Abstract
Catecholaminergic neurons in the locus coeruleus (LC) play a role in the ventilatory response to hypercapnia. Here, we show evidence for the involvement of transient receptor potential (TRP) channels. We found that the input resistance was reduced during an exposure to 8% CO2 in ∼35% LC neurons in mouse brain slices, accompanied by depolarization and higher firing activity. The neuronal responses suggest the opening of Na+ or nonselective cationic channels instead of the closure of K+ channels. As a major group of cationic channels, the TRP channels are expressed in the brain, some of which are activated by acidic pH. We therefore screened all representative TRP channels using the quantitative real-time PCR analysis. High levels of mRNA expression of TRPC5, TRPM2, and TRPM7 were found in the LC tissue. Of them, the TRPC5 transcript was the most abundant. The TRPC5 channel was activated by extracellular acidification when expressed in human embryonic kidney (HEK) cells. The TRPC5 currents started to be activated at pH 7.4 with pKa 6.9. The TRPC5 currents were also activated by isohydric hypercapnic and intracellular acidosis in a Ca2+-dependent manner. Consistently, the LC neurons were stimulated by both extra- and intracellular acidosis. The stimulatory effect of hypercapnia on LC neurons was eliminated by selective TRPC inhibitor SKF-96365 with and without the blockade of synaptic transmission. Single-cell PCR analysis indicated that TRPC5 mRNAs existed in the LC neurons. Thus these results strongly suggest that the TRP channels are likely to play a role in the CO2 chemosensitivity of LC neurons, especially TRPC5.
Keywords: breathing, norepinephrine, brain stem, transient receptor potential channel, TRPC5
breathing activity is automatically regulated by central and peripheral chemoreceptors providing feedback to the brain stem respiratory neuronal networks with the information of Pco2 and Po2 levels in the blood stream. Several groups of brain stem neurons, including those in the locus coeruleus (LC), play a role in the central CO2 chemosensitivity (Ballantyne and Scheid 2000; Gargaglioni et al. 2010; Jiang et al. 2005; Putnam 2010). The LC neurons are CO2 chemosensitive (Erlichman et al. 2009; Johnson et al. 2008; Oyamada et al. 1998; Pineda and Aghajanian 1997; Stunden et al. 2001). These norepinephrinergic (NE) neurons modulate firing activity of brain stem respiratory neurons (Biancardi et al. 2008). Breathing activity is affected by local injection of NE to the pedunculopontine tegmental nucleus (Saponjic et al. 2005). NE has excitatory effects on neurons in the pre-Bötzinger complex (PBC) (Doi and Ramirez 2010), a population of cells critical for respiratory control. NE interacts with other excitatory neuromodulators affecting the modulation of PBC neurons by neurokinin-1 and noradrenaline (Doi and Ramirez 2010). NE system lesions increase postsigh respiratory pattern disturbance (Saponjic et al. 2007). In both humans and rats, breathing is more irregular, and apnea is more frequently seen during REM sleep, a state of relative inactivity of noradrenergic neurons (Radulovacki and Carley 2003). Since the LC contains the overwhelming majority of NE-ergic neurons in the brain stem, these cells may be important for breathing modulations.
The NE neurons in the LC are intrinsically CO2 chemosensitive (Johnson et al. 2008; Nichols et al. 2008; Oyamada et al. 1998). An ionic mechanism has been shown to underlie the CO2 chemosensitivity of these neurons. In response to high CO2, pH-sensitive inward rectifier K+ (Kir) channels are inhibited, leading to depolarization and an increase in neuronal firing activity (Pineda and Aghajanian 1997). Besides the mechanism of K+ channel inhibition, openings of cationic channels also can lead to depolarization and higher firing activity as suggested by Tiwari et al. (2000). The transient receptor potential (TRP) channels are a major group of cationic channels allowing Na+, K+, and Ca2+ to cross plasma membranes in various permeabilities depending on the channel species (Clapham 2003). These channels are expressed in the central nervous system (Clapham 2003). Some members in the TRP superfamily are also activated by acidic pH, including TRPC4 and TRPC5 channels (Semtner et al. 2007). Hence, it is possible that the TRP channel activation may be produced by hypercapnic acidosis, leading to the increase in membrane excitability of the LC neurons, which may be another ionic mechanism for the CO2 chemosensitivity of these neurons. Therefore, we performed this study to test the hypothesis.
MATERIALS AND METHODS
Brain slice preparation.
All animal procedures were conducted in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals (NIH Publication No. 86-23) and were approved by the Georgia State University Institutional Animal Care and Use Committee. Brain slices were prepared as described previously (Zhang et al. 2010a). In brief, C57BL/6 mice at age 2–3 wk were decapitated after anesthetized by inhalation of saturated isoflurane. The brain stem was obtained rapidly and placed in an ice-cold, sucrose-rich artificial cerebrospinal fluid (sucrose-aCSF) containing (in mM) 200 sucrose, 3 KCl, 2 CaCl2, 2 MgCl2, 26 NaHCO3, 1.25 NaH2PO4, and 10 d-glucose. The solution was bubbled with 95% O2-5% CO2 (pH 7.40). A vibratome sectioning system was used to obtain transverse pontine sections (300 μM) containing LC. The slices were transferred to normal aCSF in which sucrose was substituted with 124 mM NaCl, allowed to recover at 33°C for 1 h, and then kept in room temperature (<6 h) before being used for recording. The slices were transferred into a recording chamber and perfused with oxygenated aCSF at a rate of 2 ml/min and maintained at 32–35°C. The LC was identified with the fourth ventricle as an anatomic landmark. Individual LC neurons were visualized with a ×40 water-immersion lens in a Zeiss microscope (Axioskop 2 Plus; Göttingen, Germany) and a near-infrared charge-coupled device (CCD) camera.
Acute dissociation of LC neurons.
LC-containing brain slices were obtained from C57BL/6 mice at age 2–3 wk as described above (Zhang et al. 2010a). The slices were then digested at 35°C for 1 h with trypsin (0.25%, type XI; Sigma) in oxygenated HEPES buffer containing (in mM) 140 NaCl, 2.5 KCl, 1 MgCl2, 1 CaCl2, 25 d-glucose, and 10 HEPES, pH 7.40. The slices were transferred to oxygenated HEPES buffer containing 1 mg/ml trypsin inhibitor and 1 mg/ml BSA for 1 min and washed twice with HEPES buffer. The LC area was micropunched and gently triturated in HEPES buffer with fire-polished Pasteur pipettes. The HEPES buffer containing triturated LC was dropped into 35-mm petri dishes and kept at room temperature for 10 min before being observed with Hoffman modulation optics.
Expression TRPC4 and TRPC5 in HEK-293 cells.
Mouse TRPC5 cDNA (GenBank acc. no. AF029983) in the eukaryotic expression vector pCI-neo was a gift from Dr. David E. Clapham (Harvard University, Boston, MA). Human TRPC4 cDNA (GenBank acc. no. AF175406) in pcDNA3.1 vector was a gift from Dr. James Putney (National Institute of Environmental Health Sciences-NIH, Research Triangle Park, NC). The human embryonic kidney (HEK-293) cells were cultured in DMEM/F-12 medium containing 10% FBS and penicillin-streptomycin at 37°C with 5% CO2. A 35-mm petri dish of cells was transfected with 1 μg of TRPC5 or TRPC4 using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). Green fluorescent protein (GFP) cDNA (in pEGFP-N2; Clontech, Palo Alto, CA) was cotransfected (0.5 μg) to identify positively transfected cells. After 24 h, the transfected cells were disassociated with 0.25% trypsin, split, and transferred to coverslips. Patch-clamp recordings were performed on the cells in the following 12–48 h.
