Abstract
Hypoxic events often occur in waters contaminated with toxic chemicals, including agonists of the aryl hydrocarbon receptor (AhR). HIF-1α, the mediator of cellular responses to hypoxia, shares a dimerization partner (ARNT) with AhR and reciprocal crosstalk may occur. Studies addressing AhR/hypoxia crosstalk in mammalian cells have produced contradictory results regarding whether reciprocal crosstalk actually occurs between these pathways and the role ARNT plays in this interaction. We assessed hypoxia-AhR crosstalk in fish cells (PLHC-1) treated with hypoxia (1% O2) or normoxia (21% O2) and AhR agonists (benzo[a]pyrene (BaP), 3,3′,4,4′,5-pentachlorobiphenyl (PCB-126), and benzo[k]fluoranthene (BkF)) with and without overexpression of ARNT. Hypoxia limited induction of a transiently transfected AhR reporter by all three of the AhR agonists; overexpression of ARNT eliminated this effect. PCB-126 had no effect on induction of a transiently transfected hypoxia reporter. BkF caused a minor increase in basal and induced hypoxia reporter activity. BaP decreased basal and induced hypoxia reporter activity; overexpression of ARNT did not alter this effect indicating that this interference with hypoxia pathway activity occurs through an alternate mechanism. Reduced hypoxia pathway activity with BaP treatment may be the result of a metabolite. This study supports the hypothesis that HIF-1α is able to sequester ARNT from AhR and limit the activity of the AhR pathway, but suggests that the converse is not true.
Keywords: Aryl hydrocarbon receptor, ARNT, hypoxia inducible factor 1α, hypoxia, polycyclic aromatic hydrocarbons, multiple stressors, crosstalk
Introduction
Environmental hypoxia is a common occurrence in coastal waters and estuaries. Hypoxic zones occur naturally in stratified waters such as areas with strong temperature and/or salinity gradients or in coastal upwelling zones; however anthropogenic influences can increase the frequency, severity, and extent of hypoxic events (Schiedek et al. 2007; Diaz et al. 2008). The geographical distribution of hypoxic events follows the distribution of areas more heavily affected by human activities and regions such as the Chesapeake Bay, the Gulf of Mexico, and the Black Sea undergo severe hypoxic or anoxic events during the summer months (Diaz et al. 2008). Hypoxia may also be more periodic, coinciding with low tide events, rainfall events, or changes in wind and tide patterns. The number and frequency of hypoxic events in coastal ecosystems have been increasing since the 1960s and most models of climate change predict that hypoxia will become more severe in the future (Schiedek et al. 2007; Diaz et al. 2008).
Because hypoxic events are more common in areas with higher human impact, they are likely to occur in waters that are also impacted by pollution from urban runoff. Polycyclic aromatic hydrocarbons (PAHs) are a major component of petroleum and byproducts of combustion, the most common sources of which are vehicular, wood/coal burning and industrial processes (Ravindra et al. 2008). While levels of PAHs have been decreasing in Europe in recent decades, sediment concentrations of PAHs have been increasing in recent years in the United States and are highest in areas with high vehicular traffic and paved roads associated with urban sprawl (Van Metre et al. 2000; Lima et al. 2003; Gigliotti et al. 2005; Mahler et al. 2005; Van Metre et al. 2005; Ravindra et al. 2008). In order to maintain the health of the ecosystems in urban coastal waters, it is essential to understand how hypoxia affects the fate and toxicity of the pollutants—such as PAHs—found in these hypoxia-prone waters.
The molecular responses to cellular hypoxia are primarily mediated through hypoxia-inducible factor 1α (HIF-1α) (Wenger 2002; Bracken et al. 2003). HIF-1α mRNA is constitutively expressed, but the protein is targeted for ubiquitin-mediated degradation under normoxic conditions, leading to very low levels of HIF-1α protein in the normoxic cell. When the cellular environment becomes hypoxic, the hydroxylation event that targets HIF-1α for ubiquitination is inhibited and HIF-1α accumulates in the cell. It then translocates to the nucleus and dimerizes with HIF-1β. This dimer recognizes hypoxia response elements (HREs) in the promoters of hypoxia responsive genes and initiates the cellular response to hypoxia. HIF-1β is also known as the aryl hydrocarbon receptor nuclear translocator (ARNT) due to its capacity to dimerize with the aryl hydrocarbon receptor (AhR) (Wenger 2002). AhR is a cellular receptor that recognizes a variety of xenobiotics including some chlorinated dibenzo dioxins and furans, polychlorinated biphenyls (PCBs) and PAHs. On binding of these ligands, AhR translocates to the nucleus and dimerizes with ARNT. This dimer recognizes xenobiotic response elements (XREs) on AhR responsive genes and upregulates a number of genes involved in xenobiotic metabolism and AhR regulation (Poellinger 2000). Because AhR and HIF-1α share a common dimerization partner, it is possible that if they are activated simultaneously they may compete for the shared pool of ARNT and interfere with one another’s action.
This hypothesis has been addressed in a number of mammalian cell culture systems. Most report that hypoxia or hypoxia mimics are capable of reducing AhR activity measured as XRE reporter activity, mRNA induction of AhR-inducible gene, CYP1A, or its activity, 7-ethoxyresorufin-O-deethlyase (EROD) activity (Gradin et al. 1996; Gassmann et al. 1997; Chan et al. 1999; Park 1999; Pollenz et al. 1999; Kim et al. 2000; Nie et al. 2001; Allen et al. 2005; Khan et al. 2007; Zhang et al. 2007) while fewer report that AhR agonists (most commonly TCDD) limit hypoxia pathway activity measured as HRE reporter activity or mRNA levels of hypoxia-inducible genes (Chan et al. 1999; Park 1999; Nie et al. 2001).
