Abstract
Effects of i.c.v. and i.t. administration of (3SR, 4aRS,6RS,8aRS)-6-[2-(1H-tetrazol-5-yl)ethyl]decahydroisoquinoline-3-carboxylic acid (LY215490), a competitive α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptor antagonist and MK-801, a non-competitive N-methyl-D-aspartate (NMDA) receptor antagonist on the micturition reflex were evaluated in urethane-anesthetized rats, to determine if glutamatergic mechanisms in brain as well as spinal cord are important for the control of micturition. I.c.v. or i.t. injection of LY215490 in low doses (0.01–0.03 μg) did not change rhythmic bladder or external urethral sphincter (EUS) electromyogram (EMG) activity during continuous cystometrograms (CMGs; 0.21 ml/min), whereas higher doses (0.1–1 μg) markedly suppressed these responses. During single CMGs (0.04 ml/min), 0.1–1 μg i.c.v. or 0.1–10 μg i.t. doses increased volume threshold and pressure threshold for inducing micturition, and decreased bladder contraction amplitude and voiding efficiency. MK-801 in low doses (0.6 μg i.c.v. or 0.6–1.8 μg for i.t.) did not change bladder contraction amplitude or EUS EMG activity during continuous CMGs, whereas higher doses 6–60 μg markedly suppressed these responses. During single CMGs, MK-801 (6–60 μg i.c.v. or 60 μg i.t.) increased volume threshold and pressure threshold, and decreased voiding efficiency and bladder contraction amplitude. Pretreatment i.c.v. with MK-801 in a dose 1.8 μg which alone had little effect on bladder contraction amplitude and EUS EMG activity, markedly enhanced depressant effects of LY215490 (0.03 μg i.c.v.) on these responses. Administration of same doses of drugs by i.t. route did not elicit a similar synergistic interaction. These data indicate that in urethane-anesthetized rats glutamatergic mechanisms in brain and spinal cord are essential for controlling micturition and that interactions between AMPA and NMDA glutamatergic transmission are important at supraspinal but not spinal sites.
Keywords: external urethral sphincter, urinary bladder, periaqueductal gray, pontine micturition center, descending pathways, afferent limb
Previous studies demonstrated that in urethane-anesthetized rats, i.v. administration of an N-methyl-D-aspartate (NMDA) receptor antagonist (MK-801 or LY274614) decreased voiding pressure and increased bladder capacity (Maggi et al., 1990; Tillig and Constantinou, 2003; Yoshiyama et al., 1991, 1993a, b). This inhibitory effect on bladder activity is due to the block of NMDA glutamatergic transmission in the central nervous system (CNS), but not due to an effect at peripheral sites (Maggi et al., 1990; Yoshiyama et al., 1991). I.v. administration of α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA)/kainate receptor antagonists (GYKI 52466 or (3SR,4aRS,6RS,8aRS)-6-[2-(1H-tetrazol-5-yl)ethyl] decahydroisoquinoline-3-carboxylic acid [LY215490]) also suppressed the amplitude of reflex bladder contractions. This inhibitory effect is also due to the blockade of AMPA/kainate receptors in the CNS (Yoshiyama et al., 1995a, b, 1997). These studies revealed that central glutamatergic transmission mediated by NMDA and AMPA/kainate receptors in the lumbosacral spinal cord plays an essential role in the micturition reflex pathway.
Subsequent studies revealed that the drugs acted at least in part in the spinal cord. The i.t. injection of an NMDA receptor antagonist or AMPA/kainate receptor antagonist inhibits micturition (i.e. decrease of bladder contraction amplitude and increase of volume threshold for micturition; Yoshiyama et al., 1993a, b, 1997). Furthermore, bladder contractions evoked by the electrical stimulation of the pontine micturition center (PMC) were suppressed by i.v. or i.t. injection of NMDA or AMPA/kainate receptor antagonists (Matsumoto et al., 1995a, b) indicating that spinal glutamatergic transmission is important in the descending limb of the spinobulbospinal micturition reflex pathway (de Groat and Yoshimura, 2001). In addition, the i.t. administration of an NMDA receptor antagonist or AMPA/kainate receptor antagonist reduced the evoked potentials in the periaqueductal gray (PAG) elicited by electrical stimulation of afferent axons in the pelvic nerve (Kakizaki et al., 1998), suggesting that glutamatergic transmission is also involved in the ascending afferent limb of the micturition reflex at the level of the spinal cord. Taken together, it is evident that spinal glutamatergic transmission is important in both afferent and efferent limbs of the spinal neural pathways controlling bladder activity.
Indirect evidences have implicated glutamate in both inhibitory and facilitatory mechanisms in brain circuitry controlling the lower urinary tract (Chen et al., 1993; Mallory et al., 1991; Yokoyama et al., 2000). On the other hand, the recent study demonstrated that supraspinal inhibition of NMDA receptors by LY274614 suppressed micturition reflex (Tillig and Constantinou, 2003). However, the contribution of supraspinal AMPA/kainate receptors to the control of voiding is less clear. In the present study, the effects of i.c.v. administration of LY215490, an AMPA/kainate receptor antagonist and also MK-801, an NMDA receptor antagonist on the micturition reflex were evaluated in the urethane-anesthetized rat, to determine if glutamatergic mechanisms via AMPA/kainite receptors as well as NMDA receptors in the brain are important for the control of micturition. In addition the effects of i.c.v. administration were compared with the effects of i.t. administration of drugs. The interactions of the two drugs at both sites were also evaluated.
Preliminary results have been presented in an abstract (Yoshiyama and de Groat, 1996).
EXPERIMENTAL PROCEDURES
Animal preparation
The protocol in this study was approved by Institutional Animal Care and Use Committee, University of Pittsburgh and all experiments conformed to named international guidelines on the ethical use of animals. All efforts were made to minimize the number of animals used and their suffering. Female Sprague–Dawley rats (n=62) weighing 240–300 g were anesthetized with urethane (1.2 g/kg s.c.). Additional urethane up to 0.3 g/kg was given i.p. when the animals were incompletely anesthetized. The trachea was cannulated with a polyethylene tube (PE-240) to facilitate respiration.
