Abstract
This study evaluated the wound healing activity of hydroalcoholic leaf extract of Oncidium flexuosum Sims. (Orchidaceae), an important native plant of Brazil, combined or not with microcurrent stimulation. Wistar rats were randomly divided into four groups of nine animals: control (C), topical application of the extract (OF), treated with a microcurrent (10 μA/2 min) (MC), and topical application of the extract plus microcurrent (OF + MC). Tissue samples were obtained 2, 6, and 10 days after injury and submitted to structural and morphometric analysis. The simultaneous application of OF + MC was found to be highly effective in terms of the parameters analyzed (P < .05), with positive effects on the area of newly formed tissue, number of fibroblasts, number of newly formed blood vessels, and epithelial thickness. Morphometric data confirmed the structural findings. The O. flexuosum leaf extract contains active compounds that speed the healing process, especially when applied simultaneously with microcurrent stimulation.
1. Introduction
Medicinal plants have been used since ancient times for the treatment of various skin and dermatological disorders, especially cuts, wounds, and burns [1]. Current phytotherapics agents have also been shown to be highly effective in the treatment of these diseases [2–4]. Oncidium flexuosum Sims. (family Orchidaceae), popularly known as the “dancing doll orchid,” is one of the most important species of the Oncidium genus in Brazil [5, 6]. This plant is an epiphyte that can reach a height of 1 m and possesses a large number of flowers that form branched inflorescences of attractive medium-sized flowers. The sepals and petals are yellow, with brown-reddish transverse stripes, and the lip is highlighted with small red dots [7, 8]. The Orchidaceae family is one of the largest angiosperm families, and more than 25,000 species have been described so far [9, 10], including important species with medicinal potential [11]. In traditional medicine, the species of this family have been used for the treatment of abscesses, inflammation, wounds, external bleedin, and chapped skin [12, 13].
Wound healing constitutes a complex, dynamic, and well-orchestrated process that is activated whenever disruption of skin tissue occurs. The wound healing process is characterized by a broad spectrum of events, including platelet aggregation and activation of the coagulation cascade, inflammatory infiltration, cell differentiation, and tissue remodeling. Although the cascade of these events seems to be well discriminated and can be divided into three phases, namely, inflammation, proliferation, and wound contraction and remodeling, these phases actually overlap and continued or renewed tissue damage may reinitiate the sequence at affected sites within an existing wound. The application of low-amperage electrical stimuli modifies the healing process in living organisms, especially factors that delay or impair this process [14–16]. Electrical stimulation of different amplitudes and frequencies has been shown to promote modifications in cell and tissue responses in experimentally induced injuries [17–19] and can be used to promote tissue repair. Stimulation of live cells with low-intensity electrical currents directly affects the membrane potential and is associated with changes in ion gradients across the cell membrane, causing an increase in the synthesis of ATP followed by increased protein synthesis [14, 19, 20]. Numerous taxonomic reviews of the Oncidium genus are available in the literature [7–9], but there are no scientific data regarding the pharmacological activities of Oncidium flexuosum Sims. (Orchidaceae). Therefore, the scope of the present study was to evaluate the effects of leaf extract of this important Brazilian plant species combined with microcurrent stimulation or alone on wound healing.
2. Materials and Methods
2.1. Plant Material
Fresh Oncidium flexuosum leaves were collected in the medicinal plant garden of Uniararas (Projeto Saúde & Harmonia), Araras, São Paulo, Brazil, between December 2008 and February 2009, and identified by MSc. Cristiano Pedroso de Moraes. A voucher specimen (45341) was deposited at Herbário Rioclarense (HRCB), Instituto de Biociências, Universidade Estadual Paulista, Rio Claro/São Paulo, Brazil.
2.2. Preparation of the Hydroalcoholic Leaf Extract
Fresh leaves (50 g) were selected, cleaned, and macerated dynamically with 300 mL of an aqueous ethanol solution (7 : 3, v/v) for 4 h at room temperature. This procedure was repeated 3 times with the same powder and the same solvent. After filtration, the solvent was completely evaporated under vacuum at 40°C in a rotary evaporator, and the hydroalcoholic Oncidium flexuosum leaf extract (OF) was obtained after lyophilization [21]. The yield of the lyophilized extract was 9%.
