Summary
Leishmania parasites infect macrophages, cells normally involved in innate defense against pathogens. L. amazonensis and L. major cause severe or mild disease, respectively, consistent with each parasite’s ability to survive within activated macrophages. The mechanisms underlying increased virulence of L. amazonensis are mostly unknown. We show that L. amazonensis promotes its own survival by inducing expression of CD200, an immunoregulatory molecule that inhibits macrophage activation. L. amazonensis does not form typical non-healing lesions in CD200−/− mice and cannot replicate in CD200−/− macrophages, an effect reversed by exogenous administration of soluble CD200-Fc. The less virulent L. major does not induce CD200 expression and forms small, self-healing lesions in both wild type and CD200−/− mice. Notably, CD200-Fc injection transforms the course of L. major infection to one resembling L. amazonensis, with large, non-healing lesions. CD200-dependent iNOS inhibition allows parasite growth in macrophages, identifying a mechanism for the increased virulence of L. amazonensis.
Introduction
The parasitic protozoan Leishmania infects millions of people worldwide. Leishmaniasis is a growing public health threat, due to recent increases in transmission by infected sand flies. Different species of Leishmania cause a wide spectrum of human disease, from self-healing cutaneous lesions to lethal visceralizing infections. The parasite factors responsible for the different forms of clinical disease have not been elucidated. In this study we investigate this issue by comparing two species that cause cutaneous lesions, L. amazonensis and L. major (Afonso and Scott, 1993; Jones et al., 2002; Jones et al., 2000). Infections with L. amazonensis and L. major cause severe or mild disease respectively (Barral et al., 1991; McMahon-Pratt and Alexander, 2004) and these differences in virulence have been proposed to be associated with the ability of each pathogen to survive within activated macrophages (Barral et al., 1991; Gomes et al., 2003; McMahon-Pratt and Alexander, 2004; Qi et al., 2004; Scott and Sher, 1986). However, the molecular mechanisms responsible for the resistance of L. amazonensis to microbicidal responses and for its enhanced virulence remain largely unknown.
Immune responses of myeloid cells can be controlled by a number of inhibitory receptors (Ravetch and Lanier, 2000). Among the best characterized immune inhibitory ligand-receptors pairs is CD200 and its receptor CD200R. CD200 (OX2) is a cell surface glycoprotein from the immunoglobulin superfamily that is expressed in several cell types, particularly lymphoid cells, neurons, and endothelium (Barclay et al., 2002). CD200 has a very short intracellular tail that does not contain any known signaling motif. By engaging CD200R, a cell surface receptor that contains three conserved tyrosines in its cytosolic domain, CD200 generates an inhibitory signal that prevents local macrophage activation (Foster-Cuevas et al., 2004; Hoek et al., 2000; Wright et al., 2000). CD200R is widely expressed in myeloid cells, but resting macrophages express low, or undetectable levels of CD200 ligand (Mukhopadhyay et al., 2010). In this study we show that L. amazonensis, but not L. major, has the ability to induce macrophages to express CD200, resulting in a dampening of macrophage microbicidal responses and an increase in parasite intracellular survival and virulence.
Results
L. amazonensis-induced expression of CD200 in host macrophages is required for intracellular replication of the parasites
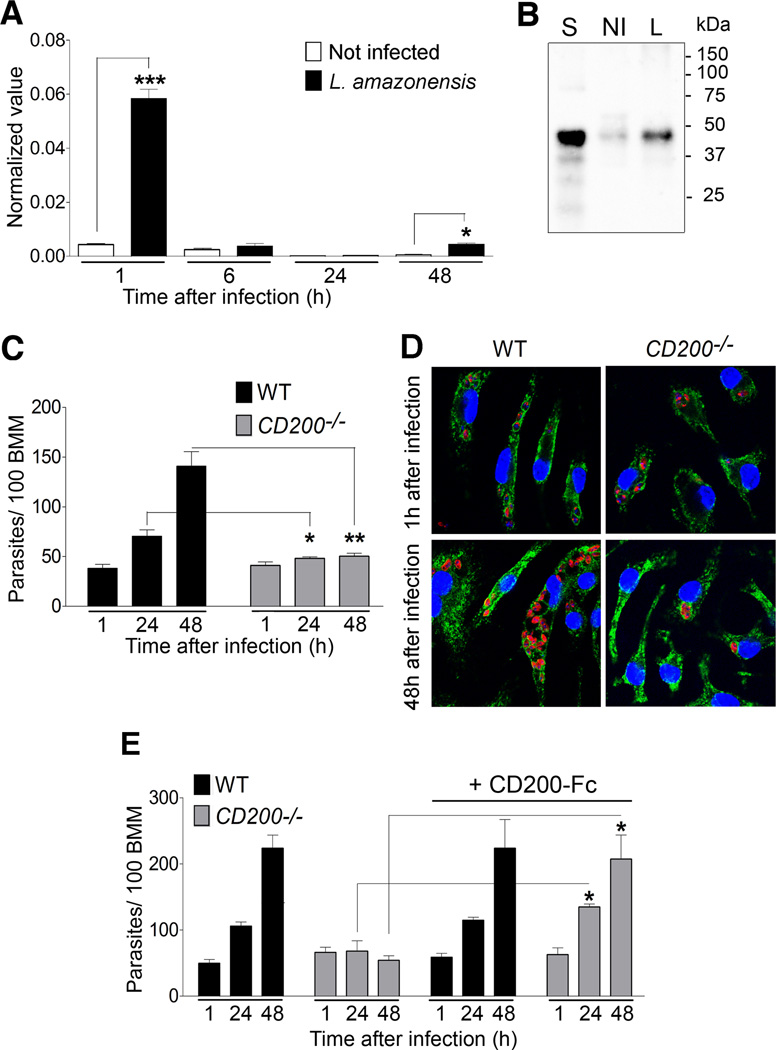
Transcriptome analysis of C57BL/6 bone marrow macrophages (BMM) infected with Leishmania amazonensis detected an increase in CD200 mRNA levels. Real Time PCR analysis confirmed a marked increase in CD200 mRNA 1 h after infection of BMM with L. amazonensis axenic amastigotes (Fig. 1A), with a concomitant increase in CD200 protein levels (Fig. 1B). Interestingly, at 6 or 24 h after infection CD200 levels were again reduced, indicating that the signals responsible for the activation of CD200 expression are restricted to early stages of interaction the parasites with the macrophage. The small but significant increase in CD200 expression detected at 48 h after infection (Fig. 1A) may reflect re-infection of macrophages by parasites released from neighboring cells.
Figure 1. CD200 expression is required for L. amazonensis growth in macrophages.
(A) CD200 transcript levels measured by qPCR in BMM infected for 1 h with L. amazonensis axenic amastigotes, and either processed immediately or after incubation for 24 or 48 h. Results correspond to mean +/− SD of triplicates. *** p<0.0001; ** p<0.0075 (Student’s t test). (B) Immunoblot of CD200 protein levels in BMM infected (I) or not (NI) for 1 h with axenic amastigotes. Mouse splenocytes (S) were used as a positive control. (C) Intracellular L. amazonensis over time in WT and CD200−/− BMM infected for 1 h with axenic amastigotes and either fixed immediately or after incubation for 24 or 48 h. Results correspond to mean +/− SD of triplicates. * p<0.0270; ** p<0.0035 (Student’s t test). (D) Images of L. amazonensis-infected WT or CD200−/− BMM, 1 and 48h after infection. Nuclei were stained with DAPI (blue); parasitophorous vacuole membranes were stained with antibodies to the lysosomal glycoprotein Lamp1 (green); parasites were stained with anti-Leishmania antibodies (red). (E) Intracellular L. amazonensis in WT and CD200−/− BMM infected for 1 h in the presence or not of CD200-Fc. Results correspond to mean +/− SD of triplicates. * p<0.0138, 24 h; *p<0.0150, 48 h (Student’s t test).
