Abstract
Young songbirds produce vocal “babbling,” and the variability of their songs is thought to underlie a process of trial-and-error vocal learning. It is known that this exploratory variability requires the “cortical” component of a basal ganglia (BG) thalamocortical loop, but less understood is the role of the BG and thalamic components in this behavior. We found that large bilateral lesions to the songbird BG homolog Area X had little or no effect on song variability during vocal babbling. In contrast, lesions to the BG-recipient thalamic nucleus DLM (medial portion of the dorsolateral thalamus) largely abolished normal vocal babbling in young birds and caused a dramatic increase in song stereotypy. These findings support the idea that the motor thalamus plays a key role in the expression of exploratory juvenile behaviors during learning.
Keywords: learning, birdsong, speech, subcortical
exploratory variability in behavior is an important component of trial-and-error learning (Doya and Sejnowski 1995; Sutton and Barto 1998), but its neural origins remain poorly understood. Vocal exploration is prevalent in juvenile songbirds, and may be necessary for the gradual process of song learning (Andalman and Fee 2009; Charlesworth et al. 2011; Tumer and Brainard 2007). In the earliest phase of vocal learning, male juvenile zebra finches sing highly variable vocalizations, akin to human babbling, called “subsong” (Marler 1970). Subsong is followed by “plastic song,” in which discrete identifiable syllables begin to appear, although the song retains a high degree of variability. Over weeks of continued practice, variability continues to decrease as the song evolves toward the “crystallized song”—a fixed sequence of stereotyped syllables imitated from a tutor's song (Price 1979).
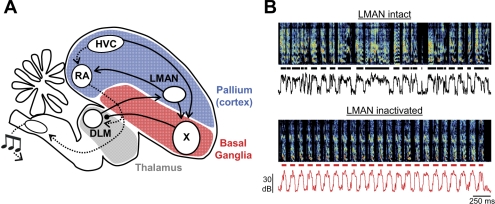
Song learning requires a specialized basal ganglia (BG) thalamocortical loop known as the anterior forebrain pathway (AFP; Fig. 1) (Bottjer et al. 1984; Doupe et al. 2005; Scharff and Nottebohm 1991). Importantly, vocal variability in young birds is not simply a consequence of immature circuitry in the motor pathway. Rather, it is actively injected into the premotor song-control region robust nucleus of the arcopallium (RA) by the lateral magnocellular nucleus of the anterior nidopallium (LMAN), the output station of the AFP (Fig. 1) (Kao et al. 2005; Olveczky et al. 2005). Notably, LMAN and RA exhibit some anatomic and functional analogies with mammalian frontal cortex and primary motor cortex, respectively (Jarvis 2004). The importance of LMAN in vocal exploration has been demonstrated by a number of lines of evidence. First, bilateral inactivation or lesion of LMAN abolishes vocal babbling in subsong birds and largely eliminates vocal variability in plastic song and adult birds (Aronov et al. 2008; Bottjer et al. 1984; Kao and Brainard 2006; Olveczky et al. 2005; Scharff and Nottebohm 1991). Second, RA-projecting neurons in LMAN exhibit highly variable spiking patterns in young birds (Olveczky et al. 2005) and show premotor bursts of activity prior to syllable onsets and offsets during subsong (Aronov et al. 2008). Finally, electrical stimulation of LMAN during singing causes immediate perturbation of ongoing song (Kao et al. 2005). These studies suggest that LMAN provides a source of premotor drive that actively generates vocal exploration required for song learning.
Fig. 1.
Schematic of the avian song system. A: lateral magnocellular nucleus of the anterior nidopallium (LMAN), Area X, and medial portion of the dorsolateral thalamus (DLM) constitute the pallial (cortexlike), basal ganglia, and thalamic portions of the anterior forebrain pathway (AFP) (solid lines). LMAN forms the output of the AFP through its projection to the robust nucleus of the arcopallium (RA), which in turn projects to motor neurons in the brain stem. A separate motor pathway is formed by the high vocal center (HVC)→RA projection (dotted lines). B: song spectrograms, syllable segments (horizontal lines), and sound amplitudes from a juvenile bird [45 days post hatch (dph)] before and during LMAN inactivation. Note that variability in syllable and gap durations, as well as in the acoustic structure within syllables, is reduced during inactivation of LMAN (bottom).
What is the origin of the highly variable neural activity in LMAN that generates exploratory vocal behavior? LMAN receives an excitatory projection from the portion of the thalamic nucleus DLM (medial portion of the dorsolateral thalamus) that in turn receives an inhibitory pallidal-like input from BG homolog Area X (Boettiger and Doupe 1998; Farries and Perkel 2002; Luo and Perkel 1999). This pathway forms a basal ganglia thalamocortical circuit homologous to those in mammals (Doupe et al. 2005, Reiner et al. 2004). Note that DLM contains not just this Area X-recipient region but also a surrounding area that forms part of a parallel BG-thalamo-“cortical” pathway outside the traditional song system (Bottjer and Altenau 2010; Bottjer et al. 1989, 2000; Feenders et al. 2008). The Area X-recipient subregion of DLM has been referred to variously as DLM, anterior DLM, vocal DLM, or dorsolateral DLM (Bottjer 2004; Wada et al. 2004); here, we simply use the term DLM, consistent with the notation of several previous studies (Boettiger and Doupe 1998; Luo and Perkel 1999; Person and Perkel 2005).
Does subsong and song variability, driven by LMAN, depend on the inputs from earlier stages in the AFP? Interestingly, in contrast to LMAN lesions, elimination of Area X in juvenile birds leads to protracted song variability in adulthood (Scharff and Nottebohm 1991; Sohrabji et al. 1990). The dramatic difference between the effects of LMAN lesions (increased stereotypy) and Area X lesions (protracted variability) suggests that Area X may not play a central role in generating subsong and plastic song variability. On the other hand, infusion of a dopamine antagonist near Area X alters the changes in song variability that occur in different social contexts (Leblois et al. 2010). In addition, Area X lesions block singing-induced activation of immediate early genes in LMAN, suggesting that Area X influences LMAN activity during singing (Kubikova et al. 2007). Finally, we have recently shown (Goldberg et al. 2010; Goldberg and Fee 2010) that neurons within Area X, including the DLM-projecting pallidal neurons, exhibit highly variable firing patterns during singing, consistent with a possible role in driving variability in the downstream DLM→LMAN circuit. These somewhat contradictory findings raise questions about the role of Area X in the generation of exploratory variability, and also about the role of DLM, a central function of which is thought to be the relay of information from Area X to LMAN (Kimpo et al. 2003; Kojima and Doupe 2009).
To examine the role of thalamic and BG structures of the AFP in generating vocal exploration, we carried out bilateral Area X and DLM lesions in juvenile birds and quantitatively examined the effect of lesions on song. Area X lesions left vocal exploration largely intact, while DLM lesions produced a dramatic increase in song stereotypy. Our findings support a key role of a BG-recipient motor thalamic nucleus in the expression of motor exploration that is surprisingly independent of innervation from the BG.
METHODS
Subjects.
Subjects were 32 juvenile male zebra finches, 38–69 days post hatch (dph). Birds with exponentially distributed syllable durations were categorized as subsong birds (Aronov et al. 2008), while those with obvious peaks in their syllable duration distribution were categorized as plastic song birds. Birds were obtained from the Massachusetts Institute of Technology (MIT) zebra finch breeding facility (Cambridge, MA). The care and experimental manipulation of the animals were carried out in accordance with guidelines of the National Institutes of Health and were reviewed and approved by the MIT Committee on Animal Care.
Lesion methods and histological confirmation.
