Abstract
Rodent models of fatty liver disease are essential research tools that provide a window into disease pathogenesis and a testing ground for prevention and treatment. Models come in many varieties involving dietary and genetic manipulations, and sometimes both. High-energy diets that induce obesity do not uniformly cause fatty liver disease; this has prompted close scrutiny of specific macronutrients and nutrient combinations to determine which have the greatest potential for hepatotoxicity. At the same time, diets that do not cause obesity or the metabolic syndrome but do cause severe steatohepatitis have been exploited to study factors important to progressive liver injury, including cell death, oxidative stress, and immune activation. Rodents with a genetic predisposition to overeating offer yet another model in which to explore the evolution of fatty liver disease. In some animals that overeat, steatohepatitis can develop even without resorting to a high-energy diet. Importantly, these models and others have been used to document that aerobic exercise can prevent or reduce fatty liver disease. This review focuses primarily on lessons learned about steatohepatitis from manipulations of diet and eating behavior. Numerous additional insights about hepatic lipid metabolism, which have been gained from genetically engineered mice, are also mentioned. Antioxid. Redox Signal. 15, 535–550.
Introduction
Animal models of human diseases are important resources because they can be used to monitor complex interactions among cells and tissues during the evolution of an illness. The ultimate value of any animal model depends on its reproducibility and the degree to which it mimics disease in human beings. Most animal models of disease fall short of a faithful replication of their human counterparts; nevertheless, they are instructive because their unique features stimulate hypotheses about disease pathogenesis. In the case of fatty liver disease, rodents have been widely used as animal models. This is due in part to their ready availability and in part to the investigative advantage provided by genetically engineered strains. Although large animals are being used on a limited basis to study fatty liver disease, they will not be discussed here. A major objective of fatty liver disease research in rodents is to find the right combination of diet, exercise, and genetic background that will cause a syndrome identical to human nonalcoholic fatty liver disease (NAFLD). In advance of this goal, there are still lessons to be learned from rodent models that reproduce some, if not all, attributes of the human disease. One of the great benefits of animal models is that they are rigorously controlled. Scrutinizing the unique features of animal models of fatty liver disease that lead to diverse outcomes will likely uncover information critical to disease pathogenesis.
Using High-Energy Diets to Induce NAFLD: The Importance of Diet Composition
Interest in the pathogenesis of obesity and the metabolic syndrome has led to the marketing of scores of high-energy diets. While these diets all have excess calories, they are not standardized in macronutrient content or composition. Researchers studying fatty liver disease have not undertaken a concerted effort to employ a single dietary regimen as an experimental model; consequently, it is important to keep the diet in mind when evaluating animal studies in which liver disease is an outcome variable. High-energy diets can be logically grouped by their relative carbohydrate (CHO) and fat content (Table 1). On one end of the spectrum are diets very high in sucrose but very low in fat (Table 1, VLF). These diets rapidly induce fatty liver (72) but their ability to cause liver injury is unproven. High-sucrose diets that contain a bit more fat (Table 1, LF) also induce hepatic steatosis; by themselves, however, they do not cause hepatic inflammation or raise serum alanine aminotransferase (ALT) (28, 72). On the other end of the nutrient spectrum are ketogenic diets, which are very high in fat and contain almost no carbohydrate (Table 1, KG). These diets modestly raise hepatic triglyceride content but do not raise ALT outside the normal range (44). A large intermediate group comprises “high-fat” and “very high-fat” diets with fat content ranging from 35% to 70% of total calories (Table 1, HF, VHF). These diets typically provoke fatty liver and in some instances, steatohepatitis. Viewing these diets as a group, a trend emerges. Diets on either end of the spectrum cause steatosis but tend not to cause steatohepatitis. Diets in the middle, which contain a mix of nutrients, seem to have the greatest potential to cause liver injury (Fig. 1A). Still, there are important exceptions to the rule, as discussed below.
Table 1.
Dietary Formulas Used in Studies of Experimental Fatty Liver
| Formula category | Fat cal (%) | CHO cal (%) | Fat source (%) | CHO source (%) | Duration (wk) | Formula # | Ref. |
|---|---|---|---|---|---|---|---|
| Chow | 10 | 78 | Soybean oil (100) | Sucrose:starch:maltodextrin (14:65:21) | N/A | AIN-93M | American Institute of Nutrition |
| VLF | 2.6 | 77 | Corn oil (100) | Sucrose:starch:maltodextrin (72:6:22) | 2 | TD03045 | (72) |
| LF | 12 | 70 | Corn oil (100) | Sucrose:starch (96:4) | 8 | TD02366 | (28) |
| 37 | 39 | Corn oil (100) | Dextrose (100) | 9 | Custom liquid | (19) | |
| 42 | 47 | Milkfat:cholesterol (99:1) | Sucrose:starch (70:30) | 18–26 | TD88137c | (73, 80) | |
| HF | 45 | 35 | Lard:soybean oil (88:12) | Sucrose:starch:maltodextrin (50:21:29) | 19 | RD12451 | (44, 88) |
| 45 | 37 | Lard (100) | Sucrose:starch:maltodextrin (48:19:33) | 16 | TD03350 | (118) | |
| 59 | 27 | Lard (100) | Sucrose (100) | 20 | F3282 | (34, 86, 120) | |
| VHF | 60 | 20 | Lard:soybean oil (91:9) | Sucrose:maltodextrin (65:35) | 18 | RD12492 | (72) |
| 70 | 11 | Corn oil (100) | Glucose:maltodextrin (75:25) | 9 | Custom liquid | (7) | |
| KG | 91 | 0.4 | Lard:butter:veg oil (48:20:11) | Starch (100) | 9 | F3666 | (44) |
| 45 | 37 | HVO:soybean oil (96:4)a | Sucrose:starch:maltodextrin (50:18:32) | 16 | TD06303 | (118) | |
| Trans-fat | 60 | 21 | HVO:coconut oil (73:27)b | Sucrose:starch (62:38) | 14 | TD97070 | (83) |
30% of fat is trans-fat.
24% of fat is trans-fat.
“Western diet.”
Table shows an extensive yet incomplete list of dietary formulas that have been used in rodents to model obesity, metabolic syndrome, and fatty liver. Formulas are segregated along a spectrum of carbohydrate and fat content; standard chow is listed as a reference. Diets that incorporate trans-fat are also listed. HF, high fat; HVO, hydrogenated vegetable oil (trans-fat); KG, ketogenic (all fat); LF, low fat; VHF, very high fat; VLF, very low fat. Commercial formulas are made by the following vendors: F, Bio-Serv; RD, Research Diets; TD, Harlan Teklad. The table highlights the wide variation in macronutrient content and composition among individual formulas.
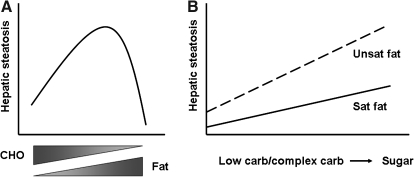
FIG. 1.
Impact of diet content and composition on hepatic steatosis. Schematized diagrams demonstrate the influence of diet content (A) and composition (B) on the evolution of hepatic steatosis in experimental animals. (A) Diets enriched to the extreme in either carbohydrate or fat tend not to provoke as much fatty liver as diets that contain a mixture of carbohydrate and fat. The outcome also depends upon the composition of carbohydrate and fat. (B) Diets that contain unsaturated fat tend to provoke hepatic steatosis even if paired with low amounts of carbohydrate or complex carbohydrate (e.g., starch). The degree of steatosis increases if unsaturated fat is paired with simple sugar. Diets that contain saturated fat cause relatively little steatosis when paired with low carbohydrate or complex carbohydrate. Steatosis increases when saturated fat is paired with sugar, but the slope of the increase may be smaller than that for unsaturated fat. It should be noted that most experimental formulas contain a mixture of carbohydrates and fats.
A popular mixed formula is the “Western diet” (TD88137) enriched in saturated fat, cholesterol, and sucrose (30%). This diet induces obesity, insulin resistance, and hepatic steatosis, along with hepatic activation of cJun N-terminal kinase (JNK) and nuclear factor-κB (NF-κB) (73, 80). A similar diet (RD12451) induces hepatic steatosis and upregulates hepatic tumor necrosis factor (TNF) and interleukin-6 (IL-6) expression (88); the degree of actual liver injury caused by these diets, however, has not been rigorously studied. Svegliati–Baroni and colleagues (117) carefully evaluated liver injury in rats fed a high-saturated fat diet (58% fat, 24% CHO) for 1–6 months. They found evidence of progressive biochemical and histologic liver injury, culminating in mild fibrosis at the 6-month time point. This was in association with obesity, hyperglycemia, elevated serum TNF, and decreased serum adiponectin, similar to human beings with nonalcoholic steatohepatitis (NASH) and the metabolic syndrome. Singh et al. (111) reported that a similar diet (RD12492) also caused steatohepatitis in mice after 4 months. The murine disease was characterized by steatosis, liver cell death, ALT elevation, and mild histologic inflammation in conjunction with marked activation of JNK.
The findings of Svegliati–Baroni and Singh are in sharp contrast to the experience of two other groups who fed rats high-saturated-fat diets for 4 months and achieved little in the way of liver injury. Tetri and colleagues (118) found that a lard-based diet (TD03350) provoked obesity and insulin resistance but caused only mild hepatic steatosis without any elevation in serum ALT. Likewise, Romestaing et al. (101) reported that high-saturated fat diets comprising butter or coconut oil as opposed to lard (67% fat calories) induced little or no liver disease. Why the lard diet failed to induce more steatosis is uncertain, given the similarity of the diet to one that caused significant steatosis in mice (88). The lack of hepatic steatosis in rodents fed butter or coconut oil, however, could be due in part to the fact that these fats contain small- and medium-chain fatty acids that utilize more energy for their metabolism than long-chain fatty acids. Although intriguing, this cannot be the sole explanation, because diets prepared exclusively with long-chain saturated fat also cause less hepatic steatosis than those prepared exclusively with unsaturated fat (105). In fact, mice fed diets with nothing but long-chain saturated fat as a fat source experience less weight gain, less insulin resistance, and less hepatic steatosis than mice fed identical formulas containing pure monounsaturated fat (105) (Fig. 2). The reason is that saturated fat stimulates fatty acid oxidation in the liver, driven by PPARγ co-activator-1α (PGC-1α) and peroxisome proliferator activated receptor-α (PPARα) (13, 105).
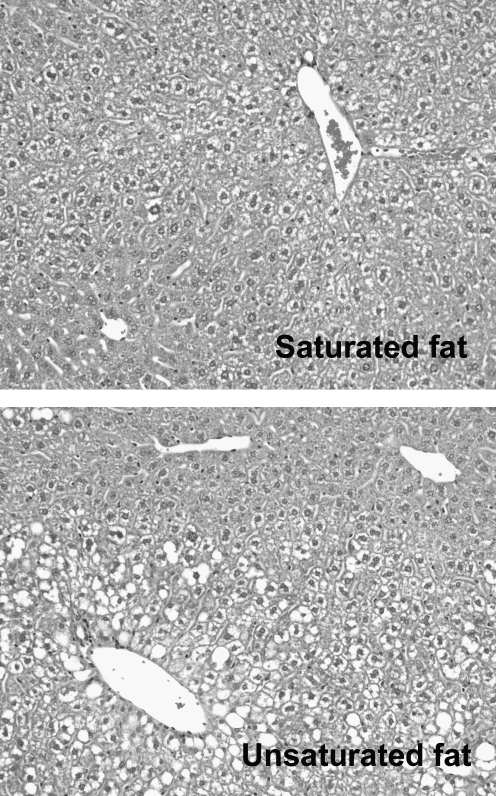
FIG. 2.
Saturated fat induces less steatosis than unsaturated fat. Photomicrographs depict liver histology in mice fed diets identical in nutrient composition except for fat. One diet was formulated with pure long-chain saturated fat (palmitate); the other was formulated with pure unsaturated fat (oleate). Diets were administered for 3 weeks.
Although saturated fat induces fatty acid oxidation in the liver, it also stimulates hepatic lipogenesis (64, 105). Therefore, when saturated fat is consumed together with sugar, any potential benefit of the nutrient-induced increase in fatty acid oxidation is likely to be offset by enhanced de novo lipid synthesis. On the other hand, when saturated fat is consumed without sugar, its pro-oxidative effects go unopposed. Indeed, this is the situation created in the experiment by Romestaing et al. (101), whose diet was highly enriched in saturated fat but contained no simple sugar (only complex carbohydrate). In the absence of dietary sugar, even a 70% saturated fat diet caused minimal hepatic steatosis.