Electrophysiology.
Whole cell current-clamp recording was performed in brain slices. Patch pipettes were pulled with a Sutter pipette puller (Model P-97; Novato, CA). The pipette-tip resistance was 3–5 MΩ. The internal (pipette) solution for current-clamp recording contained (in mM) 130 K-gluconate, 10 KCl, 10 HEPES, 2 Mg-ATP, 0.3 Na-GTP, and 0.4 EGTA (pH 7.30). The external (bath) solution was normal aCSF as described above. Whole cell voltage-clamp recording was performed in HEK cells transfected with TRPC4 and TRPC5 DNAs. The bath solutions contained (in mM) 140 NaCl, 5 CsCl, 2 CaCl2, 1 MgCl2, 10 d-glucose, and 10 PIPES, and pH was adjusted between 6.0 and 7.6 with NaOH and HCl. For patch-clamp in HEK cells, the pipette solution contained (in mM) 110 cesium methanesulfonate, 25 CsCl, 2 MgCl2, 0.362 CaCl2, 1 EGTA, and 30 HEPES (pH 7.2 with CsOH), 10 μM NaF and 30 μM AlCl3 were added in the pipette solution to activate TRPC5 currents, and 500 μM GTPγS was used to induce TRPC4 currents. The free Ca2+ concentration was calculated with Maxchelator (Chris Patton, Stanford University, Pacific Grove, CA) with 10 mM EGTA, and necessary CaCl2 added to achieve 100 nM free Ca2+. The voltage-clamp recording was performed at −60-mV holding potential. Synaptic blockade was performed using 100 μM picrotoxin, 50 μM (6)-2-amino-5-phosphonopentanoic acid, and 10 μM 6-cyano-7-nitroquinoxaline-2,3-dione. Recorded signals were amplified with an Axopatch 200B amplifier (Molecular Devices, Union City, CA), digitized at 10 kHz, filtered at 2 kHz, and collected with the Clampex 8.2 data acquisition system (Molecular Devices). Only neurons with stable resting membrane potentials (Vm) more negative than −40 mV and action potential amplitude >65 mV were used in the studies, and they can be recorded for >45 min, which was adequate for our experimental protocol. The current-voltage (I–V) relationship was obtained by injecting a series of pulse hyper- and depolarizing currents. The input resistance (Rm) of individual neurons was calculated by averaging the ratio of Vm at the last 100 ms of each command current.
Reverse-transcription PCR and real-time PCR.
The LC regions were obtained by micropuncture of the pontine sections from mice. The tissue obtained from two mice was homogenized for 2 min in solution provided in the RNeasy Mini Kit (Qiagen). The total mRNAs from the tissue were extracted according to the manufacturer's instructions (Qiagen). The concentration of the mRNA obtained was determined using a spectrophotometer (absorption at 260 and 280 nm). The first strand of cDNA from the total mRNA was synthesized with random hexamers as primers. The cDNAs obtained were used either for quantitative PCR (qPCR) or regular PCR. The qPCR primers were designed for the target genes using Primer 3.0 software (Applied Biosystems, Warrington, United Kingdom; Supplemental Table S1, available in the data supplement online at the Journal of Neurophysiology web site). The qPCR was performed using Platinum SYBR Green qPCR SuperMix-UDG (Invitrogen) on the ABI PRISM 7500 (Applied Biosystems) in the fast mode for the amplification of cDNAs according to the manufacturer's instructions with 500 nM prime. The endogenous control used in the quantitation of target genes was a housekeeping gene, β-actin. The endogenous reference gene was run parallel with the targeted genes. Each gene was performed in quadruplets obtained from four animals. The ΔΔCT method (where CT is threshold cycle) and GeneAmp 5700 SDS software were used for obtaining the data. The expression levels of the targeted genes were normalized to that of β-actin (ΔCT). The same set of primers was used for both qPCR and regular RT-PCR with the difference in concentrations (Zhang et al. 2010a,b).
PCR was performed to study the expression levels of the different TRP channels. The PCR reaction mixture contained 1.25 μl of dNTP, 2.5 μl of DMSO, 4 μl of MgCl2, 10 μl of 5× green GoTaq Flexi Buffer, 0.25 μl of Taq polymerase enzyme (5 U/μl), 2 μl of primer mix (1.0 μg/μl), 2 μl of template, and 28 μl of double-distilled water (ddH2O) for 2 tubes. The thermal cycling included initial activation at 95°C for 2 min followed by denature, annealing, and extension at 95°C for 30 s, 60°C for 30 s, and 72°C for 2 s, respectively. The final extension was at 70°C for 10 min, and the number of cycles was 40.
Single-cell PCR.
Single-cell PCR was performed for LC neurons obtained by acute dissociation as described above. The cell contents were harvested from individual neurons with patch pipettes and put into Eppendorf tubes with solution containing 10× RT buffer, RNase-free water, and RNase OUT (4.5:4.5:1). The above mixtures were immediately frozen with liquid nitrogen and kept at −80°C for future analysis. Two sets of primers were designed for the targeted genes using Primer 3.0 software (Supplemental Table S2). The 1st PCR was performed using the OneStep RT-PCR Kit (Qiagen) for obtaining the cDNAs from the LC neuron cells. The 2nd PCR was performed using HotStarTaq DNA Polymerase (Qiagen). The OneStep RT-PCR reaction mix contained 10 μl of 5× OneStep RT-PCR buffer [containing Tris·HCl, KCl, (NH4)2SO4, 12.5 mM MgCl2, DTT; pH 8.7], 10 μl of 5× Q-Solution, 2 μl of dNTP mix (containing 10 mM each dNTP), 1 μl of primer mix (1.0 μg/μl), 2 μl of OneStep RT-PCR Enzyme Mix [1 mM DTT, 0.1 mM EDTA, 0.5% (vol/vol) Nonidet P-40, 0.5% (vol/vol) Tween 20, 50% glycerol (vol/vol), stabilizer; pH 9.0], and 15 μl of RNase-free H2O, and finally 10 μl of template was added to the mixture. The thermal cycling conditions included 30 min of reverse transcription at 50°C followed by initial PCR activation at 95°C for 15 min. The 3-step cycling included denature and annealing at 94°C for 45 s and 53°C for 45 s, respectively, followed by extension at 72°C for 1 min and the final extension at 72°C for 10 min. The number of cycles performed was 30. The primers used were specific for the targeted genes. The HotStar PCR reaction mixture contained 5 μl of 10× PCR buffer, 1 μl of dNTP mix (containing 10 mM each dNTP), 0.5 μl of HotStarTaq DNA Polymerase, 10 μl of 5× Q-Solution, 1 μl of primer mix (1.0 μg/μl), and 0.5 μl of template (cDNA) and made to 50 μl with ddH2O. The thermal cycling included 40 cycles with initial PCR activation at 95°C for 15 min followed by denature, annealing, and extension steps at 94°C for 45 s, 53°C for 45 s, and 72°C for 1 min, respectively. The final extension was at 72°C for 10 min.
Electrophysiological data were analyzed with Clampfit 9.2 software (Molecular Devices). Data are presented as means ± SE. Statistical analysis of other parameters was performed with ANOVA or a two-tailed Student's t-test. Difference was considered significant when P ≤ 0.05.
RESULTS
High CO2 produced depolarization and an increase in firing activity in LC neurons.