Routes for drug administrations
To administer drugs by the i.c.v. route, the rat was placed in a stereotaxic apparatus and a small craniotomy was performed in order to insert a 30-gauge needle into the right lateral ventricle. The needle connected to a PE-10 polyethylene tube for i.c.v. injection was introduced stereotaxically: the tip of the needle located in the right lateral ventricle was at 0.8 mm posterior to the bregma, 1.4 mm lateral to midline, and 3.8 mm below the surface of the skull, according to the coordinates of Paxinos and Watson (1986). To minimize the increase in the intracranial pressure produced by multiple i.c.v. injections, the occipital crest of the skull was exposed and the atlanto-occipital membrane was incised on its midline using the tip of a 16-gauge needle as a cutting edge. After each experiment, blue food color or black India ink was injected to the ventricle to identify the location of the needle tip.
An i.t. catheter was inserted according to the technique of Yaksh and Rudy, (1976). The occipital crest of the skull was exposed and the atlanto-occipital membrane was incised at the midline using the tip of a 16-gauge needle as a cutting edge. A catheter (PE-10) was inserted through the slit and passed caudally to the L6-level of the spinal cord. At the end of the experiment a laminectomy was performed to verify the location of the catheter tip. Cumulative dose-response curves were obtained by administering the drugs in 0.6–6 μl injections followed by a 7 μl flush with artificial cerebrospinal fluid (CSF). The volume of fluid within the catheter was kept constant at 6 μl in all animals with i.c.v. or i.t. route.
Cystometrograms (CMGs)
A transurethral bladder catheter (PE-50) connected to a pressure transducer was used to record the bladder pressure during CMGs when the bladder was filled with a constant infusion of physiological saline and allowed to empty around the catheter.
Continuous infusion CMGs were performed to examine the relations between bladder and external urethral sphincter (EUS) activity in 40 rats using constant, rapid infusion (0.21 ml/min) of saline into the bladder to elicit repetitive voidings, which allowed collection of data for a large number of voiding cycles (Maggi et al., 1986).
Single infusion CMGs were applied in 22 animals to evaluate urodynamic parameters such as voided volume, residual volume, volume threshold (i.e. bladder capacity) and pressure threshold for inducing micturition, voiding efficiency (i.e. percent of bladder volume voided), and bladder contraction amplitude and duration. Saline was infused into the bladder at slower rate (0.04 ml/min) until the peak of a voiding bladder contraction; then the infusion was stopped and the saline voided from the bladder was collected and measured to determine voided volume. The bladder was then emptied to measure residual volume. This procedure was performed one to two times before any drug injection and after each drug dose. An NMDA receptor antagonist or AMPA receptor antagonist is known to dose-dependently increases volume threshold for inducing micturition before abolishing bladder activity at high doses (Yoshiyama et al., 1993a, 1997). Thus, when bladder contractions were completely suppressed, the volume to allow overflow incontinence was used for statistical analysis as a surrogate of volume threshold although this ‘structural’ bladder capacity was different from the ‘functional’ one obtained before disturbance of micturition.
EUS electromyogram (EMG) recording
To record the EUS EMG activity during continuous infusion CMGs, fine epoxy-coated stainless steel wire electrodes (50 μm; M. T. Giken Co., Ltd., Tokyo, Japan) were inserted percutaneously into the EUS to record the electrical activity of the striated muscle (n=40). This was performed using a 30-gauge needle with a hooked EMG electrode positioned at the tip. The needle was inserted into sphincter approximately 5–10 mm lateral to the urethra and then withdrawn leaving the EMG wires embedded in the muscle (Kruse et al., 1990). The EMG activity was passed through a discriminator/ratemeter, the output of which was recorded on chart recorder. The peak firing rate during each micturition contraction was measured.
Evaluation and statistical analysis
Single doses of LY215490 (i.v. or i.t.) were injected to evaluate the onset time of drug effects as previously reported using decerebrate unanesthetized rats (Yoshiyama et al., 1997). It is known that MK-801 has a short onset time to exhibit the maximal effects on micturition and its effects last for a long time (Yoshiyama et al., 1991, 1993a, 1994).
The effects of cumulative doses of LY215490 or MK-801 injected at 20 min intervals on bladder contraction amplitude and duration, intercontraction interval and EUS EMG activity were evaluated during continuous infusion (0.21 ml/min) CMGs. Bladder contractions 5 min before the first dose and 15–20 min after each dose were averaged as values of control and maximal effect, respectively. During single infusion (0.04 ml/min) CMGs, each dose of LY215490 or MK-801 was given 10 min prior to the first CMG. One to two CMGs were obtained after each dose. The effects of the drug on the volume threshold for inducing micturition and voided volume during each voiding reflex were measured. Based on these values, voiding efficiency (%) was estimated as (voided volume/volume threshold)×100.
All values are expressed as mean ± S.E.M. Repeated measures one-way analysis of variance (ANOVA) followed by the Dunnett multiple comparisons test and two-way ANOVA were used for analyzing the dose-relations and the pharmacological synergy between MK-801 and LY215490, respectively. For all statistical tests, P<0.05 was considered significant. ED50s were calculated based on sigmoidal dose-response (four-parameter logistic) by using Prism statistical program (GraphPad Software, Inc., San Diego, CA, USA).
Drugs
Drugs used in these studies included urethane (ethyl carbamate; Sigma Chemical Co., St. Louis, MO, USA), halothane (Ayerst Laboratory. Inc., Philadelphia, PA, USA), LY215490 (Lilly Res. Laboratories, Indianapolis, IN, USA) and MK-801 (Merck, Sharp & Dohme Res. Laboratories, West Point, PA, USA). LY215490 and MK-801 were dissolved in artificial CSF (Feldberg and Fleischhauer, 1960) for i.c.v. and i.t. injections and these solutions were then adjusted to pH 7.4. Drugs doses were calculated for the salts of each compound.
RESULTS
Before drug administration, baseline urodynamic parameters were measured during continuous and single infusion CMGs as presented in Tables 1 and 2, respectively.
Table 1.
Baseline values for parameters of continuous infusion CMGa
| Parameter | LY215490 (i.c.v.) | LY215490 (i.t.) | MK-801 (i.c.v.) | MK-801 (i.t.) | Vehicle+ LY215490 (i.c.v.) | MK-801+ LY215490 (i.c.v.) | Vehicle+ LY215490 (i.t.) | MK-801+ LY215490 (i.t.) | Total (n=40) |
|---|---|---|---|---|---|---|---|---|---|
| BCA (cm H2O) | 24.0±0.4 | 28.9±2.4 | 24.4±3.2 | 28.2±2.0 | 27.7±1.2 | 27.7±2.8 | 25.2±2.2 | 31.3±2.2 | 27.2±0.8 |
| ICI (s) | 137±62 | 121±37 | 56±14 | 100±33 | 65±11 | 74±21 | 62±9 | 52±9 | 83±11 |
| BCD (s) | 41±5 | 41±4 | 34±1 | 39±6 | 34±2 | 35±3 | 37±3 | 31±2 | 37±1 |
| EUS EMG (pulses/s) | 147±20 | 185±40 | 148±17 | 162±27 | 177±42 | 199±39 | 167±38 | 152±28 | 167±11 |
BCA, bladder contraction amplitude; BCD, bladder contraction duration; ICI, intercontraction interval.
