Abstract
Previous studies demonstrate that response gene to complement 32 (RGC-32) mediates transforming growth factor-β1-induced epithelial-mesenchymal transition (EMT) of human renal proximal tubular cells. However, the mechanisms underlying RGC-32 function remain largely unknown. In the present study, we found that RGC-32 function in EMT is associated with Smad3. Coexpression of RGC-32 and Smad3, but not Smad2, induces a higher mesenchymal marker α-smooth muscle actin (α-SMA) protein expression as compared with RGC-32 or Smad3 alone, while knockdown of Smad3 using short hairpin interfering RNA blocks RGC-32-induced α-SMA expression. These data suggest that RGC-32 interacts with Smad3, but not Smad2, in the regulation of EMT. In addition to α-SMA, RGC-32 and Smad3 also synergistically activate the expression of extracellular matrix protein fibronectin and downregulate the epithelial marker E-cadherin. RGC-32 colocalizes with Smad3 in the nuclei of renal proximal tubular cells. Coimmunoprecipitation assays showed that Smad3, but not Smad2, physically interacts with RGC-32 in renal proximal tubular cells. Mechanistically, RGC-32 and Smad3 coordinate the induction of EMT by regulating the EMT regulators Slug and Snail. Taken together, our data demonstrate for the first time that RGC-32 interacts with Smad3 to mediate the EMT of human renal proximal tubular cells.
Keywords: RGC-32, human renal proximal tubular cells
chronic progressive fibrosis of the kidney remains an unsolved challenge and it almost inevitably leads to end-stage renal failure, which requires dialysis or transplantation (11). Renal fibrosis can be initiated by a variety of primary insults, such as diabetes mellitus, hypertension, and autoimmune diseases (22, 25). It always involves massive deposition of matrix. Recent studies have shown that fibroblasts/myofibroblasts, usually present in large numbers in sites of fibrosis, are major matrix-producing cells (21, 23). Fibroblasts that are quiescent in healthy tissues can be activated and converted into myofibroblasts in fibrosis. Myofibroblasts are morphological intermediates between fibroblasts and smooth muscle cells, characterized by expression of smooth muscle cell (SMC) marker α-smooth muscle actin (α-SMA) and extracellular matrix proteins such as fibronectin. They are also characterized by the loss of epithelial cell marker E-cadherin or N-cadherin (9, 12). Although proliferation of preexisting fibroblasts may account for a portion of them during active fibrosis formation, their origin has not been definitely determined. Accumulating evidence indicates that a number of fibroblasts/myofibroblasts with advanced fibrosis arise through epithelial-mesenchymal transition (EMT). It is a process in which epithelial cells translocate from the epithelial compartment into the interstitium and gain the mesenchymal phenotype to participate in the synthesis of the fibrosis matrix (17).
The signaling generated by transforming growth factor-β (TGF-β) plays an essential role in EMT (19). TGF-β signaling is initiated by binding of the ligand to the type II receptor, which recruits and phosphorylates the type I receptor, resulting in its activation. The activated type I receptor further phosphorylates downstream signaling mediators Smad2 and Smad3 proteins, which then bind to Smad4 protein and are translocated into the nucleus where they act as transcription factors to activate expression of the TGF-β response gene involved in EMT (4, 15, 20). Although a number of experiments have demonstrated the importance of TGF-β and its Smad mediators in EMT, the downstream effectors of Smad signaling and their regulation that mediates EMT, however, remain largely unknown. Response gene to complement 32 (RGC-32) was initially reported as a gene induced by complement and involved in cell cycle activation (3). RGC-32 mRNA is abundantly expressed in the placenta, skeletal muscle, kidney, pancreas, liver, and aortic endothelial cells and is also overexpressed in colon cancer and several other tumors (2, 10). It can be induced by a variety of stimuli, such as complement activation and hypoxia (1, 2), luteinizing hormones (16), and growth factors. Our previous studies have shown that RGC-32 is important in TGF-β-induced EMT of human proximal tubular epithelial cells (HPTCs) (13). In the present study, we explored the interaction of RGC-32 with Smad signaling in the induction of EMT. We found that RGC-32 interacts with Smad3 to mediate TGF-β1-induced EMT of HPTCs.
EXPERIMENTAL PROCEDURES
Cell culture and reagents.
