoptosis, and to differentiate. It is well established that myoblast cell number increases before differentiation during myogenesis (12); cell number increased an equivalent amount until 72 h after transfer of C2 cells to DM. Cell number reached a plateau in the shMBP control cell line by 72 h (cell number at 72 and 96 h were not significantly different) whereas it continued to increase in both Krp1 knockdown lines until 96 h (Fig. 5A). A change in cell number could be due to alterations in either cell proliferation or death, and therefore both of these were assessed. Apoptosis, assessed by cleaved caspase-3 activity, was similar at all time points between control and Krp1 knockdown cells (data not shown). However, proliferation, measured by BrdU incorporation, increased significantly at 120 h in DM in both shKrp1 lines, when compared with the control shMBP line (Fig. 5B).
Fig. 5.
Phenotypic analysis of C2 cells with shRNA-mediated knockdown of Krp1. A: cell number in GM and during differentiation for the times indicated. *P < 0.05, **P < 0.01, significantly different from shMBP cells by ANOVA; n = 3. B: bromodeoxyuridine (BrdU) incorporation into stable shRNA cells lines at 120 h. Data are means ± SE. Bars with different letters are significantly different by ANOVA (P < 0.05); n = 3. C, top: myogenin and MHC and β-actin (loading control) protein levels in stable shRNA cell lines at 120 h. C, bottom: quantification of MHC protein levels displayed at top. Bars with different letters are significantly different as assessed by t-test (P < 0.05); n = 3. D: assessment of cell fusion in stable shRNA cells. D, top: images of stable shRNA cell lines that coexpress ZsGreen; objective ×400. D, bottom: fusion index, as calculated in the materials and methods. Bars with different letters are significantly different by ANOVA (P < 0.01); n = 3.
Increased proliferation can delay differentiation in myoblasts, and therefore the effects of Krp1 knockdown on indexes of differentiation were investigated. Myogenin levels were similar between shMBP and shK1 and shK2 cell lines between 48 and 120 h after transfer to DM (levels at 120 h are shown in Fig. 5C, top); this is not surprising as upregulation of myogenin precedes that for Krp1. However, MHC protein levels were reduced at both 96 and 120 h (shown for 120 h in Fig. 5C, top quantified at bottom), with the phenotype being stronger for the shK2 than the shK1 line, thus correlating with their relative abilities to reduce Krp1 levels. Differentiation was further assessed by determining the fusion index of myotubes at 120 h. The proportion of nuclei in ZsGreen-expressing cells that were within multinucleated structures was determined for shMBP, shK1, and shK2 cell lines (Fig. 5D, top quantified at bottom). The morphology of the control shMBP cells differed from that for the two Krp1 knockdown lines, the control myocytes/myotubes being “thick” and the Krp1 knockdown cells appearing less hypertrophied, though aligned. The fusion index was ∼0.65 for the control line but was reduced significantly for both Krp1 knockdown lines to ∼0.5.
An established scratch wound assay (9, 11), as described in Carter et al. (7), was used to investigate whether Krp1 knockdown would change the ability of myoblasts to migrate. Myoblasts were cultured in DM for 48 h and then “scratched”; the distance migrated across the scratch was measured over the next 6 h. No differences were observed between the shMBP, shK1, and shK2 lines (data not shown). This was pursued using individual live cell imaging, but again, no differences were observed (data not shown).
Taken together, these findings support a role for endogenous Krp1 in stimulating cell cycle exit and in accelerating differentiation; however, we could find no evidence for a role for Krp1 in myoblast migration.
Overexpression of Krp1.