Because hypoxia is such a prevalent environmental stressor, it is important to understand how AhR agonists (such as some environmentally relevant PAHs) may interact with hypoxia in aquatic organisms. There have been far fewer studies reported on the interactions between AhR agonists and hypoxia in fish and of those only a few address the occurrence of reciprocal crosstalk. AhR agonist, PCB-77 has been shown to prevent induction of glycolytic enzyme activity by hypoxia in killifish (Fundulus heteroclitus), however it is unknown whether this effect resulted from interference with HIF-1α activity or if it was a direct effect on the enzymes (Kraemer et al. 2004). There was a decrease in the activities of glycolytic enzymes in fish dosed with PCB-77 alone as well, indicating that while this may be a result of crosstalk, it could also be a direct effect of PCB-77 on glycolytic metabolism. Hypoxia has been reported to decrease induction of CYP1A mRNA and activity in zebrafish (Danio rerio) (Prasch et al. 2004; Matson et al. 2008); however in orange spotted grouper (Epinephelus coioides) liver explants, no effect of hypoxia on induction of CYP1A mRNA was observed (Yu et al. 2008). There was no effect seen of AhR agonists TCDD and pyrene on expression of the hypoxia-inducible genes heme oxygenase and VEGF respectively (Prasch et al. 2004; Hendon et al. 2008); however another AhR agonist, benzo[a]pyrene (BaP) increased induction of hypoxia-inducible genes VEGF, LDH-A, EPO, and IGFBP (Yu et al. 2008). Thus, the pattern of interaction between AhR agonists and hypoxia in fish remains unclear and the role of ARNT in such interactions has not been directly addressed. This study utilizes a topminnow hepatocarcinoma cell line to address interactions between the hypoxia and AhR pathways due to competition for a shared dimerization partner (ARNT). We hypothesized that activation of the AhR pathway would result in decreased induction of the hypoxia pathway under low oxygen conditions and that hypoxia would result in decreased induction of the AhR pathway by AhR agonists. We also hypothesized that this interaction was due to competition for ARNT and would be eliminated when ARNT expression was artificially increased.
Materials and Methods
Vectors
The pAHRDtkLuc3 vector (subsequently referred to as AhR reporter) was a gift from Alvaro Puga (University of Cincinnati) and consists of the mouse Cyp1a1 AhRD enhancer, which contains three XREs, inserted into a modified pGL2 vector containing the herpes simplex virus type 1 thymidine kinase minimum promoter driving the firefly (Photinus pyralis) luciferase gene (Chang et al. 1998). The N449pGL3 vector (subsequently referred to as HIF reporter) was a gift from Bernard Rees (University of New Orleans) and Trish Schulte (University of British Columbia) and consists of the Fundulus heteroclitus ldh-b promoter driving a pGL3 luciferase reporter vector (Rees et al. 2009). The pcDNA-FhARNT2 vector (subsequently referred to as ARNT expression vector) was a gift from Mark Hahn (Woods Hole Oceanographic Institution) and consists of the pcDNA3.1 vector with the Fundulus heteroclitus ARNT2 gene driven by the CMV promoter (Karchner et al. 2002). The Renilla control vector is available from Promega (Madison, WI, USA) as pRL-CMV and is the sea pansy (Renilla reniformis) luciferase gene driven by the CMV enhancer and early promoter elements and provides constitutive expression of sea pansy luciferase as an internal control for transfection efficiency in dual luciferase assays. The empty pcDNA3.1 vector is available from Invitrogen (Carlsbad, CA, USA).
Cell culture and transfection
The PLHC-1 cell line (ATCC #CRL-2406), a topminnow (Poeciliopsis lucida) hepatocellular carcinoma line, was cultured in phenol-free L-15 media (Gibco, Invitrogen, Carlsbad, CA, USA) with 10% FBS and 50 mg/L gentamicin (hereafter referred to as L15-complete) in sealed plug-cap T-75 culture flasks at 28 °C and split 1 to 10 every 3-4 days as needed to maintain the cells below 90% confluency. For each experiment, cells from 90% confluent flasks were trypsinized and plated in 24-well tissue culture plates at a concentration of 4*105 cells/mL and 500 μL total volume/well and allowed to grow overnight. Cells were then transfected using Lipofectamine 2000 (Invitrogen) following manufacturer’s protocol. Transfection mastermixes for each combination of vectors were prepared by adding the following components in order: 100 μL L15 (no serum or antibiotics added); 0.5 μg luciferase reporter vector (HIF reporter or AhR reporter); 0.025 μg Renilla control vector; 0, 0.25, 0.5 or 1.0 μg ARNT expression vector or empty pcDNA3.1; and 2 μL Lipofectamine 2000 for each well to be transfected. Cells were transfected in antibiotic-free media at 28 °C for 6 h then returned to L15-complete.
Cell dosing
Transfected cells were dosed 24 hours after the end of the transfection with benzo[a]pyrene (BaP), benzo[k]fluoranthene (BkF) or 3,3′4,4′,5-pentachlorobiphenyl (PCB-126) in L15-complete. Stock solutions of BaP, BkF and PCB-126 were in DMSO and appropriate solvent controls were used. Final concentrations of DMSO in the media were below 0.05%. For each experiment, four replicate wells were dosed for each treatment and at least three replicate experiments were run for each combination of vector and chemical insult. Immediately following dosing, plates to be treated with hypoxia were placed in a Heraeus Heracell 150 Tri-Gas Cell Culture Incubator (Thermo Scientific, Waltham, MA, USA) set to 1% oxygen and 28°C. Normoxic plates were maintained at 28°C in an incubator with unaltered atmospheric composition (~21% oxygen). While oxygen levels were not measured in experimental plates due to the need for rapid cell lysis prior to reoxygenation, dissolved oxygen was measured in cell culture media exposed to 1% oxygen for 24 h. Dissolved oxygen was measured with an OM-4 oxygen meter (Microelectrodes, Inc., Bedford, NH, USA) calibrated with saturation equal to 21% (the same setting as the Heracell incubator). Oxygen levels at the end of 24 h were 1-1.4% (which is within the error of the OM-4 oxygen meter). Removal of the media to normal oxygen resulted in rapid equilibration with the air and oxygen levels were back in the range of 1-1.4% after 1 h.
Dual-luciferase assay
Immediately following the end of the 24 h dosing period, media was removed from the plates and they were rinsed with phosphate buffered saline solution (PBS). They were passively lysed in 100 μL Passive Lysis Buffer (Promega) on a shaker at 150 rpm for 20 min. Lysate was processed immediately or stored at −80 °C. Activity of the firefly luciferase reporter vector and the Renilla luciferase control vector were measured in a DL Ready TD 20/20 Luminometer (Turner Biosystems, Sunnyvale, CA, USA) using the Dual-Luciferase Reporter Assay System (Promega) according to manufacturer protocol.
Data analysis and Statistics
For each sample, a ratio of the firefly luciferase reporter vector activity and the Renilla luciferase control vector was calculated. Results for the HIF reporter were normalized to normoxia DMSO values; results for the AhR reporter were normalized to oxygen-matched DMSO values. Statistical testing was performed in SPSS (SPSS, Inc., Chicago, IL, USA). For each set of experimental conditions (vector(s)/AhR agonist combination) a two-way ANOVA was performed. There were differences in degree of induction between replicate experiments (p<0.05) which may have resulted from slight differences in the composition of the transfection mixes prepared for each replicate experiment. Replicate experiments were treated as a covariate where needed to account for this systematic effect. In all cases where there was a significant effect of replicate experiment, the effects of oxygen level and AhR agonist were the same with only the degree of vector induction impacted.