We found that CD200 expression was required for replication in macrophages. The number of intracellular parasites doubled approximately every 24 h in wild type (WT) BMM, while no significant growth was observed in BMM isolated from CD200−/− mice (Fig. 1C and D). Importantly, treatment of CD200−/− BMM with a CD200-Fc fusion protein (Snelgrove et al., 2008) rescued their ability to support the intracellular replication of L. amazonensis, while not affecting parasite growth in WT BMM (Fig. 1E). A control IgG1-Fc fusion protein lacking the CD200 sequence had no effect on the intracellular growth of L. amazonensis in BMM (S1).
CD200 expression inhibits L. amazonensis-induced iNOS and NO production
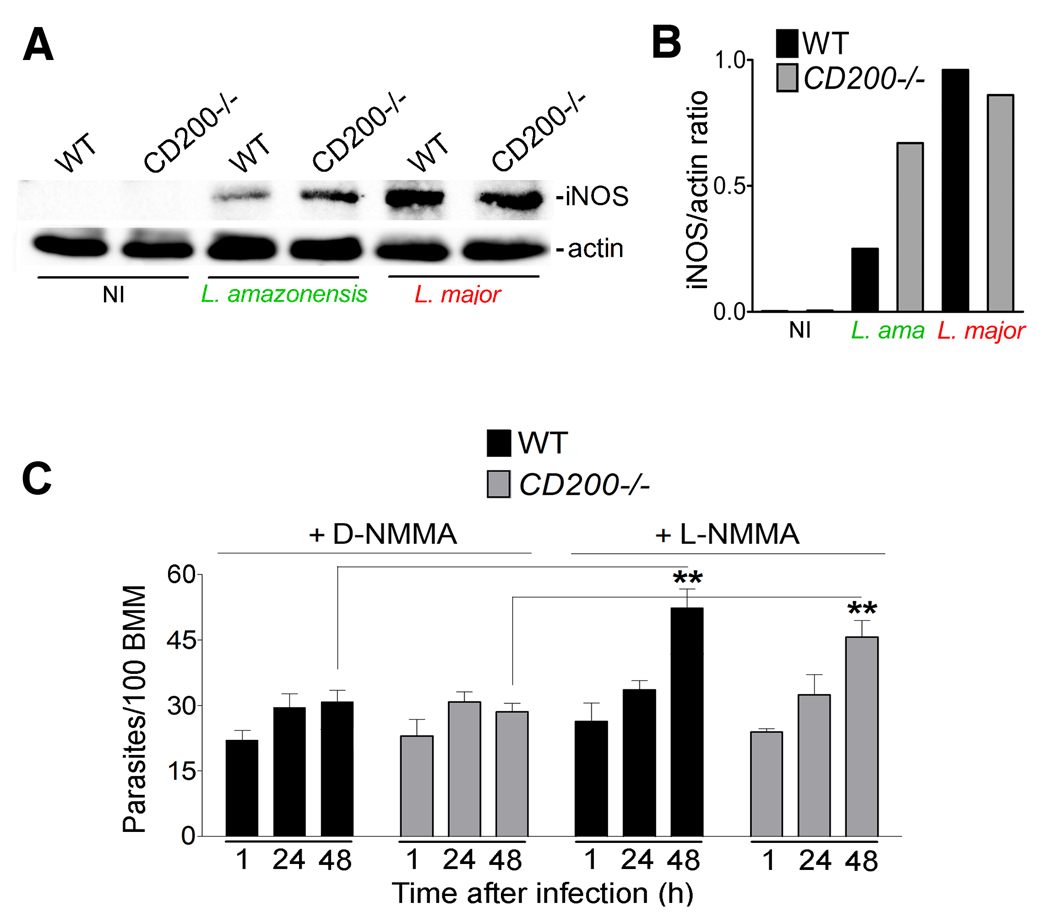
Nitric oxide (NO) production by inducible nitric oxide synthase (iNOS) is a major mechanism used by macrophages to kill intracellular Leishmania (Liew et al., 1990; Qadoumi et al., 2002; Wei et al., 1995), and to prevent reactivation of latent leishmaniasis (Stenger et al., 1996). Of particular relevance for the present studies, iNOS/NO production by macrophages also plays a prominent role during early stages of the innate immune response against L. major, (Diefenbach et al., 1998). Since CD200 signaling was shown to suppress iNOS-mediated generation of NO (Banerjee and Dick, 2004; Cameron et al., 2005; Copland et al., 2007), we investigated if this mechanism was involved in the enhanced survival of L. amazonensis after infection of CD200−/− BMM. Low levels of iNOS expression was detected by western blot in wild type BMM exposed to L. amazonensis axenic amastigotes, whereas higher levels were consistently observed in CD200−/− BMM (Fig. 2A and B). In agreement with an involvement of iNOS in the restriction of the intracellular growth of L. amazonensis in macrophages lacking CD200, the specific iNOS inhibitor L-NMMA rescued parasite replication in CD200−/− BMM. In contrast, the inactive isomer D-NMMA had no effect (Fig. 2C and D). A sensitive fluorometric assay revealed that NO produced by CD200−/− BMM infected with L. amazonensis were elevated, when compared to infected wild type BMM (Fig. 2E). These NO levels were markedly lower than what is observed in infected or non-infected BMM exposed to LPS and IFNgamma (S2), consistent with the observed inhibition in parasite replication instead of parasite death. No differences in iNOS transcript levels were observed between CD200+/+ and CD200−/− infected BMM (results not shown), consistent with previous reports suggesting that L. amazonensis inhibits iNOS expression in host cells by a post-transcriptional mechanism (Balestieri et al., 2002; Wilkins-Rodriguez et al., 2010).
Figure 2. CD200 promotes L. amazonensis intracellular growth by down-regulating iNOS.