These findings are reminiscent of observations made in experimental models of alcoholic liver disease, in which ethanol-containing diets prepared with saturated fat cause less liver injury than those prepared with unsaturated fat (79, 102, 132). Ethanol-containing diets are very low in carbohydrate (12%) due to the substitution of ethanol for carbohydrate. Furthermore, ethanol diets are typically formulated with maltodextrin, a starch-like polymer of glucose. Therefore, using saturated fat in an ethanol diet should provoke more fatty acid oxidation than using an unsaturated fat such as corn oil. This is indeed the case: ethanol diets containing saturated fat cause greater hepatic fatty acid oxidation than those containing unsaturated fat, in association with the induction of PGC-1α and AMP-activated protein kinase (AMPK) (132). The end result is the prevention of alcoholic steatosis and reduced release of ALT into the circulation. In experimental alcoholic liver disease, the beneficial effect of dietary saturated fat has been attributed to upregulation of adiponectin, which drives fatty acid oxidation in hepatocytes and thus counteracts the tendency for ethanol to promote steatosis by upregulating the lipogenic transcription factor sterol regulatory element binding protein-1 (SREBP1) (134). At present, the mechanism by which saturated fat induces adiponectin is unclear. Also uncertain is whether adiponectin is absolutely required for the saturated fat effect, or whether saturated fat could still be effective in its absence.
Taken together, these experimental observations suggest that dietary saturated fat, if consumed in the absence of simple sugar, is not a strong inducer of fatty liver disease (Fig. 1B). Of course, in most instances, saturated fat is consumed together with at least some simple sugar, and thus diets that incorporate both macronutrients are likely to be most representative of the situation in human beings. Still, from an experimental point of view, it is of great interest to study the influence of custom diets with defined macronutrient composition on fatty liver disease. In this fashion we can discern the specific effects of individual macronutrients on fatty liver disease, alone and in combination.
If dietary saturated fat does not by itself promote hepatic steatosis, then what is the experience with unsaturated fat? Unsaturated fat, if incorporated into a diet in an amount sufficient to provide 35% or more of total calories, tends to induce hepatic steatosis even if paired exclusively with complex carbohydrate (62, 69, 125). Such diets cause hepatic mitochondrial abnormalities and oxidant stress, but in limited experience, have not induced ER stress or raised serum ALT levels (62, 69, 125). In contrast, when high-unsaturated fat diets are paired with at least some simple sugar, more serious liver injury ensues. This is particularly true when rodents are intentionally overfed high-unsaturated fat diets by intragastric infusion. Indeed, using an intragastric overfeeding approach with a high-unsaturated fat formula containing either glucose or glucose-maltodextrin, Deng and colleagues (19) and Baumgardner et al. (7) successfully produced hepatic steatosis, liver cell death and ALT release, hepatic inflammation, and even hepatic fibrosis. Notably, high-unsaturated fat formulas are uncommonly used outside of liquid feeding paradigms because the relatively low melting points of unsaturated fats make them suboptimal for compounding into pellets. In addition, the expertise and care required to accomplish intragastric overfeeding is beyond the capability of many research groups.
Aside from carbohydrate and fat in general, specific macronutrients are now being scrutinized for their ability to promote fatty liver disease. Diets containing 15% or more of calories as trans-fats, for example, cause more hepatic steatosis and ALT elevation than those lacking trans-fats (49, 118). One group found that a trans-fat-enriched diet provoked several features of steatohepatitis in mice, including hepatocyte ballooning, neutrophil infiltration, and induction of collagen type 1 mRNA (81). Others, though, have failed to see hepatic inflammation or fibrosis in response to a trans-fat diet, even after 6 months of continuous feeding (49). Supplementing diets with glucose- or fructose-sweetened water has also been tested for its ability to accentuate steatohepatitis in experimental animals. Bergheim and colleagues (8) found that supplementing a chow diet with fructose, but not glucose, for 8 weeks promoted hepatic steatosis in association with portal endotoxemia, hepatic induction of TNF, and mild neutrophilic infiltration of the liver. Despite this, ALT levels remained normal. Likewise, supplementation of a trans-fat diet with a fructose-containing gel increased overall caloric intake but did not significantly worsen liver injury (118). Our group has recently tested the impact of fructose on liver injury by comparing it directly to glucose as the sole carbohydrate in a methionine-choline-deficient diet (see below). In this setting, fructose stimulates more hepatic lipogenesis than glucose and causes significantly more liver cell death than glucose (90).
Is there a unifying message that can be crafted from this plethora of information on dietary nutrients? Some conclusions appear valid. One is that sugar amplifies hepatic steatosis, regardless of the fat composition of the diet. A second is that fructose appears more toxic to the liver than other dietary sugars, at least in limited studies. A third is that all types of dietary fat contribute to hepatic steatosis, although perhaps not to an equal degree. Saturated fat may have the weakest impact on steatosis, with unsaturated and trans-fats having a greater influence. It must be emphasized that these features predict steatosis, which often, but not always, goes hand-in-hand with steatohepatitis (40). Further study is necessary to assess whether specific nutrient mixtures cause liver injury out of proportion to their ability to induce hepatic steatosis.
Pathophysiologic Insights from High-Energy Feeding
Although not all high-energy diets cause significant liver injury, those that do can be exploited to identify diet-induced events that are critical to disease pathogenesis. For example, high-energy diets that cause fatty liver disease typically do so in association with obesity and systemic insulin resistance (17, 83, 111, 117) and are accompanied by the activation of intrahepatic stress signaling cascades (111). The role of these factors is been explored using a combination of pharmacologic and genetic approaches.
Obesity and systemic insulin resistance
Several recent studies have demonstrated that obesity is associated with a pro-inflammatory state in adipose tissue, and that this state of heightened inflammation can either promote or enhance hepatic steatosis. Inflamed adipose tissue appears to influence hepatic steatosis through the release of pro-inflammatory cytokines such as monocyte chemoattractant protein-1 (MCP-1) and TNF. Of the two cytokines, MCP-1 appears particularly important; mice deficient in either MCP-1 or its receptor are resistant to diet-induced hepatic steatosis (43, 126). Inflamed adipose tissue also produces adiponectin, but at lower levels than normal adipose tissue (37). This relative deficiency of adiponectin in obesity may contribute to hepatic steatosis by reducing fatty acid oxidation (119) or enhancing lipogenesis in the liver (128, 140). In support of this concept, adiponectin-deficient mice develop worse liver disease than wild-type mice when fed a high-energy diet (5, 140). In addition, adiponectin treatment (128) or overexpression (45) limits fatty liver disease due to excessive dietary intake. To address the role of peripheral insulin resistance in promoting fatty liver disease, rodents fed high-energy diets have been treated with insulin-sensitizing agents to assess their impact on hepatic outcome. In one study, mice fed a high-energy diet for 23 weeks and then treated with pioglitazone for an additional 8 weeks exhibited normal insulin sensitivity at the end of the study but showed no improvement in hepatic steatosis or serum ALT (17). By contrast, mice fed a high-energy diet for 8 weeks and then treated with an adenovirus expressing exendin-4 (a long-acting agonist of glucagon-like peptide receptor-1) while continuing the diet for 15 more weeks showed improvements in both glucose utilization and hepatic steatosis (106). Anti-inflammatory drugs have also been used to reduce insulin resistance in the setting of high-energy feeding, based on evidence that insulin resistance is the consequence of a diet-induced pro-inflammatory state (11). Unfortunately, the impact of these agents on hepatic steatosis was not directly assessed. Overall, experiments with insulin sensitizers show mixed results in their ability to reduce diet-induced hepatic steatosis.
Endoplasmic reticulum stress
High-energy feeding causes endoplasmic reticulum (ER) stress in the liver (86), and ER stress in turn stimulates hepatic steatosis (42, 57, 103). The role of ER stress in the pathogenesis of fatty liver disease is the subject of another article in this Forum (30a), and thus will not be covered in detail here. Briefly, ER stress activates several intracellular signaling cascades that culminate in hepatic lipogenesis (57, 103). Interventions that block ER stress, in turn, reduce diet-induced hepatic lipid accumulation (42, 85, 115). From the point of view of liver disease, it is important to note that ER stress clearly influences hepatic lipid homeostasis and may also provoke hepatocellular injury (125). In the setting of ER stress, liver injury can be mediated by molecules such as JNK and C/EBP homologous protein (CHOP). JNK undoubtedly contributes to cell death in the setting of diet-induced fatty liver (111), but the role of CHOP is less clear (87). Another means by which ER stress could induce liver injury is through induction of the ER-bound transcription factor, CREBH (137). CREBH activates the transcription of two pro-inflammatory proteins, serum amyloid P and C-reactive protein, which may play a role in the inflammation seen in fatty livers.
Using MCD or CD Diets to Induce NAFLD: Reproducible Liver Injury
Certain dietary manipulations cause rapid-onset steatohepatitis, which makes them attractive for investigative studies. This is true of diets lacking methionine and/or choline, which are critical precursors of phosphatidylcholine (Fig. 3). Phosphatidylcholine is essential for the assembly of very low density lipoprotein (VLDL) particles; therefore, animals deficient in methionine or choline develop hepatic steatosis because they cannot efficiently secrete hepatic triglyceride. Methionine and choline deprivation also induces the uptake of fatty acids by the liver (96). Taken together, these dual consequences (increased fatty acid uptake and impaired triglyceride disposal) set the stage for marked hepatic steatosis. Many investigators use a methionine-choline-deficient (MCD) diet, which is completely devoid of both nutrients. Recently there has been an increase in the use of diets deficient in choline alone (48). Choline-deficient (CD) formulas also cause steatohepatitis, but the time-course of disease is protracted in comparison to that caused by MCD formulas. CD diets are also known for their ability to provoke hepatic fibrosis and hepatocellular carcinoma (136). Importantly, many commercial CD and MCD formulas not only lack methionine and choline but also contain excess sucrose and fat. These lipogenic substrates accelerate disease to the point that steatohepatitis can develop within 2 weeks (16).
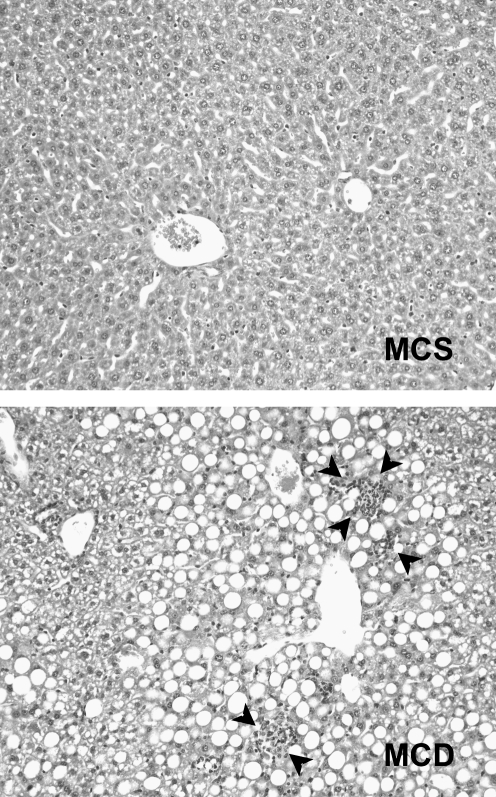
FIG. 3.
Steatohepatitis induced by an MCD diet. Photomicrographs illustrate liver histology in mice fed a control diet (methionine-choline-sufficient, MCS) and a nutrient-matched MCD diet for 3 weeks. Mice fed the MCS diet have normal histology, whereas mice fed the MCD diet have steatosis and foci of hepatic inflammation.
Importantly, unlike high-calorie diets, MCD and CD diets do not cause fatty liver disease as part of the metabolic syndrome. Instead, mice fed MCD and CD formulas remain insulin-sensitive in peripheral tissues and normoglycemic when compared to nutritionally matched controls (48, 97), although they exhibit hepatic insulin resistance (108). MCD feeding also causes weight loss due to muscle wasting and a diet-induced hypermetabolic state (100); CD diets do not cause weight loss (48). Although the inability of MCD and CD diets to induce the metabolic syndrome prevents them from being touted as models of human fatty liver disease, their ability to cause severe and progressive steatohepatitis suits them well to mechanistic studies of fat-related liver injury. Accordingly, MCD and CD diets have been extensively utilized to address the pathophysiology of fatty liver disease.
Pathophysiologic Insights from CD and MCD Feeding
Involvement of JNK1 in disease pathogenesis
Several highlights have emerged from recent studies of MCD or CD-fed rodents. One is that the activation of JNK1 in the liver is critical to the development and progression of fatty liver disease. Global knockout of JNK1 ameliorates many of the features of MCD-mediated steatohepatitis, including hepatocellular apoptosis, ALT release, and hepatic inflammation (107); importantly, JNK1 deficiency also reduces MCD-mediated hepatic steatosis, implying that hepatic lipid accumulation in this model is more complex than the net result of increased fatty acid uptake and reduced triglyceride secretion. Curiously, JNK1−/− mice fed a CD rather than MCD diet do not have reduced hepatic steatosis, but all other features of fatty liver disease are moderately suppressed including—in this more severe model—hepatic fibrosis.(48) Although fatty acids activate JNK1 in hepatocytes (66), Kupffer cell-derived JNK1 appears important to the pathogenesis of fatty liver disease. This is based on experiments in which chimeric mice with wild-type hepatocytes but JNK1-deficient Kupffer cells developed less liver disease than wild-type mice in response to a CD diet (48).
