Abstract
Marker genes are used to monitor chondrogenic differentiation, but little is known about the turnover of their mRNA during this process. We set out to measure the half life of mRNA encoding the transcription factor SOX9, an important marker of chondrocytic phenotype. We dedifferentiated human articular chondrocytes in monolayer culture before placing them in chondrogenic three-dimensional pellet cultures. At the same time, we induced chondrocytic differentiation of human bone marrow–derived mesenchymal stem cells under the same three-dimensional conditions. Pellets were cultured in standard chondrogenic media with and without BMP7. We found that SOX9 mRNA half life exhibited an inverse correlation with total SOX9 mRNA levels in both dedifferentiating human articular chondrocytes and chondrogenic pellet cultures. There was no evidence for a specific effect of BMP7 on SOX9 mRNA decay. Our findings provide an insight into a level of gene control rarely explored in regenerative medicine, which could be important in the optimization of in vitro cartilage production.
Introduction
Articular cartilage is an attractive target for tissue engineering approaches due to its lack of vascularization and single-resident cell type, the chondrocyte.1 An underlying requirement for cartilage tissue engineering is the generation of suitable numbers of chondrocytes expressing abundant cartilage-specific extracellular matrix (ECM) molecules. Many studies have been able to demonstrate cartilage matrix synthesis from different cell sources, achieved on a number of scaffold materials as well as within gels and in pellet culture systems.2,3 Examples range from juvenile chondrocytes that have been freshly isolated from tissue,4 osteoarthritic chondrocytes which have had a period of monolayer culture to expand their numbers,5 and chondrocytes derived from in vitro differentiation of adult stem cells.6 The presence of transforming growth factor β (TGFβ) stimulation appears to be necessary for efficient cartilage ECM formation in these systems7–9, and further benefits can be achieved by stimulating the cells mechanically and by changing physiochemical properties of the cultures.10–12 There is also evidence that application of other members of the TGFβ superfamily, such as bone morphogenetic proteins (BMPs)-2, 4 or 7, can enhance the formation of cartilage ECM by chondrocytes in vitro.13–15 BMP7, in particular, has been demonstrated to improve the performance of TGFβ-based chondrogenic media in chondrocytes and stem cells-derived adipose tissue.16
The success of the constructs generated by these methods is often determined by examining the phenotype of the cells contained within the newly generated tissue and the tissue composition. Two of the most commonly used molecular markers for assessing chondrocyte phenotype are the COL2A1 gene, which encodes the cartilage specific collagen type II, and the transcription factor SOX9. The latter is known to act on cis-elements within genes encoding cartilage matrix proteins, such as COL2A1, and promote their transcription.17 Chimeric mice studies have underlined the important role SOX9 has in promoting the expression of cartilage matrix genes by showing that SOX9 null cells are unable to differentiate into chondrocytes.18 Various gene therapy approaches employing viral methods to over-express SOX9 have led to significant improvements in the production of cartilaginous matrix by articular chondrocytes, bone marrow–derived stem cells and nucleus pulposus cells.8,19,20
The steady-state levels of mRNA molecules are determined not only by their rate of transcription but also by their rate of decay.21 Control of mRNA decay is complex, involving binding of microRNAs and RNA binding proteins to regions within the 3′ untranslated region.22 Due to this, the rate of decay of individual mRNAs varies widely from transcript to transcript and can be regulated in response to external cues, for instance, stress stimuli.23 Clearly, when investigating chondrocyte phenotype, measuring mRNA levels for marker genes only provides information on the steady-state level of mRNA but does not provide information on how this steady state arrived. We have previously demonstrated that SOX9 mRNA exhibits a moderately short half life in human articular chondrocytes (HACs) which can be extended if the cells are exposed to environmental stresses such as cycloheximide treatment or hyperosmolarity.24,25 However, no studies have examined how its post-transcriptional gene regulation might vary at different stages of chondrocytic differentiation. To address this, we measured the half life of SOX9 mRNA in freshly isolated and de-differentiated chondrocytes in monolayer culture and compared them with those found in chondrogenic pellet cultures formed from passaged chondrocytes or bone marrow–derived stem cells.
Materials and Methods
Isolation and growth of HACs and bone marrow–derived stem cells
Human articular cartilage was obtained from patients undergoing total knee arthroplasty due to osteoarthritis. Tissue was obtained with informed consent after approval from the Cheshire Research Ethics Committee. Cartilage was dissected from areas that appeared macroscopically intact and digested overnight in Dulbecco's modified Eagles medium containing 10% fetal bovine serum, Gentamicin, Fungazone, and 0.08% Collagenase type II (all from Invitrogen). HACs were plated on standard tissue culture plastic at high density (100,000 cells/cm2), were analyzed 24 h later for passage 0 (P0) samples, or were plated at 50,000 cells/cm2 and grown for 1 or 2 passages. Human bone marrow mononuclear cells were obtained commercially (Stem Cell Technologies), and an entire vial containing 25×106 cells was thawed and cultured overnight in a 175 cm2 flask containing medium from the mesenchymal stem cell bullet kit (Lonza). The next day, the media and nonadherent cells were removed, and the flask was washed thrice with Hanks buffered saline solution (Invitrogen). The remaining adherent bone marrow–derived mesenchymal stem cells (BMSC) were subsequently cultured in mesenchymal stem cell bullet kit media supplemented with 5 ng/mL fibroblast growth factor 2 (R&D Systems) for up to three passages using a 1:3 split ratio. All HAC and BMSC cultures were incubated at 37°C in 5% CO2 and atmospheric O2.