Bilateral excitoxic lesions of DLM were made by injecting 100 nl of 4% N-methyl-dl-aspartic acid (NMA; Sigma, St Louis, MO) into the center of the Area X-recipient portion of DLM. The antero-posterior and dorso-ventral extent of this region was determined by electrophysiologically mapping the high-frequency-firing, thin-spiking pallidal axon terminals that constitute Area X outputs (Goldberg et al. 2010; Person and Perkel 2007). Our experience in recording from DLM in singing birds has revealed that there is a medial zone of the pallidal terminal region that does not exhibit singing-related activity; thus we do not use the mapping described above to determine the lateral position of the injection. Instead, we have found that the center of the singing-related Area X-recipient zone is reliably located at 1.07 mm lateral to the midline, and all injections were made at this lateral coordinate. Thus, with a head angle of 65° relative to the flat anterior portion of the skull, injections were typically between +0.9 and 1.5 mm anterior to the bifurcation of the midsagittal sinus (lambda), 1.07 mm lateral to midline, and 4.3 mm ventral to the brain surface. While injections were targeted to the center of the Area X-recipient region, subsequent histology showed that the lesioned area encompassed all of DLM. Consistent with a role for portions of DLM in nonsinging behaviors, after DLM lesions many birds exhibited brief episodes of hemiparesis and/or discoordination of ambulation and flight. Recovery from these symptoms typically occurred within 24 h, after which the birds appeared completely normal in their mobility and behavior. For bilateral Area X lesions, 200 nl of 4% NMA was injected into the center of each Area X (head angle 20°, 5.8 mm anterior, 1.5 mm lateral, and 2.85 mm ventral) and an additional 50 nl into each medial Area X, using stereotactic coordinates (head angle 20°, 5.8 mm anterior, 1.2 mm lateral, 2.6 mm ventral) (Kubikova et al. 2007). For medial magnocellular nucleus of the anterior nidopallium (MMAN) lesions, 100 nl of 4% NMA was injected into the center of MMAN with stereotactic coordinates (head angle 20°, 5.2 mm anterior, 0.75 mm lateral, 1.9 mm ventral). Finally, the high vocal center (HVC) was lesioned bilaterally with large electrolytic lesions, as described in detail elsewhere (Aronov et al. 2008). Lesions were confirmed histochemically with a fluorescent antibody to neuronal nuclei (mouse anti-Neu-N, Millipore, Temecula, CA); song nuclei stained brighter than background in Neu-N staining, and lesion boundaries were clearly visible (Supplemental Fig. S1).1 In some birds, bilateral DLM lesions were additionally verified (n = 4) by the absence of retrogradely labeled neurons after injection of fluorescent tracer into LMAN (Alexa-conjugated 10-kDa dextran, Molecular Probes, Eugene, OR) (Supplemental Fig. S1).
To examine the effect of lesions on singing, the full day of song immediately preceding the lesion was compared with the first full day of singing following the lesion. In the Area X lesion data set, this resulted in a median of 17.6 min (range: 3.8–52.8) of prelesion singing, containing 3,095 syllables (range: 839–12,598), and 14.7 min (range: 4.0–32.5) of postlesion singing, consisting of 2,300 syllables (range 718–5,584) (data here and below presented as median and range over all birds). In the DLM lesion data set, this resulted in 19.0 min (range 7.8–91.7) of prelesion singing, containing 3,910 syllables (range 1,520–2,472), and 10.7 min (range: 1.4–61.9) of postlesion singing, consisting of 1,408 syllables (range: 89–1,632). In the MMAN lesion data set, this resulted in 9.2 min (range: 2.1–40.2) of prelesion singing, containing 1,589 syllables (range: 311–11,128), and 3.8 min (range: 1.5–13.1) of postlesion singing, consisting of 881 syllables (range: 271–2,370). LMAN inactivations were carried out as described in detail elsewhere (Olveczky et al. 2005). The present data set included birds that were aged 45–56 dph and were thus old enough such that LMAN inactivations did not block singing (Aronov et al. 2008) but were significantly younger than birds examined in previous studies (Olveczky et al. 2005; Stepanek and Doupe 2010).
Song analysis.
Birds were placed in custom-made sound isolation chambers, and vocalizations were recorded with custom-written Matlab software or with Sound Analysis Pro (Tchernichovski et al. 2000), which was configured to ensure triggering of recordings on all quiet vocalizations of young birds (Aronov et al. 2008). All recordings were manually examined to eliminate calls and cage noise from the data set. Syllable duration distributions were calculated by first identifying syllable onsets and offsets (syllable segmentation). We calculated a sound threshold as the Fisher discriminant of two Gaussian modes (corresponding to noise and sound) fit to the values of log-amplitude. We detected crossings of this threshold and defined sound onsets and offsets as the closest points to these crossings where amplitude deviated from noise by 2 SD. Sound segments separated by <7 ms of silence were merged into a single syllable, and segments <7 ms long were eliminated (Aronov et al. 2008). In assessing the amount of song (or calls) after HVC lesions, a full day of recording was partitioned into 2-s segments, and the numbers of segments containing calls or songs were estimated by visual inspection. Discrimination was based on the principle that calls typically have stereotyped structure and are produced in single renditions and vocalized with several hundreds of millisecond spacing between them. Song, on the other hand, typically consists of five or more complex syllables repeated in rapid succession, typically with 60- to 100-ms gaps between syllables.
Quantifying song variability.
Song variability was computed in four ways. First, we used an entropy-based measure of variability (Ve) to quantify lesion-induced changes to syllable duration distributions. For each day of singing, we calculated the log (base 10) of syllable durations. We used log-based bins because the syllable duration distributions of subsong birds are exponential, and thus linear in log-space (Aronov et al. 2008). While the peak in the syllable duration distribution plotted on a linear axis represents the preponderance of short-duration syllables, a peak in the syllable durations plotted on a log axis represents a repeated syllable. Histograms of log duration were calculated in evenly spaced bins from −2.5 (0.00316 s) to 0 (1 s) in log bins of 0.05 (N = 50 bins). Histograms were normalized over all bins to produce probability density function pi, where the value of the ith bin indicated the probability that a syllable will have a duration that resides in that time bin. Thus the sum of all points in this vector of length N was equal to 1
We then computed an entropy-based measure of the variability of the distribution as follows:
| (1) |
Ve is equal to 1 (maximal variability) when syllable durations are distributed across all bins and goes to 0 (maximal stereotypy) when all syllable durations are restricted to a single bin. Thus the bin size was chosen to correspond roughly to the width of syllable duration peaks in adult song.
Second, we computed variability for gap duration distributions, proceeding exactly as we did for syllables. Third, we analyzed song rhythmicity, computed as the power spectrum of sound amplitude during singing. Rhythmicity does not rely on syllable segmentation and is thus an unbiased measure of song temporal structure (Saar and Mitra 2008). To compute song rhythm, we first extracted the sound amplitude of song bouts, defined as a sequence of four or more syllables separated by at least 350 ms of silence. The sound amplitude of each bout was mean-subtracted and multiplied by a Hanning window, and the frequency spectrum was computed with the FFT function in Matlab. The frequency spectrum was normalized by the bout length and squared to obtain the power spectrum. Power spectra for all bouts were then averaged and plotted as in Figs. 2 and 3, F and K. Song rhythmicity was quantified as the height of the largest peak in the normalized power spectrum at frequencies greater than 3 Hz. Only peaks above 3 Hz were considered because these correspond to the typical frequency at which syllables occur during singing (Saar and Mitra 2008).
Fig. 2.
