Abstract
Vascularization is a major challenge in tissue engineering. The purpose of this study is to expedite the formation of blood vessels in porous polycaprolactone (PCL) scaffolds by the delivery of endothelial progenitor cells (EPCs). To establish a pro-angiogenic and pro-vasculogenic microenvironment, we employed EPCs seeded in PCL scaffold with surface-immobilized heparin and vascular endothelial growth factor (VEGF). EPCs seeded on scaffolds with VEGF exhibited phosphorylation of the receptor. After 7 days of subcutaneous implantation in immunodeficient mice, heparin-immobilized PCL scaffolds with VEGF induced significantly high density of blood vessel formation. The anastomosis of EPC-derived vessels with the host circulatory system was evident by the presence of murine erythrocytes in the lumen of human-CD31 positive vessels. A more uniform distribution of blood vessels was achieved within 2-mm thick scaffolds by seeding an optimal density of EPCs. The seeding of a higher density of EPC resulted in an increase in apoptosis and a concomitant decline in blood vessel formation at the scaffold's inner core. When co-seeded with other cells, the EPCs maintained the ability to accelerate vessel formation. The excessive expansion of EPCs in vitro was associated with a decline in their in vivo vasculogenic potential. EPCs accelerated the vascularization of heparin-immobilized PCL scaffolds in the presence of VEGF.
Introduction
The delay in the blood vessel formation is a bottleneck for tissue-engineering scaffolds of larger dimensions. In the absence of the vessel network during early stages, the limited diffusion of nutrients restricts the cell survival to the periphery of implanted scaffolds. The in-growth of host vessels can be improved by the delivery of angiogenic growth factors or gene-encoding angiogenic factors.1,2 However, angiogenesis alone is not adequate to rapidly vascularize thicker scaffolds. An attractive option to augment scaffold vascularization is the delivery of endothelial cells (ECs). Scaffolds seeded with human umbilical vein EC (HUVEC) and human microvasculature ECs were able to form vessels in the implanted scaffolds.3–5 The major impediments in clinical use of adult ECs are their insufficient supply, limited in vitro expansion, and in vivo vessel-formation potential. Embryonic and adult stem cells have been recognized as a promising source of ECs, as they can differentiate into ECs in vitro.6,7 ECs derived from human embryonic stem cells and bone marrow stromal cells formed vessels in tissue-engineered scaffolds in vivo. Nonetheless, the application of stem cells for therapeutic purposes is subject to attaining thorough understanding and control over the differentiation process.
More recently, the vessel-forming endothelial progenitor cell (EPC) population has been identified in the human umbilical cord and circulating adult blood.8,9 EPCs expressed vascular cell surface markers such as CD31, vWF, and KDR and lacked hematopoietic specific CD45 and CD14 antigens.10,11 EPCs showed robust proliferation, self-renewal, and superior vessel-forming ability compared with the matured ECs.11,12 EPCs are clinically safer to use than embryonic stem cells, as they differentiate exclusively into the endothelial lineage. The process of blood vessel formation from the undifferentiated ECs is defined as vasculogenesis. There are two common strategies to vascularize scaffolds using EC precursors. The in vitro vasculogenesis involves prevascularization of the scaffold before implantation so that the preformed graft vessels could readily anastomose with the recipient vessels.4,5 The second approach entails implantation of scaffolds seeded with EPCs to induce in vivo vasculogenesis. Other researchers have tested the latter approach in scaffolds based on collagen10,13 and Matrigel.9,14,15
In this study, we used human umbilical cord-derived EPCs to induce in vivo vasculogenesis in porous polycaprolactone (PCL) scaffolds in 7 days. The distribution of the vessels formed by EPCs was assessed as a function of the scaffold thickness. We also evaluated the effect of heparin immobilization and vascular endothelial growth factor (VEGF) delivery with the aim of improving blood vessel formation by the EPCs. We examined the optimal EPCs seeding density and the effect of passaging on vessel-forming ability of EPCs.
Materials and Methods
Animals
Eight-week-old nonobese diabetic/severe combined immunodeficient mice (NOD-SCID; Charles River Laboratories) were used as recipients for scaffolds with EPCs. Autoclaved cages with irradiated diet and acidified water were used to house NOD-SCID mice. Transgenic mice strain FVB.Cg-Tg (GFPU) 5 Nagy/J (Jackson Laboratory) were maintained as a breeding colony and used as donors for hepatocytes and bone marrow stromal cells. The animals were maintained and handled in compliance with the institutional regulations established and approved by the Animal Research Committee at the University of California, Los Angeles.
Culture of EPCs
EPCs isolated from human umbilical cord blood were kindly provided by Dr. Mervin C. Yoder, Indiana University, Indianapolis. The EPCs were cultured in EBM-2 media (Lonza) supplemented with EGM-2-SingleQuots and 10% fetal bovine serum (FBS). The cells were maintained at 37°C in a humidified incubator containing 10% carbon dioxide, and the culture medium was changed every 2–3 days. The cells were trypsinized on reaching confluency and were re-plated for the next passage.
Isolation of hepatocytes and bone marrow stromal cells
Two-month-old transgenic mice were used for the isolation of green fluorescent protein (GFP)-positive primary hepatocytes and bone marrow stromal cells. The hepatocytes were harvested by a modified two-step perfusion procedure.16 Briefly, the liver was perfused successively with two solutions via portal vein. The first solution, containing 20 mM HEPES, 7 mM KCl, 142 mM NaCl, and 0.1 mM EDTA at pH 7.4 and 37°C, was perfused for 10 min at 3 mL/min. The second solution, containing collagenase type IV (0.5 mg/mL; Sigma) in 20 mM HEPES, 20 mM CaCl2, 7 mM KCl, and 142 mM NaCl at pH 7.4 and 37°C, was perfused till the liver was sufficiently disrupted. The excised liver was placed in cold Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum on ice. The liver capsule was removed gently to free the hepatocytes and was filtered through 70 μm nylon mesh. The cells were centrifuged at 50 g for 2 min at 4°C and the cell pellet was re-suspended in Percoll (1.05 g/mL; Sigma) and Hank's balanced salt solution. The solution was centrifuged at 50 g for 5 min. The obtained cell pellet was re-suspended in cold DMEM containing 10% FBS and used immediately for the experiments.