2.3. Phytochemical Screening Method
The qualitative identification of the chemical constituents was carried out in the same extract as that used in the wound repair tests using chemical methods and thin-layer chromatography according to the methods proposed by Marini-Bettòlo et al. [22], Harborne [21], and Matos [23]. The dried extract (100 mg) was used for each test as described below. The presence of polyphenolic compounds was analyzed with 1% ferric chloride solution. Tannins were identified using the dried extract dissolved in water with 2 mL sodium chloride (2%), filtered, and mixed with 5 mL 1% gelatin solution. The presence of flavonoids was determined using 1% aluminum chloride solution in methanol, concentrated hydrochloric acid, magnesium turnings, and potassium hydroxide solution. Dragendorff's reagent was used to evaluate the presence of total alkaloids. Saponins were analyzed based on their capacity to produce foam. For the detection of triterpenes, the extract was mixed with 5 mL chloroform, warmed at 80°C for 30 min, and then treated with a small volume of concentrated sulfuric acid. Additionally, the extract (OF) was analyzed by thin-layer chromatography on silica gel using chloroform : methanol (98 : 2) and hexane : ethyl acetate (80 : 20) as eluent. Flavonoid components were visualized first under UV light and then by spraying the chromatographic plates separately with a solution of vanillin in sulfuric acid and ammonium vapor, followed by incubation at 100°C for 5 min.
2.4. Experimental Groups
Thirty-six male Wistar rats (Rattus norvegicus), each weighing 250–300 g, obtained from the Experimental Animal Center “Prof. Dr. Luiz Edmundo de Magalhães,” Uniararas, were housed individually in cages at a constant temperature (23 ± 2°C) under a 12 : 12 h light/dark cycle, with free access to food and water. No difference in the average weight or behavior of the animals was observed between the beginning and the end of the study. This study was permitted by the Ethics Committee of Uniararas (protocol number 806/2006) and was performed according to international rules considering the animal experiments and biodiversity right [24, 25].
2.5. Preparation of Test Samples for the Bioassay
Each animal's back was depilated 48 h before surgical intervention. After local asepsis with 0.4% chlorhexidine digluconate, the animals were anesthetized by intraperitoneal injection of xylazine hydrochloride (20 mg/kg body weight) and ketamine hydrochloride (50 mg/kg). After the position was marked with a dermographic pen and pachymeter, a 2 cm long and 0.2 cm deep surgical incision was made (with sterile surgical blade marked previously) through the full thickness of the skin in the craniocaudal direction, according to methods described by Mustoe et al. [26] and Mendonça et al. [27]. The incision was not sutured. In view of the similar genetic background of the animals [28] and following the guidelines of the Ethics Committee of Uniararas, groups of nine animals each were used: control group, C, receiving a topical application of saline solution 0.9%; OF group receiving a topical application of the extract of Oncidium flexuosum (60 mg/mL); MC group, treated with microcurrent (10 μA/2 min), and OF + MC group, receiving a topical application of the extract of Oncidium flexuosum plus microcurrent (10 μA/2 min), according to the protocol of Mendonça et al. [27]. A transcutaneous electrical stimulator (Physiotonus Microcurrent, Bioset, Rio Claro, São Paulo, Brazil) was used for electrical stimulation of the microgalvanic type. The applications involved the use of two metal electrodes with a spherical tip (10 mm) positioned on the wound. The treatments were started 24 h after surgical intervention and were continued daily for 10 days. From the dose-response curve using doses of 20, 40, and 60 mg/mL of the extract of Oncidium flexuosum, 60 mg/mL was selected from the evaluation of the cell count in the reparative tissue.
2.6. Collection and Preparation of Wound Samples for Structural Analysis
In 2, 6, and 10 days after the injury, three animals in each group were killed under anesthesia; the total area of the wound was removed and submitted to structural and morphometric analysis. Each sample was removed and fixed in 10% formalin in Millonig buffer, pH 7.4, for 24 h at room temperature. Next, the specimens were washed in buffer and processed for embedding in Paraplast (Merck). Longitudinal sections (7 μm) were stained with hematoxylin/eosin for routine histology, with picrosirius-hematoxylin for the observation of collagen fibers. The specimens were examined and documented using a Leica DM 2000 photomicroscope at the Laboratory of Micromorphology, Centro Universitário Hermínio Ometto, Uniararas.
2.7. Morphometric Analysis
Cross-sections of the midregion of the experimental wound were used for the determination of the following morphometric parameters: tissue repair area (×103 μm2), total number of cells (fibroblastic and inflammatory cells) (n/103 μm2), number of newly formed blood vessels (n/103 μm2), and thickness of the regenerating epithelium (μm). For this purpose, three samples were randomly selected among the sections obtained. All images were captured and digitalized using a Leica DM 2000 photomicroscope. The measurements were made on the digitalized images using the Leica Image Measure and Sigma Scan Pro 6.0 programs.
The results were compared by ANOVA and the Tukey posttest, with the level of significance set at 5%. The results were entered into spreadsheets of the Biostat for Windows XP program.
3. Results
3.1. Phytochemical Screening
Preliminary phytochemical analysis of the extract (OF) using chemical methods showed a predominance of phenolic compounds, such as flavonoids and tannins, and the presence of triterpenes. However, no total alkaloids or saponins were detected.
The best separation of the components of the hydroalcoholic extract was achieved using chloroform : methanol (98 : 2) as the mobile phase. Thin-layer chromatography also revealed the presence of polyphenols, tannins, and triterpenoids.