Three-dimensional pellet cultures
Pellet cultures of HAC or BMSC were formed by centrifuging 5×105 cells at 150 g for 5 min in a 15 mL polypropylene tube and culturing the cell aggregate in serum-free Dulbecco's modified Eagles medium supplemented with ITS1+, 50 μg/mL ascorbate 2-phosphate, 10 nM dexamethasone (all three from Sigma), and 10 ng/mL TGFβ-1 (obtained from Peprotech) for up to 14 days with media changes every 2–3 days. In some cultures, the media was further supplemented with 100 ng/mL BMP7(obtained from Peprotech). The wet weight of pellets was determined at the end of the culture period. All pellet cultures were incubated at 37°C in 5% CO2 and atmospheric O2.
Histological analysis
Pellet cultures were fixed in phosphate-buffered saline containing 4% formaldehyde, processed into paraffin wax blocks, sectioned, and mounted on glass slides. Sections were stained with safranin-O and examined using a Nikon Eclipse 80i microscope.
Real-time polymerase chain reaction analysis
Total RNA was isolated from monolayer and pellet cultures using Tri Reagent (Ambion). Pellet cultures were ground up in the Tri Reagent in a 1.5 mL centrifuge tube using a plastic pestle. cDNA was generated by reverse transcription using MMLV reverse transcriptase primed with random hexamers (Promega). Real-time polymerase chain reaction (PCR) was performed on an Applied Biosystems 7300 instrument using SYBR Green or Taqman PCR Mastermix (Applied Biosystems) and previously described primers and Taqman probes specific to either SOX9 (Taqman), the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH–Taqman/SYBR), COL2A1 (SYBR), or COLXA1 (SYBR).25,26 PCR efficiencies were determined by examining change in cycle threshold (ΔCt) value over six serial twofold dilutions. When ΔCt and log2(dilution) were plotted, GAPDH, COL2A1, and COLXA1 primer sets demonstrated a linear relationship with a slope close to 1 as has been previously described.26 The SOX9 PCR reaction is of our own design, and its efficiency was comparable to the other reactions (slope=0.944). Due to the similar reaction efficiencies, expression levels were able to be determined using the 2^Ct method, normalized to GAPDH. Melt curve analysis was performed for all of the SYBR green measurements, and only one product was observed in each instance. All primers and probes were synthesized by Eurogentec.
mRNA decay analysis
To measure mRNA decay, 1 μM Actinomycin D, an RNA polymerase inhibitor that prevents transcription, was added to the culture media; and RNA samples were obtained at a number of time points for up to 5 h. SOX9 mRNA copy number was determined throughout the decay series using reverse transcription and real-time PCR; and then half life was determined by regression analysis as previously described.24
Statistical tests
When measuring total RNA levels in pellet cultures, pellet measurements were made from two pellets from each donor with the mean expression used. Expression was measured in three donors for each cell type. Half-life measurements were generated for each cell type and donor from decay curves generated using 7–10 pellets per donor. HAC and BMSC monolayer expression and half-life analysis were carried out using cells from three donors. Analysis of changes in total mRNA levels and half-life levels was determined by one-way analysis of variance with Dunnett's post-hoc test or by unpaired student's t-tests using SPSS software. 2−Ct values representing total mRNA levels were logarithmically transformed to base 10 before analysis to ensure that the data followed a normal distribution. Correlation between mRNA levels and half life was determined by linear regression using online analytical tools at http://zunzun.com.
Results
Loss of chondrocyte phenotype is associated with stabilization of SOX9 mRNA
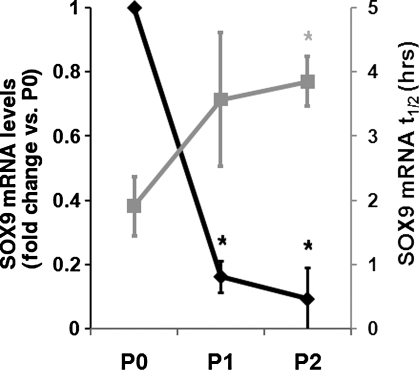
Since articular chondrocytes are grown in monolayer culture, they undergo a well-categorized process of dedifferentiation that includes loss of marker gene expression, including SOX9. In the present study, expression of SOX9 did indeed drop markedly as HAC were expanded in monolayer culture. Compared with freshly isolated HAC (P0), those grown to passages 1 or 2 exhibited 6.3 or 10-fold lower levels of SOX9 mRNA, respectively (Fig. 1). Interestingly, examination of the post-transcriptional regulation revealed that as levels of the SOX9 mRNA were falling, its half life was increased, with the average level rising from 1.9 h at P0 to 3.9 h at P2 (Fig. 1).
