Abstract
Objective
To determine if longer breastfeeding is associated with higher infant lead concentrations.
Study design
Data were analyzed from 3 studies of developmental effects of iron deficiency in infancy: Costa Rica (1981–1984), Chile (1991–1996), and Detroit (2002–2003). Pearson product-moment or partial correlation coefficients assessed the relation between duration of breastfeeding and lead levels.
Results
Over 93% of Costa Rica and Chile samples was breastfed, n = 179 and 323 breastfed infants, respectively (mean weaning age, 8–10 months), as was 35.6% of the Detroit sample, n = 53 breastfed infants (mean weaning age, 4.5 months). Lead concentrations averaged 10.8 μg/dL (Costa Rica, 12–23 months), 7.8 μg/dL (Chile, 12 months), and 2.5 μg/dL (Detroit, 9–10 months). Duration of breastfeeding as sole milk source and total breastfeeding correlated with lead concentration in all samples (r values = .14 to .57, p values = .06 to <.0001).
Conclusions
Longer breastfeeding was associated with higher infant lead concentration in 3 countries, in 3 different decades, in settings differing in breastfeeding patterns, environmental lead sources, and infant lead levels. The results suggest that monitoring lead concentrations in breastfed infants be considered.
Keywords: human, lactation, feeding, metals
INTRODUCTION
The marked mobilization of maternal bone lead stores during lactation(1;2) raises the issue of lead transfer to the infant via breast milk.(3–6) In 1994 we made the incidental observation that blood lead concentration was higher with longer breastfeeding in a sample of healthy full-term Costa Rican infants.(7) Here we pursued the finding in two other samples and analyzed the Costa Rica data in more detail. These samples in 3 different countries were used to test the hypothesis that infants breastfed longer are at risk for higher lead concentrations.
METHODS
Settings and subjects
Data on lead concentration and breastfeeding were obtained in the course of research on the behavioral and developmental effects of iron deficiency in infancy. The settings of all 3 studies – in Costa Rica, Chile, and the US – were urban or peri-urban. The study in Costa Rica was conducted between 1981 and 1984.(7) Lead-based paints were not in use but gasoline was leaded. As established by the US Centers for Disease Control (CDC), the lead level of concern at that time was 25 μg/dL. The study in Chile was conducted between 1991 and 1996 as Chile made the conversion to unleaded gasoline.(8) Housing was of recent construction and generally unpainted. The study in Detroit was conducted in 2002–2003 in an inner-city population. Leaded paint and gasoline had been legally banned for several decades.(9) The CDC’s cutoff for concern was 10 μg/dL during the time of the Chile and Detroit studies.
Subject enrollment was restricted to full-term healthy infants in the Costa Rica and Chile studies. The Costa Rica sample consisted of 12- to 23-month-old infants enrolled through community-based door-to-door screening (n = 191).(10) The sample in Chile was enrolled in community clinics at well child care visits at 4 to 6 months. Lead concentrations were available for 331 infants.(11) In the US, a sample of African-American infants was enrolled at 9-month health maintenance visits at an inner-city hospital. All such infants were invited to participate over a one-year period; lead concentrations were available for 167 of 198 infants.(9) For Costa Rica and Detroit, lead concentrations were determined on enrollment; for Chile, they were determined when infants were 12 months old.
Signed informed consent was obtained from parents in each study. Study protocols were approved by the Institutional Review Boards (IRB) of Case Western Reserve University for the Costa Rica study and the University of Michigan for the Chile and Detroit studies. IRBs of the Hospital Nacional de Niños, the University of Chile, and Wayne State University also approved protocols for Costa Rica, Chile, and Detroit, respectively. Details of the studies have been previously reported.(9–11)
Procedures
Two variables reflected the duration of breastfeeding: weeks of breastfeeding as the sole source of milk and weeks of total breastfeeding (calculated from the age at complete weaning from the breast). In each sample, project personnel obtained information on infant feeding from the infant’s mother. For the Detroit sample, the duration of breastfeeding as the sole source of milk was also determined from chart review of health care visits; pediatricians recorded feeding data from birth on. Some data from mothers entailed recall, especially in the Costa Rica sample in which infants were enrolled at 12–23 months of age. For Chile, contact with infants started at about 4 months, and feeding information was obtained prospectively thereafter during weekly home visits. Because of the strength of this data collection, that is, weekly and in-person, we will emphasize results from this sample. Data on age at starting juice and solids were not sufficiently complete across studies to be analyzed.