3.2. Structural and Morphometric Analysis of Wound Repair
Wound healing was studied in the different groups by comparing inflammatory processes (leukocytosis, hemorrhage and exudate), proliferative processes (fibroblastic, hyperplasia, epithelization, and angiogenesis), and tissue reorganization. This was completed in all animals within the 10-day observation period. Structural analysis was performed on days 2, 6, and 10.
The proliferative phase was observed early on day 2 after experimental injury in animals of the groups treated with microcurrent (MC) and with the combination of microcurrent and O. flexuosum extract (OF + MC) when compared to the control group and group treated with extract OF alone.
The structural characteristics of the fibrous matrix were also evaluated in the different groups. A predominance of thin and poorly compacted collagen fibers was observed in samples collected on day 6 after experimental injury in all groups. However, compact and medium-thick fibers were observed on day 10 when compared to intact tissue at the border of the wound (Figures 1 and 2). These results were supported by morphometric analysis comparing the different groups (Figures 3, 4, 5, and 6).
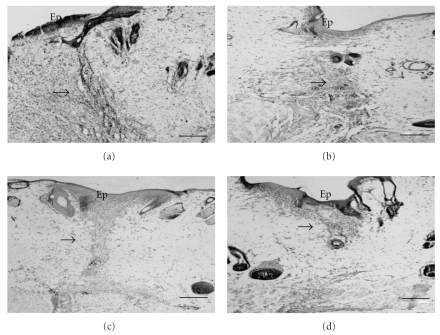
Figure 1.
Photomicrographs of cross-sections of skin obtained from the back of Wistar rats on day 10 after surgically induced injury. (a) Control group; (b) group topically treated with Oncidium flexuosum extract; (c) group treated with microcurrent (10 μA/2 min) (MC); (d) group treated with O. flexuosum extract plus microcurrent (10 μA/2 min) (OF + MC). Ep: epidermis; (→): tissue repair area. The sections were stained with toluidine blue. Bar = 100 μm.
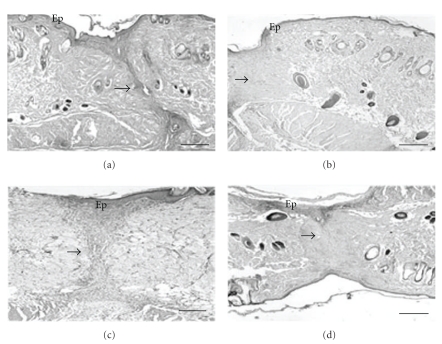
Figure 2.
Photomicrographs of cross-sections of skin obtained from the back of Wistar rats on day 10 after surgically induced injury. (a) Control group; (b) group topically treated with Oncidium flexuosum extract; (c) group treated with microcurrent (10 μA/2 min) (MC); (d) group treated with O. flexuosum extract plus microcurrent (10 μA/2 min) (OF + MC). Ep: epidermis; (→): tissue repair area. The sections were stained with picrosirius-hematoxylin. Bar = 100 μm.
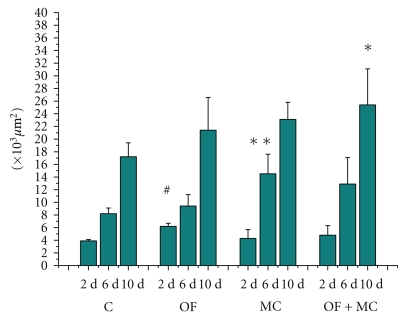
Figure 3.
Size of the tissue repair area (×103 μm2) in the region of the experimental wound. C: control group; OF: group topically treated with Oncidium flexuosum extract; MC: group treated with microcurrent (10 μA/2 min); OF + MC: group treated with O. flexuosum extract plus microcurrent (10 μA/2 min). Samples collected on days 2 (2 d), 6 (6 d), and 10 (10 d) after injury were analyzed. The results are reported as the mean and standard deviation obtained for each group and were compared by ANOVA and the Tukey posttest (P < .05). Significant differences between control and treated groups in the distinct times of sampling are indicated by #, ∗∗, and ∗.
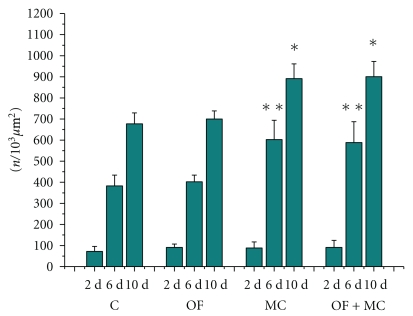
Figure 4.