FIG. 1.
SOX9 mRNA decay during HAC dedifferentiation. The black data points and left hand y-axis illustrate the expression level of SOX9 mRNA in freshly isolated (P0) and passages 1 and 2 (P1 and P2) HAC. Gray data points and the right hand y-axis show the half life of SOX9 mRNA in hours at the same stages, determined as described in the materials and methods. In all instances, SOX9 mRNA levels were measured using real-time PCR. *p<0.05 (one-way ANOVA with Dunnett's post hoc test)–the shade of the asterisk indicates which dataset it relates to. Data are presented as mean and standard deviation of the expression measured in three donors. HAC, human articular chondrocyte; ANOVA, analysis of variance.
Chondrogenic differentiation of passaged HAC and BMSCs
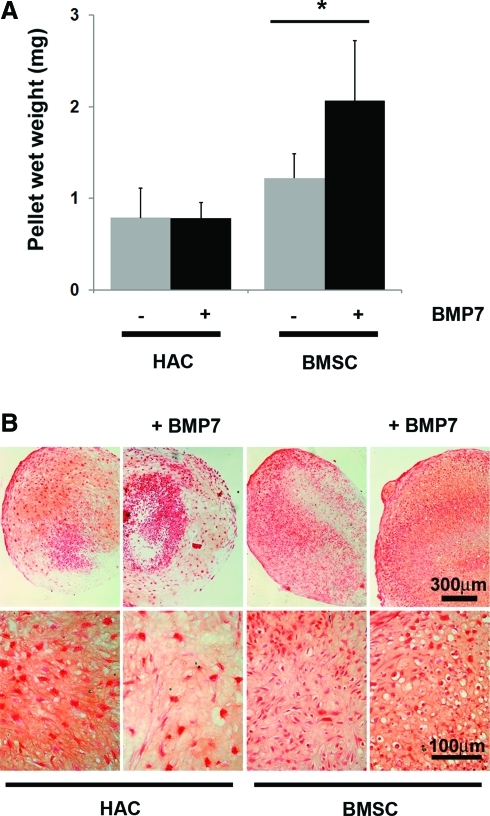
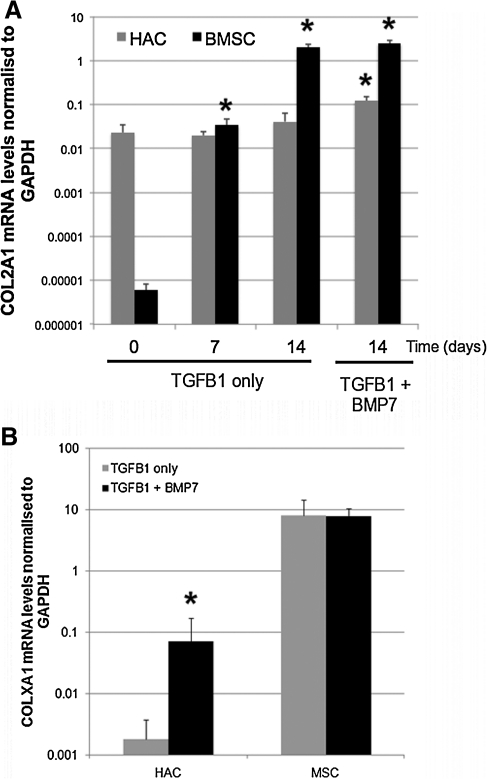
We set up pellet cultures using previously defined protocols to encourage chondrogenic differentiation of either P2 HAC or P3 BMSCs. We conducted these cultures either in standard chondrogenic media that contained TGFβ-1 or in media that had been further supplemented with the chondrogenic factor BMP7. Measurement of the wet weight of pellets after 14 days of culture showed that the BMSC were slightly heavier than the HAC in the standard culture media (Fig. 2A). The HAC pellets' mass was no larger when they had been cultured in the BMP7 supplemented media; but BMSC pellets were significantly heavier after BMP7 stimulation. We also histologically examined the morphology of 14 day pellet cultures (Fig. 2B). Safranin-O staining of sections showed that deposition of a glycosaminoglycan-rich ECM had occurred in all cultures, although the distribution was more uniform in BMSC than in HAC. HAC pellets contained large, irregular-shaped cells; whereas those formed from BMSCs had a greater proportion of smaller, rounded cells. Pellet cultures from both cell types exhibited a greater number of rounded cells in BMP7 containing media. Real-time PCR analysis of COL2A1 expression was performed to further characterize the phenotype of cells within the pellets (Fig. 3A). COL2A1 levels were not significantly altered from levels in passage 2 monolayer culture by culturing HAC in pellets for up to 14 days in standard chondrogenic media but were increased threefold, however, in HAC pellet cultures supplemented with BMP7. Monolayer cultures of BMSCs expressed barely detectable levels of COL2A1; but by 7 days of chondrogenic pellet culture, the levels were raised 5000-fold to a level comparable with monolayer HAC. By 14 days, the levels of COL2A1 had risen to 33,000-fold higher than was seen in the monolayer cultures and higher than even the 14 day HAC pellets grown with BMP7. Supplementation with BMP7 did not significantly affect the COL2A1 mRNA levels after 14 days in BMSC pellet culture. Analysis of 14-day pellet cultures using primers to COLXA1 showed that MSCs expressed significantly higher levels of this terminal differentiation marker in comparison to HAC (Fig. 3B). The levels in MSCs were not affected by treatment with BMP7, unlike HAC, whose COLXA1 expression was increased 40-fold in BMP7 supplemented pellets.
