Abstract
Hypertension elicited by chronic intermittent hypoxia (CIH) is associated with elevated activity of the thoracic sympathetic nerve (tSN) that exhibits an enhanced respiratory modulation reflecting a strengthened interaction between respiratory and sympathetic networks within the brain stem. Expiration is a passive process except for special metabolic conditions such as hypercapnia, when it becomes active through phasic excitation of abdominal motor nerves (AbN) in late expiration. An increase in CO2 evokes late-expiratory (late-E) discharges phase-locked to phrenic bursts with the frequency increasing quantally as hypercapnia increases. In rats exposed to CIH, the late-E discharges synchronized in AbN and tSN emerge in normocapnia. To elucidate the possible neural mechanisms underlying these phenomena, we extended our computational model of the brain stem respiratory network by incorporating a population of presympathetic neurons in the rostral ventrolateral medulla that received inputs from the pons, medullary respiratory compartments, and retrotrapezoid nucleus/parafacial respiratory group (RTN/pFRG). Our simulations proposed that CIH conditioning increases the CO2 sensitivity of RTN/pFRG neurons, causing a reduction in both the CO2 threshold for emerging the late-E activity in AbN and tSN and the hypocapnic threshold for apnea. Using the in situ rat preparation, we have confirmed that CIH-conditioned rats under normal conditions exhibit synchronized late-E discharges in AbN and tSN similar to those observed in control rats during hypercapnia. Moreover, the hypocapnic threshold for apnea was significantly lowered in CIH-conditioned rats relative to that in control rats. We conclude that CIH may sensitize central chemoreception and that this significantly contributes to the neural impetus for generation of sympathetic activity and hypertension.
Keywords: chronic intermittent hypoxia, hypertension, modeling, respiration
recurrent episodes of hypoxia, such as those observed in obstructive sleep apnea (OSA), are a risk factor for the development of cardiovascular diseases, including hypertension (Caples et al. 2005; Dempsey et al. 2010). In rats, it was demonstrated that exposure to chronic intermittent hypoxia (CIH) produced a sustained increase in arterial pressure (Fletcher 2003; Fletcher et al. 1992a, 1992b; Lesske et al. 1997). The fact that coexposure to intermittent hypoxia and hypercapnia produced no additional increment on arterial pressure of rats (Lesske et al. 1997) indicates that intermittent hypoxia is the main factor contributing to the development of arterial hypertension. Previous studies in vivo have reported that CIH-induced hypertension is associated with elevation of the sympathetic vasomotor tone (Zoccal et al. 2007, 2009a), indicating that the sympathetic nervous system plays a major role in the etiology of CIH-induced hypertension. Moreover, the elevated sympathetic nerve activity of CIH-conditioned rats was shown to exhibit an enhanced respiratory modulation that was not dependent on the afferent inputs from lungs or peripheral chemoreceptors (Zoccal et al. 2008). This raises the possibility that central coupling between brain stem respiratory and sympathetic neurons provides a significant contribution to the development of hypertension in CIH-conditioned animals (Zoccal et al. 2009b).
Central coupling of respiratory and sympathetic neurons may occur at the level of the ventrolateral medulla, where many of the neurons involved in the generation of respiratory and sympathetic activities are located (Barman and Gebber 1980; Dampney 1994; Guyenet 2006; Häbler et al. 1994; Haselton and Guyenet 1989; Koshiya and Guyenet 1996; McAllen 1987; Richter and Spyer 1990; Zhong et al. 1997). Specifically in this region, the inspiratory and expiratory neurons of the ventral respiratory column (VRC) interact with the presympathetic neurons of rostral ventrolateral medulla (RVLM) as well as with inhibitory interneurons of caudal ventrolateral medulla (CVLM) (Guyenet 2006; Haselton and Guyenet 1989; Mandel and Schreihofer 2006; Richter and Spyer 1990; Sun et al. 1997). The pons was also shown to play a role in the respiratory modulation of sympathetic nerve activity, since pontine transection significantly attenuated this modulation (Baekey et al. 2008, 2010). In addition, the activities of many medullary and pontine neurons involved in sympathorespiratory functions are modulated by central chemoreceptors located in the retrotrapezoid nucleus/parafacial respiratory group (RTN/pFRG) (Abdala et al. 2009b; Guyenet 2006; Guyenet et al. 2008, 2009; Moreira et al. 2006; Nattie and Li 2009).
We have previously found that hypercapnia evokes late-expiratory (late-E) discharges in the abdominal nerve (AbN) that occur just before phrenic bursts (Abdala et al. 2009a; Molkov et al. 2010). With increasing CO2, the frequency of these AbN late-E discharges is quantally increased until reaching a 1:1 ratio to the phrenic burst frequency (Abdala et al. 2009a; Molkov et al. 2010). Feldman and colleagues (Feldman and Del Negro 2006; Janczewski and Feldman 2006; Janczewski et al. 2002) suggested that the abdominal preinspiratory (or late-E) activity originates in the pFRG, a region originally described by Onimaru and Homma (1987, 2003; Onimaru et al. 1988) that appears to anatomically overlap with the RTN. Moreover, neurons were found in the RTN/pFRG region whose rhythmic discharges emerged coincidently with late-E bursts recorded from the AbN during hypercapnic conditions (Abdala et al. 2009a; Molkov et al. 2010). Also, pharmacological inactivation of the RTN/pFRG abolished the hypercapnia-induced late-E bursts in the AbN without affecting the activity of nearby expiratory neurons of the Bötzinger complex (Abdala et al. 2009a; Molkov et al. 2010), supporting the notion that the RTN/pFRG is a site generating late-E activity.
Sympathetic activity was also found to exhibit a late-E discharge during hypercapnia that coincided with AbN late-E activity. Moreover, CIH-conditioned rats also exhibit late-E sympathetic discharges synchronized with late-E bursts in the AbN (Abdala et al. 2009b; Zoccal et al. 2008, 2009a, 2009b). This supports the idea that the observed late-E activities in both the AbN and thoracic sympathetic nerve (tSN) originate in the RTN/pFRG. On the other hand, the RTN is known as a major site for central chemoreception, because it contains neurons activated by CO2 and projecting to many brain stem areas including VRC compartments and may also project to presympathetic neurons of the RVLM (Guyenet et al. 2008; Nattie and Li 2009; Takakura et al. 2011). The latter is important for the CO2-evoked sympathoexcitation and the corresponding increase of arterial pressure during hypercapnia (Moreira et al. 2006; Takakura et al. 2011).
To explain the presence of sympathetic (and abdominal) late-E activity in CIH-conditioned rats under normal conditions (normocapnia), which is similar to that observed in naive rats during hypercapnia (Abdala et al. 2009a; Molkov et al. 2010), we hypothesized that the chemosensitive RTN/pFRG neurons involved in the generation of late-E activity are sensitized during CIH conditioning, thereby reducing the CO2 threshold for the emergence of late-E activity in the RTN/pFRG and hence for its appearance in both abdominal and sympathetic motor outflows.
To explore these hypotheses, we extended our large-scale computational model of the respiratory central pattern generator (Molkov et al. 2010) and combined it with the model of sympathetic-respiratory coupling (Baekey et al. 2010). The resultant integrated model was used for simulation of the effects of sensitizing RTN/pFRG neurons by CIH conditioning on sympathetic nerve activity and on its coupling to respiratory motor activity in hypo-, normo-, and hypercapnic states. Several modeling predictions were subsequently tested in the arterially perfused in situ brain stem-spinal cord preparation (Paton 1996; Zoccal et al. 2008). Our results provide important novel insights into the role of central sympathorespiratory interactions in developing the hypertension observed in CIH-treated rats.
METHODS
Animals
Juvenile male Wistar rats (19–21 days) were divided in two experimental groups: rats exposed to CIH (n = 5) and rats maintained under normoxic conditions (control, n = 7). All rats were obtained from the Animal Care of the University of São Paulo at Ribeirão Preto, Brazil. Our experimental approaches were approved by the Ethical Committee on Animal Experimentation of the School of Medicine of Ribeirão Preto, University of São Paulo (protocol 019/2006).
Chronic Intermittent Hypoxia
CIH and control rats were housed in Plexiglas chambers (volume: 210 liters) equipped with gas injectors as well as sensors of O2, CO2, humidity, and temperature. The CIH group was exposed to a protocol of 5 min of normoxia [fraction of inspired O2 (FiO2) of 20.8%] followed by 4 min of pure N2 injection to reduce FiO2 from 20.8 to 6%, remaining at this level for 40 s. After this hypoxic period, pure O2 was injected to return FiO2 back to 20.8%. This 9-min cycle was repeated 8 h per day (from 9:30 AM to 5:30 PM) for 10 days. During the remaining 16 h, the animals were maintained at a FiO2 of 20.8%. The injections of N2 and O2 (White Martins, Sertãozinho, Brazil) into the chambers were regulated by a solenoid valve system whose opening-closing control was operated by a computerized system (Oxycycler; Biospherix, Lacona, New York). In an identical chamber in the same room, the control group of rats was exposed to a FiO2 of 20.8% for 24 h per day for 10 days. The control rats were also exposed to a similar valve noise due to the frequent injection of O2 to maintain the FiO2 at 20.8%. In both CIH and control chambers, the gas injections were performed at the upper level of the chamber to avoid direct jets of gas impacting on the animals, which could cause stress.