The bone marrow stromal cells were harvested from the tibia and femur by flushing the marrow plug with DMEM and 10% FBS under sterile conditions. The cell suspension was filtered through 70 μm nylon mesh and plated. The cells were maintained at 37°C in a humidified incubator, and the culture media was changed after 3 days. The cells were cultured for ∼20 days, and the confluent cells were trypsinized immediately before seeding on the scaffold.
Preparation of scaffold
Porous PCL (Lactel Absorbable Polymers) scaffolds were prepared by solvent casting and particulate leaching technique. Briefly, PCL (10% w/w) was dissolved in a mixed solution of chloroform and methanol (65 chloroform: 35 methanol w/w). Sieved sucrose particles of 212–300 μm diameter were added to the PCL solution. The ratio of sucrose:PCL was 96:4 (w/w). The blend was briefly mixed to form homogeneous slurry and was cast into Teflon molds with inner diameter of 6 mm and height of 2 mm. Scaffolds were freeze dried, and the sucrose was leached out in deionized water. The scaffolds were sterilized in 70% ethanol for 30 min and washed several times with phosphate-buffered saline (PBS).
The heparin was immobilized on the surface of the porous PCL scaffold by cross-linking heparin using carbodiimide reaction. The chemicals 2-(N-Morpholino)ethanesulfonic acid (MES), N-(3-Dimethylaminopropyl)-N'-ethylcarbodiimide hydrochloride (EDC), N-hydroxysuccinimide (NHS), and unfractionated heparin sodium salt derived from porcine intestinal mucosa were purchased from Sigma. The scaffolds were saturated with 0.05 M MES buffer (pH 5.5) for 15 min before the reaction. Subsequently, the scaffolds were immersed in freshly prepared solution of heparin (1% w/v), 0.5 M EDC, and 0.5 M NHS in MES buffer and briefly vortexed. After 15 h of reaction at room temperature, the scaffolds were extensively washed with distilled water to remove the byproducts.
The human rhVEGF165 (R&D Systems) was loaded on the scaffold by applying a solution of 10 μg of growth factor in 60 μL PBS in three aliquots onto the scaffold. The VEGF-loaded scaffold was allowed to dry in laminar hood for 40 min at room temperature and immersed overnight in 1 mL of PBS at 4°C to remove freely diffusible VEGF.
In vitro bioactivity of scaffolds
The scaffolds were assessed for their ability to phosphorylate the receptor for VEGF, VEGFR2, in the seeded EPCs. Scaffolds loaded with 1 μg of VEGF were washed several times with either PBS or heparin/PBS solution (1 mg/mL) for 1 h followed by several washes with PBS. Confluent EPCs in a T75 flask were incubated overnight in serum-free EBM-2 basal media. The cells were trypsinized and were suspended in serum-free media containing 0.1 mM Na3VO4 (Sigma) to inhibit phosphatase activity while centrifuging at 1200 rpm for 5 min. The cell pellet was re-suspended in serum-free media, and ∼1 million cell/50 μL were seeded in the scaffolds for 5 min at 37°C. The negative and positive controls were EPCs incubated without VEGF and with 100 ng of soluble VEGF, respectively. The reaction was stopped by adding 1 mL of cold solution of 0.2 mM Na3VO4 in PBS to each group. The cells were collected by centrifuging at 1200 rpm for 5 min, and the obtained cell pellet was lysed with RIPA buffer supplemented with Halt protease and phosphatase inhibitor (Thermo Scientific) on ice for 10 min. The lysate was centrifuged at 14,000 g for 15 min, and the supernatant was collected. The lysate proteins were separated by SDS-PAGE and transferred to nitrocellulose membrane for detection of phosphorylated VEGFR2 and VEGFR2 using anti-phospho-VEGFR2 (Tyr1175) and anti-VEGFR2 (Cell Signaling).
Scaffold implantation
The scaffolds were seeded with a specified number of EPCs suspended in 50 μL media and incubated for 2 h at 37°C in a humidified incubator before implantation. For co-seeding experiments, the heparin-modified PCL scaffolds loaded with 10 μg of VEGF were used. There were 500,000 hepatocytes seeded alone or along with an equal number of EPCs in 50 μL of media. 100,000 bone marrow stromal cells were seeded alone or along with 500,000 EPCs.
Mice were anesthetized with isofluorane, and a 1-cm-long dorsal midline incision was made to create a subcutaneous pocket. The scaffold was inserted in the pocket, and the skin was sutured with 3–0 Vicryl (Ethicon). One scaffold was implanted in each mouse. The animals were sacrificed at 7 days postimplantation. The scaffolds were harvested, fixed in 10% formalin overnight, and processed for histology.
Immunohistochemistry and analysis
The in vitro cultured EPCs were stained with live/dead stain (Invitrogen). The cells, after being fixed with 10% formalin for 10 min, were stained for hematoxylin and eosin or with mouse anti-von Willebrand factor (vWF) antibody (DAKO), followed by a fluorescein isothiocyanate-conjugated goat anti-mouse secondary antibody (Jackson ImmunoResearch Laboratories).
