Abstract
Invasive microelectrode recordings measure neuronal spikes, which are commonly considered inaccessible through standard surface electroencephalogram (EEG). Yet high-frequency EEG potentials (hf-EEG, f > 400 Hz) found in somatosensory evoked potentials of primates may reflect the mean population spike responses of coactivated cortical neurons. Since cortical responses to electrical nerve stimulation vary strongly from trial to trial, we investigated whether the hf-EEG signal can also echo single-trial variability observed at the single-unit level. We recorded extracellular single-unit activity in the primary somatosensory cortex of behaving macaque monkeys and identified variable spike burst responses following peripheral stimulation. Each of these responses was classified according to the timing of its spike constituents, conforming to one of a discrete set of spike patterns. We here show that these spike patterns are accompanied by variations in the concomitant epidural hf-EEG. These variations cannot be explained by fluctuating stimulus efficacy, suggesting that they were generated within the thalamocortical network. As high-frequency EEG signals can also be reliably recorded from the scalp of human subjects, they may provide a noninvasive window on fluctuating cortical spike activity in humans.
Keywords: electroencephalogram, somatosensory cortex, spike patterns, single-unit activity
eight decades after Berger (1929) first described the electroencephalogram (EEG), this noninvasive measure still serves as the main tool for recording human brain activity at high temporal resolution. However, standard EEG recordings (f < 100 Hz) primarily reflect mass postsynaptic potentials rather than spikes, which are the basic output of cortical computations. Since not all synaptic inputs lead to the initiation of action potentials, measurements of summed postsynaptic potentials alone cannot show the net computational effect on neuronal output. Standard EEG methods do not, therefore, provide definitive conclusions about the contribution of neuromodulatory, feedforward, and feedback connections to neural processing, and may even confound excitation and inhibition (Speckmann and Elger 2004).
Critically, noninvasive EEG recordings also contain signals at a high-frequency range (f > 400 Hz), which may offer the temporal resolution required to catch short-lived action potentials; unfortunately, such signals are usually much lower in amplitude than the summed slow potentials related to postsynaptic activity (Buzsaki and Draguhn 2004). High-frequency EEG components have, therefore, at times been neglected or even regarded as noise. However, averaging of scalp EEG responses over repeated electrical stimulations of a peripheral nerve can reveal a distinct high-frequency burst of EEG oscillations (hf-EEG; f > 400 Hz) superimposed on the much larger primary postsynaptic response (Cracco and Cracco 1976). Generators of both burst and postsynaptic responses have been localized to the primary somatosensory cortex (Curio et al. 1994; Hashimoto et al. 1996; Shimazu et al. 2000). High-frequency oscillations have been also described in other brain areas (Bragin et al. 1999; Funke and Kerscher 2000; Hanajima et al. 2004; Jones and Barth 1999) following both direct nerve and natural sensory stimulation, or emerging spontaneously prior to epileptic discharges (Jirsch et al. 2006).
The cellular substrates of this hf-EEG in primates have been previously investigated by invasive extracellular recordings of single-unit activity in somatosensory cortex. Neurons localized in cortical area 3b respond to median nerve stimulation either with a burst of two to four spikes separated by very short intervals or with a single spike at one of preferred latencies (Baker et al. 2003). The timing of spikes generated by both types of neuronal responses has a close relation to the hf-EEG: the peaks of the population peristimulus time histogram (PSTH) calculated from those responses align with peaks of the averaged hf-EEG (Baker et al. 2003). Mechanistically, this coupling between average single-cell and hf-EEG responses suggests that surface hf-EEG components reflect either synchronous action potentials of cortical neurons or ultrafast postsynaptic potentials (Baker et al. 2003; Barth 2003; Curio et al. 1994; Hashimoto et al. 1996; Shimazu et al. 2000; Stern et al. 1992).
In light of these findings, high-frequency EEG is the only currently available noninvasive measure of cortical neuronal spiking in human subjects. How close can it bring us to the spike response characteristics observed at the single-neuron level? To approach this topic, we studied whether and how the observed trial-to-trial variability in the timing of single-neuron spike bursts (Baker et al. 2003) is reflected in the concomitant surface hf-EEG. If indeed the macroscopic hf-EEG reflects the timing of underlying neuronal activity, there should be a significant covariation between single-cell spike burst patterns and concomitant hf-EEG. In the present study we demonstrate this to be the case, validating hf-EEG as a noninvasive probe for fluctuating cortical spike output.
