Abstract
Vancomycin (VAN) has been associated with acute kidney injury (AKI) since it has been put into clinical use in the 1950's. Early reports of AKI were likely linked to the impurities of the VAN preparation. With the advent of the more purified forms of VAN, the incidence of AKI related to VAN were limited to acute interstitial nephritis (AIN) or as a potentiating agent to other nephrotoxins such as Aminoglycosides. VAN as the sole etiologic factor for nephrotoxic acute tubular necrosis (ATN) has not been described. Here, we report a case of biopsy-proven ATN resulting from VAN.
1. Case
R. H. is a 23-year-old male with a history of childhood acute lymphocytic leukemia in remission, neuroectodermal tumor status postresection, and gamma knife therapy, who presented to our Emergency Department (ED) with complaints of fever and chills. He also noticed yellowish drainage around the site of a peripherally inserted central catheter (PICC). In the ED, patient was febrile to 101.7° Fahrenheit with a blood pressure of 113/83 mm Hg and pulse of 134 bpm. The PICC line was removed, and antibiotic therapy initiated with piperacillin-tazobactam and VAN. RH received 1 gm IV of VAN in the ED, and upon admission to the floor, received another 2 gm IV. Piperacillin-tazobactam was discontinued. Eight hours later, another 2 grams of VAN were given, with cumulative dose of 5 gm in 24 hrs (50 mg/kg in 24 hours). Patient's admission creatinine was 0.97 mg/dL. The patient was 103 kg and 72′′ tall. Attempting to use a loading dose of 15 mg/kg based on total body weight [1], the patient was initiated on 2000 mg of VAN per hospital protocol of using VAN doses in 1000 mg aliquots. Standard pharmacokinetic equations with simplified one-compartment models employing log-linear drug removal were used for estimations [2]. With a volume of distribution estimated at approximately 0.6 L/kg of actual body weight and a VAN clearance estimated to be at least 120 mL/min [3], the patient was expected to achieve an initial VAN trough of approximately 8 mg/L with steady state troughs above 10 mg/L with a dosing scheme of 2000 mg given every 12 hours. As pharmacokinetic estimates in patients above their average body weight exhibit substantial variability, the patient was initiated on the aforementioned dose with a measured VAN trough planned antecedent to the fourth dose (to capture steady-state concentrations). Goal troughs and monitoring schemes were performed according to national VAN guidelines [4].
The following day, the serum creatinine was 3.62 mg/dL. He became oligo-anuric with a urine output of 50 cc in 24 hours. Urinalysis revealed a bland urine sediment and a urine sodium of 75 meq/L and a fractional excretion of sodium of 2.77%. He had not been exposed to IV contrast, received an aminoglycoside or exposed to any other potential nephrotoxins.
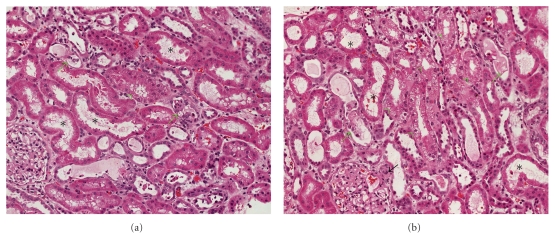
Blood cultures were negative, and the PICC line tip cultures were positive for Serratia marcescens. As the patient experienced acute nephrotoxicity, VAN was ceased after the third dose and ciprofloxacin initiated. The creatinine continued to rise (Table 1). A renal ultrasound ruled out obstruction. On day 3, a renal biopsy was performed. The main finding was ATN (Figure 1). Many tubules showed moderate degree of vacuolization of their cytoplasm (green arrows). Some of tubules contained hyaline or epithelial casts in their lumina (black arrows). The VAN serum concentration done on day 4 was 64.7 mg/L, and hemodialysis was initiated for volume overload. By day 5, his urine output increased to 1 L/day. On day 10, he was taken off hemodialysis. His creatinine improved to 1.2 mg/dL over the next few weeks.
Table 1.
Laboratory and clinical parameters.
| Day | Scr mg/dl | UO 24 hrs | VAN dose | VAN S. Conc. | HD |
|---|---|---|---|---|---|
| 0 | .97 | NR | 5 gm | ND | No |
| 1 | 3.62–4.26 | 50 cc | ND | No | |
| 2 | 6.25 | <50 cc | ND | No | |
| 3 | 8.41 | <50 cc | ND | No | |
| 4 | 9.96 | <50 cc | 64.7 | Yes | |
| 5 | 9.36 | 1000 cc | Yes | ||
| 9 | 9.21 | 2000 cc | ND | Yes | |
| 10 | 5.88 | 2500 cc | ND | No | |
| 30 | 1.24 | NR | ND | No |
Scr—serum creatinine; UO—urine output; VAN—Vancomycin; S. Conc—Serum concentration; HD—hemodialysis; ND—not done; NR—not recorded.