In Situ Arterially Perfused Preparation
Working heart-brain stem preparations (as per Paton 1996) from control and CIH-conditioned rats were made on the 11th day of the experimental protocol, as previously described (Paton 1996; Zoccal et al. 2008). Briefly, rats were deeply anesthetized with halothane (AstraZeneca, Cotia, SP, Brazil), transected caudal to the diaphragm, submerged in cooled Ringer solution, and decerebrated at the precollicular level to be made insentient. Preparations were then transferred to a recording chamber, and the descending aorta was cannulated and perfused retrogradely with a Ringer solution (in mM: 125 NaCl, 24 NaHCO3, 5 KCl, 2.5 CaCl2, 1.25 MgSO4, 1.25 KH2PO4, 10 dextrose, and 2 lactate) containing 1.25% polyethylene glycol 20,000 (an oncotic agent; Sigma, St. Louis, MO) and a neuromuscular blocker (vecuronium bromide, 3–4 μg/ml; Cristália Produtos Químicos Farmacêuticos, São Paulo, SP, Brazil) using a roller pump (Watson-Marlow 502s; Falmouth, Cornwall, UK) via a double-lumen cannula. The perfusion pressure was maintained in the range 50–70 mmHg by adjusting the flow between 21 and 25 ml/min and adding vasopressin (0.6–1.2 nM; Sigma) to the perfusate, as previously described (Pickering and Paton 2006; Zoccal et al. 2008). The perfusate was continuously gassed with 5% CO2 and 95% O2 (White Martins), warmed to 31–32°C (temperature measured at the point of entry into the aorta), and filtered using a nylon mesh (pore size: 25 μm; Millipore, Billerica, MA).
Nerve Recordings
Cardiorespiratory motor nerves were isolated and recorded simultaneously using glass suction electrodes held in three-dimensional micromanipulators (Narishige, Tokyo, Japan). Left phrenic nerve (PN) was recorded from its central end, and its rhythmic ramping activity was used as a continuous physiological index of preparation viability. The efferent activity of the left tSN was recorded from the sympathetic chain at the T8–T12 level. The AbN was isolated from the abdominal muscles on the right at lumbar level, cut distally, and its efferent activity recorded. PN, tSN, and AbN nerve activities were recorded using bipolar glass suction electrodes. All signals were amplified, band-pass filtered (0.5–5 kHz), and acquired in an analog-to-digital converter (CED micro 1401; Cambridge Electronic Design, Cambridge, UK) to a computer using Spike2 software (Cambridge Electronic Design).
Data Analyses
The phrenic burst and the late-E AbN burst frequencies were determined by the time interval between consecutive respective integrated bursts (expressed in Hz). Peak amplitude of phrenic bursts was also calculated as the difference value between baseline (noise level determined at the end of experiments 10–20 min after the perfusion ceased) and maximal activities. Thoracic sympathetic and abdominal nerve activities were evaluated using their mean values. The magnitude of alterations in phrenic amplitude, thoracic sympathetic, and abdominal activities in response to hyper- and hypocapnia were determined as the percentage of change in relation to the respective baseline activity (5% CO2). All the analyses were carried out on rectified and integrated signals (time constant of 50 ms) and were performed off-line using Spike2 software with custom-written scripts.
Hyper- and Hypocapnic Stimuli
Using a gas mixer device (GF3/MP gas mixing flowmeter; Cameron Instrument, Port Aransas, TX), the proportion of the gases in the perfusate was altered to raise or lower CO2. For hypercapnic stimuli, the concentrations were 7% CO2-93% O2 and 10% CO2-90% O2, whereas for hypocapnic stimuli, the concentrations were 3% CO2-97% O2 and 1% CO2-99% O2. The time duration of exposure for each stimulus was at least 20 min.
Statistical Analyses
Results are means ± SE and were compared, depending on the experimental design, using one-way or two-way ANOVA, followed by the Newman-Keuls or Bonferroni posttest, respectively. The comparisons were carried out on GraphPad Prism software (version 4; GraphPad Software), and differences were considered significant at P < 0.05.
Modeling and Simulations
The model was developed as a consolidation of two previous models proposed by Molkov et al. (2010) and Baekey et al. (2010). Both models were based on the model proposed by Smith et al. (2007). All neurons were modeled in the Hodgkin-Huxley style (single-compartment models) and incorporated known biophysical properties and channel kinetics characterized in respiratory neurons in vitro. Specifically, the kinetics of the fast sodium and the persistent (slowly inactivating) sodium channels were described using the experimental data obtained in studies of neurons from the rat RVLM (Rybak et al. 2003a); the kinetics of high-voltage-activated calcium current was described based on the study of calcium currents in rat medullary neurons in vitro (Elsen and Ramirez 1998); the intracellular calcium dynamics were described using data by Frermann et al. (1999). The descriptions of other ion channels, e.g., the potassium rectifier and calcium-dependent potassium ones, and all other cellular parameters were derived from our previous models (Rybak et al. 1997a, 1997b, 2003b, 2004a, 2004b). Each neuronal type was represented by a population of 20–50 neurons. Heterogeneity of neurons within each population was set by a random distribution of some parameters and the initial conditions for values of membrane potential, calcium concentrations, and channel conductances. Each neuron of a target population received synaptic inputs from all neurons of a source population and/or a corresponding source of excitatory drive in accordance with the network architecture of the model. A full description of the modeling approach and major model parameters can be found in our previous publications (Molkov et al. 2010; Smith et al. 2007).
All simulations were performed with the simulation package NSM 3.0, developed at Drexel University by S. N. Markin, I. A. Rybak, and N. A. Shevtsova and ported to multiprocessor parallel computing systems by Y. I. Molkov using the OpenMPI environment. Differential equations were solved using the exponential Euler integration method with a step of 0.1 ms. Specific details of the model and model parameters can be found in Table 1.
Table 1.
Weights of synaptic connections in the network
| Target Population (Location) | Excitatory Drive [Weight of Synaptic Input] or Source Population [Weight of Synaptic Input From Single Neuron] |
|---|---|
| Bs-E | |
| (cVRG) | Early-I(2) [−2]; late-E [0.02] |
| Ramp-I | Drive(pons) [2.0]; |
| (rVRG) | early-I(2) [−0.3]; pre-I/I [0.12];* post-I [−2.0]; aug-E [−0.1] |
| Early-I(2) | Drive(pons) [2.5]; |
| (rVRG) | post-I [−0.5];* aug-E [−0.25]; late-E [0.1] |
| Pre-I/I | Drive(pons) [0.22];* drive(RTN/pFRG) [0.65];* drive(raphé) [0.3]; |
| (pre-BötC) | pre-I/I [0.03]; post-I [−0.16]; aug-E [−0.06]; late-E [0.02] |
| Early-I(1) | Drive(pons) [1];* drive(RTN/pFRG) [1.1];* |
| (pre-BötC) | pre-I/I [0.1]; post-I [−0.45]; aug-E [−0.265] |
| Post-I | Drive(pons) [1.7];* |
| (BötC) | early-I(1) [−0.025]; aug-E [−0.01] |
| Post-I(e) | Drive(pons) [1.7];* |
| (BötC) | early-I(1) [−0.025];* aug-E [−0.01]* |
| Aug-E | Drive(pons) [2.7];* |
| (BötC) | early-I(1) [−0.135];* post-I [−0.3]* |
| Late-E | Drive(RTN/pFRG) [0.33];* |
| (RTN/pFRG) | early-I(1) [−0.025]; post-I [−0.0225]; late-E [0.04]* |
| RVLM* | Drive(VLM) [1]* |
| late-E [0.03];* IE [0.05];* early-I(2) [−0.01, 0#];* post-I [−0.1, −0.075#];* CVLM [−0.01, 0#] | |
| CVLM* | Drive(VLM) [1];* |
| post-I(e) [0.01]* | |
| IE* | |
| (pons) | Ramp-I [0.2];* post-I(e) [0.35]* |
Values in brackets represent average relative weights of synaptic inputs from the corresponding source populations or drives. Neural populations: bs-E, bulbospinal E; ramp-I, ramp inspiratory; early-I, early inspiratory; pre-I/I, preinspiratory/inspiratory; post-I, postinspiratory; post-I(e), postinspiratory (excitatory); aug-E, augmenting expiratory; late-E, late expiratory; IE, phase-spanning inspiratory-expiratory. Locations: cVRG, caudal ventral respiratory group; rVRG, rostral ventral respiratory group; pre-BötC, pre-Bötzinger complex; BötC, Bötzinger complex; RTN/pFRG, retrotrapezoid nucleus/parafacial respiratory group; RVLM, rostral ventrolateral medulla; CVLM, caudal ventrolateral medulla.
