Abstract
Poly(ethylene glycol) (PEG) hydrogels are popular for cell culture and tissue-engineering applications because they are nontoxic and exhibit favorable hydration and nutrient transport properties. However, cells cannot adhere to, remodel, proliferate within, or degrade PEG hydrogels. Methacrylated gelatin (GelMA), derived from denatured collagen, yields an enzymatically degradable, photocrosslinkable hydrogel that cells can degrade, adhere to and spread within. To combine the desirable features of each of these materials we synthesized PEG-GelMA composite hydrogels, hypothesizing that copolymerization would enable adjustable cell binding, mechanical, and degradation properties. The addition of GelMA to PEG resulted in a composite hydrogel that exhibited tunable mechanical and biological profiles. Adding GelMA (5%–15% w/v) to PEG (5% and 10% w/v) proportionally increased fibroblast surface binding and spreading as compared to PEG hydrogels (p<0.05). Encapsulated fibroblasts were also able to form 3D cellular networks 7 days after photoencapsulation only within composite hydrogels as compared to PEG alone. Additionally, PEG-GelMA hydrogels displayed tunable enzymatic degradation and stiffness profiles. PEG-GelMA composite hydrogels show great promise as tunable, cell-responsive hydrogels for 3D cell culture and regenerative medicine applications.
Introduction
An assortment of diseases and injuries can lead to tissue degeneration, organ dysfunction, and failure. Although organ transplantation is an option in some cases, potential organ donor recipients far outnumber organ donors. The emerging field of tissue engineering holds great promise to meet this outstretched demand through tissue regeneration, organ repair, and replacement.1 Presently, many of the techniques for developing engineered tissues focus on using scaffolds seeded with cells in combination with trophic factors.1–5
Hydrogels have become popular scaffold platforms in the field of tissue engineering and as materials for 3D cell culture.6,7 Hydrogels display favorable hydration properties and can typically be fabricated under cyto-compatible conditions. Development of new hydrogels has focused on replicating aspects of the extracellular matrix of native tissues to achieve the precise microenvironmental conditions for a particular cell type and/or tissue development.8–10 This has resulted in a wide variety of hydrogels developed from both synthetic and naturally derived polymers.6,11–13 Interestingly, both cell fate and cell function can be influenced by the hydrogel microenvironment.14–16 Thus, ideally the hydrogel microenvironment should mimic the mechanical and biological demands and requirements of the tissues being replicated.17
In the present study we have developed a photocrosslinkable composite hydrogel made from poly(ethylene glycol) dimethacrylate (PEGDMA MW 1000 Da) and methacrylated gelatin (GelMA). As a synthetic hydrogel, PEG has been very popular because of its many desirable properties. PEG hydrogels are nontoxic, nonimmunogenic, and favorable to nutrient and oxygen transport, can be fabricated under cell compatible conditions, and have robust mechanical properties.18–21 However, PEG is also void of bioactivity common to many natural hydrogel polymers. As a result, cells are unable to bind directly to PEG hydrogels or modify the microenvironment through enzymatic degradation. GelMA, unlike PEG, is a natural polymer, derived from hydrolyzed collagen, maintaining similar bioactivity as collagen. GelMA contains RGD binding sequences, allowing cells to bind directly to the GelMA hydrogels, and its protein structure allows cells to enzymatically remodel and degrade the hydrogel.3,22–24 Similar to PEG, GelMA has shown excellent cellular compatibility as a photocrosslinkable hydrogel for both cellular encapsulation and cell seeding.3,22–24 Although GelMA displays many desirable attributes as a hydrogel for tissue engineering, we have previously revealed an inability to tune cellular attachment of GelMA hydrogels by simply altering its concentration.23 Further, the degradation rate of GelMA hydrogels can only be altered by changing the concentration of GelMA,22 making it difficult to alter the degradation rate without affecting other characteristics, such as the mechanical properties. Therefore, pure GelMA hydrogels may be unsuitable for applications where greater tunability in cell adhesion, migration, and cell-mediated degradation are needed.
Previous reports have exploited the favorable mechanical properties and lack of bioactivity of PEG by implementing PEG as a base for the addition of bioactive polymers and peptides. Leach et al. developed a hyaluronic acid (HA)-PEG composite hydrogel, whereas Mann et al. attached peptide sequences to the acrylate groups of PEG.25–27 Although HA instills bioactive properties into the composite hydrogel, HA-PEG hydrogels lack the cell adhesive functionalities desired in many tissue engineering and 3D culture applications. Also, the addition of bioactive peptides to PEG acrylate groups is a complex multistep process and cell binding sites are limited by the number of PEG acrylate groups. We propose to exploit the lack of bioactivity of PEG by strategically adding GelMA, a bioactive polymer, with the goal of creating a group of “biologically functionalized” PEG hydrogels with properties exceeding those of either material alone. We hypothesize that the supplementation of PEG hydrogels with GelMA will create a composite photocrosslinkable hydrogel with tunable cell responsive properties, degradation, and mechanical properties, yielding a hydrogel system that can be finely tuned for many diverse applications such as creating engineered tissues or controlled in vitro micro-environments for investigating cell behaviors.
Materials and Methods
Materials
The chemicals used in the production of GelMA-fabrication (gelatin [type A, 300 bloom from porcine skin], methacrylic anhydride [MA]) and the pretreatment of glass slides 3-(trimethoxysilyl)propyl methacrylate (TMSPMA) were purchased from Sigma-Aldrich. PEGDMA with a molecular weight of 1000 DA was purchased from Polysciences, Inc. Glass slides and coverslips were purchased from Fisher Scientific. For photolithography we used printed photomasks from CADart and a UV light source (Omnicure S2000) from EXFO Photonic Solutions Inc. (Washington, DC). Electronic digital micrometer calipers from Marathon Watch Company Ltd. were used to determine spacer thickness.
GelMA synthesis
GelMA was synthesized as previously described.23,28 Briefly, gelatin was mixed at 10% (w/v) with Dulbecco's phosphate-buffered saline (DPBS; Gibco) at 50°C and stirred until completely dissolved. A high degree of methacrylation was achieved by adding 20% (w/v) of MA to the synthesis reaction as previously shown.23 MA was added at a rate of 0.5 mL/min under stirred conditions at 50°C and allowed to react for 2 h. After a 5×dilution with DPBS to stop the reaction, the mixture was dialyzed against distilled water using 12–14 kDa cutoff dialysis tubing for 1 week at 40°C to remove salts and methacrylic acid. The solution was lyophilized for 1 week to generate a white porous foam and was stored at −80°C.