All values are expressed as mean ± S.E.M (n=5 for each group).
Table 2.
Baseline values for parameters of single infusion CMGa
| Group | VV (ml) | RV (ml) | VT (ml) | VE (%) | BCA (cm H2O) | PT (cm H2O) | BCD (s) |
|---|---|---|---|---|---|---|---|
| LY215490 | |||||||
| i.c.v. (n=6) | 0.28±0.12 | 0.39±0.13 | 0.67±0.17 | 40.4±9.4 | 36.9±3.0 | 2.6±0.4 | 42±3 |
| i.t. (n=5) | 0.37±0.06 | 0.74±0.21 | 1.11±0.19 | 37.8±8.9 | 29.2±2.7 | 2.4±0.4 | 59±14 |
| MK-801 | |||||||
| i.c.v. (n=5) | 0.29±0.06 | 0.54±0.17 | 0.83±0.18 | 39.2±9.3 | 31.7±1.7 | 4.1±0.7 | 55±11 |
| i.t. (n=6) | 0.67±0.19 | 0.41±0.14 | 1.08±0.17 | 61.1±11.1 | 26.3±1.0 | 4.2±0.5 | 37±3 |
| Total (n=22) | 0.41±0.07 | 0.51±0.08 | 0.92±0.09 | 45.2±5.1 | 31.0±1.4 | 3.3±0.3 | 48±5 |
BCA, bladder contraction amplitude; BCD, bladder contraction duration; PT, pressure threshold for inducing micturition; RV, residual volume; VE, voiding efficiency; VT, volume threshold for inducing micturition; VV, voided volume.
All values are expressed as mean ± S.E.M.
Effects of i.c.v. administration of LY215490
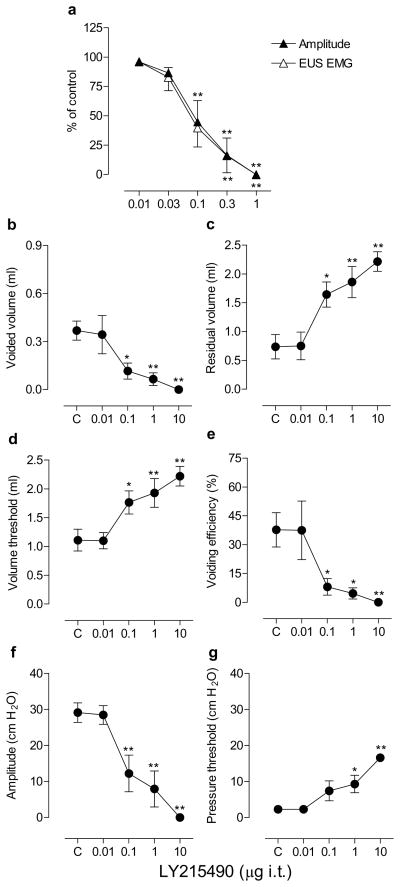
When LY215490 was tested in a range of i.c.v. doses (0.01–1 μg) administered cumulatively at 45 min intervals, low doses (0.01–0.1 μg) had small effects on bladder contraction amplitude and EUS activity, whereas the 1 μg of LY215490 produced a rapid onset (within 3 min) suppression of bladder activity (Figs. 1 and 2a). The ED50s for suppression of bladder and EUS activity were 0.17 and 0.12 μg, respectively. Maximal inhibitory effect appeared within 5 min. The 1 μg totally abolished the bladder activity producing overflow incontinence at a baseline pressure of 22 ± 3 cm H2O. LY215490 did not change bladder contraction durations before complete suppression of bladder contractions, whereas the doses 0.1–0.3 μg decreased inter-contraction intervals (28–84%).
Fig. 1.
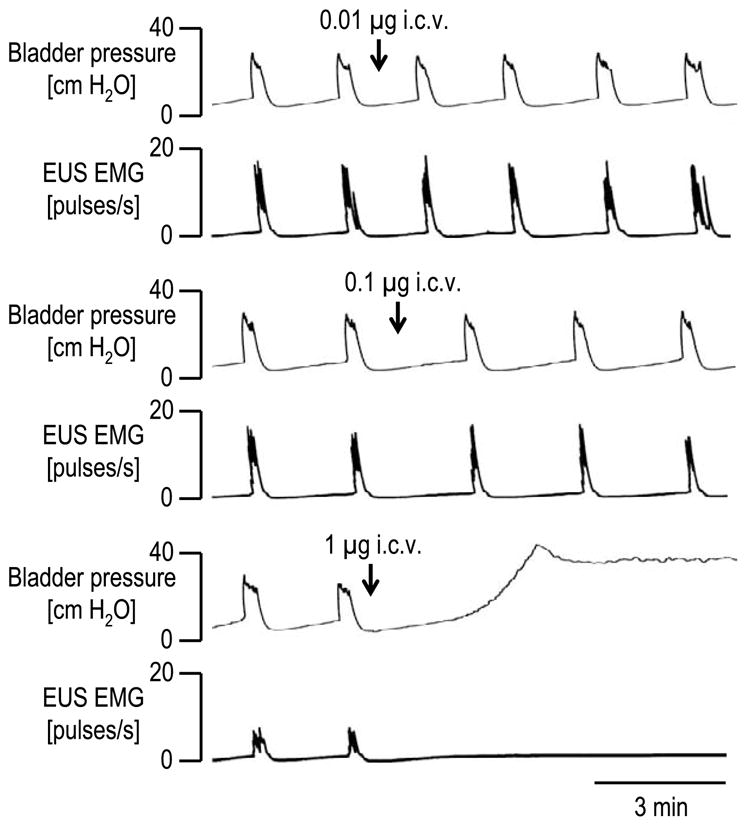
The effects of LY215490 (0.01, 0.1 and 1 μg i.c.v.) on the reflex bladder contractions and EUS EMG activity during a continuous filling (0.21 ml/min) CMG in a urethane-anesthetized rat. Doses of LY215490 (0.01, 0.03, 0.1, 0.3 and 1 μg) were injected at 20 min intervals. Note that the dose of 1 μg abolished the micturition reflex, inducing overflow incontinence. The effects of doses, 0.03 μg and 0.3 μg, are not included in the chart.