Populations not present in the model of Molkov et al. (2010) and weights of connections adjusted in the present model relative to that model. #After CIH conditioning.
This study utilized the high-performance computational capabilities of the Biowulf Linux cluster at the National Institutes of Health (Bethesda, MD; http://biowulf.nih.gov).
RESULTS
Respiratory and Sympathetic Activities in Control and CIH-Conditioned Rats In Situ Under Normal Conditions and During Hypercapnia
Control rats.
Typical patterns of respiratory and sympathetic activities from a representative control rat (1 of n = 7) are shown in Fig. 1, A1–A5. Under normal conditions (5% CO2) (see Abdala et al. 2009a; Molkov et al. 2010), the integrated burst of activity in the PN had an augmenting profile, the AbN exhibited a low-amplitude activity, and the integrated activity in the tSN expressed an augmenting inspiratory modulation with the activity profile slowly increasing during inspiration, reaching a peak at the inspiration-to-expiration transition, and rapidly falling at the beginning of expiration (Fig. 1A4).
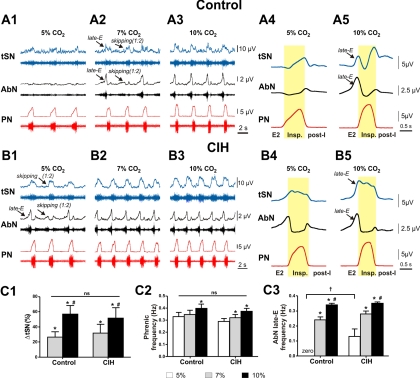
Fig. 1.
Sympathetic and respiratory responses to hypercapnia in control and chronic intermittent hypoxia (CIH)-conditioned rats. A1–A3: activity of phrenic (PN), abdominal (AbN), and thoracic sympathetic nerves (tSN) in the representative control preparation at 5% CO2 (base level, A1) and during hypercapnia (7% CO2 in A2 and 10% CO2 in A3). The activity of each nerve is represented by a raw recording (bottom trace) and integrated activity (top trace). Note the skipping of some late-expiratory (late-E) bursts in both AbN and tSN at 7% CO2 (A2). A4 and A5: profiles of PN, AbN, and tSN bursts at 5 and 10% CO2 shown on an expanded scale. B1–B3: PN, AbN, and tSN activities in the representative CIH-conditioned preparation at 5% CO2 (base level, B1) and during hypercapnia (7% CO2 in B2 and 10% CO2 in B3). Note the existence of late-E bursts in both AbN and tSN at 5% CO2 (B1). B4 and B5: profiles of PN, AbN, and tSN bursts in CIH-conditioned preparations at 5 and 10% CO2 shown on an expanded scale. The phase of inspiration (Insp.) in A4, A5, B4, and B5 is highlighted in yellow. Note a temporal shift (∼0.2-s delay) in tSN activity profiles relative to the corresponding PN profiles, which presumably results from a difference in the conducting velocities of the postganglionic tSN fibers (0.46–0.83 m/s; Häbler et al. 1999) and PN fibers (∼46 m/s; Hayashi and Fukuda 1995). C1–C3: normalized increase in tSN average activity (C1), phrenic frequency (C2), and AbN late-E frequency (C3) in control (n = 7) and CIH rats (n = 5). *P < 0.05, statistically significant change relative to 5% CO2. #P < 0.05, statistically significant change relative to 7% CO2. †P < 0.05, significantly different from control group. ns, No significant difference between CIH and control groups. post-I, postinspiration.
As described previously (Abdala et al. 2009a; Iizuka and Fregosi 2007; Molkov et al. 2010; Rubin et al. 2010), hypercapnia (an increase in CO2 level) evoked high-amplitude late-E AbN discharges in control preparations (Fig. 1, A2, A3, and A5). The emerging AbN discharges were always phase-locked to PN bursts. With progressive development of hypercapnia (increase in CO2 in the perfusate from 5 to 10%), the ratio of frequency of AbN late-E discharges to frequency of PN bursts increased stepwise from about 1:4/1:3 to 1:2 (see Fig. 1A2 at 7% CO2, where approximately each second respiratory cycle skipped an AbN discharge) and, finally, to 1:1 as the CO2 level was elevated to 10% (Fig. 1A3). Importantly, although the frequency of the late-E bursts increased with increasing hypercapnia, they were always coupled (phase-locked) to the PN bursts, evidencing the “quantal acceleration” as we described previously (Molkov et al. 2010; Rubin et al. 2010).
Hypercapnia-evoked late-E activity and its quantal acceleration with increasing CO2 were also observed in tSN of control preparations (Fig. 1, A2, A3, and A5). The tSN late-E discharges always coincided with AbN late-E bursts, and a skipping of late-E activity in the tSN (e.g., at 7% CO2) always coincided with its skipping in the AbN, suggesting a common excitatory source of late-E discharges in AbN and tSN. To make sure that the late-E bursts in AbN did not reflect the activity of sympathetic fibers contained in this nerve, in a subset of animals (n = 3) we performed activation of baroreceptors by increases in perfusion pressure. These stimuli produced a marked attenuation of tSN but not AbN late-E activity (data not shown), indicating that the late-E bursts observed in AbN were produced by separate motoneurons controlling AbN activity. The sympathoexcitatory response to hypercapnia in control rats (ΔtSN in Fig. 1C1) was associated with an increase in the average tSN activity during expiration, which was mainly because of the evoked late-E discharges but also because of an increase in activity during postinspiration (Fig. 1A5). In relation to the PN activity, hypercapnia produced an increase in both PN frequency (0.35 ± 0.03 Hz at 7% CO2, P < 0.05, and 0.40 ± 0.03 Hz at 10% CO2, P < 0.05; Fig. 1C2) and amplitude (9 ± 3% at 7% CO2, P < 0.05, and 23 ± 3% at 10% CO2, P < 0.05; Fig. 1, A1–A5).
CIH rats.
The pattern of baseline sympathetic and respiratory activities from a representative CIH preparation (1 of n = 5) is shown in Fig. 1B1. The baseline frequency of PN bursts in CIH-conditioned rats (0.29 ± 0.02 Hz) was not significantly different from that in the control rat preparations (0.33 ± 0.04 Hz) (Fig. 1C2). Similar to that in rats of the control group, the PN frequency in CIH rats increased with the increase in CO2 level in the perfusate (0.32 ± 0.03 Hz at 7% CO2 and 0.37 ± 0.02 Hz at 10% CO2; see Fig. 1C2).
In contrast to control rats, the CIH-treated preparations expressed coincident late-E discharges in AbN and tSN with a frequency ratio to PN of between 1:3 and 1:2 in normocapnic conditions (5% CO2; see Fig. 1, B1, B4, and C3). At 7% CO2 this ratio was 1:1 (Fig. 1, B2 and C3) and unlike that seen in control rats (where frequent skipping was observed; Fig. 1A2).
The tSN profile in CIH-conditioned rats in normocapnia differed from the tSN profile in control preparations. Specifically, after CIH conditioning, tSN activity was elevated during the late-E interval and maintained throughout the PN burst but not during postinspiration (Fig. 1, B4 and B5).
Modeling and Simulations
Model description.
The experimental findings described above suggest that synchronized late-E discharges observed in the AbN and tSN after CIH conditioning have a common source. On the basis of this suggestion, we combined two previous models, the one describing the origin of the late-E abdominal activity (Molkov et al. 2010) and the other simulating respiratory-sympathetic coupling (Baekey et al. 2010), and developed a united computational model. The resultant model (Fig. 2A) has the same core circuitry of the respiratory network as both of the previous models (originally proposed by Smith et al. 2007). This circuitry includes the excitatory preinspiratory/inspiratory (pre-I/I) and inhibitory early-inspiratory [early-I(1)] populations of the pre-Bötzinger complex (pre-BötC) and the inhibitory augmenting-expiratory (aug-E) and postinspiratory (post-I) [and excitatory premotor post-I(e)] populations of the BötC (see Fig. 2A). The united model also includes a ramp-inspiratory (ramp-I) population of premotor bulbospinal inspiratory neurons and an inhibitory early-inspiratory [early-I(2)] population, which are both located in the rostral ventral respiratory group (rVRG; Fig. 2A) (Smith et al. 2007).
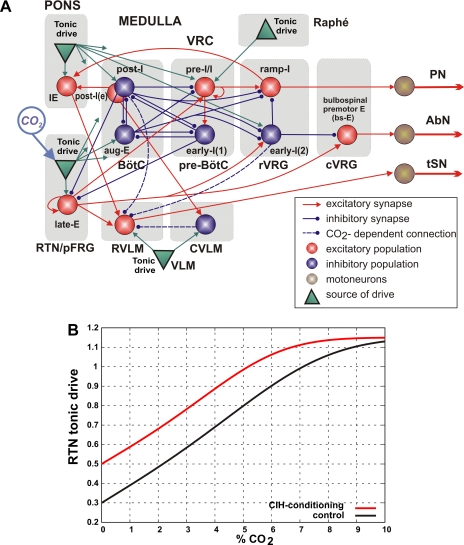
Fig. 2.