Hydrogel preparation
PEGDMA and lyophilized GelMA were mixed into DPBS with 0.5% (w/v) 2-hydroxy-1(4-(hydroxyethox)pheny)-2-methyl-1-propanone (Irgacure 2959; CIBA Chemicals) at 80°C until completely dissolved. Ten percent and 5% (w/v) PEG hydrogels were mixed with 0%, 5%, 10%, or 15% (w/v) of GelMA. PEGDMA alone did not polymerize at 5% (w/v) and was not included in experimental analyses. To achieve a homogeneous distribution, the prepolymer was placed on a vortex mixer. To prevent gelation the prepolymer was maintained at 40°C before use.
Mechanical testing
Eighty microliters of prepolymer was pipetted between two glass slides separated by a 1 mm spacer and exposed to 6.9 mW/cm2 UV light (360–480 nm) for 50 s. Samples were detached from the slide and incubated in DPBS at room temperature for 24 h. Immediately before testing, an 8 mm disc was punched from each swollen hydrogel with a biopsy punch. The disc was blotted dry and compressed at a rate of 20% strain/min on an Instron 5542 mechanical tester. The compressive modulus was determined as the slope of the linear region corresponding with 0%–10% strain. The sample size was 4–15 gels per group.
Swelling analysis
To perform swelling analysis, the gels were formed as described for mechanical testing. Samples were then detached from the slide and incubated in DPBS at room temperature for 24 h. Gels were removed from DPBS and lightly blotted dry, and the swollen weight was recorded. Samples were then lyophilized and weighed to determine the dry weight of polymer. The mass swelling ratio was then calculated as the ratio of wet mass to the mass of dry polymer. The gels were then incubated in DPBS at room temperature for 48 h, weighed, and the rehydrated ratio was calculated as the ratio of the rehydrated wet mass to the initial wet mass. The sample size for analysis was six gels per group.
Hydrogel degradation
Polymerization was performed as described for mechanical testing. Hydrogels were placed in 1.5 mL tubes with 1 mL of DPBS with 2.5 U/mL of collagenase type II (Worthington Biochemical). Gels were incubated with collagenase type II at 37°C for 3, 6, 12, 24, 36, or 48 h. After each time point, the collagenase solution was removed, without disturbing the undigested hydrogel. The remaining hydrogel was washed with DPBS, and then all liquid was removed and gels were lyophilized. The percent degradation was calculated by the dried weight after digestion divided by the weight of untreated hydrogels. The sample size was six gels per group.
Cell culture
All cells were cultured in a standard cell culture incubator (Forma Scientific) in a 5% CO2 atmosphere at 37°C. NIH3T3 fibroblasts were maintained in Dulbecco's modified Eagle's medium (DMEM; Gibco) supplemented with 10% FBS, 1% Penicillin/Streptomycin changed every 2 days, and passaged two times per week.
Cell adhesion
For cell adhesion studies, hydrogels were patterned onto PEG-coated glass slides using techniques previously demonstrated to prevent cell adhesion on the slide surface.23 Briefly, PEG prepolymer was pipetted between a TMSPMA-coated glass slide and an untreated coverslip (18 mm [w]×18 mm [l]), then exposed to 6.9 mW/cm2 UV light (360–480 nm) for 50 s. Six circular hydrogels (1 cm in diameter and 150 μm in height) for each PEG and GelMA combination were then patterned onto PEG-coated slides and placed in DMEM at 37°C overnight. NIH3T3 cells were stained with PKH67 Fluorescent Linker Kit according to the manufacturer's instructions (Sigma-Aldrich). Hydrogels were then covered with a solution containing 3.5×105 labeled NIH3T3 cells/mL to a depth of approximately 1 mm above the surface of the hydrogel and incubated for 8 h before washing twice with DPBS. PHK67 fluorescence was observed using an inverted fluorescence microscope (Nikon TE 2000-U). Six gels per group and six fluorescent images from each hydrogel were used to quantify total area occupied by PHK67-labeled fibroblasts by using NIH ImageJ software.
Cell encapsulation and viability
NIH3T3 fibroblasts were trypsinized and resuspended in prepolymer containing 0.5% photoinitiator at a concentration of 2×106 cells/mL. Two hundred microgel units (500 μm×500 μm×150 μm) were fabricated as previously described, onto PEG and TMSPMA-treated glass.23 The glass slides containing microgels were washed with DPBS and incubated for 6 and 48 h in NIH3T3 medium under standard culture conditions. The calcein-AM/ethidium homodimer Live/Dead assay (Invitrogen) was used according to the manufacturer's instructions. Calcein-AM/ethidium homodimer fluorescence was observed using an inverted fluorescence microscope (Nikon TE 2000-U). NIH ImageJ software was used to quantify the number of calcein-AM and ethidium homodimer-positive cells from 10 randomly selected microgel units from six patterns for each PEG-GelMA hydrogel combination.
Data analysis
Hydrogel data were analyzed using two- or three-way ANOVA with concentration of GelMA and PEG as well as culture time as independent variables. Post hoc analysis was performed with Fisher's LSD test. All statistical analysis was performed with GB-STAT v8.0 (Dynamic microsystems). For all statistical tests the level of significance was set at p<0.05. Data are presented as mean±standard deviation (SD).
Results
Mechanical properties
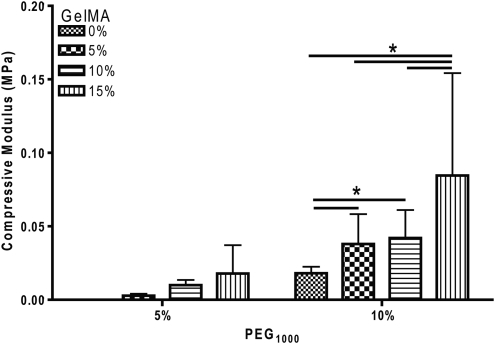
The mechanical properties of hydrogels are well documented to influence cellular behavior, function, and differentiation.29,30 Unconfined compression was used to evaluate the effect of GelMA supplementation on the mechanical properties of 5% (w/v) and 10% (w/v) PEG hydrogels. Statistical analysis illustrated a significant effect of PEG (p<0.05) and a significant effect of GelMA (p<0.05) concentration on the compressive modulus of composite hydrogels (Fig. 1). Not surprisingly, this analysis revealed that increasing the percentage of PEG from 5% (w/v) to 10% (w/v) significantly increased the compressive modulus of the composite hydrogels. Further, this analysis also illustrated that GelMA supplementation from 5% to 15% (w/v) enhanced the compressive modulus of composite hydrogels more than an increase in PEG alone. These data reveal a tunable increase in the compressive modulus of composite hydrogels. Fisher's LSD revealed significant differences between groups for the 10% (w/v) PEG supplemented with GelMA. The addition of GelMA to 10% PEG increased the compressive modulus of composite hydrogels as compared to 10% PEG alone. Interestingly, none of the samples failed before the maximum 50 N load was reached.