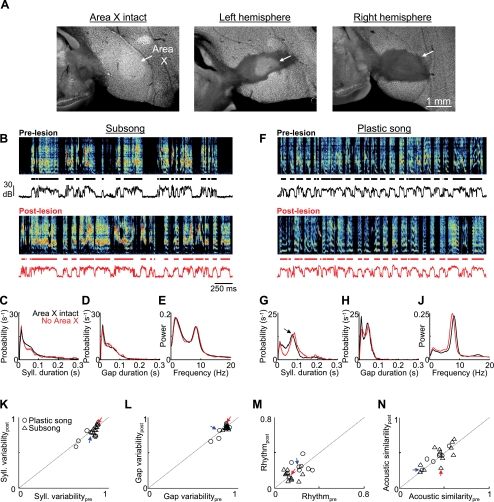
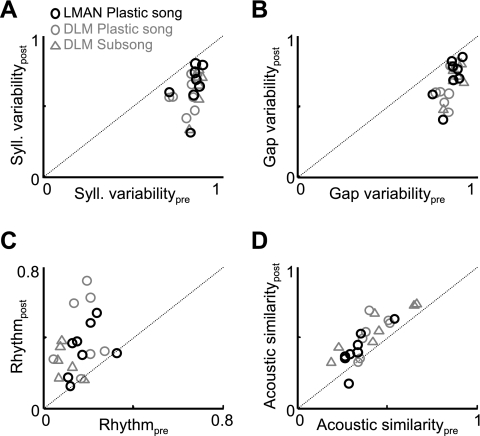
Song variability is preserved after Area X lesions. A: histological verification of Area X lesion. In a control bird (left), the boundaries of Area X are readily visible with the Neu-N stain for neuronal cell bodies (see methods). Neuronal labeling reveals bilateral elimination of Area X following injection of the excitotoxin N-methyl-dl-aspartic acid (NMA) into the center of each Area X (see methods). B–E: data from an Area X-lesioned subsong bird. B, top: prelesion song spectrogram of a subsong bird (dph 44). Bottom trace is the song amplitude. Black segments indicate individual syllables. Bottom, spectrogram taken from the first day of singing (dph 46) after bilateral Area X lesions. Note the preservation of variability in the durations of syllables and gaps and in the acoustic structure of syllables. C and D: histograms of syllable (C) and gap (D) durations before (black traces) and after (red traces) the Area X lesion. E: normalized power spectra of the sound amplitudes before and after the lesion. F–J: data from an Area X-lesioned plastic song bird (prelesion song: dph 49, postlesion song: dph 53; histology shown in A) are plotted as in B–E. Note the similarity between pre- and postlesion songs. Arrow in G points to the peak in the syllable duration distribution that is typical in plastic song birds. K–N: population data from Area X lesions in subsong (n = 12) and plastic song (n = 7) birds. K and L: for each bird, the variability in syllable and gap durations was estimated from the entropy of the duration distributions (see methods). Scatterplots show postlesion vs. prelesion syllable (K) and gap (L) duration variability. M: song rhythmicity, computed as the peak of the normalized power spectrum (as in E and J; see methods), for pre- and postlesion song. N: average pairwise spectrogram cross-correlations in pre- and postlesion song. Red and blue arrows point to the data points from the subsong and plastic song birds from B–E and F–J, respectively. Note that none of the measures shown here was significantly affected by Area X lesions.
Fig. 3.
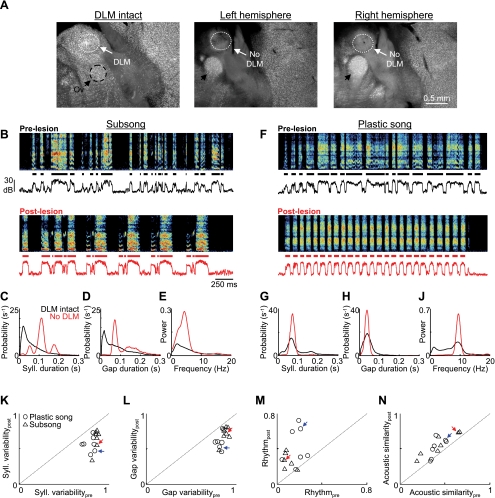
DLM lesions cause an increase in song stereotypy in the first postlesion song. A: histological verification of DLM lesions. Left: DLM and surrounding thalamus in a control bird is clearly visible in neuronal staining. Center and right: NMA injections (100 nl, 4%) were bilaterally targeted to the Area X-recipient portion of DLM by mapping out the location of Area X pallidal axon terminals. These injections resulted in the bilateral elimination of the entire DLM (see methods; see also Supplemental Fig. S1, A–H). Note that the auditory thalamic nucleus ovoidalis was preserved in our lesions (Ov, black arrows). B–E: data from a DLM-lesioned subsong bird. B, top: prelesion song spectrogram of a subsong bird (dph 41). Bottom trace is the song amplitude. Black segments indicate individual syllables. Bottom: spectrogram taken from the first day of singing following bilateral DLM lesions (dph 45; histology of this bird is shown in A). Note the increase in song stereotypy, including 4 repetitions of a highly stereotyped 3-syllable motif. C: histogram of syllable durations from singing before and after the DLM lesion, black and red, respectively. Before the lesion, syllables were randomly distributed; after the lesion, 3 distinct peaks were visible, corresponding to the 3 syllables of the motif. D: histogram of gap durations before and after lesion. E: normalized power spectra of the sound amplitudes before and after lesion. F–J: data are plotted as in B–E for a plastic song bird (prelesion dph 50, postlesion dph 54; histology of this bird is shown in Supplemental Fig. S1, C–H). Note the dramatic increase in song rhythmicity following the lesion, evident in the repetition of a highly stereotyped syllable. K–N: population data from DLM lesions in subsong (n = 7) and plastic song (n = 7) birds. K and L: scatterplots show prelesion vs. postlesion variability of syllable (K) and gap (L) durations. M: scatterplot of song rhythmicity in pre- and postlesion song. N: average pairwise spectrogram cross-correlations in pre- and postlesion song. Note the significantly decreased variability of both syllable and gap durations and the significantly increased rhythmicity and acoustic stereotypy following DLM lesions. Red and blue arrows point to the data points from the subsong and plastic song birds from B–E and F–J, respectively.
Finally, we computed the acoustic variability within syllables by performing pairwise spectrogram cross-correlations (Nelson and Marler 1994) within a set of syllables selected from postlesion singing, and also within another set of syllables selected from prelesion song. The distribution of spectrogram cross-correlations was then compared in the prelesion and postlesion sets. To ensure that lesion-related changes in syllable durations did not affect our analysis of acoustic variability within syllables, we ensured that the pre- and postlesion sets were matched for syllable duration. More specifically, we randomly selected 250 syllables from postlesion song, and then, for each one of these syllables, we selected a syllable from prelesion song that had a duration within 10 ms of the postlesion song syllable. The result was that the syllable duration distributions of the pre- and postlesion data sets were equal. Within both data sets, we then performed direct cross-correlations of the spectrograms for all pairs of syllables whose durations differed by <10 ms, allowing us to quantify acoustic similarity independent from similarity in syllable duration. We then compared the average spectrogram cross-correlation coefficient from the pre- and postlesion data sets (Table 1).
Table 1.
Lesion-induced changes to juvenile song structure
| DLMsubsong (n = 7) | DLMplastic (n = 7) | Area Xsubsong (n = 12) | Area Xplastic (n = 7) | MMANsubsong (n = 3) | LMAN (n = 8) | |
|---|---|---|---|---|---|---|
| Syllable duration variability | 0.85 ± 0.02 | 0.79 ± 0.06 | 0.86 ± 0.02. | 0.78 ± 0.08 | 0.85 ± 0.01 | 0.82 ± 0.06 |
| (0.65 ± 0.15)† | (0.60 ± 0.11)† | (0.84 ± 0.05)NS | (0.73 ± 0.07)NS | (0.87 ± 0.03)NS | (0.66 ± 0.16)* | |
| Gap duration variability | 0.87 ± 0.04 | 0.82 ± 0.04 | 0.87 ± 0.02 | 0.82 ± 0.06 | 0.88 ± 0.03 | 0.84 ± 0.05 |
| (0.72 ± 0.11)† | (0.66 ± 0.13)† | (0.85 ± 0.02)NS | (0.80 ± 0.08)NS | (0.88 ± 0.03)NS | (0.72 ± 0.14)* | |
| Rhythmicity | 0.08 ± 0.05 | 0.17 ± 0.07 | 0.15 ± 0.04 | 0.24 ± 0.05 | 0.18 ± 0.08 | 0.17 ± 0.07 |
| (0.32 ± 0.17)* | (0.42 ± 0.20)* | (0.14 ± 0.04)NS | (0.25 ± 0.06)NS | (0.14 + 0.04)NS | (0.32 ± 0.14)* | |
| Average spectrogram cross-correlation | 0.43 ± 0.18 | 0.41 ± 0.07 | 0.44 ± 0.14 | 0.41 ± 0.10 | 0.63 ± 0.03 | 0.33 ± 0.09 |
| (0.55 ± 0.16)* | (0.52 ± 0.13)* | (0.47 ± 0.16)NS | (0.44 ± 0.10)NS | (0.65 + 0.02)NS | (0.40 ± 0.13)* |
Data are mean ± SE prelesion and postlesion (in parentheses) values for n birds. Four measures of song variability were used to analyze the effect of DLM (medial portion of dorsolateral thalamus) and Area X lesions on subsong and plastic song birds, of MMAN (medial magnocellular nucleus of anterior nidopallium) lesions on subsong birds, and of LMAN (lateral magnocellular nucleus of anterior nidopallium) inactivations on young plastic song birds. 1–2) Syllable and gap duration variability measures are entropy-based metrics of variability in syllable and gap duration distributions, where 0 and 1 represent maximal stereotypy and variability, respectively. 3) Song rhythmicity was computed as the peak of the power spectrum of song amplitude, where larger values correspond to increased repetitive song temporal structure. 4) The average spectrogram cross-correlation was computed from 250 pairwise cross-correlations performed on randomly chosen syllales from pre- and postlesion data sets. Values of 0 and 1 represent dissimilar and identical syllable acoustic structure, respectively (see [scap]methods[r]).