FIG. 2.
In vitro chondrogenic pellet culture using HAC or BMSCs. (A) Wet weight and (B) Safranin O stained paraffin wax sections of HAC or BMSC pellet cultures that had been grown for 14 days culture in standard chondrogenic media with or without supplementation with 100 ng/mL BMP7. *p<0.05–effect of BMP7 on BMSC wet weight (unpaired students t-test). Data are presented as mean and standard deviation of the expression measured in three donors. BMSC, bone marrow–derived mesenchymal stem cell; BMP, bone morphogenetic protein. Color images available online at www.liebertonline.com/tea
FIG. 3.
COL2A1 and COLXA1 mRNA expression levels in HAC and BMSC chondrogenic pellet cultures. (A) Pellets from HAC and BMSC were cultured in chondrogenic media for 7 and 14 days; and COL2A1 mRNA levels were determined. Additionally, 14 day pellets whose media was supplemented with BMP7 were also examined. Day 0 refers to cell monolayers before pellet culture. *p<0.05 versus day 0 (one-way ANOVA with Dunnett's post hoc test). Note from the y-axis scales that the difference in COL2A1 expression levels was cultured in chondrogenic media with and without BMP7 for 14 days and COLXA1 between the HAC and the BMSC cultures (B) Pellets from HAC and BMSC mRNA levels determined. *p<0.05 effect of BMP7 supplementation (unpaired students t-test). Data are presented as mean and standard deviation of arbitrary 2−Ct relative expression values measured in three donors. TGFβ-1, transforming growth factor β-1; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
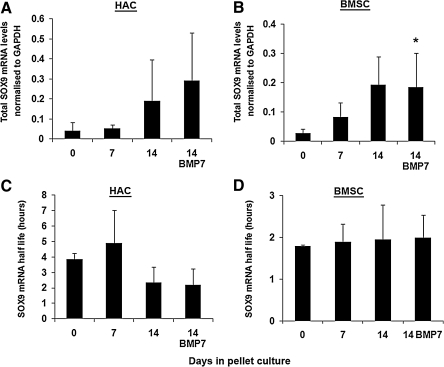
Average SOX9 mRNA decay rates are not altered during standard in vitro chondrogenesis
We next examined the change in SOX9 mRNA levels in P2 HAC grown as pellet cultures in chondrogenic media (Fig. 4A). Levels of SOX9 mRNA in the pellet cultures varied considerably from donor to donor. No significant change in expression had occurred after 7 days of pellet culture; but 14-day BMP7 supplemented HAC pellet cultures demonstrated a significant increase in SOX9 mRNA, whereas 14-day non-BMP7 cultures demonstrated a trend toward significance (p=0.082). To examine the degree to which post transcriptional gene control might be involved in this process, SOX9 mRNA half life was determined for HAC pellet cultures grown for up to 14 days in standard chondrogenic media. However, despite a drop in the mean half life in the 14-day pellet cultures, no statistically significant change was observed (Fig. 4C). BMSCs grown in pellet cultures had significantly higher levels of SOX9 mRNA after 14 days in cultures in the presence and absence of BMP7 (Fig. 4B). The half life of SOX9 mRNA in the monolayer (time 0) BMSC cultures was lower than that in HAC, and it did not change significantly through the course of the 14-day pellet culture, remaining at around 2 h (Fig. 4D).
FIG. 4.
Analysis of SOX9 mRNA levels and decay rates in HAC and BMSC chondrogenic pellet cultures. Pellets from (A, C) HAC and (B, D) BMSC were cultured in chondrogenic media for 7 and 14 days and for 14 days in media further supplemented with BMP7. Day 0 involves monolayer cultures before pellet formation. The overall levels (A, B) and the half life (C, D) of SOX9 mRNA were measured in these cultures. *p<0.05 versus Day 0 (One-way ANOVA with Dunnett's post hoc test). Data are presented as mean and standard deviation of arbitrary 2−Ct relative expression values measured in three donors.