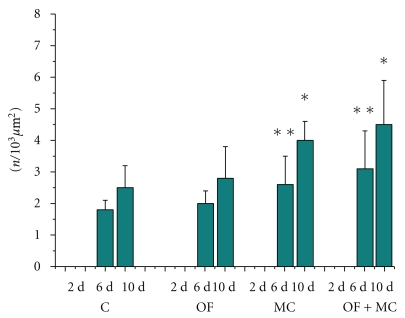
Total number of cells (n/103 μm2) in the region of the experimental wound. C: control group; OF: group topically treated with Oncidium flexuosum extract; MC: group treated with microcurrent (10 μA/2 min); OF + MC: group treated with O. flexuosum extract plus microcurrent (10 μA/2 min). Samples collected on days 2 (2 d), 6 (6 d) and 10 (10 d) after injury were analyzed. The results are reported as the mean and standard deviation obtained for each group and were compared by ANOVA and the Tukey posttest (P < .05). Significant differences between control and treated groups in the distinct times of sampling are indicated by ∗∗ and ∗.
Figure 5.
Total number of newly formed blood vessels (n/103 μm2) in the region of the experimental wound. C: control group; OF: group topically treated with Oncidium flexuosum extract; MC: group treated with microcurrent (10 μA/2 min); OF + MC: group treated with O. flexuosum extract plus microcurrent (10 μA/2 min). Samples collected on days 2 (2 d), 6 (6 d), and 10 (10 d) after injury were analyzed. The results are reported as the mean and standard deviation obtained for each group and were compared by ANOVA and the Tukey posttest (P < .05). Significant differences between control and treated groups in the distinct times of sampling are indicated by ∗∗ and ∗.
Figure 6.
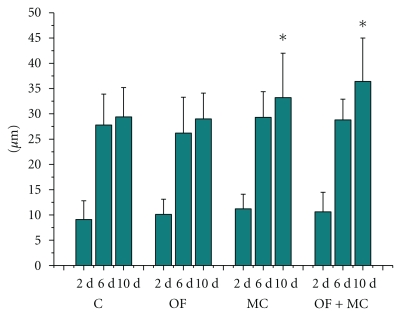
Epithelial thickness (μm) in the region of the experimental wound. C: control group; OF: group topically treated with Oncidium flexuosum extract; MC: group treated with microcurrent (10 μA/2 min); OF + MC: group treated with O. flexuosum extract plus microcurrent (10 μA/2 min). Samples collected on days 2 (2 d), 6 (6 d), and 10 (10 d) after injury were analyzed. The results are reported as the mean and standard deviation obtained for each group and were compared by ANOVA and the Tukey posttest (P < .05). Significant differences between control and treated groups in the distinct times of sampling are indicated by ∗.
Two days after experimental injury, the tissue repair area was significantly greater in the OF group compared to the other experimental groups. This is the inflammatory period of repair process and probably the application of the extract containing O. flexuosum exerted an anti-inflammatory action favoring the repair of the lesion. A significantly greater tissue repair area was observed in the MC group as early as on day 6. On day 10 after injury, the wound area was completely reepithelized in the group treated with the combination of O. flexuosum extract and microcurrent, with the difference being significant when compared to the other groups (Figure 3).
With respect to the total number of cells on day 6 and 10 after injury, similar numbers were observed in the MC and OF + MC groups, which were significantly higher than those found in the control and OF groups (Figure 4).
The same pattern was observed for the total number of cells and the number of newly formed vessels. A larger number of newly formed vessels inside the wound area were observed in the OF + MC and MC groups on days 6 and 10 after experimental injury when compared to the other groups (Figure 5).
Additionally, the thickness of newly formed epithelium was higher in the MC and OF + MC groups only on day 10 of treatment (Figure 6).
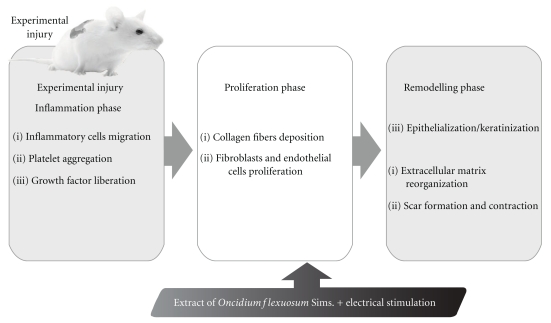
Structural and morphometric analysis suggested that the phytochemical content of the leaf extract of O. flexuosum might be responsible for collagen formation at the proliferative state, which is contributed by increased fibroblasts content (Figure 7).
Figure 7.
Hypothetical diagram demonstrating the possible effect of Oncidium flexuosum leaf extract in wound healing activity.
4. Discussion
The understanding of the biological and pathological events that occur during the healing process is of the utmost importance for the treatment of wounds. Clinical evidence shows that the repair of connective, dermal and subdermal tissues can be accelerated by the external application of a low-intensity electrical current [19, 29]. Microcurrent electrical stimulation is called biostimulation since this is compatible with that of endogenous currents that act in the organism at the cellular level [14, 17, 18, 30]. This technique is not invasive, presumably has an antioxidant effect, and it showed potential to accelerate wound healing [31].