FIG. 1.
Composite hydrogels exhibit tunable compressive moduli (MPa). Two-way ANOVA reveals a significant effect of PEG (p<0.05) and GelMA (p<0.05) concentration on the compressive modulus. Fisher's LSD. *p<0.05, n=4–15 per group. GelMA, methacrylated gelatin.
Swelling properties
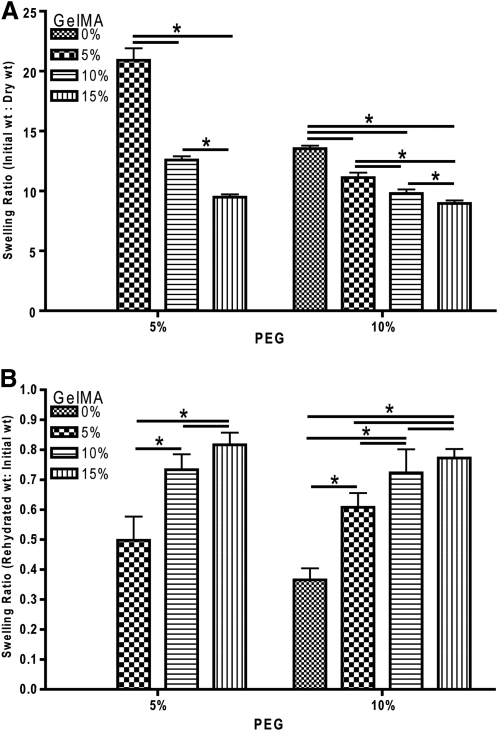
The swelling characteristics of hydrogels can greatly affect the pore size and diffusive and mechanical properties.20 As such, the effect of GelMA supplementation on the swelling profiles of PEG hydrogels was evaluated. Composite hydrogels were generated as previously described and allowed to reach equilibrium via a 24 h incubation in DPBS at room temperature. The hydrogels were then weighed and lyophilized, and the wet mass-to-dry mass ratio was compared across the composite hydrogels. Statistical analysis revealed a significant effect of GelMA (p<0.05) and a significant interaction of PEG and GelMA (p<0.05) concentration in driving the swelling properties of composite hydrogels (Fig. 2A). These data demonstrate the ability of GelMA supplementation in 5% (w/v) and 10% (w/v) PEG to reduce the swelling ratio. Further, the interaction of PEG and GelMA reveals an effect of both materials on the swelling of composite hydrogels, where the concentration of both materials has a greater effect on swelling compared to one material alone. The post hoc analysis illustrated additional significant differences between groups for both 5% (w/v) and 10% (w/v) PEG supplemented with GelMA. Hydrogels containing 5% PEG supplemented with 5%, 10%, or 15% (w/v) GelMA were found to have significantly different swelling profiles. Ten percent PEG supplemented with 0%, 5%, 10%, or 15% (w/v) of GelMA also all displayed significantly different swelling profiles.
FIG. 2.
Composite hydrogels display adjustable swelling and rehydration properties. (A) Initial wt: Dry wt. Two-way ANOVA illustrates a significant effect of GelMA concentration (p<0.05) and a PEG and GelMA interaction (p<0.05) on swelling. Fisher's LSD *p<0.05, n=6 per group. (B) Rehydrated wt: Initial wt. Analysis found a significant effect of PEG concentration (p<0.05), GelMA concentration (p<0.05) and a PEG and GelMA interaction (p<0.05). Fisher's LSD *p<0.05, n=6 per group.
For rehydration, the same lyophilized composite hydrogels used in the swelling experiment were allowed to reach equilibrium via incubation in DPBS for at room temperature for 48 h. Rehydration revealed a significant effect of PEG (p<0.05), GelMA (p<0.05) and a significant interaction of PEG and GelMA (p<0.05) concentration on the rehydration ratio (Fig. 2B). These data describe the ability of both PEG and GelMA concentration to drive the rehydration properties. The interaction of PEG and GelMA reveals a synergistic effect of both materials on the rehydration profile, where the combination of the materials has a greater effect on rehydration than either material alone. Further, Fisher's LSD found significant differences between groups for both 5% (w/v) and 10% (w/v) PEG supplemented with 0%, 5%, 10%, and 15% GelMA, respectively. In general, the data reveal that GelMA supplementation increases the recovery from dehydration as compared to PEG alone, with PEG-GelMA hydrogels reaching up to 80% of their original wet mass after 48 h in DPBS.