Impact of oxidant stress on disease outcome
Second, recent studies using the MCD diet to induce steatohepatitis have shed light on the relationship between oxidative stress and fatty liver disease. Three independent groups addressed this question by feeding an MCD diet to mice lacking the master antioxidant defense regulator nuclear factor E2-related factor-2 (nrf2) (16, 116, 139). Nrf2 controls the induction of several compounds involved in antioxidant defense, including heme oxygenase-1 (HO-1), glutamate-cysteine ligase (gclc), and NAD(P)H dehydrogenase quinone 1 (nqo1) (47); consequently, nrf2-deficient mice are unable to mount an appropriate antioxidant defense when needed. When nrf2-deficient mice were fed MCD formulas, they developed no more hepatic steatosis than wild-type mice. They did, however, display exaggerated oxidant stress in the form of increased tissue levels of oxidized glutathione, malondialdehyde, and 4-hydroxynonenal (16, 116). These changes were accompanied by a significant increase in hepatic inflammation and hepatic fibrosis. Interestingly, two of the three groups found that excess oxidant stress caused by nrf2 deficiency did not result in an increase in serum ALT (16, 139). Perhaps more importantly, one group experimentally reversed MCD-mediated oxidant stress by knocking out Kelch-like-ECH-associated protein 1 (keap1), an inhibitor of nrf2, but despite this, achieved no improvement in serum ALT (139). These findings suggest that oxidant stress in a fatty liver has a more pronounced effect on hepatic inflammation and fibrosis than it does on hepatocellular injury. This concept has some support in the form of earlier work in which elimination of oxidant stress from the livers of MCD-fed mice by altering the fat composition of the diet improved hepatic inflammation but had no effect on hepatocellular injury (58). Still, such a theory remains to be substantiated. To date, studies that have employed pharmacologic agents to inhibit oxidant stress in MCD-fed mice have demonstrated improvements in hepatic inflammation and fibrosis, without clearly distinguishing the effect from an improvement in hepatocellular injury (89, 124). Inhibiting oxidant stress by knocking out thioredoxin binding protein-2 (tbp2), however, improves hepatic inflammation and fibrosis and at the same time significantly lowers serum ALT (1). In addition, treating MCD-fed mice with hemin to induce HO-1 reduces hepatic lipid peroxidation by 50% and reduces serum ALT by 40% (135).
Factors underlying hepatocellular injury
Third, the MCD model has placed important focus on hepatocyte lipotoxicity as a component of fatty liver disease. Indeed, MCD feeding is unique in that it causes marked elevation of serum ALT in conjunction with histologic evidence of steatohepatitis (55, 58, 104). Although some of the increase in serum ALT may be due to a diet-related induction of enzyme expression in hepatocytes (65), there is independent proof that MCD feeding causes apoptotic and necrotic hepatocyte death (27, 58, 127, 130). Cell death in the MCD model of steatohepatitis appears mediated by free fatty acids or acylglycerol intermediates. This is based on evidence that inhibiting their incorporation into triglycerides markedly increases hepatocellular injury (130). Furthermore, cell death (measured by terminal deoxynucleotidyl transferase dUTP nick end labeling) is enhanced specifically when MCD-fed mice are treated with agents that inhibit diacylglycerol transferase-2 (DGAT2) rather than DGAT1 (129, 130). This suggests that fatty acids arising from de novo lipogenesis, which are preferentially handled by DGAT2 (122), are more hepatotoxic than those reaching the liver from the circulation. A similar increase in liver cell death occurs when an MCD formula is fed to mice lacking the enzyme stearoyl CoA desaturase-1 (SCD1) (61). SCD1 converts toxic long-chain saturated fatty acids to nontoxic monounsaturated fatty acids (30) and has recently been shown to have preferential activity toward fatty acids produced in the liver from dietary sugar (72). Taken together, these findings suggest that saturated fatty acids derived from de novo lipogenesis are important mediators of hepatocellular death in fatty liver disease. Recent experiments have confirmed this by showing that MCD formulas lacking sugar fail to cause liver disease (91), whereas MCD formulas that incorporate pure fructose, a highly lipogenic substrate, into the MCD formula, enhance its ability to cause hepatocyte death (90) (Fig. 4). The fact that MCD-mediated steatohepatitis is dependent on de novo lipogenesis emphasizes the potential danger of this pathway in human beings. It should be noted, however, that MCD diets will tend to accentuate the adverse effect of de novo lipogenesis on the liver, because MCD feeding also suppresses SCD1 (100), presumably in an effort to preserve hepatic phospholipid (22).
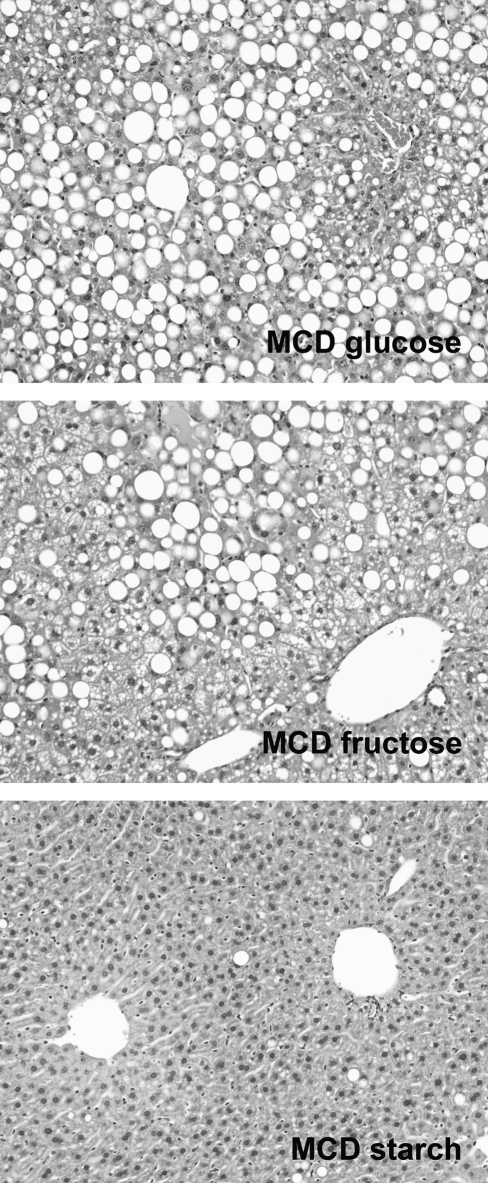
FIG. 4.
Dietary sugar is critical to the pathogenesis of steatohepatitis in the MCD model. Photomicrographs illustrate liver histology in mice fed MCD diets containing glucose, fructose, or starch as carbohydrate. Diets were administered for 3 weeks. Mice fed MCD with glucose developed macrovesicular steatosis and had a mean ALT value of 471 IU/L. Mice fed MCD with fructose had mixed macrovesicular and microvesicular steatosis and had a mean ALT value of 928 IU/L. Mice fed MCD with starch had minimal steatosis and had a mean ALT of 93 IU/L. Data from (90, 91).
Innate immune regulation of steatohepatitis
CD and MCD models of steatohepatitis have also been used to address the role of the innate immune system in disease pathogenesis. One of the first observations made in this regard was that MCD-fed animals display hepatic upregulation of Toll-like receptor-4 (TLR4) and its co-receptors CD14 and MDR2 (99). A causal role for TLR4 in MCD-mediated liver disease was demonstrated in experiments with TLR4 mutant mice, in which diet-induced hepatic inflammation and hepatocellular injury were significantly reduced (99). Similar results were obtained with Kupffer cell depletion, thus identifying Kupffer cells as an important TLR4-expressing population. Recent attention has focused on the dual intracellular signaling pathways that can be activated by engagement of TLR4. It can signal through myeloid differentiation primary response gene 88 (MyD88), which leads to the activation of NF-κB, or alternatively through TIR-domain-containing adapter-inducing interferon-β (TRIF), which leads to the activation of type I interferons. MCD feeding induces MyD88 expression in the liver (121) as does excess dietary fructose (112). Genetic deletion of MyD88 ameliorates steatohepatitis and liver fibrosis in mice fed a CD diet, assigning a causal role to the MyD88 signaling pathway (71). Interestingly, in experimental alcoholic steatohepatitis, it is the MyD88-independent pathway that appears critical in the pathogenesis of liver injury (36). This represents a unique distinction between alcoholic and nonalcoholic steatohepatitis.
Other TLRs besides TLR4 are emerging as participants in the pathogenesis of fatty liver disease. Deng et al. (20) demonstrated that activation of TLR7 on immature hepatic myeloid cells can enhance steatosis and promote ALT release in mice fed a high-fat diet. Importantly, this pathway was not active spontaneously in high-fat-fed mice (which did not exhibit baseline liver injury); instead, it required exogenous administration of a TLR7 agonist. New evidence also implicates TLR9 as an important contributor to steatohepatitis. TLR9 is an important receptor for DNA from bacteria or dying cells; in the latter case, its activation serves as a means to amplify tissue injury once a disease process has begun (76). Activation of TLR9 on Kupffer cells stimulates the secretion of IL-1β. IL-1β, in turn, promotes hepatic steatosis, hepatocyte death, and liver fibrosis (71). These effects, which require MyD88, suggest the existence of an auto-amplification mechanism that perpetuates steatohepatitis.
An important, TLR-independent aspect of immune regulation that has been investigated in CD as well as non-CD models of fatty liver disease is steatosis-related alteration of hepatic natural killer T (NKT) cells. These regulatory T cells were initially found to be reduced in number in the livers of genetically obese (ob/ob) mice (31), and a similar reduction was subsequently reported in wild-type mice fed a high-fat diet (60). One presumed mechanism of NKT cell depletion was by apoptosis, mediated by Kupffer cell-derived IL-12 (60). Adoptive transfer of NKT cells can ameliorate hepatic steatosis (24); this implies that NKT cells play an active role in preventing hepatic fat accumulation and possibly also adverse events downstream of steatosis. In the CD model of fatty liver disease, hepatic NKT cells also progressively decline in number (51). CD-fed mice that are depleted of Kupffer cells as a local source of IL-12, however, maintain their NKT cells. Unlike in the adoptive transfer study, in the CD feeding study mice that maintained normal numbers of NKT cells did not resist hepatic steatosis. One possible explanation for this is that hepatic steatosis in CD- and MCD-fed mice is influenced heavily by lipid retention, which may not be amenable to NKT cell regulation. Unfortunately, although CD feeding, which can produce progressive steatohepatitis, held promise to delineate the role of NKT cells in actual liver injury, the mice in this study did not develop significant liver disease. Consequently, the impact of hepatic NKT cell depletion on outcomes other than steatosis in fatty liver disease remains uncertain.
Dysregulation of adiponectin signaling
One notable feature of MCD-fed mice is that serum levels of adiponectin are normal or even increased compared to diet-matched controls, even in the face of hepatic steatosis (18, 54, 107). This is not entirely unexpected, since MCD-fed mice do not develop obesity, which is typically a prerequisite to a decline in circulating adiponectin. Nevertheless, because adiponectin facilitates the maintenance of lipid homeostasis in liver (128), MCD-fed mice have been characterized as being in a state of adiponectin resistance.(54) Adiponectin promotes fatty acid oxidation by activating AMPK and PPARα (41); it also has anti-inflammatory properties, which are mediated through an IL-10/HO-1 axis (68). Therefore, adiponectin resistance may play a role in both the steatosis and inflammation that occur in response to MCD feeding. This notion was proven directly in experiments in which MCD-fed mice with improved adiponectin signaling due to adenovirus-mediated overexpression of adiponectin receptor-2 were spared from MCD-mediated hepatic steatosis, oxidant stress, and fibrosis (119).
Other factors under investigation
Many factors aside from those cited above are currently under investigation in animal models of steatohepatitis. Among these are targeted studies of the involvement of nuclear receptors in the pathogenesis of experimental fatty liver disease. In addition to PPARα and PPARγ, whose expression and activity have been explored in MCD-fed mice, experiments are expanding to include retinoid X receptor (RXR), farnesoid X receptor (FXR), and constitutive androstane receptor (CAR). RXR deficiency predisposes to MCD-mediated steatohepatitis, as might be expected based on its partnership with PPARα (32). FXR agonists, which are known for their ability to induce the synthesis of molecules involved in hepatic lipid export, improve MCD-mediated liver injury but interestingly not by reducing hepatic steatosis (138). CAR agonists have met with mixed results when tested in MCD-fed mice. In one study, CAR activation worsened MCD-mediated liver disease and CAR deficiency was protective against steatohepatitis (131). This contrasts with another report in which a CAR agonist improved MCD-induced liver disease (6). Further work will be required for a clear picture to emerge from these studies.
MicroRNAs, which are known to influence a number of processes pertinent to fatty liver disease (14), are beginning to be explored in experimental animals. This work is limited, but studies indicate that miRNA regulation in experimental alcoholic fatty liver disease is distinct from that in MCD-related nonalcoholic fatty liver disease (23). Other studies have pointed to miR-21, -122, and -335 as regulators of lipid homeostasis, but these miRNAs have not been evaluated directly in the setting of experimental steatohepatitis (25, 78, 123).