Figure 1.
Representative photographs of the renal biopsy showing tubular damage secondary to drug toxicity. In both panels (a) and (b) a number of tubules show moderate degree of acute tubular necrosis (asterisks). Some of tubules contain hyaline or epithelial casts in their lumina (green arrows), while several tubules show vacuolization of their cytoplasm (green arrowheads). One of the glomerular afferent arteriole shows swollen endothelia and occlusive change (black arrow).
2. Discussion
VAN, a parenteral glycopeptide antibiotic, has been used clinically since 1956. The original preparation contained a number of impurities and was brown in color, hence, the nickname “Mississippi Mud”. It is these impurities that were thought to be responsible for certain toxicities such as anaphylaxis, toxic epidermal necrolysis, erythema multiforme, ototoxicity, and nephrotoxicity [5, 6]. Between 1956 and 1986, 57 cases of VAN-associated nephrotoxicity were described. More than 50% of the cases were identified within the first six years of VAN use when the product was relatively impure [7]. As manufacturing processes improved and VAN was purified, nephrotoxicity became less common [8], and contemporary preparations seemed to nearly eliminate the original adverse events.
In 1983, Farber andMoelleringreported the incidence of nephrotoxicity with VAN to be 5% [5]. This, however, was a retrospective study, and it failed to evaluate whether the AKI was due to AIN or ATN. VAN in association with an aminoglycoside is known to be associated with an increased incidence of nephrotoxicity presumed to be ATN [9, 10].
Other risk factors associated with nephrotoxicity include prolonged therapy for >21 days, [9] higher APACHE scores, [11] loop diuretics, and steady-state VAN concentration > or = 28 mg/L [12]. Recent studies have shown that doses greater than or equal to 4 grams per day [13] and trough concentrations greater than 15 mg/L are associated with nephrotoxicity [11, 14]. More specifically, when trough concentrations are analyzed as a linear variable with classification and regression tree modeling, a threshold of 9.9 mg/L has been identified [15]. These studies, while instructive for the association of VAN and toxicity, are limited by a failure to control for weight-adjusted dose, and the retrospective nature of the studies do not determine causality [11, 13–15]. Instead, elevated VAN concentrations could be merely an intermediate variable in the pathway to the ultimate causal event of renal failure by other mechanisms.
As VAN safety profile improved and with the emerging prevalence of methicillin-resistant Staphylococcus aureus (MRSA), VAN became one of the most frequently prescribed intravenous anti-infective for treatment of these infections [16]. With increasing the minimum inhibitory concentrations for VAN and proper testing methodologies for completing minimum inhibitory concentrations under continual debate [17–20], the dire outcomes associated with MRSA infections have lead many to seek novel treatment strategies including increasing VAN doses and exposure. Specifically, various national guidelines for serious infections have recommended that VAN troughs be maintained between 15–20 mg/L to ensure an AUC/MIC of ~400 [21–23]. Such a position has been substantiated on the basis of appropriate pharmacodynamic targets, limited clinical data, and the supposition that such a strategy is safe [21, 22, 24]. However, no study to date has prospectively evaluated the impact of VAN exposure on renal endpoints and that elevated doses (such as a 5 gram dose given in 24 hours in our patient) could predispose patients to kidney injury.
The potential mechanism of action of VAN-associated nephropathy has been studied in both humans and animals. The energy-dependent transport mechanisms found in the proximal tubular epithelium render the kidneys highly susceptible to toxicant-induced renal injury. VAN exposure in renal proximal tubule epithelial cells results in increased cell proliferation as evidenced by increased number of cells, total protein, and DNA synthesis. VAN enhances cellular ATP concentration and stimulates oxygen consumption, supporting its role as a stimulant of oxidative phosphorylation [25]. The beneficial effect of some antioxidants like DL-α lipoic acid, Melatonin, Ginkgo biloba and milrinone have been shown to reduce the renal damage, suggesting the involvement of free radicals in renal damage [26].
While it is unclear if VAN-associated acute tubular necrosis is preventable, these data argue for prompt serum VAN monitoring given the rapid functional decline of patient nephrologic function even before the patient was scheduled to receive the 4th dose of therapeutically dosed VAN. Additionally, these data may support previously published literature suggesting that VAN doses >4 g per day, and patient body weights in excess of 101.4 kg may predispose to nephrotoxicity, [27] in this case, acute tubular necrosis.
3. Conclusion
The most common VAN-associated nephrotoxicity is AIN [28–30]. Although an association with ATN has been described, to our knowledge, VAN as the sole etiologic cause of ATN has never been reported in adult patients [31, 32]. Our findings support these retrospective associations and provide evidence for contemporary VAN-associated ATN. Further studies are needed to determine the safety profile of targeting higher VAN trough levels for serious infectious in light of the potential for AKI.