The paraffin-embedded scaffolds were cut into 6-μm sections and were stained with the mouse anti-human CD31 antibody (DAKO) and biotinylated horse anti-mouse secondary antibody (antibody detail; Vector Laboratories). The staining was visualized with a diaminobenzidine substrate (Vector Laboratories) at 200× magnification. The blood vessel density and the area occupied by vessels within the scaffolds were measured using ImageScope software (Aperio Technologies). The DNA fragmentation in apoptotic cells was visualized with Terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling (TUNEL) assay using the ApopTag fluorescein detection kit (Chemicon).
For multiple immunostaining, the agarose-embedded scaffolds were cut with a Vibratome (Ted Pella) into 200-μm sections17 and stained with anti-mouse CD31 antibody (BD Biosciences), anti-human CD31 antibody, and anti-GFP antibody (Abcam), combined with fluorescent secondary antibodies (Jackson ImmunoResearch Laboratories). The samples were visualized on a Zeiss Axioskop microscope (Thornwood).
Statistics
The Student's t-test was used to evaluate the difference between the groups. The data were considered statistically significant when p<0.05.
Results
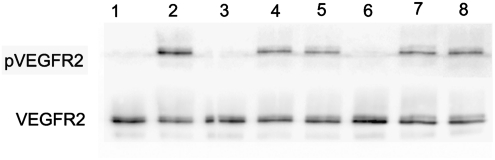
To enhance angiogenesis, VEGF was preloaded on PCL scaffolds with or without heparin immobilization. The bioactivity of the VEGF in scaffolds was confirmed by VEGFR2 phosphorylation in the seeded EPCs. The VEGF-loaded scaffolds, after washing away the unbound growth factor with PBS, were able to phosphorylate VEGFR2 in EPCs seeded onto the scaffold (Fig. 1). Moreover, after eluting the adsorbed VEGF on scaffolds with soluble heparin, the VEGF remaining on scaffolds still exhibited the ability to phosphorylate VEGFR2.
FIG. 1.
VEGFR2 phosphorylation in EPCs. The trypsinized EPCs were seeded in the scaffold for 5 min and assessed for the VEGFR2 phosphorylation. Lane 1: trypsinized EPCs as negative control; lane 2: trypsinized EPCs exposed to soluble VEGF in PBS as positive control; lane 3: cells seeded in PCL scaffold; lane 4: cells seeded in PCL scaffold loaded with VEGF and prewashed with PBS; lane 5: cells seeded in PCL scaffold loaded with VEGF and prewashed with soluble heparin and PBS; lane 6: cells seeded in heparin-PCL scaffold; lane 7: cells seeded in heparin-PCL scaffold loaded with VEGF and prewashed with PBS; lane 8: cells seeded in heparin-PCL scaffold loaded with VEGF and prewashed with soluble heparin and PBS. PCL, polycaprolactone; EPC, endothelial progenitor cell; VEGF, vascular endothelial growth factor; PBS, phosphate-buffered saline.
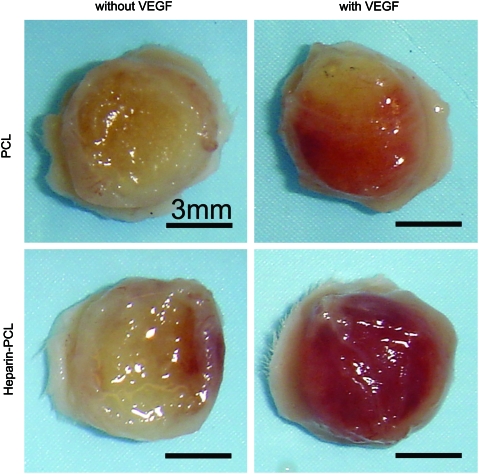
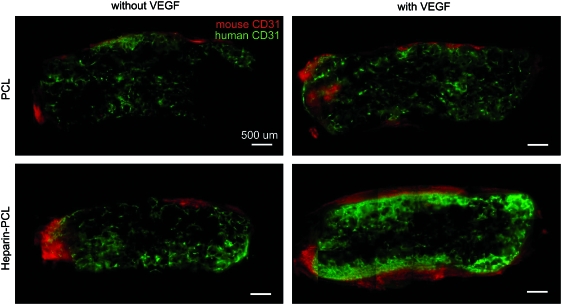
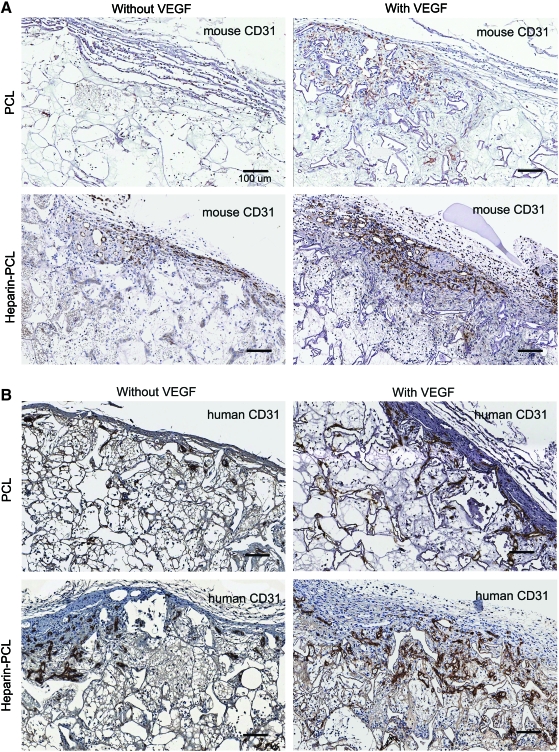
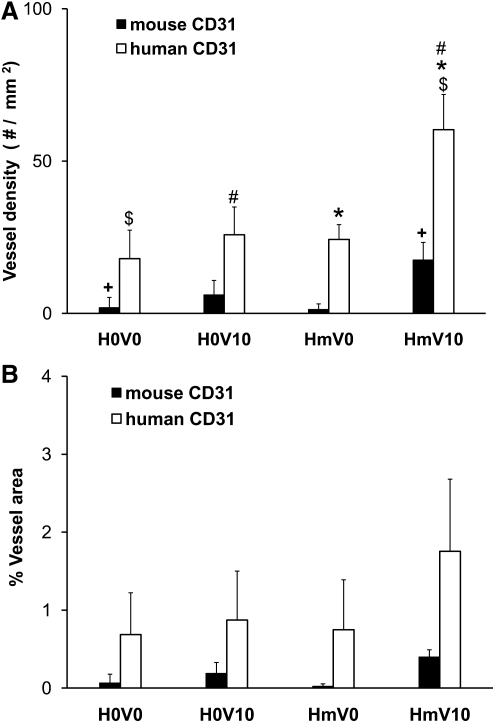
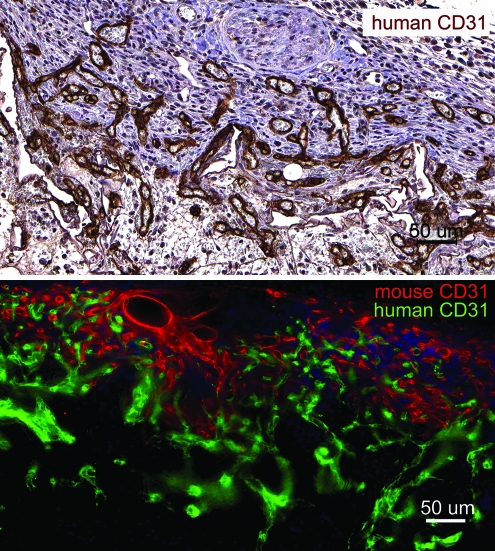
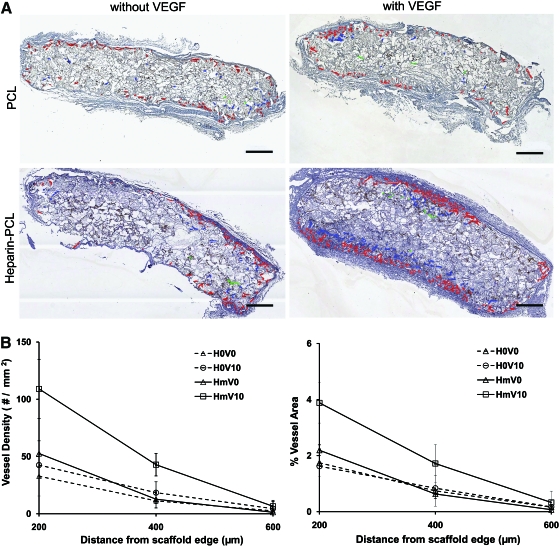