Infant lead concentrations were determined in venous blood, which was anticoagulated with EDTA and stored frozen at −20°C in lead-free vials if not assayed immediately. Laboratory procedures for lead assays in the Costa Rica and Chile samples were under direct CDC quality control specifications. All laboratories maintained strict quality control using internal and external standards. For the Costa Rica sample, lead levels were determined by a graphite furnace method using a matrix modification procedure.(12) For the Chile sample, lead concentration was determined by electrothermal atomization (graphite furnace HGA700) atomic absorption spectrophotometry (Perkin Elmer 1100B, Boston MA).(13) For the Detroit sample, the Detroit Medical Center University Laboratories performed lead assays by atomic absorption using a graphite furnace.(14) Previous analyses showed that measures of iron status did not relate to lead concentrations in these samples.(7–9)
Statistics
Information on feeding and lead levels has been previously published as part of sample descriptions for the iron deficiency studies.(8–10) In these secondary analyses, we focus on normal birth weight breastfed infants in each sample for whom lead concentrations were available. Three breastfed infants in the Detroit sample had birth weight < 2500 g and were excluded from analysis. The associations between lead concentration and the duration of breastfeeding as the sole source of milk and total breastfeeding in weeks was analyzed using SAS 9.1.(15) For infants who were still being breastfed when the lead concentration was obtained, their current age was used as the duration of total breastfeeding. A single unified analysis was not possible, since the samples did not overlap sufficiently in age, duration of breastfeeding at the longer end, or lead levels > 5 μg/dl. Therefore, the association had to be determined within sample.
Pearson product-moment correlations were calculated when background factors were not associated with lead concentration and breastfeeding duration in a given sample. Multiple linear regression was used to calculate partial correlation coefficients when a background factor might be a confounder, that is, even weakly correlated (p < .10) with both these variables. In addition, we considered as a covariate any background variable that was correlated with lead concentration (p < .10). The following background variables were considered: gender, age at testing, birth weight, gestational age, birth order, weight-for-age z-score or WAZ, weight-for-length z-score or WHZ, maternal age, maternal education, and family socioeconomic status (SES). We derived the most parsimonious models that included all background factors independently contributing to the models.
RESULTS
Breastfeeding was near universal in the Latin American samples (93.7% and 97.6% in Costa Rica and Chile samples, respectively), whereas 35.6% of infants in the Detroit sample were breastfed. Table 1 shows the characteristics of breastfed infants in each sample (Costa Rica, n = 179; Chile, n = 323; Detroit, n = 53). Regarding family characteristics, mothers in Costa Rica and Chile averaged 9.5 years of education, and 62–76% were married. Families were working- to middle-class. In the Detroit sample, maternal education averaged 12.8 years. Only 14.7% were married, and 85% of families had Medicaid insurance, indicating considerable economic stress.
Table 1.
Characteristics of breastfed infants*
| N | Costa Rica | Chile | Detroit |
|---|---|---|---|
| 179 | 323 | 53 | |
| Gender, % male (n) | 55.3 (99) | 52.6 (170) | 45.3 (24) |
| Age at testing†, months | 17.4 (3.2) | 12.4 (0.2) | 9.1 (0.5) |
| Birth weight, kg | 3.26 (0.41) | 3.61 (0.38) | 3.55 (0.57) |
| Gestational age, weeks | 39.4 (1.4) | 39.6 (1.1) | 39.5 (1.0) |
| Birth order | 2.5 (1.6) | 2.2 (1.2) | 1.8 (1.0) |
| Breastfeeding as sole milk source, weeks | 17.5 (18.1) | 22.8 (13.0) | 7.3 (11.8) |
| Total breastfeeding, weeks | 32.0 (24.1) | 41.5 (15.1) | 18.7 (13.2) |
| Lead concentration, μg/dL whole blood | 10.8 (2.6) | 7.8 (3.6) | 2.5 (1.5) |
| Weight-for-age z-score (WAZ) | −.24 (1.01) | −0.03 (1.02) | 0.12 (1.13) |
| Weight-for-length z-score (WHZ) | 0.09 (1.03) | 0.48 (1.00) | 0.56 (1.01) |
Values are mean (SD) for continuous variables and % for categorical variables.