Degradation profiles
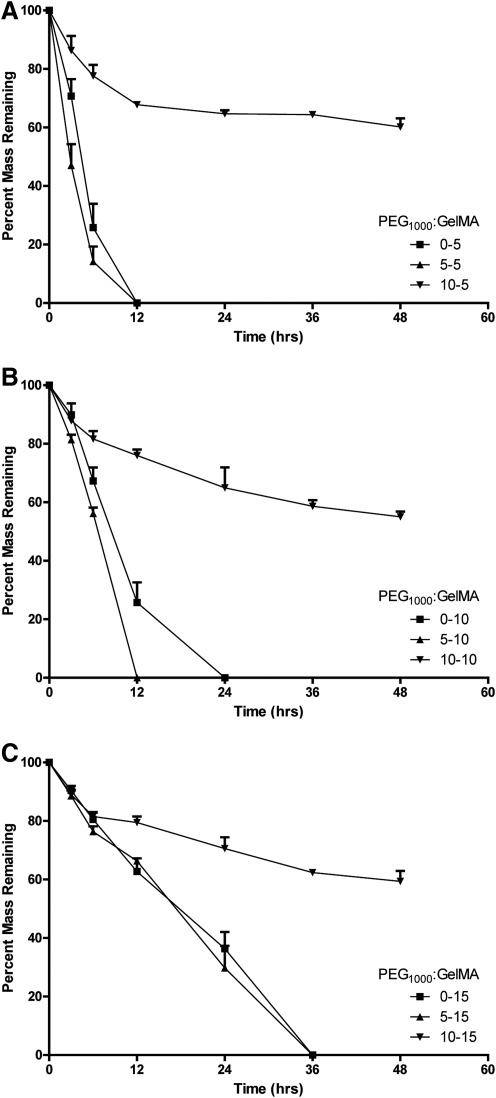
The ability of cells to degrade and remodel their hydrogel environment is crucial to the formation and development of mature tissue. GelMA, like gelatin in its native state, maintains its susceptibility to enzymatic degradation, whereas PEG hydrogels are not susceptible to enzymatic activity.3 Previously, Benton et al. used 2.5 U/mL of type II collagenase to evaluate the enzymatic degradation profile of 10% GelMA.3 In the present study, we implemented an identical method to examine the enzymatic degradation profiles of PEG-GelMA composite hydrogels as compared to GelMA alone. The degradation profiles of 5%, 10%, and 15% (w/v) GelMA supplemented with 5% and 10% (w/v) PEG resulted in similar statistical outcomes: a significant effect of PEG concentration (p<0.05), degradation time (p<0.05), and an interaction of PEG and degradation time (p<0.05) (Fig. 3A–C). The main effect of PEG concentration illustrates the ability of the PEG concentration to alter the degradation rates of composite hydrogels. As evident in the graph, the main effect of degradation time describes that the longer the hydrogels are exposed to collagenase II the greater the mass loss. The significant interaction of the PEG concentration and degradation time reveals that composite hydrogels with higher concentrations have less mass loss over time. For example, from 24 to 48 h 10% (w/v) PEG with 5% (w/v) GelMA hydrogels display less mass loss than from 0 to 12 h (Fig. 3A). Interestingly, the addition of only 5% (w/v) PEG significantly enhanced the degradation rate of 5% (w/v) and 10% (w/v) of GelMA. However, supplementation of 5% (w/v) PEG to 15% (w/v) GelMA resulted in similar degradation rate as 15% (w/v) GelMA alone (Fig. 3C). The addition of 15% (w/v) PEG significantly reduced the degradation rates of composite hydrogels with 5%, 10%, and 15% (w/v) GelMA as compared to GelMA alone. These data illustrate that PEG-GelMA composite hydrogels display tunable degradation profiles that can be altered by varying the concentration of PEG, GelMA, or both materials.
FIG. 3.
Composite hydrogels display modifiable degradation profiles. For composite hydrogels containing 5% (A), 10% (B), and 15% (C) GelMA analysis reveals a significant effect of PEG concentration (p<0.05), degradation time (p<0.05), and a PEG and degradation time interaction (p<0.05) on the rate of degradation. n=6 per group.
2D cellular surface attachment
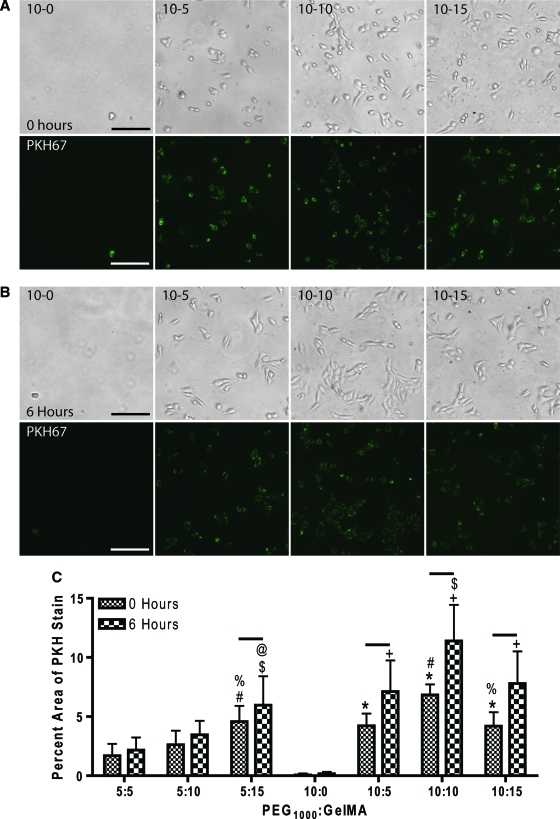
The ability of cells to attach, spread, and proliferate on hydrogels is an imperative attribute for the engineering of tissues. PEG, unlike GelMA, lacks the cell binding sequences necessary for cellular adhesion. Thus, the ability of GelMA to enhance the cell adhesion profile of PEG hydrogels was evaluated using NIH3T3 fibroblasts stained with PKH67. Composite hydrogels were then covered with a solution containing 3.5×105 NIH3T3 cells/mL to a depth of approximately 1 mm above the surface of the hydrogel and incubated for 8 h before washing twice with DPBS, with this considered time “0.” Cell attachment/spreading was analyzed at 0 and 6 h post-DPBS wash. Statistical analysis revealed a significant effect of culture time (p<0.05), GelMA concentration (p<0.05), PEG concentration (p<0.05), an interactive effect of culture time and GelMA concentration (p<0.05), an interactive effect of time and PEG (p<0.05) and an interactive effect of GelMA and PEG (p<0.05) (Fig. 4). The results reveal that an increased concentration in PEG or GelMA increased cellular attachment, suggesting stiffer hydrogels supported more attachment. Further, the significant interaction of PEG and GelMA describes a synergistic effect, increasing cellular attachment and spreading more than an increase in PEG or GelMA alone, further supporting the hypothesis that stiffness drives cellular attachment. The effect of time on cell spreading was evident, as there was a significant increase in the area of confluence from 0 to 6 h for most composite hydrogels. Post hoc analysis revealed additional differences between groups. Five percent (w/v) PEG supplemented with 15% (w/v) GelMA displayed significantly more cell attachment and spreading as compared to 5% (w/v) PEG with 5% and 10% GelMA (Fig. 4C). All composite hydrogels with 10% (w/v) PEG displayed greater cell attachment and spreading compared to PEG alone. Further, all composite hydrogels with 10% (w/v) PEG displayed significant increases in cellular spreading from 0 to 6 h. These data reveal the ability to modulate cellular attachment and spreading on PEG-GelMA composite hydrogels by altering the concentration of either PEG or GelMA.
FIG. 4.