P < 0.05;
†P < 0.01; NS, not significant. See text for details.
To compare the differential effects of Area X lesions, DLM lesions, and LMAN inactivations, for each bird we subtracted the prelesion value from the postlesion value for each of the four measures of song variability. For each measure, we then carried out a t-test on the hypothesis that the pre-post differences for DLM-lesioned birds were the same as the differences for Area X-lesioned birds. This comparison was also done between DLM lesions and LMAN inactivations.
RESULTS
In juvenile and adult birds, LMAN neurons exhibit highly variable patterns of spiking and bursting activity during singing (Aronov et al. 2008; Kao et al. 2005, 2008; Leonardo 2004; Olveczky et al. 2005). These premotor signals are thought to generate variability in vocal behavior, at the level of both acoustic and temporal structure. LMAN lesions or inactivations cause an increased stereotypy in the durations of syllables and gaps. In addition, the acoustic structure within syllables becomes more stereotyped, reducing trial-to-trial fluctuations in syllable phonology (Fig. 1B) (Bottjer et al. 1984; Kao and Brainard 2006; Olveczky et al. 2005; Scharff and Nottebohm 1991). If DLM or Area X plays a key role in driving premotor variability signals in LMAN, then lesions of these areas should resemble LMAN lesions and reduce variability in the durations of syllables and gaps, and the acoustic variability within syllables. Thus we examined how lesions in earlier stages in the Area X→DLM→LMAN pathway affect variability in the temporal and acoustic structure of early vocalizations.
Exploratory variability is preserved after Area X lesions.
Although Area X lesions in juveniles result in an abnormally variable adult song (Scharff and Nottebohm 1991; Sohrabji et al. 1990), the acute effect of Area X lesions on juvenile song has not been quantitatively examined. We performed bilateral excitotoxic lesions of Area X in subsong birds (n = 12) and in plastic song birds (n = 7) and compared the prelesion song to the first postlesion song (Fig. 2, see methods). Birds started singing from 1 to 7 days after lesion (average 3.1 ± 1.6 days after lesion). Subsequent histology confirmed that the lesions destroyed between 70% and 100% of Area X bilaterally (Fig. 2A; Supplemental Fig. S1). After Area X lesions, songs retained a high degree of variability that was clearly visible in the song spectrograms (Fig. 2, B and F; Supplemental Fig. S1). We quantified lesion-induced changes to song in four ways. First, we first analyzed the distributions of syllable durations. In intact subsong birds, syllable durations were broadly distributed, with monotonically decreasing probability of generating longer syllables (Fig. 2C) (Aronov et al. 2008). After Area X lesions, these syllable durations remained highly variable (Fig. 2C).
The pre- and postlesion syllable duration distributions were significantly different when quantified by a sensitive statistical measure such as the Kolmogorov-Smirnov test (n = 19/19 birds, P < 0.001). In some birds, the differences were subtle (Fig. 2), and in others they were more obvious (see Supplemental Fig. S1 for more examples). For several reasons, we focus here on the variability in the distribution of syllable durations rather than on the detailed shape of the distributions themselves. First, by the same statistical measures, syllable duration distributions exhibited significant changes between sequential days in the prelesion period (n = 19/19 birds, P < 0.001). Second, because of the time between lesion and first postlesion song, one cannot be sure which changes in the distribution are due to the lesion and which are due to a period of nonsinging. To specifically quantify the effect of AFP lesions on vocal variability, we developed an entropy-based measure of variability in syllable duration distributions, Ve, which approaches 1 (maximal variability) when syllable durations are distributed evenly across all intervals of the distribution (logarithmically binned) and is equal to 0 (maximal stereotypy) when all syllable durations are within one bin (see methods).
In subsong birds, Area X lesions did not cause a significant change in the variability metric of syllable durations (Table 1; Vpree = 0.86 ± 0.02, Vposte = 0.84 ± 0.05; P > 0.05 paired t-tests, n = 12 subsong birds). In plastic song birds, which have one or more peaks in their syllable duration distribution due to the presence of identifiable syllables (arrow, Fig. 2G) (Tchernichovski et al. 2004), Area X lesions similarly had no significant effect on the variability metric (Table 1, Fig. 2, G and K; Vpree = 0.78 ± 0.08; Vposte = 0.73 ± 0.07; P > 0.05, n = 7 birds).
We next examined the effect of Area X lesions on the silent intervals (gaps) between syllables. In both subsong and plastic song birds, Area X lesions did not reduce the variability of gap durations, computed as above for syllables (Fig. 2, D, H, and L, Table 1; subsong: Vpree = 0.87 ± 0.02, Vposte = 0.85 ± 0.02, P > 0.05, n = 12 birds; plastic song: Vpree = 0.82 ± 0.06, Vposte = 0.80 ± 0.08, P > 0.05, n = 7 birds).
Recently, it has been shown that zebra finch song acquires more rhythmic temporal structure as the timing of vocalizations becomes more stereotyped during development (Saar and Mitra 2008). Thus we computed a metric of rhythmicity, R, from the peak height of the normalized power spectrum of the sound amplitude during singing (Fig. 2, E and J; see methods). Area X lesions did not significantly change song rhythmicity in either subsong or plastic song birds (subsong: Rpre = 0.15 ± 0.04 vs. Rpost = 0.14 ± 0.04, P > 0.5; plastic song: Rpre = 0.24 ± 0.05 vs. Rpost = 0.25 ± 0.06, P > 0.7; Fig. 2M, Table 1).
We next wondered whether the variability of the acoustic structure within syllables was affected by Area X lesions. To quantify acoustic variability, we performed a pairwise spectrogram cross-correlation analysis (Nelson and Marler 1994; see methods) on 250 syllables randomly selected from postlesion singing, and then performed the same analysis on 250 duration-matched syllables from prelesion song (see methods). Average pairwise correlation coefficients (CC) were not significantly changed after Area X lesions in subsong birds (Fig. 2N, Table 1; CCpre = 0.44 ± 0.14, CCpost = 0.47 ± 0.16, P > 0.05). In plastic song birds there was a slight increase in syllable similarity that did not reach statistical significance (plastic song: CCpre 0.41 ± 0.10, CCpost = 0.44 ± 0.10, P > 0.05).
Finally, while Area X lesions did not reduce aspects of song variability captured by any of our variability metrics, in many subsong birds Area X lesions subtly affected song structure, as evidenced by some change in the contour of the syllable duration distributions (Supplemental Fig. S1). In five birds, Area X lesions reduced the occurrence of short syllables and gaps, resulting in song that contained abnormally long and variable syllables (Supplemental Fig. S1, A–E). In contrast, in three other birds Area X lesions resulted in an increase in the production of brief syllables (Supplemental Fig. S1, F–Q). These observations suggest that Area X may play a role in patterning normal subsong. However, in all cases songs following Area X lesions remained highly variable in syllable and gap durations, song temporal structure, and syllable phonology (Fig. 2; Supplemental Fig. S1). Thus our findings suggest that the expression of vocal variability in juvenile birds does not require Area X.