Results
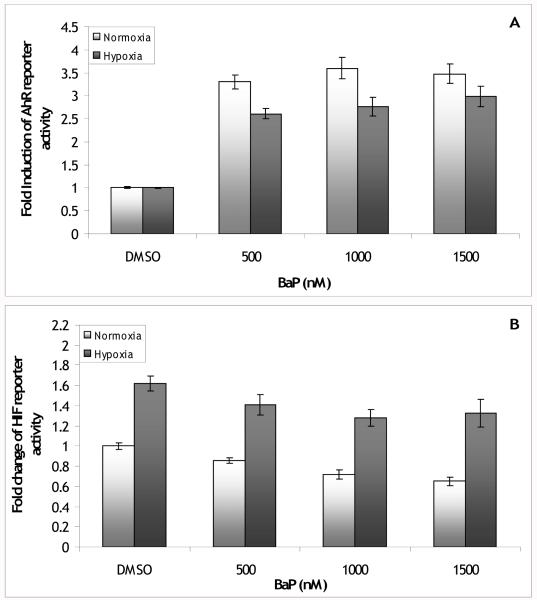
In order to test for crosstalk between the AhR and hypoxia pathways in fish, PLHC-1 cells were transfected with the AhR reporter vector or the HIF reporter vector and dosed with 0, 500, 1000, or 1500 nM BaP and hypoxia or normoxia. The AhR reporter activity was induced 3.3 to 3.6-fold over DMSO by these doses of BaP (Figure 1A). Hypoxia slightly increased basal AhR reporter activity 1.26 ± 0.06 fold over normoxia DMSO (data not shown, p<0.001). Under hypoxia, the level of induction of AhR reporter activity by BaP was reduced to 2.6 to 3.0-fold over hypoxia DMSO with a significant interaction between BaP and oxygen level (p<0.02). HIF reporter activity was induced by hypoxia to 1.6-fold over normoxia DMSO (Figure 1B). BaP decreased this induction to 1.3 to 1.4-fold over normoxia DMSO however this was not characteristic of crosstalk because BaP also reduced HIF reporter activity in the normoxic samples to 0.7 to 0.9-fold below normoxia DMSO. While there was a significant main effect of BaP (p<0.001) there was no significant interaction between BaP and oxygen level (p=0.8).
Figure 1.
(A) Fold induction of AhR reporter vector activity in PLHC-1 cells exposed to 500, 1000 or 1500 nM BaP. Hypoxia inhibited induction of the AhR reporter by BaP (hypoxia main effect p<0.001; hypoxia*BaP effect p<0.02). Bars are means normalized to DMSO +/− SE, n (replicate wells) = 20-28. (B) Fold change of the HIF reporter vector activity in PLHC-1 cells exposed to 500, 1000, or 1500 nM BaP. BaP decreased activity of the HIF reporter (BaP main effect p<0.001) but did not interact with hypoxia (hypoxia*BaP interaction p>0.05). Bars are means normalized to normoxia DMSO ± SE, n (replicate wells) = 20-22.
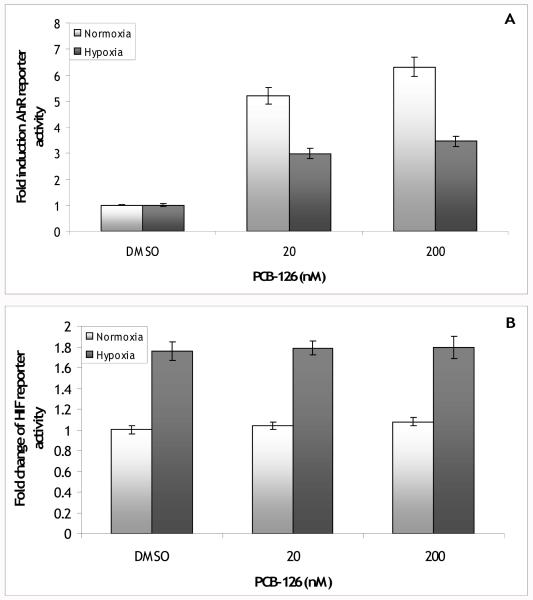
Because most of the published studies on AhR-hypoxia pathway crosstalk have used TCDD as the AhR agonist, this experiment was repeated with a dioxin-like AhR agonist (PCB-126) to determine if the same patterns would be seen with this type of agonist. Similar to BaP, the level of induction of AhR reporter activity by PCB-126 was reduced in hypoxic cells (Figure 2A). PCB-126 induced AhR reporter activity 5.2 to 6.3-fold over DMSO under normoxia and 3.0 to 3.5-fold over DMSO under hypoxia; there was a significant interaction between PCB-126 and oxygen level (p<0.001). Again, there was a slight induction of basal AhR activity under hypoxia (1.39 ± 0.10 fold over normoxia DMSO, data not shown, p<0.001). Contrary to what was seen with BaP, induction of HIF reporter activity by hypoxia was unaffected by PCB-126 (Figure 2B). In these experiments, HIF reporter activity was induced by hypoxia to 1.8-fold over normoxia DMSO samples; the PCB-126/hypoxia samples were also induced 1.8-fold over normoxia DMSO. There was neither a significant main effect of PCB-126 (p=0.7) nor a significant interaction between PCB-126 and oxygen level (p=0.96).
Figure 2.
(A) Fold induction of AhR reporter vector activity in PLHC-1 cells exposed to 20 or 200 nM PCB-126. Hypoxia inhibited induction of the AhR reporter by PCB-126 (hypoxia main effect p<0.001; hypoxia*PCB effect p<0.001). Bars are means normalized to DMSO +/− SE, n (replicate wells) = 12. (B) Fold change of the HIF reporter vector activity in PLHC-1 cells exposed to 20 or 200 nM PCB-126. PCB-126 failed to affect induction of the HIF reporter by hypoxia (PCB main effect p>0.05; hypoxia*PCB effect p>0.05). Bars are means normalized to normoxia DMSO ± SE, n (replicate wells) = 11-12.