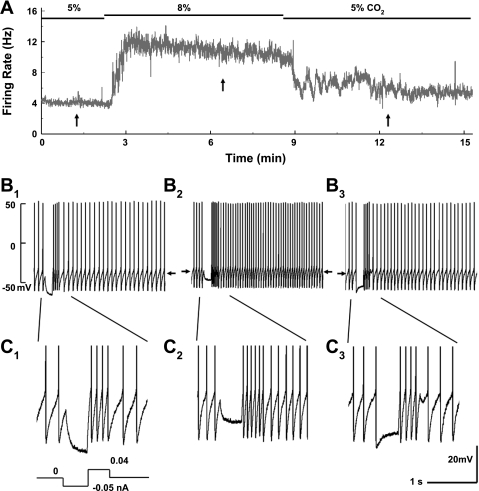
Whole cell current clamp was performed in >200 LC neurons. As shown previously (Johnson et al. 2008; Zhang et al. 2010a), most LC neurons fired spontaneously at the basal condition in the presence of 5% CO2. In this study, we were focused on the LC neurons in which activity was stimulated by hypercapnia. The spontaneous firing rate (FR) of these neurons increased clearly when the CO2 level was raised to 8%, accompanied by depolarization. These neuronal responses were reversible, and both FR and Vm returned to the baseline levels in a few minutes after washout.
A group of LC neurons showed a decrease in Rm with the depolarization.
The hypercapnia-stimulated neurons were not homogenous, as we found that the Rm increased in some neurons but decreased in others during the 8% CO2 exposure. The mechanism for the former neuronal responses is already known, which is produced by the inhibition of Kir channels (Pineda and Aghajanian 1997), whereas the latter or the Kir channel-independent neuronal response remains elusive. Therefore, studies were undertaken to investigate the Kir channel-independent neuronal response. Ba2+ (100 μM) was added to the extracellular solution so that the Kir channels were mostly blocked (Zhu et al. 2000). Under this condition, 56 out of 159 (35%) LC neurons were found to raise their FR by >10% when exposed to 8% CO2 (Fig. 1) compared with 86% without Kir channel blockade as reported previously (Johnson et al. 2008). Detailed data analysis of cellular responses to hypercapnia was performed in 21 LC neurons before and after the treatment with a TRPC blocker (see below). During hypercapnia, the FR of these neurons increased by 10–300% (Fig. 1, A and B), and the Rm was lowered by 10–50% (Fig. 1C). These cells also showed depolarization by a few millivolts (Fig. 1B). The small depolarization was probably due to their high-frequency spontaneous firing activity when the afterhyperpolarizations and the pacemaker depolarization were packed in a narrow interspike interval. When the threshold is reached by either high CO2 or the pacemaker channel activity, action potential is initiated. Thus no further depolarization can take place above the threshold. Quantitatively, we found that the FR of these neurons increased from 3.8 ± 0.3 to 5.9 ± 0.6 Hz (n = 21 cells) or by ∼155% with the exposure to 8% CO2 (Fig. 2A), and the Rm was lowered from 375.2 ± 26.4 to 309.8 ± 19.9 MΩ (n = 21) or by ∼18% (Fig. 2B). Meanwhile, these LC neurons showed depolarization from −45.1 ± 1.1 to −43.0 ± 1.1 mV (n = 21) with the 8% CO2 exposure (Fig. 2C). The changes in FR, Rm, and Vm in these LC neurons suggest the activation of Na+ or the nonselective cationic channels with hypercapnia in the LC neurons rather than the inhibition of K+ channels.
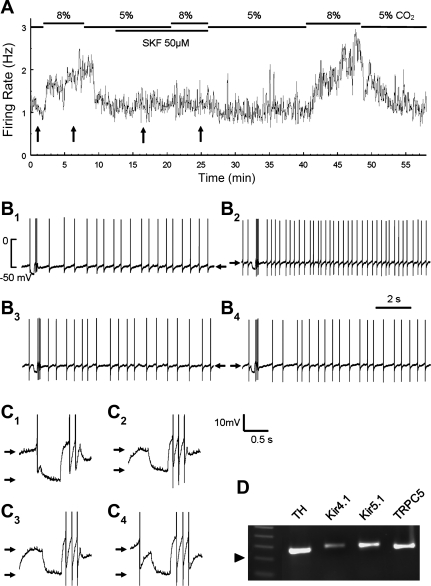
Fig. 1.
Whole cell recordings from locus coeruleus (LC) neurons in brain slice. A: neuronal firing activity was studied in instant-frequency histogram. The cell had a firing rate (FR) of 4 Hz at baseline with 5% CO2. Exposure to 8% CO2 raised the FR markedly. The peak of the FR (∼12 Hz) was reached in 1 min and then declined slightly throughout the 6-min 8% CO2 exposure. Washout with 5% CO2 led to almost complete recovery of the FR in 4–5 min. B: membrane potentials (Vm) and action potentials of the neuron. B1, B2, and B3 were obtained from A at the arrows from left to right, respectively. The Vm of each trace is indicated by the arrows. Hypercapnia augmented the FR and depolarized the cell (B2). The input resistance (Rm) was monitored by injecting a −0.05-nA current pulse every 20 s during the experiment and then calculated with Ohm's law, which is better seen in C with expanded displays. The Rm dropped by ∼50% during the high-CO2 exposure (C2). C1 and C3 are obtained from B1 and B2, respectively.
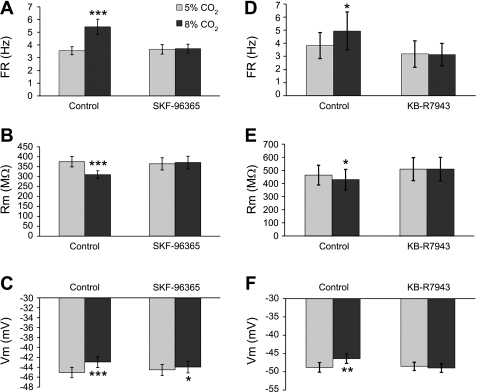
Fig. 2.
A–C: hypercapnia (8% CO2) produced a rise in FR (A), reduction in Rm (B), and depolarization (C). D: summary of the augmentation of neuronal FR by hypercapnia with and without changing extracellular pH (pHo). Two-tailed paired Student's t-test was used for the comparison of each group. All of these changes were statistically significant (**P < 0.01; ***P < 0.001). No significant differences were found between groups with 1-tailed Student's t-test (2-sample assuming equal variances).
Similar changes in the membrane properties were seen with intra- or extracellular acidification.
The effects of hypercapnia on LC neurons are likely to be mediated by the changes in intra- and/or extracellular pH (pHi, pHo) according to previous reports (Hartzler et al. 2008; Pineda and Aghajanian 1997). Therefore, we studied the effects of pHi and pHo on FR, Rm, and Vm of LC neurons. The FR of 6 out of 19 cells rose when the cells were exposed to an acidic perfusate with pHo 7.0, a level that resembled the pH level in the regular perfusate measured after bubbling with 8% CO2 for 30 min. Quantitatively, the FR of these cells was augmented by 127.2 ± 5.6% (n = 6; Fig. 2D). Isohydric hypercapnia (high CO2 without changing pHo) also enhanced the FR in 5 of 13 cells by 155.3 ± 7.4% (n = 5; Fig. 2D). The neuronal response to either treatment was accompanied by a decrease in Rm and depolarization (Supplemental Fig. S1). The effect of extracellular acidifications on neuronal FR was smaller than that of 8% CO2 exposure (127.2 ± 5.6%; Fig. 2D). With the intra- or extracellular acidification, the ratio of acid-stimulated cells vs. total number of cells was not significantly different from that with 8% CO2 alone (P > 0.05, χ2 test). These results thus indicate that hypercapnia seems to affect the LC neurons via both intra- and extracellular acidifications.