HPTCs were cultured as described previously (13, 18). Rat proximal tubular epithelial cells (NRK-52E) and BXCP3 pancreatic cells were cultured in Dulbecco's modified Eagle's medium (Invitrogen, Frederick, MD) supplemented with 10% fetal bovine serum and 4 mM l-glutamine. Cells were incubated in a humidified atmosphere of 5% CO2 in air at 37°C (8). TGF-β was obtained from R&D Systems (Minneapolis, MN). α-SMA, α-tubulin, Smad3 monoclonal antibodies, and fibronectin polyclonal antibody were purchased from Sigma (St. Louis, MO). Smad2 and Smad4 antibodies were purchased from Cell Signaling Technology (Beverly, MA) and Santa Cruz Biotechnology (Santa Cruz, CA), respectively. E-cadherin monoclonal antibody was from BD Biosciences (San Jose, CA). RGC-32 polyclonal antibody preparation was described previously (13, 14). RGC-32, Smad2, and Smad3 short hairpin interfering RNAs (shRNA) were purchased from Origene (Rockville, MD). RGC-32, Smad2, and Smad3 expression plasmids were described previously (6, 14, 24).
Transfection and luciferase assay.
HPTCs were transfected with various plasmids using lipofectamine 2000, as described previously. Luciferase activity measured using the luciferase assay system from Promega (Madison, MI) as described was normalized with protein concentration and is presented as means of three independent experiments (5).
RT-PCR and quantitative PCR.
Total RNA was extracted using TRIzol reagent (Invitrogen) following the manufacturer's instructions. cDNA was synthesized from 1 μg of total RNA in 20 μl using iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA). PCR and quantitative PCR (qPCR) were performed as previously described (7, 14). mRNA expression of interested genes was normalized to cyclophilin. Primer sequences for α-SMA, Slug, and Snail were published previously (13).
Western blot analysis.
Cells were washed with ice-cold PBS and incubated on ice for 20 min with lysis buffer containing protease inhibitors. Protein concentration was determined using a BCA kit (Pierce, Rockford, IL). Western blot analysis was performed as described previously (5–7, 13).
Coimmunoprecipitation assays.
Cells were lysed in 500 μl coimmunoprecipitation assay (Co-IP) lysis buffer (Pierce) on ice for 30 min. Following rapid centrifugation, the resulting supernatants were incubated with IgG, Smad2, Smad3, or Smad4 antibody at 4°C for 2 h followed by addition of 30 μl protein A/G-agarose beads (Santa Cruz, CA) and incubation at 4°C overnight. Immunoprecipitates were washed five times with Co-IP buffer. The beads were resuspended and boiled in SDS loading buffer. Western blotting was performed using anti-RGC-32 antibody.
Immunofluorescent staining.
HPTCs, NRK-52E, or BXCP3 cells were grown on glass coverslips in 24-well culture dishes and treated with vehicle or TGF-β1 (5 ng/ml) for 24 h. Immunostaining was performed as described previously (13).
Statistical analysis.
All values are expressed as means ± SD. All of the experiments were independently repeated three or four times. Data were analyzed using ANOVA with pairwise comparisons between groups. Holm-Sidak test was used for post hoc analysis. P < 0.05 was considered as a statistically significant difference.
RESULTS
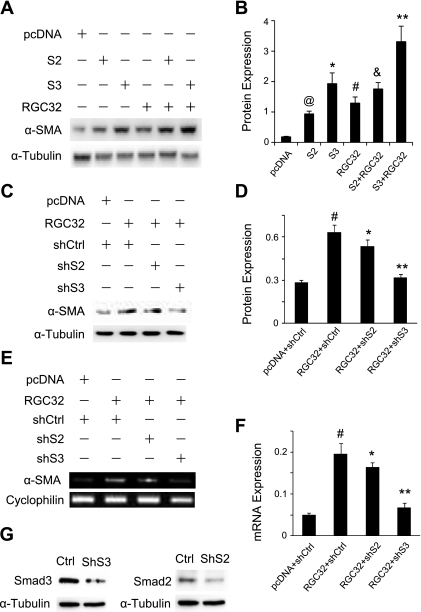
Our previous studies have shown that HPTCs were converted to myofibroblasts after TGF-β stimulation (18). RGC-32, a TGF-β downstream target, plays an essential role in the TGF-β-induced EMT (18). The mechanisms underlying RGC-32 function, however, remain largely unknown. Smad proteins are central components of TGF-β signaling pathways, so we tested whether RGC-32 interacts with Smad2 or Smad3 in the induction of EMT. We transfected Smad2, Smad3, and RGC-32 cDNA individually or in combination into HPTCs and detected the induction of α-SMA. As shown in Fig. 1, A and B, coexpression of RGC-32 with Smad3, but not Smad2, induced a significantly higher protein expression of α-SMA as compared with RGC-32 or Smad3 alone. Although Smad2 also induced α-SMA expression, Smad2 did not increase RGC-32 activity in the induction of α-SMA. To confirm the cooperative action between RGC-32 and Smad3, we blocked the endogenous Smad2 and Smad3 expression using shRNA. We found that knockdown of Smad3 significantly inhibited RGC-32-induced α-SMA expression (Fig. 1, C and D). Smad2 knockdown did not significantly inhibited α-SMA expression (Fig. 1, C and D). Smad3 shRNA also inhibited RGC-32-induced α-SMA mRNA expression (Fig. 1, E and F). These data indicate that RGC-32 interacts with Smad3, but not Smad2, in the regulation of TGF-β-induced EMT.
