Abstract
We analyzed clinical outcome of patients with an isolated central nervous system lymphoma (CNSL) relapse after systemic non-Hodgkin’s lymphoma (NHL). All 23 patients with an isolated secondary CNSL (SCNSL) treated at two institutions from 04/2003–12/2007 were included into this analysis. At cerebral relapse, 15/23 patients were treated with a regimen consisting of high-dose methotrexate (Bonn protocol). After a median follow-up of 6.5 months (range 1–68), 15/23 (65%) patients with SCNSL had relapsed or progressed. HD (high-dose)- methotrexate (MTX) chemotherapy according to the Bonn protocol is effective concerning response rates; however, overall survival of patients with SCNSL seems to be impaired in comparison to relapses in primary CNSL (PCNSL).
Keywords: lymphoma, neoplasia, chemotherapy, CNS, relapse, methotrexate
Abstract
Wir analysierten den klinischen Verlauf von Patienten mit isoliertem Zentralnervensystem (ZNS)-Rezidiv nach systemischem Non-Hogdkin-Lymphom (NHL). Alle 23 Patienten mit einem isolierten sekundären ZNS-Lymphom (SZNSL), die an unseren 2 Institutionen von 04/2003 bis 12/2007 behandelt wurden, wurden in diese Analyse eingeschlossen. Bei zerebralem Rezidiv wurden 15/23 Patienten nach dem Bonner Protokoll behandelt. Nach einem medianen Follow-up von 6,5 Monaten (zwischen 1–68) waren 15/23 (65%) mit SZNSL rezidiviert oder hatten einen Progress. Das Bonner Protokoll ist bezüglich Ansprechraten effektiv. Allerdings scheint das Gesamtüberleben der Patienten mit SZNSL gegenüber den Patienten mit primärem ZNS-Lymphom (PZNSL) eingeschränkt zu sein.
Introduction
A central nervous system relapse is a serious complication of aggressive lymphomas. Prognosis is generally regarded as poor and standard therapies of relapsed central nervous system lymphoma (CNSL) have not yet been established [1]. In contrast, therapeutic results have been much better in primary CNS lymphomas (PCNSL) with a regimen developed by our group, consisting of combined systemic and intraventricular chemotherapy with deferred radiotherapy and applied within a pilot/phase II study in 65 patients [2]. The overall response rate was 71% for the whole group, median time to treatment failure (TTF) was 21 months, and median overall survival 50 months. Results were significantly better in patients <60 years of age with a 86% overall response rate and a 75% survival fraction at five years.
CNS relapse is common in acute lymphatic leukemia (ALL) and Burkitt lymphoma (30–50%), less common (2–10%) in diffuse large B cell lymphoma (DLBCL). In indolent lymphoma CNS relapse is 0–4%, in mantle cell lymphoma 4–23% [3], [4].
In DLBCL CNS relapse occurs in median 5–12 months from original diagnosis. Leptomenigeal (33–100%) occurrence is more frequent than parenchymal (10–56%). In half of the cases of CNS relapse there is an additional systemic relapse. Median survival is only 2–6 months [3], [4], [5], [6], [7], [8], [9]. High-dose chemotherapy with stem cell transplantation leads to a median event free survival (EFS) of 0.4 to 1.5 years and an overall survival (OS) of 0.8 to 2.2 years [10]. A pilot study with MTX/Ifo has been recently performed [4].
Since data on secondary central nervous system lymphoma (SCNSL) is limited, an efficient therapy has not been established yet [1]. Therefore, we have retrospectively evaluated the clinical characteristics and outcome of SCNSL patients at our centers.
Patients and methods
Eligibility criteria, initial treatment and patient characteristics
All patients with SCNSL treated at Bonn University Hospital and Cologne University Hospital from 04/2003–12/2007 were included into the analysis. 23 patients could be identified.
60% of patients had a DLBCL, 30% a follicular lymphoma, 10% presented with other histologic subtypes.