EPCs were seeded in PCL scaffolds with or without VEGF and heparin. Scaffolds seeded with 1 million EPCs were subcutaneously implanted in NOD-SCID mice and were retrieved after 7 days. Macroscopically, the heparin-PCL scaffold loaded with 10 μg of VEGF exhibited intense redness grossly, indicating the presence of red blood cells within the scaffold (Fig. 2). The increase in angiogenesis and vasculogenesis in heparin-PCL scaffolds with VEGF was confirmed by double-immunostaining with anti-mouse CD31 and anti-human CD31 antibodies (Fig. 3). Moreover, the EPCs formed denser vessels in the regions of increased angiogenesis. The extent of vascularization was quantified by measuring the total vessel density and the percentage area occupied by blood vessels (Fig. 4). The heparin-PCL scaffolds with VEGF possessed significantly higher density of EPC-derived vessels than other scaffolds. There was a threefold increase in vessel density in heparin-PCL scaffolds with VEGF as compared with PCL scaffolds without heparin and VEGF, and a >twofold increase in vessel density was observed compared with PCL scaffolds with either VEGF or heparin (Fig. 5). The heparin-PCL scaffolds with VEGF exhibited improved angiogenesis and vasculogenesis. The anastomosis between the host vessels and EPC-derived vessels was evident by the presence of red blood cells in human CD31-positive vessels and the close proximity of mouse and human cells-derived vessels (Fig. 6). The spatial distribution of the EPC-derived vessels was quantified as a function of scaffold thickness at intervals of 200 μm from the scaffold-tissue interface (Fig. 7). Most of the vessels formed by EPCs were restricted to the periphery of the scaffold.
FIG. 2.
Macroscopic evaluation of the scaffolds harvested at day 7. The scaffolds were seeded with one million EPCs. The scaffolds with VEGF were loaded with 10 μg of the growth factor; the scaffolds with heparin were cross-linked for 15 h. Scale bar=3 mm. Color images available online at www.liebertonline.com/tea
FIG. 3.
Immuno-histochemical evaluation of the scaffolds harvested at day 7. Angiogenesis and vasculogenesis visualized with anti-mouse and anti-human CD31 antibodies, respectively, in 200-μm thick scaffold sections. One million EPCs were seeded in each scaffold. The scaffolds with VEGF were loaded with 10 μg of the growth factor; the scaffolds with heparin were cross-linked for 15 h. Scale bar=500 μm. Color images available online at www.liebertonline.com/tea
FIG. 4.
Immuno-histochemical evaluation of the scaffolds harvested at day 7. Angiogenesis and vasculogenesis quantified by diaminobenzidine staining of the scaffold sections with anti-mouse CD31 (A) and anti-human CD31 (B) antibodies, respectively. One million EPCs were seeded in each scaffold. The scaffolds with VEGF were loaded with 10 μg of the growth factor. The scaffolds with heparin were cross-linked for 15 h. Scale bar=100 μm. Color images available online at www.liebertonline.com/tea
FIG. 5.