Specific TRP channels were expressed in the LC neurons.
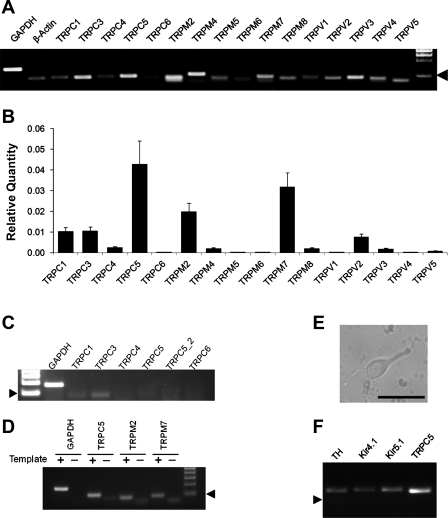
The pH-sensitive TRP channels may play a role in the LC neuronal responses to high CO2 if they are expressed in the LC neurons. Therefore, we screened all representative TRP channels in the LC tissue using real-time qPCR. The mRNA expression of several TRP channels was readily detected in the LC tissue with regular RT-PCR (Fig. 3A). In the qPCR analysis based on 16–20 samples from 5 experiments, TRPC5 was the most abundant TRP channels in the LC area. The expression of TRPC1, TRPC3, TRPM2, TRPM7, and TRPV2 was substantial, although not as high as TRPC5. The mRNAs of other TRP channels were either modest or undetectable (Fig. 3B). Control experiments were performed on the myocardium as previous studies indicate the lack of TRPC5 expression in the cardiac ventricular muscle tissue (Kunert-Keil et al. 2006). Consistently, TRPC5 mRNA was not seen in the heart (Fig. 3C). In the absence of cDNA template, weak primer bands were observed. These fussy bands were greater than primers, suggesting the primer multimerization. In the presence of cDNA templates, strong bands were found (Fig. 3D). These bands of expected sizes for each PCR product cannot be missed with the primer bands because they were clearly larger than the primer bands, and no primer bands were seen in the presence of cDNA templates. To prove the TRPC5 expression in LC neurons, we performed single-cell PCR analysis on cells that were acutely dissociated from the LC nucleus. Single-cell PCR analysis showed that the cells with positive staining for tyrosine hydroxylase (TH) expressed the TRPC5 mRNA as well as Kir4.1 and Kir5.1 mRNAs (Fig. 3, E and F).
Fig. 3.
Expression of transient receptor potential (TRP) channels (TRPC) in LC neurons. A: all representative members in the TRPC, TRPM, and TRPV families were included in the RT-PCR analysis. Several TRP channels were clearly detected in the mRNA level. Arrowhead indicates 100 bp. B: analysis of TRP mRNA expression in the LC tissue with quantitative PCR indicated that the expression of TRPC5, TRPM2, and TRPM7 were severalfold higher than other TRP channels. Data are presented as means ± SE (n = 16–20 samples from 5 experiments). C and D: negative PCR controls. C: expression levels of various TRPC mRNAs in cardiac ventricular muscle. Of representative members in the TRPC families, only TRPC3 and TRPC1 mRNAs were detected in RT-PCR. Note that TRPC5 was studied with 2 different sets of primers, none of which produced a detectable band. Arrowhead indicates 100 bp (n = 3 experiments). D: another negative control for TRPC5, TRPM2, and TRPM7 expressions, the 3 most abundant TRPs in the LC (B). In the absence of template cDNAs, a weak and fuzzy band was found. The sizes of these bands were greater than primers, suggesting primer multimerization. In the presence of cDNA templates, a strong band was produced in each TRP, which was clearly larger than the primer band with expected size. More importantly, none of the primer bands was seen in the presence of cDNA templates. E and F: single-cell PCR analysis on the expression of TRPC5 in neurons. E: a cell acutely dissociated from the LC tissue. Calibration: 50 μm. F: single-cell PCR analysis showed that this cell was a tyrosine hydroxylase (TH)-positive neuron and expressed the TRPC5 mRNA. Inward rectifier K+ 4.1 (Kir4.1) and Kir5.1 mRNAs were also seen in the cell.
TRPC5 was sensitive to extra- and intracellular acidifications.
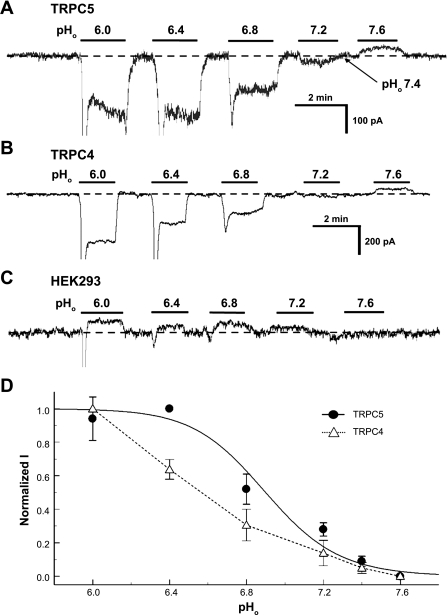
The high level of expression of TRPC5 mRNAs in the LC is interesting because TRPC5 and TRPC4 have been previously reported to be activated and inhibited by acidic pH (Kim et al. 2008; Semtner et al. 2007). Therefore, whether the pH sensitivity allows them to act in neuronal CO2 chemosensitivity is uncertain. If it does, the channels should be activated by moderate intra- or extracellular acidification. To address this issue, we studied the titration properties of TRPC5 and TRPC4. Whole cell currents were recorded from HEK cells expressing either channel in voltage clamp. The cells showed rather small basal inward currents at Vm of −60 mV. The current amplitude of both TRPC5 and TRPC4 increased markedly when the cell was exposed to acidic perfusates with clear pHo dependence (Fig. 4, A and B). TRPC5 was partially open at pHo 7.4. Alkalization to pHo 7.6 inhibited the channel. The maximum channel activation was reached at pHo 6.4. In contrast, the maximum activation of the TRPC4 currents was not reached even at pHo 6.0. The pHo-current relationship was described using the Hill equation for TRPC5, showing pKa 6.9 and the Hill coefficient (nH) 2.4 (Fig. 4D).
Fig. 4.
Sensitivity of TRPC5 and TRPC4 to pHo. The TRPC channels were expressed in human embryonic kidney (HEK) cells. Whole cell currents were studied in voltage clamp with a holding potential of −60 mV. A: small inward currents were seen in an HEK cell transfected with TRPC5 at pHo 7.4. Extracellular acidifications produced pH-dependent activation of the inward currents. B: similar results were obtained from another cell transfected with TRPC4. Note that the maximum channel activation was not reached at pHo 6.0. C: the same pH exposures did not activate the inward currents in a nontransfected HEK cell. Indeed, the inward currents were slightly inhibited during acid exposures, indicating that the acid-activated currents are mediated by the exogenous TRP channels. D: relationships of pHo vs. TRPC currents (I). The TRPC5 currents were described using the Hill equation with pKa 6.9 and Hill coefficient (nH) 2.4. Similar data fitting was not attempted for TRPC4 as the maximum channel activation was not reached. Despite that, the pHo-current relationship was at least 0.4 pH units lower than that of the TRPC5.