Fig. 1.
Response gene to complement 32 (RGC-32) and Smad3 cooperatively regulate α-smooth muscle actin (α-SMA) expression in the epithelial-mesenchymal transition (EMT) of human proximal tubular epithelial cells (HPTCs). A: coexpression of RGC-32 and Smad3 cooperatively increases α-SMA protein expression. HPTCs were cotransfected with empty vector (pcDNA), Smad2 (S2), Smad3 (S3), or RGC-32 (RGC32), as indicated. α-SMA protein expression was examined by Western blotting. α-Tubulin was used as an internal control. B: quantification of α-SMA expression by normalization to α-tubulin. @,*,#P < 0.01 compared with pcDNA-transfected groups; &P > 0.05 compared with RGC-32 groups; **P < 0.01 compared with Smad3 or RGC-32 alone groups. C: short hairpin interfering RNA (shRNA) knockdown of Smad3 blocks RGC-32-induced α-SMA protein expression. RGC-32 cDNA was cotransfected with control (shCtrl), Smad2 (shS2), or Smad3 (ShS3) shRNA into HPTCs. α-SMA protein expression was examined by Western blotting. D: quantification of α-SMA protein expression induced by RGC-32, normalized to α-tubulin. #P < 0.01 compared with pcDNA with shCtrl groups; *P > 0.05, **P < 0.01 compared with RGC-32 with shCtrl or shS2 groups. E and F: shRNA knockdown of Smad3 blocks RGC-32-induced α-SMA mRNA expression. The cells were treated similarly as in C, and RT-PCR (E) and quantitative PCR (qPCR; F) were performed and normalized to cyclophilin. #P < 0.01 compared with pcDNA with shCtrl groups; *P > 0.05, **P < 0.01 compared with RGC-32 with shCtrl or shS2 groups. G: Smad2 (shS2) and Smad3 shRNA (shS3) efficiently knock down endogenous Smad2 and Smad3 expression, respectively.
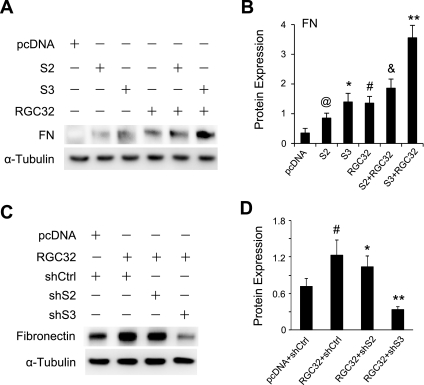
A hallmark of EMT process is the production of extracellular matrix (ECM) components, such as collagen I and fibronectin. To test whether RGC-32 interacts with Smad3 in inducing ECM production in the EMT, we detected fibronectin expression after coexpression of RGC-32 and/or Smad2 and Smad3 in HPTCs. As shown in Fig. 2, A and B, RGC-32 and Smad3 synergistically activated the expression of fibronectin. Although Smad2 also induced fibronectin expression, it did not enhance RGC-32 activity (Fig. 2, A and B), suggesting that RGC-32 does not functionally interact with Smad2 in inducing ECM matrix. Moreover, shRNA knockdown of Smad3, but not Smad2, blocked RGC-32-induced fibronectin expression (Fig. 2, C and D). These data further demonstrate that RGC-32 interacts with Smad3 in inducing ECM production in the EMT of HPTCs.
Fig. 2.