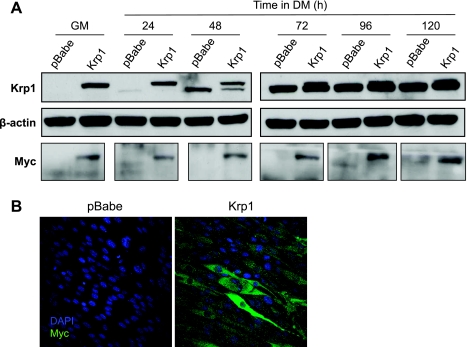
Our next aim was to overexpress rat Krp1 (which should be resistant to the shRNA Krp1 targets because of a lack of identity between the rat and mouse sequences) in the mouse Krp1 knockdown lines to determine whether their phenotype could be reversed; however, overexpression was not sustained for unidentified reasons. We therefore generated stable C2 cell lines that simply overexpressed COOH-terminal myc-tagged Krp1, as described in the materials and methods. Expression of transfected and endogenous Krp1 is presented in Fig. 6A, top. The myc-tagged Krp1 resolved, as predicted, at a slower mobility than endogenous Krp1, and its apparent molecular weight coincided with that for myc (Fig. 6A, bottom). Transfected Krp1 was strongly expressed in GM, and this was maintained to 120 h in DM. Expression of endogenous Krp1 was delayed slightly till 72 h in cells stably expressing Krp1-myc. Transfected Krp1 was readily detected by immunofluorescence (Fig. 6B), which demonstrated that nearly all puromycin-selected cells overexpressed Krp1.
Fig. 6.
Stable overexpression of Krp1-myc in C2 cells. A, top: Krp1 protein levels in C2 cells stably expressing vector alone (pBabe) or Krp1-myc (Krp1) in GM and in DM for the times indicated. Endogenous Krp1 is the bottom band, and Krp1-myc is the top band. A, middle: β-actin (loading control). A, bottom: myc to display the transfected Krp1. B: immunofluorescence to show the localization of transfected Krp1-myc by myc IF. Representative of n = 3 separate experiments.
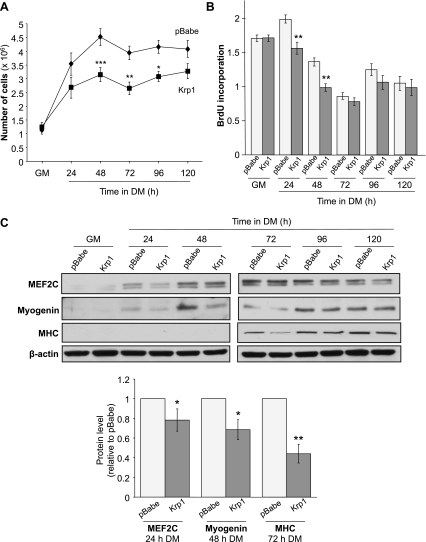
The phenotype of Krp1-myc overexpressing myoblasts was assessed for comparison with the Krp1 knockdown lines (Fig. 7). In agreement with Krp1 knockdown inducing increased cell number, Krp1-myc overexpressing cells exhibited a significant decrease in cell number compared with empty vector-transfected cells from 48 h after transfer to DM (Fig. 7A). Again this was not due to changes in cell death, as assessed by cleaved caspase-3 activity (data not shown), but was instead via decreased proliferation, as indicated by significantly reduced BrdU incorporation at 24 and 48 h and a tendency for reduced incorporation thereafter (Fig. 7B). Further, overexpression of Krp1-myc inhibited differentiation, as assessed by myogenin, MEF2C, and MHC protein levels by 20–50% (Fig. 7C, top, Western blot, and bottom, quantified data at the key time point in their upregulation). Moreover, fusion index was also inhibited significantly (P < 0.001) to 50% of vector-transfected cells by Krp1 overexpression (data not shown). The effects of Krp1 overexpression on cell migration, indicated by the scratch wound assay, were investigated; in agreement with the knockdown data, no differences were observed between control and Krp1 overexpressing lines (data not shown). Since differentiation is in part regulated by cell-cell contact, the reduced proliferation in Krp1-overexpressing cells could have inhibited subsequent differentiation. Therefore, the Krp1-overexpressing and pBabe empty vector myoblasts were cultured at four times increased density, as described in Lovett et al. (21). Krp1 overexpression still induced a significant decrease in cell number and also reduced protein abundances of MEF2C, myogenin, and MHC (data not shown). Although not unequivocal, these findings suggest that a primary effect of Krp1 overexpression is of inhibited proliferation.