Special considerations with CD and MCD diets
A major selling point for the use of CD or MCD diets, in addition to their ability to cause serious liver injury, is that they ideally lead to a consistent outcome in comparison to that seen with the array of commercial high-energy diets. This is only partly true, for two reasons. First, there are species, strain, and sex differences in the response of rodents to MCD feeding (46, 92). Second, the macronutrient composition of MCD and CD formulas varies among vendors, which may influence experimental results (Table 2). A third issue is that in an effort to offset the weight loss caused by MCD feeding, some investigators have fed MCD formulas to rodents with background predispositions to obesity and fatty liver (84, 104, 129). This permits more prolonged study, with the evolution of hepatic fibrosis, but can cause difficulty in deciphering experimental results. These limitations are not meant to discourage the use of CD or MCD formulas, but instead to encourage the informed design and interpretation of future studies.
Table 2.
CD and MCD Formulas
| Formula category | Fat cal (%) | CHO cal (%) | Fat source (%) | CHO source (%) | Formula # | Manufacturer | Ref. |
|---|---|---|---|---|---|---|---|
| MCS, MCD | 31 | 55 | HVO:corn oil (67:33)a | Sucrose:starch (67:33) | 518811, 518810 | Dyets | (139) |
| MCS, MCD | 21 | 62 | Corn oil (100) | Sucrose:starch (69:31) | 960441, 960439 | MP Biomedicals | (1, 96, 100, 130) |
| MCS, MCD | 22 | 63 | Corn oil (100) | Sucrose:starch (69:31) | 94149, 90262 | Harlan Teklad | |
| CSAA, CDAA | 32 | 54 | HVO:corn oil (67:33) | Sucrose:starch (66:34) | 518754, 518753 | Dyets | (48, 71) |
21% of fat as trans-fat.
Table illustrates the macronutrient composition of CD and MCD formulas, as well as their matched control formulas, where available. CDAA, choline-deficient, amino acid-defined; CSAA, choline-sufficient, amino acid-defined; MCD, methionine-choline-deficient; MCS, methionine-choline-sufficient. Note the use of trans-fat in some MCS and MCD formulas.
Overeating as a Means of Inducing NAFLD
Some investigators argue that simple overeating, rather than more extreme manipulation of dietary macronutrients, constitutes the best experimental approach to studying steatohepatitis because it most closely reflects a major risk factor for fatty liver disease in human beings. Overeating can be accomplished in wild-type animals by intragastric infusion of a liquid diet. When normal rats are overfed chow-equivalent diets at 117% of their necessary calories, they gain weight and become hyperglycemic but develop little evidence of steatohepatitis (7). On the other hand, mice fed 130%–185% of daily calorie requirements for 9 weeks develop clinical and biochemical evidence of fatty liver disease (19). Interestingly, some mutant rodents that spontaneously overeat develop fatty liver even on a chow formula. This is true of leptin-deficient (ob/ob) mice, cholecystokinin-1 (CCK1) receptor-deficient (Otsuka Long–Evans Tokushima Fatty; OLETF) rats and Alms1-deficient (foz/foz) mice, which eat at least 20% more calories per day than their wild-type counterparts (Table 3). Leptin deficiency causes far broader phenotypic abnormalities than overeating, which limits the use of ob/ob mice as a faithful model of human steatohepatitis (26, 56, 59). Still, ob/ob mice are continuing to provide critical information regarding the role of leptin in hepatic lipid metabolism. In this regard, leptin has been recently shown to avert hepatic steatosis not by increasing local fat oxidation, but instead by reducing fatty acid synthesis and enhancing hepatic VLDL secretion (110). This has prompted the notion that leptin enables the liver to rid itself of fat for catabolism at another, yet unspecified site.
Table 3.
Rodent Models of Overfeeding
| Animal model | Genetic defect | Phenotype | Ref. |
|---|---|---|---|
| Intragastric overfeeding | None (wild-type) | Steatohepatitis in 9 weeks at 130%–185% of calories | (19) |
| Ob/ob mice | Leptin-null | Steatohepatitis in 10-14 weeks on standard diet | (63) |
| Foz/foz mice | Alms1-null | Steatohepatitis in 300 days on high-fat diet | (3) |
| OLETF rats | CCKR1-null | Steatohepatitis in 40 weeks on chow diet | (95) |
Table depicts the characteristics of four rodent models of overfeeding that have been used in the study of fatty liver disease.
Unlike the metabolic derangements seen in ob/ob mice, those in OLETF rats are entirely attributable to excess food intake (74). These rats have a defect in the satiety signal mediated by CCK1 as well as the loss of CCK1-mediated inhibition of the orexigenic neuropeptide Y in the brain. These combined defects cause the rats to eat larger meals as well as eat more frequently. Interestingly, CCK1 receptor-deficient mice do not have the same phenotype as rats; the reason is that mice do not express CCK1 receptors in the brain, and thus there is no central nervous system cross-talk between CCK1 and neuropeptide Y in this species to influence appetite (9). OLETF rats develop hepatic steatosis beginning at 8 weeks of age that progresses steadily to week 40 (95). By week 40, the rats exhibit several signs of steatohepatitis, including hepatocyte ballooning, ALT elevation, hepatic TNF induction, inflammatory infiltrates, and pericentral fibrosis (95, 109). Rector and colleagues (95) recently examined the hepatic mitochondria of OLETF rats at 40 weeks of age and found them to have reduced protein content and abnormal ultrastructure. They also noted that hepatocytes from OLETF rats of all ages have a reduced ability to oxidize fatty acids completely to CO2. Incomplete fatty acid oxidation can cause oxidative stress (75); this was also evident in the livers of OLETF rats. These observations suggest that incomplete fatty acid oxidation is an important mechanism underlying experimental fatty liver disease. The data supplement findings from other groups that implicate incomplete fatty acid oxidation as a cause of muscle insulin resistance (50). Importantly, if the theory of incomplete fatty acid oxidation holds true in liver, it will resolve an apparent paradox in experimental models of alcoholic and nonalcoholic fatty liver disease, namely that despite a strong association between steatohepatitis and oxidative stress, stimulating fatty acid oxidation in a fatty liver improves, rather than exacerbates, steatosis and liver injury (29, 38, 77, 109).
The newest model of hyperphagia is the foz/foz mouse, a spontaneous mutant with an 11-base-pair deletion in the alms1 gene on chromosome 6 (4). Alms1 muations in humans cause Alström syndrome, an autosomal recessive disorder with a multi-organ phenotype, including stunted growth, cardiomyopathy, obesity, diabetes, and fatty liver. Why the mutation causes increased food intake is unknown. Foz/foz mice are not hyperphagic from birth, but acquire this behavior within 60 days of age. Adult males eat 40% more food than their wild-type littermates. Foz/foz mice fed standard chow for 300 days develop pericentral steatosis but exhibit no further biochemical or histologic signs of liver disease. When foz/foz mice are fed a high-fat diet, however, they develop more severe liver disease than wild-type mice, including severe steatosis, hepatocyte ballooning, ALT elevation, hepatic inflammation, and pericellular and perivenular fibrosis (3). Genes governing fatty acid uptake and lipogenesis are expressed at high levels in the livers of foz/foz mice regardless of diet. Genes regulating fatty acid oxidation and secretion are also upregulated, but only when the mice are fed a chow diet. Although some of the changes may be nutrient-related, the data suggest that foz/foz mice cannot adapt to diet-induced steatosis by upregulating pathways of fat oxidation or disposal in the liver. Such a defect could be prompted by low circulating adiponectin, which is present in foz/foz mice.
The Role of Exercise in Modulating Experimental NAFLD
The fact that aerobic exercise can reduce hepatic steatosis and improve serum ALT levels in humans with fatty liver disease (39, 113) has stimulated interest in the role of physical activity in the pathogenesis of steatohepatitis. The impact of exercise on fatty liver disease in experimental animals was first addressed in rats that were forced into sedentary behavior through hindlimb unloading (98). Rats rendered sedentary for 3 weeks while eating a sucrose- and fat-enriched diet developed three times as much hepatic steatosis and slightly more hepatic inflammation as rats with unrestricted ambulation. This was associated with endotoxemia, which occurred in sedentary rats regardless of diet. Why sedentary behavior would lead to endotoxemia was unexplained. Although intriguing, this finding has yet to be reproduced. Sedentary behavior was also examined by Tetri and colleagues (118), together with a high-fat, fructose-supplemented diet in an effort to model the American Lifestyle-Induced Obesity Syndrome (ALIOS). Although sedentary behavior may have contributed to liver disease in this study, physical activity was not directly tested as an outcome variable. Rector and colleagues (94) employed a different strategy by adding, rather than subtracting, the opportunity for physical activity to rats with a predisposition to fatty liver disease. They used OLETF rats, which, as mentioned above, develop steatohepatitis due to overeating even if fed a chow diet. OLETF rats that were offered a running wheel for 16 weeks were less obese and had fewer features of the metabolic syndrome than rats maintained in standard caging. Active rats also had significantly less hepatic steatosis than control rats even though serum ALT levels were comparable. Importantly, physical activity prompted more complete oxidation of fatty acids in the liver and reduced the expression of several genes involved in hepatic lipogenesis. In a followup study, the same group examined whether the cessation of voluntary exercise in OLETF rats would prompt the redevelopment of fatty liver disease (93). They showed that the beneficial effects of 16 weeks of physical activity on fatty acid oxidation and hepatic lipogenesis could be completely reversed by one week of inactivity, even though gross changes in hepatic steatosis or liver injury were not apparent. These findings underscore the degree to which exercise can modulate metabolic activity in the liver and its potential to reduce the risk of fatty liver disease.
Genetic Manipulation of Hepatic Lipid Homeostasis
Although many animal studies addressing fatty liver disease are based on manipulations of diet and exercise, others have focused on genetic manipulation of metabolic or disease-related pathways in an effort to identify events that leading to hepatic steatosis—or worse. A comprehensive summary of these studies is beyond the scope of this review, but select examples are provided to highlight recent observations.
Endocannabinoids as mediators of fatty liver disease
Endocannabinoids can contribute to fatty liver disease in part through their orexigenic effects that are mediated through CB1 receptors in the brain and peripheral nervous system (52). Endocannabinoids also promote hepatic steatosis directly by stimulating CB1 receptors in the liver that enhance lipogenesis and suppress fatty acid oxidation (82). Blockade of CB1 signaling in the liver protects mice from diet-induced fatty liver and ALT elevation by increasing energy expenditure and fatty acid oxidation (83). CB2 receptors, which are present in adipose tissue, also contribute to fatty liver disease. Through these receptors, endocannabinoids promote adipose tissue inflammation, which modulates hepatic lipid accumulation and pro-inflammatory cytokine expression (21). The role of endocannabinoids in fatty liver is the subject of a recent review (67).
Sirtuins as master regulators of lipid synthesis and oxidation
Sirtuins are a family of NAD+-dependent protein deacetylases that play a central role in the regulation of metabolism (10). In the liver, Sirtuin 1 (SIRT1) deacetylates peroxisome proliferator activated receptor-γ co-activator-1α (PGC-1α), which is critical for the activation of gluconeogenesis during fasting. PGC-1α is also a co-activator for peroxisome proliferator activated receptor-α (PPARα) (108). In the deacetylated (active) state, PGC-1α partners with PPARα to promote the transcription of PPARα-dependent genes. Mice with a liver-specific deletion of SIRT1 have impaired PPARα signaling, which results in a reduction in fatty acid oxidation (81). Consequently, when liver-specific SIRT1-null mice are fed a high-fat diet, they develop hepatic steatosis. In addition, they develop signs of ER stress and modest, but significant, increases in hepatic pro-inflammatory cytokines (81). The results are similar to those reported previously in mice with PPARα deficiency (reviewed in (86)).
SIRT1 also plays a role in hepatic steatosis due to excessive alcohol consumption. Ethanol reduces SIRT1 activity, which leads to hyperacetylation of PPARα, decreased PPARα activity, and hepatic steatosis (133). Reduced SIRT1 activity in ethanol-fed animals also affects SREBP1; in this case, however, hyperacetylation leads to increased SREBP1 activity and increased hepatic lipogenesis. SIRT1, therefore, is in a pivotal position to regulate all aspects of lipid metabolism in the alcoholic and nonalcoholic fatty liver. Interestingly, resveratrol, a polyphenol present in the skin of red grapes, activates SIRT1 (53). A recent study demonstrated that activation of SIRT1 underlies the ability of resveratrol to limit experimental alcoholic fatty liver (2).
SIRT3 also influences lipid metabolism in the liver. It is located in the mitochondrial matrix, where it regulates the acetylation of mitochondrial enzymes. A major target of SIRT3 is long-chain acyl-CoA dehydrogenase (LCAD). In the absence of SIRT3, LCAD is hyperacetylated and in an inactive state. Mice deficient in SIRT3 have impaired ability to oxidize fatty acids during fasting (35). Whether SIRT3 influences fatty liver disease is currently unknown.