The combination of the low levels of antioxidants and raised levels of free radical played a major role in delaying wound healing in aged rats and diabetic rats [32]. Microcurrent has been used in the treatment of chronic wounds [33–35]. Lee et al. [31] used a 100 nA current 3 μA in the treatment of chronic wounds and ulcers associated with chronic diseases and found that the application of such currents supposedly provides electrons to tissues saturating free radicals and facilitating tissue repair.
In the present study, it was evident from the morphometric analysis application that microcurrent alone or combined with OF was significant in promoting an increase in the total number of cells, blood vessels, and thickness of the epithelium in the damaged area in all experimental groups subjected to this treatment at 6 and 10 days after the injury. The stimulation of wound healing by low-intensity electrical currents has been reported by various investigators [15, 18, 36, 37]. Biedeback [38] proposed that transmembrane currents open voltage-controlled calcium channels in fibroblasts, causing ATP resynthesis, activation of protein kinase mechanisms to synthesize new cellular protein, and DNA replication necessary for mitotic cell division. Mendonça et al. [27] suggested that microcurrent application to tissue injuries might be used as a coadjuvant to accelerate the healing process. Variations in cell metabolism as well as fibroblast proliferation, neovascularization, and collagen deposition in the wound area have been observed after microcurrent application [39, 40].
Topical application of the O. flexuosum extract (OF) promoted effects similar to those observed with microcurrent therapy, but to a lesser extent. Various studies have shown the efficacy of phytotherapics agents in wound healing [41–45]. The phytochemical compounds effective in this process and which also present marked anti-inflammatory activity include flavonoids and tannins, also present in the extract OF. Additionally, these compounds exert antioxidant and antibacterial activity by interacting with a wide variety of enzymatic and biochemical systems [42, 46–48]. Moreover, phytochemical studies of species of the Orchidaceae family have shown the presence of structures which produce and store substances that are probably responsible for these pharmacological actions, such as flavonoids and terpenes [49, 50]. Fiallo et al. [50] demonstrated that flavonoids were detected in the leaves of the Oncidium luridum Lindl. The presence of these compounds in the Orchidaceae family is widely known [51] and was also present in the extract of O. flexuosum.
Flavonoids are used for therapeutic purposes because of their anti-inflammatory, antifungal, antioxidant, and wound healing properties [52]. Moreover, flavonoids and their derivatives are known to decrease lipid peroxidation by improving vascularity and preventing or slowing down the progress of cell necrosis in Colutea cilicica Boiss. & Bal. [53]. Flavonoids have also been shown to enhance wound healing processes primarily owing to their antimicrobial and astringent properties, which appear to be responsible for wound contraction and an elevated epithelization rate [54]. Nayak et al. [55] observed a significant wound healing potential after topical application of Vanda roxburghii R.Br. extract (Orchidaceae), an epiphytic orchid, to wounds surgically induced in Wistar rats.
Tannins also have a role in wound healing processes, according to Bedi and Shenefelt [43] and Neto et al. [56]; these compounds precipitate proteins in damaged tissues, forming a protective lining that favors repair and reduces wound permeability and exudation.
The combination of microcurrent and the extract OF was advantageous in terms of all parameters studied when compared to the control group and to either treatment alone. Soares [57] combined the application of vitamin C and physical agents for the healing of experimental wounds and demonstrated that the combination of antioxidant agents and photodynamic or low-amperage electrical therapy accelerates wound healing. Filho et al. [58] also investigated the effects of simultaneous application of physical and phytotherapics agents to wounds using ultrasound and Aloe vera gel on an experimental model of induction of tendinitis in rats and demonstrated that this type of treatment is effective in terms of both skin repair and reduction of the inflammatory process. Mendonça et al. [27] showed that the simultaneous application of Aloe vera and microcurrent was effective in the treatment of open wounds, potentiating wound healing in Wistar rats. This seems to indicate a synergistic action between these two applications. Mendonça et al. [59] associated the low-power GaAlAs laser (830 nm) with aqueous solution curative of Stryphnodendron barbatimao in the treatment of septic wounds of sheep and found complete epithelization at 15 days by daily application of these agents combined. These reports suggest that this methodology where physical agents associated with herbal medicines is effective in the treatment of wounds.
5. Conclusion
The present results show that the extract of O. flexuosum was effective in experimental models of wound healing, accelerating this process. However, a better performance was observed when the extract was combined with microcurrent stimulation compared to the other treatments and to the control group, suggesting a synergistic action of these agents. The application of this treatment showed advantages in terms of all parameters studied.
Acknowledgments
The authors thank Marta Yabase for donation of the Oncidium flexuosum Sims. seedlings used in this study, Projeto Saúde & Harmonia (Uniararas) for seedling maintenance, and support from FAPESP and CNPq.