United computational model of the brain stem sympatho-respiratory network. A: schematic of the model showing interactions between different populations of respiratory neurons within major brain stem compartments involved in the control of breathing and sympathetic activity. Brain stem compartments: BötC, Bötzinger complex; CVLM, caudal ventrolateral medulla; cVRG, caudal ventral respiratory group; pre-BötC, pre-Bötzinger complex; RTN/pFRG, retrotrapezoid nucleus/parafacial respiratory group; RVLM, rostral ventrolateral medulla; rVRG, rostral ventral respiratory group; VRC, ventral respiratory column. Neural populations: aug-E, augmenting expiratory; early-I, early inspiratory; IE, phase-spanning inspiratory-expiratory; late-E, late expiratory; post-I, postinspiratory; post-I(e), postinspiratory (excitatory); pre-I/I, preinspiratory/inspiratory; ramp-I, ramp inspiratory. Key is shown at bottom right. Each population (large sphere) consists of 20–50 neurons modeled in the Hodgkin-Huxley style. All tonic drive sources (green triangles) provide constant drives, except for RTN/pFRG, whose drive is CO2 dependent (see B). Not all connections from pontine and RTN/pFRG drive sources are shown. For details see text and Table 1. B: RTN/pFRG tonic drive as a function of CO2 for the control and CIH-conditioning cases.
Similar to the model of Molkov et al. (2010), a late-E population of neurons with the intrinsic bursting properties has been included in the RTN/pFRG. This population represents a putative source of late-E oscillations and projects to the bulbospinal premotor expiratory (bulbospinal E, or bs-E) population located in the caudal ventral respiratory group (cVRG), which drives AbN motor output (Fig. 2A).
Following the model of Baekey et al. (2010), we have incorporated excitatory RVLM and inhibitory CVLM populations of the ventrolateral medulla (Barman and Gebber 1980; Dampney 1994; Guyenet 2000, 2006), as well as a phase-spanning inspiratory-expiratory (IE) population in the pons (Cohen and Shaw 2004; Dick et al. 1994, 2008; Mörschel and Dutschmann 2009; Segers et al. 2008). The latter was suggested to project to RVLM, hence providing a pontine-dependent inspiratory modulation of tSN (Baekey et al. 2010) (see Fig. 2A).
In our model, both the pre-I/I population of pre-BötC and the late-E population of RTN/pFRG consist of neurons with the persistent (slowly inactivating) sodium current (INaP; defining endogenous bursting properties of neurons that can be expressed under certain conditions) and mutual excitatory interactions within the corresponding population (Molkov et al. 2010; Rybak et al. 2007; Smith et al. 2007). The neurons in the post-I, post-I(e), aug-E, early-I(1), and early-I(2) populations are characterized by adapting firing properties defined by high-voltage-activated calcium (ICaL) and calcium-activated potassium (IK,Ca) currents. The remaining neurons (bs-E, ramp-I, IE, RVLM, and CVLM) have only a minimal set of ionic currents (fast sodium, INa; potassium rectifier, IK; and leakage, IL) necessary for generating spiking activity.
The behavior of respiratory and sympathetic networks and their interactions depend on a variety of external inputs (or drives) to all neural populations involved in network operations. These inputs allow the system to maintain the appropriate homeostatic levels of O2 and CO2 and adaptively respond to various metabolic demands (Fortuna et al. 2008; Guyenet 2006; Guyenet et al. 2005, 2008, 2009; Moreira et al. 2006; Mulkey et al. 2007; Nattie 1999; Nattie and Li 2009; Richerson 2004). These drives have been conditionally modeled as excitatory “tonic drives” from multiple sources distributed within the brain stem (pons, RTN, raphé; see Fig. 2A). Although currently undefined, these drives seem to have specific mapping on the spatial organization of the brain stem networks (note that only some connection from drive sources in the pons and RTN/pFRG are shown in Fig. 2A; all connections with their weights are specified in Table 1).
Among the drive sources mentioned above, the RTN/pFRG is considered to be a major central chemoreceptor site whose drive is sensitive to CO2 (Guyenet 2006; Guyenet et al. 2005, 2008, 2009; Mulkey et al. 2007). To explicitly simulate this property, the RTN tonic drive was considered not constant, as other tonic drives in the model, but dependent on the CO2 level. The CO2 dependence of the RTN drive was modeled as a sharply saturating function:
where CO2 is the CO2 content in perfusate (in %), σ(x) = tanh (x + x3), β = 9.5% is the saturation level, and α is the CIH sensitization parameter: α = 0 for the control case and α = 2% after CIH conditioning. This hypothetical dependence of RTN/pFRG tonic drive on CO2 is shown in Fig. 2B (black curve). As hypothesized above, the CO2 sensitivity of RTN/pFRG increases due to CIH conditioning. This was simulated by the horizontal shift of the curve representing CO2-dependent RTN drive by α = 2% CO2 to the direction of lower CO2 values (i.e., to the left; see red curve in Fig. 2B). In addition, we suggest that all synaptic weights of inhibitory inputs to the RVLM population are reduced as a result of CIH conditioning (see Table 1). The implementation of this suggestion in the model allowed us to reproduce some specific changes in the tSN activity profile in CIH-conditioned preparations described below.
Regarding the activity of the pre-I/I and late-E populations, the previous theoretical studies have demonstrated that such neural populations (i.e., populations comprising neurons with INaP-dependent busting properties and mutual excitatory interconnections) can be silent (at a low level of neuronal excitability), operate in a population-bursting mode (at the increased excitability or external drive, exceeding some threshold), or switch to a sustained asynchronous activity (when the excitability or drive is additionally increased, exceeding a higher threshold) so that with the increasing neuronal excitability or external drive, the firing behavior of the population switches sequentially from a silent state to bursting and then to a sustained firing (Butera et al. 1999; Rybak et al. 2003b). Importantly, although the pre-I/I and late-E populations are almost identical in the model, their behavior is different because of the different levels of basal excitability. Specifically, under normal conditions, the pre-I/I population receives a strong total excitatory drive from the pons, RTN/pFRG and raphé that keeps this population in the operation regime not critically dependent on INaP (Rybak et al. 2003b, 2007; Smith et al. 2007). In contrast, the late-E population during normocapnia is silent and can be switch to the bursting regime either by hypercapnia (Abdala et al. 2009a; Molkov et al. 2010) or as a result of sensitization of RTN/pFRG during CIH conditioning as hypothesized in this study.
Simulations and Modeling Predictions
Control Conditions.
Figure 3, A1 and A2, shows the integrated activity of the PN, AbN, and tSN outputs in the model (A1) and the corresponding burst profiles (A2) in control conditions, when CO2 was stepwise increasing from 1% CO2 (hypocapnia) through 5% CO2 (normocapnia) to 10% CO2 (hypercapnia), as shown in Fig. 3B. Progressive hypercapnia led to the emergence and quantal acceleration of late-E bursts in both AbN and tSN (Fig. 3A1), which was consistent with our experimental data described above. The late-E discharges appeared at about 7% CO2 and reached a 1:1 ratio to the PN bursts at 9% CO2. The profiles of PN and tSN activity with and without preceding AbN late-E burst in the model (Fig. 3A2) closely reproduced the profiles of activity obtained from corresponding experimental recordings (Fig. 1, A4 and A5). Figure 3A1 also shows that a reduction of CO2 below 3% (an apneic threshold for hypocapnia in the control conditions) caused apnea (lack of PN activity), a phenomenon well known from previous experimental investigations (e.g., Boden et al. 1998).
Fig. 3.
Model performance in simulated control (A1 and A2) and CIH cases (C1 and C2). In both cases, integrated activity for PN, AbN, and tSN outputs are shown. The CO2 level was changed stepwise from 1% (hypocapnia) to 10% (hypercapnia) as shown in B. A2 represents offset from A1 (gray highlighted rectangle) shown on an expanded scale. Correspondingly, C2 represents offset from C1 (gray highlighted rectangle) shown on an expanded scale. In A2 and C2, the inspiratory phase is highlighted in yellow. The black and red dashed vertical arrows indicate CO2 levels for emerging late-E activity and for hypocapnic apnea, respectively. See text for details.
CIH CONDITIONING.
As described above, we hypothesize that CIH conditioning augments the CO2 sensitivity of central chemoreceptors. To simulate this phenomenon, the curve reflecting the CO2 dependence of RTN drive was shifted to the left (to lower values of CO2) by 2% (see red curve in Fig. 2B). In addition, weights of inhibitory synaptic connections to RVLM were reduced (see above and Table 1).
The results of the corresponding simulation of the effects of CIH conditioning are shown in Fig. 3, C1 and C2. In this simulation, CO2 was again increased stepwise from 1% CO2 (hypocapnia) through 5% CO2 (normocapnia) to 10% CO2 (hypercapnia; Fig. 3B). However, in contrast to the control scenario (Fig. 3A1), the late-E bursts in AbN and tSN emerged at 3% CO2, and at the normocapnic state (5% CO2) they showed a stable 1:2 ratio to the PN bursts (see Fig. 3C1), which was consistent with our experimental observations (Fig. 1B1). At 7% CO2, this ratio reached 1:1 (Fig. 3C1), a ratio not seen until 10% CO2 in control conditions (Figs. 1B2 and 3A1). Note also that after CIH, the profiles of PN and tSN activity with and without preceding AbN late-E burst in the model (Fig. 3C2) closely reproduced the profiles of activity obtained from the corresponding experimental recordings (Fig. 1, B4 and B5).