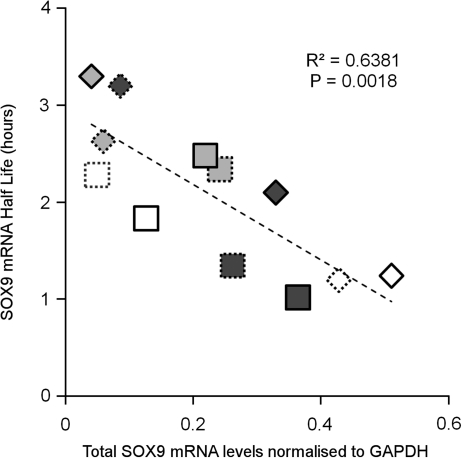
SOX9 mRNA decay rates correlate with total levels of the transcript
Despite no change in average SOX9 mRNA decay rates under the different pellet culture conditions, we did observe considerable variations in total SOX9 mRNA level and half life of the transcript between donors in pellet cultures from both cell types. We, therefore, compared SOX9 expression levels with SOX9 mRNA half lives for all 14-day pellet cultures from both HAC or BMSC (Fig. 5). This involved pooling data from pellets grown in both standard chondrogenic media and BMP7-supplemented media from each cell type. Interestingly, we found that there was a significant inverse relationship between overall SOX9 mRNA levels and the half life of SOX9 mRNA (R2=0.6381, p<0.002) in the chondrocytic pellet cultures.
FIG. 5.
Correlation of SOX9 mRNA turnover with SOX9 mRNA expression. Linear regression analysis of SOX9 half-life values versus total SOX9 mRNA levels for pooled data of 14-day pellet cultures formed from HAC or BMSC, cultured both with and without BMP7. Total mRNA represented as arbitrary 2−Ct relative expression values. Diamond-shaped data points represent HAC, whereas square points represent BMSC. Dashed border=chondrogenic media, solid border=chondrogenic media+BMP7. Different fill shades (white, light gray, and dark gray) define different donors within each cell type. Note that MSCs and HAC were already from separate donors.
Discussion
In the vast majority of tissue engineering studies, there is no examination of marker gene regulation beyond the measurement of the steady-state levels that exist at a given time point. This is understandable given the additional number of experimental samples required to determine rates of mRNA decay. However, with our increased understanding of the importance of post-transcriptional gene regulation, through processes controlled by RNA binding proteins and microRNAs, it is interesting to examine how this tier of control may be altered during cellular differentiation. In this study, we began to investigate whether post-transcriptional control of SOX9 mRNA was linked to the chondrocyte differentiation state. We have shown that the rate of decay of SOX9 mRNA is more rapid in freshly isolated HAC than in those that have been allowed to dedifferentiate in monolayer culture. This is despite a drop in the overall levels of SOX9 mRNA as the cells adjust to a more fibrotic phenotype. When the passaged HAC were placed in chondrogenic pellet culture conditions, they did not perform as well as BMSCs with regard to their COL2A1 gene expression and wet weight. Total levels of SOX9 mRNA and the rate of its decay varied considerably from donor to donor, although increases in total SOX9 mRNA levels were observed after 14 days of culture. The BMSCs demonstrated strong chondrogenic induction with a large degree of terminal differentiation as demonstrated by their high COLXA1 expression. The BMSCs exhibit a rapid turnover of SOX9 in both their undifferentiated state in monolayer culture and as they differentiate into chondrocytic cells in pellet culture. This was despite a 16-fold difference in SOX9 expression between the undifferentiated monolayers and the chondrocytic pellet cultures. Taken together, our results indicate that although a relationship between SOX9 mRNA levels and SOX9 mRNA turnover exists, it is limited in this study to those cells expressing chondrocytic characteristics.
Mouse transgenic studies have indicated that absolute levels of SOX9 might play a very important role during embryonic development.27 It is possible that fine tuning of SOX9 mRNA decay rates, through some form of feedback mechanism, keeps overall levels of SOX9 mRNA within a certain threshold, even as transcriptional rates change. This form of feedback control through regulation of RNA turnover has already been shown to limit the levels of tumor necrosis factor α mRNA.28 There is evidence that feedback loops that employ post-transcriptional regulation control transcriptional regulation of microRNA genes by proteins whose transcripts are targeted by the genes' miRNA products.29 The effect of altering the half life of RNAs is complex. Given a constant rate of transcription, a long half life will lead to an increase in accumulation of the mRNA. However, a longer half life will also cause a slower increase in fold levels of an mRNA in response to increased transcription. Conversely, mRNAs that have short half lives will exhibit more rapid fold inductions in response to enhanced transcription and will also be down-regulated more rapidly when the transcription rates return to starting levels.30 The BMSC and freshly isolated HAC maintain a rapid decay of SOX9 mRNA, which would allow them greater flexibility to rapidly alter SOX9 transcript levels as conditions demanded. The dedifferentiated HAC, on the other hand, display an extended half life of SOX9 mRNA, which is likely to lead to a pool of transcript that is less responsive to altered transcriptional input with regard to fold change.