References
- 1.Govindarajan R, Kumar B, Vijayakumar M, Pushpangadan P. Ethnopharmacological approaches to wound healing-exploring medicinal plants of India. Journal of Ethnopharmacology. 2007;114(2):103–113. doi: 10.1016/j.jep.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 2.Rao SG, Udupa AL, Udupa SL. Calendula and hypericum: two homeopathic drugs promoting wound healing in rats. Fitoterapia. 1991;62(6):508–510. [Google Scholar]
- 3.Schempp CM, Winghofer B, Lüdtke R, Simon-Haarhaus B, Schöpf E, Simon JC. Topical application of St John’s wort (Hypericum perforatum L.) and of its metabolite hyperforin inhibits the allostimulatory capacity of epidermal cells. British Journal of Dermatology. 2000;142(5):979–984. doi: 10.1046/j.1365-2133.2000.03482.x. [DOI] [PubMed] [Google Scholar]
- 4.Martins PS, Alves ALG, Hussni CA, Sequeira JL, Nicoletti JLM, Thomassian A. Comparação entre fitoterápicos de uso tópico na cicatrização de pele em eqüinos. Archives of Veterinary Science. 2003;8(2):1–7. [Google Scholar]
- 5.Joly AB. Botânica—Introdução à Taxonomia Vegetal. 11th edition. Companhia Editora Nacional; 1993. [Google Scholar]
- 6.Raven PH, Everest RF, Eichhorn SE. Biologia Vegetal. 6th edition. Guanabara Koogan; 2001. [Google Scholar]
- 7.Pabst GJ. Estudos no gênero Oncidium Sw. (Orchidaceae)-IV. Bradea. 1972;1(15):137–143. [Google Scholar]
- 8.Garay LA, Stacy JE. Synopsis of the genus Oncidium . Bradea. 1974;1(40):393–427. [Google Scholar]
- 9.Dressler RL. The Orchids: Natural History and Classification. Harvard University Press; 1990. [Google Scholar]
- 10.Arditti J. Fundamentals of Orchid Biology. New York, NY, USA: John Wiley; 1992. [Google Scholar]
- 11.Kong JM, Goh NK, Chia LS, Chia TF. Recent advances in traditional plant drugs and orchids. Acta Pharmacologica Sinica. 2003;24(1):7–21. [PubMed] [Google Scholar]
- 12.The Committee on Scholarly Communication with the People's Republic of China. Herbal Pharmacology in the People's Republic of China. Washington, DC, USA: National Academy of Sciences; 1975. [Google Scholar]
- 13.Ward H. Herbal Manual: The Medicinal, Toilet, Culinary and other Uses of 130 of the most Commonly Used Herbs. London, UK: LN Fowler & Co; 1936. [Google Scholar]
- 14.Cheng N, Van Hoof H, Bocks E. The effects of electric currents on ATP generation, protein synthesis, and membrane transport in rat skin. Clinical Orthopaedics and Related Research. 1982;171:264–272. [PubMed] [Google Scholar]
- 15.Houghton PE, Kincaid CB, Lovell M. Effect of electrical stimulation on chronic leg ulcer size and appearance. Physical Therapy. 2003;83(1):17–28. [PubMed] [Google Scholar]
- 16.Kloth LC, Feedar JA. Acceleration of wound healing with high voltage, monophasic, pulsed current. Physical Therapy. 1988;68(4):503–508. doi: 10.1093/ptj/68.4.503. [DOI] [PubMed] [Google Scholar]
- 17.Kloth LC. Electrical stimulation for wound healing: a review of evidence from in vitro studies, animal experiments, and clinical trials. International Journal of Lower Extremity Wounds. 2005;4(1):23–44. doi: 10.1177/1534734605275733. [DOI] [PubMed] [Google Scholar]
- 18.Bayat M, Asgari-Moghadam Z, Maroufi M, Rezaie FS, Bayat M, Rakhshan M. Experimental wound healing using microamperage electrical stimulation in rabbits. Journal of Rehabilitation Research and Development. 2006;43(2):219–226. doi: 10.1682/jrrd.2005.05.0089. [DOI] [PubMed] [Google Scholar]
- 19.Santos VNS, Ferreira LM, Horibe EK, Duarte IS. Electric microcurrent in the restoration of the skin undergone a trichloroacetic acid peeling in rats. Acta Cirurgica Brasileira. 2004;19(5):466–470. [Google Scholar]
- 20.Valle KKR, Reis LL, Bouvent JJ, Shida CS. Efeito da aplicação de microcorrente elétrica na restauração de pele de ratos exposta à ação de radicais livres. In: Proceedings of the 21th Congresso Brasileiro de Engenharia Biomédica; 2008; pp. 247–249. [Google Scholar]
- 21.Harborne JB. Phytochemical Methods. A Guide to Modern Techniques of Plant Analysis. 2nd edition. Chapman and Hall; 1984. [Google Scholar]
- 22.Marini-Bettòlo GB, Nicoletti M, Patamia M. Plant screening by chemical and chromatographic procedures under field conditions. Journal of Chromatography. 1981;218:113–217. [Google Scholar]