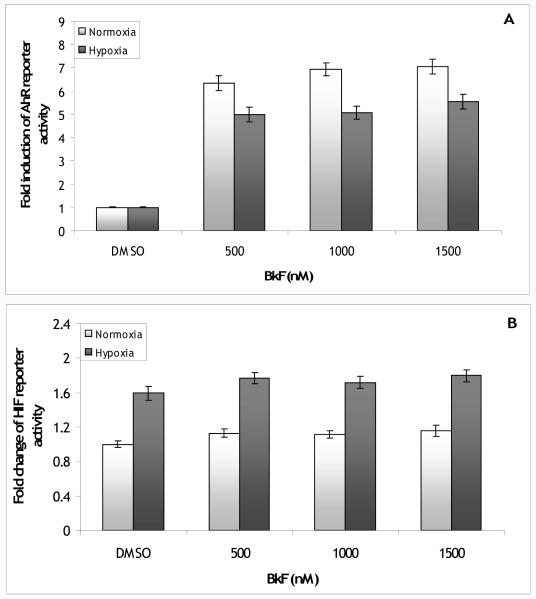
To determine whether the inhibitory effect of BaP on hypoxia pathway activity was a trait of PAH AhR agonists or more specific to BaP, another PAH AhR agonist (BkF) was used. BkF induced AhR reporter activity 6.4 to 7.1-fold over DMSO levels; this induction was decreased under hypoxia to 5.0 to 5.5-fold over DMSO levels (Figure 3A). There was a significant interaction between BkF and oxygen level (p<0.01). Hypoxia slightly increased basal AhR activity to 1.29 ± 0.06 fold over normoxia DMSO (data not shown, p<0.001). In these experiments, hypoxia induced HIF reporter activity 1.6-fold over normoxia DMSO levels (Figure 3B). This induction was slightly increased by BkF to 1.7 to 1.8-fold over normoxia DMSO levels and normoxic HIF reporter activity was increased to 1.1 to 1.2-fold over DMSO. There was a significant main effect of Bkf (p<0.02), but no interaction between BkF and oxygen level (p=0.97).
Figure 3.
(A) Fold induction of AhR reporter vector activity in PLHC-1 cells exposed to 500, 1000 or 1500 nM BkF. Hypoxia inhibited induction of the AhR reporter by BkF (hypoxia main effect p<0.001; hypoxia*BkF effect p<0.01). Bars are means normalized to DMSO +/− SE, n (replicate wells) = 15-16. (B) Fold change of HIF reporter vector activity in PLHC-1 cells exposed to 500, 1000 or 1500 nM BkF. BkF significantly, but slightly increased HIF reporter activity (BkF main effect p<0.02) but failed to interact with hypoxia (hypoxia*BkF effect p>0.05). Bars are means normalized to normoxia DMSO ± SE, n (replicate wells) = 12.
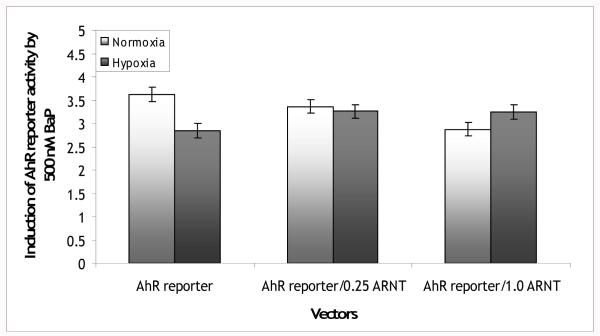
If the effects of BaP on HIF reporter activity and hypoxia on AhR reporter activity were based on competition for ARNT, overexpression of ARNT in these cells should eliminate these effects. Cells were transfected as in the previously described BaP experiments, but with the addition of 0, 0.25, or 1.0 μg of the ARNT expression vector. In these experiments, AhR reporter activity was induced by 500 nM BaP to 3.6-fold over DMSO levels; this induction was reduced to 2.8-fold under hypoxia (Figure 4). With the addition of 0.25 μg of the ARNT expression vector to the transfection mix the effect of hypoxia was eliminated, with a 3.4-fold induction under normoxia and a 3.3-fold induction under hypoxia. The addition of 1.0 μg of the ARNT expression vector reversed the trend with a 2.9-fold induction under normoxia and a 3.2-fold induction under hypoxia. There was a significant global oxygen*BaP*ARNT interaction (p<0.001). In ANOVAs run on the data split by ARNT concentration, there was a significant oxygen*BaP interaction in the cells with no added ARNT (p<0.001). However the oxygen*BaP interaction term in the cells with 0.25 μg of the ARNT expression vector added was not significant (p=0.6) and the interaction term with 1.0 μg of the ARNT expression vector added was marginally non-significant (p=0.07). The effect of overexpression of ARNT was found to be specific to ARNT as co-transfection of empty pcDNA3.1 vector in the place of the ARNT expression vector yielded results similar to those with the AhR reporter vector alone. Induction of the AhR reporter was reduced under hypoxia (data not shown, p<0.001).
Figure 4.
Fold induction of AhR reporter vector activity in PLHC-1 cells exposed to 500 nM BaP and transfected with 0, 0.25, or 1 μg of the ARNT expression vector. The effect of hypoxia on AhR reporter activity was eliminated by ARNT overexpression (global hypoxia*BaP*ARNT interaction p<0.001; oxygen*BaP interaction for AhR-Luc p<0.001; oxygen*BaP interaction for AhR-Luc/0.25ARNT p>0.05; oxygen*BaP interaction for AhR-Luc/1.0ARNT p>0.05). Bars are means normalized to DMSO ± SE, n (replicate wells) = 12-16.
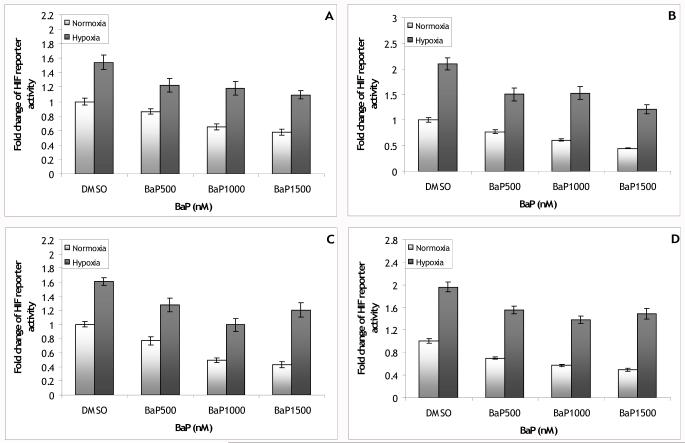
The role of ARNT in the effects of BaP on HIF reporter activity was also assessed. Cells were transfected as in the previously described BaP experiments, but with the addition of 0, 0.25, 0.5, or 1.0 μg of the ARNT expression vector. Results were similar regardless of whether or not ARNT was added, indicating that the effects of BaP on HIF reporter activity are not mediated by ARNT. In these experiments, hypoxia induced HIF reporter activity 1.5-fold over normoxia DMSO and this level of induction was reduced to 1.1 to 1.2-fold over normoxia DMSO by co-exposure to BaP; under normoxia, BaP reduced HIF reporter activity to 0.6 to 0.9-fold less than normoxia DMSO (Figure 5A). The effects of BaP on HIF activity were unchanged when ARNT was overexpressed in these cells. Hypoxic induction of HIF reporter activity in cells with 0.25 μg of the ARNT expression vector added to the transfection mix was 2.1-fold over normoxia DMSO with BaP reducing this induction to 1.2 to 1.5-fold over normoxia DMSO; under normoxia, BaP reduced HIF reporter activity to 0.4 to 0.8-fold below normoxia DMSO (Figure 5B). In cells with 0.5 μg of the ARNT expression vector added, hypoxic induction of HIF reporter activity was 1.6-fold over normoxia DMSO with BaP reducing this induction to 1.0 to 1.3-fold over normoxia DMSO; under normoxia, BaP reduced HIF reporter activity to 0.4 to 0.8-fold below normoxia DMSO (Figure 5C). In cells with 1.0 μg of the ARNT expression vector added, hypoxic induction of HIF reporter activity was 2.0-fold over normoxia DMSO with BaP reducing this induction to 1.4 to 1.6-fold over normoxia DMSO; under normoxia, BaP reduced HIF reporter activity to 0.5 to 0.7-fold over normoxia DMSO (Figure 5D). There was no global ARNT*BaP*oxygen interaction (p=0.4). However, in the global ANOVA, there was a significant main effect of ARNT (p<0.001), indicative of the different levels of induction seen with the addition of different levels of ARNT. It is likely that these differences were caused by slight differences in the transfection mixes prepared for each ARNT concentration; this is supported by the lack of an ARNT dose response in the induction levels. Thus it may be more appropriate to treat each ARNT concentration as a separate experiment. Doing so, we find with no ARNT expression vector added there is a significant main effect of BaP (p<0.001) but no BaP*oxygen interaction (p=0.5). Similarly, with 0.25, 0.5, or 1.0 μg of the ARNT expression vector added, there is a significant main effect of BaP (p<0.001 for each ARNT concentration), but no BaP*oxygen interaction (p=0.1, 0.2, and 0.36 respectively). Thus, overexpression of ARNT failed to alter the effects of BaP on HIF reporter activity.