Fig. 2.
Dose-response curves showing the effects of graded doses of LY215490 (0.01–1 μg) by i.c.v. injections on the bladder contraction amplitude and EUS EMG activity during continuous filling (0.21 ml/min) CMGs in urethane-anesthetized rats (n=5; a) and the effects of LY215490 (0.01, 0.1 and 1 μg i.c.v.) on micturition reflex during single slow infusion (0.04 ml/min) CMGs in urethane-anesthetized rats (n=6; b–g). Abscissa, the doses of LY215490 plotted on a log scale. Ordinate, bladder contraction amplitude or EUS EMG activity as a % of control (a), voided volume (ml; b), residual volume (ml; c), volume threshold for inducing micturition (ml; d), voiding efficiency (%; e), bladder contraction amplitude (cm H2O; f) and pressure threshold for inducing micturition (cm H2O; g). Mean± S.E.M. is plotted for each point The raw values at individual doses are compared with those of controls using Dunnett’s multiple comparisons test (* P<0.05, ** P<0.01) following repeated measures ANOVA. C, control.
In six animals in which urinary bladder activity was recorded during single CMGs (Fig. 2b–g), even the lowest dose, LY215490 (0.01 μg i.c.v.) increased residual volume 151% and decreased voiding efficiency by 66% (Fig. 2c, e). Higher doses (0.1–1 μg) decreased voided volume (71–100%), voiding efficiency (84–100%) and bladder contraction amplitude (84–100%), whereas these doses increased residual volume (up to 20-fold), volume threshold (up to nine-fold) and pressure threshold (up to 26-fold; Fig. 2b–2g). The drug had no effect on bladder contraction durations. A higher dose (10 μg) killed all animals (n=6), 8–24 min (mean±16 min) after the administration.
Effects of i.t. administration of LY215490
The i.t. injection of LY215490 produced a rapid onset suppression of bladder and EUS activity in this study as noted in a previous report using unanesthetized rats (Yoshiyama et al., 1997). Fig. 3a shows that the LY215490 injected in a cumulative manner (0.01–1 μg) suppressed bladder contraction amplitude and EUS activity with ED50s of 0.09 μg and 0.08 μg, respectively. Maximal inhibitory effect appeared within 5–20 min. Doses 0.1 μg and 0.3 μg significantly decreased bladder contraction amplitude by 31%, and 1 μg totally abolished the bladder activity in all animals, producing overflow incontinence at a baseline pressure of 19±3 cm The inhibitory effect of H2O. LY215490 (0.1 μg<) on bladder and EUS persisted for at least 120 min. LY215490 did not change bladder contraction durations before complete suppression of bladder contractions, whereas the doses 0.03–0.3 μg decreased intercontraction intervals (10–88%).
Fig. 3.
Dose-response curves showing the effects of graded doses of LY215490 (0.01–1 μg) by i.t. injections on the bladder contraction amplitude and EUS EMG activity during continuous filling (0.21 ml/min) CMGs in urethane-anesthetized rats (n=5; a) and the effects of LY215490 (0.01, 0.1, 1 and 10 μg i.t.) on micturition reflex during single slow infusion (0.04 ml/min) CMGs in urethane-anesthetized rats (n=5; b–g). Abscissa, the doses of LY215490 plotted on a log scale. Ordinate, bladder contraction amplitude or EUS EMG activity as a % of control (a), voided volume (ml; b), residual volume (ml; c), volume threshold for inducing micturition (ml; d), voiding efficiency (%; e), bladder contraction amplitude (cm H2O; f) and pressure threshold for inducing micturition (cm H2O; g). Mean± S.E.M. is plotted for each point. The raw values at individual doses are compared with those of controls using Dunnett’s multiple comparisons test (* P<0.05, ** P<0.01) following repeated measures ANOVA. C, control.
In five animals in which urinary bladder activity was recorded during single CMGs (Fig. 3b–g), LY215490 was tested over a range of doses (0.01–10 μg i.t.). The 0.1–1 μg doses decreased voided volume (10–100%), voiding efficiency (12–100%) and bladder contraction amplitude (14–100%), and increased residual volume (up to 8.5-fold) and volume threshold (up to three-fold). The drug, however, did not change bladder contraction durations. The 10 μg dose completely abolished bladder activity and induced overflow incontinence at a baseline pressure of 15–18 cm H2O. Bladder capacity measured when continuous leakage of infused saline occurred ranged from 1.69–2.68 ml (mean±2.22 ml).
Effects of i.c.v. administration of MK-801
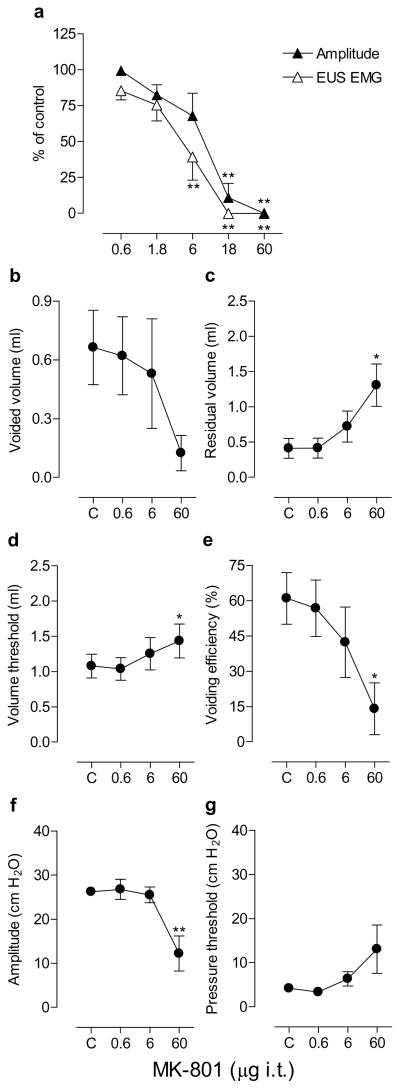
The i.c.v. injection of MK-801 (6–18 μg) produced a rapid onset (within 3 min) suppression of bladder and EUS activity in a pilot study. Fig. 4a shows the effects of MK-801 injected at 20–30 min intervals in a cumulative manner (0.6–60 μg) on bladder contraction amplitude and EUS activity, exhibiting ED50s of 3.3 and 1.9 μg, respectively. The 6 μg dose significantly decreased bladder contraction amplitude and EUS EMG by 83% and 91%, respectively, and 18–60 μg totally abolished the bladder and EUS activity in all animals (n=5) producing overflow incontinence at a baseline pressure of 19± 5 cm H2O. MK-801 had no effect on bladder contraction durations before complete suppression of bladder contractions, whereas the doses 1.8–6 μg decreased intercontraction intervals (22–50%).