- 23.Matos FJA. Introdução à Fitoquímica Experimental. Fortaleza, Brazil: Ediçães UFC; 1997. [Google Scholar]
- 24.International Guiding Principles for Biomedical Research Involving Animals. CIOMS, 1985. [PubMed]
- 25.Guide for the Care and Use of Laboratory Animals. NIH; 1996. [Google Scholar]
- 26.Mustoe TA, Pierce GF, Thomason A, Gramates P, Sporn MB, Deuel TF. Accelerated healing of incisional wounds in rats induced by transforming growth factor-beta. Sciense. 1987;237(4820):1333–1336. doi: 10.1126/science.2442813. [DOI] [PubMed] [Google Scholar]
- 27.Mendonça FAS, Passarini JR, Jr., Esquisatto MAM, Mendonça JS, Franchini CC, Santos GMT. Effects of the application of Aloe vera (L.) and microcurrent on the healing of wounds surgically induced in Wistar rats (Rattus norvegicus) Acta Cirurgica Brasileira. 2009;24(1):150–155. doi: 10.1590/s0102-86502009000200013. [DOI] [PubMed] [Google Scholar]
- 28.Gill TJ, Smith GJ, Wissler RW. The rat as an experimental animal. Science. 1989;245(4915):269–276. doi: 10.1126/science.2665079. [DOI] [PubMed] [Google Scholar]
- 29.Frick A, McCauley D. Microcurrent electrical therapy. Journal of Equine Veterinary Science. 2005;25(11):418–422. [Google Scholar]
- 30.Barker AT, Jaffe LF, Vanable JW., Jr. The glabrous epidermis of cavies contains a powerful battery. The American Journal of Physiology. 1982;242(3):358–366. doi: 10.1152/ajpregu.1982.242.3.R358. [DOI] [PubMed] [Google Scholar]
- 31.Lee BY, Al-Waili N, Stubbs D, et al. Ultra-low microcurrent in the management of diabetes mellitus, hypertension and chronic wounds: report of twelve cases and discussion of mechanism of action. International Journal of Medical Sciences. 2010;7(1):29–35. doi: 10.7150/ijms.7.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anamika M, Rasik AS. Antioxidant status in delayed healing type of wounds. International Journal of Experimental Pathology. 2000;81(4):257–263. doi: 10.1046/j.1365-2613.2000.00158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carley PJ, Wainapel SF. Electrotherapy for acceleration of wound healing: low intensity direct current. Archives of Physical Medicine and Rehabilitation. 1985;66(7):443–446. [PubMed] [Google Scholar]
- 34.Nessler JP, Mass DP. Direct-current electrical stimulation of tendon healing in vitro. Clinical Orthopaedics and Related Research. 1987;217:303–312. [PubMed] [Google Scholar]
- 35.Al-Waili N. Electrotherapy for chronic gum and periapical abscesses. Journal of the Pakistan Medical Association. 1989;39(6):161–162. [PubMed] [Google Scholar]
- 36.Bassett CAL. Beneficial effects of electromagnetic fields. Journal of Cellular Biochemistry. 1993;51(4):387–393. doi: 10.1002/jcb.2400510402. [DOI] [PubMed] [Google Scholar]
- 37.Sheffet A, Cytryn AS, Louria DB. Applying electric and electromagnetic energy as adjuvant treatment for pressure ulcers: a critical review. Ostomy Wound Management. 2000;46:28–33. [PubMed] [Google Scholar]
- 38.Biedebach MC. Accelerated healing of skin ulcers by electrical stimulation and the intracellular physiological mechanisms involved. Acupuncture and Electro-Therapeutics Research. 1989;14(1):43–60. doi: 10.3727/036012989816358579. [DOI] [PubMed] [Google Scholar]
- 39.Aaron RK, Ciombor DM. Therapeutic effects of electromagnetic fields in the stimulation of connective tissue repair. Journal of Cellular Biochemistry. 1993;52(1):42–46. doi: 10.1002/jcb.240520107. [DOI] [PubMed] [Google Scholar]
- 40.Macedo ACB, Simões ND. Aplicação de estimulação elétrica de baixa intensidade no tratamento de úlceras varicosas. Fisioterapia em Movimento. 2007;20(3):23–33. [Google Scholar]
- 41.Peruchi CMS, Acevedo RA, Franco SL. Efecto del propóleos en la cicatrización de lesiones subcutáneas inducidas en el dorso del ratones: estudio histológico. Revista de la Facultad de Odontología de la Universidad de Chile. 2001;19(2):23–34. [Google Scholar]
- 42.Ardisson L, Godoy JS, Ferreira LAM, Stehmann JR, Brandão MGL. Preparação e caracterização de extratos glicólicos enriquecidos em taninos a partir das cascas de Stryphnodendron adstringens (Mart.) Coville (Barbatimão) Revista Brasileira de Farmacognosia. 2002;12(1):27–34. [Google Scholar]