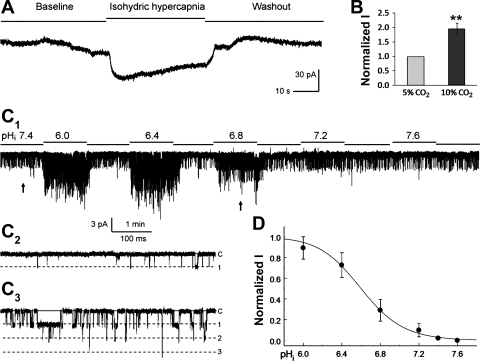
When the cells were exposed to isohydric hypercapnia, the TRPC5 currents were activated (Fig. 5, A and B), suggesting that pHi also affects the channel. Thus we studied the channel in inside-out patches. Concentration-dependent activation of the TRPC5 currents was found (Fig. 5C). The channel activation was mediated by augmentation of the channel open-state probability rather than single-channel conductance. The pHi-current relationship showed pKa 6.6 and nH 2.0 (Fig. 5D). Similar data analysis was not attempted for TRPC4 because of the lack of maximum activation.
Fig. 5.
Activation of TRPC5 by intracellular acidification. A: whole cell currents were studied in the same condition as in Fig. 4. After the expression of the TRPC5 currents was confirmed with acidic pHo, the cell was exposed to a perfusate containing 25 mM HCO3− bubbled with 5% CO2 (baseline). Isohydric hypercapnia (46 mM HCO3− with 10% CO2, pHo 7.4) produced a rapid increase in inward currents. Washout with the same perfusate for baseline recording led to clear recovery of the currents. B: summary of the effect of isohydric hypercapnia on TRPC5 currents (n = 7 cells; **P < 0.01). C: single-channel TRPC5 currents were studied in an inside-out patch with a Vm of −50 mV. Exposures to perfusates with different pH led to concentration-dependent activation of the currents. Note C2 and C3 are obtained from C1 at the arrows from left to right, respectively. D: the relationship of intracellular pH (pHi) vs. currents was described with the Hill equation showing pKa 6.6 and nH 2.0 (n = 5 patches from 5 different cells).
The pHi effect on the TRPC5 currents was Ca2+-dependent. No channel activation was found in a Ca2+-free condition with high concentration of EGTA (10 mM) in inside-out patches. When the free Ca2+ concentration was titrated to 100 nM with EGTA at each pHi level studied, we found that the TRPC5 currents were barely augmented by acidic pHi, suggesting that the pHi sensitivity of TRPC5 is extrinsic to the channel protein, and the channel activation seems to be mediated by the pHi-induced elevation of intracellular Ca2+ instead. Since the levels of pHo and pHi (6.8–7.4) can be reached in several physiological and pathophysiological conditions, these data suggest that TRPC5 seems available for activation during hypercapnia.
The neuronal responses to high CO2 were blocked by SKF-96365.
If the activation of TRPC channels during hypercapnia leads to depolarization and the increase in firing activity of LC neurons, blockade of these channels should diminish or even eliminate the CO2 effects. To test this idea, we studied the LC neuronal responses to 8% CO2 with and without the selective TRPC blocker SKF-96365. Figure 6 shows recordings from an LC neuron. The FR of this cell was ∼1 Hz at baseline and increased to ∼2 Hz with 8% CO2 (Fig. 6, A, B1, and B2). The hypercapnic exposure also led to depolarization (Fig. 6, B1 and B2) and a decrease in Rm (Fig. 6, C1 and C2). After washout with 5% CO2, the FR returned to the baseline level. SKF-96365 (50 μM) was then added to the perfusate. The SKF-96365 exposure did not have much effect on the FR in most cells but reduced FR slightly in some cells. However, the FR response of LC neurons to hypercapnia was almost totally eliminated (Fig. 6, A, B3, and B4). Similarly, the SKF-96365 exposure also blocked the depolarization (Fig. 6, B3 and B4) and the drop in Rm during hypercapnia (Fig. 6, C3 and C4). Another TRPC blocker, KB-R7943 (5 μM) (Kraft 2007; Pezier et al. 2009), had similar effects (Fig. 7, D–F).
Fig. 6.
Blockade of the hypercapnic effects by the selective TRPC inhibitor SKF-96365 (SKF). A: instant-frequency histogram of an LC neuron. A clear increase in FR was seen with the exposure to 8% CO2. The hypercapnic effect was almost totally eliminated after a treatment of the cell with SKF. The augmentation of FR by high CO2 was resumed after washout. B, B1–B4: individual traces of action potentials and Vm (arrow) obtained from places in A pointed by the arrows from the left to the right, respectively. C, C1–C4: the decrease in Rm (between arrows) occurred before but not during SKF treatment. Traces are obtained from B in the same numbers accordingly. D: single-cell PCR analysis indicates that the cell is a TH-positive neuron and expresses the TRPC5 mRNA. The size of the PCR products is ∼250 bp (arrowhead: 200 bp).
Fig. 7.
A–C: effects of the SKF-96365 treatment on electrophysiological properties of LC neurons. Data were obtained from 20 LC neurons in which hypercapnia (8% CO2) was examined before and during the SKF-96365 (50 μM) treatment as shown in Fig. 4A. Whereas the high CO2 exposure produced marked changes in FR, Rm, and Vm (*P < 0.05; ***P < 0.001), no significant changes were found for any of these cellular properties. Note the slight differences in the results of the control group from those in Fig. 2. Data are presented as means ± SE (n = 20 cells). D–F: in the presence of 5 μM KB-R7943, the hypercapnic effects on FR, Rm, and Vm were abolished (*P < 0.05; **P < 0.01; data are presented as means ± SE, n = 5 cells).
After electrophysiological studies, this LC neuron was aspirated into the recording patch pipette, and the cellular materials were then subjected to single-cell PCR to determine whether it expressed TRPC5. The nested PCR analysis showed that the cell was a catecholaminergic neuron as it expressed TH. A clear band of TRPC5 mRNA was seen (Fig. 6D). In addition to TRPC5, the neuron had positive expression of Kir4.1 and Kir5.1. Such a single-cell PCR analysis was done in another four LC neurons, all of which were stimulated by 8% CO2 and showed positive bands of TH and TRPC5. Similar single-cell PCR results were obtained from acutely dissociated LC neurons (Fig. 3, E and F).
Statistical analysis of FR, Rm, and Vm was performed with and without SKF-96365 in 20 LC neurons showing increased FR with 8% CO2. Our results indicated the hypercapnia-induced changes in all of these cellular properties were either greatly diminished or completely abolished (Fig. 7, A–C). The TRPC blocker KB-R7943 (5 μM) had similar effects (Fig. 7, D–F). These results thus strongly suggest that TRPC channels are likely to be activated by hypercapnic acidosis.
The TRPC-dependent neuronal responses to high CO2 persisted after both excitatory and inhibitory synaptic transmissions were blocked.
Of 21 LC neurons studied, 7 cells showed an increase in firing activity with 8% CO2, a ratio that resembled that of hypercapnia-responding cells (P > 0.05, χ2 test). The FR of these cells was augmented by 134.6 ± 2.7% (n = 7) as well as a fall in Rm and depolarization (Supplemental Fig. S2). Four of the cells were also tested with the TRPC inhibitor. With the synaptic blockade [100 μM picrotoxin, 50 μM (6)-2-amino-5-phosphonopentanoic acid, and 10 μM 6-cyano-7-nitroquinoxaline-2,3-dione], 50 μM SKF-96365 remained to suppress the hypercapnic effect on FR (103.0 ± 1.1%, n = 4 with SKF-96365 vs. 130.7 ± 2.9% before, n = 4; P < 0.001). SKF-96365 also had effects on Rm and Vm similar to those without synaptic blockade (Supplemental Fig. S2). These results suggest that the TRPC-dependent cellular responses appear intrinsic to the LC neurons.