Figure 5.
Fold change of the HIF reporter vector activity in PLHC-1 cells exposed to 500, 1000, or 1500 nM BaP and co-transfected with (A) 0, (B) 0.25, (C) 0.5 and (D) 1.0 μg of the ARNT expression vector. Over expression of ARNT failed to eliminate the effects of BaP on HIF reporter activity (ARNT*hypoxia*BaP interaction p>0.05; BaP main effect for HIF reporter p<0.001; BaP main effect for HIF reporter/0.025ARNT p<0.001; BaP main effect for HIF reporter/0.5ARNT p<0.001; BaP main effect for HIF reporter/1.0ARNT p<0.001). Bars are means normalized to normoxia DMSO ± SE, n (replicate wells) = 12-14.
Discussion
Because the AhR and hypoxia pathways share a common dimerization partner (ARNT), it is possible that activation of both pathways simultaneously will result in depletion of the shared pool of ARNT and crosstalk between the two pathways. Most reports of potential crosstalk in mammalian cell culture systems indicate that hypoxia or hypoxia mimics are capable of reducing AhR activity measured as XRE reporter activity, CYP1A mRNA induction or EROD activity (Gradin et al. 1996; Gassmann et al. 1997; Chan et al. 1999; Park 1999; Pollenz et al. 1999; Kim et al. 2000; Nie et al. 2001; Allen et al. 2005; Khan et al. 2007; Zhang et al. 2007) while fewer report that AhR agonists (most commonly dioxin) limit hypoxia pathway activity measured as HRE reporter activity or mRNA levels of hypoxia-inducible genes (Chan et al. 1999; Park 1999; Nie et al. 2001). While directional crosstalk appears to occur in mammals, the evidence for true bi-directional crosstalk is less conclusive. One study reported the reverse pattern of crosstalk, with TCDD reducing induction of a hypoxia reporter in MCF-7 breast cancer cells, but hypoxia having no effect on induction of an AhR reporter (Seifert et al. 2008). Additionally, two groups have reported differences in AhR/hypoxia crosstalk in different cell lines (Pollenz et al. 1999; Nie et al. 2001); Nie et al. suggest that the differences they see are due to differences in the AhR:ARNT ratios in the cells (H4IIE rat hepatoma cells and Hepa1 mouse hepatoma cells) indicating that there may be species differences in patterns of AhR/hypoxia crosstalk.
Because many of the waterways contaminated with AhR agonists, such as PAHs, PCBs and dioxins, will experience periods of hypoxia at some point during the year, it is important to understand the interactions between the hypoxia and AhR pathways in aquatic organisms such as fish. While the results of studies performed in mammalian cells are informative, the differences found in the responses of rat and mouse cells suggest that pathway interactions may be species specific and will need to be studied in fish directly. Furthermore, differences exist between the mammalian and fish hypoxia and AhR pathways which may alter interactions between the two pathways. For example, there is only one AhR gene in mammals, while fish have two AhRs (AhR1 and AhR2) as a result of a gene duplication; additionally they may have multiple forms of each AhR1 and AhR2 as a result of a genome duplication (Hahn 2002; Hahn et al. 2006). It is thought that the functions of AhR in mammals are split between the two AhRs in fish as AhR2 (the form that is most active in inducing CYP1A) lacks some sequence motifs present in human AhR and has a different tissue expression profile (Hahn 2002; Hahn et al. 2006). Both fish and mammals have HIF-1α and HIF-2α, but like AhR, additional forms of HIF have been found in fish (HIF-3α in zebrafish and HIF-4α in grass carp) (Nikinmaa et al. 2005). Additionally, the range of oxygen concentrations over which HIF-1α is activated is much wider in fish and extends toward normoxic conditions (Soitamo et al. 2001) and some genes, such as erythropoietin (EPO), which are hypoxia-inducible in mammals behave differently in fish (Ton et al. 2003; Nikinmaa et al. 2005). It is possible that differences in the expression, activity and sequences of AhR and HIF-1α in fish and mammals lead to differences in interactions between the two pathways, limiting the value of evidence from mammalian studies in understanding how hypoxia may affect the fate and toxicity of AhR agonists in fish.
In the current study, AhR activity—measured as induction of an XRE driven luciferase reporter—was induced by BaP, PCB-126 and BkF; the induction with each of these compounds was decreased by concurrent exposure to hypoxia (Figures 1A, 2A, 3A). These results are corroborated by evidence of decreased induction of CYP1A mRNA in TCDD/hypoxia treated zebrafish (Prasch et al. 2004) and decreased induction of EROD activity in BaP/hypoxia treated zebrafish (Matson et al. 2008). However another study reported no effect of hypoxia on induction of CYP1A mRNA in juvenile orange spotted grouper liver explants (Yu et al. 2008), perhaps indicating species differences between fish. This difference may also be attributable to differences in the level of hypoxia used in the studies. While the studies by Prasch et al. (2004) and Matson et al. (2008) involved exposure of whole embryos to hypoxia, the study by Yu et al. (2008) directly exposed liver explants to 3% oxygen. It is unknown how that level relates to the tissue hypoxia experienced by the zebrafish embryos; however, this level of oxygen is three times higher than the level used in the current study and may account for the lack of effect seen in their study versus the current one.