References
- 1.Vance-Bryan K, Guay DRP, Gilliland SS, Rodvold KA, Rotschafer JC. Effect of obesity on vancomycin pharmacokinetic parameters as determined by using a Bayesian forecasting technique. Antimicrobial Agents and Chemotherapy. 1993;37(3):436–440. doi: 10.1128/aac.37.3.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Winter ME. Basic Clinical Pharmacokinetics. 4th edition. Philadelphia, Pa, USA: Lippincott Williams & Wilkins; 2003. [Google Scholar]
- 3.Bearden DT, Rodvold KA. Dosage adjustments for antibacterials in obese patients: applying clinical pharmacokinetics. Clinical Pharmacokinetics. 2000;38(5):415–426. doi: 10.2165/00003088-200038050-00003. [DOI] [PubMed] [Google Scholar]
- 4.Rybak M, Lomaestro B, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. American Journal of Health-System Pharmacy. 2009;66(1):82–98. doi: 10.2146/ajhp080434. [DOI] [PubMed] [Google Scholar]
- 5.Farber BF, Moellering RC., Jr. Retrospective study of the toxicity of preparations of vancomycin from 1974 to 1981. Antimicrobial Agents and Chemotherapy. 1983;23(1):138–141. doi: 10.1128/aac.23.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woodley DW, Hall WH. The treatment of severe staphylococcal infections with vancomycin. Annals of Internal Medicine. 1961;55:235–249. doi: 10.7326/0003-4819-55-2-235. [DOI] [PubMed] [Google Scholar]
- 7.Bailie GR, Neal D. Vancomycin ototoxicity and nephrotoxicity. A review. Medical Toxicology and Adverse Drug Experience. 1988;3(5):376–386. doi: 10.1007/BF03259891. [DOI] [PubMed] [Google Scholar]
- 8.Levine DP. Vancomycin: a history. Clinical Infectious Diseases. 2006;42(supplement 1):S5–S12. doi: 10.1086/491709. [DOI] [PubMed] [Google Scholar]
- 9.Goetz MB, Sayers J. Nephrotoxicity of vancomycin and aminoglycoside therapy separately and in combination. Journal of Antimicrobial Chemotherapy. 1993;32(2):325–334. doi: 10.1093/jac/32.2.325. [DOI] [PubMed] [Google Scholar]
- 10.Rybak MJ, Abate BJ, Kang SL, Ruffing MJ, Lerner SA, Drusano GL. Prospective evaluation of the effect of an aminoglycoside dosing regimen on rates of observed nephrotoxicity and ototoxicity. Antimicrobial Agents and Chemotherapy. 1999;43(7):1549–1555. doi: 10.1128/aac.43.7.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeffres MN, Isakow W, Doherty JA, Micek ST, Kollef MH. A retrospective analysis of possible renal toxicity associated with vancomycin in patients with health care-associated methicillin-resistant Staphylococcus aureus pneumonia. Clinical Therapeutics. 2007;29(6):1107–1115. doi: 10.1016/j.clinthera.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 12.Elting LS, Rubenstein EB, Kurtin D, et al. Mississippi mud in the 1990s: risks and outcomes of vancomycin- associated toxicity in general oncology practice. Cancer. 1998;83(12):2597–2607. doi: 10.1002/(sici)1097-0142(19981215)83:12<2597::aid-cncr27>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 13.Lodise TP, Lomaestro B, Graves J, Drusano GL. Larger vancomycin doses (at least four grams per day) are associated with an increased incidence of nephrotoxicity. Antimicrobial Agents and Chemotherapy. 2008;52(4):1330–1336. doi: 10.1128/AAC.01602-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hidayat LK, Hsu DI, Quist R, Shriner KA, Wong-Beringer A. High-dose vancomycin therapy for methicillin-resistant Staphylococcus aureus infections: efficacy and toxicity. Archives of Internal Medicine. 2006;166(19):2138–2144. doi: 10.1001/archinte.166.19.2138. [DOI] [PubMed] [Google Scholar]
- 15.Lodise TP, Patel N, Lomaestro BM, Rodvold KA, Drusano GL. Relationship between initial vancomycin concentration-time profile and nephrotoxicity among hospitalized patients. Clinical Infectious Diseases. 2009;49(4):507–514. doi: 10.1086/600884. [DOI] [PubMed] [Google Scholar]