Quantification of vessels in the scaffold harvested on day 7. One million EPCs were seeded in each scaffold. The average vessel density (A) and the percentage of scaffold area occupied by vessels (B) were quantified using anti-mouse CD31 and anti -human CD31 antibodies, respectively. Values represent the mean and standard deviation (n=3). V0 and V10 represent scaffold without VEGF and loaded with 10 μg of VEGF, respectively. H0 and Hm represent scaffolds without and with cross-linked heparin, respectively. $,*p<0.0005, #p<0.005, +p<0.05.
FIG. 6.
Anastomoses between the EPC-derived vessels and murine vessels in the scaffolds harvested at day 7. The heparin cross-linked scaffold loaded with 10 μg of VEGF and seeded with one million EPCs displays human CD31-positive vessels filled with murine erythrocytes. Double immuno-fluorescence staining of 200-μm thick section shows the mouse and human CD31-positive vessel network in close proximity. Scale bar=50 μm. Color images available online at www.liebertonline.com/tea
FIG. 7.
Quantification of angiogenesis as a function of scaffold depth at day 7. One million EPCs were seeded in each scaffold. The human CD31-positive vessels in scaffold sections were outlined with red, blue, and green color for 0–200, 200–400, and 400–600 μm intervals, respectively (A). The vessel density and percentage of area occupied by vessels (B) were evaluated at 200-μm intervals. Values represent the mean and standard deviation (n=3). V0 and V10 represent scaffolds without VEGF and loaded with 10 μg of VEGF, respectively. H0 and Hm represent scaffolds without and with cross-linked heparin, respectively. Scale bar=500 μm. Color images available online at www.liebertonline.com/tea
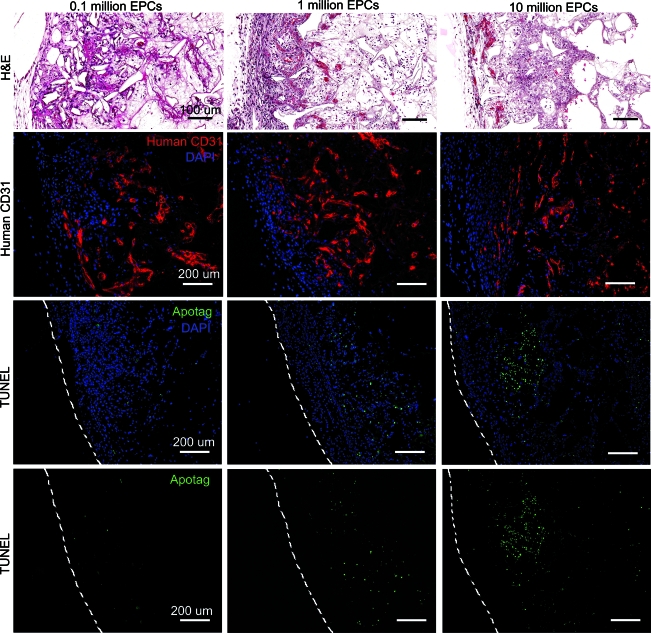
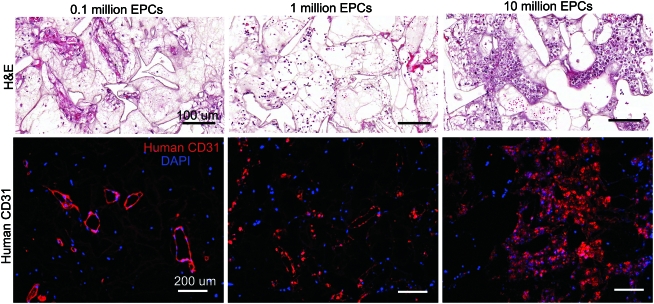
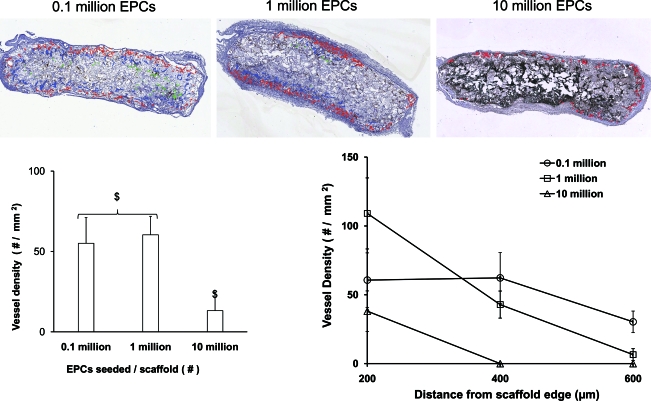
To achieve uniform vessel formation in heparin-PCL scaffolds with VEGF, we assessed the effect of varying the EPCs seeding density. The scaffolds seeded with 105, 106, or 107 EPCs in 50 μL of the media were implanted in NOD-SCID mice. After 7 days in vivo, the scaffolds were assessed for vasculogenesis and apoptosis using anti-human CD31 and TUNEL staining, respectively (Fig. 8). The periphery of scaffolds displayed more apoptotic cells as the EPC seeding density increased. There were less apoptosis and more erythrocyte-filled vessels in scaffolds seeded with 105 EPCs. The core of the scaffold had human-CD31 positive vessels when fewer EPCs were initially seeded (Fig. 9). In contrast, the core of the scaffold seeded with more EPCs lacked vessel lumens and showed cellulardebris with hematoxylin and eosin stain. The dead cells caused nonspecific adsorption of anti-human CD31 antibody. The quantification of human-CD31 positive vessels in the scaffolds demonstrated significantly higher vessel density in scaffolds seeded with 105 and 106 EPCs when compared with 107 EPCs (Fig. 10). There was no significant difference in overall vessel density between seeding with 105 and 106 EPCs; however, a more uniform spatial distribution of vessels was observed in scaffolds seeded with 105 EPCs.
FIG. 8.