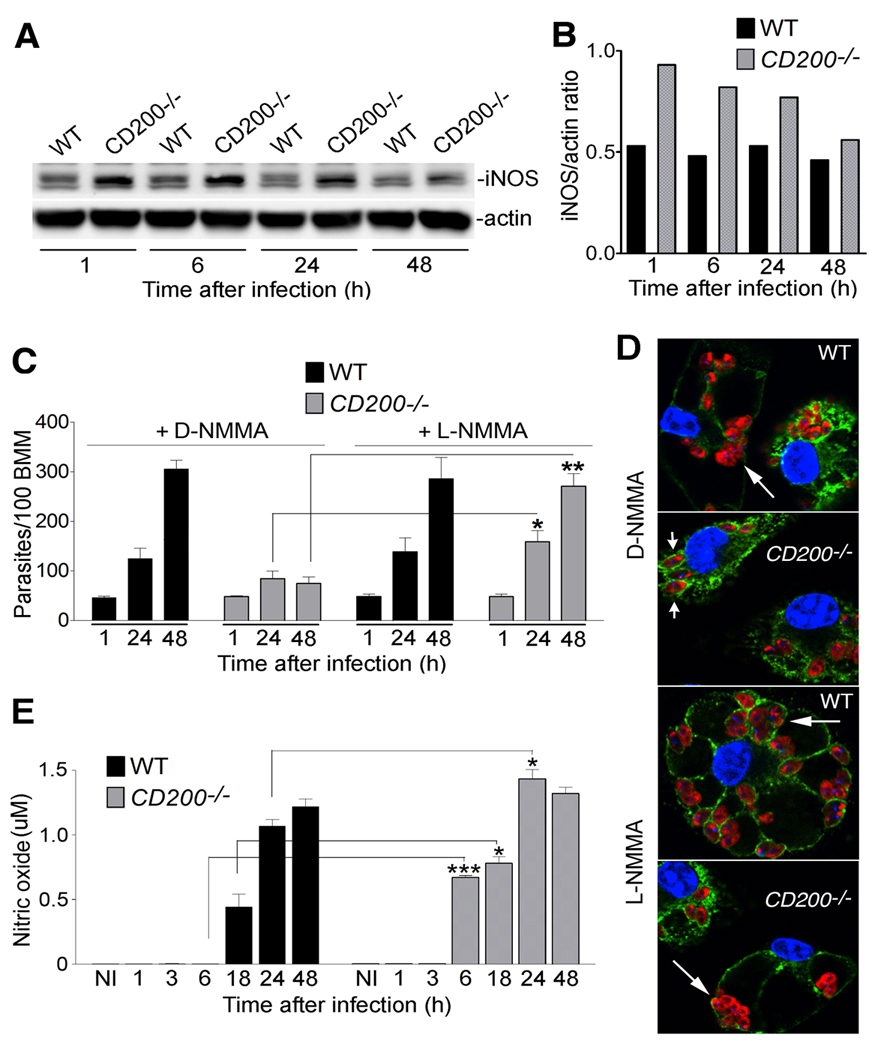
(A) iNOS immunoblot in WT and CD200−/− BMM infected for 1 h with L. amazonensis axenic amastigotes and incubated for the indicated time points. No iNOS band was detected in lysates of non-infected BMM (not shown). Blots were also probed with anti-actin as a loading control. (B) Densitometry analysis of the data in (A), expressed as iNOS/actin ratio. (C) Intracellular L. amazonensis over time in WT or CD200−/− BMM pre-treated with 500 µM D- or L-NMMA and infected for 1 h with axenic amastigotes and either fixed immediately or after incubation for 24 or 48 h. Results correspond to mean +/− SD of triplicates. * p<0.0444; ** p<0.0023 (Student’s t test). (D) Images of WT and CD200−/− BMM pre-treated with D- or L-NMMA, 48h after infection. Nuclei were stained with DAPI (blue); parasitophorous vacuole membranes were stained with anti-Lamp1 (green); parasites were stained with anti-Leishmania (red). Long arrows point to replicating amastigotes; short arrows point to single, non-replicating amastigotes. (E) NO levels in the supernatant of WT and CD200−/− BMM measured with a fluorometric assay after 1 h infection with axenic amastigotes. Results correspond to mean +/− SD of triplicates. *** p=0.0001; * p= 0.038 (18h); p=0.014 (24h) (Student’s t test).
CD200 expression is required for Leishmania virulence in vivo
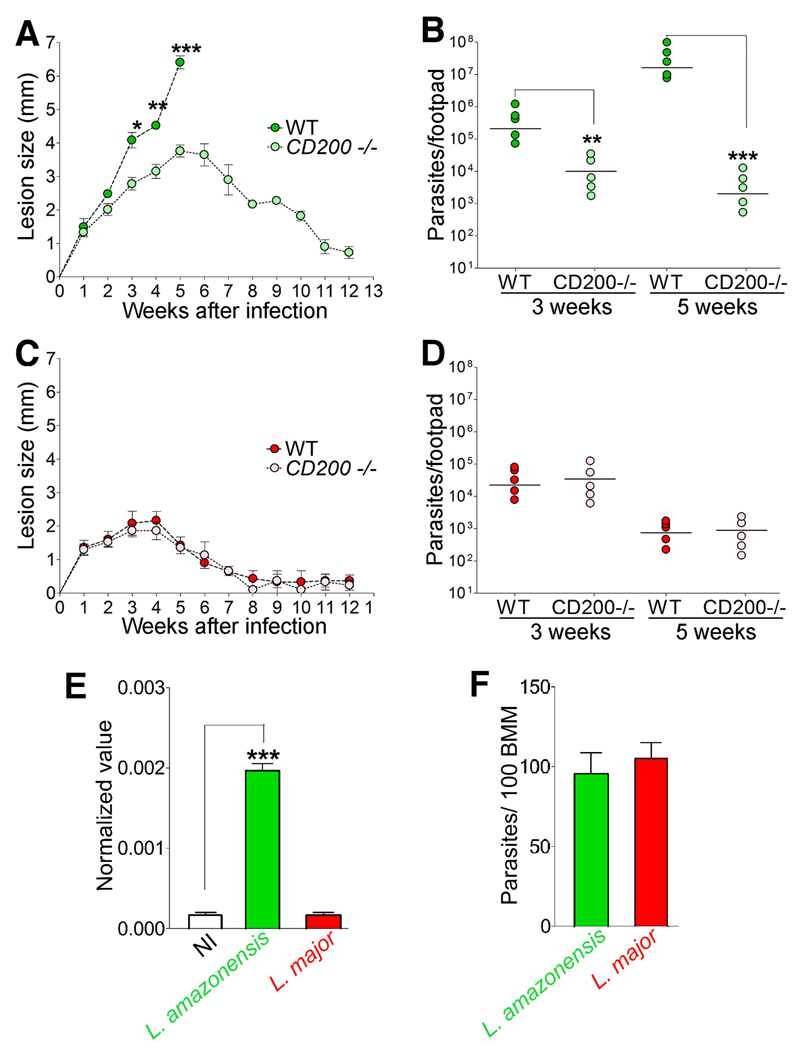
Next, we examined the progression of cutaneous lesions after inoculation of L. amazonensis into mouse footpads. Wild type CD200+/+ mice (WT) showed the rapidly growing, non-healing lesions that are typical of L. amazonensis infections in C57BL/6 mice. In sharp contrast, CD200−/− mice infected with a similar parasite dose developed smaller lesions that healed by the 12th week following inoculation (Fig. 3A). Quantification of viable L. amazonensis in infected footpads revealed a ∼100 fold expansion in the parasite population between weeks 3 and 5 in WT mice, while no significant increase was detected during the same period in CD200−/− mice (Fig. 3B, S3). Thus, CD200 expression, which promotes L. amazonensis growth in macrophages, appears to also lead to more severe cutaneous lesions in vivo.
Figure 3. The reduced virulence of L. major is associated with its inability to induce CD200 expression.
(A) Lesion size during infection of WT and CD200−/− mice with L. amazonensis. Results correspond to mean +/− SD (n=5). * p<0.021; ** p< 0.0037; *** p< 0.0006 (Student’s t test). (B) Parasite load in footpads from WT and CD200−/− mice infected with L. amazonensis. Results correspond to mean +/− SD (n=5). ** p<0.0025; *** p< 0.0001 (Student’s t test). (C) Lesion size during infection of WT and CD200−/− mice with L. major. Results correspond to mean +/− SD (n=5). (D) Parasite load in footpads from WT and CD200−/− mice infected with L. major. Results correspond to mean +/− SD (n=5). (E) CD200 transcript levels determined by qPCR in BMM infected for 1 h with L. amazonensis (green) or L. major (red) lesion-derived amastigotes. Results correspond to mean +/− SD of triplicates. *** p=0.0001 (Student’s t test). (F) Numbers of intracellular parasites in the experiment shown in 3E. Results correspond to mean +/− SD of triplicates.
The progressive, non-healing pattern of L. amazonensis lesion formation in mice (Jones et al., 2002) resembles the human disease caused by this Leishmania species in Central and South America (Barral et al., 1991; McMahon-Pratt and Alexander, 2004). L. major, an agent of cutaneous leishmaniasis in the Old World, causes a more benign, self-healing form of human disease, which can be reproduced in mice of the C57BL/6 background (Belkaid et al., 2000; Jones et al., 2002). In marked contrast to L. amazonensis, L. major caused small lesions that healed within 8–9 weeks in both WT and CD200−/− mice (Fig. 3C), and tissue load assays showed that CD200 deficiency did not result in any significant increase in the numbers of viable L. major between 3 and 5 weeks after infection (Fig. 3D, S3). These results suggested that L. major differs from L. amazonensis in its capacity to engage CD200 as a strategy for intracellular growth in macrophages.