MATERIALS AND METHODS
Experimental protocol.
Neuronal responses were evoked in the hand representation of the primary somatosensory cortex of two awake Macaca mulatta monkeys by electrical median nerve stimulation at the wrist (pulse width: 0.2 ms; repetition rate: 3 Hz; intensity: 150% motor threshold). Single-unit activity was recorded extracellularly with a 16-channel “Eckhorn” drive (Thomas Recording, Giessen, Germany; Mountcastle et al. 1991). Each of the platinum/glass electrodes (electrode impedance: 1 MΩ) was advanced into the cortex (area 3b) until a well-isolated neuron was found at one of the electrodes. The receptive field of this cell was tested by means of manual tapping with a stylus. The local macro-EEG was measured with a bipolar ball electrode placed epidurally over the central sulcus. The precise position varied from session to session, but the electrodes always spanned the posterior and anterior edges of the sulcus. Another macroelectrode was implanted in the pyramidal tract at the brain stem level, which, because of its proximity to the medial lemniscus, could be used to monitor subcortical input variations. The location of the brain stem electrode in the pyramidal tract was confirmed during surgery by the presence of an antidromic field potential recorded from the surface of the motor cortex and at postmortem by histology.
All experimental procedures were performed under appropriate licenses issued by the UK Home Office under the Animals (Scientific Procedures) Act (1986), and were also approved by the local ethical committee. Full details of the surgical protocol can be found in Baker et al. (2001).
Data preprocessing.
Field recordings (epidural cortical EEG, brain stem) were band-pass filtered (3 Hz–2 kHz) and sampled with a frequency of 5 kHz (monkey A) or 6 kHz (monkey B). The hf-EEG signals were separated from the wideband response with an acausal band-pass filter (finite impulse response, order 200, Hamming window, cutoff 450–1,100 Hz, roll-off 8 dB/decade, attenuation in stop-band 29.1 dB).
Signal-to-noise ratio.
To assess the quality of hf-EEG recordings the average signal-to-noise ratio (SNRavg) was estimated as the ratio between root mean square (RMS) amplitudes of mean signal and noise. The signal RMS was calculated in a time window aligned to the hf-EEG burst averaged over all trials (6–13 ms poststimulus); the noise RMS was calculated from single-trial activity in a later window that did not contain the hf-EEG burst (200–250 ms) and then averaged across all trials. The estimated SNR averaged over all sessions was 0.83 ± 0.10 (mean ± SE). For subsequent analysis, only recordings with SNR > 0.9 (60% of original recordings) were selected, allowing for a reliable identification of the hf-EEG burst in averages with a low number of trials (n > 10). The SNRavg of this reduced set of recordings was 1.55 ± 0.13 (mean ± SE).
The trial averaging procedure used in the estimation of signal RMS removes any contribution of induced high-frequency components that are not locked to the stimulus onset. Therefore, we also estimated the single-trial SNR (SNRst) by dividing single-trial RMS in the burst window by RMS in the noise window of the same trial and averaging these ratios across trials (see also Supplemental Material).1 Mean SNRst across the cells selected for further analysis was estimated to 2.15 ± 0.11 (mean ± SE), suggesting that non-phase-locked components also contribute to hf-EEG response (Supplemental Fig. S1). These components will be addressed in an upcoming paper (Waterstraat, Telenczuk, Burgoff, Scheer, Curio, manuscript in preparation).
Spike discrimination.
Spike waveforms were first band-pass filtered (1 kHz–10 kHz) and then sampled with a frequency of 24 kHz. Action potentials of neurons surrounding the microelectrode were detected in the extracellular recordings by means of amplitude thresholding; the threshold was chosen manually to detect spikes whose amplitude was significantly above noise level. The waveforms of the detected action potentials were parameterized by their amplitude, width, and projection coefficients on two main principal components. The spike timings of single units were determined based on these shape features with a manual cluster-cutting method that allowed for identification of clusters of arbitrary shapes (Hazan et al. 2006; Lewicki 1998). To ensure correct clustering the procedure was performed by two operators using different software packages (GetSpike, S. N. Baker; PySpikeSort, B. Telenczuk) and then checked for consistency.