RGC-32 and Smad3 synergistically regulate fibronectin (FN) production in the EMT of HPTCs. A: coexpression of RGC-32 and Smad3 results in dramatic increase of fibronectin expression. Smad2 (S2), Smad3 (S3), and RGC-32 (RGC32) cDNAs were transfected individually or in combination into HPTCs for 48 h. Fibronectin protein expression was examined by Western blotting. α-Tubulin was used as an internal control. B: quantification of fibronectin expression, normalized to α-tubulin. @,*,#P < 0.01 compared with pcDNA-transfected groups; &P > 0.05 compared with RGC32 groups; **P < 0.01 compared with Smad3 or RGC-32 alone groups. RGC-32 synergized with Smad3 in stimulating fibronectin production. C: Smad3 knockdown blocks RGC-32-induced fibronectin expression. RGC-32 cDNA was cotransfected with control (shCtrl), Smad2 (shS2), or Smad3 shRNA (shS3) into HPTCs as indicated for 48 h. Fibronectin protein expression was examined by Western blotting. D: quantification of fibronectin expression, normalized to α-tubulin. #P < 0.01 compared with pcDNA with shCtrl groups; *P > 0.05, **P < 0.01 compared with RGC-32 with shCtrl or shS2 groups. Smad3 is essential for RGC-32 activity in fibronectin production.
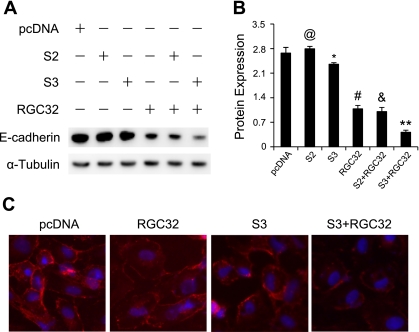
In addition to the expression of myofibroblast marker and ECM production, EMT is also characterized by the loss of epithelial markers such as E-cadherin. To characterize further the interaction between RGC-32 and Smad3 in the EMT of HPTCs, we examined the cooperative effect of RGC-32 and Smad3 on E-cadherin expression in both HPTCs and NRK-52E rat kidney epithelial cells. As shown in Fig. 3A, E-cadherin was highly expressed in HPTCs transfected with empty vector. The expression was inhibited slightly by Smad3 but markedly by RGC-32. Importantly, coexpression of RGC-32 and Smad3 synergistically suppressed E-cadherin expression (Fig. 3B). E-cadherin is a cell junction protein. Since NRK-52E showed a much better membrane staining of E-cadherin than HPTCs (18), we used NRK-52E to characterize the interaction of Smad3 and RGC-32 in maintaining epithelial cell junctions. Immunostaining of E-cadherin in NRK-52E cells showed a slight disruption of E-cadherin in adhesion junctions by Smad3, but a significant disruption by RGC-32 (Fig. 3C). However, coexpression of RGC-32 and Smad3 resulted in disruption of the majority of the adhesion junctions (Fig. 3C). These data demonstrate that RGC-32 interacts with Smad3 to block epithelial marker expression during EMT of HPTCs.
Fig. 3.
RGC-32 and Smad3 synergistically inhibit E-cadherin expression. A: coexpression of RGC-32 and Smad3 results in greater inhibition of E-cadherin than the individual expression. Empty vector (pcDNA), Smad2 (S2), Smad3 (S3), and RGC-32 (RGC32) cDNAs were transfected individually or in combination into NRK-52E cells, as indicated. E-cadherin expression was assessed by Western blotting. Coexpression of RGC-32 and Smad3 blocked epithelial marker expression. B: quantification of E-cadherin expression, normalized to α-tubulin. @P > 0.05, *P < 0.05, #P < 0.01 compared with pcDNA-transfected groups; &P > 0.05 compared with RGC32 groups; **P < 0.01 compared with Smad3 or RGC-32 alone groups. C: RGC-32 and Smad3 cooperatively disrupt E-cadherin membrane location in cell-cell junctions. RGC-32 and Smad3 cDNAs were transfected individually or in combination into NRK-52E cells for 48 h as indicated. Immunostaining was performed using E-cadherin antibody. Coexpression of RGC-32 and Smad3 almost completely prevented the membrane staining of E-cadherin.