The second observation from the above simulation was that CIH conditioning reduced the apneic threshold for hypocapnia in the model by at least 2% CO2, since the PN bursts were still generated even at 1% CO2 (see Fig. 3C1). This modeling prediction was tested experimentally (see below).
Experimental Testing of Modeling Predictions
We checked the prediction that CIH-conditioned preparations have an apneic threshold for hypocapnia reduced by about 2% CO2 relative to control rat preparations.
Control rats.
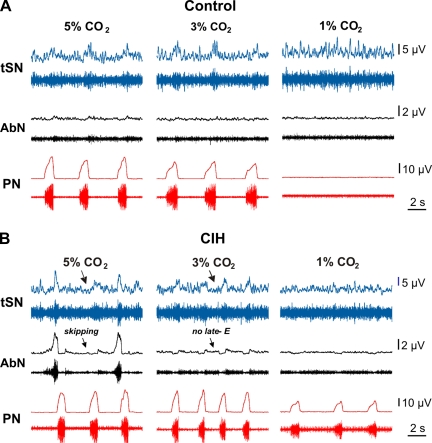
Preparations (n = 7) were exposed to hypocapnia (from normal 5% CO2 to 3 and 1% CO2). These preparations exhibited no significant changes in the integrated PN burst amplitude at 3% CO2 (PN amplitude was changed by −5 ± 13%) and a hypocapnic apnea at 1% CO2 (PN amplitude was reduced by 91 ± 4%; Fig. 4A). These preparations never expressed late-E activity in AbN or tSN in either normocapnia or hypocapnia.
Fig. 4.
Changes in the sympathetic and respiratory activities in response to hypocapnia in control and CIH-conditioned rats. Raw and integrated recordings are shown of tSN, AbN, and PN activities of representative preparations from control (A) and CIH groups (B) in normocapnic (5% CO2) and hypocapnic conditions (3 and 1% CO2). An expressed late-E activity in AbN and tSN was observed in CIH-conditioned preparations at 5% CO2 and was abolished with reduction of CO2 to 3%. Hypocapnia at 1% CO2 eliminated PN activity in control but not in CIH-conditioned preparations.
CIH-conditioned rats.
The same experimental procedure was applied to CIH-conditioned preparations (n = 5). A representative example is shown in Fig. 4B. The expressed late-E activity in both AbN and tSN in these preparations was already present with some cycle skipping during normocapnia (at 5% CO2), which corresponded to our previous modeling and experimental data (see Figs. 1B1 and 3C1). This late-E activity in both AbN and tSN disappeared at 3% CO2 (Fig. 4B, see for comparison Fig. 3C1). At the same time, PN activity with a reduced amplitude (and respiratory modulation of tSN) remained at 1% CO2 (with PN amplitude changed by 4 ± 5% at 3% CO2 and −27 ± 6% at 1% CO2; Fig. 4B). These data confirm our modeling prediction of a reduced apneic threshold during hypocapnia in CIH-conditioned rats.
DISCUSSION
In agreement with previous in vivo observations (Guyenet 2006; Guyenet et al. 2008, 2009; Nattie 1999), we demonstrated in situ that stimulation of central chemoreceptors with high CO2 produced increased sympathetic and respiratory activities. In our experiments, the increase in sympathetic activity during hypercapnia occurred preferentially within the expiratory phase and was associated with the simultaneous emergence of late-E bursts in the AbN and tSN (Fig. 1, A2 and A3). The neural mechanisms underlying the CO2-dependent sympathoexcitatory response during late expiration seem to contribute to the enhanced baseline sympathetic activity of CIH rats, because the reduction of CO2 to 3% eliminated late-E activities in AbN and tSN (Fig. 4). In addition, decreasing CO2 to 1% abolished PN activity in control but not in CIH rats (Fig. 4), supporting the hypothesis that central chemoreceptors may be sensitized after CIH exposure and contribute to the development of active expiratory pattern as well as an augmented sympathetic activity observed in CIH rats in normoxic/normocapnic conditions.
Preparation Specificity
Like other experimental preparations, such as anesthetized and decerebrate animals, the arterially perfused in situ preparation used in this study has its own technical limitations. However, accumulating evidence demonstrates that sympathetic and respiratory activities (either baseline or evoked) observed in this preparation are, in most cases, similar to those observed in vivo. A recent example is the study by Marina et al. (2010) showing that inhibition of the Phox2b-expressing neurons in the ventrolateral brain stem including the RTN produced a comparable attenuation of the hypercapnia-induced abdominal late-E activity in the in situ rat preparations and in the anesthetized and conscious rats. Moreover, in our previous studies (Zoccal et al. 2009), we demonstrated that hypertensive CIH-treated conscious rats exhibited an increased respiratory modulation of arterial pressure similar to that originally demonstrated in the in situ preparations (Zoccal et al. 2008). Therefore, our current (and previous) data obtained in situ do not seem to be exclusive to, or critically dependent on, the preparation used.
One specific technical aspect of our studies in situ concerns the evaluation of CO2 content, which in our study is conditionally considered equal to the amount of gas bubbled in the perfusate. This value is not the same as the percentage of CO2 measured at end-expiration as generally used in vivo. Also, the amount of CO2 in the preparation is likely to be greater in the blood vessels because of the additional CO2 generated by the metabolism. This can partly explain the difference in the threshold for hypocapnic apnea between our preparation and the in vivo data. Specifically, we found that this threshold was achieved in situ at lower levels of CO2 (1% CO2; Fig. 4A) than in the previous experiments performed in vivo (3–4% CO2) (see Moreira et al. 2006; Takakura et al. 2008).
Abdominal and Sympathetic Activities Evoked by Hypercapnia
It has been proposed that central chemoreception involves a cluster of Phox2b-expressing neurons located in RTN/pFRG, which play a dominant role in respiratory and sympathetic responses to central chemoreceptor activation (Guyenet 2006; Guyenet et al. 2005, 2008, 2009; Marina et al. 2010; Stornetta et al. 2006). It was demonstrated that the RTN chemosensitive neurons are predominantly glutamatergic, since they express the glutamatergic vesicular transporter, and establish connections with respiratory neurons of the VRC, parabrachial, and Kölliker-Fuse nuclei in the pons and dorsal respiratory column (Rosin et al. 2006). Through these connections, excitatory inputs from RTN/pFRG to respiratory neurons possibly mediate the respiratory response involved in central chemoreception. Our experimental study demonstrated that hypercapnia induced increases in both inspiratory and expiratory activities. In relation to phrenic inspiratory activity, both frequency and amplitude of phrenic bursts of control preparations increased with the incrementing of CO2 in the perfusate (Fig. 1), a response that may involve connections between RTN/pFRG and inspiratory neurons of pre-BötC (e.g., pre-I/I; see Fig. 2A) as suggested by our model (Abdala et al. 2009a; Molkov et al. 2010).
With respect to the expiratory response, we confirmed that high levels of CO2 result in the emergence of late-E bursts in AbN, which was not seen in control rats in basal conditions (5% CO2, Fig. 1, A1–A3). Recent studies have demonstrated that RTN/pFRG neurons are essential for the expression of abdominal late-E bursts, because pharmacological inhibition of RTN (Abdala et al. 2009a; Molkov et al. 2010) or inhibition of predominantly Phox2b-expressing neurons in this region (Marina et al. 2010) abolished the AbN late-E activity in response to hypercapnia without interfering with the activity of BötC expiratory neurons (Abdala et al. 2009a). Therefore, during hypercapnia the RTN/pFRG appears to be an important source of excitation to bulbospinal expiratory neurons located in cVRC that relay excitatory drive to the lumbar abdominal motoneurons that drive late-E bursting in the AbN (Abdala et al. 2009a; Molkov et al. 2010).
The sympathoexcitatory response to hypercapnia suggests excitatory connections from CO2-sensitive neurons of RTN/pFRG to the presympathetic RVLM neurons (Moreira et al. 2006; Takakura et al. 2011). In the present study, we demonstrated that the CO2-induced increase in sympathetic activity in control rats occurred, at least in part, due to the emerging late-E activity. The source of this activity was suggested to be in RTN/pFRG, which contains neurons that are silent during normocapnia (5% CO2) and activated during hypercapnia, exhibiting a pattern of activity that is strongly correlated with AbN late-E activity (Abdala et al. 2009a; Molkov et al. 2010). These late-E neurons of RTN/pFRG could be an excitatory source of excitation not only to cVRC bulbospinal expiratory neurons but also to presympathetic RVLM neurons, culminating in an increase of sympathetic activity correlated with late-E bursts in abdominal motor activity. The synchronous activation of abdominal and sympathetic late-E activities was more evident at 7% CO2, when sympathetic late-E activity skipped in the respiratory cycles in which abdominal late-E activity was also absent (Fig. 1, A2 and B1).