Since L. major cannot be grown axenically, we used lesion-derived amastigotes from both species for our subsequent in vitro studies of the CD200 effect. We found that L. major infection, in contrast to L. amazonensis, did not induce CD200 transcription in BMM (Fig. 3E, S4), although infection levels with both parasite species were comparable (Fig. 3F). We also compared iNOS induction in macrophages infected with either L. amazonensis or L. major. As observed in infections performed with axenic amastigotes (Fig. 2A and B), iNOS levels with L. amazonensis lesion-derived amastigotes were higher in CD200−/− BMM, when compared to WT BMM after 1 h of infection (Fig. 4A and B). However, the outcome of infection with L. major lesion-derived amastigotes was markedly different. iNOS expression was detected after 1 h of infection in both populations of BMM, independent of CD200 expression (Fig. 4A and B). These iNOS expression levels were apparently sufficient to restrict the early intracellular growth of L. major, since the specific iNOS inhibitor L-NMMA rescued parasite replication after 48 h in both WT and CD200−/− BMM, whereas the inactive isomer D-NMMA had no effect (Fig. 4C).
Figure 4. The reduced ability of L. major to replicate in macrophages is associated with induction of iNOS expression.
(A) Immunoblot of iNOS in WT and CD200−/− BMM infected for 1 h with L. amazonensis or L. major lesion-derived amastigotes. Anti-actin was used as loading control. (B) Densitometry analysis of the data in (A), expressed as iNOS/actin ratio. (C) Intracellular L. major over time in WT or CD200−/− BMM pre-treated with 500 µM D- or L-NMMA and infected for 1 h with lesion-derived amastigotes, and either fixed immediately or after incubation for 24 or 48 h. Results correspond to mean +/− SD of triplicates. ** p=0.0079 (WT); p=0.0027 (CD200−/−) (Student’s t test).
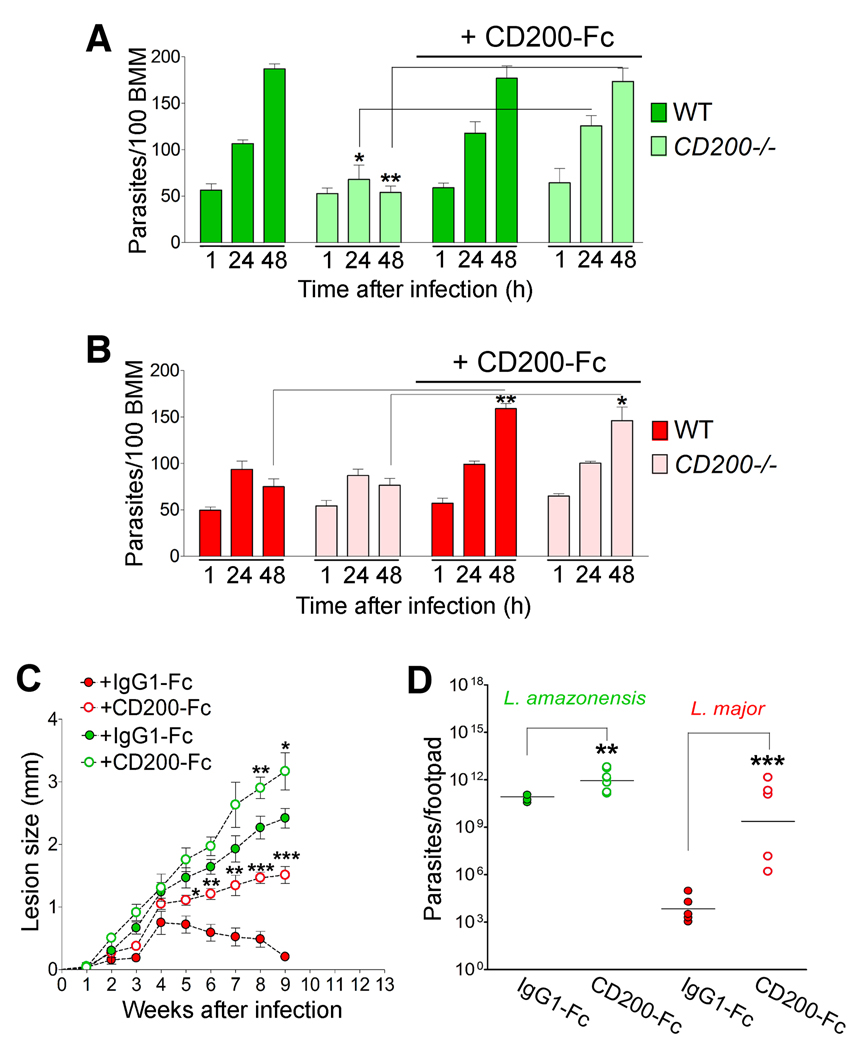
The mild, self-healing course of infection with L. major is transformed into a virulent non-healing pattern by administration of soluble CD200-Fc
As observed with axenic amastigotes (Fig. 1C and E), L. amazonensis lesion-derived amastigotes grew poorly in CD200−/− BMM, and extracellular addition of CD200-Fc rescued parasite replication (Fig. 5A). In contrast, L. major growth was limited in both WT and CD200−/− BMM (Fig. 5B), consistent with the small, self-healing lesions observed in mice (Fig. 3C). Furthermore, the addition of CD200-Fc to both WT and CD200−/− BMM promoted vigorous intracellular growth of L. major (Fig. 5B). In vivo results further supported the hypothesis that the induction of CD200 is critical for Leishmania virulence. Administration of CD200-Fc to L. amazonensis-infected WT mice resulted in slightly larger lesions and increased tissue parasite load (Fig. 5C and D). Remarkably, in vivo inoculation of CD200-Fc converted the mild L. major phenotype into a much more virulent one, resembling that of L. amazonensis including non-healing lesions and a six log increase in parasite load (Fig. 5C and D).
Figure 5. CD200 transforms the mild L. major infection into a virulent pattern typical of L. amazonensis.
(A) Intracellular parasites in WT or CD200−/− BMM infected for 1h with L. amazonensis lesion-derived amastigotes and either fixed immediately or after incubation for 24 or 48 h. Results correspond to mean +/− SD of triplicates. * p< 0.0379; ** p< 0.0018. (B) Intracellular parasites in WT or CD200−/− BMM infected for 1h with L. major lesion-derived amastigotes, in the presence or not of CD200-Fc. Results correspond to mean +/− SD of triplicates. ** p< 0.0011; * p< 0.0142 (Student’s t test). (C) Lesion size during L. amazonensis (green) or L. major (red) infection in WT mice, treated with CD200-Fc (open circles) or control IgG1-Fc (closed circles). Results correspond to mean +/− SD (n=5). L. amazonensis: ** p= 0.038; * p=0.077; L. major: * p=0.039; ** p=0.005 (6th week), p=0.0054 (7th week); *** p= 0.0002 (8th week), p=0.0001 (9th week) (Student’s t test). (D) Parasite load in footpads from the experiment in (C), 9 weeks following infection with L. amazonensis (green) or L. major (red). Results correspond to mean +/− SD (n=5). L. amazonensis * p=0.025; L. major *** p=0.0004 (Student’s t test).
Discussion
Negative regulatory mechanisms within the immune system are essential to prevent pathology driven by unrestrained inflammatory responses to self and foreign antigens. The widely expressed and inducible ligand CD200 plays an important role in this process, by suppressing inflammatory responses on myeloid cells expressing CD200 receptors (CD200R) (Barclay et al., 2002). In this report, we demonstrate that the protozoan parasite L. amazonensis manipulates this potent immune inhibitory pathway to its advantage, by inducing the expression of CD200. In the absence of CD200, the virulence of L. amazonensis is attenuated to that resembling L. major, a strain that does not induce CD200. Concordantly, exogenous CD200 is capable of increasing the virulence of L. major to levels normally observed for L. amazonensis.