Despite the difference in SOX9 mRNA levels between cultures, the high efficiency of our PCR assays over a wide range of dilutions of cDNA reassured us that the observed changes in half life had not arisen due to technical bias. Although the mean SOX9 half life of the dedifferentiated HAC was reduced after 14 days in pellet culture, the variation between samples did not allow us to conclude statistically that this was a change. However, by combining the data from 14-day pellet cultures with and without BMP7, we were able to show a negative correlation between total SOX9 mRNA levels and SOX9 mRNA half life within HAC and BMSC pellet cultures. This finding fits with the observed changes in HAC during their dedifferentiation. We noted that the average level of SOX9 mRNA half life in the 14-day HAC pellet cultures is not as low as that of freshly isolated HAC, thus suggesting that these cells have not fully regained their earlier phenotype. The inability of these cells to readjust their post-transcriptional gene regulatory patterns is, thus, linked to their expression of SOX9; and this may be a factor that limits the effectiveness of dedifferentiated chondrocytes in forming cartilage in vitro and even in vivo. Certainly, elevated expression of SOX9 is able to greatly improve ECM production by these cells in three-dimensional culture.8 Clearly, future work to investigate how manipulation of SOX9 mRNA levels at the post-transcriptional level dedifferentiated in HAC would be worthwhile. An additional factor that needs to be borne in mind is the effect of pathology on the HAC, having come from osteoarthritic cartilage and potential exposure to inflammatory factors. The potential for pathology to alter RNA decay is fascinating and is the subject of ongoing work in our laboratory.
We have utilized BMP7 as an additional chondrogenic stimulus in these studies. This factor has now been demonstrated in a number of studies to promote in vitro chondrogenic differentiation31,32 and cartilage matrix formation33–35 and to improve in vivo cartilage repair.36,37 In our experiments, BMP7 supplementation of the TGFβ-1-containing chondrogenic media led to increased COL2A1 and COLXA1 expression in HAC pellets and greater wet weight of BMSC pellets. Studies in mouse bone marrow stromal cells have demonstrated that BMP7 can promote SOX9 expression in BMP-2 knockdown cells during osteogenic differentiation.38 However, it did not significantly affect overall SOX9 mRNA levels or mRNA decay rates in our chondrogenic culture systems; and in our final correlation analysis, the BMP7 and non-BMP7 data points for each donor/cell type were, in most cases, close together. (Fig. 5).
In conclusion, we have demonstrated a novel relationship between SOX9 expression levels and SOX9 post-transcriptional control. HAC monolayer and pellet cultures, expressing lower levels of SOX9 mRNA, had half lives that were up to twice as long as freshly isolated chondrocytes expressing high levels of SOX9 mRNA. However, a shorter SOX9 half life did not define the chondrocytic cell, as the SOX9 transcript also decayed quickly in undifferentiated BMSCs. Instead, the correlation of total levels of SOX9 mRNA with its half life may be a property of the chondrocyte phenotype. This regulation adds an increased level of complexity on top of the stress-induced SOX9 mRNA half-life regulation that we have previously demonstrated in HAC.24,25 An increased understanding of how chondrocytes regulate important genes such as SOX9 at the post-transcriptional level and how important this process is to cartilage matrix homeostasis and cell phenotype will be important as therapeutic approaches to cartilage resurfacing continue to develop.
Acknowledgments
The authors wish to thank Richard Parkinson and the staff at Clatterbridge Hospital, Wirral NHS Trust, for provision of human joint tissue. This work was supported by a grant from Arthritis Research United Kingdom.
Disclosure Statement
No competing financial interests exist.
References
- 1.Hardingham T. Tew S. Murdoch A. Tissue engineering: chondrocytes and cartilage. Arthritis Res. 2002;4(Suppl 3):S63. doi: 10.1186/ar561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vinatier C. Mrugala D. Jorgensen C. Guicheux J. Noel D. Cartilage engineering: a crucial combination of cells, biomaterials and biofactors. Trends Biotechnol. 2009;27:307. doi: 10.1016/j.tibtech.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 3.Nesic D. Whiteside R. Brittberg M. Wendt D. Martin I. Mainil-Varlet P. Cartilage tissue engineering for degenerative joint disease. Adv Drug Deliv Rev. 2006;58:300. doi: 10.1016/j.addr.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 4.Hayes A.J. Hall A. Brown L. Tubo R. Caterson B. Macromolecular organization and in vitro growth characteristics of scaffold-free neocartilage grafts. J Histochem Cytochem. 2007;55:853. doi: 10.1369/jhc.7A7210.2007. [DOI] [PubMed] [Google Scholar]