DLM is required for vocal exploratory behavior.
Given the hypothesized role of thalamic nucleus DLM in relaying information from Area X to LMAN (Kojima and Doupe 2009; Person and Perkel 2007), the lack of a significant effect of Area X lesions on the variability of juvenile song suggests that DLM may also not be necessary for the expression of vocal exploration. On the other hand, lesions of the pallido-recipient thalamus in primates lead to severe deficits in many behaviors (Canavan et al. 1989), including human speech (Nadeau and Crosson 1997). To resolve this question in the context of the songbird AFP, we bilaterally targeted injections of the excitotoxin NMA into the Area X-recipient portion of DLM in juvenile birds (n = 18; see methods). Subsequent histology confirmed that in most birds the injections eliminated 100% of the entire DLM in both hemispheres [n = 7 subsong birds (39–48 dph), n = 7 plastic song birds (44–63 dph); Fig. 3A, Supplemental Fig. S2, A–H]. These birds began singing between 2 and 8 days after lesion (average 4.8 ± 2.1 days, n = 15). Songs from the first postlesion day of singing were analyzed and compared with prelesion songs. Histology revealed that in four birds one of the injections missed Area X-recipient DLM, resulting in partial (<50%) and/or unilateral lesions. Visual inspection of their songs showed that they continued to sing variably, and they are not included in the analysis presented below.
In contrast to Area X lesions, we found that DLM lesions produced an acute and dramatic increase in song stereotypy that was apparent in the song spectrograms (Fig. 3, B and F). We quantified lesion-induced changes in song, using the same four metrics described above for Area X lesions. In subsong birds, the first songs produced after DLM lesions contained stereotyped, identifiable syllables associated with distinct peaks in the syllable duration distributions (Fig. 3, B and C). DLM lesions caused a significant reduction in the variability of syllable durations in all subsong birds (Fig. 3K, Table 1; Vpree = 0.85 ± 0.02, Vposte = 0.65 ± 0.15, P < 0.01 paired t-test, n = 7 birds). In plastic song birds, DLM lesions largely eliminated syllable duration variability from the song, causing an increase in the size of the peak and a decrease in the variability metric of the distribution (Fig. 3, F, G, K, Table 1; Vpree = 0.79 ± 0.06, Vposte = 0.60 ± 0.11, P < 0.01, n = 7 birds).
DLM lesions also had a dramatic effect on the gaps between syllables. In both subsong and plastic song birds, gap durations became more stereotyped after DLM lesions, resulting in more prominent peaks in the gap duration distributions and a reduction in the gap variability metric, computed as above for syllables (Fig. 3, D, H, L, Table 1; subsong: Vpree = 0.87 ± 0.04, Vposte = 0.72 ± 0.11, P < 0.01, n = 7 birds; plastic song: Vpree = 0.82 ± 0.04, Vposte = 0.66 ± 0.13, P < 0.01, n = 7 birds).
The decreased variability in both syllable and gap durations following DLM lesions was accompanied by a dramatic increase in the rhythmicity of song temporal structure that was clearly visible in the song spectrograms and amplitude waveforms (Fig. 3, E and J). This was reflected in a significant increase in the rhythmicity measure in both subsong (Rpre = 0.08 ± 0.05 vs. Rpost = 0.32 ± 0.17, P < 0.05) and plastic song (Rpre = 0.17 ± 0.07 vs. Rpost = 0.42 ± 0.20, P < 0.05) birds (Fig. 3M, Table 1).
Finally, variability in the acoustic structure of syllables was substantially reduced after DLM lesions, as indicated by a significant increase in the average pairwise spectrogram cross-correlation coefficients in both subsong (pre 0.43 ± 0.18, post 0.55 ± 0.16; P < 0.05, n = 7) and plastic song (pre 0.41 ± 0.07, post 0.52 ± 0.12; P < 0.05, n = 7) birds (Fig. 3N, Table 1).
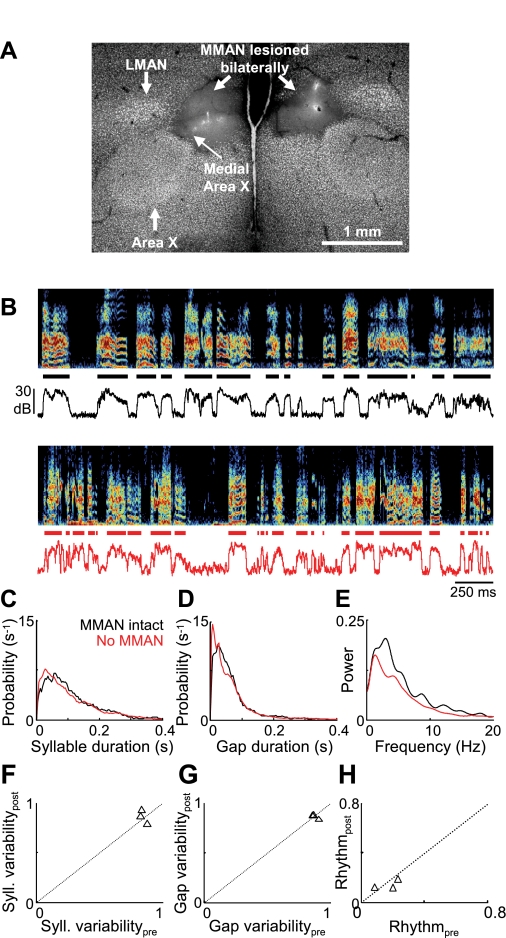
We next considered the possibility that the effect of DLM lesions was due to damage to a neighboring thalamic nucleus, DMP (dorsomedial nucleus of the posterior thalamus), part of a BG-forebrain loop parallel to the AFP. DMP damage could affect singing through its projection to MMAN, which in turn projects to HVC (Bottjer et al. 1989; Kubikova et al. 2007; Nottebohm et al. 1982). In subsong birds, we found that bilateral lesions of MMAN, the forebrain component of this parallel loop, produced no effect on subsong structure that was detectable by our measures (Fig. 4, n = 3 birds, P > 0.2 for all measures, Table 1; birds sang 2.7 ± 1.5 days after lesion, range 1–4 days). This limited data set is consistent with previous reports that MMAN lesion or inactivation does not affect song variability in plastic song birds (Foster and Bottjer 2001; Horita et al. 2008; Olveczky et al. 2005).
Fig. 4.
MMAN lesions do not impair vocal variability in subsong birds. A: Neu-N staining showing the bilateral elimination of MMAN. LMAN and lateral Area X remained intact after the lesion. Note that a portion of medial Area X was also lesioned in the left hemisphere. B–E: the song of a subsong bird (prelesion dph 44, postlesion dph 47; histology shown in A) is analyzed as in Fig. 2, B–E. F–H: population data from MMAN lesions (n = 3 subsong birds) are plotted as in Fig. 2, K and M.
In summary, DLM lesions caused a dramatic increase in song stereotypy. Notably, the effect of DLM lesions on song variability was significantly greater than the effect of Area X lesions (P < 0.01, all measures; see methods). Given the similarity between our findings and previous reports of LMAN lesions (Bottjer et al. 1984; Kao and Brainard 2006; Scharff and Nottebohm 1991), we compared the effect of DLM lesions with the effect of LMAN inactivations in birds at the earliest phase of plastic song (45–56 dph; see methods). LMAN inactivations increased the stereotypy of syllable and gap duration distributions, song rhythmicity, and the acoustic similarity of syllables (P < 0.05 for all 4 measures; Fig. 5, Table 1). In fact, the effect of LMAN inactivations was not statistically different from the effect of DLM lesions (P > 0.05 for all 4 measures). These data suggest that normal LMAN premotor function critically depends on its inputs from the thalamic nucleus DLM.
Fig. 5.