The detailed mechanism by which CD200 expression promotes Leishmania virulence in vivo remains to be fully elucidated. However, we found a role for inhibition of iNOS expression and NO production, a process known to be mediated by CD200 binding to CD200R (Banerjee and Dick, 2004; Cameron et al., 2005; Copland et al., 2007). Expression of iNOS (NOS2) in macrophages is stimulated by numerous cytokines and microbial products, which often act in synergistic pairs (MacMicking et al., 1997). The effect of LPS and IFNgamma in inducing iNOS expression and NO-mediated killing of Leishmania major (Liew et al., 1990) has been the subject of numerous studies, which have largely focused on the potent microbicidal effect associated with the establishment of a Th1 helper lymphocyte response (Reiner and Locksley, 1995). However, more recent studies revealed that the role of iNOS expression in L. major infections is not restricted to the adaptive phase of the immune response. iNOS-mediated antimicrobial activity dependent on Type 1 interferons (IFNalpha, IFNbeta) was detected within the first day after parasite inoculation into mice, and identified as part of the innate immune response to Leishmania (Bogdan et al., 2000; Buxbaum, 2010; Diefenbach et al., 1998). IFNalpha was also shown to synergize with subactivating doses of LPS, activating macrophages to produce NO and kill intracellular Leishmania major (Shankar et al., 1996). The fact that we detected iNOS expression and NO production by macrophages after short periods of infection (1–6 h) adds significant additional information to the poorly understood innate response to Leishmania, and indicates that parasite components may be sufficient to provide the molecular signals required for this early response. Despite the detectable levels of iNOS present in isolated wild type macrophages infected with L. amazonensis, NO production by these cells could only be measured 18–24 h after infection. In contrast, in infected macrophages lacking CD200, iNOS levels were elevated, and resulted in detectable NO production as early as 6 h after infection. It is important to note that the NO levels we observed in these assays, using a sensitive fluorescence based assay, are markedly lower than the levels observed after lipopolysaccharide (LPS) and IFNgamma stimulation of macrophages (see S2). This observation is consistent with our finding that CD200 deficiency in macrophages impairs parasite replication over a period of 48 h, rather than parasite killing which is observed in IFNgamma-activated macrophages and appears to require higher NO levels (Liew et al., 1990).
Our in vivo experiments show that the progressive cutaneous lesions typical of L. amazonensis infections shift to a healing pattern in CD200 deficient mice, and that L. major-induced lesions can be transformed into a non-healing pattern by CD200 inoculation. Taken together with our observations in isolated macrophages, these results suggest that the CD200-mediated suppression of iNOS expression seen shortly after cell entry may also play a role in the stimulation of lesion progression in vivo. However, important aspects of the long-term in vivo immune responses that develop in presence or absence of CD200 remain to be determined, such as whether CD200 expression affects the normal Th1 subset development that occurs in mice infected with L. major (Reiner and Locksley, 1995). It will also be of interest to determine if parasite-induced expression of CD200 plays a role in the functional impairment observed in dendritic cells infected with L. amazonensis (Boggiatto et al., 2009; Xin et al., 2008).
The increased susceptibility of Balb/c mice to L. major infection, with extensive parasite proliferation at sites of inoculation in vivo, is well documented (Nasseri and Modabber, 1979; Belosevic et al., 1989; Reiner and Locksley, 1995). In resistant mice from the C57BL/6 background, the L. major tissue load increases slowly in the first few weeks after infection, followed by a decline that coincides with lesion healing (Sacks and Noben-Trauth, 2002). However, very few studies have addressed in detail the rate of L. major replication in isolated macrophages from susceptible and resistant mouse strains. Most reports show increases in intracellular parasite numbers only after long periods of infection, such as 72 h (Blos et al., 2003), or longer. The numbers of intracellular L. major were reported to remain constant in the first 48 h of infection in Balb/c BMM (Muleme et al., 2009), and studies performed with C57BL/6 or CH3/HeNHSD BMM showed either a very small increase (Green et al., 1990; Mattner et al., 2000) or a reduction in parasite numbers during this period (Kuang et al., 2009). These observations are consistent with our present study, where we show that only L. amazonensis is able to double its numbers intracellularly every 24 h, while the number of intracellular L. major stays constant during this period. Our results suggest that CD200-mediated suppression of Leishmania intracellular growth may also influence lesion progression in vivo, although additional factors must also come into play as the acquired immune response develops in infected animals.
Consistent with the increased susceptibility of CD200−/− animals to autoimmune and inflammatory diseases (Hoek et al., 2000), deficiency in CD200 or CD200R leads to exacerbated responses to infections with microbes such as influenza (Snelgrove et al., 2008) and Neisseria meningitis (Mukhopadhyay et al., 2010). In those studies, the observed pathology was mediated by an uncurtailed systemic response against the pathogen, rather than an intrinsic increase in pathogen virulence. In sharp contrast with these observations, we found that CD200 deficient mice are more resistant to infection with virulent L. amazonensis, without any obvious evidence of additional pathology. The anti-inflammatory environment generated during Leishmania infections (Sacks and Noben-Trauth, 2002) may play a role in this process, by masking the damaging consequences of systemic CD200 deficiency.
CD200 is thought to control the activation of CD200R-expressing myeloid cells by establishing trans-cellular interactions (Barclay et al., 2002; Hatherley and Barclay, 2004). Thus, we envision that CD200 molecules expressed in response to L. amazonensis infection interact with CD200R on neighboring macrophages, dampening the overall ability of the host cell population to control intracellular parasite growth through suppression of the microbicide NO. Our experiments demonstrate that signaling mediated by CD200 can suppress NO production and promote Leishmania growth within a homogeneous population of cultured macrophages. In vivo, it is conceivable that additional cell types may also respond to L. amazonensis infection by expressing CD200, expanding the parasites’ ability to evade host microbicidal activity.
Experimental Procedures
Leishmania culture and purification
Leishmania amazonensis (IFLA/BR/67/PH8) and Leishmania major (clone VI, MHOM/IL/80/Friedlin) were propagated as promastigotes at 26°C in M199 media supplemented with 5% penicillin/ streptomycin, 0.1% hemin (25 mg/ml in 0.1N NaOH), 10 mM adenine and 10% FBS, pH 7.5. Metacyclic forms of L. amazonensis and L. major were purified by agglutination of stationary-phase promastigote cultures using the 3A.1 mAb (Pinto-da-Silva et al., 2005) and peanut agglutinin (Sigma) (Sacks and Melby, 2001), respectively. To generate L. amazonensis axenic amastigotes, cultures rich in metacyclic forms were incubated in M199 media supplemented with 0.25% glucose, 0.5% trypticase, 40 mM sodium succinate (pH 4.5), 20% FBS, 5% penicillin/ streptomycin at 2×106/ml at 32°C for a minimum of 6 days, and then cultured axenically at 32°C. Parasites were washed 3 times in PBS before use in experiments. To obtain lesion amastigotes, female Balb/c mice were infected with purified metacyclic promastigotes of L. amazonensis or L. major. After 4–5 weeks the animals were euthanized and the parasites recovered from footpad lesions, as described (Sacks and Melby, 2001). The lesion homogenate rich in amastigotes was submitted to sequential 2000×g centrifugation steps to separate parasites from tissue debris.