- 5.Katopodi T. Tew S.R. Clegg P.D. Hardingham T.E. The influence of donor and hypoxic conditions on the assembly of cartilage matrix by osteoarthritic human articular chondrocytes on Hyalograft matrices. Biomaterials. 2009;30:535. doi: 10.1016/j.biomaterials.2008.09.064. [DOI] [PubMed] [Google Scholar]
- 6.Murdoch A.D. Grady L.M. Ablett M.P. Katopodi T. Meadows R.S. Hardingham T.E. Chondrogenic differentiation of human bone marrow stem cells in transwell cultures: generation of scaffold-free cartilage. Stem Cells. 2007;25:2786. doi: 10.1634/stemcells.2007-0374. [DOI] [PubMed] [Google Scholar]
- 7.Barry F. Boynton R.E. Liu B. Murphy J.M. Chondrogenic differentiation of mesenchymal stem cells from bone marrow: differentiation-dependent gene expression of matrix components. Exp Cell Res. 2001;268:189. doi: 10.1006/excr.2001.5278. [DOI] [PubMed] [Google Scholar]
- 8.Tew S.R. Li Y. Pothacharoen P. Tweats L.M. Hawkins R.E. Hardingham T.E. Retroviral transduction with SOX9 enhances re-expression of the chondrocyte phenotype in passaged osteoarthritic human articular chondrocytes. Osteoarthritis Cartilage. 2005;13:80. doi: 10.1016/j.joca.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 9.Johnstone B. Hering T.M. Caplan A.I. Goldberg V.M. Yoo J.U. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998;238:265. doi: 10.1006/excr.1997.3858. [DOI] [PubMed] [Google Scholar]
- 10.Schulz R.M. Bader A. Cartilage tissue engineering and bioreactor systems for the cultivation and stimulation of chondrocytes. Eur Biophys J. 2007;36:539. doi: 10.1007/s00249-007-0139-1. [DOI] [PubMed] [Google Scholar]
- 11.Kisiday J.D. Jin M. DiMicco M.A. Kurz B. Grodzinsky A.J. Effects of dynamic compressive loading on chondrocyte biosynthesis in self-assembling peptide scaffolds. J Biomech. 2004;37:595. doi: 10.1016/j.jbiomech.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Negoro K. Kobayashi S. Takeno K. Uchida K. Baba H. Effect of osmolarity on glycosaminoglycan production and cell metabolism of articular chondrocyte under three-dimensional culture system. Clin Exp Rheumatol. 2008;26:534. [PubMed] [Google Scholar]
- 13.Sekiya I. Larson B.L. Vuoristo J.T. Reger R.L. Prockop D.J. Comparison of effect of BMP-2, −4, and −6 on in vitro cartilage formation of human adult stem cells from bone marrow stroma. Cell Tissue Res. 2005;320:269. doi: 10.1007/s00441-004-1075-3. [DOI] [PubMed] [Google Scholar]
- 14.Miljkovic N.D. Cooper G.M. Marra K.G. Chondrogenesis, bone morphogenetic protein-4 and mesenchymal stem cells. Osteoarthritis Cartilage. 2008;16:1121. doi: 10.1016/j.joca.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 15.Masuda K. Pfister B.E. Sah R.L. Thonar E.J. Osteogenic protein-1 promotes the formation of tissue-engineered cartilage using the alginate-recovered-chondrocyte method. Osteoarthritis Cartilage. 2006;14:384. doi: 10.1016/j.joca.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 16.Kim H.J. Im G.I. Combination of transforming growth factor-beta2 and bone morphogenetic protein 7 enhances chondrogenesis from adipose tissue-derived mesenchymal stem cells. Tissue Eng Part A. 2009;15:1543. doi: 10.1089/ten.tea.2008.0368. [DOI] [PubMed] [Google Scholar]
- 17.de Crombrugghe B. Lefebvre V. Behringer R.R. Bi W. Murakami S. Huang W. Transcriptional mechanisms of chondrocyte differentiation. Matrix Biol. 2000;19:389. doi: 10.1016/s0945-053x(00)00094-9. [DOI] [PubMed] [Google Scholar]
- 18.Bi W. Deng J.M. Zhang Z. Behringer R.R. de Crombrugghe B. Sox9 is required for cartilage formation. Nat Genet. 1999;22:85. doi: 10.1038/8792. [DOI] [PubMed] [Google Scholar]
- 19.Paul R. Haydon R.C. Cheng H. Ishikawa A. Nenadovich N. Jiang W., et al. Potential use of Sox9 gene therapy for intervertebral degenerative disc disease. Spine. 2003;28:755. [PMC free article] [PubMed] [Google Scholar]
- 20.Tsuchiya H. Kitoh H. Sugiura F. Ishiguro N. Chondrogenesis enhanced by overexpression of sox9 gene in mouse bone marrow-derived mesenchymal stem cells. Biochem Biophys Res Commun. 2003;301:338. doi: 10.1016/s0006-291x(02)03026-7. [DOI] [PubMed] [Google Scholar]