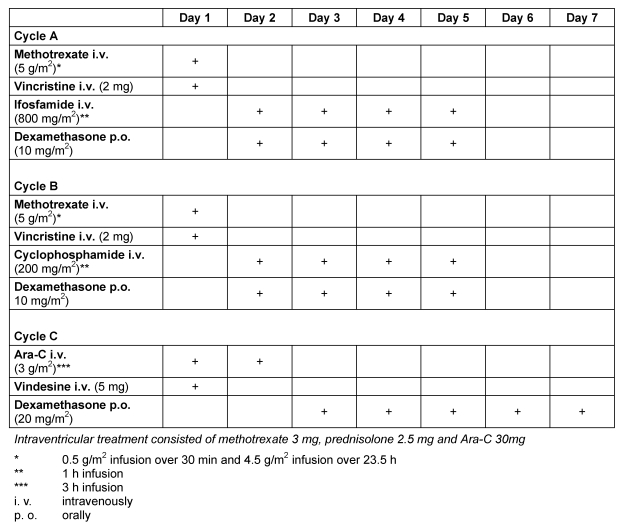
Patients with SCNSL had received 6–8 cycles of CHOP (Cyclophosphamide-Hydroxydaunorubicin-Oncovin-Prednisone) or CHOP-like combination chemotherapy as initial treatment. At cerebral relapse, 4/23 patients received an acute leukemia regimen (GMALL-B-ALL protocol), 3/23 patients received whole brain irradiation to a total of 40 Gy in 2 Gy fractions alone, one was treated with a chemotherapy according to the BEAM protocol and autologous bone marrow transplantation and 15/23 patients received a combined systemic and intraventricular polychemotherapy according to the Bonn protocol (Table 1 (Tab. 1)). Systemic high-dose MTX was administered as a 24 h infusion under vigorous hydration, urine alkalization and preconditions as well as dose modifications as described in [2]. Ifosfamide, cyclophosphamide, ARA-C, vinca-alkaloids and dexamethasone (cycles 3 to 6) were administered as described in [2]. In patients developing a peripheral neuropathy under treatment, application of vinca-alkaloids was omitted in subsequent cycles. Dexamethasone, if given postoperatively, was tapered and omitted during the first cycle.
Table 1. Modified Bonn Chemotherapy Protocol for Primary CNS Lymphoma .
Sequence of cycles: A1 (d. 1–5), B1 (d. 22–26), C1 (d. 43–49); A2 (d. 64–68), B2 (d. 85–89), C2 (d. 106–112). Cycles A to C (A1 to C1) are repeated once (A2 to C2).
18/23 patients (78%) were male with 13 /23 patients (57%) being older than 60 years. The median age at diagnosis was 60 years (range 41–77).
Evaluation of response and toxicity
Response criteria were used in line with recommendations of the International Primary Central Nervous System Lymphoma Collaborative Group (IPCG) consensus (for primary CNS lymphomas), and therefore all respective magnetic resonance imaging (MRI) studies were re-evaluated for this analysis according to these criteria [11]: Complete response (CR) was defined as disappearance of all enhancing lesions on MRI of the brain in patients being off steroids, unconfirmed complete response (CRu) was defined as a minimal residual enhancing lesion in the region of biopsy/hemorrhage without change or with involution during follow up, partial response (PR) as reduction of enhancing tumor volume by more than 50%, progressive disease (PD) as increase of tumor volume of more than 25% or occurrence of new lesions and stable disease (SD) as any other situation [12]. Treatment failure was defined as PD or SD, relapse after initial response, death or discontinuation of chemotherapy due to any cause. Acute toxicity was graded according to the WHO system [13].
Statistics
Students T-test was used to analyse for statistical significance. A p-value <0.05 was considered significant. For probability of survival calculations, the Kaplan-Meier method was used and was presented graphically by SPSS (SPSS Inc., Chicago, Illinois, U.S.A. Version 11.5.1).
Results
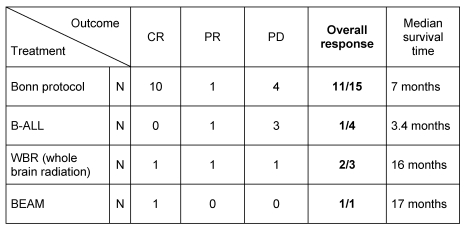
The response rates of the pooled cohort of patients with SCNSL were as follows: CR in 12 cases, PR in 3 cases, and PD in 8 cases (overall response: 65%). Interestingly, response rates were higher with the Bonn protocol as compared to the ALL protocol (Table 2 (Tab. 2)).