DISCUSSION
In addition to the Kir channel-dependent mechanism (Pineda and Aghajanian 1997), activation of cation-permeable channels can also produce depolarization and increase the membrane excitability. After eliminating the Kir channel-mediated excitation, ∼35% LC neurons show an increase in their FR with a decrease in Rm, suggesting that a good number of LC neurons respond to hypercapnia by activation of the cation-permeable channels, although more cells seem to rely on other mechanisms, including Kir channels, for their hypercapnic responses as 86% LC neurons are stimulated by hypercapnic without Kir channel blockade (Johnson et al. 2008).
Several previous studies have demonstrated that the LC neurons are intrinsically sensitive to hypercapnic acidosis (Johnson et al. 2008; Nichols et al. 2008; Oyamada et al. 1998). Consistent with these previous reports, our data have shown that a proportion (7 of 23) of LC neurons is stimulated by 8% CO2 after the majority of excitatory and inhibitory synaptic inputs as well as Kir channels is blocked. The ratio of the hypercapnia-stimulated cells is about the same as that without synaptic blockade during 8% CO2 exposure (56 of 159). Statistically, there is no significant difference between the 2 groups. This as well as the response patterns of FR, Rm, and Vm strongly suggests that neurons in these tests are very likely to be sampled from the same group of cells. The magnitude of neuronal firing response to hypercapnia, however, is slightly lower after synaptic blockade, suggesting that presynaptic signals also contribute to a small fraction of the CO2 chemosensitivity of LC neurons, consistent with several recent studies (Gourine et al. 2005, 2010; Nichols et al. 2008; Su et al. 2007; Yang et al. 2008). Therefore, our results suggest that the cationic channel-dependent mechanism is most likely intrinsic to the LC neurons.
In medullary raphé neurons, nonselective cationic channels are activated with high CO2 exposure augmenting neuronal excitability (Tiwari et al. 2000), although the molecular identity and biophysical properties of the cationic channels are still unknown. In our current studies, we have found that a group of LC neurons show a decrease in Rm with depolarization and increase in firing activity during high CO2 exposure. The effects can be eliminated by the selective TRPC blocker SKF-96365, suggesting that cation-permeable TRPC channels may be involved in the CO2 response of LC neurons. Consistently, we have found several TRP mRNAs in the LC area. One of them is TRPC5 found in the TH-positive neurons dissociated from the LC area. Indeed, our qPCR analysis indicates that TRPC5 is the most abundant TRP mRNAs expressed in the LC. The negative expression of TRPC5 in the heart tissue consistent with the previous studies shows that TRPC5 mRNAs are highly expressed in the LC area (Kunert-Keil et al. 2006). The absence of bands of expected sizes in the negative control experiments without template reassures the absence of contamination from primers.
There are several lines of evidence supporting that TRPC channels are involved in the hypercapnic responses of LC neurons. First, the augmentation of LC neuronal activity is likely to be mediated by both intra- and extracellular acidifications, whereas TRPC5 is activated in this way. Second, although several TRP channels are pH-sensitive, most of them are inhibited by acidic pH (Clapham 2003). TRPC4 and TRPC5 are the only known TRP channels that are activated at pH 7.5–6.5 and inhibited when pH is <6.0 (Kim et al. 2008; Semtner et al. 2007). We have found evidence for the apparent differences in their potential contribution to the hypercapnic responses of LC neurons. Although both TRPC5 and TRPC4 have been shown to be activated by acid pHo, the pHo sensitivity appears to allow TRPC5 but not TRPC4 to be activated by a drop in pHo under the physiological or pathophysiological conditions. Third, the LC neurons are rich in TRPC5 mRNAs but have a very low level of TRPC4 expression. Despite these lines of supporting evidence, it is still unclear how the TRPC channels work in the central CO2 chemosensitivity because both TRPC5 and TRPC4 are expressed in several brain regions in addition to the brain stem (Philipp et al. 1998; Riccio et al. 2002) where neuronal activity does not seem to be modulated by hypercapnia. Since similar situations are seen in TASK channels, voltage-gated K+ (Kv) channels, large-conductance Ca2+-activated K+ (BK) channels, gap junctions, and l-type Ca2+ channels (Putnam 2010), it is possible that the modulation of these channels is subject to other signaling systems such as protein phosphorylation and second messenger systems through surface receptors, affecting the channel availability for activation by acid pH.
The pHo sensitivity appears to be intrinsic to TRPC5 (Semtner et al. 2007). The pHi sensitivity of the channel, however, might be mediated by intracellular Ca2+, as the channel activation is blunted in a Ca2+-free condition or when Ca2+ concentrations are maintained constant at various pH levels. Similarly, firing activity of LC neurons was also significantly reduced in the presence of BAPTA in pipette solution (Supplemental Fig. S3), consistent with the necessity of a rise in intracellular Ca2+. Interestingly, previous studies have also suggested the activation of certain unidentified pH-sensitive cationic channels in raphé neurons depends on intracellular Ca2+ (Tiwari et al. 2000), suggesting that such a mechanism may exist in other chemosensitive areas of the brain stem as well.
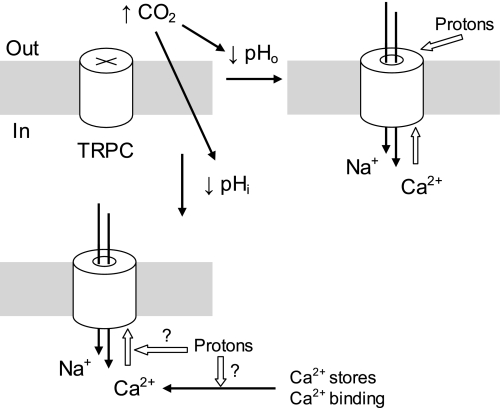
Based on these observations, we have proposed a working hypothesis to explain the channel activation during hypercapnia (Fig. 8). The TRPC channels are partially inactivated at physiological pH. Hypercapnia causes decreases in both pHo and pHi. Whereas the low pHo activates TRPC directly (Semtner et al. 2007), acidic pHi activates the channels in a Ca2+-dependent manner. The latter effect may result from Ca2+ release from intracellular store, Ca2+ dissociation from Ca2+-binding proteins, and/or the enhancement of the channel sensitivity to intracellular Ca2+. Certainly, further studies are needed to pinpoint the molecular mechanisms for the pHi-mediated channel activation using different methodologies such as chimerical construction, mutagenesis, etc. Nevertheless, our results showing the isohydric hypercapnic effect and the Ca2+ dependence of pHi should benefit the studies.
Fig. 8.
Schematics for the hypercapnic effects on TRPC channels. The TRPC channels are mostly closed at physiological pH levels. Both pHo and pHi are reduced with high CO2. The low pHo activates TRPC directly, allowing both Na+ and Ca2+ to enter the cell. The increase in intracellular Ca2+ concentration around the channel protein leads to further opening of TRPC5. Acidic pHi activates TRPC5 in a Ca2+-dependent manner. This effect may result from Ca2+ release from intracellular store, Ca2+ dissociation from Ca2+-binding proteins, or the enhancement of the channel sensitivity to Ca2+. Open arrows indicate stimulation.