- 21.Ross J. mRNA stability in mammalian cells. Microbiol Rev. 1995;59:423. doi: 10.1128/mr.59.3.423-450.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Asirvatham A.J. Magner W.J. Tomasi T.B. miRNA regulation of cytokine genes. Cytokine. 2009;45:58. doi: 10.1016/j.cyto.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhattacharyya S.N. Habermacher R. Martine U. Closs E.I. Filipowicz W. Relief of microRNA-mediated translational repression in human cells subjected to stress. Cell. 2006;125:1111. doi: 10.1016/j.cell.2006.04.031. [DOI] [PubMed] [Google Scholar]
- 24.Tew S.R. Peffers M.J. McKay T.R. Lowe E.T. Khan W.S. Hardingham T.E., et al. Hyperosmolarity regulates SOX9 mRNA posttranscriptionally in human articular chondrocytes. Am J Physiol Cell Physiol. 2009;297:C898. doi: 10.1152/ajpcell.00571.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tew S.R. Hardingham T.E. Regulation of SOX9 mRNA in human articular chondrocytes involving p38 MAPK activation and mRNA stabilization. J Biol Chem. 2006;281:39471. doi: 10.1074/jbc.M604322200. [DOI] [PubMed] [Google Scholar]
- 26.Martin I. Jakob M. Schafer D. Dick W. Spagnoli G. Heberer M. Quantitative analysis of gene expression in human articular cartilage from normal and osteoarthritic joints. Osteoarthritis Cartilage. 2001;9:112. doi: 10.1053/joca.2000.0366. [DOI] [PubMed] [Google Scholar]
- 27.Akiyama H. Lyons J.P. Mori-Akiyama Y. Yang X. Zhang R. Zhang Z., et al. Interactions between Sox9 and beta-catenin control chondrocyte differentiation. Genes Dev. 2004;18:1072. doi: 10.1101/gad.1171104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carballo E. Lai W.S. Blackshear P.J. Feedback inhibition of macrophage tumor necrosis factor-alpha production by tristetraprolin. Science. 1998;281:1001. doi: 10.1126/science.281.5379.1001. [DOI] [PubMed] [Google Scholar]
- 29.Fazi F. Rosa A. Fatica A. Gelmetti V. De Marchis M.L. Nervi C., et al. A minicircuitry comprised of microRNA-223 and transcription factors NFI-A and C/EBPalpha regulates human granulopoiesis. Cell. 2005;123:819. doi: 10.1016/j.cell.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 30.Yang E. van Nimwegen E. Zavolan M. Rajewsky N. Schroeder M. Magnasco M., et al. Decay rates of human mRNAs: correlation with functional characteristics and sequence attributes. Genome Res. 2003;13:1863. doi: 10.1101/gr.1272403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee S.Y. Nakagawa T. Reddi A.H. Induction of chondrogenesis and expression of superficial zone protein (SZP)/lubricin by mesenchymal progenitors in the infrapatellar fat pad of the knee joint treated with TGF-beta1 and BMP-7. Biochem Biophys Res Commun. 2008;376:148. doi: 10.1016/j.bbrc.2008.08.138. [DOI] [PubMed] [Google Scholar]
- 32.Knippenberg M. Helder M.N. Zandieh Doulabi B. Wuisman P.I. Klein-Nulend J. Osteogenesis versus chondrogenesis by BMP-2 and BMP-7 in adipose stem cells. Biochem Biophys Res Commun. 2006;342:902. doi: 10.1016/j.bbrc.2006.02.052. [DOI] [PubMed] [Google Scholar]
- 33.Flechtenmacher J. Huch K. Thonar E.J. Mollenhauer J.A. Davies S.R. Schmid T.M., et al. Recombinant human osteogenic protein 1 is a potent stimulator of the synthesis of cartilage proteoglycans and collagens by human articular chondrocytes. Arthritis Rheum. 1996;39:1896. doi: 10.1002/art.1780391117. [DOI] [PubMed] [Google Scholar]
- 34.Nishida Y. Knudson C.B. Eger W. Kuettner K.E. Knudson W. Osteogenic protein 1 stimulates cells-associated matrix assembly by normal human articular chondrocytes: up-regulation of hyaluronan synthase, CD44, and aggrecan. Arthritis Rheum. 2000;43:206. doi: 10.1002/1529-0131(200001)43:1<206::AID-ANR25>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 35.Nishida Y. Knudson C.B. Kuettner K.E. Knudson W. Osteogenic protein-1 promotes the synthesis and retention of extracellular matrix within bovine articular cartilage and chondrocyte cultures. Osteoarthritis Cartilage. 2000;8:127. doi: 10.1053/joca.1999.0281. [DOI] [PubMed] [Google Scholar]
- 36.Cook S.D. Patron L.P. Salkeld S.L. Rueger D.C. Repair of articular cartilage defects with osteogenic protein-1 (BMP-7) in dogs. J Bone Joint Surg Am. 2003;85-A(Suppl 3):116. doi: 10.2106/00004623-200300003-00018. [DOI] [PubMed] [Google Scholar]
- 37.Louwerse R.T. Heyligers I.C. Klein-Nulend J. Sugihara S. van Kampen G.P. Semeins C.M., et al. Use of recombinant human osteogenic protein-1 for the repair of subchondral defects in articular cartilage in goats. J Biomed Mater Res. 2000;49:506. doi: 10.1002/(sici)1097-4636(20000315)49:4<506::aid-jbm9>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 38.Bais M.V. Wigner N. Young M. Toholka R. Graves D.T. Morgan E.F., et al. BMP2 is essential for post natal osteogenesis but not for recruitment of osteogenic stem cells. Bone. 2009;45:254. doi: 10.1016/j.bone.2009.04.239. [DOI] [PMC free article] [PubMed] [Google Scholar]