Composite hydrogels display tunable cellular attachment. Representative images of NIH 3T3 cells stained with PKH67 at 0 h (A) and 6 h (B) post-attachment on composite hydrogels. (C) Statistical analysis displayed a significant effect of culture time (p<0.05), GelMA concentration (p<0.05), PEG concentration (p<0.05), culture time and GelMA interaction (p<0.05), culture time and PEG interaction (p<0.05), and GelMA and PEG interaction (p<0.05). Fisher's LSD: p<0.05 at 0 h: * versus 0%, # versus 5%, and % 10% GelMA. p<0.05 at 6 h: + versus 0%, $ versus 5% and @ versus 10% GelMA. n=6 per group. Scale bar=250 μm. Color images available online at www.liebertonline.com/tea
3D encapsulation and viability
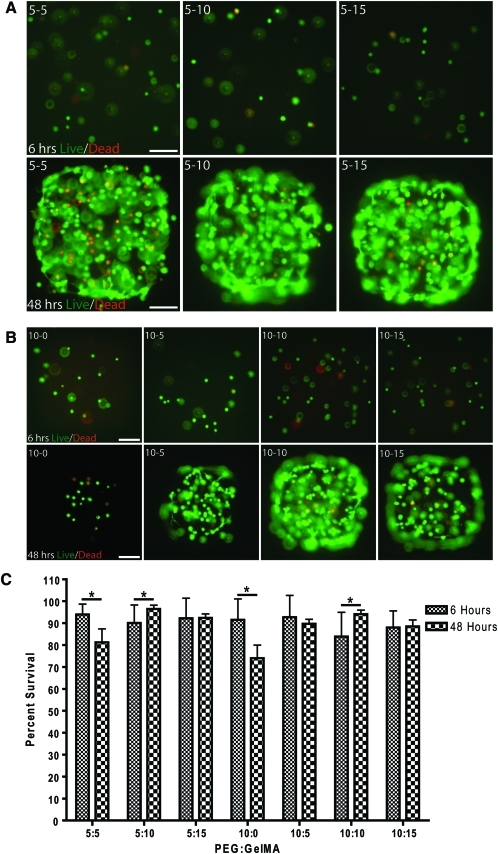
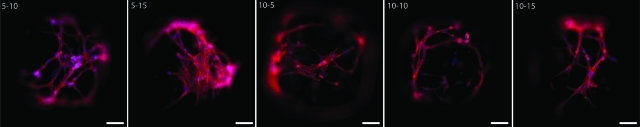
Separately, PEG and GelMA are documented to display favorable cell viability after cellular encapsulation.24,31 NIH3T3 fibroblasts were encapsulated in composite hydrogels and the percent viability was analyzed at 6 and 48 h post-encapsulation. For cell viability in composite hydrogels there was a significant effect of GelMA concentration (p<0.05), PEG concentration (p<0.05), an interactive effect of time and GelMA (p<0.05), an interactive effect of PEG and GelMA (p<0.05), and an interactive effect of time, GelMA, and PEG (p<0.05) (Fig. 5). In general, the addition of GelMA increased the survival from 6 to 48 h postencapsulation, as compared to PEG alone. Post hoc analysis revealed the only significant reduction in viability was in 5% (w/v) PEG with 5% (w/v) GelMA and 10% (w/v) PEG alone, whereas 10% (w/v) and 15% (w/v) GelMA typically enhanced or maintained cell viability from 6 to 48 h at both PEG concentrations (Fig. 5C). By 7 days post-encapsulation, NIH3T3 fibroblasts remained viable and were able to substantially remodel the composite hydrogels, which did not occur in PEG hydrogels. Cellular staining with phalloidin, which labels F-actin, revealed multicellular networks in most of the composite hydrogels (Fig. 6). NIH3T3 fibroblasts encapsulated in 5% (w/v) PEG with 5% (w/v) GelMA and 10% (w/v) PEG only hydrogels did not stain for phalloidin or DAPI. DAPI stains double-stranded DNA and the lack of DAPI staining suggests degradation of cell nuclei and substantial levels of cell death.
FIG. 5.
Viability of NIH3T3 cell encapsulation in composite hydrogels at 6 h and 48 h. (A, B) Representative images of live and dead assay. Scale bar=200 μm. (C). ANOVA reveals a significant effect of GelMA concentration (p<0.05), PEG concentration (p<0.05), a culture time and GelMA interaction (p<0.05), a PEG and GelMA interaction (p<0.05), and an interaction of culture time, GelMA, and PEG (p<0.05), n=6 per group. Fisher's LSD *p<0.05: 6 h versus 48 h. Color images available online at www.liebertonline.com/tea
FIG. 6.
3T3 cells form interconnected networks in composite hydrogels after 7 days. Cells encapsulated in 5%GelMA-5%PEG and 10%PEG alone did not stain for DAPI (blue) or phalloidin (red) and were presumed non-viable. The numbers on each panel represent the percentage of PEG-GelMA (w/v) in each hydrogel. Scale bar=200 μm. Color images available online at www.liebertonline.com/tea
Discussion
Materials used as hydrogels for tissue engineering must meet specific characteristics to promote the generation of new tissue. It is imperative that hydrogels maintain their shape and provide the necessary mechanical support while being nontoxic and nonimmunogenic. They should mimic the extracellular matrix and promote cellular proliferation and differentiation, desired cell functions, and interactions. Similar to the extracellular matrix, hydrogels should be enzymatically degradable, allowing cells to remodel their environment. Therefore, the importance of developing a hydrogel with tunable mechanical and biological properties is crucial when considering the wide range of optimal mechanical and micro-environmental requirements for each cell and tissue type.
PEG is one of the most widely used materials for biomedical applications and has arguably become the standard for hydrogel biomaterials. PEG is biologically inert, being both nontoxic and nonimmunogenic, and has been approved by the U.S. Food and Drug Administration for a variety of clinical applications. Numerous methods have been developed to produce PEG hydrogels through covalent crosslinking of PEG prepolymer; free radical polymerization of PEG acrylates, Michael-type addition, enzymatic reaction, and radiation.32–39 Although PEG displays many desirable features as a biomaterial, the inability for cells to degrade, remodel, and attach to PEG make it difficult to implement as a hydrogel for tissue engineering, where such characteristics are critical. The lack of bioactivity of PEG has spurred the development of functionalized PEG polymers, where peptide sequences are attached to PEG acrylate groups or polymerized to 4-arm PEG using Michael-type addition.27,40–43 These functionalized PEG hydrogels are able to support cellular attachment and migration in vitro, and cell infiltration and integration in vivo. When engineering tissues, each cell type may require a different concentration of peptide sequences for optimal cellular attachment to and enzymatic degradation of the hydrogel. Further, each cell and tissue type will have specific mechanical demands of the hydrogel to promote proper tissue development. The development and manufacturing of these functionalized PEG hydrogels are achieved through complicated, multistep processes that typically cannot be completed using only commonly available chemicals and equipment. In addition, simultaneously customizing the biological and mechanical properties of these types of PEG hydrogels is difficult to achieve without significant experience and training. Further, the production of these types of PEG polymers can be expensive.