Quantitative comparison of DLM lesions and LMAN inactivations. Inactivation of LMAN was carried out as described in detail elsewhere (Olveczky et al. 2005). Data in A–D are plotted as in Fig. 3, K–N, comparing data from DLM lesions (n = 14, gray) side by side with data from LMAN inactivations from young plastic song birds (dph 45–56, black). A and B: scatterplots showing prelesion vs. postlesion variability of syllable (A) and gap (B) durations. C: scatterplot of song rhythmicity in pre- and postlesion song. D: average pairwise spectrogram cross-correlations in pre- and postlesion song.
Role of HVC in singing after thalamic and BG lesions.
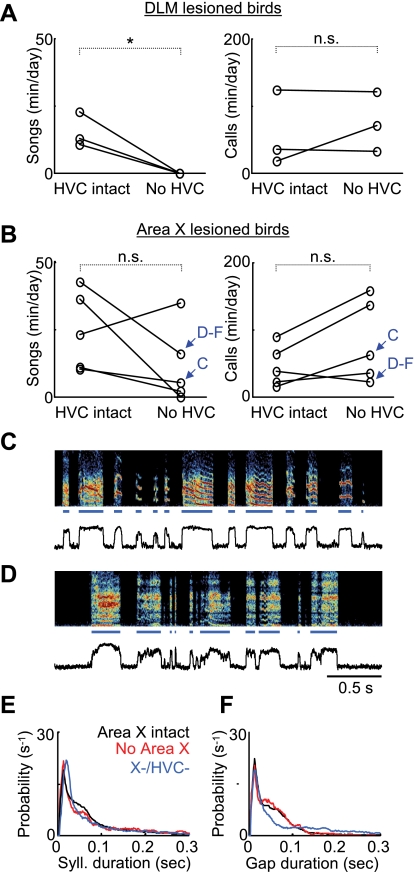
Stereotyped song structure at all developmental stages, from subsong to adult song, is dependent on the projection to RA from premotor nucleus HVC (Fig. 1) (Aronov et al. 2008; Nottebohm et al. 1976). We wondered whether the stereotyped songs produced by juvenile birds after DLM lesions were generated by HVC. We performed bilateral HVC lesions in birds that had already received DLM lesions as subsong birds (DLM lesion age: 40.3 ± 2.3 dph, HVC lesion age: 56.0 ± 4.6 dph; see methods). After HVC lesions, birds were continuously recorded for at least 2 wk, and all of their vocalizations were monitored daily. Although they continued to make forebrain-independent contact calls (Simpson and Vicario 1990), birds with bilateral HVC and DLM lesions did not produce any songlike vocalizations (Fig. 6A; prelesion song rate: 15.2 ± 6.4 min/day, postlesion = 0.0 min/day, P < 0.05, n = 3 birds, paired t-test). In contrast, in five birds in which the exact same HVC lesion procedure was carried out after Area X lesions, three continued to sing at normal, although somewhat reduced, rates of singing; one increased its singing rate; and in one bird singing was abolished (Fig. 6, B–F; Area X lesion age: 43.2 ± 4.2 dph, HVC lesion age: 55.2 ± 5.7 dph; data across birds: prelesion song rate: 24.6 ± 14.6 min/day, postlesion: 11.6 ± 14.4 min/day, P > 0.05). These findings suggest that the stereotyped songs produced after DLM lesion were driven from HVC and did not result from a lesion-induced pathological state of LMAN. Moreover, the findings that dual HVC/Area X-lesioned birds can sing but HVC/DLM-lesioned birds cannot suggest that the LMAN→RA pathway cannot generate subsonglike vocalizations independent of DLM.
Fig. 6.
Song after DLM lesions is driven by HVC. Average rates of song and call production in dual Area X/HVC and DLM/HVC lesions are shown. For rate measurement, a full day of recording was partitioned into 2-s segments, and the numbers of segments containing calls or songs were estimated (see methods) (Aronov et al. 2008). A: in DLM-lesioned birds, subsequent HVC lesions abolished singing (left, *P < 0.05, paired t-test) but not calls [right, not significant (n.s.)], which are known not to require the song system (Simpson and Vicario 1990). B: HVC lesions in Area X-lesioned birds did not abolish the production of songs (left) or calls (right). Blue arrows point to the birds whose data are shown in C–F. C and D: example of singing from 2 birds that sustained dual Area X/HVC lesions (see Supplemental Fig. S1 for histological verification of HVC lesion in the bird whose postlesion song appears in C). E and F: syllable and gap distributions for the bird from D. Black, red, and blue curves represent data from before the Area X lesion and after the Area X lesion and subsequent HVC lesion, respectively.
DISCUSSION
To further understand the origin of exploratory variability in motor circuitry, we have quantitatively analyzed the singing behavior of young zebra finches following lesions of several nuclei in the AFP, a BG-thalamocortical circuit known to be important for the generation of vocal variability. Previous studies of the AFP have found that whereas early LMAN lesions caused increased song stereotypy and premature song crystallization, Area X lesions led to protracted song variability and a failure to imitate (Bottjer et al. 1984; Scharff and Nottebohm 1991; Sohrabji et al. 1990). In fact, it was reported that in the 10 days following Area X lesions in juveniles, song remained variable (Scharff and Nottebohm 1991), suggesting that Area X was not required for the expression of vocal variability.
An alternative view was suggested by the observation that neurons in Area X discharge highly variable spike patterns with respect to song timing in singing juvenile birds (Goldberg et al. 2010; Goldberg and Fee 2010; Hessler and Doupe 1999a, 1999b). There is also evidence that Area X can regulate or bias variability during singing: infusion of a dopamine antagonist into the region of Area X affects the changes in song variability that occur in different social contexts (Leblois et al. 2010). To help resolve these different views of AFP function, we carried out lesions of Area X in subsong and plastic song birds. Consistent with the earlier lesion studies, we found that variability in syllable and gap durations, as well as in syllable acoustic structure, was preserved after Area X lesions. Notably, even in those subsong birds in which Area X lesions caused subtle changes to the song that were visible in the syllable duration distributions, songs retained their variability (Supplemental Fig. S1). Thus our findings suggest that Area X is not necessary for the generation of variability by LMAN. They also suggest that the variability of spiking patterns in Area X is not the origin of variability in downstream AFP nuclei, but may instead be driven by LMAN via the projection from LMAN to Area X (Farries et al. 2005; Vates and Nottebohm 1995).
Of course, we should note several caveats. Our observations cannot rule out a contribution of Area X to the generation of variability in acoustic or temporal song features we did not analyze. They also cannot rule out the possible role of Area X in the modulation of variability during singing (Leblois et al. 2010).
The overall preservation of the vocal variability following Area X lesions raises the question of what role the basal ganglia play during song learning. Consistent with previous studies (Scharff and Nottebohm 1991; Sohrabji et al. 1990), two birds receiving Area X lesions as juveniles and subsequently followed into adulthood exhibited severely impaired imitation as well as abnormally variable song (Supplemental Fig. S3). Poor imitation and persistent variability, while distinct phenomena, may have a common underlying cause: a loss of Area X-driven learning in the HVC→RA pathway. Specifically, we speculate that Area X controls plasticity in the motor pathway via signals sent to RA through the AFP. Consistent with this hypothesis, Area X lesions block the normal singing-related induction of immediate-early genes in RA (Kubikova et al. 2007). In addition, during learning LMAN may bias ongoing song to reduce vocal errors (Andalman and Fee 2009), a process that likely requires Area X. Together, these findings suggest that Area X may be necessary for normal motor pathway development, including song imitation and crystallization.
Given that one hypothesized function of the pallidal-recipient thalamic nucleus DLM is to relay signals from Area X to LMAN (Kojima and Doupe 2009; Person and Perkel 2007), we wondered what role DLM would have in the expression of vocal variability. In contrast to the minimal effect of Area X lesions on juvenile song variability, lesions of DLM abolished normal vocal babbling, causing a pathological reduction in the variability of syllable and gap durations, song rhythm, as well as syllable acoustic structure (Figs. 3 and 5). Furthermore, after DLM lesions, song could not be driven by the LMAN→RA pathway alone but required the premotor (HVC→RA) pathway that generates adult song (Figs. 1 and 6). Thus our findings support a central role for the BG-recipient thalamus in the expression of motor exploration that is surprisingly independent of its inputs from the BG.