To validate the spike discrimination we checked the extracellular action potentials generated by a putative single cell for the consistency of the waveform and amplitude. Additionally, we searched for the interspike intervals (ISIs) shorter than 1 ms; if such short intervals were found the clustering procedure was repeated. Spike trains with evidence of poor spike sorting (inconsistent waveforms or ISIs < 1 ms) were excluded from subsequent analysis.
The quality of spike sorting was evaluated by means of spike SNR (SNRspk) and an “isolation score.” The SNRspk was calculated as the peak-to-peak amplitude of the spike waveform averaged across all spikes divided by the three standard deviations of concatenated residuals obtained after subtracting the average from the individual spike waveforms. The mean SNRspk of spikes from a data set evaluated in the present study (see below) was 2.66 ± 0.85 (mean ± SD, range 1.09–5.04). This measure estimates the amplitude of the spike relative to the noise floor, but it is not sensitive to spike sorting errors (spike omissions). Therefore, in addition, we calculated a spike “isolation score,” which evaluates how well the spikes are discriminated (Joshua et al. 2007). This score estimated the probability that an event classified as a spike belonged to the spike cluster of a single unit as opposed to the background activity. The background activity containing noise and spikes of other cells was extracted by amplitude thresholding of the raw microelectrode record. To obtain a conservative estimate of the background activity the waveforms of only the 2% of spikes with smallest amplitude were first averaged, then the peak amplitude of the average was calculated, and the threshold was set to half of its value. For perfectly isolated cells the spike isolation score takes a value of 1, whereas in the case of complete overlap between background activity and spike cluster it will be smaller than 0.5. According to an evaluation of this “isolation score” on simulated data (not shown), a value > 0.9 corresponds to spike discrimination with <5% of errors. In addition, the “isolation score” calculated between spike waveforms elicited by two different cells recorded in independent penetrations was found equal to 1. The average isolation score of the single-cell spike trains analyzed in the present study (see below) was 0.958 ± 0.041 (mean ± SD, range 0.825–0.998; see also Supplemental Table S1).
Spike pattern classification.
After spike sorting, a total of 46 cells were identified in both monkeys. From this data set only those cells that responded with bursts of spikes separated by short ISIs were taken for the subsequent analysis of spike pattern variability. The receptive fields of the cells were identified to lie within the lateral part of the palm and palmar surface of thumb, index, and ring finger, i.e., in the territory innervated by the stimulated median nerve. Cells were classified as bursting based on two criteria: a response with more than one spike following at least 4% of stimuli, and a mode of the ISI histogram shorter than 1.8 ms. In the complete data set responses of 17 cells (14 and 3 in each monkey, respectively) fulfilled these stringent criteria.
After summing responses from these bursting cells over all trials, prominent peaks were identified in the PSTH (bin width 0.2 ms; Fig. 1C). As the within-burst spike composition varied from trial to trial, each trial was described with a binary string whose entries (1 or 0) represented the occurrence or nonoccurrence of a spike in a sequence of bins bracketing the major peaks of the overall PSTH: the borders between the bins were placed manually in the troughs of the PSTH (Fig. 2, B and C, vertical lines). Each string corresponded to one spike pattern; the length of the string equaled the total number of peaks in the PSTH (depending on the cell: 1–4 digits; cf. Supplemental Table S1). The hf-EEG signals measured simultaneously with the cellular spike bursts (6–13 ms after stimulus; cf. Fig. 1B) were averaged separately over trial sets defined by the neuronal spike patterns. To obtain a sufficient SNR for reliable identification of hf-EEG in these averages, only spike patterns that were found in at least 10 trials were further analyzed.