Table 2. Outcome in patients with recurrent or refractory SCNSL.
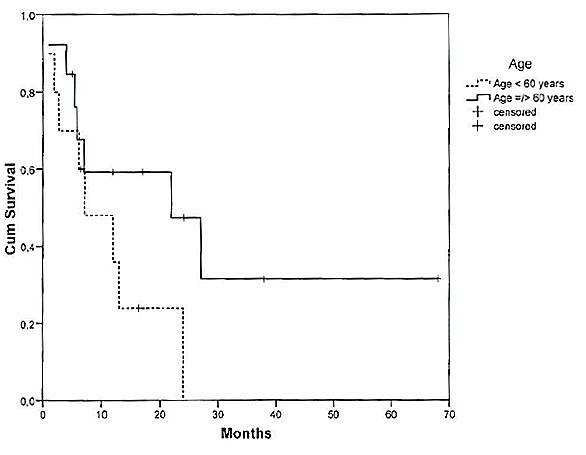
Median overall survival was 7 months (Figure 1 (Fig. 1)). The predictive value of age on the overall survival time was analysed. Among the patients who suffered from SCNSL, median OS averaged 7 months for patients <60 years (n=10) and 12 months for the group =60 years (n=13), respectively (Figure 2 (Fig. 2)).
Figure 1. Overall survival of patients with SCNSL.
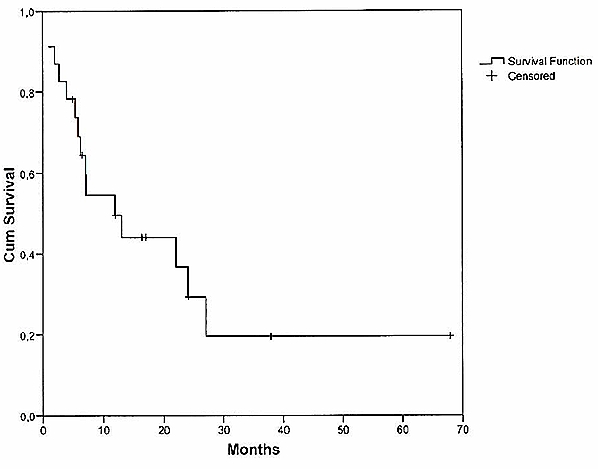
Figure 2. Overall survival of patients with SCNSL according to age of patients.
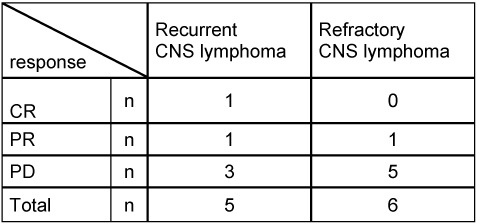
After a median follow-up of 6.5 months (range 1–68), 15/23 (65%) patients with SCNSL relapsed a second time (n=5) or progressed (n=10). The respective five patients with recurrence all showed a relapsed within the CNS. Median time to relapse was 20 months. Eleven cases were submitted to salvage therapy at time of second relapse or PD, whereas 4 patients with a PD did not receive another therapy because of an “early death” or a decline in general condition. For 4/5 patients, treatment at relapse consisted of radiotherapy and 1/5 patients received a polychemotherapy according to the Bonn protocol for PCNSL. Response to relapse treatment was in one case CR, in one case PR, and in 3 cases PD (Table 2 (Tab. 2), Table 3 (Tab. 3)). Treatment at second PD was radiotherapy alone (n=4), radiotherapy in combination with temozolomide (n=1), and BEAM chemotherapy (n=1). Response to treatment was in one case PR and in 5 cases PD (Table 3 (Tab. 3)). Patients without specific treatment (n=4) suffered from PD, as expected.
Table 3. Response rates in patients with recurrent or refractory SCNSL.