For Costa Rica and Detroit, age of enrollment and age at testing for lead, WAZ, and WHZ were the same. For Chile, infants were enrolled at 4–6 months in a clinical trial of preventing iron deficiency anemia; blood lead levels were determined at the conclusion of the trial at 12 months; WAZ and WHZ at that age are shown.
The patterns of breastfeeding differed markedly across samples (Table 1). The mean duration of breastfeeding as the sole source of milk was 4–5 months in the Latin American samples, in contrast to less than 2 months in the Detroit sample. Complete weaning from the breast (duration of total breastfeeding) occurred on average at about 8 –10 months in Costa Rica and Chile samples and 4.5 months in Detroit. In Costa Rica, 9.5% of the infants were still nursing when enrolled at 12 to 23 months;(10) 29.8% of the Chilean infants were still nursing when they completed the study at 12 months.(11) In the Detroit sample, < 8% infants were nursing when enrolled at 9–10 months.
The mean lead concentrations were also distinctly different across studies (Table 1). The mean in the Costa Rica sample was close to 11 μg/dL, and 53% of infants had concentrations of 10 μg/dL or higher. The mean in the Chile sample was close to 8 μg/dL, and 26% were above the cutoff. The highest concentrations in both Latin American samples were 21–22 μg/dL. In contrast, the mean lead concentration in the Detroit sample was < 3 μg/dL; the highest concentration was 8.0 μg/dL, and only 7.8% of values were above 5 μg/dL.
Table 2 shows background factors and their correlations with lead concentration and the duration of breastfeeding as the sole milk source in each sample (correlations with duration of total breastfeeding available on request). For the Costa Rica sample, the most parsimonious model included infant age (potential confounder), birth weight, and SES. For Detroit, birth order was a potential confounder; there were no other covariates. For Chile, no background variable related to either breastfeeding or lead concentration.
Table 2.
Correlations between lead, breastfeeding, and background factors *
| Lead r (p-value) |
Breastfeeding as sole milk source r (p-value) |
||
|---|---|---|---|
| Gender | Costa Rica | .15 (.04) | .09 (.24) |
| Chile | .07 (.18) | −.02 (.78) | |
| Detroit | .05 (.73) | .03 (.82) | |
| Age at testing | Costa Rica | .19 (.01) | .17 (.02) |
| Chile | .01 (.89) | .04 (.48) | |
| Detroit | −.15 (.28) | .08 (.56) | |
| Birth weight | Costa Rica | −.16 (.04) | .04 (.58) |
| Chile | .08 (.14) | .05 (.37) | |
| Detroit | −.02 (.90) | .04 (.75) | |
| Gestational age | Costa Rica | .05 (.52) | −.09 (.23) |
| Chile | .08 (.15) | −.04 (.48) | |
| Detroit | −.01 (.95) | −.003 (.98) | |
| Birth order | Costa Rica | .04 (.55) | .07 (.37) |
| Chile | .04 (.49) | .02 (.74) | |
| Detroit | .23 (.10) | .30 (.03) | |
| WAZ | Costa Rica | −.20 (.007) | −.24 (.001) |
| Chile | −.01 (.93) | −.05 (.40) | |
| Detroit | −.07 (.61) | .11 (.44) | |
| WHZ | Costa Rica | −.17 (.02) | −.22 (.004) |
| Chile | −.01 (.86) | −.004 (.95) | |
| Detroit | −.005 (.97) | .002 (.99) | |
| Maternal age | Costa Rica | −.15 (.05) | .003 (.97) |
| Chile | .05 (.38) | .05 (.37) | |
| Detroit | −.03 (.84) | .53 (<.0001) | |
| Maternal education | Costa Rica | −.20 (.007) | .04 (.56) |
| Chile | .03 (.56) | −.06 (.25) | |
| Detroit | −.06 (.68) | −.08 (.58) | |
| Family SES | Costa Rica | −.23 (.002) | .05 (.51) |
| Chile | .06 (.28) | .02 (.67) | |
| Detroit† | NA | NA |
Values in bold indicate a potential confounder, i.e., correlated with both breastfeeding and lead; those in italics indicate a potential covariate, i.e., correlated with lead. We included these variables in the appropriate multiple regression analyses and retained those that were statistically significant in the most parsimonious models.