Novel findings from manipulation of lipogenic genes
The influence of lipogenic enzymes on hepatic steatosis and steatohepatitis has been the subject of extensive study, using mice with genetic under- or overexpression of lipogenic genes or their transcriptional activators. This topic has been elegantly reviewed by Strable and Ntamb (114). Some experiments emphasize specific points: for example, mice lacking acetyl-CoA carboxylase-1 (ACC1) or SCD1 are protected from carbohydrate-induced hepatic steatosis but not fat-induced hepatic steatosis (33, 70, 72), which underscores the independent contributions of dietary carbohydrate and fat to fatty liver. Second, mice with a liver-specific knockout of fatty acid synthase (FAS) paradoxically develop fatty liver in response to a high-carbohydrate diet, rather than being spared from it (13). FAS deficiency is accompanied by decreased PPARα activity and decreased fatty acid oxidation, which is the likely reason for steatosis. This prompted the theory that newly synthesized fatty acids are natural ligands for PPARα, and one relevant fatty acid species has just been identified as 1-palmitoyl-2-oleoyl-sn-glycerol-3-phosphocholine (12). Third, silencing of DGAT2 in the liver prevents triglyceride synthesis, as expected, but also suppresses hepatic lipogenesis and stimulates fatty acid oxidation. This emphasizes the degree of interconnection among metabolic pathways in hepatocytes (15). Finally, liver-specific overexpression of DGAT2 provokes hepatic steatosis but does not cause insulin resistance or glucose intolerance (73), which argues that hepatic steatosis is insufficient by itself to induce the metabolic syndrome. These represent only a few of many insights gained from basic studies of hepatic lipid metabolism. Even though these works may not address hepatic steatosis as a clinical entity, they serve to inform other studies that focus on hepatic steatosis as a liver disease with the potential to progress to steatohepatitis, hepatic fibrosis, and hepatocellular carcinoma.
Conclusion
Rodent models have enabled many important discoveries about hepatic steatosis and steatohepatitis that would not be possible in humans. These studies have detected differences in the ability of individual dietary macronutrients to induce steatosis and liver injury; they have also shown that factors provoking lipotoxicity may not be the same as those that induce oxidant stress or hepatic fibrosis. In addition, experiments in mice have permitted fine dissection of pathogenic events that are liver-specific from those that involve bone marrow-derived cells. They are continually evolving to reveal which cellular derangements (e.g., in lipid synthesis or lipid oxidation or both) are key to the pathogenesis of liver injury. Rodent studies of fatty liver are useful because each one accentuates the impact of an individual variable (e.g., diet, inflammation, exercise) on liver-related outcome. Their true value emerges when data from numerous studies are viewed in aggregate, creating a composite picture of the effects of multiple variables as may be faced by humans at risk for fatty liver disease.
Abbreviations Used
- ACC1
acetyl CoA carboxylase-1
- ALIOS
American Lifestyle-Induced Obesity Syndrome
- ALT
alanine aminotransferase
- AMPK
AMP-activated protein kinase
- CAR
constitutive androstane receptor
- CCK
cholecystokinin
- CD
choline-deficient
- CDAA
choline-deficient, amino acid-supplemented
- CHO
carbohydrate
- CHOP
C/EBP homologous protein
- CSAA
choline-sufficient, amino acid-supplemented
- DGAT
diacylglycerol transferase
- ER
endoplasmic reticulum
- FAS
fatty acid synthase
- FXR
Farnesoid X receptor
- Gclc
glutamate-cysteine ligase
- HF
high fat
- HO-1
heme oxygenase-1
- IL
interleukin
- JK
cJun N-terminal kinase
- Keap1
Kelch-like-ECH-associated protein 1
- KG
ketogenic
- LF
low fat
- MCD
methionine-choline-deficient
- MCP-1
monocyte chemoattractant protein-1
- MCS
methionine-choline-sufficient
- miRNA
microRNA
- MyD88
myeloid differentiation primary response gene 88
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- NF-κB
nuclear factor-κB
- Nfr2
nuclear factor E2-related factor-2
- NKT
natural killer T
- Nqo1
NAD(P)H dehydrogenase quinone 1
- OLETF
Otsuka Long-Evans Tokushima Fatty
- PGC1α
PAR gamma co-activator-1α
- PPR
peroxisome proliferator activated receptor
- RXR
retinoid X receptor
- SCD1
stearoyl CoA desaturase-1
- SIRT
sirtuin
- SREBP1
sterol regulatory element binding protein-1
- TLR
Toll-like receptor
- TNF
tumor necrosis factor
- TRIF
TIR-domain-containing adapter-inducing interferon-β
- VLDL
very low density lipoprotein
- VLF
very low fat
Acknowledgments
This work was supported by NIH grants R01 DK068450 and P30 DK026743.
References
- 1.Ahsan MK. Okuyama H. Hoshino Y. Oka S. Masutani H. Yodoi J. Nakamura H. Thioredoxin-binding protein-2 deficiency enhances methionine-choline deficient diet-induced hepatic steatosis but inhibits steatohepatitis in mice. Antioxid Redox Signal. 2009;11:2573–2584. doi: 10.1089/ars.2009.2385. [DOI] [PubMed] [Google Scholar]
- 2.Ajmo JM. Liang X. Rogers CQ. Pennock B. You M. Resveratrol alleviates alcoholic fatty liver in mice. Am J Physiol Gastrointest Liver Physiol. 2008;295:G833–842. doi: 10.1152/ajpgi.90358.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arsov T. Larter CZ. Nolan CJ. Petrovsky N. Goodnow CC. Teoh NC. Yeh MM. Farrell GC. Adaptive failure to high-fat diet characterizes steatohepatitis in Alms1 mutant mice. Biochem Biophys Res Commun. 2006;342:1152–1159. doi: 10.1016/j.bbrc.2006.02.032. [DOI] [PubMed] [Google Scholar]
- 4.Arsov T. Silva DG. O'Bryan MK. Sainsbury A. Lee NJ. Kennedy C. Manji SS. Nelms K. Liu C. Vinuesa CG. de Kretser DM. Goodnow CC. Petrovsky N. Fat aussie—A new Alstrom syndrome mouse showing a critical role for ALMS1 in obesity, diabetes, and spermatogenesis. Mol Endocrinol. 2006;20:1610–1622. doi: 10.1210/me.2005-0494. [DOI] [PubMed] [Google Scholar]
- 5.Asano T. Watanabe K. Kubota N. Gunji T. Omata M. Kadowaki T. Ohnishi S. Adiponectin knockout mice on high fat diet develop fibrosing steatohepatitis. J Gastroenterol Hepatol. 2009;24:1669–1676. doi: 10.1111/j.1440-1746.2009.06039.x. [DOI] [PubMed] [Google Scholar]
- 6.Baskin–Bey ES. Anan A. Isomoto H. Bronk SF. Gores GJ. Constitutive androstane receptor agonist, TCPOBOP, attenuates steatohepatitis in the methionine choline-deficient diet-fed mouse. World J Gastroenterol. 2007;13:5635–5641. doi: 10.3748/wjg.v13.i42.5635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baumgardner JN. Shankar K. Hennings L. Badger TM. Ronis MJ. A new model for nonalcoholic steatohepatitis in the rat utilizing total enteral nutrition to overfeed a high-polyunsaturated fat diet. Am J Physiol Gastrointest Liver Physiol. 2008;294:G27–38. doi: 10.1152/ajpgi.00296.2007. [DOI] [PubMed] [Google Scholar]
- 8.Bergheim I. Weber S. Vos M. Kramer S. Volynets V. Kaserouni S. McClain CJ. Bischoff SC. Antibiotics protect against fructose-induced hepatic lipid accumulation in mice: role of endotoxin. J Hepatol. 2008;48:983–992. doi: 10.1016/j.jhep.2008.01.035. [DOI] [PubMed] [Google Scholar]
- 9.Bi S. Scott KA. Kopin AS. Moran TH. Differential roles for cholecystokinin a receptors in energy balance in rats and mice. Endocrinology. 2004;145:3873–3880. doi: 10.1210/en.2004-0284. [DOI] [PubMed] [Google Scholar]
- 10.Bishop NA. Guarente L. Genetic links between diet and lifespan: Shared mechanisms from yeast to humans. Nat Rev Genet. 2007;8:835–844. doi: 10.1038/nrg2188. [DOI] [PubMed] [Google Scholar]
- 11.Cai D. Yuan M. Frantz DF. Melendez PA. Hansen L. Lee J. Shoelson SE. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med. 2005;11:183–190. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chakravarthy MV. Lodhi IJ. Yin L. Malapaka RR. Xu HE. Turk J. Semenkovich CF. Identification of a physiologically relevant endogenous ligand for PPARα in liver. Cell. 2009;138:476–488. doi: 10.1016/j.cell.2009.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chakravarthy MV. Pan Z. Zhu Y. Tordjman K. Schneider JG. Coleman T. Turk J. Semenkovich CF. “New” hepatic fat activates PPARalpha to maintain glucose, lipid, and cholesterol homeostasis. Cell Metab. 2005;1:309–322. doi: 10.1016/j.cmet.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 14.Cheung O. Sanyal AJ. Role of microRNAs in non-alcoholic steatohepatitis. Curr Pharm Des. 2010;16:1952–1957. doi: 10.2174/138161210791208866. [DOI] [PubMed] [Google Scholar]
- 15.Choi CS. Savage DB. Kulkarni A. Yu XX. Liu ZX. Morino K. Kim S. Distefano A. Samuel VT. Neschen S. Zhang D. Wang A. Zhang XM. Kahn M. Cline GW. Pandey SK. Geisler JG. Bhanot S. Monia BP. Shulman GI. Suppression of diacylglycerol acyltransferase-2 (DGAT2), but not DGAT1, with antisense oligonucleotides reverses diet-induced hepatic steatosis and insulin resistance. J Biol Chem. 2007;282:22678–22688. doi: 10.1074/jbc.M704213200. [DOI] [PubMed] [Google Scholar]
- 16.Chowdhry S. Nazmy MH. Meakin PJ. Dinkova–Kostova AT. Walsh SV. Tsujita T. Dillon JF. Ashford ML. Hayes JD. Loss of Nrf2 markedly exacerbates nonalcoholic steatohepatitis. Free Radic Biol Med. 2010;48:357–371. doi: 10.1016/j.freeradbiomed.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 17.Cong WN. Tao RY. Tian JY. Liu GT. Ye F. The establishment of a novel non-alcoholic steatohepatitis model accompanied with obesity and insulin resistance in mice. Life Sci. 2008;82:983–990. doi: 10.1016/j.lfs.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 18.Da Silva Morais A. Lebrun V. Abarca-Quinones J. Brichard S. Hue L. Guigas B. Viollet B. Leclercq IA. Prevention of steatohepatitis by pioglitazone: Implication of adiponectin-dependent inhibition of SREBP-1c and inflammation. J Hepatol. 2009;50:489–500. doi: 10.1016/j.jhep.2008.10.027. [DOI] [PubMed] [Google Scholar]
- 19.Deng QG. She H. Cheng JH. French SW. Koop DR. Xiong S. Tsukamoto H. Steatohepatitis induced by intragastric overfeeding in mice. Hepatology. 2005;42:905–914. doi: 10.1002/hep.20877. [DOI] [PubMed] [Google Scholar]
- 20.Deng ZB. Liu Y. Liu C. Xiang X. Wang J. Cheng Z. Shah SV. Zhang S. Zhang L. Zhuang X. Michalek S. Grizzle WE. Zhang HG. Immature myeloid cells induced by a high-fat diet contribute to liver inflammation. Hepatology. 2009;50:1412–1420. doi: 10.1002/hep.23148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deveaux V. Cadoudal T. Ichigotani Y. Teixeira–Clerc F. Louvet A. Manin S. Nhieu JT. Belot MP. Zimmer A. Even P. Cani PD. Knauf C. Burcelin R. Bertola A. Le Marchand–Brustel Y. Gual P. Mallat A. Lotersztajn S. Cannabinoid CB2 receptor potentiates obesity-associated inflammation, insulin resistance and hepatic steatosis. PLoS ONE. 2009;4:e5844. doi: 10.1371/journal.pone.0005844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dobrzyn A. Dobrzyn P. Miyazaki M. Sampath H. Chu K. Ntambi JM. Stearoyl-CoA desaturase 1 deficiency increases CTP:choline cytidylyltransferase translocation into the membrane and enhances phosphatidylcholine synthesis in liver. J Biol Chem. 2005;280:23356–23362. doi: 10.1074/jbc.M502436200. [DOI] [PubMed] [Google Scholar]