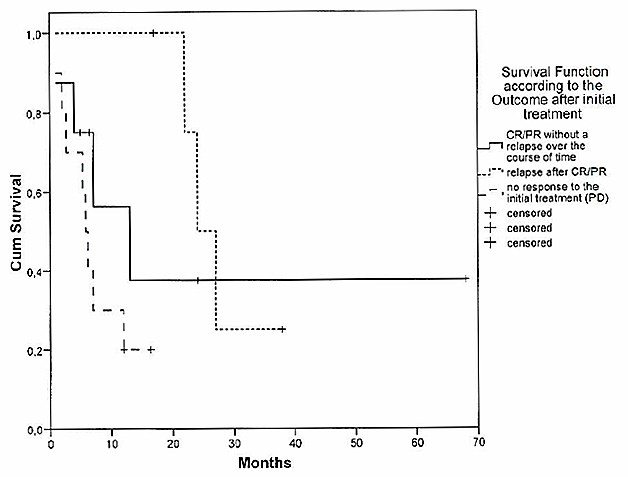
Prognosis of relapsed/progressive SCNSL was dismal. Median OS was short with only 7 months survival time after relapse. 15/23 patients (65%) with a relapse or a PD died within the observation period due to complications of CNS relapse (Figure 3 (Fig. 3)).
Figure 3. Survival function according to the outcome after initial treatment.
Discussion
CNS relapse in patients with aggressive lymphoma is uncommon. The cumulative incidence is only 2.8% and 2.2%, respectively [14], [15]. However, the prognosis of patients with CNS relapse is dismal.
In contrast to clinical trials in PCNSL, the rarity of secondary central nervous system lymphoma is one of the reasons why prospective trials on this entity are difficult to perform. Therefore, there is only limited data on treatment strategies for SCNSL. At present there is a clinical trial running in Germany.
However, since in our centers results with a combined systemic and intraventricular MTX-based chemotherapy has resulted in very promising results in PCNSL [2], we favored this regimen in the majority of patients with SCNSL that came to our institutions.
Our series includes 23 patients diagnosed and treated for SCNSL between 2003 and 2007. 15/23 patients (65%) received the same therapy at cerebral relapse as patients with PCNSL (data not shown), which consisted of high-dose methotrexate (MTX) in combination with vincristine, ifosfamide, prednisolone and cytarabine (Bonn protocol). Yet, compared to other studies on SCNSL that reported median survival times from 1–5 months [14], [15], [16] and response rates under 50%, our series confirms the view of different authors, that early introduction of HD-methotrexate is important when treating secondary CNS lymphoma as well as PCNSL and is associated with longer survival [1], [16].
The age of the patients at the time of diagnosis was predictive for the survival time among the patients with PCNSL. Patients younger than 60 years old had a significantly longer survival time than patients who were more than 60 years old (37 months/13 months) (p<0.001). In contrast to PCNSL, a statistically significant association between the age of the patients and long-term survival for the SCNSL-collective could not be verified. This is most likely due to the comparatively small group of only 23 evaluated patients with SCNSL in our series. However, most reported studies, with a collective of over 100 patients, recognized age as a predictor for longer survival times [1], [17]. Therefore, treatment strategies for SCNSL must be adjusted to the patient’s age, in a similar fashion to the previously modified treatments for PCNSL.
A median follow-up of 6.5 months (range 1–68) showed that 15 patients with a SCNSL (65%) relapsed after initial response or progressed within the CNS. Treatment at relapse/progression was heterogeneous dependent of the regimens applied at first relapse and due to the lack of approved and established treatment strategies for such clinical courses. 56% of the patients with second cerebral relapse achieved CR or PR after salvage therapy, while the response rate of patients with a refractory disease was only 14%, respectively. Relapse or PD of the lymphoma dims the high overall response rates after initial treatment for SCNSL in both groups and leads to the respectively poor prognosis. Therefore, new treatment strategies for PCNSL and SCNSL are needed to prevent the risk of relapse and refractory disease.
In conclusion, HD-MTX chemotherapy according to the Bonn Protocol is effective in SCNSL patients although the prognosis of patients with SCNSL seems to be worse as compared to PCNSL patients. Prognosis of relapsed and refractory CNSL patients is dismal. Median overall survival was only 7.1 months in patients with SCNSL. Therefore, new treatment strategies are urgently needed.
Notes
Competing interests
The authors declare that they have no competing interests.
Remark
Secondary CNS lymphoma data were presented in part at the ASH Meeting 2008 in San Francisco.