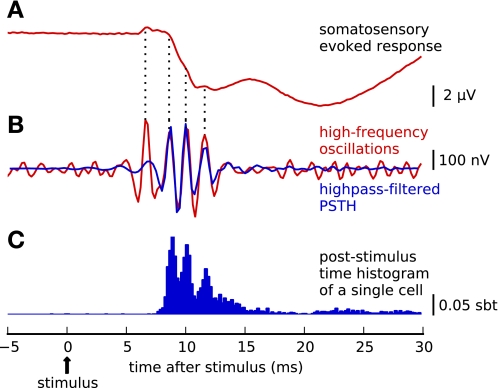
Fig. 1.
Neural responses to electrical median nerve stimulation. A: evoked EEG potentials recorded epidurally over the central sulcus (average of 956 trials, band pass 3 Hz–2 kHz). The primary somatosensory evoked response consists of slow potential deflections (peaking at ∼10 ms) related to postsynaptic activity, on top of which small-amplitude ripples are superimposed. B: application of a high-pass filter (450–1,100 Hz) reveals that the ripples form a short train of high-frequency oscillations (hf-EEG, red line; note change of amplitude scaling). PSTH, poststimulus time histogram. C: PSTH of single-neuron spike responses recorded simultaneously with hf-EEG in somatosensory cortex (normalized PSTH of a sample cell, blue bars; sbt, spikes per bin per trial). Single neurons respond to the stimulation with a burst of spikes that, after trial averaging, sum into a multipeaked PSTH. Peaks of the PSTH align to the peaks of hf-EEG, which becomes more apparent when the high-pass filtered PSTH (B, blue line) is superimposed on the hf-EEG waveform (B, red line).
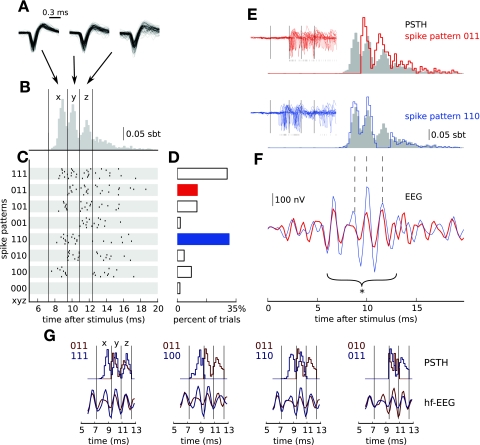
Fig. 2.
Variable single-unit responses in primary somatosensory cortex can be classified into a reduced set of spike patterns. A: extracellular spike waveforms are consistent across multiple repetitions of the stimulus (superimposed traces of 100 spikes) and 3 positions within a burst (each panel displays superimposed spikes from 1 particular latency window delimited in B and indicated by the arrow), indicating that they all were generated by the same cell. B: single-cell responses averaged over all trials (PSTH, same as Fig. 1C; sbt, spikes per bin per trial) reveal that spikes occur preferentially at discrete latencies (delimited by vertical lines). C: in single trials multiple spikes are elicited in diverse combinations of preferred latencies resulting in significant trial-to-trial response variability. Spike combinations are classified into spike patterns: Each trial is assigned a binary string (spike pattern “xyz” from “000” to “111”) whose entries (0 or 1) represent the (non-)occurrence of a spike in a sequence of 3 bins bracketing the peaks of the overall PSTH. Spike timings of 8 representative sample responses assigned to each pattern are shown as raster plots. D: frequency at which the spike patterns occurred over repeated trials (patterns shown in blue and red are further analyzed in E and F). E: normalized subgroup PSTH of 2 sample spike patterns highlighted in D (red: pattern 011; blue: pattern 110; gray bars: all trials). As expected, spike activity is visible only in windows corresponding to the 1s in the binary string. Similarly, raw microelectrode voltage traces (insets; 100 sample traces, ticks represent timings of identified action potentials) do not contain action potentials in the remaining “empty” windows, confirming that the observed spike pattern variability was not due to spike sorting errors (omissions). F: trial-to-trial variability of single-cell activity is associated with differences in the high-frequency surface EEG recordings: Mean hf-EEG wavelets (red: 114 trials, blue: 293 trials) concomitant with the 2 different spike patterns (same color code as in D and E) differ significantly with respect to their root mean square (RMS) amplitude (calculated over the bracket interval; *P < 0.05, see materials and methods). The main differences between the waveforms are localized at the peaks of hf-EEG coincident with the major upstrokes of single-cell activity (dashed lines delineate the positions of PSTH peaks). Notably, the first hf-EEG peak at 7 ms is almost identical, reflecting comparable thalamocortical input (Ikeda et al. 2002). G: comparison of normalized PSTHs (top) and corresponding hf-EEG averages (bottom) for all pairs of spike patterns (color-coded labels in the top left corner of each subplot) that yielded significant hf-EEG RMS differences for this particular cell.