- 23.Dolganiuc A. Petrasek J. Kodys K. Catalano D. Mandrekar P. Velayudham A. Szabo G. MicroRNA expression profile in Lieber–DeCarli diet-induced alcoholic and methionine choline deficient diet-induced nonalcoholic steatohepatitis models in mice. Alcohol Clin Exp Res. 2009;33:1704–1710. doi: 10.1111/j.1530-0277.2009.01007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elinav E. Pappo O. Sklair–Levy M. Margalit M. Shibolet O. Gomori M. Alper R. Thalenfeld B. Engelhardt D. Rabbani E. Ilan Y. Adoptive transfer of regulatory NKT lymphocytes ameliorates non-alcoholic steatohepatitis and glucose intolerance in ob/ob mice and is associated with intrahepatic CD8 trapping. J Pathol. 2006;209:121–128. doi: 10.1002/path.1950. [DOI] [PubMed] [Google Scholar]
- 25.Esau C. Davis S. Murray SF. Yu XX. Pandey SK. Pear M. Watts L. Booten SL. Graham M. McKay R. Subramaniam A. Propp S. Lollo BA. Freier S. Bennett CF. Bhanot S. Monia BP. miR-122 regulation of lipid metabolism revealed by in vivo antisense targeting. Cell Metab. 2006;3:87–98. doi: 10.1016/j.cmet.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 26.Faggioni R. Feingold KR. Grunfeld C. Leptin regulation of the immune response and the immunodeficiency of malnutrition. FASEB J. 2001;15:2565–2571. doi: 10.1096/fj.01-0431rev. [DOI] [PubMed] [Google Scholar]
- 27.Farrell GC. Larter CZ. Hou JY. Zhang RH. Yeh MM. Williams J. dela Pena A. Francisco R. Osvath SR. Brooling J. Teoh N. Sedger LM. Apoptosis in experimental NASH is associated with p53 activation and TRAIL receptor expression. J Gastroenterol Hepatol. 2009;24:443–452. doi: 10.1111/j.1440-1746.2009.05785.x. [DOI] [PubMed] [Google Scholar]
- 28.Feldstein AE. Canbay A. Guicciardi ME. Higuchi H. Bronk SF. Gores GJ. Diet associated hepatic steatosis sensitizes to Fas-mediated liver injury in mice. J Hepatol. 2003;39:978–983. doi: 10.1016/s0168-8278(03)00460-4. [DOI] [PubMed] [Google Scholar]
- 29.Fischer M. You M. Matsumoto M. Crabb DW. Peroxisome proliferator-activated receptor alphaα (PPARα) agonist treatment reverses PPARα dysfunction and abnormalities in hepatic lipid metabolism in ethanol-fed mice. J Biol Chem. 2003;278:27997–28004. doi: 10.1074/jbc.M302140200. [DOI] [PubMed] [Google Scholar]
- 30.Flowers MT. Ntambi JM. Role of stearoyl-coenzyme A desaturase in regulating lipid metabolism. Curr Opin Lipidol. 2008;19:248–256. doi: 10.1097/MOL.0b013e3282f9b54d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30a.Gentile C. Frye M. Pagliassotti MJ. Endoplasmic reticulum stress and the unfolded protein response in nonalcoholic fatty liver disease. Antioxid Redox Signal. 2011;15:505–521. doi: 10.1089/ars.2010.3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guebre–Xabier M. Yang S. Lin HZ. Schwenk R. Krzych U. Diehl AM. Altered hepatic lymphocyte subpopulations in obesity-related murine fatty livers: Potential mechanism for sensitization to liver damage. Hepatology. 2000;31:633–640. doi: 10.1002/hep.510310313. [DOI] [PubMed] [Google Scholar]
- 32.Gyamfi MA. Tanaka Y. He L. Klaassen CD. Wan YJ. Hepatic effects of a methionine-choline-deficient diet in hepatocyte RXRα-null mice. Toxicol Appl Pharmacol. 2009;234:166–178. doi: 10.1016/j.taap.2008.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harada N. Oda Z. Hara Y. Fujinami K. Okawa M. Ohbuchi K. Yonemoto M. Ikeda Y. Ohwaki K. Aragane K. Tamai Y. Kusunoki J. Hepatic de novo lipogenesis is present in liver-specific ACC1-deficient mice. Mol Cell Biol. 2007;27:1881–1888. doi: 10.1128/MCB.01122-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hirosumi J. Tuncman G. Chang L. Gorgun CZ. Uysal KT. Maeda K. Karin M. Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 35.Hirschey MD. Shimazu T. Goetzman E. Jing E. Schwer B. Lombard DB. Grueter CA. Harris C. Biddinger S. Ilkayeva OR. Stevens RD. Li Y. Saha AK. Ruderman NB. Bain JR. Newgard CB. Farese RV., Jr. Alt FW. Kahn CR. Verdin E. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature. 2010;464:121–125. doi: 10.1038/nature08778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hritz I. Mandrekar P. Velayudham A. Catalano D. Dolganiuc A. Kodys K. Kurt–Jones E. Szabo G. The critical role of toll-like receptor (TLR) 4 in alcoholic liver disease is independent of the common TLR adapter MyD88. Hepatology. 2008;48:1224–1231. doi: 10.1002/hep.22470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu E. Liang P. Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem. 1996;271:10697–10703. doi: 10.1074/jbc.271.18.10697. [DOI] [PubMed] [Google Scholar]
- 38.Ip E. Farrell G. Hall P. Robertson G. Leclercq I. Administration of the potent PPARalpha agonist, Wy-14,643, reverses nutritional fibrosis and steatohepatitis in mice. Hepatology. 2004;39:1286–1296. doi: 10.1002/hep.20170. [DOI] [PubMed] [Google Scholar]
- 39.Johnson NA. Sachinwalla T. Walton DW. Smith K. Armstrong A. Thompson MW. George J. Aerobic exercise training reduces hepatic and visceral lipids in obese individuals without weight loss. Hepatology. 2009;50:1105–1112. doi: 10.1002/hep.23129. [DOI] [PubMed] [Google Scholar]
- 40.Jou J. Choi SS. Diehl AM. Mechanisms of disease progression in nonalcoholic fatty liver disease. Semin Liver Dis. 2008;28:370–379. doi: 10.1055/s-0028-1091981. [DOI] [PubMed] [Google Scholar]
- 41.Kadowaki T. Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005;26:439–451. doi: 10.1210/er.2005-0005. [DOI] [PubMed] [Google Scholar]
- 42.Kammoun HL. Chabanon H. Hainault I. Luquet S. Magnan C. Koike T. Ferre P. Foufelle F. GRP78 expression inhibits insulin and ER stress-induced SREBP-1c activation and reduces hepatic steatosis in mice. J Clin Invest. 2009;119:1201–1215. doi: 10.1172/JCI37007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kanda H. Tateya S. Tamori Y. Kotani K. Hiasa K. Kitazawa R. Kitazawa S. Miyachi H. Maeda S. Egashira K. Kasuga M. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116:1494–1505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kennedy AR. Pissios P. Otu H. Xue B. Asakura K. Furukawa N. Marino FE. Liu FF. Kahn BB. Libermann TA. Maratos–Flier E. Roberson R. A high-fat, ketogenic diet induces a unique metabolic state in mice. Am J Physiol Endocrinol Metab. 2007;292:E1724–1739. doi: 10.1152/ajpendo.00717.2006. [DOI] [PubMed] [Google Scholar]
- 45.Kim JY. van de Wall E. Laplante M. Azzara A. Trujillo ME. Hofmann SM. Schraw T. Durand JL. Li H. Li G. Jelicks LA. Mehler MF. Hui DY. Deshaies Y. Shulman GI. Schwartz GJ. Scherer PE. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest. 2007;117:2621–2637. doi: 10.1172/JCI31021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kirsch R. Clarkson V. Shephard EG. Marais DA. Jaffer MA. Woodburne VE. Kirsch RE. Hall Pde L. Rodent nutritional model of non-alcoholic steatohepatitis: Species, strain and sex difference studies. J Gastroenterol Hepatol. 2003;18:1272–1282. doi: 10.1046/j.1440-1746.2003.03198.x. [DOI] [PubMed] [Google Scholar]
- 47.Klaassen CD. Reisman SA. Nrf2 the rescue: Effects of the antioxidative/electrophilic response on the liver. Toxicol Appl Pharmacol. 2010;244:57–65. doi: 10.1016/j.taap.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kodama Y. Kisseleva T. Iwaisako K. Miura K. Taura K. De Minicis S. Osterreicher CH. Schnabl B. Seki E. Brenner DA. c-Jun N-terminal kinase-1 from hematopoietic cells mediates progression from hepatic steatosis to steatohepatitis and fibrosis in mice. Gastroenterology. 2009;137:1467–1477. doi: 10.1053/j.gastro.2009.06.045. e1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koppe SW. Elias M. Moseley RH. Green RM. Trans fat feeding results in higher serum alanine aminotransferase and increased insulin resistance compared with a standard murine high-fat diet. Am J Physiol Gastrointest Liver Physiol. 2009;297:G378–384. doi: 10.1152/ajpgi.90543.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koves TR. Ussher JR. Noland RC. Slentz D. Mosedale M. Ilkayeva O. Bain J. Stevens R. Dyck JR. Newgard CB. Lopaschuk GD. Muoio DM. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 2008;7:45–56. doi: 10.1016/j.cmet.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 51.Kremer M. Thomas E. Milton RJ. Perry AW. van Rooijen N. Wheeler MD. Zacks S. Fried M. Rippe RA. Hines IN. Kupffer cell and interleukin-12-dependent loss of natural killer T cells in hepatosteatosis. Hepatology. 2010;51:130–141. doi: 10.1002/hep.23292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kunos G. Understanding metabolic homeostasis and imbalance: What is the role of the endocannabinoid system? Am J Med. 2007;120:S18–24. doi: 10.1016/j.amjmed.2007.06.007. discussion S24. [DOI] [PubMed] [Google Scholar]
- 53.Lagouge M. Argmann C. Gerhart–Hines Z. Meziane H. Lerin C. Daussin F. Messadeq N. Milne J. Lambert P. Elliott P. Geny B. Laakso M. Puigserver P. Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 54.Larter CZ. Yeh MM. Williams J. Bell–Anderson KS. Farrell GC. MCD-induced steatohepatitis is associated with hepatic adiponectin resistance and adipogenic transformation of hepatocytes. J Hepatol. 2008;49:407–416. doi: 10.1016/j.jhep.2008.03.026. [DOI] [PubMed] [Google Scholar]
- 55.Leclercq IA. Farrell GC. Field J. Bell DR. Gonzalez FJ. Robertson GR. CYP2E1 and CYP4A as microsomal catalysts of lipid peroxides in murine nonalcoholic steatohepatitis. J Clin Invest. 2000;105:1067–1075. doi: 10.1172/JCI8814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leclercq IA. Farrell GC. Schriemer R. Robertson GR. Leptin is essential for the hepatic fibrogenic response to chronic liver injury. J Hepatol. 2002;37:206–213. doi: 10.1016/s0168-8278(02)00102-2. [DOI] [PubMed] [Google Scholar]
- 57.Lee AH. Scapa EF. Cohen DE. Glimcher LH. Regulation of hepatic lipogenesis by the transcription factor XBP1. Science. 2008;320:1492–1496. doi: 10.1126/science.1158042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee GS. Yan JS. Ng RK. Kakar S. Maher JJ. Polyunsaturated fat in the methionine-choline-deficient diet influences hepatic inflammation but not hepatocellular injury. J Lipid Res. 2007;48:1885–1896. doi: 10.1194/jlr.M700181-JLR200. [DOI] [PubMed] [Google Scholar]
- 59.Levin N. Nelson C. Gurney A. Vandlen R. de Sauvage F. Decreased food intake does not completely account for adiposity reduction after ob protein infusion. Proc Natl Acad Sci USA. 1996;93:1726–1730. doi: 10.1073/pnas.93.4.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li Z. Soloski MJ. Diehl AM. Dietary factors alter hepatic innate immune system in mice with nonalcoholic fatty liver disease. Hepatology. 2005;42:880–885. doi: 10.1002/hep.20826. [DOI] [PubMed] [Google Scholar]
- 61.Li ZZ. Berk M. McIntyre TM. Feldstein AE. Hepatic lipid partitioning and liver damage in nonalcoholic fatty liver disease: Role of stearoyl-CoA desaturase. J Biol Chem. 2009;284:5637–5644. doi: 10.1074/jbc.M807616200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lieber CS. Leo MA. Mak KM. Xu Y. Cao Q. Ren C. Ponomarenko A. DeCarli LM. Model of nonalcoholic steatohepatitis. Am J Clin Nutr. 2004;79:502–509. doi: 10.1093/ajcn/79.3.502. [DOI] [PubMed] [Google Scholar]
- 63.Lin HZ. Yang SQ. Chuckaree C. Kuhajda F. Ronnet G. Diehl AM. Metformin reverses fatty liver disease in obese, leptin-deficient mice. Nat Med. 2000;6:998–1003. doi: 10.1038/79697. [DOI] [PubMed] [Google Scholar]