Scaffolds seeded with varying number of EPCs harvested at day 7. The heparin cross-linked scaffold loaded with 10 μg VEGF were seeded with 0.1, 1, and 10 million EPCs. H&E-stained scaffold sections depict erythrocyte-filled vessels in the edges of the scaffold. The EPC-derived vessels were visualized by immuno-fluorescence staining for human CD31. The TUNEL staining was used to visualize apoptotic cells in the scaffold. The dashed line represents the edge of the scaffold. Scale bar=100 μm (H&E), 200 μm (CD31, TUNEL). H&E, hematoxylin and eosin. Color images available online at www.liebertonline.com/tea
FIG. 9.
Core of the scaffolds seeded with varying number of EPCs after 7 days in vivo. The heparin cross-linked scaffolds loaded with 10 μg of VEGF were seeded with 0.1, 1, and 10 million EPCs. The blood vessels in the core of the scaffold were visualized with H&E and anti-human CD31 stain. Scale bar=100 μm (H&E), 200 μm (CD31). Color images available online at www.liebertonline.com/tea
FIG. 10.
Quantification of angiogenesis as a function of scaffold depth at day 7. The heparin cross-linked scaffolds loaded with 10 μg VEGF were seeded with 0.1, 1, and 10 million EPCs. Human CD31-positive vessels in scaffold sections were outlined with red, blue, and green color for 0–200, 200–400, and 400–600 μm intervals, respectively. The total vessel density and the vessel density at 200-μm intervals were evaluated. Values represent the mean and standard deviation (n=3). $p<0.0005. Color images available online at www.liebertonline.com/tea
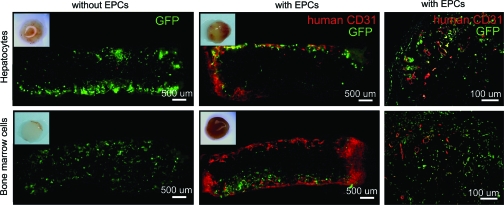
We tested whether the EPCs maintained the vasculogenesis potential in the presence of other seeded cells. The heparin-PCL scaffolds with 10 μg of VEGF and 105 EPCs were co-seeded with GFP-positive primary hepatocytes or bone marrow stromal cells and were implanted in NOD-SCID mice. After 1 week, the retrieved implants were sectioned and immunostained for anti-human CD31 and anti-GFP antibodies. The results indicated that the EPCs continued to form vessels in the presence of other cell types; however, the vessels were confined to the edge of scaffolds (Fig. 11).
FIG. 11.
Scaffolds seeded with hepatocytes or bone marrow cells harvested at day 7. The heparin cross-linked scaffold loaded with 10 μg of VEGF, either without or with EPCs. The EPC-derived vessels and transplanted cells were visualized with anti-human CD31 and anti-GFP antibodies, respectively. Color images available online at www.liebertonline.com/tea
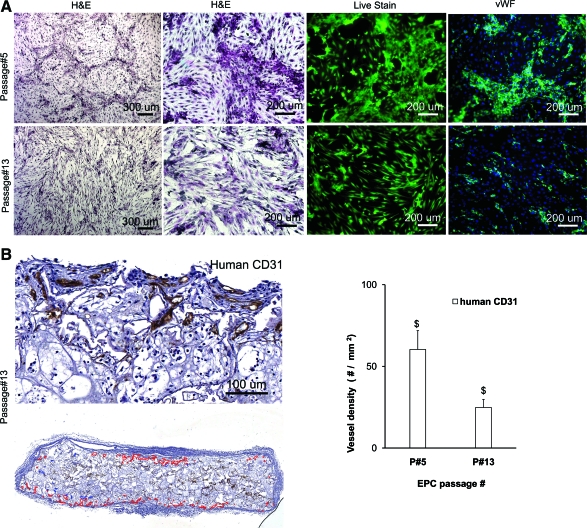
To examine the effect of the in vitro expansion of EPCs on their in vivo vaculogenic potential, the EPCs were maintained in culture for 90 days. The EPCs at passage 13 looked morphologically distinct from the cells at passage 5 (Fig. 12). The EPCs at a lower passage number had several large clusters of cobble-stone shaped cells that stained intensely with anti-vWF antibody. In contrast, EPCs at a higher passage number had few anti-vWF positive cells inter-dispersed among predominantly spindle-shaped cells. When scaffolds seeded with high passage number EPCs were implanted in vivo for 1 week, there was a significant decline in anti-human CD31 positive vessels as compared with those seeded with EPCs at an earlier passage number.
FIG. 12.
Effect of in vitro expansion of EPCs. The cultured EPCs at passage 5 and passage 13 were stained with H&E, live-dead stain, and anti-von Willebrand factor antibody (A). The heparin cross-linked scaffolds loaded with 10 μg of VEGF were seeded with one million EPCs at passage 13 and harvested after 7 days. The vessels in the scaffold were stained with anti-human CD31 antibody to quantify the total vessel density in the scaffold (B). Values represent the mean and standard deviation (n=3). $p<0.0005. Color images available online at www.liebertonline.com/tea
Discussion
In our previous study, PCL scaffolds with surface cross-linked heparin were shown to have an increase in VEGF-mediated angiogenesis after 14 days of implantation.18 However, even under the most optimal conditions, there was insufficient angiogenesis in 2-mm thick scaffolds at an earlier time point of 7 days. In this study, we employed human umbilical cord blood-derived EPCs to induce blood vessel formation at day 7 in vivo. Since VEGF enhances the EPCs proliferation in vitro11,14 and vasculogenesis in vivo,19 we hypothesized that delivery of VEGF from the heparin-immobilized scaffold will improve angiogenesis and vasculogenesis, simultaneously. This was indeed the case, as heparin-PCL scaffolds with VEGF and EPCs had the most vascularization compared with other scaffolds with EPCs.