- 16.Kirst HA, Thompson DG, Nicas TI. Historical yearly usage of vancomycin. Antimicrobial Agents and Chemotherapy. 1998;42(5):1303–1304. doi: 10.1128/aac.42.5.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mason EO, Lamberth LB, Hammerman WA, Hulten KG, Versalovic J, Kaplan SL. Vancomycin MICs for Staphylococcus aureus vary by detection method and have subtly increased in a pediatric population since 2005. Journal of Clinical Microbiology. 2009;47(6):1628–1630. doi: 10.1128/JCM.00407-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sader HS, Rhomberg PR, Jones RN. Nine-hospital study comparing broth microdilution and Etest method results for vancomycin and daptomycin against methicillin-resistant Staphylococcus aureus. Antimicrobial Agents and Chemotherapy. 2009;53(7):3162–3165. doi: 10.1128/AAC.00093-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swenson JM, Anderson KF, Lonsway DR, et al. Accuracy of commercial and reference susceptibility testing methods for detecting vancomycin-intermediate Staphylococcus aureus. Journal of Clinical Microbiology. 2009;47(7):2013–2017. doi: 10.1128/JCM.00221-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sader HS, Fey PD, Fish DN, et al. Evaluation of vancomycin and daptomycin potency trends (MIC creep) against methicillin-resistant Staphylococcus aureus isolates collected in nine U.S. Medical Centers from 2002 to 2006. Antimicrobial Agents and Chemotherapy. 2009;53(10):4127–4132. doi: 10.1128/AAC.00616-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rybak MJ, Lomaestro BM, Rotscahfer JC, et al. Vancomycin therapeutic guidelines: a summary of consensus recommendations from the infectious diseases Society of America, the American Society of Health-System Pharmacists, and the Society of Infectious Diseases Pharmacists. Clinical Infectious Diseases. 2009;49:325–327. doi: 10.1086/600877. [DOI] [PubMed] [Google Scholar]
- 22.Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society Consensus Guidelines on the management of community-acquired pneumonia in adults. Clinical Infectious Diseases. 2007;44(supplement 2):S27–S72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tunkel AR, Hartman BJ, Kaplan SL, et al. Practice guidelines for the management of bacterial meningitis. Clinical Infectious Diseases. 2004;39(9):1267–1284. doi: 10.1086/425368. [DOI] [PubMed] [Google Scholar]
- 24.Scheetz MH, Wunderink RG, Postelnick MJ, Noskin GA. Potential impact of vancomycin pulmonary distribution on treatment outcomes in patients with methicillin-resistant Staphylococcus aureus pneumonia. Pharmacotherapy. 2006;26(4):539–550. doi: 10.1592/phco.26.4.539. [DOI] [PubMed] [Google Scholar]
- 25.King DW, Smith MA. Proliferative responses observed following vancomycin treatment in renal proximal tubule epithelial cells. Toxicology in Vitro. 2004;18(6):797–803. doi: 10.1016/j.tiv.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 26.Celik I, Cihangiroglu M, Ilhan N, Akpolat N, Akbulut HH. Protective effects of different antioxidants and amrinone on vancomycin-induced nephrotoxicity. Basic and Clinical Pharmacology and Toxicology. 2005;97(5):325–332. doi: 10.1111/j.1742-7843.2005.pto_153.x. [DOI] [PubMed] [Google Scholar]
- 27.Lodise TP, Lomaestro B, Graves J, Drusano GL. Larger vancomycin doses (at least four grams per day) are associated with an increased incidence of nephrotoxicity. Antimicrobial Agents and Chemotherapy. 2008;52(4):1330–1336. doi: 10.1128/AAC.01602-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bergman MM, Glew RH, Ebert TH. Acute interstitial nephritis associated with vancomycin therapy. Archives of Internal Medicine. 1988;148(10):2139–2140. [PubMed] [Google Scholar]
- 29.Codding CE, Ramseyer L, Allon M, Pitha J, Rodriguez M. Tubulointerstitial nephritis due to vancomycin. American Journal of Kidney Diseases. 1989;14(6):512–515. doi: 10.1016/s0272-6386(89)80152-0. [DOI] [PubMed] [Google Scholar]
- 30.Wai AO, Lo AMS, Abdo A, Marra F. Vancomycin-induced acute interstitial nephritis. Annals of Pharmacotherapy. 1998;32(11):1160–1164. doi: 10.1345/aph.17448. [DOI] [PubMed] [Google Scholar]
- 31.Fiaccadori E, Maggiore U, Arisi A, et al. Outbreak of acute renal failure due to cefodizime-vancomycin association in a heart surgery unit. Intensive Care Medicine. 2001;27(11):1819–1822. doi: 10.1007/s00134-001-1123-3. [DOI] [PubMed] [Google Scholar]
- 32.Sokol H, Vigneau C, Maury E, Guidet B, Offenstadt G. Biopsy-proven anuric acute tubular necrosis associated with vancomycin and one dose of aminoside. Nephrology Dialysis Transplantation. 2004;19(7):1921–1922. doi: 10.1093/ndt/gfh170. [DOI] [PubMed] [Google Scholar]