Central Chemoreceptor Sensitization and Sympathetic Nerve Activity After CIH Exposure
According to previous studies (Abdala et al. 2009a; Janczewski and Feldman 2006; Janczewski et al. 2002; Molkov et al. 2010), late-E activity originates in RTN/pFRG and can be evoked by hypercapnia (Abdala et al. 2009a; Molkov et al. 2010). In the present study, we have demonstrated that this hypercapnia-evoked late-E activity can substantially contribute to the sympathoexcitatory response. We have also provided evidence for a previous conclusion (Zoccal et al. 2008) that CIH-treated rats exhibit late-E activity in AbN and tSN under normocapnic conditions (5% CO2; see Figs. 1B1 and 4B). These observations provide important insights into possible mechanisms involved in the elevation of sympathetic activity and development of arterial hypertension observed after CIH conditioning.
We hypothesize that the excitability and hence CO2 sensitivity of a subset of RTN/pFRG neurons increases after CIH conditioning. This CIH-evoked increase in the neuronal excitability or CO2 sensitivity results in a lowering of the CO2 threshold for generation of late-E activity within the RTN/pFRG. This hypothesis was explicitly incorporated in our computational model.
Computational Model
The model developed in the present study represents our first attempt to integrate sympathorespiratory circuits. This model was developed based on direct combination of two previous models describing the emergence of late-E activity in the RTN/pFRG (Molkov et al. 2010) and the sympathetic-respiratory coupling (Baekey et al. 2010). The integrated model could reproduce all the experimental data that each of the two basic models reproduced. In addition, we explicitly incorporated CO2 concentration as a model variable to simulate the CO2-dependent central chemoreceptor drive in the model.
The model supports the important role of chemosensitive RTN/pFRG neurons for activation of presympathetic neurons in the RVLM. It hypothesizes specific interaction between the pons (IE population), respiratory populations of VRC, central chemoreceptors located in RTN/pFRG (late-E population), and RVLM and CVLM populations (Fig. 2A). These interactions and connectivities predicted by the model await further experimental testing. However, the role of CVLM in these interactions in the present model is, so far, minimal and does not account for the different respiratory-modulated patterns observed in CVLM neurons (Mandel and Schreihofer 2006). Clarification of the respiratory network interactions with the RVLM and CVLM are the focus of our future experimental and modeling studies.
The model reproduces the experimentally observed effects of hypercapnia and CIH conditioning on AbN and tSN motor activities. The central assumption (hypothesis) of our model is that CIH-conditioning increases the excitability of RTN/pFRG (late-E) neurons or their CO2 sensitivity, leading to the emergence of late-E activity at a normal level of CO2 (after CIH conditioning) and resulting in elevation of the average tSN activity contributing to the CIH-evoked hypertension. In the model, this was reproduced by the simulated shift of the curve representing CO2-dependent RTN drive by 2% CO2 to the direction of lower CO2 values (Fig. 2B). The mechanism(s) for this shift and the nature of the plastic changes in the excitability or CO2 sensitivity of RTN/pFRG neurons after CIH conditioning have not been considered so far and remain to be determined. One possible explanation for this may be based on a reduction of inhibitory inputs from the BötC post-I neurons to the RTN/pFRG late-E neurons (see Fig. 2A), which could increase the excitability in the latter neurons (see Molkov et al. 2010). The other mechanism could be based on the increased excitatory drive from peripheral chemoreceptors (mediated by corresponding neurons in the nucleus of solitary tract) to central chemoreceptors (late-E neurons) (Guyenet et al. 2009; Takakura et al. 2006) and/or on the peripheral chemoreceptor control of the gain of central chemoreceptors (Blain et al. 2010). However, the suggestion of the involvement of peripheral chemoreceptor drive would contradict our previous data showing that the carotid body denervation after CIH exposure did not eliminate the late-E discharges from sympathetic activity (Zoccal et al. 2008). Nevertheless, peripheral-central chemoreceptor interaction may be involved in the development of plastic changes in the excitability of central chemoreceptors after CIH conditioning via activation of neuromodulators that enhance the activity of RTN chemosensitive neurons, such as serotonin (Mulkey et al. 2007), ATP (Mulkey et al. 2006), or locally produced oxidative stress (Jurado-Gamez et al. 2011). These or other, currently unknown mechanisms may be involved in the observed CIH-evoked sensitization of RTN/pFRG chemoreceptors, which requires additional studies.
The important prediction of our model is a CIH-dependent lowering of the threshold for hypocapnic apnea (Fig. 3). This prediction has been confirmed experimentally in the in situ preparations of CIH-treated rats exposed to hypocapnia (Fig. 4). The latter confirms the plausibility of the model per se, as well as the plausibility of the neural mechanisms and interactions suggested and incorporated in the model.
Our model also predicts a CIH-evoked reduction in the synaptic inhibition to the RVLM. Incorporation of this feature allowed the model to reproduce the experimentally observed changes in the tSN burst profile induced by CIH conditioning. This prediction is consistent with raised sympathetic activity and reflex sympathetic responses in CIH rats (Braga et al. 2006) and also awaits experimental testing.
In conclusion, our combined experimental and modeling studies support an important role of late-E oscillations originated in the RTN/pFRG in the generation of forced expiration and their significant contribution to the elevated sympathetic activity after CIH treatment. The neurons responsible for these effects may provide a novel target for correcting the hypertension in conditions of CIH and perhaps sleep apneic patients.
At the same time, our study does not dismiss or diminish a possible role of other mechanisms in the CIH-evoked hypertension not necessarily connected with the increasing respiratory-sympathetic interactions and/or emerging late-E activity. For example, the Prabhakar group (Peng and Prabhakar 2004; Peng et al. 2003) reported an increase in the sensitivity of peripheral chemoreceptors in CIH treated rats, which can also be the case in OSA patients (Narkiewicz et al. 1998). Other hypertension mechanisms independent of peripheral or central chemoreceptor sensitization may also be involved (Xing and Pilowsky 2010). Therefore, our finding that respiratory-sympathetic interactions and sympathetic late-E activity may significantly contribute to the development of hypertension in CIH-conditioned animals does not exclude a possibility that there are other factors involved in CIH-evoked hypertension.
GRANTS
The part of study performed in the U.S. was supported by National Institutes of Health Grants R01 NS057815, R01 NS069220, and R33 HL087379 (to I. A. Rybak). In Brazil, this study was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico Grant 472704/2004-4 and Fundação de Amparo a Pesquisa do Estado de São Paulo Grants 2004/03285-7, 2006/51159-6, and 2009/50113-0. J. F. R. Paton is the recipient of a Royal Society Wolfson Research Merit Award.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Dr. Mogens Lesner Glass (Brazil) for help with the gas mixer device.