EPCs have a much higher vasculogenic potential than mature ECs, but additional strategies are required to enhance the efficacy of EPC-based therapies. Majority of the studies in literature have focused on improving vasculogenic potential of EPCs by co-delivery of supporting cells that can functions as pericytes, such as smooth muscle cells and fibroblasts.3,9,14,15,20 Although the presence of supporting cells improves the vessel formation by ECs, they also increase the metabolic burden during the vessel remodeling stages in the scaffold. Recently, investigators have started exploring the role of matrix microenviroment in modulating the vessel formation by ECs. Cooper and Sefton reported that the fibronectin coating increases the vessels formation by HUVECs in collagen scaffolds in vivo.21 Besides, Critser et al. reported a decline in the in vivo vasculogenesis of EPCs with an increase in collagen fiber density and scaffold stiffness.13
An important finding in our study was the significant increase in the formation of vessels by EPCs in heparin-immobilized scaffold with VEGF. Previously, we reported that the release rate of VEGF from the heparin cross-linked scaffold was 100 ng/day.18 The significant increase in vasculogenesis and angiogenesis may be due to the sustained delivery of VEGF by the scaffolds. A similar improvement in vasculogenesis of EPCs was observed in vivo in Matrigel plug with microsphere delivering multiple angiogenic growth factors.22
On assessing the spatial distribution of blood vessels, the EPCs formed denser vessels in the scaffold periphery, in close proximity to the infiltrated murine vessels. The nonuniform distribution of vessels suggests that the nutrients availability was restricted to EPCs seeded near the scaffold edge. We attribute the decline in vessel density at the core of the scaffold to the hypoxic environment. It has been demonstrated that hypoxic EPCs exhibit impaired neo-vascularization capacity in the chronic hind limb ischemia.23 On increasing the EPCs seeding density, the scaffolds displayed an increase in the apoptosis and a significant decline in the EPC-derived vessel density. The observation is consistent with our assumption that higher density of cell would produce a more hypoxic interior. In contrast, there were no apoptotic cells in the scaffolds seeded with fewer EPCs, indicating less hypoxia. As expected, the lower seeding density of EPCs displayed a more uniform distribution of vessels; there were erythrocyte-filled vessel lumens visible at the core of the 2-mm thick scaffold. To test the clinical applicability of EPCs, they were co-seeded with hepatocytes or bone marrow cells. The vessel formation by EPCs was limited to the scaffold edges at day 7. Implantation of scaffolds for longer time points would be required to evaluate the contribution of EPCs in liver and bone regeneration. A strategy to minimize hypoxia in the scaffold core could improve efficacy of EPCs delivery in large-sized scaffolds. The oxygen-generating scaffolds reported by Oh et al. may improve the vessel formation by the EPCs.24 Alternatively, it could be possible to delay the onset of hypoxia in the scaffold by prevascularization with EPCs before implantation. It has been shown that scaffolds prevasculariized with HUVEC formed vessels much earlier than non-prevascularized scaffolds.4
We observed a significant decline in the in vivo vessel forming ability of EPCs at a higher passage after 60 days of in vitro expansion. Our result suggests that the EPCs undergo cell maturation after extended in vitro culture. Others have noticed a decline in the EPCs growth kinetics and proliferative response toward VEGF in vitro at a high passage number.14 There was an eightfold decline in the vessel density in the implanted Matrigel between the EPCs at passage 3 and passage 12. We employed human umbilical cord blood-derived EPCs in our current studies. However, the human adult peripheral blood would be a valuable source for autologous EPCs in clinical applications. Melero-Martin et al. reported a comparable in vivo vasculogenic potential of the peripheral blood and cord blood-derived EPCs.14
In summary, our results demonstrate that the vessel-forming ability of EPCs was significantly improved by the delivery of VEGF from scaffolds with immobilized heparin. In future, the strategies to reduce hypoxia in scaffold during the EPC vasculogenesis should be implemented for more uniform vascularization.
Acknowledgments
This work was funded by the National Institutes of Health R01 DK083319 and the Fubon Foundation.
Disclosure Statement
No competing financial interests exist.