References
- 1.Van Besien K, Gisselbrecht C, Pfreundschuh M, Zucca E. Secondary lymphomas of the central nervous system: risk, prophylaxis and treatment. Leuk Lymphoma. 2008;49 Suppl 1:52–58. doi: 10.1080/10428190802311458. Available from: http://dx.doi.org/10.1080/10428190802311458. [DOI] [PubMed] [Google Scholar]
- 2.Pels H, Schmidt-Wolf IG, Glasmacher A, Schulz H, Engert A, Diehl V, Zellner A, Schackert G, Reichmann H, Kroschinsky F, Vogt-Schaden M, Egerer G, Bode U, Schaller C, Deckert M, Fimmers R, Helmstaedter C, Atasoy A, Klockgether T, Schlegel U. Primary central nervous system lymphoma: results of a pilot and phase II study of systemic and intraventricular chemotherapy with deferred radiotherapy. J Clin Oncol. 2003;21(24):4489–4495. doi: 10.1200/JCO.2003.04.056. Available from: http://dx.doi.org/10.1200/JCO.2003.04.056. [DOI] [PubMed] [Google Scholar]
- 3.Hollender A, Kvaloy S, Nome O, Skovlund E, Lote K, Holte H. Central nervous system involvement following diagnosis of non-Hodgkin's lymphoma: a risk model. Ann Oncol. 2002;13(7):1099–1107. doi: 10.1093/annonc/mdf175. Available from: http://dx.doi.org/10.1093/annonc/mdf175. [DOI] [PubMed] [Google Scholar]
- 4.Jahnke K, Thiel E, Martus P, Schwartz S, Korfel A. Retrospective study of prognostic factors in non-Hodgkin lymphoma secondarily involving the central nervous system. Ann Hematol. 2006;85(1):45–50. doi: 10.1007/s00277-005-1096-3. Available from: http://dx.doi.org/10.1007/s00277-005-1096-3. [DOI] [PubMed] [Google Scholar]
- 5.Keldsen N, Michalski W, Bentzen SM, Hansen KB, Thorling K. Risk factors for central nervous system involvement in non-Hodgkins-lymphoma--a multivariate analysis. Acta Oncol. 1996;35(6):703–708. doi: 10.3109/02841869609084002. Available from: http://dx.doi.org/10.3109/02841869609084002. [DOI] [PubMed] [Google Scholar]
- 6.Bollen EL, Brouwer RE, Hamers S, Hermans J, Kluin M, Sankatsing SU, A-Tjak RV, Charvat MV, Kluin-Nelemans JC. Central nervous system relapse in non-Hodgkin lymphoma. A single-center study of 532 patients. Arch Neurol. 1997;54(7):854–859. doi: 10.1001/archneur.1997.00550190044013. [DOI] [PubMed] [Google Scholar]
- 7.Haioun C, Besson C, Lepage E, Thieblemont C, Simon D, Rose C, Tilly H, Sonet A, Lederlin P, Attal M, Brière J, Reyes F. Incidence and risk factors of central nervous system relapse in histologically aggressive non-Hodgkin's lymphoma uniformly treated and receiving intrathecal central nervous system prophylaxis: a GELA study on 974 patients. Groupe d'Etudes des Lymphomes de l'Adulte. Ann Oncol. 2000;11(6):685–690. doi: 10.1023/A:1008394827806. Available from: http://dx.doi.org/10.1023/A:1008394827806. [DOI] [PubMed] [Google Scholar]
- 8.Tilly H, Lepage E, Coiffier B, Blanc M, Herbrecht R, Bosly A, Attal M, Fillet G, Guettier C, Molina TJ, Gisselbrecht C, Reyes F Groupe d'Etude des Lymphomes de l'Adulte. Intensive conventional chemotherapy (ACVBP regimen) compared with standard CHOP for poor-prognosis aggressive non-Hodgkin lymphoma. Blood. 2003;102(13):4284–4289. doi: 10.1182/blood-2003-02-0542. Available from: http://dx.doi.org/10.1182/blood-2003-02-0542. [DOI] [PubMed] [Google Scholar]