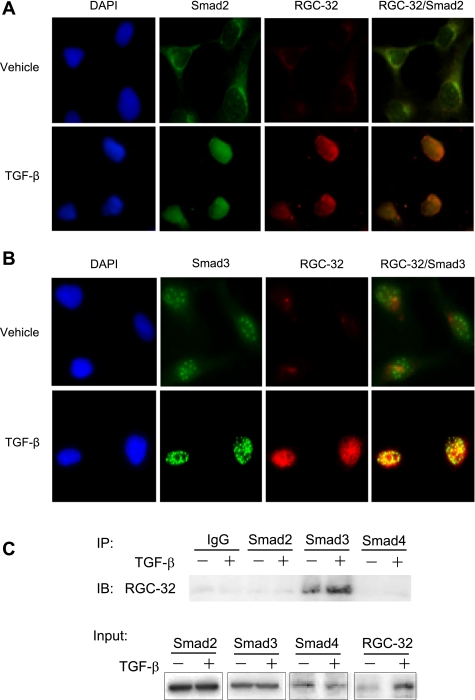
Since RGC-32 functionally interacts with Smad3 in inducing EMT of HPTCs, we sought to determine whether RGC-32 physically interacts with Smad3. We first detected RGC-32 and Smad3 colocalization in HPTCs. As shown in Fig. 4A, RGC-32 was weakly expressed in vehicle-treated HPTCs, but TGF-β strongly induced RGC-32 expression. Interestingly, most of RGC-32 induced by TGF-β was located in the nuclei. TGF-β also induced Smad2 and Smad3 nuclear translocation. The colocalization between RGC-32 and Smad2 appeared to be weak even with TGF-β treatment (Fig. 4A). In vehicle-treated HPTCs, The colocalization between RGC-32 and Smad3 was also weak, probably because of the weak expression of RGC-32 (Fig. 4B). However, strong colocalization of RGC-32 with Smad3 (yellow dots) was observed in TGF-β-treated cells (Fig. 4B), suggesting that RGC-32 may physically interact with Smad3. To verify the physical interaction between RGC-32 and Smad3, Co-IP was performed using endogenous proteins extracted from HPTCs treated with vehicle or TGF-β. As shown in Fig. 4C, RGC-32 did not coprecipitate with either Smad2 or Smad4, suggesting that RGC-32 does not physically interact with Smad2 or Smad4. However, Smad3 antibody specifically pulled down RGC-32 even with proteins from vehicle-treated cells, suggesting that RGC-32 weakly interacts with Smad3 in basal status. TGF-β treatment greatly enhanced the interaction (Fig. 4C).
Fig. 4.
RGC-32 physically interacts with Smad3 in HPTCs. A and B: RGC-32 colocalization with Smad 2 and Smad3 in the nuclei of HPTCs. Cells were treated with vehicle or transforming growth factor-β (TGF-β; 5 ng/ml) for 24 h. Dual immunostaining was performed using Smad2 (green; A), Smad3 (green; B), and RGC-32 (red) antibodies. Images were captured using confocal microscopy. RGC-32 and Smad3 colocalization (yellow dots; B) was observed in cells with vehicle treatment, and TGF-β enhanced the colocalization. C: RGC-32 physically interacts with Smad3 in HPTCs. HPTCs were treated with vehicle or TGF-β for 24 h, and coimmunoprecipitation was performed. Control IgG, Smad2, Smad3, and Smad4 antibodies were used for immunoprecipitation (IP), and RGC-32 antibody was used for immunoblotting (IB). Only Smad3 physically interacted with RGC-32 in HPTCs, and TGF-β enhanced the interaction.
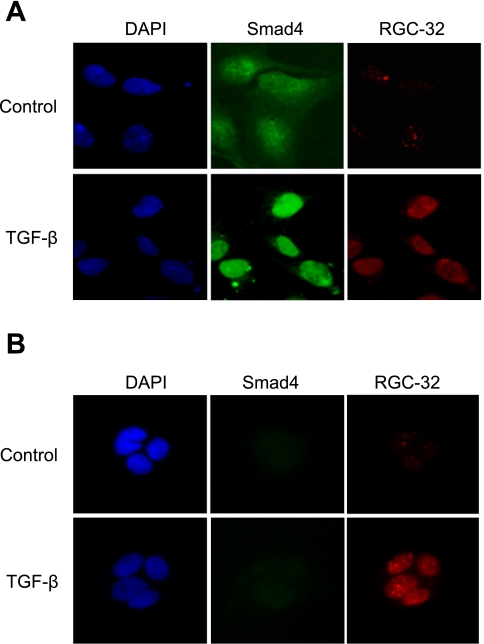
Smad2 or Smad3 nuclear location is mediated by Smad4 (15). Since RGC-32 interacts with Smad3, it is reasonable to speculate that Smad4 may play a role in RGC-32 nuclear location. To determine whether or not RGC-32 nuclear location is Smad4 dependent, we used Smad4-null BXCP3 cells to test whether TGF-β-induced RGC-32 locates in the nuclei when Smad4 is not present in the cells. A shown in Fig. 5A, Smad4 was translocated into nuclei of HPTCs by TGF-β. RGC-32 was expressed in the nuclei as well. However, in BXCP3 cells, although there was no Smad4 expression, TGF-β-induced RGC-32 was still located in the nuclei of the cells (Fig. 5B). These data demonstrate that RGC-32 nuclear location is Smad4 independent.