REFERENCES
- Abdala et al., 2009a. Abdala AP, Rybak IA, Smith JC, Paton JF. Abdominal expiratory activity in the rat brainstem-spinal cord in situ: patterns, origins and implications for respiratory rhythm generation. J Physiol 587: 3539–3559, 2009a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdala et al., 2009b. Abdala AP, Rybak IA, Smith JC, Zoccal DB, Machado BH, St-John WM, Paton JF. Multiple pontomedullary mechanisms of respiratory rhythmogenesis. Respir Physiol Neurobiol 168: 19–25, 2009b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baekey et al., 2008. Baekey DM, Dick TE, Paton JF. Pontomedullary transection attenuates central respiratory modulation of sympathetic discharge, heart rate and the baroreceptor reflex in the in situ rat preparation. Exp Physiol 93: 803–816, 2008 [DOI] [PubMed] [Google Scholar]
- Baekey et al., 2010. Baekey DM, Molkov YI, Paton JF, Rybak IA, Dick TE. Effect of baroreceptor stimulation on the respiratory pattern: insights into respiratory-sympathetic interactions. Respir Physiol Neurobiol 174: 135–145, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barman and Gebber, 1980. Barman SM, Gebber GL. Sympathetic nerve rhythm of brain stem origin. Am J Physiol Regul Integr Comp Physiol 239: R42–R47, 1980 [DOI] [PubMed] [Google Scholar]
- Blain et al., 2010. Blain GM, Smith CA, Henderson KS, Dempsey JA. Peripheral chemoreceptors determine the respiratory sensitivity of central chemoreceptors to CO2. J Physiol 588: 2455–2471, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boden et al., 1998. Boden AG, Harris MC, Parkes MJ. Apneic threshold for CO2 in the anesthetized rat: fundamental properties under steady-state conditions. J Appl Physiol 85: 898–907, 1998 [DOI] [PubMed] [Google Scholar]
- Braga et al., 2006. Braga VA, Soriano RN, Machado BH. Sympathoexcitatory response to peripheral chemoreflex activation is enhanced in juvenile rats exposed to chronic intermittent hypoxia. Exp Physiol 91: 1025–1031, 2006 [DOI] [PubMed] [Google Scholar]
- Butera et al., 1999. Butera RJ, Rinzel J, Smith JC. Models of respiratory rhythm generation in the pre-Bötzinger complex. II. Populations of coupled pacemaker neurons. J Neurophysiol 82: 398–415, 1999 [DOI] [PubMed] [Google Scholar]
- Caples et al., 2005. Caples SM, Gami AS, Somers VK. Obstructive sleep apnea. Ann Intern Med 142: 187–197, 2005 [DOI] [PubMed] [Google Scholar]
- Cohen and Shaw, 2004. Cohen MI, Shaw CF. Role in the inspiratory off-switch of vagal inputs to rostral pontine inspiratory-modulated neurons. Respir Physiol Neurobiol 143: 127–140, 2004 [DOI] [PubMed] [Google Scholar]
- Dampney, 1994. Dampney RA. Functional organization of central pathways regulating the cardiovascular system. Physiol Rev 74: 323–364, 1994 [DOI] [PubMed] [Google Scholar]
- Dempsey et al., 2010. Dempsey JA, Veasey SC, Morgan BJ, O'Donnell CP. Pathophysiology of sleep apnea. Physiol Rev 90: 47–112, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick et al., 1994. Dick TE, Bellingham MC, Richter DW. Pontine respiratory neurons in anesthetized cats. Brain Res 636: 259–269, 1994 [DOI] [PubMed] [Google Scholar]
- Dick et al., 2008. Dick TE, Shannon R, Lindsey BG, Nuding SC, Segers LS, Baekey DM, Morris KF. Pontine respiratory-modulated activity before and after vagotomy in decerebrate cats. J Physiol 586: 4265–4282, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsen and Ramirez, 1998. Elsen FP, Ramirez JM. Calcium currents of rhythmic neurons recorded in the isolated respiratory network of neonatal mice. J Neurosci 18: 10652–10662, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman and Del Negro, 2006. Feldman JL, Del Negro CA. Looking for inspiration: new perspectives on respiratory rhythm. Nat Rev Neurosci 7: 232–242, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher, 2003. Fletcher EC. Sympathetic over activity in the etiology of hypertension of obstructive sleep apnea. Sleep 26: 15–19, 2003 [DOI] [PubMed] [Google Scholar]
- Fletcher et al., 1992a. Fletcher EC, Lesske J, Behm R, Miller CC, 3rd, Stauss H, Unger T. Carotid chemoreceptors, systemic blood pressure, and chronic episodic hypoxia mimicking sleep apnea. J Appl Physiol 72: 1978–1984, 1992a [DOI] [PubMed] [Google Scholar]
- Fletcher et al., 1992b. Fletcher EC, Lesske J, Culman J, Miller CC, Unger T. Sympathetic denervation blocks blood pressure elevation in episodic hypoxia. Hypertension 20: 612–619, 1992b [DOI] [PubMed] [Google Scholar]
- Fortuna et al., 2008. Fortuna MG, West GH, Stornetta RL, Guyenet PG. Botzinger expiratory-augmenting neurons and the parafacial respiratory group. J Neurosci 28: 2506–2515, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frermann et al., 1999. Frermann D, Keller BU, Richter DW. Calcium oscillations in rhythmically active respiratory neurones in the brainstem of the mouse. J Physiol 515: 119–131, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet, 2000. Guyenet PG. Neural structures that mediate sympathoexcitation during hypoxia. Respir Physiol 121: 147–162, 2000 [DOI] [PubMed] [Google Scholar]
- Guyenet, 2006. Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci 7: 335–346, 2006 [DOI] [PubMed] [Google Scholar]
- Guyenet et al., 2009. Guyenet PG, Bayliss DA, Stornetta RL, Fortuna MG, Abbott SB, DePuy SD. Retrotrapezoid nucleus, respiratory chemosensitivity and breathing automaticity. Respir Physiol Neurobiol 168: 59–68, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet et al., 2005. Guyenet PG, Mulkey DK, Stornetta RL, Bayliss DA. Regulation of ventral surface chemoreceptors by the central respiratory pattern generator. J Neurosci 25: 8938–8947, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet et al., 2008. Guyenet PG, Stornetta RL, Bayliss DA. Retrotrapezoid nucleus and central chemoreception. J Physiol 586: 2043–2048, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häbler et al., 1999. Häbler HJ, Bartsch T, Jänig W. Rhythmicity in single fiber postganglionic activity supplying the rat tail. J Neurophysiol 81: 2026–2036, 1999 [DOI] [PubMed] [Google Scholar]
- Häbler et al., 1994. Häbler HJ, Jänig W, Michaelis M. Respiratory modulation in the activity of sympathetic neurones. Prog Neurobiol 43: 567–606, 1994 [DOI] [PubMed] [Google Scholar]
- Haselton and Guyenet, 1989. Haselton JR, Guyenet PG. Central respiratory modulation of medullary sympathoexcitatory neurons in rat. Am J Physiol Regul Integr Comp Physiol 256: R739–R750, 1989 [DOI] [PubMed] [Google Scholar]
- Hayashi and Fukuda, 1995. Hayashi F, Fukuda Y. Electrophysiological properties of phrenic motoneurons in adult rats. Jpn J Physiol 45: 69–83, 1995 [DOI] [PubMed] [Google Scholar]
- Iizuka and Fregosi, 2007. Iizuka M, Fregosi RF. Influence of hypercapnic acidosis and hypoxia on abdominal expiratory nerve activity in the rat. Respir Physiol Neurobiol 157: 196–205, 2007 [DOI] [PubMed] [Google Scholar]
- Janczewski and Feldman, 2006. Janczewski WA, Feldman JL. Distinct rhythm generators for inspiration and expiration in the juvenile rat. J Physiol 570: 407–420, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janczewski et al., 2002. Janczewski WA, Onimaru H, Homma I, Feldman JL. Opioid-resistant respiratory pathway from the preinspiratory neurones to abdominal muscles: in vivo and in vitro study in the newborn rat. J Physiol 545: 1017–1026, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurado-Gámez et al., 2011. Jurado-Gámez B, Fernandez-Marin MC, Gómez-Chaparro JL, Muñoz-Cabrera L, Lopez-Barea J, Perez-Jimenez F, Lopez-Miranda J. Relationship of oxidative stress and endothelial dysfunction in sleep apnoea. Eur Respir J 37: 873–879, 2011 [DOI] [PubMed] [Google Scholar]
- Koshiya and Guyenet, 1996. Koshiya N, Guyenet PG. Tonic sympathetic chemoreflex after blockade of respiratory rhythmogenesis in the rat. J Physiol 491: 859–869, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesske et al., 1997. Lesske J, Fletcher EC, Bao G, Unger T. Hypertension caused by chronic intermittent hypoxia—influence of chemoreceptors and sympathetic nervous system. J Hypertens 15: 1593–1603, 1997 [DOI] [PubMed] [Google Scholar]