Almost all families received Medicaid insurance; data were not collected for a SES scale.
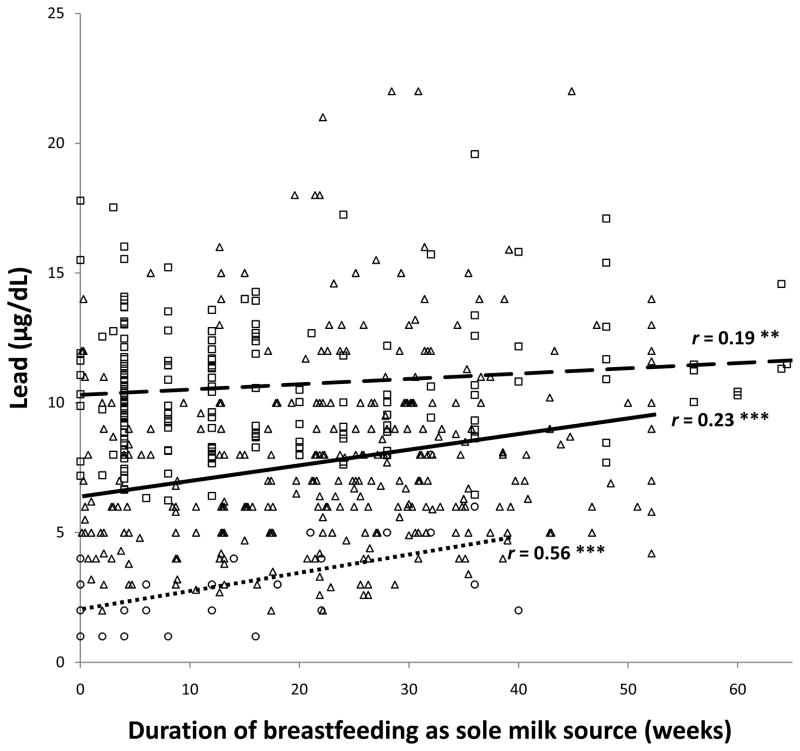
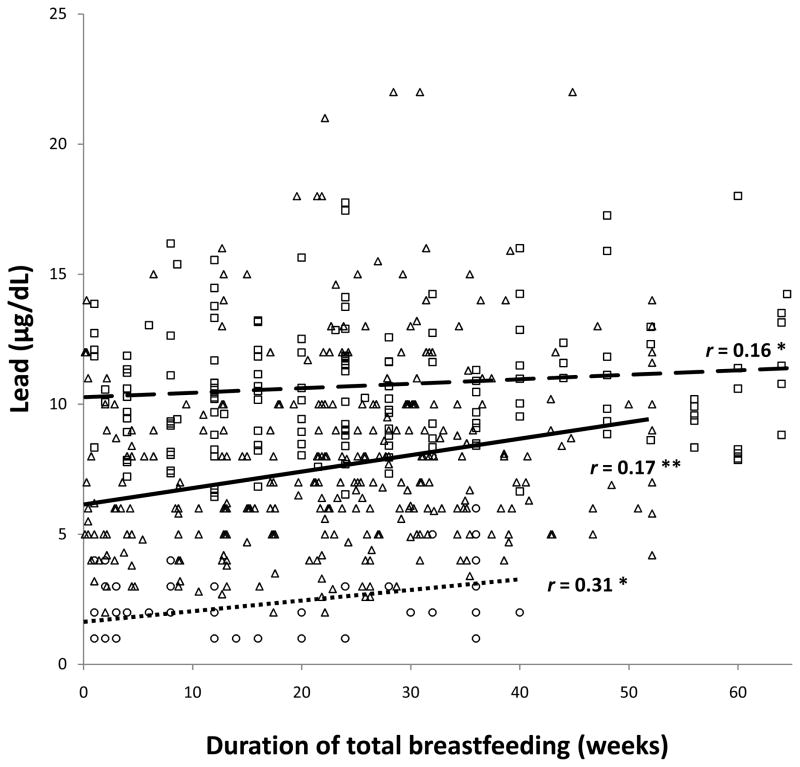
As reported previously as an incidental finding,(7) there was a positive correlation between the total duration of breastfeeding and infant lead levels in the Costa Rica sample. The partial correlations between lead and the duration of breastfeeding as the sole milk source and total breastfeeding were .19 and .16, respectively, controlling for infant age, birth weight, and SES. Even stronger correlations were observed in the Chile and Detroit samples for the duration of breastfeeding as the sole source of milk, r values = .23 and .56, respectively (controlling for birth order in the Detroit sample). The corresponding r values for total breastfeeding were .17 and .31. All data points and the regression lines in the 3 samples are shown for the associations between lead and duration of breastfeeding as the sole source of milk (Figure 1) and total breastfeeding (Figure 2), controlling for background factors as indicated.
Figure 1.
Relation between lead concentration and duration of breastfeeding as the sole source of milk. All data points are shown: Costa Rica □, Chile △, Detroit ○. Regression lines are shown for each sample (Costa Rica
 , Chile
, Chile
 , Detroit
, Detroit
 ) along with the Pearson correlation coefficient for Chile and partial correlation coefficients for Costa Rica and Detroit. ** p < .01, *** p < .001
) along with the Pearson correlation coefficient for Chile and partial correlation coefficients for Costa Rica and Detroit. ** p < .01, *** p < .001
Figure 2.
Relation between lead concentration and total duration of breastfeeding. All data points are shown: Costa Rica □, Chile △, Detroit ○. Regression lines are shown for each sample (Costa Rica