- 43.Bedi MK, Shenefelt PD. Herbal therapy in dermatology. Archives of Dermatology. 2002;138(2):232–242. doi: 10.1001/archderm.138.2.232. [DOI] [PubMed] [Google Scholar]
- 44.Lee WR, Park JH, Kim KH, et al. The biological effects of topical alginate treatment in an animal model of skin wound healing. Wound Repair and Regeneration. 2009;17(4):505–510. doi: 10.1111/j.1524-475X.2009.00496.x. [DOI] [PubMed] [Google Scholar]
- 45.d'Acampora AJ, Tramonte R, Bürger D, Bürger PJ. Efeitos da quercetina na cicatrização de ferida cirúrgica contaminada em ratos Wistar. Arquivos Catarinenses de Medicina. 2007;36(1):69–75. [Google Scholar]
- 46.Araújo TAS, Alencar NL, Amorim ELC, Albuquerque UP. A new approach to study medicinal plants with tannins and flavonoids contents from the local knowledge. Journal of Ethnopharmacology. 2008;120(1):72–80. doi: 10.1016/j.jep.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 47.Ermertcan AT, Inan S, Ozturkcan S, Bilac C, Cilaker S. Comparison of the effects of collagenase and extract of Centella asiatica in an experimental model of wound healing: an immunohistochemical and histopathological study. Wound Repair and Regeneration. 2008;16(5):674–681. doi: 10.1111/j.1524-475X.2008.00417.x. [DOI] [PubMed] [Google Scholar]
- 48.Chifa C, Ricciardi AIA. Especies del género Oncidium (Orchidaceae) utilizadas como diuréticas por los nativos del Chaco argentino. Ciencia & Tecnica—Comunicaciones Científicas y Tecnológicas. 2001;7(T-066) [Google Scholar]
- 49.Fernandes MFG, Oliveira CS, Mercadante-Simões MO, Mota BCF. III Fórum Gestão, Pesquisa, Ensino e Extensão. Montes Claros, Brazil: Unimontes Universidade Estadual de Montes Claros; 2009. Aplicaçães terapêuticas de orquidáceas. [Google Scholar]
- 50.Fiallo VRF, Lorenzo MMG, Hernádez CML, Ferradá CAR. Estudios fenológicos en plantas medicinales. Revista Cubana de Plantas Medicinais. 1998;3(1):7–11. [Google Scholar]
- 51.Williams CA, Greenham J, Harborne JB, Kong JM, Goh NK, Chia LS. Acylated anthocyanins and flavonols from purple flowers of Dendrobium cv. ’Pompadour’. Biochemical Systematics and Ecology. 2002;30(7):667–675. [Google Scholar]
- 52.Nayak SB, Sandiford S, Maxwell A. Evaluation of the wound-healing activity of ethanolic extract of Morinda citrifolia L. leaf. Evidence-based Complementary and Alternative Medicine. 2009;6(3):351–356. doi: 10.1093/ecam/nem127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Süntar IS, Koca U, Akkol EK, Yilmazer D, Alper M. Assessment of wound healing activity of the aqueous extracts of Colutea cilicica Boiss. & Bal. fruits and leaves. Evidence-Based Complementary and Alternative Medicine. 2011;2011:7 pages. doi: 10.1093/ecam/nep190. Article ID 758191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pesin I, Koca U, Keles H, Akkol EK. Wound healing activity of Rubus sanctus Schreber (Rosaceae): preclinical study in animal models. doi: 10.1093/ecam/nep137. Evidence-Based Complementary and Alternative Medicine. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nayak BS, Suresh R, Rao AV, et al. Evaluation of wound healing activity of Vanda roxburghii R. Br (Orchidaceae): a preclinical study in a rat model. International Journal of Lower Extremity Wounds. 2005;4(4):200–204. doi: 10.1177/1534734605282994. [DOI] [PubMed] [Google Scholar]
- 56.Neto JJ, Fracasso JF, Neves MCLC, Santos LE, Banuth VL. Tratamento de úlcera varicosa e lesões de pele com Calendula officinalis e/ou com Stryphnodendron barbatiman (vellozo) martius . Revista de Ciências Farmacêuticas. 1996;17:181–186. [Google Scholar]
- 57.Soares FRL. Reparação de feridas cutâneas tratadas com vitamina C, laser e a associação de vitamina C e laser: estudo histológico em ratos. Unimar; 2005. M.S. thesis. [Google Scholar]
- 58.Filho ALMM, VilaVerda AGB, Araújo KS, et al. Análise do efeito do ultra-som terapêutico associado ao Aloe vera no tratamento de tendinite experimental. In: Proceedings of the 21th Congresso Brasileiro de Engenharia Biomédica; 2008; pp. 1359–1362. [Google Scholar]
- 59.Mendonça GBN, Moraes JM, Ferreira J, et al. Laser As-Ga-Al de baixa potência associado com solução aquosa de barbatimão (Stryphynodendron barbatiman Martius) na reparação tecidual de ferida cutânea séptica de ovino. Universidade Federal de Goiás, 2008.