Fig. 4.
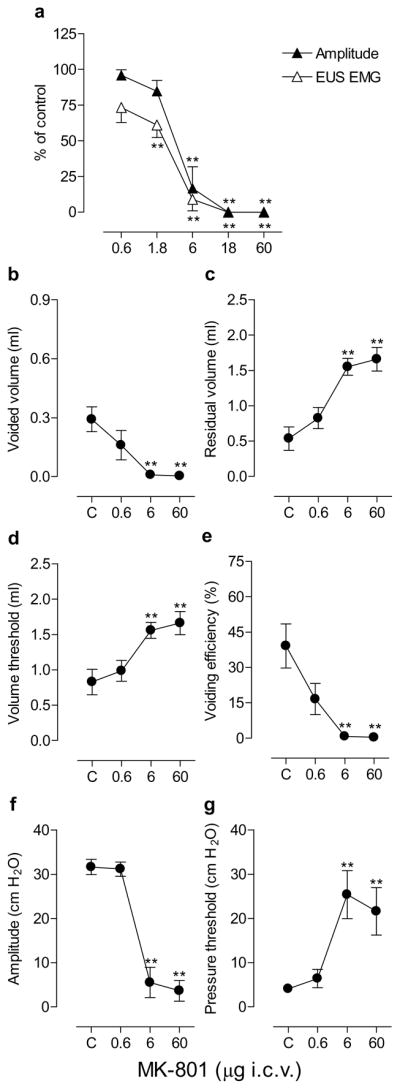
Dose-response curves showing the effects of graded doses of MK-801 (0.6–60 μg) by i.c.v. injections on the bladder contraction amplitude and EUS EMG activity during continuous filling (0.21 ml/min) CMGs in urethane-anesthetized rats (n=5; a) and the effects of MK-801 (0.6, 6 and 60 μg i.c.v.) on micturition reflex during single slow infusion (0.04 ml/min) CMGs in urethane-anesthetized rats (n=5; b–g). Abscissa, the doses of MK-801 plotted on a log scale. Ordinate, bladder contraction amplitude or EUS EMG activity as a % of control (a), voided volume (ml; b), residual volume (ml; c), volume threshold for inducing micturition (ml; d), voiding efficiency (%; e), bladder contraction amplitude (cm H2O; f) and pressure threshold for inducing micturition (cm H2O; g). Mean± S.E.M. is plotted for each point. The raw values at individual doses are compared with those of controls using Dunnett’s multiple comparisons test (* P<0.05, ** P<0.01) following repeated measures ANOVA. C, control.
In five animals in which urinary bladder activity was recorded during single CMGs (Fig. 4b–g), MK-801 was administered i.c.v. in a range of doses (0.6, 6 and 60 μg). The lowest dose 0.6 μg had no effect on any parameters, whereas the 6–60 μg doses increased residual volume (up to eight-fold), volume threshold (29–250%) and pressure threshold (up to 16-fold), and decreased voided volume (87–100%), voiding efficiency (96–100%) and bladder contraction amplitude (42–100%). The drug doses, however, had no effect on bladder contraction durations.
Effects of i.t. administration of MK-801
MK-801 injected in a cumulative manner (0.6–60 μg) dose-dependently decreased bladder contraction amplitude and EUS activity during continuous infusion CMGs, (Fig. 5a). exhibiting ED50s of 7.8 and 3.8 μg, respectively The 18 or 60 μg doses totally abolished the bladder and EUS activity and produced overflow incontinence at a baseline pressure of 23± 3 cm H2O. MK-801 had no effect on bladder contraction durations before micturition was completely suppressed, whereas the doses 1.8–6 μg decreased intercontraction intervals (60–94%).
Fig. 5.
Dose-response curves showing the effects of graded doses of MK-801 (0.6–60 μg) by i.t. injections on the bladder contraction amplitude and EUS EMG activity during continuous filling (0.21 ml/min) CMGs in urethane-anesthetized rats (n=5; a) and the effects of MK-801 (0.6, 6 and 60 μg i.t.) on micturition reflex during single slow infusion (0.04 ml/min) CMGs in urethane-anesthetized rats (n=6; b–g). Abscissa, the doses of MK-801 plotted on a log scale. Ordinate, bladder contraction amplitude or EUS EMG activity as a % of control (a), voided volume (ml; b), residual volume (ml; c), volume threshold for inducing micturition (ml; d), voiding efficiency (%; e), bladder contraction amplitude (cm H2O; f) and pressure threshold for inducing micturition (cm H2O; g) Mean ± S.E.M. is plotted for each point. The. raw values at individual doses are compared with those of controls using Dunnett’s multiple comparisons test (* P<0.05, ** P<0.01) following repeated measures ANOVA. C, control.
In six animals in which urinary bladder activity was recorded during single CMGs (Fig. 5b–g), i.t. administration of lower doses of MK-801, 0.6 μg and 6 μg did not alter any parameter. The 60 μg dose increased residual volume (four-fold), volume threshold (8–94%), and decreased voiding efficiency (5–100%) and bladder contraction amplitude (7–100%). Voided volume, pressure threshold and bladder contraction duration were not changed by MK-801. The 60 μg dose totally abolished voiding reflex in two of six rats.