 , Chile
, Chile
 , Detroit
, Detroit
 ) along with the Pearson correlation coefficient for Chile and partial correlation coefficients for Costa Rica and Detroit. * p < .05, ** p < .01
) along with the Pearson correlation coefficient for Chile and partial correlation coefficients for Costa Rica and Detroit. * p < .05, ** p < .01
DISCUSSION
Longer breastfeeding was associated with higher infant lead concentrations in 3 different countries, in 3 different decades, in settings that differed in the prevalence and duration of breastfeeding, the use of unleaded gasoline, and mean infant lead levels. The findings in the Chile and Detroit samples confirm our earlier observation in the sample from Costa Rica.
The association between lead concentration and duration of breastfeeding as the sole milk source was weakest in Costa Rica, perhaps because the infants were considerably older than in Detroit and Chile samples. This difference is plausible because breast milk is likely to contribute less to infants’ lead burden as they get older and have longer periods of environmental exposure and normal developmental behaviors, such as mouthing and independent locomotion. The Costa Rica sample was also the only one with a wide age range, compared to the other studies, and largely retrospective data on breastfeeding, which could introduce inaccuracy and potential bias. We also emphasize that the Detroit sample, in particular, was small, and breastfeeding was shorter and less intense than the other samples. Thus, we have the most confidence in the associations observed in the Chile sample. It was not only the largest but also the one with the best data on breastfeeding, i.e., obtained prospectively beginning at 4–6 months during weekly home visits.