While induction of AhR activity by all three AhR agonists used in this study was inhibited by hypoxia, the three did not affect hypoxia pathway activity in the same manner. The HIF reporter vector, which is driven by the Fundulus heteroclitus LDH-B promoter, was induced by 1% oxygen between 1.5 and 2.1-fold over normoxic controls (Figures 1B, 2B, 3B). This level of induction is comparable to the ~2.7-fold induction of LDH-A in hypoxia-exposed zebrafish reported by Ton et al. (2003). PCB-126 had no effect on induction of the HIF reporter vector (Figure 2B). Similarly, Prasch et al. (2004) reported no effect of TCDD on hypoxic induction of heme oxygenase mRNA in developing zebrafish. Induction of HIF reporter activity was reduced by BaP as expected under the crosstalk hypothesis, however it also reduced HIF reporter activity under normoxic conditions (Figure 1B). In contrast, Yu et al. (2008) reported that BaP and hypoxia act synergistically to induce the hypoxia responsive genes vascular endothelial growth factor (VEGF), lactate dehydrogenase-a (LDH-A), EPO and insulin-like growth factor binding protein (IGFBP) in juvenile orange spotted grouper liver explants. It has been reported that a BaP metabolite, BaP-3,6-dione (BPQ), decreased VEGF expression in human A549 cells through increased degradation of HIF-1α protein while another metabolite, BaP-diol-epoxide (BPDE), had no effect (Li et al. 2007). While Li et al. (2007) found no effect of BPDE, another study reported induction of VEGF by BPDE in Cl41 mouse cells in a HIF-1α independent, AP-1 dependent manner (Ding et al. 2006). It is possible that the inhibition of HIF reporter activity by BaP was due to BPQ-mediated HIF-1α degradation. If so, conditions that shift metabolism of BaP toward producing more BPDE and less BPQ might eliminate this effect, and activation of AP-1 by BPDE may confuse results regarding whether HIF-1α activity is affected by AhR activation.
BkF exhibited the opposite effect of that exerted by BaP on HIF reporter activity, with activity increased under both normoxic and hypoxic conditions (Figure 3B). This effect was very minor in scale and may have no biological implications. The reason for this increase in HIF reporter activity is unclear, but may be related to production of reactive oxygen species (ROS) during BkF metabolism. In juvenile orange spotted grouper liver explants, treatment with hypoxia and an ROS inducer, DDC (diethyldithiocarbamate), led to synergistic induction of two hypoxia responsive genes (EPO and IGFBP) that did not occur with DDC alone (Yu et al. 2008). While this may help explain why there is elevated HIF activity in the hypoxia/BkF cotreatments, it is unclear why basal activity is increased by BkF.
It appears that similar to mammals, most of the evidence in fish points to directional crosstalk in which activation of the hypoxia pathway limits activity of the AhR pathway, but not vice versa, though there may be some differences seen with individual AhR agonists. It is possible that this pattern indicates that HIF-1α is able to sequester ARNT from AhR while the converse does not occur. In vitro, HIF-1α protein has been shown to inhibit AhR/ARNT dimer formation (Gradin et al. 1996) and AhR/ARNT DNA binding (Chan et al. 1999). In the same studies, AhR protein had no effect on HIF-1α/ARNT dimer formation (Gradin et al. 1996) but inhibited HIF-1α/ARNT DNA binding (Chan et al. 1999). This supports the idea that HIF-1α may be able to sequester ARNT from AhR, while AhR cannot sequester ARNT from HIF-1α. However the effects on HIF-1α/ARNT DNA binding suggest that other mechanisms of crosstalk may be involved. The hypoxia and AhR pathways also share coactivators (SRC-1 and p300) which are required for transcription (Wenger 2002; Bracken et al. 2003; Fujii-Kuriyama et al. 2005; Hankinson 2005); competition for these factors may also be occurring and would account for decreased activity of the hypoxia pathway in absence of an effect on HIF-1α/ARNT dimer formation. Contrary to Gradin et al. (1996) and Chan et al. (1999), Pollenz et al. (1999) reported no effect of hypoxia on AhR/ARNT dimer formation in nuclear extracts from several cell lines from different species. They also report that the cellular response to hypoxia uses only 12-15% of the total ARNT pool, suggesting that ARNT may not be limiting; a caveat of this is that it remains unknown what portion of the total ARNT pool is unbound and available for dimerization. Further supporting the idea that ARNT may not be involved in interactions between hypoxia and the AhR pathway, overexpression of ARNT in ZR-75 breast cancer cells failed to eliminate the effects of hypoxia on induction of EROD, DRE reporter activity, and CYP1A protein levels (Khan et al. 2007).
The current study is the first report of the role of ARNT in AhR-hypoxia crosstalk in fish. The effect of hypoxia on induction of AhR reporter activity was eliminated by overexpression of ARNT (Figure 4A), supporting the hypothesis that competition for ARNT is responsible for the interference between hypoxia and the AhR pathway. Importantly, the AhR reporter vector consists of the herpes simplex virus type 1 thymidine kinase minimum promoter with three XREs preceding it. Therefore, induction of AhR reporter activity represents action of the AhR at the XREs and not activity of any specific AhR responsive gene. Therefore although these data support the hypothesis that HIF-1α may be able to sequester ARNT from active AhR in fish cells, it does not predict the activity of a particular AhR-responsive gene as other aspects of promoter structure may affect transcription. In a microarray experiment with Hep3B cells exposed to a hypoxia-mimic (CoCl2) and dioxin, complex patterns of crosstalk were reported and analysis of the promoter regions of genes in which crosstalk was observed revealed associations with binding sites of many transcription factors including HIF-1α, AhR, SRF, Sp-1, NF-kB and Ap-2 (Lee et al. 2006). Therefore, while this study supports the hypothesis that HIF-1α may compete with AhR for a shared pool of ARNT and thus interfere with the expression of AhR-regulated genes, other mechanisms of crosstalk may also be occurring and further study is necessary to understand how this will affect the expression of specific AhR-regulated genes and the toxicity of AhR agonists to fish.