In the present study we chose to use GelMA to biologically functionalize PEG hydrogels.3,23 Gelatin is an inexpensive, natural, edible, biocompatible, nontoxic material derived from acid or alkaline hydrolysis of collagen.6 Type A gelatin is produced by the acid processing of collagen and exhibits an isoelectric point of 7–9.23 Type B gelatin produced by the alkaline process has an isoelectric point of 4.6–5.2.23 For the present study, type A gelatin was chosen as it has a higher number of reactive amino groups, resulting in a greater potential degree of methacrylation.44 Although there are many reported methods for covalently crosslinking gelatin, photocrosslinking GelMA has been shown to be both compatible with cellular encapsulation and microfabrication.3,23 Aside from the above-mentioned biological qualities that gelatin displays, previous research has highlighted the trophic effects of gelatin, exhibiting pro-angiogenic properties and promotion of valvular interstitial cell function.3,44
The supplementation of PEG hydrogels with GelMA has led to the development of a photocrosslinkable hydrogel that is inexpensive, easily produced with common equipment and chemicals, and biologically and mechanically tunable. GelMA enhanced the compressive modulus of 5% (w/v) and 10% (w/v) PEG, exceeding the compressive modulus of PEG and GelMA alone.22,23 The addition of GelMA also affected the swelling and rehydration properties, where GelMA significantly reduced the swelling of hydrogels while simultaneously increasing the ability of hydrogels to rehydrate closer to their initial hydration mass in 48 h. Typically, PEG hydrogels are not degradable; however, the addition of GelMA created a composite hydrogel with tunable degradation profiles outside the range of pure GelMA hydrogels. Interestingly, supplementation of 5% (w/v) GelMA in PEG hydrogels resulted in composite hydrogels that had the greatest rate of degeneration as compared to both 10% (w/v) and 15% (w/v) GelMA supplementation. GelMA supplementation also allowed NIH3T3 fibroblasts to attach to and spread on the composite hydogels, a phenomenon not possible with PEG alone. The PEG-GelMA composite hydrogels displayed adjustable cellular attachment, an event that may not be easily possible with PEG or GelMA alone.23 The addition of GelMA greatly improved cellular viability after hydrogel encapsulation as compared to PEG alone. These data are in line with previous data highlighting the trophic effects of gelatin on cellular behavior.3,44 The addition of GelMA allowed NIH3T3 fibroblasts to remodel their environment, creating cellular networks within the composite hydrogels at 7 days post-encapsulation.
Although PEG was functionalized in this study through the addition of GelMA, conceivably any water-soluble hydrogel that polymerizes using acrylate chemistry could be functionalized with the addition of GelMA. Many other hydrogels currently used for cell encapsulation that lack cell-responsive elements, such as methacrylated HA, could potentially be made to have tunable cell-binding and degradation properties similar to PEG-GelMA. In our group we have confirmed that GelMA mixes well with, and improves some of the cell-responsive properties of pullulan methacrylate (PulMA) and methacrylated HA; however, a thorough characterization of the resulting polymers was not performed to the extent that is presented in this article.45,46 Interestingly, PEG with a molecular weight of 4000 Da would force GelMA to precipitate out of solution at PEG concentrations of 5% (w/v) or greater. Previous research has demonstrated that PEG 4000 precipitates proteins with high affinity, whereas PEG 1000 does not.47 This could pose a potential hindrance to functionalizing some acrylated hydrogels using GelMA.
PEG-GelMA composite hydrogels exhibit a range of biological and mechanically tunable profiles. Thus, these hydrogels have the potential to meet an assortment of cellular and mechanical demands required for engineering tissues. In the present study we only manipulated the concentration of PEG or GelMA when evaluating the properties of these hydrogels. Yet these gels have the potential for further biological and mechanical tuning by varying the degree of methacrylation of the GelMA, the concentration of the photoinitiator, or UV exposure.23,28 Nichol et al. previously illustrated the effects that the degree of methacrylation can have on the compressive modulus and the swelling ratio.23 Increasing the degree of substitution, with GelMA concentration held constant, augmented the compressive modulus while reducing the swelling ratio of hydrogels.23 Reducing the degree of substitution had the opposite effect on GelMA hydrogels.23 In the case of the PEG-GelMA composite hydrogels, reducing the degree of methacrylation in GelMA would allow for the addition of GelMA to enhance cellular attachment while not increasing the stiffness of the composite hydrogel. Van Den Bulcke et al. have highlighted the effects of altering UV exposure and photointiator on GelMA, with the increase in UV time and/or photointiator resulting in stiffer hydrogels.28 Thus, the PEG-GelMA composite hydrogels can be customized to meet the wide range of biological and mechanical profiles required for tissue engineering.
Conclusion
The present study highlights the ability of GelMA to biologically functionalize and alter the mechanical properties of PEG hydrogels in a cost-effective and reproducible manner. The mechanical and biological properties of PEG-GelMA hydrogels can be controlled by altering the concentration of PEG, GelMA, or both. Unlike PEG alone, PEG-GelMA composite hydrogels are enzymatically degradable hydrogels that cells can attach to, and create cellular networks within. These data suggest that PEG-GelMA composite hydrogels can be customized to meet the necessary requirements of a variety of tissue engineering applications.
Acknowledgments
We thank Dr. Ian Wheeldon, Dr. Shilpa Sant, Dr. Nezam Kachouie, Dr. Robert Gauvin, Daniela Coutinho, Hyeongho Shin, and Majid Ghodoosi for their scientific discussions. This article was supported by the National Institutes of Health (DE019024; HL099073; EB012597; AR057837; HL092836), the National Science Foundation CAREER award (DMR0847287), and the Office of Naval Research Young Investigator award. J.W.N. was supported by the U.S. Army Construction Engineering Research Laboratory, Engineering Research and Development Center (USACERL/ERDC).
Disclosure Statement
We have no commercial associations that might create a conflict of interest in connection with this article.