The finding that DLM lesions caused a dramatic decrease in song variability in the first postlesion song (Fig. 3) suggests that DLM may play a role in generating the premotor signals observed in LMAN (Aronov et al. 2008). How might DLM contribute to these signals? One possibility is that they are directly driven by DLM, such that DLM triggers syllable onsets and offsets in subsong and drives vocal variability in plastic song. Another possibility is that, as the sole known glutamatergic projection to LMAN, DLM simply provides a general, tonic drive to LMAN during singing, and that the premotor correlations to subsong syllables and variability arise within LMAN. Recordings from LMAN-projecting DLM neurons in juvenile birds may elucidate the mechanisms of thalamocortical activation in the AFP. Of course, it remains possible that the loss of LMAN functionality in DLM-lesioned birds may not derive from the loss of excitatory inputs but simply from the loss of a trophic influence of DLM neurons in LMAN (Johnson et al. 1997). However, after DLM lesions, LMAN and its projection to RA appeared intact (Supplemental Fig. S1H), suggesting that the behavioral effect of DLM lesions was not primarily due to a secondary LMAN lesion.
The differential effects of Area X and DLM lesions appear counterintuitive from classical models in which thalamic function critically depends on BG inputs (DeLong 1990). Indeed, in anesthetized birds, DLM neurons discharge at low rates (<3 Hz), because of tonic inhibition by Area X outputs, and DLM neuronal activity is patterned primarily by Area X (Leblois et al. 2009; Person and Perkel 2007). After Area X inactivation, however, DLM neurons are still active, and even discharge at higher rates (>20 Hz) that cause increased activation of LMAN (Kojima and Doupe 2009). Thus the loss of Area X inputs to DLM leaves neurons there with some level of functionality. An intriguing possibility is that nonpallidal inputs to DLM, such as from RA or neuromodulatory regions, could contribute to the generation of exploratory variability following Area X lesions (Vates et al. 1997; Wada et al. 2004; Wild 1993).
Our findings are reminiscent of what has been observed in stroke patients. In humans, thalamic lesions are strongly correlated with dramatic speech impairment while lesions restricted to the BG are not (Nadeau and Crosson 1997). Interestingly, verbal repetition—the rote execution of a learned motor program—is preserved in thalamic strokes. In contrast, spontaneous speech, which has an improvisational and exploratory component, is severely impaired in these conditions. This pattern shows a striking similarity to our findings that thalamic lesions leave stereotyped components of the song intact but abolish exploratory behaviors. More generally, these parallels suggest that in both songbirds and mammals a given module of cortex critically depends on its thalamic inputs for normal motor function, and raise the possibility that conserved thalamocortical circuits enable motor exploration and variability across species (Jarvis 2007).
GRANTS
Funding was provided to M. S. Fee by National Institutes of Health (NIH) Grant R01-DC-009183 and to J. H. Goldberg by the Damon Runyon and Charles King Trust Postdoctoral Fellowships and NIH grant K99-NS-067062.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We thank Dmitriy Aronov and Tatsuo Okubo for helpful comments.
Footnotes
Supplemental Material for this article is available online at the Journal website.
REFERENCES
- Andalman and Fee, 2009. Andalman AS, Fee MS. A basal ganglia-forebrain circuit in the songbird biases motor output to avoid vocal errors. Proc Natl Acad Sci USA 106: 12518–12523, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronov et al., 2008. Aronov D, Andalman AS, Fee MS. A specialized forebrain circuit for vocal babbling in the juvenile songbird. Science 320: 630–634, 2008 [DOI] [PubMed] [Google Scholar]
- Boettiger and Doupe, 1998. Boettiger CA, Doupe AJ. Intrinsic and thalamic excitatory inputs onto songbird LMAN neurons differ in their pharmacological and temporal properties. J Neurophysiol 79: 2615–2628, 1998 [DOI] [PubMed] [Google Scholar]
- Bottjer, 2004. Bottjer SW. Developmental regulation of basal ganglia circuitry during the sensitive period for vocal learning in songbirds. Ann NY Acad Sci 1016: 395–415, 2004 [DOI] [PubMed] [Google Scholar]
- Bottjer and Altenau, 2010. Bottjer SW, Altenau B. Parallel pathways for vocal learning in basal ganglia of songbirds. Nat Neurosci 13: 153–155, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottjer et al., 2000. Bottjer SW, Brady JD, Cribbs B. Connections of a motor cortical region in zebra finches: relation to pathways for vocal learning. J Comp Neurol 420: 244–260, 2000 [PubMed] [Google Scholar]
- Bottjer et al., 1989. Bottjer SW, Halsema KA, Brown SA, Miesner EA. Axonal connections of a forebrain nucleus involved with vocal learning in zebra finches. J Comp Neurol 279: 312–326, 1989 [DOI] [PubMed] [Google Scholar]
- Bottjer et al., 1984. Bottjer SW, Miesner EA, Arnold AP. Forebrain lesions disrupt development but not maintenance of song in passerine birds. Science 224: 901–903, 1984 [DOI] [PubMed] [Google Scholar]
- Canavan et al., 1989. Canavan AG, Nixon PD, Passingham RE. Motor learning in monkeys (Macaca fascicularis) with lesions in motor thalamus. Exp Brain Res 77: 113–126, 1989 [DOI] [PubMed] [Google Scholar]
- Charlesworth et al., 2011. Charlesworth JD, Tumer EC, Warren TL, Brainard MS. Learning the microstructure of successful behavior. Nat Neurosci 14: 373–380, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong, 1990. DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci 13: 281–285, 1990 [DOI] [PubMed] [Google Scholar]
- Doupe et al., 2005. Doupe AJ, Perkel DJ, Reiner A, Stern EA. Birdbrains could teach basal ganglia research a new song. Trends Neurosci 28: 353–363, 2005 [DOI] [PubMed] [Google Scholar]
- Doya and Sejnowski, 1995. Doya K, Sejnowski T. A novel reinforcement model of birdsong vocalization learning. Adv Neural Information Processing Syst 7: 101–108, 1995 [Google Scholar]
- Farries et al., 2005. Farries MA, Ding L, Perkel DJ. Evidence for “direct” and “indirect” pathways through the song system basal ganglia. J Comp Neurol 484: 93–104, 2005 [DOI] [PubMed] [Google Scholar]
- Farries and Perkel, 2002. Farries MA, Perkel DJ. A telencephalic nucleus essential for song learning contains neurons with physiological characteristics of both striatum and globus pallidus. J Neurosci 22: 3776–3787, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feenders et al., 2008. Feenders G, Liedvogel M, Rivas M, Zapka M, Horita H, Hara E, Wada K, Mouritsen H, Jarvis ED. Molecular mapping of movement-associated areas in the avian brain: a motor theory for vocal learning origin. PLoS One 3: e1768, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster and Bottjer, 2001. Foster EF, Bottjer SW. Lesions of a telencephalic nucleus in male zebra finches: influences on vocal behavior in juveniles and adults. J Neurobiol 46: 142–165, 2001 [DOI] [PubMed] [Google Scholar]
- Goldberg et al., 2010. Goldberg JH, Adler A, Bergman H, Fee MS. Singing-related neural activity distinguishes two putative pallidal cell types in the songbird basal ganglia: comparison to the primate internal and external pallidal segments. J Neurosci 30: 7088–7098, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg and Fee, 2010. Goldberg JH, Fee MS. Singing-related neural activity distinguishes four classes of putative striatal neurons in the songbird basal ganglia. J Neurophysiol 103: 2002–2014, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessler and Doupe, 1999a. Hessler NA, Doupe AJ. Singing-related neural activity in a dorsal forebrain-basal ganglia circuit of adult zebra finches. J Neurosci 19: 10461–10481, 1999a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessler and Doupe, 1999b. Hessler NA, Doupe AJ. Social context modulates singing-related neural activity in the songbird forebrain. Nat Neurosci 2: 209–211, 1999b [DOI] [PubMed] [Google Scholar]