The inhibition of K+ channel can increase cellular excitability during hypercapnia. Several putative CO2-chemosensitive K+ channels have been suggested in previous studies. The pH-sensitive Kir channels were first described in LC neurons (Pineda and Aghajanian 1997). Experimental evidence suggests that the hypercapnic responses of several brain stem neurons are mediated by pH- and Ba2+-sensitive Kir channels as well (Mulkey et al. 2004; Schultz et al. 2003). The Kir2.2 channel is expressed in the brain stem, and Kir2.2-null mice show transient impair in their hypercapnic ventilatory response (Oyamada et al. 2005). The Kir4.1-Kir5.1 heteromeric channel is highly sensitive to CO2/pH, allowing cells expressing the channel to be modulated by both hypercapnia and hypocapnia (Pessia et al. 2001; Xu et al. 2000). Both Kir4.1 and Kir5.1 are expressed in brain stem neurons, including the LC (Wu et al. 2004). They are also expressed in glial cells where they play a role in regulating pH-dependent K+ buffering and recycling (Neusch et al. 2006). The leak-conductance two-pore domain TASK-1 channels are expressed in hypoglossal motoneurons and midbrain raphé neurons (Talley et al. 2000; Washburn et al. 2002). However, the hypercapnic response is rather normal in TASK-knockout mice in vivo (Mulkey et al. 2007), suggesting that either function of the channels is compensated by others, or they do not play a critical role in CO2 chemosensitivity. Interestingly, the Ca2+-activated K+ channels may be activated during hypercapnic acidosis, whereas the functional significance of the channel activation is still unclear (Putnam et al. 2004).
Acid-sensing ion channels (ASICs) are also expressed in the mammalian nervous system. They are generally closed at physiological pH. The open probability of the ASICs increases when the pH drops with pKa ∼6.2 (Simard et al. 2007). Since such a low pH level may not be reached even during severe hypercapnia, whether and how the ASICs are involved in central CO2 chemosensitivity is still unknown. Moreover, Kv and gap junction channels may also play a role in neuronal responses to intra- and/or extracellular acidification (Putnam 2010), such as shaping action potentials and cell-to-cell communications.
The existence of multiple pH-sensitive ion channels may be attributed to different functional needs in neuronal responses to hypercapnia. Some of the pH-sensitive ion channels regulate Vm, and some shape action potentials. Also, neurons may respond to a range of changes in pHo and pHi. The distinct pH sensitivity of these channels may allow each to respond to the pH change more effectively than a single or a few sensors when a modest change occurs at a given pH level (Jiang et al. 2005).
In conclusion, a group of LC neurons depolarizes, lowers their Rm, and fires more action potentials in response to moderate hypercapnia. Such cellular responses can be blocked by specific TRPC inhibitor. Consistent with the involvement of TRP channels, several TRP channels are expressed in the LC at the mRNA level, with TRPC5 the most abundant. The TRPC5 currents are activated by extracellular acidification with the pKa value in the physiological pH range. The channel is also activated by intracellular acidosis in a Ca2+-dependent manner. Since the activation of the TRP channels can produce depolarization and augment membrane excitability with a decrease in Rm, it is very likely that the modulation of the pH-sensitive TRP channels by CO2 and acidic pH may contribute to the CO2 response of a good number of LC neurons.
GRANTS
This work was supported by NIH Grant HD-060959 and the American Heart Association Grant 09GRNT2010037. X. Zhang was a fellow of the Molecular Basis of Disease Program of Georgia State University.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
REFERENCES
- Ballantyne and Scheid, 2000. Ballantyne D, Scheid P. Mammalian brainstem chemosensitive neurones: linking them to respiration in vitro. J Physiol 525: 567–577, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biancardi et al., 2008. Biancardi V, Bicego KC, Almeida MC, Gargaglioni LH. Locus coeruleus noradrenergic neurons and CO2 drive to breathing. Pflügers Arch 455: 1119–1128, 2008 [DOI] [PubMed] [Google Scholar]
- Clapham, 2003. Clapham DE. TRP channels as cellular sensors. Nature 426: 517–524, 2003 [DOI] [PubMed] [Google Scholar]
- Doi and Ramirez, 2010. Doi A, Ramirez JM. State-dependent interactions between excitatory neuromodulators in the neuronal control of breathing. J Neurosci 30: 8251–8262, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlichman et al., 2009. Erlichman JS, Boyer AC, Reagan P, Putnam RW, Ritucci NA, Leiter JC. Chemosensory responses to CO2 in multiple brain stem nuclei determined using a voltage-sensitive dye in brain slices from rats. J Neurophysiol 102: 1577–1590, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargaglioni et al., 2010. Gargaglioni LH, Hartzler LK, Putnam RW. The locus coeruleus and central chemosensitivity. Respir Physiol Neurobiol 173: 264–273, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourine et al., 2005. Gourine AV, Llaudet E, Dale N, Spyer KM. ATP is a mediator of chemosensory transduction in the central nervous system. Nature 436: 108–111, 2005 [DOI] [PubMed] [Google Scholar]
- Gourine et al., 2010. Gourine AV, Kasymov V, Marina N, Tang F, Figueiredo MF, Lane S, Teschemacher AG, Spyer KM, Deisseroth K, Kasparov S. Astrocytes control breathing through pH-dependent release of ATP. Science 329: 571–575, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzler et al., 2008. Hartzler LK, Dean JB, Putnam RW. The chemosensitive response of neurons from the locus coeruleus (LC) to hypercapnic acidosis with clamped intracellular pH. Adv Exp Med Biol 605: 333–337, 2008 [DOI] [PubMed] [Google Scholar]
- Jiang et al., 2005. Jiang C, Rojas A, Wang R, Wang X. CO2 central chemosensitivity: why are there so many sensing molecules? Respir Physiol Neurobiol 145: 115–126, 2005 [DOI] [PubMed] [Google Scholar]
- Johnson et al., 2008. Johnson SM, Haxhiu MA, Richerson GB. GFP-expressing locus ceruleus neurons from Prp57 transgenic mice exhibit CO2/H+ responses in primary cell culture. J Appl Physiol 105: 1301–1311, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim et al., 2008. Kim MJ, Jeon JP, Kim HJ, Kim BJ, Lee YM, Choe H, Jeon JH, Kim SJ, So I. Molecular determinant of sensing extracellular pH in classical transient receptor potential channel 5. Biochem Biophys Res Commun 365: 239–245, 2008 [DOI] [PubMed] [Google Scholar]
- Kraft, 2007. Kraft R. The Na+/Ca2+ exchange inhibitor KB-R7943 potently blocks TRPC channels. Biochem Biophys Res Commun 361: 230–236, 2007 [DOI] [PubMed] [Google Scholar]
- Kunert-Keil et al., 2006. Kunert-Keil C, Bisping F, Kruger J, Brinkmeier H. Tissue-specific expression of TRP channel genes in the mouse and its variation in three different mouse strains. BMC Genomics 7: 159–166, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey et al., 2004. Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA, Guyenet PG. Respiratory control by ventral surface chemoreceptor neurons in rats. Nat Neurosci 7: 1360–1369, 2004 [DOI] [PubMed] [Google Scholar]