- 9.Feugier P, Virion JM, Tilly H, Haioun C, Marit G, Macro M, Bordessoule D, Recher C, Blanc M, Molina T, Lederlin P, Coiffier B. Incidence and risk factors for central nervous system occurrence in elderly patients with diffuse large-B-cell lymphoma: influence of rituximab. Ann Oncol. 2004;15(1):129–133. doi: 10.1093/annonc/mdh013. Available from: http://dx.doi.org/10.1093/annonc/mdh013. [DOI] [PubMed] [Google Scholar]
- 10.Van Besien K, Forman A, Champlin R. Central nervous system relapse of lymphoid malignancies in adults: the role of high-dose chemotherapy. Ann Oncol. 1997;8(6):515–524. doi: 10.1023/A:1008248315859. Available from: http://dx.doi.org/10.1023/A:1008248315859. [DOI] [PubMed] [Google Scholar]
- 11.Abrey LE, Batchelor TT, Ferreri AJ, Gospodarowicz M, Pulczynski EJ, Zucca E, Smith JR, Korfel A, Soussain C, DeAngelis LM, Neuwelt EA, O'Neill BP, Thiel E, Shenkier T, Graus F, van den Bent M, Seymour JF, Poortmans P, Armitage JO, Cavalli F International Primary CNS Lymphoma Collaborative Group. Report of an international workshop to standardize baseline evaluation and response criteria for primary CNS lymphoma. J Clin Oncol. 2005;23(22):5034–5043. doi: 10.1200/JCO.2005.13.524. Available from: http://dx.doi.org/10.1200/JCO.2005.13.524. [DOI] [PubMed] [Google Scholar]
- 12.Macdonald DR, Cascino TL, Schold SC, Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8(7):1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization. WHO Handbook for Reporting results of Cancer Treatment. Geneva, Switzerland: World Health Organization; 1979. pp. 14–21. (WHO offset publication; no. 48). Available from: http://whqlibdoc.who.int/offset/WHO_OFFSET_48.pdf. [Google Scholar]
- 14.Arkenau HT, Chong G, Cunningham D, Watkins D, Agarwal R, Sirohi B, Trumper M, Norman A, Wotherspoon A, Horwich A. The role of intrathecal chemotherapy prophylaxis in patients with diffuse large B-cell lymphoma. Ann Oncol. 2007;18(3):541–545. doi: 10.1093/annonc/mdl434. Available from: http://dx.doi.org/10.1093/annonc/mdl434. [DOI] [PubMed] [Google Scholar]
- 15.Boehme V, Zeynalova S, Kloess M, Loeffler M, Kaiser U, Pfreundschuh M, Schmitz N German High-Grade Non-Hodgkin's Lymphoma Study Group (DSHNHL) Incidence and risk factors of central nervous system recurrence in aggressive lymphoma--a survey of 1693 patients treated in protocols of the German High-Grade Non-Hodgkin's Lymphoma Study Group (DSHNHL) Ann Oncol. 2007;18(1):149–157. doi: 10.1093/annonc/mdl327. Available from: http://dx.doi.org/10.1093/annonc/mdl327. [DOI] [PubMed] [Google Scholar]
- 16.Doolittle ND, Abrey LE, Shenkier TN, Tali S, Bromberg JE, Neuwelt EA, Soussain C, Jahnke K, Johnston P, Illerhaus G, Schiff D, Batchelor T, Montoto S, Kraemer DF, Zucca E. Brain parenchyma involvement as isolated central nervous system relapse of systemic non-Hodgkin lymphoma: an International Primary CNS Lymphoma Collaborative Group report. Blood. 2008;111(3):1085–1093. doi: 10.1182/blood-2007-07-101402. Available from: http://dx.doi.org/10.1182/blood-2007-07-101402. [DOI] [PubMed] [Google Scholar]
- 17.Bernstein SH, Unger JM, Leblanc M, Friedberg J, Miller TP, Fisher RI. Natural history of CNS relapse in patients with aggressive non-Hodgkin's lymphoma: a 20-year follow-up analysis of SWOG 8516 -- the Southwest Oncology Group. J Clin Oncol. 2009;27(1):114–119. doi: 10.1200/JCO.2008.16.8021. Available from: http://dx.doi.org/10.1200/JCO.2008.16.8021. [DOI] [PMC free article] [PubMed] [Google Scholar]