Interaction between LY215490 and MK-801 following i.c.v. or i.t. administration
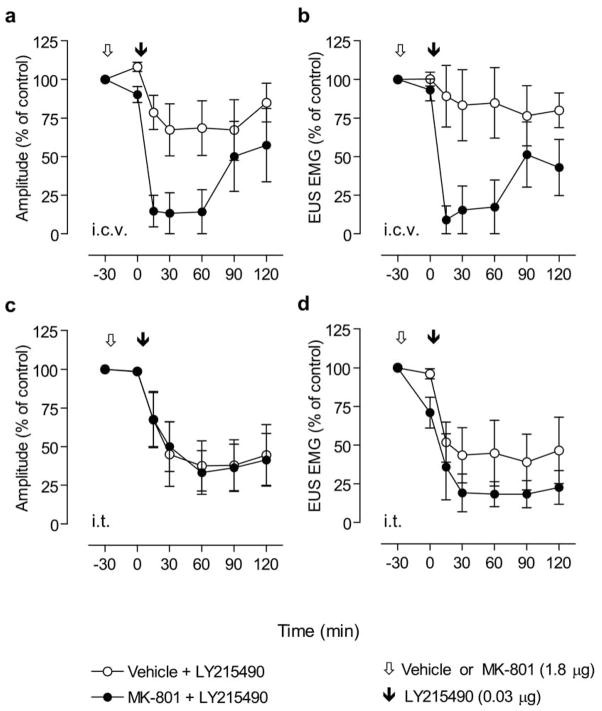
Experiments were conducted to determine if the combined i.c.v. or i.t. administration of low doses of MK-801 (1.8 μg) and LY215490 (0.03 μg) which individually had little effect (Fig. 2a, 3a, 4a and 5a), had a synergistic effect on the bladder and EUS activity. As shown in Fig. 6, MK-801 was administered 30 min prior to LY215490. I.c.v. pretreatment with MK-801 dose significantly enhanced the inhibitory effect of i.c.v. LY215490 dose on bladder contraction amplitude and EUS EMG activity (Fig. 6a, b; P=0.0020 and P=0.0008, respectively, by two-way repeated measures ANOVA), whereas i.t. administration of MK-801 dose did not significantly alter the response to i.t. LY215490 dose (Fig. 6c, d; bladder contraction amplitude, P=0.1305; EUS, P=0.7732, by two-way repeated measures ANOVA).
Fig. 6.
Graphs showing the time course of the effects of i.c.v. or i.t. combined administration of MK-801 (1.8 μg) and (LY215490 (0.03 μg; n=5 for each treatment group), on the bladder contraction amplitude ((a) for i.c.v. and (c) for i.t.) and EUS EMG activity ((b) for i.c.v. and (d) for i.t.) during continuous filling (0.21 ml/min) CMGs. Opened, and closed arrowheads indicate (i.c.v. or i.t.) injection of vehicle (artificial CSF only) or MK-801 (1.8 μg), and LY215490 (0.03 μg), respectively. Abscissa, time scale (min). Ordinates, bladder contraction amplitude (% of control; a, c) or EUS EMG activity (% of control; b, d).
No synergistic effect on bladder contraction duration and intercontraction interval was detected when these drugs given i.t. On the other hand, statistics to analyze a synergy at supraspinal sites could not be performed because an immediate suppression of bladder activity after i.c.v. injection of LY215490 did not allow obtaining values of these parameters in four of five animals.
DISCUSSION
These experiments revealed that the i.c.v. as well as i.t. administration, of an AMPA or NMDA glutamatergic receptors antagonist (LY215490 or MK-801, respectively) dose-dependently increased the volume threshold and pressure threshold for voiding, reduced the efficiency of voiding and in high doses completely suppressed bladder and EUS EMG activity in urethane-anesthetized rats. Pretreatment with a low dose of MK-801 enhanced the inhibitory effects of LY215490 when injected i.c.v. but not i.t., suggesting that an interaction between AMPA and NMDA synaptic mechanisms is important for the control of micturition in the brain but not in the spinal cord.
It has been proposed that glutamatergic transmission mediated by both AMPA and NMDA receptors is important in the spinal processing of afferent input from the bladder (Kakizaki et al., 1998) and in the descending limb of the spinobulbospinal micturition reflex pathway (Matsumoto et al., 1995a, b). Previous pharmacological studies also revealed that i.t. injection of MK-801 (Yoshiyama et al., 1993a) or LY215490 (Yoshiyama et al., 1997) suppressed reflex bladder contractions and increased the volume threshold for inducing voiding in anesthetized or unanesthetized rats, suggesting that the glutamatergic receptors in the spinal cord are essential for control of micturition reflexes.
Tillig and Constantinou (2003) described that i.c.v. injection of LY274614, a competitive NMDA receptor antagonist, suppressed reflex bladder contractions and increased volume threshold for inducing micturition. Although the present experiments, taken together with their report, indicated that both types of receptors are also involved in the brain circuitry controlling reflex micturition, it is unclear where the receptors are located in the brain. Previous studies reported that L-glutamate microinjected into the dorsolateral pontine tegmentum, in the region of the PMC evoked an increase in bladder pressure in the anesthetized rat (Willette et al., 1988) and lowered the volume threshold for inducing micturition in the anesthetized cat (Mallory et al., 1991). Matsuura et al. (2000) reported that the microinjection of L-glutamate into caudal regions of the PAG evoked bladder contractions with coordinated EUS activity in the urethane-anesthetized rat. They also suggested that the pathway from the PAG to the PMC is functionally important for producing micturition reflexes. Thus, it is likely that PMC and PAG are the important sites for the supraspinal inhibitory effects of LY215490 or MK-801 on the bladder and EUS activity. A possible action of LY215490 on forebrain structures such as the diencephalon and cerebral cortex, which are known to modulate the micturition reflex (Kuru, 1965; de Groat et al., 1993), remains to be elucidated, although our previous study (Yoshiyama et al., 1993a) demonstrated that an action of MK-801 on these areas was not essential for producing bladder inhibition because in urethane-anesthetized animals in which the forebrain was removed rostral to the superior colliculus, MK-801 i.v. produced its usual inhibitory effects on bladder reflexes. Similar dose-response curves were obtained in urethane-anesthetized rats with an intact neuraxis and after decerebration.
The present studies during slow infusion (0.04 ml/min) CMGs revealed that glutamatergic transmission in the brain is more sensitive to LY215490 and MK-801 than that in the spinal cord. Even the lowest dose 0.01 μg of LY215490 via i.c.v. but not i.t. significantly increased the residual volume and decreased the voiding efficiency. The i.c.v. administration of LY215490 (0.1–1 μg) was also more effective in increasing the pressure threshold for inducing voiding than i.t. injections. The differences in the inhibitory effects of MK-801 elicited by i.c.v. and i.t. injections were more prominent. The i.c.v. dose 6 μg of MK-801 significantly altered all micturition parameters measured in this study, whereas the i.t. injection of this dose was ineffective. Thus, glutamatergic transmission in micturition reflex pathways via AMPA receptors and NMDA receptors in the brain is more susceptible to antagonists than that in the spinal cord.
In addition, LY215490 or MK-801 via either i.c.v. or i.t. reduced the intercontraction interval measured during continuous infusion CMGs. The interpretation of this parameter, when it stands alone, is complicated since it is, like voiding efficiency, directly influenced by bladder capacity (i.e. afferent processing) and residual volume (or voided volume; i.e. efferent and somatic pathways to the bladder and urethra, respectively). In the present studies, thus, changes by either drug in the intercontraction interval were evaluated to be substantially supported by dose-relations of micturition parameters obtained during single infusion CMGs; except for the results by i.t. MK-801 in which the threshold dose to produce significant effects was consistently higher.