Mouse infections and quantification of parasite load
Female wild type (WT) C57BL/6 mice (obtained from Jackson Laboratories) and CD200−/− mice generated in the C57BL/6 background (Hoek et al., 2000) (obtained from Schering-Plough BioPharma) were bred and used under protocols approved by the Institutional Animal Care and Use Committee at the Yale University School of Medicine. Female WT or CD200−/− 8 week old C57BL/6 mice were injected in the left hind footpad with 106 purified metacyclic promastigotes of L. amazonensis or L. major, and lesion progression was followed by blinded weekly measurements with a caliper. Inoculations of CD200-Fc or IgG1-Fc were done as described (Snelgrove et al., 2008), with some modifications. Mice were injected intraperitoneally with 10 µg of recombinant CD200-Fc chimera (3355-CD, R&D Systems) or control human IgG1-Fc (110-HG, R&D Systems) in PBS on days 1, 3 and 6 after infection, followed by weekly injections throughout the experiment. The total number of parasites in the injected footpad was estimated by a limiting dilution assay as previously described (Sacks and Melby, 2001; Titus et al., 1985), with minor modifications. Footpad tissues were ground in a Medimachine (Beckton Dickinson) and mechanically dissociated using a pellet pestle in 100 µl of Dulbecco’s modified Eagle’s medium containing 100 U/ml penicillin, 100 µg/ml streptomycin. Tissue homogenates were filtered in a 70 µm pore-size cell strainer (Falcon Products, Inc.) and serially diluted in a 96-well flat-bottom microtiter plate containing biphasic medium prepared using 30 µl of NNN medium containing 20% defibrinated rabbit blood and overlaid with 100 µl of M199 medium. The number of viable parasites was calculated from the highest dilution at which promastigote growth could be observed after 10 days of incubation at 26°C.
Affymetrix Gene Chip analysis
C57BL/6 BMM plated at 2.5×105 in 10 cm dishes were either not infected or infected with L. amazonensis amastigotes at MOI=10 for 30 min at 34°C. After infection, cells were washed 3 times in PBS and further incubated for the appropriate periods. Total RNA was isolated using Trizol (Invitrogen) and analyzed using an Affymetrix Gene Chip protocol, as previously described (Wilson et al., 2008). The Affymetrix protocol, used to analyze RNA extracted from BMM infected for 48 h with L. amazonensis amastigotes, was essentially as described (GeneChip Expression Analysis Technical Manual, Affymetrix). cRNA was hybridized for 16 h to Affymetrix Genechip Mouse Genome 430 2.0 Array (Affymetrix), which contain 45,000 probe sets for the analysis of 39,000 transcripts and variants from over 34,000 mouse genes. Normalization was performed using G-C-corrected robust multiarray average (GC-RMA). Identification of significantly perturbed genes was done using significance analysis of microarrays. The false positive rate was 0.1%.
Quantitative Real Time PCR (qPCR)
Real-time PCR was performed using a BioRad iQ icycler Detection system (BioRad Laboratories) using SYBR green fluorophore (BioRad Laboratories) according to the manufacturer’s instructions. Oligonucleotide primers 5’-GAA TCA AAC AAT ACA GAA TG-3’ and 5’-TGC CCC CCA CCA GTA ACA TGG- 3’ were used to amplify a portion of the CD200 cDNA or 5’-TCA GTC AAC GGG GGA CAT AAA-3’ and 5’-GGG GCT GTA CTG CTT AAC CAG-3’ to amplify the control cDNA HPRT1. The reaction was incubated for 3 min at 95°C, and then for 45 cycles of 20 s at 95°C followed by 30 s at 55°C. Fluorescence was detected at each annealing step and the cycle threshold (Ct) was calculated by determining the point at which the fluorescence exceeded a threshold limit. All reactions were performed in triplicate and negative controls (no template cDNA) were included in each experiment. The data was normalized by the level of HPRT1 expression in individual samples.
Quantification of Leishmania intracellular growth in macrophages
Mouse bone marrow macrophages (BMM) were prepared as previously described (Becker et al., 2009). 1×105 BMM were plated on glass coverslips in 3 cm dishes 24 h prior to experiments. Purified recombinant CD200-Fc (3355-CD, R&D Systems) or the control construct IgG1-Fc (110-HG, R&D Systems) were added at 2 µg/ml. The nitric oxide synthase inhibitors N-methyl-L-arginine (L-NMMA, active form) or N-methyl-D-arginine (D-NMMA, inactive form) were added at 500 µM (Sigma) (Iniesta et al., 2001; Koblish et al., 1998) for 30 min, followed by a PBS wash prior to infection. Axenic or lesion amastigotes were then added at a MOI=2 in RPMI 10% FBS for 1 h at 34°C. After invasion, cells were washed 3 times in PBS and incubated for the indicated times at 34°C. Coverslips were then fixed in 4% paraformaldehyde (PFA), and host cell and parasite DNA were stained with 10 µg/ml DAPI for 1 h, after permeabilization with 0.1% Triton-X 100 for 10 min. The number of intracellular parasites was quantified by scoring the total number of macrophages and the total number of intracellular parasites (clearly identifiable by visualization of the parasitophorous vacuole by phase microscopy) per microscopic field (100x N.A. 1.3 oil immersion objective, Nikon E200 epifluorescence microscope). The results were expressed as intracellular parasites per 100 macrophages. At least 300 host cells, in triplicate, were analyzed for each time point. For immunofluorescence, PFA fixed cells were washed with PBS, quenched with 15 mM NH4Cl for 15 min and permeabilized with saponin/PBS (0.1 %), prior to staining with rat anti-mouse Lamp1 mAb (Developmental Studies Hybridoma Bank) for 1 h, followed by 1 h incubation with anti–rabbit IgG AlexaFluor 488. For parasite staining, coverslips were permeabilized with 0.1 % Triton-X 100 for 3 min and incubated with mouse polyclonal antibodies prepared against axenic amastigotes of L. amazonensis, followed by anti-mouse IgG Texas red. All samples were incubated with 10 µg/ml DAPI for nuclei staining. Secondary antibodies were purchased from Molecular Probes. Images were either acquired through a 100x objective using a Zeiss Axiovert microscope equipped with a Hamamatsu Orca II cooled CCD camera controlled by Metamorph Software (Molecular Devices), or with a Leica SPX5 confocal microscope using a 63x/1.4 objective.
Western Blot and Immunoprecipitation
15 µg of protein/well were separated under reducing conditions on a 10% SDS polyacrylamide gel, blotted onto nitrocellulose membranes using a Trans-blot Transfer system (Bio-Rad Laboratories) overnight at 40V, and probed with rabbit anti-mouse iNOS (Abcam), and mouse anti-mouse actin (Sigma) followed by peroxidase-conjugated secondary antibodies. CD200 protein detection was performed using the Pierce Crosslink Immunoprecipitation Kit as described by the manufacturer (Thermo Scientific). Briefly, samples were added to columns containing goat anti-mouse CD200 antibodies (R&D Systems) crosslinked to protein G/A beads. After several washes elution buffer (20 µl) was added, and the eluate samples were separated under reducing conditions on a 10% SDS polyacrylamide gel, blotted onto nitrocellulose membranes, and probed with goat anti-CD200 antibodies followed by peroxidase-conjugated secondary antibodies. Immunoblots were developed using Supersignal West Pico Chemiluminescent Substrate (Thermo Scientific) and detected using a Fuji LAS-3000 Imaging System and Image Reader LAS-3000 software.
Nitric oxide measurement
Nitrite concentrations in BMM culture supernatants were used as a measure of nitric oxide production, and quantified using a Fluorometric Assay Kit (Biovision) or modified Griess reagent (Sigma), as described by the manufacturers. Before quantification, all samples were centrifuged at 800×g to remove cellular debris, and filtered in Amicon Ultra device filters (Millipore). Absorbance was measured at 450 nm after excitation at 360 nm for the Fluorometric Assay Kit, and at 540 nm for the modified Griess reagent. The concentration of nitric oxide (total nitrite concentration) in each sample was calculated from standard curve determinations as provided by the manufacturers.
Statistical analysis
Statistical significance between means of various groups was determined using an unpaired Student t test for independent samples (GraphPad Prism software). Values of 0.05 or less were considered significant.
Highlights
L. amazonensis induces expression of the immunoregulatory ligand CD200 in macrophages.
CD200 inhibits host iNOS expression and NO production, and is required for L. amazonensis virulence.
L. major grows slowly in macrophages and mice, and does not induce CD200 expression.
Mild L. major infections are transformed into a virulent pattern by soluble CD200.