References
- 1.Khademhosseini A. Langer R. Borenstein J. Vacanti J.P. Microscale technologies for tissue engineering and biology. Proc Natl Acad Sci U S A. 2006;103:2480. doi: 10.1073/pnas.0507681102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Langer R. Vacanti J.P. Tissue engineering. Science. 1993;260:920. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 3.Benton J.A. DeForest C.A. Vivekanandan V. Anseth K.S. Photocrosslinking of gelatin macromers to synthesize porous hydrogels that promote valvular interstitial cell function. Tissue Eng Part A. 2009;15:3221. doi: 10.1089/ten.tea.2008.0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khademhosseini A. Vacanti J.P. Langer R. Progress in tissue engineering. Sci Am. 2009;300:64. doi: 10.1038/scientificamerican0509-64. [DOI] [PubMed] [Google Scholar]
- 5.Chiu Y.N. Norris R.A. Mahler G. Recknagel A. Butcher J.T. Transforming growth factor beta, bone morphogenetic protein, and vascular endothelial growth factor mediate phenotype maturation and tissue remodeling by embryonic valve progenitor cells: relevance for heart valve tissue engineering. Tissue Eng Part A. 2010;16:3375. doi: 10.1089/ten.tea.2010.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee K.Y. Mooney D.J. Hydrogels for tissue engineering. Chem Rev. 2001;101:1869. doi: 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]
- 7.Slaughter B.V. Kurshid S.S. Fisher O.Z. Khademhosseini A. Peppas N.A. Hydrogels in Regenerative Medicine. Adv Mater. 2009;21:3307. doi: 10.1002/adma.200802106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rehfeldt F. Engler A.J. Eckhardt A. Ahmed F. Discher D.E. Cell responses to the mechanochemical microenvironment—implications for regenerative medicine and drug delivery. Adv Drug Deliv Rev. 2007;59:1329. doi: 10.1016/j.addr.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burdick J.A. Vunjak-Novakovic G. Engineered microenvironments for controlled stem cell differentiation. Tissue Eng Part A. 2009;15:205. doi: 10.1089/ten.tea.2008.0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murtuza B. Nichol J.W. Khademhosseini A. Micro- and nanoscale control of the cardiac stem cell niche for tissue fabrication. Tissue Eng Part B Rev. 2009;15:443. doi: 10.1089/ten.teb.2009.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brigham M.D. Bick A. Lo E. Bendali A. Burdick J.A. Khademhosseini A. Mechanically robust and bioadhesive collagen and photocrosslinkable hyaluronic acid semi-interpenetrating networks. Tissue Eng Part A. 2009;15:1645. doi: 10.1089/ten.tea.2008.0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buxton A.N. Zhu J. Marchant R. West J.L. Yoo J.U. Johnstone B. Design and characterization of poly(ethylene glycol) photopolymerizable semi-interpenetrating networks for chondrogenesis of human mesenchymal stem cells. Tissue Eng. 2007;13:2549. doi: 10.1089/ten.2007.0075. [DOI] [PubMed] [Google Scholar]
- 13.Deng C. Zhang P. Vulesevic B. Kuraitis D. Li F. Yang A.F. Griffith M. Ruel M. Suuronen E.J. A collagen-chitosan hydrogel for endothelial differentiation and angiogenesis(*) Tissue Eng Part A. 2010;16:3099. doi: 10.1089/ten.tea.2009.0504. [DOI] [PubMed] [Google Scholar]
- 14.Hwang Y.S. Chung B.G. Ortmann D. Hattori N. Moeller H.C. Khademhosseini A. Microwell-mediated control of embryoid body size regulates embryonic stem cell fate via differential expression of WNT5a and WNT11. Proc Natl Acad Sci U S A. 2009;106:16978. doi: 10.1073/pnas.0905550106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hesse E. Hefferan T.E. Tarara J.E. Haasper C. Meller R. Krettek C. Lu L. Yaszemski M.J. Collagen type I hydrogel allows migration, proliferation, and osteogenic differentiation of rat bone marrow stromal cells. J Biomed Mater Res A. 2010;94:442. doi: 10.1002/jbm.a.32696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fisher O.Z. Khademhosseini A. Langer R. Peppas N.A. Bioinspired materials for controlling stem cell fate. Acc Chem Res. 2010;43:419. doi: 10.1021/ar900226q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerecht S. Burdick J.A. Ferreira L.S. Townsend S.A. Langer R. Vunjak-Novakovic G. Hyaluronic acid hydrogel for controlled self-renewal and differentiation of human embryonic stem cells. Proc Natl Acad Sci U S A. 2007;104:11298. doi: 10.1073/pnas.0703723104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hill-West J.L. Chowdhury S.M. Sawhney A.S. Pathak C.P. Dunn R.C. Hubbell J.A. Prevention of postoperative adhesions in the rat by in situ photopolymerization of bioresorbable hydrogel barriers. Obstet Gynecol. 1994;83:59. [PubMed] [Google Scholar]
- 19.Alcantar N.A. Aydil E.S. Israelachvili J.N. Polyethylene glycol-coated biocompatible surfaces. J Biomed Mater Res. 2000;51:343. doi: 10.1002/1097-4636(20000905)51:3<343::aid-jbm7>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 20.Peppas N. Hilt J. Khademhosseini A. Langer R. Hydrogels in biology and medicine: from molecular principles to bionanotechnology. Adv Mater. 2006;18:1345. [Google Scholar]
- 21.Tibbitt M.W. Anseth K.S. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol Bioeng. 2009;103:655. doi: 10.1002/bit.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu X. Ma L. Wang C. Gao C. Gelatin hydrogel prepared by photo-initiated polymerization and loaded with TGF-beta1 for cartilage tissue engineering. Macromol Biosci. 2009;9:1194. doi: 10.1002/mabi.200900275. [DOI] [PubMed] [Google Scholar]
- 23.Nichol J.W. Koshy S.T. Bae H. Hwang C.M. Yamanlar S. Khademhosseini A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials. 2010;31:5536. doi: 10.1016/j.biomaterials.2010.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aubin H. Nichol J.W. Hutson C.B. Bae H. Sieminski A.L. Cropek D.M. Akhyari P. Khademhosseini A. Directed 3D cell alignment and elongation in microengineered hydrogels. Biomaterials. 2010;31:6941. doi: 10.1016/j.biomaterials.2010.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leach J.B. Bivens K.A. Collins C.N. Schmidt C.E. Development of photocrosslinkable hyaluronic acid-polyethylene glycol-peptide composite hydrogels for soft tissue engineering. J Biomed Mater Res A. 2004;70:74. doi: 10.1002/jbm.a.30063. [DOI] [PubMed] [Google Scholar]