Fig. 5.
RGC-32 nuclear location is Smad4-independent. Smad4-expressing HPTCs (A) and Smad4-null BXCP3 (B) were treated with vehicle or TGF-β for 24 h, and immunostaining was performed using Smad4 (green) and RGC-32 (red). RGC-32 was induced by TGF-β and was located in the nuclei of both HPTCs and BXCP3.
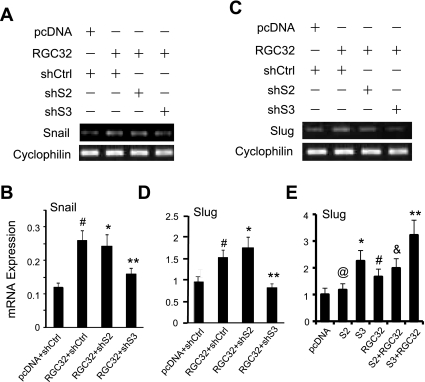
Previous studies have shown that RGC-32 acts as an upstream regulator of the EMT genes Snail and Slug in renal tubular EMT. Additional studies found that RGC-32 significantly increased Slug and Snail expression (Fig. 6). To determine whether RGC-32 activity in inducing EMT genes was associated with Smad3, we used shRNA to block Smad2 or Smad3 expression and then determined whether RGC-32 was still able to induce Snail or Slug mRNA expression in HPTCs. As shown in Fig. 6, A–D, Smad3 shRNA blocked RGC-32-induced Snail (Fig. 6, A and B) as well as Slug (Fig. 6, C and D) expression, while Smad2 shRNA showed no effect. To confirm the cooperative effect of Smad3 and RGC-32 on Slug expression, we coexpressed Smad2 or Smad3 with RGC-32 in HPTCs. As shown in Fig. 6E, Smad2 did not induce Slug expression, while both RGC-32 and Smad3 significantly increased Slug expression. Coexpression of RGC-32 and Smad3 achieved a higher induction of Slug, suggesting that Smad3 enhanced RGC-32 function in inducing Slug. Taken together, these results indicate that RGC-32 and Smad3 work together to induce EMT by cooperatively regulating EMT regulators Slug and Snail.
Fig. 6.
Smad3 is essential for RGC-32-induced Snail and Slug expression. RGC-32 cDNA was cotransfected with control (shCtrl), Smad2 (shS2), or Smad3 shRNA (shS3) for 48 h. A and C: Snail (A) and Slug (C) expression was examined by RT-PCR. B and D: quantitative analyses of Snail (B) and Slug (D) expression by qPCR. #P < 0.01 compared with pcDNA with shCtrl groups; *P > 0.05, **P < 0.01 compared with RGC-32 with shCtrl or shS2 groups. E: coexpression of RGC-32 and Smad3 cooperatively increases Slug expression. HPTCs were cotransfected with empty vector (pcDNA), Smad2 (S2), Smad3 (S3), or RGC-32 (RGC32) as indicated. Slug mRNA expression was examined by qPCR. @P > 0.05, *P < 0.01, #P < 0.01 compared with pcDNA-transfected groups; &P > 0.05 compared with RGC32 groups; **P < 0.05 compared with Smad3 or RGC-32 alone groups.
DISCUSSION
TGF-β signaling plays a critical role in renal tubular EMT. The molecular mechanisms underlying TGF-β function in EMT, however, remain largely unknown. Previous studies demonstrate that the TGF-β downstream target gene RGC-32 is important for EMT of HPTCs. Our data from the current studies have provided more detailed insight about RGC-32-induced EMT, i.e., RGC-32 partners with Smad3 to induce EMT in HPTCs. First, RGC-32 and Smad3 cooperatively regulate the activation myofibroblast marker (e.g., α-SMA) gene including both the protein and mRNA expression. Second, RGC-32 and Smad3 cooperatively regulate ECM production, as shown by fibronectin expression studies. Third, RGC-32 and Smad3 work together to block the expression of an epithelial marker, facilitating the phenotype conversion. Fourth, RGC-32 physically interacts with Smad3; they also colocalize in the nuclei of HPTCs. Finally, RGC-32 interacts with Smad3 to regulate the expression of EMT genes Snail and Slug.