The present studies revealed that in urethane-anesthetized rats, pretreatment with MK-801 enhanced the inhibitory effects of LY215490 on bladder and EUS EMG activity when these drugs were given i.c.v., whereas combined i.t. injections did not produce such synergistic effects. Our previous studies demonstrated that i.t. injection of these glutamatergic antagonists to unanesthetized decerebrate rats produced synergistic inhibitory effects on the activities of bladder and EUS EMG (Yoshiyama et al., 1997). Furthermore, combined i.t. administration of MK-801 and LY215490 in low doses which individually had no effect, markedly suppressed the potentials in the PAG evoked by electrical stimulation of the pelvic nerve (Kakizaki et al., 1998). On the other hand, i.v. administration of GYKI 52466, an AMPA/kainate receptor antagonist reduced the amplitude of isovolumetric bladder contractions elicited by electrical stimulation in the PMC in urethane-anesthetized rats, in a similar dose-dependent manner with and without MK-801 (i.v.) pretreatment (Matsumoto et al., 1995b), demonstrating no synergistic interaction between these drugs. Based on the present and previous studies, it seems likely that synergistic interactions between NMDA and AMPA glutamatergic excitatory transmission can occur at multiple sites: (1) synapses in the spinal ascending limb of the micturition reflex pathway (Kakizaki et al., 1998), (2) in the brainstem (PMC and/or PAG; present studies) and (3) in the descending limb of micturition pathway (Yoshiyama et al., 1997; i.e. studies in the unanesthetized decerebrate rat). It seems, however, that urethane-anesthesia masks the interactions at the spinal level in the descending micturition pathways (Matsumoto et al., 1995b), suggesting that a polysynaptic descending pathway utilizes different synapses at which excitatory transmission via NMDA and AMPA/kainate receptors is mediated.
Previous studies have revealed that urethane anesthesia affects glutamatergic signaling in the control of micturition (Matsumoto et al., 1995b; Yoshiyama et al., 1994, 1995a). To avoid interactions associated with anesthesia, using conscious animals can be alternative. On the other hand, it has been described that an AMPA receptor antagonist or an NMDA receptor antagonist has anxiolytic properties and alters behavior (Chase et al., 2003; Clineschmidt et al., 1982a, b), suggesting that either drug potentially affects conscious level and voluntary micturition (i.e. micturition behavior) of animals (Tillig and Constantinou, 2003). Thus, for evaluating the effects of the drug on the involuntary ‘reflex’ micturition, which is considered different from the ‘conscious’ voiding (Maggi and Meli, 1986a), the use of anesthetized or decerebrate unanesthetized animal is inevitable. Either animal also allows studying EUS EMG activity, with minimizing its suffering. However, decerebration limits the route of drug administrations and the method to evaluate the supraspinal involvement. Herein, in view of studies on the ‘reflex’ micturition mechanisms, anesthesia such as urethane remains choice in in vivo neurophysiological and pharmacological experiments (Maggi and Meli, 1986b).
In conclusion, the present studies using urethane-anesthetized rats suggested that AMPA as well as NMDA glutamatergic transmission at the supraspinal sites plays an essential role in the control of micturition reflexes and that synergistic interaction between AMPA and NMDA transmissions can occur at the synapses in the brain. These interactions seem to be masked by urethane anesthesia at the synapses in the spinal components of the micturition reflex pathway.
Acknowledgments
We are grateful to Eli Lilly and Company (Indianapolis, IN, USA) and Merck, Sharp and Dohme Research Laboratories for gifts of LY215490 and MK-801, respectively. This work was supported by National Institutes of Health Grant DK-49430 (W.D.).
Abbreviations
- AMPA
α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid
- ANOVA
analysis of variance
- CMGs
cystometrograms
- CNS
central nervous system
- CSF
cerebrospinal fluid
- EMG
electromyogram
- EUS
external urethral sphincter
- LY215490
(3SR,4aRS,6RS,8aRS)-6-[2-(1H-tetrazol-5-yl)ethyl]decahydroisoquinoline-3-carboxylic acid
- MK-801
dizocilpine, (5R,10S)-(+)-5-methyl-10,11-dihydro-5H-dibenzo-[a, d]cyclohepten-5,10-imine
- NMDA
N-methyl-D-aspartate
- PAG
periaqueductal gray
- PMC
pontine micturition center
References
- Chase TN, Bibbiani F, Oh JD. Striatal glutamatergic mechanisms and extrapyramidal movement disorders. Neurotoxicity Res. 2003;5:139–146. doi: 10.1007/BF03033378. [DOI] [PubMed] [Google Scholar]
- Chen SY, Wang SD, Cheng CL, Kuo JS, de Groat WC, Chai CY. Glutamate activation of neurons in CV-reactive areas of cat brain stem afferents urinary bladder motility. Am J Physiol. 1993;265:F520–F529. doi: 10.1152/ajprenal.1993.265.4.F520. [DOI] [PubMed] [Google Scholar]
- Clineschmidt BV, Martin GE, Bunting PR, Papp NL. Central sympathomimetic activity of (+)-5-methyl-10,11-dihydro-5H-dibenzo-[a,d]cyclohepten-5,10,-imime (MK-801): a substance with potent anticonvulsant, central sympathomimetic, and apparent anxiolytic properties. Drug Dev Res. 1982a;2:135–145. [Google Scholar]
- Clineschmidt BV, Williams M, Witoslawski JJ, Bunting PR, Risley EA, Totaro JA. Restoration of shock-suppressed behavior by treatment with (+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10,-imime (MK-801): a substance with potent anticonvulsant, central sympathomimetic, and apparent anxiolytic properties. Drug Dev Res. 1982b;2:147–163. [Google Scholar]
- de Groat WC, Booth AM, Yoshimura N. Neurophysiology of micturition and its modification in animal models of human disease. In: Maggi CA, editor. The autonomic nervous system: nervous control of the urogenital system. Vol. 3. London: Harwood Academic Publishers; 1993. pp. 227–290. [Google Scholar]