Although we saw no evidence of bi-directional crosstalk in which AhR activation would interfere with induction of hypoxia pathway activity, BaP was found to reduce both basal and induced activity of the HIF reporter (Figure 1B). This pattern was not seen with the two other AhR agonists used in this study. Given that this effect was not seen with a stronger AhR agonist, PCB-126, it seems unlikely that it was due to AhR sequestering ARNT from the very small basal levels of HIF-1α protein in the normoxic cell, suggesting some other mechanism is involved. As mentioned previously the unique effects of BaP may be due to effects of one of its metabolites. BPQ has been reported to increase degradation of HIF-1α protein (Li et al. 2007) which would result in the observed pattern of reduced basal and induced hypoxia pathway activity.
Conclusions
These data support the hypothesis that HIF-1α can compete with AhR for a shared ARNT pool in fish cells, limiting the activation of the AhR pathway. We did not, however, see evidence that AhR can sequester ARNT from HIF-1α, so this may be one-sided crosstalk. One caveat of this is that the AhR construct used in this study was an XRE reporter and was not driven by the full promoter of an AhR-responsive gene. Thus while we have shown the possibility of an effect of hypoxia on AhR pathway activity, it is possible that other motifs in the promoters of specific AhR-responsive genes will interfere with this crosstalk yielding gene-specific results. Measurement of expression of hypoxia and AhR inducible genes in whole fish is necessary to determine the potential consequences of this crosstalk.
Acknowledgements
We would like to acknowledge Mark Hahn, Alvaro Puga, Bernard Rees, and Trish Schulte for the generous gifts of the plasmids used in this paper. This work was funded by the NIEHS Superfund Basic Research Program (#P42ES010356), the Integrated Toxicology and Environmental Health Program (T32-ES-007031), and an EPA STAR Fellowship (#FP-91671701).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen JW, Johnson RS, Bhatia SN. Hypoxic inhibition of 3-methylcholanthrene-induced CYP1A1 expression is independent of HIF-1alpha. Toxicol Lett. 2005;155:151–159. doi: 10.1016/j.toxlet.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Bracken CP, Whitelaw ML, Peet DJ. The hypoxia-inducible factors: key transcriptional regulators of hypoxic responses. Cell Mol Life Sci. 2003;60:1376–1393. doi: 10.1007/s00018-003-2370-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan WK, Yao G, Gu YZ, Bradfield CA. Cross-talk between the aryl hydrocarbon receptor and hypoxia inducible factor signaling pathways. Demonstration of competition and compensation. J Biol Chem. 1999;274:12115–12123. doi: 10.1074/jbc.274.17.12115. [DOI] [PubMed] [Google Scholar]
- Chang CY, Puga A. Constitutive activation of the aromatic hydrocarbon receptor. Mol Cell Biol. 1998;18:525–535. doi: 10.1128/mcb.18.1.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz RJ, Rosenberg R. Spreading dead zones and consequences for marine ecosystems. Science. 2008;321:926–929. doi: 10.1126/science.1156401. [DOI] [PubMed] [Google Scholar]
- Ding J, Li J, Chen J, Chen H, Ouyang W, Zhang R, Xue C, Zhang D, Amin S, Desai D, Huang C. Effects of polycyclic aromatic hydrocarbons (PAHs) on vascular endothelial growth factor induction through phosphatidylinositol 3-kinase/AP-1-dependent, HIF-1alpha-independent pathway. J Biol Chem. 2006;281:9093–9100. doi: 10.1074/jbc.M510537200. [DOI] [PubMed] [Google Scholar]
- Fujii-Kuriyama Y, Mimura J. Molecular mechanisms of AhR functions in the regulation of cytochrome P450 genes. Biochem Biophys Res Commun. 2005;338:311–317. doi: 10.1016/j.bbrc.2005.08.162. [DOI] [PubMed] [Google Scholar]
- Gassmann M, Kvietikova I, Rolfs A, Wenger RH. Oxygen- and dioxin-regulated gene expression in mouse hepatoma cells. Kidney Int. 1997;51:567–574. doi: 10.1038/ki.1997.81. [DOI] [PubMed] [Google Scholar]
- Gigliotti CL, Totten LA, Offenberg JH, Dachs J, Reinfelder JR, Nelson ED, Glenn T.R.t., Eisenreich SJ. Atmospheric concentrations and deposition of polycyclic aromatic hydrocarbons to the Mid-Atlantic East Coast region. Environ Sci Technol. 2005;39:5550–5559. doi: 10.1021/es050401k. [DOI] [PubMed] [Google Scholar]
- Gradin K, McGuire J, Wenger RH, Kvietikova I, fhitelaw ML, Toftgard R, Tora L, Gassmann M, Poellinger L. Functional interference between hypoxia and dioxin signal transduction pathways: competition for recruitment of the Arnt transcription factor. Mol Cell Biol. 1996;16:5221–5231. doi: 10.1128/mcb.16.10.5221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn ME. Aryl hydrocarbon receptors: diversity and evolution. Chem Biol Interact. 2002;141:131–160. doi: 10.1016/s0009-2797(02)00070-4. [DOI] [PubMed] [Google Scholar]
- Hahn ME, Karchner SI, Evans BR, Franks DG, Merson RR, Lapseritis JM. Unexpected diversity of aryl hydrocarbon receptors in non-mammalian vertebrates: insights from comparative genomics. J Exp Zoolog A Comp Exp Biol. 2006;305:693–706. doi: 10.1002/jez.a.323. [DOI] [PubMed] [Google Scholar]
- Hankinson O. Role of coactivators in transcriptional activation by the aryl hydrocarbon receptor. Arch Biochem Biophys. 2005;433:379–386. doi: 10.1016/j.abb.2004.09.031. [DOI] [PubMed] [Google Scholar]
- Hendon LA, Carlson EA, Manning S, Brouwer M. Molecular and developmental effects of exposure to pyrene in the early life-stages of Cyprinodon variegatus. Comp Biochem Physiol C Toxicol Pharmacol. 2008;147:205–215. doi: 10.1016/j.cbpc.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Karchner SI, Franks DG, Powell WH, Hahn ME. Regulatory interactions among three members of the vertebrate aryl hydrocarbon receptor family: AHR repressor, AHR1, and AHR2. J Biol Chem. 2002;277:6949–6959. doi: 10.1074/jbc.M110779200. [DOI] [PubMed] [Google Scholar]