Some investigators have reported low lead levels in breast milk or noted that formula contributes similar, if not more, lead to the infant’s diet(3;4) and concluded that increased lead levels in breastfed babies are not a significant public health concern. However, the physiologic rationale for concern is strong. Even with no current environmental exposure, lead accumulates in maternal bones from past exposure. During lactation, a period of heightened bone turnover, maternal bone lead redistributes from bone into plasma and into breast milk.(1;2) Our results are consistent with this physiology mechanism. With a longer duration of breastfeeding, the infant would have an opportunity to accumulate more lead as maternal blood lead is redistributed into breast milk. Our findings support the conclusion of Ettinger et al.(5) that “[t]his phenomenon constitutes a potential public health problem in areas where environmental lead exposure is continuing as well as in areas where environmental lead exposure has recently declined.”(p. 926) Our observations should be confirmed and extended in future studies. Longitudinal intergenerational studies are needed to understand how maternal lead burden in infancy and childhood affects lead in breast milk and infant lead levels. Interactions between minerals, such as calcium and lead, also warrant further investigation.
Our study has important limitations. As the figures show, there was insufficient overlap between samples in lead concentrations or breastfeeding duration to support a unified analysis. Rather, each sample had to be analyzed separately. Furthermore, we could not analyze cumulative lead burden, because lead was measured at a single time point in the Chile and Detroit samples and only over a 3-month period for the Costa Rica sample. The Costa Rica data were collected at what is typically the age period for peak lead levels, but infants in the Chile and Detroit samples were younger, and we have no data on their lead levels in the second year of life. We also had no data on maternal lead levels in bone or blood. Thus, we could not directly test the hypothesized mechanism or address fundamental questions about the relations between lead in maternal bone, maternal blood, breast milk, and infant blood. Our results point to a need for further research on these relations and the mechanisms that account for them. We also could not determine if maternal blood lead concentrations during pregnancy could be used in a selective screening process, i.e., to identify infants at particular risk for higher lead concentrations with longer breastfeeding.
Our findings do not detract from the many known benefits of breastfeeding. Rather, they suggest that monitoring lead concentrations in breastfed infants should be considered. There is increasing concern about adverse developmental effects of lead at even very low levels,(16;17) which has contributed to updated policy statements. Some recommend lowering the blood lead action level.(18) The American Academy of Pediatrics recommends that pediatric health care providers consider universal screening if there are no state guidelines other than for infants participating in Medicaid or WIC.(19) However, state guidelines vary, and many breastfed infants do not meet state criteria for screening. If our results are born out in other samples, additional monitoring of breastfed infants may be indicated until there are generations of mothers who grow up with little lead exposure and infants with little environmental exposure themselves.
Acknowledgments
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Child Health and Human Development or the National Institutes of Health in the US or FONDECYT in Chile. We are grateful to Yuezhou Jing, MA, for assistance in preliminary data analyses, to the laboratories that performed the lead assays, to project staff members in each country who so carefully collected the data, and to study families for their generous participation.
Support: Grant numbers R01 HD14122, R37 HD31606, R01 HD33487, and P01 HD039386, B. Lozoff, principal investigator, from the US National Institute of Child Health and Human Development and 195-0772, P. Pino, principal investigator, from the Chilean Fondo Nacional de Desarrollo Cientifico y Technológico (FONDECYT).
Role of the study sponsors: The study sponsors had no role in the study design, collection, analysis, and interpretation of data, writing of the report, or decision to submit for publication.
Footnotes
Conflict of interest statement: No author had a conflict of interest.