- 26.Leach J.B. Schmidt C.E. Characterization of protein release from photocrosslinkable hyaluronic acid-polyethylene glycol hydrogel tissue engineering scaffolds. Biomaterials. 2005;26:125. doi: 10.1016/j.biomaterials.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 27.Mann B.K. Gobin A.S. Tsai A.T. Schmedlen R.H. West J.L. Smooth muscle cell growth in photopolymerized hydrogels with cell adhesive and proteolytically degradable domains: synthetic ECM analogs for tissue engineering. Biomaterials. 2001;22:3045. doi: 10.1016/s0142-9612(01)00051-5. [DOI] [PubMed] [Google Scholar]
- 28.Van Den Bulcke A.I. Bogdanov B. De Rooze N. Schacht E.H. Cornelissen M. Berghmans H. Structural and rheological properties of methacrylamide modified gelatin hydrogels. Biomacromolecules. 2000;1:31. doi: 10.1021/bm990017d. [DOI] [PubMed] [Google Scholar]
- 29.Engler A.J. Sen S. Sweeney H.L. Discher D.E. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 30.Engler A.J. Sweeney H.L. Discher D.E. Schwarzbauer J.E. Extracellular matrix elasticity directs stem cell differentiation. J Musculoskelet Neuronal Interact. 2007;7:335. [PubMed] [Google Scholar]
- 31.Zamanian B. Masaeli M. Nichol J.W. Khabiry M. Hancock M.J. Bae H. Khademhosseini A. Interface-directed self-assembly of cell-laden microgels. Small. 2010;6:937. doi: 10.1002/smll.200902326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fernandez J.G. Khademhosseini A. Micro-masonry: construction of 3D structures by microscale self-assembly. Adv Mater. 2010;22:2538. doi: 10.1002/adma.200903893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Du Y. Ghodousi M. Lo E. Vidula M.K. Emiroglu O. Khademhosseini A. Surface-directed assembly of cell-laden microgels. Biotechnol Bioeng. 2010;105:655. doi: 10.1002/bit.22552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Metters A. Hubbell J. Network formation and degradation behavior of hydrogels formed by Michael-type addition reactions. Biomacromolecules. 2005;6:290. doi: 10.1021/bm049607o. [DOI] [PubMed] [Google Scholar]
- 35.Park Y. Lutolf M.P. Hubbell J.A. Hunziker E.B. Wong M. Bovine primary chondrocyte culture in synthetic matrix metalloproteinase-sensitive poly(ethylene glycol)-based hydrogels as a scaffold for cartilage repair. Tissue Eng. 2004;10:515. doi: 10.1089/107632704323061870. [DOI] [PubMed] [Google Scholar]
- 36.Sanborn T.J. Messersmith P.B. Barron A.E. In situ crosslinking of a biomimetic peptide-PEG hydrogel via thermally triggered activation of factor XIII. Biomaterials. 2002;23:2703. doi: 10.1016/s0142-9612(02)00002-9. [DOI] [PubMed] [Google Scholar]
- 37.Ehrbar M. Rizzi S.C. Hlushchuk R. Djonov V. Zisch A.H. Hubbell J.A. Weber F.E. Lutolf M.P. Enzymatic formation of modular cell-instructive fibrin analogs for tissue engineering. Biomaterials. 2007;28:3856. doi: 10.1016/j.biomaterials.2007.03.027. [DOI] [PubMed] [Google Scholar]
- 38.Keys K.B. Andreopoulos F. Peppas N.A. Poly(ethylene glycol) star polymer hydrogels. Macromolecules. 1998;31:8149. [Google Scholar]
- 39.Peppas N.A. Keys K.B. Torres-Lugo M. Lowman A.M. Poly(ethylene glycol)-containing hydrogels in drug delivery. J Control Release. 1999;62:81. doi: 10.1016/s0168-3659(99)00027-9. [DOI] [PubMed] [Google Scholar]
- 40.Lutolf M.P. Lauer-Fields J.L. Schmoekel H.G. Metters A.T. Weber F.E. Fields G.B. Hubbell J.A. Synthetic matrix metalloproteinase-sensitive hydrogels for the conduction of tissue regeneration: engineering cell-invasion characteristics. Proc Natl Acad Sci U S A. 2003;100:5413. doi: 10.1073/pnas.0737381100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lutolf M.P. Weber F.E. Schmoekel H.G. Schense J.C. Kohler T. Muller R. Hubbell J.A. Repair of bone defects using synthetic mimetics of collagenous extracellular matrices. Nat Biotechnol. 2003;21:513. doi: 10.1038/nbt818. [DOI] [PubMed] [Google Scholar]
- 42.Raeber G.P. Lutolf M.P. Hubbell J.A. Molecularly engineered PEG hydrogels: a novel model system for proteolytically mediated cell migration. Biophys J. 2005;89:1374. doi: 10.1529/biophysj.104.050682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kraehenbuehl T.P. Zammaretti P. Van der Vlies A.J. Schoenmakers R.G. Lutolf M.P. Jaconi M.E. Hubbell J.A. Three-dimensional extracellular matrix-directed cardioprogenitor differentiation: systematic modulation of a synthetic cell-responsive PEG-hydrogel. Biomaterials. 2008;29:2757. doi: 10.1016/j.biomaterials.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 44.Dreesmann L. Ahlers M. Schlosshauer B. The pro-angiogenic characteristics of a cross-linked gelatin matrix. Biomaterials. 2007;28:5536. doi: 10.1016/j.biomaterials.2007.08.040. [DOI] [PubMed] [Google Scholar]
- 45.Bae H. Ahari A. Shin H. Nichol J.W. Hutson C. Masaeli M. Kim S. Aubin H. Khademhosseini A. Cell-laden microengineered pullulan methacrylate hydrogels promote cell proliferation and 3D cluster formation. Soft Matter. 2011;7:1903. doi: 10.1039/C0SM00697A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Camci-Unal G. Aubin H. Ahari A.F. Bae H. Nichol J.W. Khademhosseini A. Surface-modified hyaluronic acid hydrogels to capture endothelial progenitor cells. Soft Matter. 2010;6:5120. doi: 10.1039/c0sm00508h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Honig W. Kula M.R. Selectivity of protein precipitation with polyethylene glycol fractions of various molecular weights. Anal Biochem. 1976;72:502. doi: 10.1016/0003-2697(76)90560-1. [DOI] [PubMed] [Google Scholar]