Fig. 7.
Phenotype of C2 cells stably overexpressing Krp1-myc. A: cell number in GM and during differentiation for the times indicated. *P < 0.05, **P < 0.01, ***P < 0.001, significantly different from pBabe cells by ANOVA; n = 3. B: BrdU incorporation pBabe and Krp1-myc cells lines in GM and in DM. Data are means ± SE. **P < 0.01, significantly different from pBabe cells assessed by ANOVA; n = 3. C, top: MEF2C, myogenin and MHC and β-actin (loading control) protein levels in pBabe and Krp1-myc cell lines in GM and DM at the time points indicated. C, bottom: quantification of MEF2C, myogenin, and MHC protein levels displayed at top at the critical time points when they are upregulated in control pBabe cells. Data are means ± SE relative to pBabe lines. *P < 0.05, **P < 0.01 as assessed by t-test; n = 3.
DISCUSSION
In this study, we demonstrate upregulation of Krp1 relatively early in myogenesis after myogenin expression but before that for later differentation structural proteins such as MHC. Examination of murine Krp1 putative promoter regions revealed two pairs of consensus myogenic E boxes; a luciferase promoter-reporter containing a region −2,000 bp to the Krp1 start site and encompassing these elements was responsive to both MyoD and myogenin, suggesting that upregulation of Krp1 is part of an orchestrated myogenic program. Knockdown and overexpression of Krp1 indicated that it has direct or indirect functions in myogenesis before myofibrillar formation.
Endogenous Krp1 protein appeared to be evenly distributed in the cytoplasm of differentiating myoblasts and was always extranuclear; when overepressed, its localization remained apparently evenly cytoplasmic. Mainly cytoplasmic Krp1 was observed previously in normal and transformed fibroblasts (26); however, in transformed cells, or those transfected with Krp1 and treated with epidermal growth factor, Krp1 is additionally concentrated at tips of lamellipodia (26, 27), leading to the hypothesis that it is involved with a cell migration and invasion phenotype. In cardiomyocytes, Krp1 has a punctate cytoplasmic distribution before myofibril formation (16). Further investigation, in which cardiomyocytes were detergent extracted to separate insoluble (i.e., cytoskeletal) and soluble components of the cytoplasm, revealed that up to 40% of Krp1 was associated with the cytoskeletal fraction (16); in preliminary studies, we observed a similar proportion of Krp1 in the insoluble fraction (data not shown). Mayven is localized cytoplasmically and to the leading edges during process formation in oligodendrocytes; however, these are destined to wrap around axons rather than induce cell migration (29). Thus it is possible that the regulation of cell invasiveness is not a normal physiological function for Krp1.
Myogenesis is orchestrated in a strictly temporal manner by a family four basic helix-loop-helix transcription factors: MyoD, Myf5, myogenin, and MRF4. All of these form heterodimers with constitutively expressed E proteins and bind to the core E box sequence CAnnTG (32). E boxes are widely distributed, and the specificity of the myogenic bHLH proteins to only activate myogenic targets is likely due to further interaction with nearby elements or to association with other proteins such as the MEF2 family. While myogenic bHLH proteins have unique targets, they can in certain circumstances exhibit considerable overlap (2, 3). The Krp1 promoter has not been examined previously, and we identified two pairs of consensus E boxes in a region 2,000-bp upstream of the start of the Krp1 coding sequence that are compatible with the hypothesis that they could be myogenic targets. The increased activity of a luciferase promoter-reporter encompassing these E boxes both during normal differentiation of C2 myoblasts and after transfection of C2 cells with myogenin and/or MyoD supports their functional significance in the context of high Krp1 expression in muscle. A specific myogenic stimulus for Krp1 expression is also supported by our observation that Krp1 was only upregulated after myoblasts were subjected to a differentiation stimulus: cell-cell contact/increased cell density was not sufficient. Whether all of the four of the identified Krp1 E boxes are of equal importance in regulating its expression was not determined. However, multiple functional E boxes have been identified in other myogenic promoters; for example, MyoD binds to up to eight E boxes within the Akt2 promoter, ranging from approximately −50 to −1,850 bp from the Akt2 start site (18).