Comparison of hf-EEG wavelets.
The spike pattern-related hf-EEG averages were compared by means of a RMS measure. First, hf-EEG RMS values were calculated separately from each averaged hf-EEG waveform in the interval covering the entire wavelet (6–13 ms after stimulus onset, cf. bracket in Fig. 2F). The resulting spread of RMS values reflects the overall hf-EEG amplitude variability related to spike pattern variability. Next, these hf-EEG RMS amplitudes related to different spike patterns were compared pairwise; the significance of the calculated differences was tested by means of a nonparametric bootstrap test (Efron and Tibshirani 1994). This was implemented by counting how often surrogate hf-EEG RMS differences (calculated after randomly shuffling the trials between patterns) were greater than the original difference. The fraction of such cases in n = 1,000 random shuffles was taken as the significance level (p value). As multiple comparisons were performed, the significance level was corrected for each cell separately with the false discovery rate (FDR, q value) method (Benjamini and Hochberg 1995).
Detailed information on the number of spike patterns detected in all cells and the number of independent comparisons is available in the Supplemental Material for this article (Supplemental Table S1).
Variance explained by spike patterns.
We estimated the fraction of the total hf-EEG response variance that could be explained by the spike pattern-related differences. This fraction was calculated as variance in the set of RMS values that were calculated from selective hf-EEG averages related to different spike patterns (cf. previous paragraph) divided by the total variance in single-trial hf-EEG RMS values calculated in the same time window.
Correction for subcortical input variations.
We tested how much the identified hf-EEG RMS differences might relate to changes in the brain stem responses to the stimulus, which could occur because of changing stimulus efficacy in the periphery or neural gating of the responses at the brain stem level. All trials were ranked based on the single-trial brain stem hf-EEG power and then partitioned into 20 subsets each containing 50 trials. From these data, we obtained a recruitment relation between the RMS values of subset-averaged brain stem responses (time window 3–7 ms poststimulus) and the corresponding epidural cortical hf-EEG responses. This relation was fitted with a polynomial (degree 3). Then, for each pair of spike patterns, we determined the corresponding brain stem responses and used the polynomial fit to calculate an hf-EEG RMS difference expected from the brain stem responses. This was subtracted from the measured epidural hf-EEG RMS difference, yielding the component of the difference that was unlikely to be explained solely by differences in brain stem responsiveness (Fig. 3C). Finally, we tested the significance of the residuals using the bootstrap test described in the previous paragraph, for which surrogate RMS values were constructed for both hf-EEG and corresponding brain stem responses.
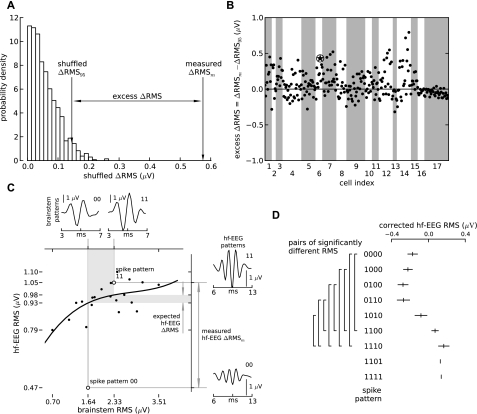
Fig. 3.