References
- 1.Manton W, Angle C, Stanek K, Kuntzelman D, Reese Y, Kuehnemann T. Release of lead from bone in pregnancy and lactation. Environ Res. 2003;92:139–51. doi: 10.1016/s0013-9351(03)00020-3. [DOI] [PubMed] [Google Scholar]
- 2.Gulson B, Mizon K, Korsch M, Palmer J, Donnelly J. Mobilization of lead from human bone tissue during pregnancy and lactation--a summary of long-term research. Sci Total Environ. 2003;303:79–104. doi: 10.1016/s0048-9697(02)00355-8. [DOI] [PubMed] [Google Scholar]
- 3.Rabinowitz M, Leviton A, Needleman H. Lead in milk and infant blood: a dose-response model. Arch Environ Health. 1985;40(5):283–6. doi: 10.1080/00039896.1985.10545933. [DOI] [PubMed] [Google Scholar]
- 4.Gundacker C, Pietschnig B, Wittmann KJ, Lischka A, Salzer H, Hohenauer L, et al. Lead and mercury in breast milk. Pediatrics. 2002;110(5):873–8. doi: 10.1542/peds.110.5.873. [DOI] [PubMed] [Google Scholar]
- 5.Ettinger AS, Tellez-Rojo MM, Amarasiriwardena C, Bellinger D, Peterson K, Schwartz J, et al. Effect of breast milk lead on infant blood lead levels at 1 month of age. Environ Health Perspect. 2004;112(14):1381–5. doi: 10.1289/ehp.6616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Counter SA, Buchanan LH, Ortega F. Lead concentrations in maternal blood and breast milk and pediatric blood of Andean villagers: 2006 follow-up investigation. J Occup Environ Med. 2007;49(3):302–9. doi: 10.1097/jom.0b013e31803225b0. [DOI] [PubMed] [Google Scholar]
- 7.Wolf AW, Jimenez E, Lozoff B. No evidence of developmental ill effects of low-level lead exposure in a developing country. J Dev Behav Pediatr. 1994;15:224–31. [PubMed] [Google Scholar]
- 8.Pino P, Walter T, Oyarzun M, Lozoff B. Rapid drop in infant blood lead levels during the transition to unleaded gasoline use in Santiago, Chile. Arch Environ Health. 2004;59(4):182–7. doi: 10.3200/AEOH.59.4.182-187. [DOI] [PubMed] [Google Scholar]
- 9.Lozoff B, Angelilli ML, Zatakia J, Jacobson SW, Calatroni A, Beard JL. Iron status of inner-city African-American infants. Am J Hematol. 2007;82:112–21. doi: 10.1002/ajh.20782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lozoff B, Brittenham GM, Wolf AW, McClish DK, Kuhnert PM, Jimenez E, et al. Iron deficiency anemia and iron therapy: effects on infant developmental test performance. Pediatrics. 1987;79:981–95. [PubMed] [Google Scholar]
- 11.Lozoff B, De Andraca I, Castillo M, Smith J, Walter T, Pino P. Behavioral and developmental effects of preventing iron-deficiency anemia in healthy full-term infants. Pediatrics. 2003;112:846–54. [PubMed] [Google Scholar]
- 12.Fernandez FJ, Hillegos D. An improved graphite furnace method for the determination of lead in blood using matrix modification and the L’vov platform. Atomic Spectroscopy. 1982;3:1300–11. [Google Scholar]
- 13.Ballew C, Khan LK, Kaufmann R, Mokdad A, Miller DT, Gunter EW. Blood lead concentration and children’s anthropometric dimensions in the Third National Health and Nutrition Examination Survey (NHANES III), 1988–1994. J Pediatr. 1999;134(5):623–30. doi: 10.1016/s0022-3476(99)70250-7. [DOI] [PubMed] [Google Scholar]
- 14.Slavin W. Graphite furnace AAS for biological materials. Sci Total Environ. 1988;71:17–35. [Google Scholar]
- 15.SAS 9.1. Cary, NC: SAS Institute Inc; 2003. [Google Scholar]
- 16.Lanphear BP, Hornung R, Khoury J, Yolton K, Baghurst P, Bellinger DC, et al. Low-level environmental lead exposure and children’s intellectual function: an international pooled analysis. Environ Health Perspect. 2005;113(7):894–9. doi: 10.1289/ehp.7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Canfield RL, Henderson CR, Jr, Cory-Slechta DA, Cox C, Jusko TA, Lanphear BP. Intellectual impairment in children with blood lead concentrations below 10 microg per deciliter. New Eng J Med. 2003;348:1517–26. doi: 10.1056/NEJMoa022848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gilbert SG, Weiss B. A rationale for lowering the blood lead action level from 10 to 2 microg/dL. Neuro Toxicology. 2006;27(5):693–701. doi: 10.1016/j.neuro.2006.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.American Academy of Pediatrics Committee on Environmental Health. Lead exposure in children: prevention, detection, and management. Pediatrics. 2005;116(4):1036–46. doi: 10.1542/peds.2005-1947. [DOI] [PubMed] [Google Scholar]