A role for Krp1 in the early stages of myogenesis, before myofibril formation, was investigated by both its shRNA-mediated knockdown and its overexpression. To try and avoid artefactual data generated by off-target effects, two different Krp1 shRNA targets were characterized that decreased Krp1 protein levels throughout the period of differentiation studied; in general they yielded compatible data that were related to the reduction of Krp1 protein expression. Krp1 overexpression was mediated via stable transfection with a myc-tagged rat Krp1 construct, and its expression was also maintained throughout the period studied. Knockdown of Krp1 increased C2 myoblast cell number and overexpression reduced cell number. No effect of changed Krp1 on apoptosis was observed at any time point during differentiation, a finding that is supported by studies in cardiomyocytes (16). However, effects of Krp1 on cell proliferation were observed, with knockdown inducing increased proliferation and overexpression inhibiting proliferation and suggesting that this was the principal mechanism by which Krp1 regulated cell number. Inhibition of proliferation following overexpression of the Kelch domain only Klhdc2 protein in C2C12 myoblasts has been reported; its knockdown induced a minimal phenotype (24). Further, HCF-1, also a Kelch domain only protein, interacts with both E2F1 and E2F4 to increase or decrease cell proliferation, depending on conditions (19). However, its localization is nuclear, so this is unlikely to be a mechanism by which Krp1 regulates proliferation.
Paradoxically, both Krp1 knockdown and overexpression inhibited C2 myoblast differentiation, as assessed by relative protein levels of markers for key stages of myogenesis and also by determination of cell fusion. Overexpression of Klhdc2 in C2C12 myoblasts also inhibited differentiation by unidentified mechanisms (24). However, the temporal aspects of Krp1 regulation of C2 cell myogenesis differed, depending on whether its overexpression was increased or decreased. In the Krp1 knockdown studies, myogenin protein levels were equivalent to those for control cells, which was consistent with the earlier observation of myogenin upregulation prior increased Krp1 expression. Thus Krp1 only inhibited later stages of differentiation, exemplified by reduced MHC levels. In the overexpression studies, C2 cells were exposed to considerably increased levels Krp1 before they normally would have been, even in GM before the onset of differentiation. Therefore, it must be questioned whether the consequences of premature Krp1 overexpression on differentiation truly reflect physiological phenomena or artefact.
Given the importance of cell migration and elongation in all myogenic precursers, the specific and high expression of Krp1 in myogenic lineages, and its reported role in cell morphology and migration, we had predicted that Krp1 might have a key role in myoblast migration. However, no effects of Krp1 overexpression or knockdown were observed on cell motility, either via a scratch wound assay or using live cell imaging. Changes in myoblast “thickness” were detected, but these were probably secondary to inhibited differentiation and therefore structural protein accumulation. Greenberg and colleagues also observed no effects of Krp1 on cardiomyocyte cell spreading (16), and paradoxical effects of Klhdc2 were not detected in C2C12 cells (24). siRNA targeting of Mayven had no effect on oligodendrocyte migration (29). When considered together with observations made in transformed cells (25), it seems likely that Krp1, and possibly related BTB-Kelch domain proteins, do not regulate cell motility unless they are transformed, in which case Krp1 upregulation and actions are part of a wider coordinated response involving other cytoskeletal-interacting proteins.