- de Groat WC, Yoshimura N. Pharmacology of the lower urinary tract. Annu Rev Pharmacol Toxicol. 2001;41:691–721. doi: 10.1146/annurev.pharmtox.41.1.691. [DOI] [PubMed] [Google Scholar]
- Feldberg W, Fleischhauer K. Penetration of bromophenol blue from the perfused cerebral ventricles into the brain tissue. J Physiol (Lond) 1960;150:451–462. doi: 10.1113/jphysiol.1960.sp006397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakizaki H, Yoshiyama M, Roppolo JR, Booth AM, de Groat WC. Role of spinal glutamatergic transmission in the ascending limb of the micturition reflex pathway in the rat. J Pharmacol Exp Ther. 1998;285:22–27. [PubMed] [Google Scholar]
- Kruse MN, Noto H, Roppolo JR, de Groat WC. Pontine control of the urinary bladder and external urethral sphincter in the rat. Brain Res. 1990;532:182–190. doi: 10.1016/0006-8993(90)91758-9. [DOI] [PubMed] [Google Scholar]
- Kuru M. Nervous control of micturition. Physiological Rev. 1965;45:425–494. doi: 10.1152/physrev.1965.45.3.425. [DOI] [PubMed] [Google Scholar]
- Maggi CA, Giuliani S, Giachetti A, Meli A. The effect of MK-801 on the micturition reflex in anesthetized rats. Eur J Pharmacol. 1990;181:105–109. doi: 10.1016/0014-2999(90)90250-a. [DOI] [PubMed] [Google Scholar]
- Maggi CA, Meli A. Do “conscious” and “reflex” micturition have a separate sensory input? Implications for clinical urodynamics. Neurourol Urodyn. 1986a;5:563–571. [Google Scholar]
- Maggi CA, Meli A. Suitability of urethane anesthesia for physiopharmacological investigations in various systems: Part 1. General considerations. Experientia. 1986b;42:109–210. doi: 10.1007/BF01952426. [DOI] [PubMed] [Google Scholar]
- Maggi CA, Santicioli P, Meli A. The nonstop transvesical cystometrogram in urethane-anesthetized rats. J Pharmacol Methods. 1986;15:157–167. doi: 10.1016/0160-5402(86)90064-1. [DOI] [PubMed] [Google Scholar]
- Mallory BS, Roppolo JR, de Groat WC. Pharmacological modulation of the pontine micturition center. Brain Res. 1991;546:310–320. doi: 10.1016/0006-8993(91)91495-m. [DOI] [PubMed] [Google Scholar]
- Matsumoto G, Hisamitsu T, de Groat WC. Role of glutamate and NMDA receptors in the descending limb of the spinobulbospinal micturition reflex pathway of the rat. Neurosci Lett. 1995a;183:58–61. doi: 10.1016/0304-3940(94)11114-x. [DOI] [PubMed] [Google Scholar]
- Matsumoto G, Hisamitsu T, de Groat WC. Non-NMDA glutamatergic excitatory transmission in the descending limb of the spinobulbospinal micturition reflex pathway of the rat. Brain Res. 1995b;693:246–250. doi: 10.1016/0006-8993(95)00738-c. [DOI] [PubMed] [Google Scholar]
- Matsuura S, Downie JW, Allen GV. Micturition evoked by glutamate microinjection in the ventrolateral periaqueductal gray is mediated through Barrington’s nucleus in the rat. Neuroscience. 2000;101:1053–1061. doi: 10.1016/s0306-4522(00)00404-8. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 2. New York: Academic Press; 1986. [DOI] [PubMed] [Google Scholar]
- Tillig B, Constantinou CE. Supraspinal N-methyl-D-aspartate receptor inhibition influences the micturition reflex and function of the upper urinary tract of anesthetized and conscious rats. Neurourol Urodyn. 2003;22:164–175. doi: 10.1002/nau.10080. [DOI] [PubMed] [Google Scholar]
- Willette RN, Morrison S, Sapru HN, Reis DJ. Stimulation of opiate receptors in the dorsal pontine tegmentum inhibits reflex contraction of the urinary bladder. J Pharmacol Exp Ther. 1988;244:403–409. [PubMed] [Google Scholar]
- Yaksh TL, Rudy TA. Chronic catheterization of the spinal subarachnoid space. Physiol Behav. 1976;17:1031–1036. doi: 10.1016/0031-9384(76)90029-9. [DOI] [PubMed] [Google Scholar]
- Yokoyama O, Yoshiyama M, Namiki M, de Groat WC. Role of the forebrain in bladder overactivity following cerebral infarction in the rat. Exp Neurol. 2000;163:469–476. doi: 10.1006/exnr.2000.7391. [DOI] [PubMed] [Google Scholar]
- Yoshiyama M, de Groat WC. Supraspinal AMPA and NMDA glutamatergic transmission in the micturition reflex in the rat. Soc Neurosci Abstr. 1996;22:93. [Google Scholar]
- Yoshiyama M, Roppolo JR, de Groat WC. Effects of MK-801 on the micturition reflex in the rat: possible sites of action. J Pharmacol Exp Ther. 1993a;265:844–850. [PubMed] [Google Scholar]
- Yoshiyama M, Roppolo JR, de Groat WC. Alteration by urethane of glutamatergic control of micturition. Eur J Pharmacol. 1994;264:417–425. doi: 10.1016/0014-2999(94)00505-2. [DOI] [PubMed] [Google Scholar]
- Yoshiyama M, Roppolo JR, de Groat WC. Effects of GYKI 52466 and CNQX, AMPA/kainate receptor antagonists, on the micturition reflex in the rat. Brain Res. 1995a;691:185–194. doi: 10.1016/0006-8993(95)00671-c. [DOI] [PubMed] [Google Scholar]
- Yoshiyama M, Roppolo JR, de Groat WC. Interactions between NMDA and AMPA/kainate receptors in the control of micturition in the rat. Eur J Pharmacol. 1995b;287:73–78. doi: 10.1016/0014-2999(95)00615-7. [DOI] [PubMed] [Google Scholar]
- Yoshiyama M, Roppolo JR, de Groat WC. Effects of LY215490, a competitive α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptor antagonist, on the micturition reflex in the rat. J Pharmacol Exp Ther. 1997;280:894–904. [PubMed] [Google Scholar]
- Yoshiyama M, Roppolo JR, Rihmland J, Blastos B, de Groat WC. The effects of MK-801, an NMDA receptor antagonist, on the micturition reflex in the rat. Neurosci Lett. 1991;126:141–144. doi: 10.1016/0304-3940(91)90539-6. [DOI] [PubMed] [Google Scholar]
- Yoshiyama M, Roppolo JR, Thor KB, de Groat WC. Effects of LY274614, a competitive NMDA receptor antagonist, on the micturition reflex in the urethane-anaesthetized rat. Br J Pharmacol. 1993b;110:77–86. doi: 10.1111/j.1476-5381.1993.tb13774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]