References
- 1.Richardson T.P. Peters M.C. Ennett A.B. Mooney D.J. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;19:1029. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 2.Mao Z. Shi H. Guo R. Ma L. Gao C. Han C. Shen J. Enhanced angiogenesis of porous collagen scaffolds by incorporation of TMC/DNA complexes encoding vascular endothelial growth factor. Acta Biomater. 2009;5:2983. doi: 10.1016/j.actbio.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Levenberg S. Rouwkema J. Macdonald M. Garfein E.S. Kohane D.S. Darland D.C. Marini R. van Blitterswijk C.A. Mulligan R.C. D'Amore P.A. Langer R. Engineering vascularized skeletal muscle tissue. Nat Biotechnol. 2005;23:879. doi: 10.1038/nbt1109. [DOI] [PubMed] [Google Scholar]
- 4.Chen X. Aledia A.S. Ghajar C.M. Griffith C.K. Putnam A.J. Hughes C.C. George S.C. Prevascularization of a fibrin-based tissue construct accelerates the formation of functional anastomosis with host vasculature. Tissue Eng Part A. 2009;15:1363. doi: 10.1089/ten.tea.2008.0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montaño I. Schiestl C. Schneider J. Pontiggia L. Luginbühl J. Biedermann T. Böttcher-Haberzeth S. Braziulis E. Meuli M. Reichmann E. Formation of human capillaries in vitro: the engineering of prevascularized matrices. Tissue Eng Part A. 2010;16:269. doi: 10.1089/ten.TEA.2008.0550. [DOI] [PubMed] [Google Scholar]
- 6.Levenberg S. Golub J.S. Amit M. Itskovitz-Eldor J. Langer R. Endothelial cells derived from human embryonic stem cells. Proc Natl Acad Sci USA. 2002;99:4391. doi: 10.1073/pnas.032074999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valarmathi M.T. Davis J.M. Yost M.J. Goodwin R.L. Potts J.D. A three-dimensional model of vasculogenesis. Biomaterials. 2009;30:1098. doi: 10.1016/j.biomaterials.2008.10.044. [DOI] [PubMed] [Google Scholar]
- 8.Mead L.E. Prater D. Yoder M.C. Ingram D.A. Isolation and characterization of endothelial progenitor cells from human blood. Curr Protoc Stem Cell Biol. 2008;6 doi: 10.1002/9780470151808.sc02c01s6. 2C.1.1. [DOI] [PubMed] [Google Scholar]
- 9.Fedorovich N.E. Haverslag R.T. Dhert W.J. Alblas J. The role of endothelial progenitor cells in prevascularized bone tissue engineering: development of heterogeneous constructs. Tissue Eng Part A. 2010;16:2355. doi: 10.1089/ten.TEA.2009.0603. [DOI] [PubMed] [Google Scholar]
- 10.Yoder M.C. Mead L.E. Prater D. Krier T.R. Mroueh K.N. Li F. Krasich R. Temm C.J. Prchal J.T. Ingram D.A. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ingram D.A. Mead L.E. Tanaka H. Meade V. Fenoglio A. Mortell K. Pollok K. Ferkowicz M.J. Gilley D. Yoder M.C. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104:2752. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- 12.Decaris M.L. Lee C.I. Yoder M.C. Tarantal A.F. Leach J.K. Influence of the oxygen microenvironment on the proangiogenic potential of human endothelial colony forming cells. Angiogenesis. 2009;12:303. doi: 10.1007/s10456-009-9152-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Critser P. Kreger S. Voytik-Harbin S. Yoder M. Collagen matrix physical properties modulate endothelial colony forming cell-derived vessels in vivo. Microvasc Res. 2010;80:23. doi: 10.1016/j.mvr.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Melero-Martin J.M. Khan Z.A. Picard A. Wu X. Paruchuri S. Bischoff J. In vivo vasculogenic potential of human blood-derived endothelial progenitor cells. Blood. 2007;109:4761. doi: 10.1182/blood-2006-12-062471. [DOI] [PubMed] [Google Scholar]
- 15.Melero-Martin J.M. De Obaldia M.E. Kang S.Y. Khan Z.A. Yuan L. Oettgen P. Bischoff J. Engineering robust and functional vascular networks in vivo with human adult and cord blood-derived progenitor cells. Circ Res. 2008;103:194. doi: 10.1161/CIRCRESAHA.108.178590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith M.K. Peters M.C. Richardson T.P. Garbern J.C. Mooney D.J. Locally enhanced angiogenesis promotes transplanted cell survival. Tissue Eng. 2004;10:63. doi: 10.1089/107632704322791709. [DOI] [PubMed] [Google Scholar]
- 17.Lee S. Jilani S.M. Nikolova G.V. Carpizo D. Iruela-Arispe M.L. Processing of VEGF-A by matrix metalloproteinases regulates bioavailability and vascular patterning in tumors. J Cell Biol. 2005;169:681. doi: 10.1083/jcb.200409115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh S. Wu B.M. Dunn J.C. The enhancement of VEGF-mediated angiogenesis by polycaprolactone scaffolds with surface cross-linked heparin. Biomaterials. 2011;32:2059. doi: 10.1016/j.biomaterials.2010.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li B. Sharpe E.E. Maupin A.B. Teleron A.A. Pyle A.L. Carmeliet P. Young P.P. VEGF and PlGF promote adult vasculogenesis by enhancing EPC recruitment and vessel formation at the site of tumor neovascularization. FASEB J. 2006;20:1495. doi: 10.1096/fj.05-5137fje. [DOI] [PubMed] [Google Scholar]
- 20.Wu X. Rabkin-Aikawa E. Guleserian K.J. Perry T.E. Masuda Y. Sutherland F.W. Schoen F.J. Mayer J.E., Jr. Bischoff J. Tissue-engineered microvessels on three-dimensional biodegradable scaffolds using human endothelial progenitor cells. Am J Physiol Heart Circ Physiol. 2004;287:H480. doi: 10.1152/ajpheart.01232.2003. [DOI] [PubMed] [Google Scholar]
- 21.Cooper T. Sefton M. Fibronectin coating of collagen modules increases in vivo HUVEC survival and vessel formation in SCID mice. Acta Biomater. 2011;7:1072. doi: 10.1016/j.actbio.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saif J. Schwarz T.M. Chau D.Y. Henstock J. Sami P. Leicht S.F. Hermann P.C. Alcala S. Mulero F. Shakesheff K.M. Heeschen C. Aicher A. Combination of injectable multiple growth factor-releasing scaffolds and cell therapy as an advanced modality to enhance tissue neovascularization. Arterioscler Thromb Vasc Biol. 2010;30:1897. doi: 10.1161/ATVBAHA.110.207928. [DOI] [PubMed] [Google Scholar]
- 23.Marsboom G. Pokreisz P. Gheysens O. Vermeersch P. Gillijns H. Pellens M. Liu X. Collen D. Janssens S. Sustained endothelial progenitor cell dysfunction after chronic hypoxia-induced pulmonary hypertension. Stem Cells. 2008;26:1017. doi: 10.1634/stemcells.2007-0562. [DOI] [PubMed] [Google Scholar]
- 24.Oh S.H. Ward C.L. Atala A. Yoo J.J. Harrison B.S. Oxygen generating scaffolds for enhancing engineered tissue survival. Biomaterials. 2009;30:757. doi: 10.1016/j.biomaterials.2008.09.065. [DOI] [PubMed] [Google Scholar]