- Horita et al., 2008. Horita H, Wada K, Jarvis ED. Early onset of deafening-induced song deterioration and differential requirements of the pallial-basal ganglia vocal pathway. Eur J Neurosci 28: 2519–2532, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis, 2004. Jarvis ED. Learned birdsong and the neurobiology of human language. Ann NY Acad Sci 1016: 749–777, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis, 2007. Jarvis ED. Neural systems for vocal learning in birds and humans: a synopsis. J Ornithol 148: 35–44, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson et al., 1997. Johnson F, Hohmann SE, DiStefano PS, Bottjer SW. Neurotrophins suppress apoptosis induced by deafferentation of an avian motor-cortical region. J Neurosci 17: 2101–2111, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson et al., 1995. Johnson F, Sablan MM, Bottjer SW. Topographic organization of a forebrain pathway involved with vocal learning in zebra finches. J Comp Neurol 358: 260–278, 1995 [DOI] [PubMed] [Google Scholar]
- Kao and Brainard, 2006. Kao MH, Brainard MS. Lesions of an avian basal ganglia circuit prevent context-dependent changes to song variability. J Neurophysiol 96: 1441–1455, 2006 [DOI] [PubMed] [Google Scholar]
- Kao et al., 2005. Kao MH, Doupe AJ, Brainard MS. Contributions of an avian basal ganglia-forebrain circuit to real-time modulation of song. Nature 433: 638–643, 2005 [DOI] [PubMed] [Google Scholar]
- Kao et al., 2008. Kao MH, Wright BD, Doupe AJ. Neurons in a forebrain nucleus required for vocal plasticity rapidly switch between precise firing and variable bursting depending on social context. J Neurosci 28: 13232–13247, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimpo et al., 2003. Kimpo RR, Theunissen FE, Doupe AJ. Propagation of correlated activity through multiple stages of a neural circuit. J Neurosci 23: 5750–5761, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima and Doupe, 2009. Kojima S, Doupe AJ. Activity propagation in an avian basal ganglia-thalamocortical circuit essential for vocal learning. J Neurosci 29: 4782–4793, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubikova et al., 2007. Kubikova L, Turner EA, Jarvis ED. The pallial basal ganglia pathway modulates the behaviorally driven gene expression of the motor pathway. Eur J Neurosci 25: 2145–2160, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblois et al., 2009. Leblois A, Bodor AL, Person AL, Perkel DJ. Millisecond timescale disinhibition mediates fast information transmission through an avian basal ganglia loop. J Neurosci 29: 15420–15433, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblois et al., 2010. Leblois A, Wendel BJ, Perkel DJ. Striatal dopamine modulates basal ganglia output and regulates social context-dependent behavioral variability through D1 receptors. J Neurosci 30: 5730–5743, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardo, 2004. Leonardo A. Experimental test of the birdsong error-correction model. Proc Natl Acad Sci USA 101: 16935–16940, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo and Perkel, 1999. Luo M, Perkel DJ. Long-range GABAergic projection in a circuit essential for vocal learning. J Comp Neurol 403: 68–84, 1999 [PubMed] [Google Scholar]
- Marler, 1970. Marler P. Birdsong and speech development: could there be parallels? Am Sci 58: 669–673, 1970 [PubMed] [Google Scholar]
- Nadeau and Crosson, 1997. Nadeau SE, Crosson B. Subcortical aphasia. Brain Lang 58: 355–402, 1997 [DOI] [PubMed] [Google Scholar]
- Nelson and Marler, 1994. Nelson DA, Marler P. Selection-based learning in bird song development. Proc Natl Acad Sci USA 91: 10498–10501, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nottebohm et al., 1982. Nottebohm F, Kelley DB, Paton JA. Connections of vocal control nuclei in the canary telencephalon. J Comp Neurol 207: 344–357, 1982 [DOI] [PubMed] [Google Scholar]
- Nottebohm et al., 1976. Nottebohm F, Stokes TM, Leonard CM. Central control of song in the canary, Serinus canarius. J Comp Neurol 165: 457–486, 1976 [DOI] [PubMed] [Google Scholar]
- Olveczky et al., 2005. Olveczky BP, Andalman AS, Fee MS. Vocal experimentation in the juvenile songbird requires a basal ganglia circuit. PLoS Biol 3: e153, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Person and Perkel, 2007. Person AL, Perkel DJ. Pallidal neuron activity increases during sensory relay through thalamus in a songbird circuit essential for learning. J Neurosci 27: 8687–8698, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Person and Perkel, 2005. Person AL, Perkel DJ. Unitary IPSPs drive precise thalamic spiking in a circuit required for learning. Neuron 46: 129–140, 2005 [DOI] [PubMed] [Google Scholar]
- Price, 1979. Price P. Developmental determinants of structure in zebra finch song. J Comp Physiol Psychol 93: 260–277, 1979 [Google Scholar]
- Reiner et al., 2004. Reiner A, Perkel DJ, Bruce LL, Butler AB, Csillag A, Kuenzel W, Medina L, Paxinos G, Shimizu T, Striedter G, Wild M, Ball GF, Durand S, Gunturkun O, Lee DW, Mello CV, Powers A, White SA, Hough G, Kubikova L, Smulders TV, Wada K, Dugas-Ford J, Husband S, Yamamoto K, Yu J, Siang C, Jarvis ED. Revised nomenclature for avian telencephalon and some related brainstem nuclei. J Comp Neurol 473: 377–414, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saar and Mitra, 2008. Saar S, Mitra PP. A technique for characterizing the development of rhythms in bird song. PLoS One 3: e1461, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharff and Nottebohm, 1991. Scharff C, Nottebohm F. A comparative study of the behavioral deficits following lesions of various parts of the zebra finch song system: implications for vocal learning. J Neurosci 11: 2896–2913, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson and Vicario, 1990. Simpson HB, Vicario DS. Brain pathways for learned and unlearned vocalizations differ in zebra finches. J Neurosci 10: 1541–1556, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohrabji et al., 1990. Sohrabji F, Nordeen EJ, Nordeen KW. Selective impairment of song learning following lesions of a forebrain nucleus in the juvenile zebra finch. Behav Neural Biol 53: 51–63, 1990 [DOI] [PubMed] [Google Scholar]
- Stepanek and Doupe, 2010. Stepanek L, Doupe AJ. Activity in a cortical-basal ganglia circuit for song is required for social context-dependent vocal variability. J Neurophysiol 104: 2474–2486, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton and Barto, 1998. Sutton RS, Barto AG. Reinforcement learning: an introduction. IEEE Trans Neural Netw 9: 1054, 1998 [Google Scholar]
- Tchernichovski et al., 2004. Tchernichovski O, Lints TJ, Deregnaucourt S, Cimenser A, Mitra PP. Studying the song development process: rationale and methods. Ann NY Acad Sci 1016: 348–363, 2004 [DOI] [PubMed] [Google Scholar]
- Tchernichovski et al., 2000. Tchernichovski O, Nottebohm F, Ho CE, Pesaran B, Mitra PP. A procedure for an automated measurement of song similarity. Anim Behav 59: 1167–1176, 2000 [DOI] [PubMed] [Google Scholar]
- Tumer and Brainard, 2007. Tumer EC, Brainard MS. Performance variability enables adaptive plasticity of “crystallized” adult birdsong. Nature 450: 1240–1244, 2007 [DOI] [PubMed] [Google Scholar]
- Vates and Nottebohm, 1995. Vates GE, Nottebohm F. Feedback circuitry within a song-learning pathway. Proc Natl Acad Sci USA 92: 5139–5143, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vates et al., 1997. Vates GE, Vicario DS, Nottebohm F. Reafferent thalamo-“cortical” loops in the song system of oscine songbirds. J Comp Neurol 380: 275–290, 1997 [PubMed] [Google Scholar]
- Wada et al., 2004. Wada K, Sakaguchi H, Jarvis ED, Hagiwara M. Differential expression of glutamate receptors in avian neural pathways for learned vocalization. J Comp Neurol 476: 44–64, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild, 1993. Wild JM. Descending projections of the songbird nucleus robustus archistriatalis. J Comp Neurol 338: 225–241, 1993 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.