- Mulkey et al., 2007. Mulkey DK, Talley EM, Stornetta RL, Siegel AR, West GH, Chen X, Sen N, Mistry AM, Guyenet PG, Bayliss DA. TASK channels determine pH sensitivity in select respiratory neurons but do not contribute to central respiratory chemosensitivity. J Neurosci 27: 14049–14058, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neusch et al., 2006. Neusch C, Papadopoulos N, Müller M, Maletzki I, Winter SM, Hirrlinger J, Handschuh M, Bähr M, Richter DW, Kirchhoff F, Hülsmann S. Lack of the Kir4.1 channel subunit abolishes K+ buffering properties of astrocytes in the ventral respiratory group: impact on extracellular K+ regulation. J Neurophysiol 95: 1843–1852, 2006 [DOI] [PubMed] [Google Scholar]
- Nichols et al., 2008. Nichols NL, Hartzler LK, Conrad SC, Dean JB, Putnam RW. Intrinsic chemosensitivity of individual nucleus tractus solitarius (NTS) and locus coeruleus (LC) neurons from neonatal rats. Adv Exp Med Biol 605: 348–352, 2008 [DOI] [PubMed] [Google Scholar]
- Oyamada et al., 1998. Oyamada Y, Ballantyne D, Muckenhoff K, Scheid P. Respiration-modulated membrane potential and chemosensitivity of locus coeruleus neurones in the in vitro brainstem-spinal cord of the neonatal rat. J Physiol 513: 381–398, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyamada et al., 2005. Oyamada Y, Yamaguchi K, Murai M, Hakuno H, Ishizaka A. Role of Kir2.2 in hypercapnic ventilatory response during postnatal development of mouse. Respir Physiol Neurobiol 145: 143–151, 2005 [DOI] [PubMed] [Google Scholar]
- Pessia et al., 2001. Pessia M, Imbrici P, D'Adamo MC, Salvatore L, Tucker SJ. Differential pH sensitivity of Kir4.1 and Kir4.2 potassium channels and their modulation by heteropolymerisation with Kir5.1. J Physiol 532: 359–367, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezier et al., 2009. Pezier A, Bobkov YV, Ache BW. The Na+/Ca2+ exchanger inhibitor, KB-R7943, blocks a nonselective cation channel implicated in chemosensory transduction. J Neurophysiol 101: 1151–1159, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipp et al., 1998. Philipp S, Hambrecht J, Braslavski L, Schroth G, Freichel M, Murakami M, Cavalie A, Flockerzi V. A novel capacitative calcium entry channel expressed in excitable cells. EMBO J 17: 4274–4282, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineda and Aghajanian, 1997. Pineda J, Aghajanian GK. Carbon dioxide regulates the tonic activity of locus coeruleus neurons by modulating a proton- and polyamine-sensitive inward rectifier potassium current. Neuroscience 77: 723–743, 1997 [DOI] [PubMed] [Google Scholar]
- Putnam et al., 2004. Putnam RW, Filosa JA, Ritucci NA. Cellular mechanisms involved in CO2 and acid signaling in chemosensitive neurons. Am J Physiol Cell Physiol 287: C1493–C1526, 2004 [DOI] [PubMed] [Google Scholar]
- Putnam, 2010. Putnam RW. CO2 chemoreception in cardiorespiratory control. J Appl Physiol 108: 1796–1802, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radulovacki and Carley, 2003. Radulovacki M, Carley WD. The laboratory rat model of sleep-related breathing disorders. In: Sleep-Related Breathing Disorders: Experimental Models and Therapeutic Potential, edited by Carley WD, Radulovacki M. New York: Dekker, 2003, p. 265– 1796–288 [Google Scholar]
- Riccio et al., 2002. Riccio A, Medhurst AD, Mattei C, Kelsell RE, Calver AR, Randall AD, Benham CD, Pangalos MN. mRNA distribution analysis of human TRPC family in CNS and peripheral tissues. Brain Res Mol Brain Res 109: 95–104, 2002 [DOI] [PubMed] [Google Scholar]
- Richerson, 2004. Richerson GB. Serotonergic neurons as carbon dioxide sensors that maintain pH homeostasis. Nat Rev Neurosci 5: 449–461, 2004 [DOI] [PubMed] [Google Scholar]
- Saponjic et al., 2005. Saponjic J, Cvorovic J, Radulovacki M, Carley DW. Serotonin and noradrenaline modulate respiratory pattern disturbances evoked by glutamate injection into the pedunculopontine tegmentum of anesthetized rats. Sleep 28: 560–570, 2005 [DOI] [PubMed] [Google Scholar]
- Saponjic et al., 2007. Saponjic J, Radulovacki M, Carley DW. Monoaminergic system lesions increase post-sigh respiratory pattern disturbance during sleep in rats. Physiol Behav 90: 1–10, 2007 [DOI] [PubMed] [Google Scholar]
- Schultz et al., 2003. Schultz JH, Czachurski J, Volk T, Ehmke H, Seller H. Central sympathetic chemosensitivity and Kir1 potassium channels in the cat. Brain Res 963: 113–120, 2003 [DOI] [PubMed] [Google Scholar]
- Semtner et al., 2007. Semtner M, Schaefer M, Pinkenburg O, Plant TD. Potentiation of TRPC5 by protons. J Biol Chem 282: 33868–33878, 2007 [DOI] [PubMed] [Google Scholar]
- Simard et al., 2007. Simard JM, Tarasov KV, Gerzanich V. Non-selective cation channels, transient receptor potential channels and ischemic stroke. Biochim Biophys Acta 1772: 947–957, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stunden et al., 2001. Stunden CE, Filosa JA, Garcia AJ, Dean JB, Putnam RW. Development of in vivo ventilatory and single chemosensitive neuron responses to hypercapnia in rats. Respir Physiol 127: 135–155, 2001 [DOI] [PubMed] [Google Scholar]
- Su et al., 2007. Su J, Yang L, Zhang X, Rojas A, Shi Y, Jiang C. High CO2 chemosensitivity versus wide sensing spectrum: a paradoxical problem and its solutions in cultured brainstem neurons. J Physiol 578: 831–841, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talley et al., 2000. Talley EM, Lei Q, Sirois JE, Bayliss DA. TASK-1, a two-pore domain K+ channel, is modulated by multiple neurotransmitters in motoneurons. Neuron 25: 399–410, 2000 [DOI] [PubMed] [Google Scholar]
- Tiwari et al., 2000. Tiwari JK, Zaykin AV, Cruadhlaoich MI, Wang W, Richerson GB. A novel pH sensitive cation current is present in putative central chemoreceptors of the medullary raphe (Abstract). Soc Neurosci Abstr 26: 423, 2000 [Google Scholar]
- Washburn et al., 2002. Washburn CP, Sirois JE, Talley EM, Guyenet PG, Bayliss DA. Serotonergic raphe neurons express TASK channel transcripts and a TASK-like pH- and halothane-sensitive K+ conductance. J Neurosci 22: 1256–1265, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu et al., 2004. Wu J, Xu H, Shen W, Jiang C. Expression and coexpression of CO2-sensitive Kir channels in brainstem neurons of rats. J Membr Biol 197: 179–191, 2004 [DOI] [PubMed] [Google Scholar]
- Xu et al., 2000. Xu H, Cui N, Yang Z, Qu Z, Jiang C. Modulation of Kir4.1 and Kir5.1 by hypercapnia and intracellular acidosis. J Physiol 524: 725–735, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang et al., 2008. Yang L, Su J, Zhang X, Jiang C. Hypercapnia modulates synaptic interaction of cultured brainstem neurons. Respir Physiol Neurobiol 160: 147–159, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al., 2010a. Zhang X, Cui N, Wu Z, Su J, Tadepalli JS, Sekizar S, Jiang C. Intrinsic membrane properties of locus coeruleus neurons in Mecp2-null mice. Am J Physiol Cell Physiol 298: C635–C646, 2010a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al., 2010b. Zhang X, Su J, Rojas A, Jiang C. Pontine norepinephrine defects in Mecp2-null mice involve deficient expression of dopamine beta-hydroxylase but not a loss of catecholaminergic neurons. Biochem Biophys Res Commun 394: 285–290, 2010b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu et al., 2000. Zhu G, Liu C, Qu Z, Chanchevalap S, Xu H, Jiang C. CO2 inhibits specific inward rectifier K+ channels by decreases in intra- and extracellular pH. J Cell Physiol 183: 53–64, 2000 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.