- 64.Lin J. Yang R. Tarr PT. Wu PH. Handschin C. Li S. Yang W. Pei L. Uldry M. Tontonoz P. Newgard CB. Spiegelman BM. Hyperlipidemic effects of dietary saturated fats mediated through PGC-1beta coactivation of SREBP. Cell. 2005;120:261–273. doi: 10.1016/j.cell.2004.11.043. [DOI] [PubMed] [Google Scholar]
- 65.Liu R. Pan X. Whitington PF. Increased hepatic expression is a major determinant of serum alanine aminotransferase elevation in mice with nonalcoholic steatohepatitis. Liver Int. 2009;29:337–343. doi: 10.1111/j.1478-3231.2008.01862.x. [DOI] [PubMed] [Google Scholar]
- 66.Malhi H. Bronk SF. Werneburg NW. Gores GJ. Free fatty acids induce JNK-dependent hepatocyte lipoapoptosis. J Biol Chem. 2006;281:12093–12101. doi: 10.1074/jbc.M510660200. [DOI] [PubMed] [Google Scholar]
- 67.Mallat A. Lotersztajn S. Endocannabinoids and their role in fatty liver disease. Dig Dis. 2010;28:261–266. doi: 10.1159/000282100. [DOI] [PubMed] [Google Scholar]
- 68.Mandal P. Park PH. McMullen MR. Pratt BT. Nagy LE. The anti-inflammatory effects of adiponectin are mediated via a heme oxygenase-1-dependent pathway in rat Kupffer cells. Hepatology. 2010;51:1420–1429. doi: 10.1002/hep.23427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mantena SK. Vaughn DP. Andringa KK. Eccleston HB. King AL. Abrams GA. Doeller JE. Kraus DW. Darley–Usmar VM. Bailey SM. High fat diet induces dysregulation of hepatic oxygen gradients and mitochondrial function in vivo. Biochem J. 2009;417:183–193. doi: 10.1042/BJ20080868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mao J. DeMayo FJ. Li H. Abu–Elheiga L. Gu Z. Shaikenov TE. Kordari P. Chirala SS. Heird WC. Wakil SJ. Liver-specific deletion of acetyl-CoA carboxylase 1 reduces hepatic triglyceride accumulation without affecting glucose homeostasis. Proc Natl Acad Sci USA. 2006;103:8552–8557. doi: 10.1073/pnas.0603115103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Miura K. Kodama Y. Inokuchi S. Schnabl B. Aoyama T. Ohnishi H. Olefsky JM. Brenner DA. Seki E. Toll-like receptor 9 promotes steatohepatitis by induction of interleukin-1β in mice. Gastroenterology. 2010;139:323–334. doi: 10.1053/j.gastro.2010.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Miyazaki M. Flowers MT. Sampath H. Chu K. Otzelberger C. Liu X. Ntambi JM. Hepatic stearoyl-CoA desaturase-1 deficiency protects mice from carbohydrate-induced adiposity and hepatic steatosis. Cell Metab. 2007;6:484–496. doi: 10.1016/j.cmet.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 73.Monetti M. Levin MC. Watt MJ. Sajan MP. Marmor S. Hubbard BK. Stevens RD. Bain JR. Newgard CB. Farese RV., Sr. Hevener AL. Farese RV., Jr. Dissociation of hepatic steatosis and insulin resistance in mice overexpressing DGAT in the liver. Cell Metab. 2007;6:69–78. doi: 10.1016/j.cmet.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 74.Moran TH. Unraveling the obesity of OLETF rats. Physiol Behav. 2008;94:71–78. doi: 10.1016/j.physbeh.2007.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Muoio DM. Koves TR. Skeletal muscle adaptation to fatty acid depends on coordinated actions of the PPARs and PGC1 alpha: Implications for metabolic disease. Appl Physiol Nutr Metab. 2007;32:874–883. doi: 10.1139/H07-083. [DOI] [PubMed] [Google Scholar]
- 76.Muruve DA. Petrilli V. Zaiss AK. White LR. Clark SA. Ross PJ. Parks RJ. Tschopp J. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature. 2008;452:103–107. doi: 10.1038/nature06664. [DOI] [PubMed] [Google Scholar]
- 77.Nagasawa T. Inada Y. Nakano S. Tamura T. Takahashi T. Maruyama K. Yamazaki Y. Kuroda J. Shibata N. Effects of bezafibrate, PPAR pan-agonist, and GW501516, PPARdelta agonist, on development of steatohepatitis in mice fed a methionine- and choline-deficient diet. Eur J Pharmacol. 2006;536:182–191. doi: 10.1016/j.ejphar.2006.02.028. [DOI] [PubMed] [Google Scholar]
- 78.Nakanishi N. Nakagawa Y. Tokushige N. Aoki N. Matsuzaka T. Ishii K. Yahagi N. Kobayashi K. Yatoh S. Takahashi A. Suzuki H. Urayama O. Yamada N. Shimano H. The up-regulation of microRNA-335 is associated with lipid metabolism in liver and white adipose tissue of genetically obese mice. Biochem Biophys Res Commun. 2009;385:492–496. doi: 10.1016/j.bbrc.2009.05.058. [DOI] [PubMed] [Google Scholar]
- 79.Nanji AA. Jokelainen K. Tipoe GL. Rahemtulla A. Dannenberg AJ. Dietary saturated fatty acids reverse inflammatory and fibrotic changes in rat liver despite continued ethanol administration. J Pharmacol Exp Ther. 2001;299:638–644. [PubMed] [Google Scholar]
- 80.Newberry EP. Xie Y. Kennedy SM. Luo J. Davidson NO. Protection against Western diet-induced obesity and hepatic steatosis in liver fatty acid-binding protein knockout mice. Hepatology. 2006;44:1191–1205. doi: 10.1002/hep.21369. [DOI] [PubMed] [Google Scholar]
- 81.Obara N. Fukushima K. Ueno Y. Wakui Y. Kimura O. Tamai K. Kakazu E. Inoue J. Kondo Y. Ogawa N. Sato K. Tsuduki T. Ishida K. Shimosegawa T. Possible involvement and the mechanisms of excess trans-fatty acid consumption in severe NAFLD in mice. J Hepatol. 2010;53:326–334. doi: 10.1016/j.jhep.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 82.Osei–Hyiaman D. DePetrillo M. Pacher P. Liu J. Radaeva S. Batkai S. Harvey–White J. Mackie K. Offertaler L. Wang L. Kunos G. Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. J Clin Invest. 2005;115:1298–1305. doi: 10.1172/JCI23057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Osei–Hyiaman D. Liu J. Zhou L. Godlewski G. Harvey–White J. Jeong WI. Batkai S. Marsicano G. Lutz B. Buettner C. Kunos G. Hepatic CB1 receptor is required for development of diet-induced steatosis, dyslipidemia, and insulin and leptin resistance in mice. J Clin Invest. 2008;118:3160–3169. doi: 10.1172/JCI34827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ota T. Takamura T. Kurita S. Matsuzawa N. Kita Y. Uno M. Akahori H. Misu H. Sakurai M. Zen Y. Nakanuma Y. Kaneko S. Insulin resistance accelerates a dietary rat model of nonalcoholic steatohepatitis. Gastroenterology. 2007;132:282–293. doi: 10.1053/j.gastro.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 85.Oyadomari S. Harding HP. Zhang Y. Oyadomari M. Ron D. Dephosphorylation of translation initiation factor 2alpha enhances glucose tolerance and attenuates hepatosteatosis in mice. Cell Metab. 2008;7:520–532. doi: 10.1016/j.cmet.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ozcan U. Cao Q. Yilmaz E. Lee AH. Iwakoshi NN. Ozdelen E. Tuncman G. Gorgun C. Glimcher LH. Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 87.Pfaffenbach KT. Gentile CL. Nivala AM. Wang D. Wei Y. Pagliassotti MJ. Linking endoplasmic reticulum stress to cell death in hepatocytes: Roles of C/EBP homologous protein and chemical chaperones in palmitate-mediated cell death. Am J Physiol Endocrinol Metab. 2010;298:E1027–1035. doi: 10.1152/ajpendo.00642.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pfluger PT. Herranz D. Velasco–Miguel S. Serrano M. Tschop MH. Sirt1 protects against high-fat diet-induced metabolic damage. Proc Natl Acad Sci USA. 2008;105:9793–9798. doi: 10.1073/pnas.0802917105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Phung N. Pera N. Farrell G. Leclercq I. Hou JY. George J. Pro-oxidant-mediated hepatic fibrosis and effects of antioxidant intervention in murine dietary steatohepatitis. Int J Mol Med. 2009;24:171–180. doi: 10.3892/ijmm_00000220. [DOI] [PubMed] [Google Scholar]
- 90.Pickens MK. Ogata H. Soon RK. Grenert JP. Maher JJ. Dietary fructose exacerbates hepatocellular injury when incorporated into a methionine-choline-deficient diet. Liver Int. 2010;30:1229–1239. doi: 10.1111/j.1478-3231.2010.02285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pickens MK. Yan JS. Ng RK. Ogata H. Grenert JP. Beyson C. Turner SM. Maher JJ. Dietary sucrose is essential to the development of liver injury in the MCD model of steatohepatitis. J Lipid Res. 2009;50:2072–2082. doi: 10.1194/jlr.M900022-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rangnekar AS. Lammert F. Igolnikov A. Green RM. Quantitative trait loci analysis of mice administered the methionine-choline deficient dietary model of experimental steatohepatitis. Liver Int. 2006;26:1000–1005. doi: 10.1111/j.1478-3231.2006.01314.x. [DOI] [PubMed] [Google Scholar]
- 93.Rector RS. Thyfault JP. Laye MJ. Morris RT. Borengasser SJ. Uptergrove GM. Chakravarthy MV. Booth FW. Ibdah JA. Cessation of daily exercise dramatically alters precursors of hepatic steatosis in Otsuka Long–Evans Tokushima Fatty (OLETF) rats. J Physiol. 2008;586:4241–4249. doi: 10.1113/jphysiol.2008.156745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rector RS. Thyfault JP. Morris RT. Laye MJ. Borengasser SJ. Booth FW. Ibdah JA. Daily exercise increases hepatic fatty acid oxidation and prevents steatosis in Otsuka Long–Evans Tokushima Fatty rats. Am J Physiol Gastrointest Liver Physiol. 2008;294:G619–626. doi: 10.1152/ajpgi.00428.2007. [DOI] [PubMed] [Google Scholar]
- 95.Rector RS. Thyfault JP. Uptergrove GM. Morris EM. Naples SP. Borengasser SJ. Mikus CR. Laye MJ. Laughlin MH. Booth FW. Ibdah JA. Mitochondrial dysfunction precedes insulin resistance and hepatic steatosis and contributes to the natural history of non-alcoholic fatty liver disease in an obese rodent model. J Hepatol. 2010;52:727–736. doi: 10.1016/j.jhep.2009.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rinella ME. Elias MS. Smolak RR. Fu T. Borensztajn J. Green RM. Mechanisms of hepatic steatosis in mice fed a lipogenic methionine choline-deficient diet. J Lipid Res. 2008;49:1068–1076. doi: 10.1194/jlr.M800042-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rinella ME. Green RM. The methionine-choline deficient dietary model of steatohepatitis does not exhibit insulin resistance. J Hepatol. 2004;40:47–51. doi: 10.1016/j.jhep.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 98.Rivera CA. Abrams SH. Tcharmtchi MH. Allman M. Ziba TT. Finegold MJ. Smith CW. Feeding a corn oil/sucrose-enriched diet enhances steatohepatitis in sedentary rats. Am J Physiol Gastrointest Liver Physiol. 2006;290:G386–393. doi: 10.1152/ajpgi.00229.2005. [DOI] [PubMed] [Google Scholar]
- 99.Rivera CA. Adegboyega P. van Rooijen N. Tagalicud A. Allman M. Wallace M. Toll-like receptor-4 signaling and Kupffer cells play pivotal roles in the pathogenesis of non-alcoholic steatohepatitis. J Hepatol. 2007;47:571–579. doi: 10.1016/j.jhep.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rizki G. Arnaboldi L. Gabrielli B. Yan J. Lee GS. Ng RK. Turner SM. Badger TM. Pitas RE. Maher JJ. Mice fed a lipogenic methionine-choline-deficient diet develop hypermetabolism coincident with hepatic suppression of SCD-1. J Lipid Res. 2006;47:2280–2290. doi: 10.1194/jlr.M600198-JLR200. [DOI] [PubMed] [Google Scholar]
- 101.Romestaing C. Piquet MA. Bedu E. Rouleau V. Dautresme M. Hourmand–Ollivier I. Filippi C. Duchamp C. Sibille B. Long term highly saturated fat diet does not induce NASH in Wistar rats. Nutr Metab (Lond) 2007;4:4. doi: 10.1186/1743-7075-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ronis MJ. Korourian S. Zipperman M. Hakkak R. Badger TM. Dietary saturated fat reduces alcoholic hepatotoxicity in rats by altering fatty acid metabolism and membrane composition. J Nutr. 2004;134:904–912. doi: 10.1093/jn/134.4.904. [DOI] [PubMed] [Google Scholar]