- Khan S, Liu S, Stoner M, Safe S. Cobaltous chloride and hypoxia inhibit aryl hydrocarbon receptor-mediated responses in breast cancer cells. Toxicol Appl Pharmacol. 2007;223:28–38. doi: 10.1016/j.taap.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JE, Sheen YY. Inhibition of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-stimulated Cyp1a1 promoter activity by hypoxic agents. Biochem Pharmacol. 2000;59:1549–1556. doi: 10.1016/s0006-2952(00)00283-5. [DOI] [PubMed] [Google Scholar]
- Kraemer LD, Schulte PM. Prior PCB exposure suppresses hypoxia-induced up-regulation of glycolytic enzymes in Fundulus heteroclitus. Comp Biochem Physiol C Toxicol Pharmacol. 2004;139:23–29. doi: 10.1016/j.cca.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Lee KA, Burgoon LD, Lamb L, Dere E, Zacharewski TR, Hogenesch JB, LaPres JJ. Identification and characterization of genes susceptible to transcriptional cross-talk between the hypoxia and dioxin signaling cascades. Chem Res Toxicol. 2006;19:1284–1293. doi: 10.1021/tx060068d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ZD, Liu LZ, Shi X, Fang J, Jiang BH. Benzo[a]pyrene-3,6-dione inhibited VEGF expression through inducing HIF-1alpha degradation. Biochem Biophys Res Commun. 2007;357:517–523. doi: 10.1016/j.bbrc.2007.03.178. [DOI] [PubMed] [Google Scholar]
- Lima AL, Eglinton TI, Reddy CM. High-resolution record of pyrogenic polycyclic aromatic hydrocarbon deposition during the 20th century. Environ Sci Technol. 2003;37:53–61. doi: 10.1021/es025895p. [DOI] [PubMed] [Google Scholar]
- Mahler BJ, Van Metre PC, Bashara TJ, Wilson JT, Johns DA. Parking lot sealcoat: an unrecognized source of urban polycyclic aromatic hydrocarbons. Environ Sci Technol. 2005;39:5560–5566. doi: 10.1021/es0501565. [DOI] [PubMed] [Google Scholar]
- Matson CW, Timme-Laragy AR, Di Giulio RT. Fluoranthene, but not benzo[a]pyrene, interacts with hypoxia resulting in pericardial effusion and lordosis in developing zebrafish. Chemosphere. 2008;74:149–154. doi: 10.1016/j.chemosphere.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie M, Blankenship AL, Giesy JP. Interactions between aryl hydrocarbon receptor (AhR) and hypoxia signaling pathways. Environ Toxicol Pharmacol. 2001;10:17–27. doi: 10.1016/s1382-6689(01)00065-5. [DOI] [PubMed] [Google Scholar]
- Nikinmaa M, Rees BB. Oxygen-dependent gene expression in fishes. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1079–1090. doi: 10.1152/ajpregu.00626.2004. [DOI] [PubMed] [Google Scholar]
- Park H. Aromatic hydrocarbon nuclear translocator as a common component for the hypoxia- and dioxin-induced gene expression. Mol Cells. 1999;9:172–178. [PubMed] [Google Scholar]
- Poellinger L. Mechanistic aspects--the dioxin (aryl hydrocarbon) receptor. Food Addit Contam. 2000;17:261–266. doi: 10.1080/026520300283333. [DOI] [PubMed] [Google Scholar]
- Pollenz RS, Davarinos NA, Shearer TP. Analysis of aryl hydrocarbon receptor-mediated signaling during physiological hypoxia reveals lack of competition for the aryl hydrocarbon nuclear translocator transcription factor. Mol Pharmacol. 1999;56:1127–1137. doi: 10.1124/mol.56.6.1127. [DOI] [PubMed] [Google Scholar]
- Prasch AL, Andreasen EA, Peterson RE, Heideman W. Interactions between 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and hypoxia signaling pathways in zebrafish: hypoxia decreases responses to TCDD in zebrafish embryos. Toxicol Sci. 2004;78:68–77. doi: 10.1093/toxsci/kfh053. [DOI] [PubMed] [Google Scholar]
- Ravindra K, Sokhi R, Van Grieken R. Atmospheric polycyclic aromatic hydrocarbons: Source attribution, emission factors and regulation. Atmospheric Environment. 2008;42:2895–2921. [Google Scholar]
- Rees BB, Figueroa YG, Wiese TE, Beckman BS, Schulte PM. A novel hypoxia-response element in the lactate dehydrogenase-b gene of the killifish Fundulus heteroclitus. Comp Biochem Physiol A. 2009 doi: 10.1016/j.cbpa.2009.05.001. doi:10.1016/j.cbpa.2009.1005.1001. [DOI] [PubMed] [Google Scholar]
- Schiedek D, Sundelin B, Readman JW, Macdonald RW. Interactions between climate change and contaminants. Mar Pollut Bull. 2007;54:1845–1856. doi: 10.1016/j.marpolbul.2007.09.020. [DOI] [PubMed] [Google Scholar]
- Seifert A, Katschinski DM, Tonack S, Fischer B, Navarrete Santos A. Significance of prolyl hydroxylase 2 in the interference of aryl hydrocarbon receptor and hypoxia-inducible factor-1 alpha signaling. Chem Res Toxicol. 2008;21:341–348. doi: 10.1021/tx7001838. [DOI] [PubMed] [Google Scholar]
- Soitamo AJ, Rabergh CM, Gassmann M, Sistonen L, Nikinmaa M. Characterization of a hypoxia-inducible factor (HIF-1alpha ) from rainbow trout. Accumulation of protein occurs at normal venous oxygen tension. J Biol Chem. 2001;276:19699–19705. doi: 10.1074/jbc.M009057200. [DOI] [PubMed] [Google Scholar]
- Ton C, Stamatiou D, Liew CC. Gene expression profile of zebrafish exposed to hypoxia during development. Physiol Genomics. 2003;13:97–106. doi: 10.1152/physiolgenomics.00128.2002. [DOI] [PubMed] [Google Scholar]
- Van Metre PC, Mahler BJ. Trends in hydrophobic organic contaminants in urban and reference lake sediments across the United States, 1970-2001. Environ Sci Technol. 2005;39:5567–5574. doi: 10.1021/es0503175. [DOI] [PubMed] [Google Scholar]
- Van Metre PC, Mahler BJ, Furlong ET. Urban sprawl leaves its PAH signature. Environ Sci Technol. 2000;34:4064–4070. [Google Scholar]
- Wenger RH. Cellular adaptation to hypoxia: O2-sensing protein hydroxylases, hypoxia-inducible transcription factors, and O2-regulated gene expression. FASEB J. 2002;16:1151–1162. doi: 10.1096/fj.01-0944rev. [DOI] [PubMed] [Google Scholar]
- Yu RM, Ng PK, Tan T, Chu DL, Wu RS, Kong RY. Enhancement of hypoxia-induced gene expression in fish liver by the aryl hydrocarbon receptor (AhR) ligand, benzo[a]pyrene (BaP) Aquat Toxicol. 2008;90:235–242. doi: 10.1016/j.aquatox.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Zhang N, Walker MK. Crosstalk between the aryl hydrocarbon receptor and hypoxia on the constitutive expression of cytochrome P4501A1 mRNA. Cardiovasc Toxicol. 2007;7:282–290. doi: 10.1007/s12012-007-9007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]