Supplementary Material
Acknowledgements
We thank Dr. A. Vignery (Yale University) for helpful discussions and Amy Beaven (University of Maryland) and the Department of Cell Biology and Molecular Genetics Imaging Core for assistance with confocal microscopy. This work was supported by NIH grants R01AI067979 and R37AI34867 to N.W.A., and R01AI025032 and R01AI032972 to A.A. Author Contributions: M.C., C.H., A.A. and N.W.A. conceived and designed the project. M.C., C.H., M.C.F and K.A.K. performed experiments. M.C., K.A.K., A.A. and N.W.A. wrote the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors do not have any conflicts of interest to declare.
References
- Afonso LC, Scott P. Immune responses associated with susceptibility of C57BL/10 mice to Leishmania amazonensis. Infect Immun. 1993;61:2952–2959. doi: 10.1128/iai.61.7.2952-2959.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balestieri FM, Queiroz AR, Scavone C, Costa VM, Barral-Netto M, Abrahamsohn Ide A. Leishmania (L.) amazonensis-induced inhibition of nitric oxide synthesis in host macrophages. Microbes Infect. 2002;4:23–29. doi: 10.1016/s1286-4579(01)01505-2. [DOI] [PubMed] [Google Scholar]
- Banerjee D, Dick AD. Blocking CD200-CD200 receptor axis augments NOS-2 expression and aggravates experimental autoimmune uveoretinitis in Lewis rats. Ocul Immunol Inflamm. 2004;12:115–125. doi: 10.1080/09273940490895326. [DOI] [PubMed] [Google Scholar]
- Barclay AN, Wright GJ, Brooke G, Brown MH. CD200 and membrane protein interactions in the control of myeloid cells. Trends Immunol. 2002;23:285–290. doi: 10.1016/s1471-4906(02)02223-8. [DOI] [PubMed] [Google Scholar]
- Barral A, Pedral-Sampaio D, Grimaldi Junior G, Momen H, McMahon-Pratt D, Ribeiro de Jesus A, Almeida R, Badaro R, Barral-Netto M, Carvalho EM, et al. Leishmaniasis in Bahia, Brazil: evidence that Leishmania amazonensis produces a wide spectrum of clinical disease. Am J Trop Med Hyg. 1991;44:536–546. doi: 10.4269/ajtmh.1991.44.536. [DOI] [PubMed] [Google Scholar]
- Becker SM, Delamarre L, Mellman I, Andrews NW. Differential role of the Ca(2+) sensor synaptotagmin VII in macrophages and dendritic cells. Immunobiology. 2009;214:495–505. doi: 10.1016/j.imbio.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkaid Y, Mendez S, Lira R, Kadambi N, Milon G, Sacks D. A natural model of Leishmania major infection reveals a prolonged "silent" phase of parasite amplification in the skin before the onset of lesion formation and immunity. J Immunol. 2000;165:969–977. doi: 10.4049/jimmunol.165.2.969. [DOI] [PubMed] [Google Scholar]
- Belosevic M, Finbloom DS, Van Der Meide PH, Slayter MV, Nacy CA. Administration of monoclonal anti-IFN-gamma antibodies in vivo abrogates natural resistance of C3H/HeN mice to infection with Leishmania major. J Immunol. 1989;143:266–274. [PubMed] [Google Scholar]
- Blos M, Schleicher U, Soares Rocha FJ, Meissner U, Rollinghoff M, Bogdan C. Organ-specific and stage-dependent control of Leishmania major infection by inducible nitric oxide synthase and phagocyte NADPH oxidase. Eur J Immunol. 2003;33:1224–1234. doi: 10.1002/eji.200323825. [DOI] [PubMed] [Google Scholar]
- Bogdan C, Rollinghoff M, Diefenbach A. The role of nitric oxide in innate immunity. Immunol Rev. 2000;173:17–26. doi: 10.1034/j.1600-065x.2000.917307.x. [DOI] [PubMed] [Google Scholar]
- Boggiatto PM, Jie F, Ghosh M, Gibson-Corley KN, Ramer-Tait AE, Jones DE, Petersen CA. Altered dendritic cell phenotype in response to Leishmania amazonensis amastigote infection is mediated by MAP kinase, ERK. Am J Pathol. 2009;174:1818–1826. doi: 10.2353/ajpath.2009.080905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxbaum LU. Type I IFNs promote the early IFN-gamma response and the IL-10 response in Leishmania mexicana infection. Parasite Immunol. 2010;32:153–160. doi: 10.1111/j.1365-3024.2009.01167.x. [DOI] [PubMed] [Google Scholar]
- Cameron CM, Barrett JW, Liu L, Lucas AR, McFadden G. Myxoma virus M141R expresses a viral CD200 (vOX-2) that is responsible for down-regulation of macrophage and T-cell activation in vivo. J Virol. 2005;79:6052–6067. doi: 10.1128/JVI.79.10.6052-6067.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copland DA, Calder CJ, Raveney BJ, Nicholson LB, Phillips J, Cherwinski H, Jenmalm M, Sedgwick JD, Dick AD. Monoclonal antibody-mediated CD200 receptor signaling suppresses macrophage activation and tissue damage in experimental autoimmune uveoretinitis. Am J Pathol. 2007;171:580–588. doi: 10.2353/ajpath.2007.070272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diefenbach A, Schindler H, Donhauser N, Lorenz E, Laskay T, MacMicking J, Rollinghoff M, Gresser I, Bogdan C. Type 1 interferon (IFNalpha/beta) and type 2 nitric oxide synthase regulate the innate immune response to a protozoan parasite. Immunity. 1998;8:77–87. doi: 10.1016/s1074-7613(00)80460-4. [DOI] [PubMed] [Google Scholar]
- Foster-Cuevas M, Wright GJ, Puklavec MJ, Brown MH, Barclay AN. Human herpesvirus 8 K14 protein mimics CD200 in down-regulating macrophage activation through CD200 receptor. J Virol. 2004;78:7667–7676. doi: 10.1128/JVI.78.14.7667-7676.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes IN, Calabrich AF, Tavares Rda S, Wietzerbin J, de Freitas LA, Veras PS. Differential properties of CBA/J mononuclear phagocytes recovered from an inflammatory site and probed with two different species of Leishmania. Microbes Infect. 2003;5:251–260. doi: 10.1016/s1286-4579(03)00025-x. [DOI] [PubMed] [Google Scholar]
- Green SJ, Crawford RM, Hockmeyer JT, Meltzer MS, Nacy CA. Leishmania major amastigotes initiate the L-arginine-dependent killing mechanism in IFN-gamma-stimulated macrophages by induction of tumor necrosis factor-alpha. J Immunol. 1990;145:4290–4297. [PubMed] [Google Scholar]
- Hatherley D, Barclay AN. The CD200 and CD200 receptor cell surface proteins interact through their N-terminal immunoglobulin-like domains. Eur J Immunol. 2004;34:1688–1694. doi: 10.1002/eji.200425080. [DOI] [PubMed] [Google Scholar]
- Hoek RM, Ruuls SR, Murphy CA, Wright GJ, Goddard R, Zurawski SM, Blom B, Homola ME, Streit WJ, Brown MH, et al. Down-regulation of the macrophage lineage through interaction with OX2 (CD200) Science. 2000;290:1768–1771. doi: 10.1126/science.290.5497.1768. [DOI] [PubMed] [Google Scholar]
- Iniesta V, Gomez-Nieto LC, Corraliza I. The inhibition of arginase by N(omega)-hydroxy-l-arginine controls the growth of Leishmania inside macrophages. J Exp Med. 2001;193:777–784. doi: 10.1084/jem.193.6.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DE, Ackermann MR, Wille U, Hunter CA, Scott P. Early enhanced Th1 response after Leishmania amazonensis infection of C57BL/6 interleukin-10-deficient mice does not lead to resolution of infection. Infect Immun. 2002;70:2151–2158. doi: 10.1128/IAI.70.4.2151-2158.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DE, Buxbaum LU, Scott P. IL-4-independent inhibition of IL-12 responsiveness during Leishmania amazonensis infection. J Immunol. 2000;165:364–372. doi: 10.4049/jimmunol.165.1.364. [DOI] [PubMed] [Google Scholar]