- 103.Rutkowski DT. Wu J. Back SH. Callaghan MU. Ferris SP. Iqbal J. Clark R. Miao H. Hassler JR. Fornek J. Katze MG. Hussain MM. Song B. Swathirajan J. Wang J. Yau GD. Kaufman RJ. UPR pathways combine to prevent hepatic steatosis caused by ER stress-mediated suppression of transcriptional master regulators. Dev Cell. 2008;15:829–840. doi: 10.1016/j.devcel.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sahai A. Malladi P. Pan X. Paul R. Melin–Aldana H. Green RM. Whitington PF. Obese and diabetic db/db mice develop marked liver fibrosis in a model of nonalcoholic steatohepatitis: Role of short-form leptin receptors and osteopontin. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1035–1043. doi: 10.1152/ajpgi.00199.2004. [DOI] [PubMed] [Google Scholar]
- 105.Sampath H. Miyazaki M. Dobrzyn A. Ntambi JM. Stearoyl-CoA desaturase-1 mediates the pro-lipogenic effects of dietary saturated fat. J Biol Chem. 2007;282:2483–2493. doi: 10.1074/jbc.M610158200. [DOI] [PubMed] [Google Scholar]
- 106.Samson SL. Gonzalez EV. Yechoor V. Bajaj M. Oka K. Chan L. Gene therapy for diabetes: Metabolic effects of helper-dependent adenoviral exendin 4 expression in a diet-induced obesity mouse model. Mol Ther. 2008;16:1805–1812. doi: 10.1038/mt.2008.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Schattenberg JM. Singh R. Wang Y. Lefkowitch JH. Rigoli RM. Scherer PE. Czaja MJ. JNK1 but not JNK2 promotes the development of steatohepatitis in mice. Hepatology. 2006;43:163–172. doi: 10.1002/hep.20999. [DOI] [PubMed] [Google Scholar]
- 108.Schattenberg JM. Wang Y. Singh R. Rigoli RM. Czaja MJ. Hepatocyte CYP2E1 overexpression and steatohepatitis lead to impaired hepatic insulin signaling. J Biol Chem. 2005;280:9887–9894. doi: 10.1074/jbc.M410310200. [DOI] [PubMed] [Google Scholar]
- 109.Seo YS. Kim JH. Jo NY. Choi KM. Baik SH. Park JJ. Kim JS. Byun KS. Bak YT. Lee CH. Kim A. Yeon JE. PPAR agonists treatment is effective in a nonalcoholic fatty liver disease animal model by modulating fatty-acid metabolic enzymes. J Gastroenterol Hepatol. 2008;23:102–109. doi: 10.1111/j.1440-1746.2006.04819.x. [DOI] [PubMed] [Google Scholar]
- 110.Singh A. Wirtz M. Parker N. Hogan M. Strahler J. Michailidis G. Schmidt S. Vidal–Puig A. Diano S. Andrews P. Brand MD. Friedman J. Leptin-mediated changes in hepatic mitochondrial metabolism, structure, and protein levels. Proc Natl Acad Sci USA. 2009;106:13100–13105. doi: 10.1073/pnas.0903723106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Singh R. Wang Y. Xiang Y. Tanaka KE. Gaarde WA. Czaja MJ. Differential effects of JNK1 and JNK2 inhibition on murine steatohepatitis and insulin resistance. Hepatology. 2009;49:87–96. doi: 10.1002/hep.22578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Spruss A. Kanuri G. Wagnerberger S. Haub S. Bischoff SC. Bergheim I. Toll-like receptor 4 is involved in the development of fructose-induced hepatic steatosis in mice. Hepatology. 2009;50:1094–1104. doi: 10.1002/hep.23122. [DOI] [PubMed] [Google Scholar]
- 113.St George A. Bauman A. Johnston A. Farrell G. Chey T. George J. Independent effects of physical activity in patients with nonalcoholic fatty liver disease. Hepatology. 2009;50:68–76. doi: 10.1002/hep.22940. [DOI] [PubMed] [Google Scholar]
- 114.Strable MS. Ntambi JM. Genetic control of de novo lipogenesis: Role in diet-induced obesity. Crit Rev Biochem Mol Biol. 2010;45:199–214. doi: 10.3109/10409231003667500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Su Q. Tsai J. Xu E. Qiu W. Bereczki E. Santha M. Adeli K. Apolipoprotein B100 acts as a molecular link between lipid-induced endoplasmic reticulum stress and hepatic insulin resistance. Hepatology. 2009;50:77–84. doi: 10.1002/hep.22960. [DOI] [PubMed] [Google Scholar]
- 116.Sugimoto H. Okada K. Shoda J. Warabi E. Ishige K. Ueda T. Taguchi K. Yanagawa T. Nakahara A. Hyodo I. Ishii T. Yamamoto M. Deletion of nuclear factor-E2-related factor-2 leads to rapid onset and progression of nutritional steatohepatitis in mice. Am J Physiol Gastrointest Liver Physiol. 2010;298:G283–294. doi: 10.1152/ajpgi.00296.2009. [DOI] [PubMed] [Google Scholar]
- 117.Svegliati–Baroni G. Candelaresi C. Saccomanno S. Ferretti G. Bachetti T. Marzioni M. De Minicis S. Nobili L. Salzano R. Omenetti A. Pacetti D. Sigmund S. Benedetti A. Casini A. A model of insulin resistance and nonalcoholic steatohepatitis in rats: role of peroxisome proliferator-activated receptor-alpha and n-3 polyunsaturated fatty acid treatment on liver injury. Am J Pathol. 2006;169:846–860. doi: 10.2353/ajpath.2006.050953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tetri LH. Basaranoglu M. Brunt EM. Yerian LM. Neuschwander–Tetri BA. Severe NAFLD with hepatic necroinflammatory changes in mice fed trans fats and a high-fructose corn syrup equivalent. Am J Physiol Gastrointest Liver Physiol. 2008;295:G987–995. doi: 10.1152/ajpgi.90272.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tomita K. Oike Y. Teratani T. Taguchi T. Noguchi M. Suzuki T. Mizutani A. Yokoyama H. Irie R. Sumimoto H. Takayanagi A. Miyashita K. Akao M. Tabata M. Tamiya G. Ohkura T. Hibi T. Hepatic AdipoR2 signaling plays a protective role against progression of nonalcoholic steatohepatitis in mice. Hepatology. 2008;48:458–473. doi: 10.1002/hep.22365. [DOI] [PubMed] [Google Scholar]
- 120.Uysal KT. Wiesbrock SM. Marino MW. Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature. 1997;389:610–614. doi: 10.1038/39335. [DOI] [PubMed] [Google Scholar]
- 121.Velayudham A. Dolganiuc A. Ellis M. Petrasek J. Kodys K. Mandrekar P. Szabo G. VSL#3 probiotic treatment attenuates fibrosis without changes in steatohepatitis in a diet-induced nonalcoholic steatohepatitis model in mice. Hepatology. 2009;49:989–997. doi: 10.1002/hep.22711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Villanueva CJ. Monetti M. Shih M. Zhou P. Watkins SM. Bhanot S. Farese RV., Jr. Specific role for acyl CoA:diacylglycerol acyltransferase 1 (Dgat1) in hepatic steatosis due to exogenous fatty acids. Hepatology. 2009;50:434–442. doi: 10.1002/hep.22980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Vinciguerra M. Sgroi A. Veyrat–Durebex C. Rubbia–Brandt L. Buhler LH. Foti M. Unsaturated fatty acids inhibit the expression of tumor suppressor phosphatase and tensin homolog (PTEN) via microRNA-21 up-regulation in hepatocytes. Hepatology. 2009;49:1176–1184. doi: 10.1002/hep.22737. [DOI] [PubMed] [Google Scholar]
- 124.Vizzutti F. Provenzano A. Galastri S. Milani S. Delogu W. Novo E. Caligiuri A. Zamara E. Arena U. Laffi G. Parola M. Pinzani M. Marra F. Curcumin limits the fibrogenic evolution of experimental steatohepatitis. Lab Invest. 2010;90:104–115. doi: 10.1038/labinvest.2009.112. [DOI] [PubMed] [Google Scholar]
- 125.Wang D. Wei Y. Pagliassotti MJ. Saturated fatty acids promote endoplasmic reticulum stress and liver injury in rats with hepatic steatosis. Endocrinology. 2006;147:943–951. doi: 10.1210/en.2005-0570. [DOI] [PubMed] [Google Scholar]
- 126.Weisberg SP. Hunter D. Huber R. Lemieux J. Slaymaker S. Vaddi K. Charo I. Leibel RL. Ferrante AW., Jr. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006;116:115–124. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Witek RP. Stone WC. Karaca FG. Syn WK. Pereira TA. Agboola KM. Omenetti A. Jung Y. Teaberry V. Choi SS. Guy CD. Pollard J. Charlton P. Diehl AM. Pan-caspase inhibitor VX-166 reduces fibrosis in an animal model of nonalcoholic steatohepatitis. Hepatology. 2009;50:1421–1430. doi: 10.1002/hep.23167. [DOI] [PubMed] [Google Scholar]
- 128.Xu A. Wang Y. Keshaw H. Xu LY. Lam KS. Cooper GJ. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J Clin Invest. 2003;112:91–100. doi: 10.1172/JCI17797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yamaguchi K. Yang L. McCall S. Huang J. Yu XX. Pandey SK. Bhanot S. Monia BP. Li YX. Diehl AM. Diacylglycerol acyltranferase 1 anti-sense oligonucleotides reduce hepatic fibrosis in mice with nonalcoholic steatohepatitis. Hepatology. 2008;47:625–635. doi: 10.1002/hep.21988. [DOI] [PubMed] [Google Scholar]
- 130.Yamaguchi K. Yang L. McCall S. Huang J. Yu XX. Pandey SK. Bhanot S. Monia BP. Li YX. Diehl AM. Inhibiting triglyceride synthesis improves hepatic steatosis but exacerbates liver damage and fibrosis in obese mice with nonalcoholic steatohepatitis. Hepatology. 2007;45:1366–1374. doi: 10.1002/hep.21655. [DOI] [PubMed] [Google Scholar]
- 131.Yamazaki Y. Kakizaki S. Horiguchi N. Sohara N. Sato K. Takagi H. Mori M. Negishi M. The role of the nuclear receptor constitutive androstane receptor in the pathogenesis of non-alcoholic steatohepatitis. Gut. 2007;56:565–574. doi: 10.1136/gut.2006.093260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.You M. Considine RV. Leone TC. Kelly DP. Crabb DW. Role of adiponectin in the protective action of dietary saturated fat against alcoholic fatty liver in mice. Hepatology. 2005;42:568–577. doi: 10.1002/hep.20821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.You M. Liang X. Ajmo JM. Ness GC. Involvement of mammalian sirtuin 1 in the action of ethanol in the liver. Am J Physiol Gastrointest Liver Physiol. 2008;294:G892–898. doi: 10.1152/ajpgi.00575.2007. [DOI] [PubMed] [Google Scholar]
- 134.You M. Matsumoto M. Pacold CM. Cho WK. Crabb DW. The role of AMP-activated protein kinase in the action of ethanol in the liver. Gastroenterology. 2004;127:1798–1808. doi: 10.1053/j.gastro.2004.09.049. [DOI] [PubMed] [Google Scholar]
- 135.Yu J. Chu ES. Wang R. Wang S. Wu CW. Wong VW. Chan HL. Farrell GC. Sung JJ. Heme oxygenase-1 protects against steatohepatitis in both cultured hepatocytes and mice. Gastroenterology. 2010;138:694–704. doi: 10.1053/j.gastro.2009.09.058. , 704 e691. [DOI] [PubMed] [Google Scholar]
- 136.Zeisel SH. da Costa KA. Albright CD. Shin OH. Choline and hepatocarcinogenesis in the rat. Adv Exp Med Biol. 1995;375:65–74. doi: 10.1007/978-1-4899-0949-7_6. [DOI] [PubMed] [Google Scholar]
- 137.Zhang K. Shen X. Wu J. Sakaki K. Saunders T. Rutkowski DT. Back SH. Kaufman RJ. Endoplasmic reticulum stress activates cleavage of CREBH to induce a systemic inflammatory response. Cell. 2006;124:587–599. doi: 10.1016/j.cell.2005.11.040. [DOI] [PubMed] [Google Scholar]
- 138.Zhang S. Wang J. Liu Q. Harnish DC. Farnesoid X receptor agonist WAY-362450 attenuates liver inflammation and fibrosis in murine model of non-alcoholic steatohepatitis. J Hepatol. 2009;51:380–388. doi: 10.1016/j.jhep.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 139.Zhang YK. Yeager RL. Tanaka Y. Klaassen CD. Enhanced expression of Nrf2 in mice attenuates the fatty liver produced by a methionine- and choline-deficient diet. Toxicol Appl Pharmacol. 2010;245:326–334. doi: 10.1016/j.taap.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhou M. Xu A. Tam PK. Lam KS. Chan L. Hoo RL. Liu J. Chow KH. Wang Y. Mitochondrial dysfunction contributes to the increased vulnerabilities of adiponectin knockout mice to liver injury. Hepatology. 2008;48:1087–1096. doi: 10.1002/hep.22444. [DOI] [PMC free article] [PubMed] [Google Scholar]