GRANTS
We thank the UK Biotechnology and Biological Sciences Research Council for Competitive Strategic funding for this work and the Gates Cambridge Trust for studentship funding to C. W. Paxton.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Adams J, Kelso R, Cooley L. The kelch repeat superfamily of proteins: propellers of cell function. Trends Cell Biol 10: 17–24, 2000 [DOI] [PubMed] [Google Scholar]
- 2. Bergstrom DA, Penn BH, Strand A, Perry RL, Rudnicki MA, Tapscott SJ. Promoter-specific regulation of MyoD binding and signal transduction cooperate to pattern gene expression. Mol Cell 9: 587–600, 2002 [DOI] [PubMed] [Google Scholar]
- 3. Blais A, Tsikitis M, Acosta-Alvear D, Sharan R, Kluger Y, Dynlacht BD. An initial blueprint for myogenic differentiation. Genes Dev 19: 553–569, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bork P, Doolittle RF. Drosophila kelch motif is derived from a common enzyme fold. J Mol Biol 236: 1277–1282, 1994 [DOI] [PubMed] [Google Scholar]
- 5. Buckingham M, Vincent SD. Distinct and dynamic myogenic populations in the vertebrate embryo. Curr Opin Genet Dev 19: 444–453, 2009 [DOI] [PubMed] [Google Scholar]
- 6. Cao Y, Kumar RM, Penn BH, Berkes CA, Kooperberg C, Boyer LA, Young RA, Tapscott SJ. Global and gene-specific analyses show distinct roles for Myod and Myog at a common set of promoters. EMBO J 25: 502–511, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carter EJ, Cosgrove RA, Gonzalez I, Eisemann JH, Lovett FA, Cobb LJ, Pell JM. MEK5 and ERK5 are mediators of the pro-myogenic actions of IGF-2. J Cell Sci 122: 3104–3112, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Das M, Wilson K, Molnar P, Hickman JJ. Differentiation of skeletal muscle and integration of myotubes with silicon microstructures using serum-free medium and a synthetic silane substrate. Nat Protoc 2: 1795–1801, 2007 [DOI] [PubMed] [Google Scholar]
- 9. DeBiasio R, Bright GR, Ernst LA, Waggoner AS, Taylor DL. Five-parameter fluorescence imaging: wound healing of living Swiss 3T3 cells. J Cell Biol 105: 1613–1622, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dietrich S, Abou-Rebyeh F, Brohmann H, Bladt F, Sonnenberg-Riethmacher E, Yamaai T, Lumsden A, Brand-Saberi B, Birchmeier C. The role of SF/HGF and c-Met in the development of skeletal muscle. Development 126: 1621–1629, 1999 [DOI] [PubMed] [Google Scholar]
- 11. Etienne-Manneville S, Hall A. Integrin-mediated activation of Cdc42 controls cell polarity in migrating astrocytes through PKCzeta. Cell 106: 489–498, 2001 [DOI] [PubMed] [Google Scholar]
- 12. Florini JR, Ewton DZ, Coolican SA. Growth hormone and the insulin-like growth factor system in myogenesis. Endocr Rev 17: 481–517, 1996 [DOI] [PubMed] [Google Scholar]
- 13. Florini JR, Magri KA, Ewton DZ, James PL, Grindstaff K, Rotwein PS. “Spontaneous” differentiation of skeletal myoblasts is dependent upon autocrine secretion of insulin-like growth factor-II. J Biol Chem 266: 15917–15923, 1991 [PubMed] [Google Scholar]
- 14. Gonzalez I, Tripathi G, Carter EJ, Cobb LJ, Salih DA, Lovett FA, Holding C, Pell JM. Akt2, a novel functional link between p38 mitogen-activated protein kinase and phosphatidylinositol 3-kinase pathways in myogenesis. Mol Cell Biol 24: 3607–3622, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gray CH, McGarry LC, Spence HJ, Riboldi-Tunnicliffe A, Ozanne BW. Novel beta-propeller of the BTB-Kelch protein Krp1 provides a binding site for Lasp-1 that is necessary for pseudopodial extension. J Biol Chem 284: 30498–30507, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Greenberg CC, Connelly PS, Daniels MP, Horowits R. Krp1 (Sarcosin) promotes lateral fusion of myofibril assembly intermediates in cultured mouse cardiomyocytes. Exp Cell Res 314: 1177–1191, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jacobson C, Duggan D, Fischbach G. Neuregulin induces the expression of transcription factors and myosin heavy chains typical of muscle spindles in cultured human muscle. Proc Natl Acad Sci USA 101: 12218–12223, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kaneko S, Feldman RI, Yu L, Wu Z, Gritsko T, Shelley SA, Nicosia SV, Nobori T, Cheng JQ. Positive feedback regulation between Akt2 and MyoD during muscle differentiation. Cloning of Akt2 promoter. J Biol Chem 277: 23230–23235, 2002 [DOI] [PubMed] [Google Scholar]