TGF-β signaling is mediated by both Smad2 and Smad3 (4, 15, 20), but RGC-32 appears to interact only with Smad3 in inducing EMT of HPTCs. Although Smad2 is involved in the activation of α-SMA, it has no effect on RGC-32 activity. Overexpression of Smad2 did not enhance RGC-32 induction of α-SMA protein expression (Fig. 1, A and B). Knockdown of Smad2 did not significantly block RGC-32 induction of α-SMA expression (Fig. 1, C−F). Smad2 had no effect on RGC-32-induced ECM production or E-cadherin expression (Figs. 2 and 3). The definite evidence showing the absence of interaction between Smad2 and RGC-32 comes from the biochemical studies. Co-IP data demonstrate that RGC-32 does not physically interact with Smad2 in the EMT of HPTCs.
Interestingly, Smad3 and TGF-β-induced RGC-32 are both located in the nuclei of HPTCs. Smad3 is known to be translocated into nuclei by Smad4. However, Smad4 appears to have no role in RGC-32 nuclear location because, in Smad4-null cells, TGF-β-induced RGC-32 is also located in the nuclei. Therefore, the nuclear location mechanism for RGC-32 is different from that of Smad although RGC-32 interacts with Smad3 to regulate the EMT process of HPTCs. Previous studies have shown that RGC-32 can be phosphorylated by p34CDC2-cyclin B1 in vitro (2), but it is unclear whether this phosphorylation contributes to RGC-32 nuclear location in vivo. Thus, the RGC-32 nuclear location mechanisms may be a subject for future studies.
We showed previously that RGC-32 is required for Slug and Snail expression. In this study we found that RGC-32 can induce Slug and Snail expression, further demonstrating that RGC-32 is upstream of these two genes in the pathway of TGF-β-induced EMT. Interestingly, the induction of Slug and Snail requires a cooperative action between RGC-32 and Smad3, but not Smad2, because knockdown of Smad3 significantly inhibits RGC-32-induced expression of Slug or Snail. Moreover, Smad3 enhances RGC-32 activity in inducing Slug. Therefore, RGC-32 and Smad3 are likely to work together to regulate the transcription of the EMT genes. How they regulate the Slug or Snail promoter is a subject for future study.
Taken together, our data provide novel information about the mechanism underlying RGC-32 function in inducing EMT of renal tubular cells, i.e., RGC-32 physically interacts with Smad3 to regulate the expression of myofibroblast marker α-SMA, ECM components, and E-cadherin in HPTCs. The effect of this interaction on EMT of HPTCs is achieved through the regulation of EMT-related genes Slug and Snail.
GRANTS
This work was supported, in whole or in part, by National Institutes of Health Grants HL-093429 (to S. Y. Chen) and DK-039308, HL-092196, and HL-23081 (to P. A. Jose).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
REFERENCES
- 1. An X, Jin Y, Guo H, Foo SY, Cully BL, Wu J, Zeng H, Rosenzweig A, Li J. Response gene to complement 32, a novel hypoxia-regulated angiogenic inhibitor. Circulation 120: 617–627, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Badea T, Niculescu F, Soane L, Fosbrink M, Sorana H, Rus V, Shin ML, Rus H. RGC-32 increases p34CDC2 kinase activity and entry of aortic smooth muscle cells into S-phase. J Biol Chem 277: 502–508, 2002 [DOI] [PubMed] [Google Scholar]
- 3. Badea TC, Niculescu FI, Soane L, Shin ML, Rus H. Molecular cloning and characterization of RGC-32, a novel gene induced by complement activation in oligodendrocytes. J Biol Chem 273: 26977–26981, 1998 [DOI] [PubMed] [Google Scholar]
- 4. Bottinger EP, Bitzer M. TGF-beta signaling in renal disease. J Am Soc Nephrol 13: 2600–2610, 2002 [DOI] [PubMed] [Google Scholar]
- 5. Chen S, Crawford M, Day RM, Briones VR, Leader JE, Jose PA, Lechleider RJ. RhoA modulates Smad signaling during transforming growth factor-beta-induced smooth muscle differentiation. J Biol Chem 281: 1765–1770, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen S, Kulik M, Lechleider RJ. Smad proteins regulate transcriptional induction of the SM22alpha gene by TGF-beta. Nucleic Acids Res 31: 1302–1310, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen S, Lechleider RJ. Transforming growth factor-beta-induced differentiation of smooth muscle from a neural crest stem cell line. Circ Res 94: 1195–1202, 2004 [DOI] [PubMed] [Google Scholar]