- Koblish HK, Hunter CA, Wysocka M, Trinchieri G, Lee WM. Immune suppression by recombinant interleukin (rIL)-12 involves interferon gamma induction of nitric oxide synthase 2 (iNOS) activity: inhibitors of NO generation reveal the extent of rIL-12 vaccine adjuvant effect. J Exp Med. 1998;188:1603–1610. doi: 10.1084/jem.188.9.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang Z, Yao S, Xu Y, Lewis RS, Low A, Masters SL, Willson TA, Kolesnik TB, Nicholson SE, Garrett TJ, et al. SPRY domain-containing SOCS box protein 2: crystal structure and residues critical for protein binding. J Mol Biol. 2009;386:662–674. doi: 10.1016/j.jmb.2008.12.078. [DOI] [PubMed] [Google Scholar]
- Liew FY, Millott S, Parkinson C, Palmer RM, Moncada S. Macrophage killing of Leishmania parasite in vivo is mediated by nitric oxide from L-arginine. J Immunol. 1990;144:4794–4797. [PubMed] [Google Scholar]
- MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- Mattner J, Schindler H, Diefenbach A, Rollinghoff M, Gresser I, Bogdan C. Regulation of type 2 nitric oxide synthase by type 1 interferons in macrophages infected with Leishmania major. Eur J Immunol. 2000;30:2257–2267. doi: 10.1002/1521-4141(2000)30:8<2257::AID-IMMU2257>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- McMahon-Pratt D, Alexander J. Does the Leishmania major paradigm of pathogenesis and protection hold for New World cutaneous leishmaniases or the visceral disease? Immunol Rev. 2004;201:206–224. doi: 10.1111/j.0105-2896.2004.00190.x. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay S, Pluddemann A, Hoe JC, Williams KJ, Varin A, Makepeace K, Aknin ML, Bowdish DM, Smale ST, Barclay AN, et al. Immune inhibitory ligand CD200 induction by TLRs and NLRs limits macrophage activation to protect the host from meningococcal septicemia. Cell Host Microbe. 2010;8:236–247. doi: 10.1016/j.chom.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Muleme HM, Reguera RM, Berard A, Azinwi R, Jia P, Okwor IB, Beverley S, Uzonna JE. Infection with arginase-deficient Leishmania major reveals a parasite number-dependent and cytokine-independent regulation of host cellular arginase activity and disease pathogenesis. J Immunol. 2009;183:8068–8076. doi: 10.4049/jimmunol.0803979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasseri M, Modabber FZ. Generalized infection and lack of delayed hypersensitivity in BALB/c mice infected with Leishmania tropica major. Infect Immun. 1979;26:611–614. doi: 10.1128/iai.26.2.611-614.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto-da-Silva LH, Fampa P, Soares DC, Oliveira SM, Souto-Padron T, Saraiva EM. The 3A1-La monoclonal antibody reveals key features of Leishmania (L) amazonensis metacyclic promastigotes and inhibits procyclics attachment to the sand fly midgut. Int J Parasitol. 2005;35:757–764. doi: 10.1016/j.ijpara.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Qadoumi M, Becker I, Donhauser N, Rollinghoff M, Bogdan C. Expression of inducible nitric oxide synthase in skin lesions of patients with american cutaneous leishmaniasis. Infect Immun. 2002;70:4638–4642. doi: 10.1128/IAI.70.8.4638-4642.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi H, Ji J, Wanasen N, Soong L. Enhanced replication of Leishmania amazonensis amastigotes in gamma interferon-stimulated murine macrophages: implications for the pathogenesis of cutaneous leishmaniasis. Infect Immun. 2004;72:988–995. doi: 10.1128/IAI.72.2.988-995.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravetch JV, Lanier LL. Immune inhibitory receptors. Science. 2000;290:84–89. doi: 10.1126/science.290.5489.84. [DOI] [PubMed] [Google Scholar]
- Reiner SL, Locksley RM. The regulation of immunity to Leishmania major. Annu Rev Immunol. 1995;13:151–177. doi: 10.1146/annurev.iy.13.040195.001055. [DOI] [PubMed] [Google Scholar]
- Sacks D, Noben-Trauth N. The immunology of susceptibility and resistance to Leishmania major in mice. Nat Rev Immunol. 2002;2:845–858. doi: 10.1038/nri933. [DOI] [PubMed] [Google Scholar]
- Sacks DL, Melby PC. Animal models for the analysis of immune responses to leishmaniasis. Curr Protoc Immunol. 2001 doi: 10.1002/0471142735.im1902s28. Chapter 19, Unit 19 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott P, Sher A. A spectrum in the susceptibility of leishmanial strains to intracellular killing by murine macrophages. J Immunol. 1986;136:1461–1466. [PubMed] [Google Scholar]
- Shankar AH, Morin P, Titus RG. Leishmania major: differential resistance to infection in C57BL/6 (high interferon-alpha/beta) and congenic B6.C-H-28c (low interferon-alpha/beta) mice. Exp Parasitol. 1996;84:136–143. doi: 10.1006/expr.1996.0099. [DOI] [PubMed] [Google Scholar]
- Snelgrove RJ, Goulding J, Didierlaurent AM, Lyonga D, Vekaria S, Edwards L, Gwyer E, Sedgwick JD, Barclay AN, Hussell T. A critical function for CD200 in lung immune homeostasis and the severity of influenza infection. Nat Immunol. 2008;9:1074–1083. doi: 10.1038/ni.1637. [DOI] [PubMed] [Google Scholar]
- Stenger S, Donhauser N, Thuring H, Rollinghoff M, Bogdan C. Reactivation of latent leishmaniasis by inhibition of inducible nitric oxide synthase. J Exp Med. 1996;183:1501–1514. doi: 10.1084/jem.183.4.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titus RG, Marchand M, Boon T, Louis JA. A limiting dilution assay for quantifying Leishmania major in tissues of infected mice. Parasite Immunol. 1985;7:545–555. doi: 10.1111/j.1365-3024.1985.tb00098.x. [DOI] [PubMed] [Google Scholar]
- Wei XQ, Charles IG, Smith A, Ure J, Feng GJ, Huang FP, Xu D, Muller W, Moncada S, Liew FY. Altered immune responses in mice lacking inducible nitric oxide synthase. Nature. 1995;375:408–411. doi: 10.1038/375408a0. [DOI] [PubMed] [Google Scholar]
- Wilkins-Rodriguez AA, Escalona-Montano AR, Aguirre-Garcia M, Becker I, Gutierrez-Kobeh L. Regulation of the expression of nitric oxide synthase by Leishmania mexicana amastigotes in murine dendritic cells. Exp Parasitol. 2010;126:426–434. doi: 10.1016/j.exppara.2010.07.014. [DOI] [PubMed] [Google Scholar]
- Wilson J, Huynh C, Kennedy KA, Ward DM, Kaplan J, Aderem A, Andrews NW. Control of parasitophorous vacuole expansion by LYST/Beige restricts the intracellular growth of Leishmania amazonensis. PLoS Pathog. 2008;4:e1000179. doi: 10.1371/journal.ppat.1000179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright GJ, Puklavec MJ, Willis AC, Hoek RM, Sedgwick JD, Brown MH, Barclay AN. Lymphoid/neuronal cell surface OX2 glycoprotein recognizes a novel receptor on macrophages implicated in the control of their function. Immunity. 2000;13:233–242. doi: 10.1016/s1074-7613(00)00023-6. [DOI] [PubMed] [Google Scholar]
- Xin L, Li K, Soong L. Down-regulation of dendritic cell signaling pathways by Leishmania amazonensis amastigotes. Mol Immunol. 2008;45:3371–3382. doi: 10.1016/j.molimm.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.