- 19. Knez J, Piluso D, Bilan P, Capone JP. Host cell factor-1 and E2F4 interact via multiple determinants in each protein. Mol Cell Biochem 288: 79–90, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Lassar AB, Davis RL, Wright WE, Kadesch T, Murre C, Voronova A, Baltimore D, Weintraub H. Functional activity of myogenic HLH proteins requires hetero-oligomerization with E12/E47-like proteins in vivo. Cell 66: 305–315, 1991 [DOI] [PubMed] [Google Scholar]
- 21. Lovett FA, Gonzalez I, Salih DA, Cobb LJ, Tripathi G, Cosgrove RA, Murrell A, Kilshaw PJ, Pell JM. Convergence of Igf2 expression and adhesion signalling via RhoA and p38 MAPK enhances myogenic differentiation. J Cell Sci 119: 4828–4840, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Lu S, Borst DE, Horowits R. Expression and alternative splicing of N-RAP during mouse skeletal muscle development. Cell Motil Cytoskeleton 65: 945–954, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lu S, Carroll SL, Herrera AH, Ozanne B, Horowits R. New N-RAP-binding partners alpha-actinin, filamin and Krp1 detected by yeast two-hybrid screening: implications for myofibril assembly. J Cell Sci 116: 2169–2178, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Neuhaus P, Jaschinsky B, Schneider S, Neuhaus H, Wolter A, Ebelt H, Braun T. Overexpression of Kelch domain containing-2 (mKlhdc2) inhibits differentiation and directed migration of C2C12 myoblasts. Exp Cell Res 312: 3049–3059, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Ozanne BW, Spence HJ, McGarry LC, Hennigan RF. Transcription factors control invasion: AP-1 the first among equals. Oncogene 26: 1–10, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Spence HJ, Johnston I, Ewart K, Buchanan SJ, Fitzgerald U, Ozanne BW. Krp1, a novel kelch related protein that is involved in pseudopod elongation in transformed cells. Oncogene 19: 1266–1276, 2000 [DOI] [PubMed] [Google Scholar]
- 27. Spence HJ, McGarry L, Chew CS, Carragher NO, Scott-Carragher LA, Yuan Z, Croft DR, Olson MF, Frame M, Ozanne BW. AP-1 differentially expressed proteins Krp1 and fibronectin cooperatively enhance Rho-ROCK-independent mesenchymal invasion by altering the function, localization, and activity of nondifferentially expressed proteins. Mol Cell Biol 26: 1480–1495, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Taylor A, Obholz K, Linden G, Sadiev S, Klaus S, Carlson KD. DNA sequence and muscle-specific expression of human sarcosin transcripts. Mol Cell Biochem 183: 105–112, 1998 [DOI] [PubMed] [Google Scholar]
- 29. Williams SK, Spence HJ, Rodgers RR, Ozanne BW, Fitzgerald U, Barnett SC. Role of Mayven, a kelch-related protein in oligodendrocyte process formation. J Neurosci Res 81: 622–631, 2005 [DOI] [PubMed] [Google Scholar]
- 30. Xue F, Cooley L. kelch encodes a component of intercellular bridges in Drosophila egg chambers. Cell 72: 681–693, 1993 [DOI] [PubMed] [Google Scholar]
- 31. Yaffe D, Saxel O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature 270: 725–727, 1977 [DOI] [PubMed] [Google Scholar]
- 32. Yun K, Wold B. Skeletal muscle determination and differentiation: story of a core regulatory network and its context. Curr Opin Cell Biol 8: 877–889, 1996 [DOI] [PubMed] [Google Scholar]