- 8. De Larco JE, Todaro GJ. Epithelioid and fibroblastic rat kidney cell clones: epidermal growth factor (EGF) receptors and the effect of mouse sarcoma virus transformation. J Cell Physiol 94: 335–342, 1978 [DOI] [PubMed] [Google Scholar]
- 9. Ehrlich HP, Allison GM, Leggett M. The myofibroblast, cadherin, alpha smooth muscle actin and the collagen effect. Cell Biochem Funct 24: 63–70, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Fosbrink M, Cudrici C, Niculescu F, Badea TC, David S, Shamsuddin A, Shin ML, Rus H. Overexpression of RGC-32 in colon cancer and other tumors. Exp Mol Pathol 78: 116–122, 2005 [DOI] [PubMed] [Google Scholar]
- 11. Hewitson TD, Ono T, Becker GJ. Small animal models of kidney disease: a review. Methods Mol Biol 466: 41–57, 2009 [DOI] [PubMed] [Google Scholar]
- 12. Hinz B, Pittet P, Smith-Clerc J, Chaponnier C, Meister JJ. Myofibroblast development is characterized by specific cell-cell adherens junctions. Mol Biol Cell 15: 4310–4320, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huang WY, Li ZG, Rus H, Wang X, Jose PA, Chen SY. RGC-32 mediates transforming growth factor-beta-induced epithelial-mesenchymal transition in human renal proximal tubular cells. J Biol Chem 284: 9426–9432, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li F, Luo Z, Huang W, Lu Q, Wilcox CS, Jose PA, Chen S. Response gene to complement 32, a novel regulator for transforming growth factor-beta-induced smooth muscle differentiation of neural crest cells. J Biol Chem 282: 10133–10137, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Massague J, Wotton D. Transcriptional control by the TGF-beta/Smad signaling system. EMBO J 19: 1745–1754, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Park ES, Choi S, Muse KN, Curry TE, Jr, Jo M. Response gene to complement 32 expression is induced by the luteinizing hormone (LH) surge and regulated by LH-induced mediators in the rodent ovary. Endocrinology 149: 3025–3036, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Radisky DC, Kenny PA, Bissell MJ. Fibrosis and cancer: do myofibroblasts come also from epithelial cells via EMT? J Cell Biochem 101: 830–839, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sanada H, Jose PA, Hazen-Martin D, Yu PY, Xu J, Bruns DE, Phipps J, Carey RM, Felder RA. Dopamine-1 receptor coupling defect in renal proximal tubule cells in hypertension. Hypertension 33: 1036–1042, 1999 [DOI] [PubMed] [Google Scholar]
- 19. Sato M, Muragaki Y, Saika S, Roberts AB, Ooshima A. Targeted disruption of TGF-beta1/Smad3 signaling protects against renal tubulointerstitial fibrosis induced by unilateral ureteral obstruction. J Clin Invest 112: 1486–1494, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schnaper HW, Hayashida T, Hubchak SC, Poncelet AC. TGF-β signal transduction and mesangial cell fibrogenesis. Am J Physiol Renal Physiol 284: F243–F252, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Shirai K, Sera Y, Bulkeley W, Mehrotra M, Moussa O, LaRue AC, Watson DK, Stuart RK, Lazarchick J, Ogawa M. Hematopoietic stem cell origin of human fibroblasts: cell culture studies of female recipients of gender-mismatched stem cell transplantation and patients with chronic myelogenous leukemia. Exp Hematol 37: 1464–1471, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Simonson MS. Phenotypic transitions and fibrosis in diabetic nephropathy. Kidney Int 71: 846–854, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol 214: 199–210, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang Y, Feng XH, Derynck R. Smad3 and Smad4 cooperate with c-Jun/c-Fos to mediate TGF-beta-induced transcription. Nature 394: 909–913, 1998 [DOI] [PubMed] [Google Scholar]
- 25. Zhao W, Chen SS, Chen Y, Ahokas RA, Sun Y. Kidney fibrosis in hypertensive rats: role of oxidative stress. Am J Nephrol 28: 548–554, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.