- Mandel and Schreihofer, 2006. Mandel DA, Schreihofer AM. Central respiratory modulation of barosensitive neurones in rat caudal ventrolateral medulla. J Physiol 572: 881–896, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marina et al., 2010. Marina N, Abdala APL, Trapp S, Li A, Nattie EE, Hewinson J, Smith JC, Paton JF, Gourine AV. Essential role of Phox2b-expressing ventrolateral brainstem neurons in the chemosensory control of inspiration and expiration. J Neurosci 30: 12466–12473, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllen, 1987. McAllen RM. Central respiratory modulation of subretrofacial bulbospinal neurones in the cat. J Physiol 388: 533–545, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molkov et al., 2010. Molkov YI, Abdala AP, Bacak BJ, Smith JC, Paton JF, Rybak IA. Late-expiratory activity: emergence and interactions with the respiratory CPG. J Neurophysiol 104: 2713–2729, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira et al., 2006. Moreira TS, Takakura AC, Colombari E, Guyenet PG. Central chemoreceptors and sympathetic vasomotor outflow. J Physiol 577: 369–386, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mörschel and Dutschmann, 2009. Mörschel M, Dutschmann M. Pontine respiratory activity involved in inspiratory/expiratory phase transition. Philos Trans R Soc Lond B Biol Sci 364: 2517–2526, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey et al., 2006. Mulkey DK, Mistry AM, Guyenet PG, Bayliss DA. Purinergic P2 receptors modulate excitability but do not mediate pH sensitivity of RTN respiratory chemoreceptors. J Neurosci 26: 7230–7233, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey et al., 2007. Mulkey DK, Rosin DL, West G, Takakura AC, Moreira TS, Bayliss DA, Guyenet PG. Serotonergic neurons activate chemosensitive retrotrapezoid nucleus neurons by a pH-independent mechanism. J Neurosci 27: 14128–14138, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narkiewicz et al., 1998. Narkiewicz K, van de Borne PJH, Montano N, Dyken ME, Phillips BG, Somers VK. Contribution of tonic chemoreflex activation to sympathetic activity and blood pressure in patients with obstructive sleep apnea. Circulation 97: 943–945, 1998 [DOI] [PubMed] [Google Scholar]
- Nattie, 1999. Nattie E. CO2, brainstem chemoreceptors and breathing. Prog Neurobiol 59: 299–331, 1999 [DOI] [PubMed] [Google Scholar]
- Nattie and Li, 2009. Nattie E, Li A. Central chemoreception is a complex system function that involves multiple brain stem sites. J Appl Physiol 106: 1464–1466, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onimaru et al., 1988. Onimaru H, Arata A, Homma I. Primary respiratory rhythm generator in the medulla of brainstem-spinal cord preparation from newborn rat. Brain Res 445: 314–324, 1988 [DOI] [PubMed] [Google Scholar]
- Onimaru and Homma, 2003. Onimaru H, Homma I. A novel functional neuron group for respiratory rhythm generation in the ventral medulla. J Neurosci 23: 1478–1486, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onimaru and Homma, 1987. Onimaru H, Homma I. Respiratory rhythm generator neurons in medulla of brainstem-spinal cord preparation from newborn rat. Brain Res 403: 380–384, 1987 [DOI] [PubMed] [Google Scholar]
- Paton, 1996. Paton JF. A working heart-brainstem preparation of the mouse. J Neurosci Methods 65: 63–68, 1996 [DOI] [PubMed] [Google Scholar]
- Pickering and Paton, 2006. Pickering AE, Paton JF. A decerebrate, artificially-perfused in situ preparation of rat: utility for the study of autonomic and nociceptive processing. J Neurosci Methods 155: 260–271, 2006 [DOI] [PubMed] [Google Scholar]
- Peng et al., 2003. Peng YJ, Overholt JL, Kline D, Kumar GK, Prabhakar NR. Induction of sensory long-term facilitation in the carotid body by intermittent hypoxia: implications for recurrent apneas. Proc Natl Acad Sci USA 100: 10073–10078, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng and Prabhakar, 2004. Peng YJ, Prabhakar NR. Effect of two paradigms of chronic intermittent hypoxia on carotid body sensory activity. J Appl Physiol 96: 1236–1242, 2004 [DOI] [PubMed] [Google Scholar]
- Richerson, 2004. Richerson GB. Serotonergic neurons as carbon dioxide sensors that maintain pH homeostasis. Nat Rev Neurosci 5: 449–461, 2004 [DOI] [PubMed] [Google Scholar]
- Richter and Spyer, 1990. Richter DW, Spyer KM. Cardiorespiratory control. In: Central Regulation of Autonomic Functions, edited by Loewy AD, Spyer KM. Oxford: Oxford University Press, 1990, p. 189–207 [Google Scholar]
- Rosin et al., 2006. Rosin DL, Chang DA, Guyenet PG. Afferent and efferent connections of the rat retrotrapezoid nucleus. J Comp Neurol 499: 64–89, 2006 [DOI] [PubMed] [Google Scholar]
- Rubin et al., 2010. Rubin JE, Bacak BJ, Molkov YI, Shevtsova NA, Smith JC, Rybak IA. Interacting oscillations in neural control of breathing: modeling and qualitative analysis. J Comput Neurosci 2010, doi:10.1007/s10827-010-0281-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybak et al., 2007. Rybak IA, Abdala APL, Markin SN, Paton JFR, Smith JC. Spatial organization and state-dependent mechanisms for respiratory rhythm and pattern generation. Prog Brain Res 165: 201–220, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybak et al., 1997a. Rybak IA, Paton JF, Schwaber JS. Modeling neural mechanisms for genesis of respiratory rhythm and pattern. I. Models of respiratory neurons. J Neurophysiol 77: 1994–2006, 1997a [DOI] [PubMed] [Google Scholar]
- Rybak et al., 1997b. Rybak IA, Paton JF, Schwaber JS. Modeling neural mechanisms for genesis of respiratory rhythm and pattern. II. Network models of the central respiratory pattern generator. J Neurophysiol 77: 2007–2026, 1997b [DOI] [PubMed] [Google Scholar]
- Rybak et al., 2003a. Rybak IA, Ptak K, Shevtsova NA, McCrimmon DR. Sodium currents in neurons from the rostroventrolateral medulla of the rat. J Neurophysiol 90: 1635–1642, 2003a [DOI] [PubMed] [Google Scholar]
- Rybak et al., 2004a. Rybak IA, Shevtsova NA, Paton JF, Dick TE, St-John WM, Mörschel M, Dutschmann M. Modeling the ponto-medullary respiratory network. Respir Physiol Neurobiol 143: 307–319, 2004a [DOI] [PubMed] [Google Scholar]
- Rybak et al., 2004b. Rybak IA, Shevtsova NA, Ptak K, McCrimmon DR. Intrinsic bursting activity in the pre-Bötzinger complex: role of persistent sodium and potassium currents. Biol Cybern 90: 59–74, 2004b [DOI] [PubMed] [Google Scholar]
- Rybak et al., 2003b. Rybak IA, Shevtsova NA, St-John WM, Paton JFR, Pierrefiche O. Endogenous rhythm generation in the pre-Bötzinger complex and ionic currents: modelling and in vitro studies. Eur J Neurosci 18: 239–257, 2003b [DOI] [PubMed] [Google Scholar]
- Segers et al., 2008. Segers LS, Nuding SC, Dick TE, Shannon R, Baekey DM, Solomon IC, Morris KF, Lindsey BG. Functional connectivity in the pontomedullary respiratory network. J Neurophysiol 100: 1749–1769, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith et al., 2007. Smith JC, Abdala AP, Koizumi H, Rybak IA, Paton JF. Spatial and functional architecture of the mammalian brain stem respiratory network: a hierarchy of three oscillatory mechanisms. J Neurophysiol 98: 3370–3387, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stornetta et al., 2006. Stornetta RL, Moreira TS, Takakura AC, Kang BJ, Chang DA, West GH, Brunet JF, Mulkey DK, Bayliss DA, Guyenet PG. Expression of Phox2b by brainstem neurons involved in chemosensory integration in the adult rat. J Neurosci 26: 10305–10314, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun et al., 1997. Sun QJ, Minson J, Llewellyn-Smith IJ, Arnolda L, Chalmers J, Pilowsky P. Bötzinger neurons project towards bulbospinal neurons in the rostral ventrolateral medulla of the rat. J Comp Neurol 388: 23–31, 1997 [DOI] [PubMed] [Google Scholar]
- Takakura et al., 2011. Takakura AC, Colombari E, Menani JV, Moreira TS. Ventrolateral medulla mechanisms involved in cardiorespiratory responses to central chemoreceptor activation in rats. Am J Physiol Regul Integr Comp Physiol 300: R501–R510, 2011 [DOI] [PubMed] [Google Scholar]
- Takakura et al., 2006. Takakura AC, Moreira TS, Colombari E, West GH, Stornetta RL, Guyenet PG. Peripheral chemoreceptor inputs to retrotrapezoid nucleus (RTN) CO2-sensitive neurons in rats. J Physiol 572: 503–523, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakura et al., 2008. Takakura AC, Moreira TS, Stornetta RL, West GH, Gwilt JM, Guyenet PG. Selective lesion of retrotrapezoid Phox2b-expressing neurons raises the apnoeic threshold in rats. J Physiol 586: 2975–2991, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing and Pilowsky, 2010. Xing T, Pilowsky PM. Acute intermittent hypoxia in rat in vivo elicits a robust increase in tonic sympathetic nerve activity that is independent of respiratory drive. J Physiol 588: 3075–3088, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong et al., 1997. Zhong S, Zhou SY, Gebber GL, Barman SM. Coupled oscillators account for the slow rhythms in sympathetic nerve discharge and phrenic nerve activity. Am J Physiol Regul Integr Comp Physiol 272: R1314–R1324, 1997 [DOI] [PubMed] [Google Scholar]
- Zoccal et al., 2007. Zoccal DB, Bonagamba LG, Oliveira FR, Antunes-Rodrigues J, Machado BH. Increased sympathetic activity in rats submitted to chronic intermittent hypoxia. Exp Physiol 92: 79–85, 2007 [DOI] [PubMed] [Google Scholar]
- Zoccal et al., 2009a. Zoccal DB, Bonagamba LG, Paton JF, Machado BH. Sympathetic-mediated hypertension of awake juvenile rats submitted to chronic intermittent hypoxia is not linked to baroreflex dysfunction. Exp Physiol 94: 972–983, 2009a [DOI] [PubMed] [Google Scholar]
- Zoccal et al., 2009b. Zoccal DB, Paton JF, Machado BH. Do changes in the coupling between respiratory and sympathetic activities contribute to neurogenic hypertension? Clin Exp Pharmacol Physiol 36: 1188–1196, 2009b [DOI] [PubMed] [Google Scholar]
- Zoccal et al., 2008. Zoccal DB, Simms AE, Bonagamba LG, Braga VA, Pickering AE, Paton JF, Machado BH. Increased sympathetic outflow in juvenile rats submitted to chronic intermittent hypoxia correlates with enhanced expiratory activity. J Physiol 586: 3253–3265, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]