The difference between hf-EEG RMS amplitudes associated with different spike patterns is significantly larger than the difference between random groups of trials. A: the distribution of RMS amplitude differences between spike pattern-related hf-EEG waveforms after randomly shuffling the trials (see materials and methods) in an example cell. The RMS difference between the spike patterns before shuffling (measured ΔRMSm) is larger than the 95th percentile of the RMS differences calculated from random shuffles (shuffled ΔRMS95). B: the excess of the measured ΔRMS over the critical value ΔRMS95 (excess ΔRMS) for all other identified pattern pairs pooled over all analyzed 17 cells (dots; the encircled star denotes the sample pair shown in A). Gray and white stripes delimit spike pattern pairs identified in different cells. A sizeable fraction of points (100 of 188; 53%) lie above the zero line, which delimits the significant differences from insignificant ones (P < 0.05, no correction for multiple comparisons; see results for corrected values). C: correction of the measured ΔRMS for subcortical input variations. Two sample spike patterns (open circles in main panel) were different not only in terms of cortical (sample hf-EEG traces shown in right insets) but also subcortical (sample brain stem traces shown in top insets) responses. To correct for the subcortical variations, the relation between RMS power of brain stem response and hf-EEG response (dots) was first fitted with a 3rd-order polynomial (recruitment curve; main panel, solid line). The recruitment curve was used to transform the difference in RMS of brain stem activations related to 2 different spike patterns (main panel, vertical part of gray-shaded band) into expected hf-EEG ΔRMS that was explained by the subcortical variations (horizontal part of gray-shaded band). This value was then subtracted from the total hf-EEG ΔRMS estimated from real hf-EEG recordings (measured hf-EEG ΔRMSm) to obtain a corrected measure independent of brain stem variations. D: after correction for brain stem variations hf-EEG RMS related to specific spike patterns (patterns shorter than 4 windows were suffixed with 0s to bring them up to a length of 4) were averaged across all cells (ticks and whiskers: mean ± SE). For some pairs of spike patterns the obtained RMS amplitudes were significantly different [left brackets, pairwise t-test corrected for multiple comparison: false discovery rate (FDR) q < 0.05]. Note that patterns 1101 and 1111 were identified in only 1 cell so that standard error could not be determined.
Timing of differences in hf-EEG compared with timing of spike pattern differences.
To investigate how the timing of differences in the hf-EEG related to the timing of spike patterns, we repeated the analysis above using a more narrowly circumscribed temporal window for the calculation of RMS amplitude. Windows were chosen to encompass single peaks of the hf-EEG response, corresponding to single digits of the spike pattern. Statistical testing of differences and correction for brain stem input fluctuations were carried out as described above.
RESULTS
In two awake monkeys the median nerve was stimulated electrically to evoke brief (10 ms) epidural hf-EEG wavelets, which were recorded together with extracellular spike responses from primary somatosensory cortex. Since the focus of this study was on the relation between response patterns of single neurons and hf-EEG, we manually discriminated clusters of spike waveforms from extracellular traces and selected only clusters that clearly corresponded to single-unit activity (see materials and methods). From these well-discriminated units we selected 17 bursting cells (14 and 3 in each monkey; for exact selection criteria see materials and methods) recorded in 15 independent sessions (2 pairs of neurons were recorded in the same session but from different microelectrodes).
When averaged across all trials the single-cell PSTHs exhibited multiple peaks well aligned with peaks of the concomitant hf-EEG (Fig. 1, B and C), but the repeated presentation of the same stimulus elicited single-cell spike responses with large trial-to-trial variability (Fig. 2C). These variations were contained in a narrow distribution of spike times around the mean latencies of PSTH peaks; this type of variability could be driven partially by, e.g., first-spike latency jitter of the burst response or channel noise (Faisal et al. 2008). Critically, we observed that the exact spike composition within a burst was variable across trials, forming different spike response patterns. In the following analysis, we examined this spike pattern variability by reducing the single-cell responses to binary strings whose digits encode the presence or absence of a spike in one of several (up to 4) consecutive nonoverlapping bins. The edges of these bins were located in the troughs of PSTH so that the variability due to spike jitter was substantially reduced (Fig. 2B). Consequently, for every cell studied, the variable burst responses were classified into a reduced set (≤16) of spike patterns labeled by distinct binary strings. Sample spike trains of each class are shown in the raster plot (Fig. 2C), and the percentage of trials belonging to each class is depicted by the adjacent bars (Fig. 2D). In the example shown, the most frequent patterns are burst of different lengths: doublets (110) and triplets (111). Besides, the cell also produced other forms of doublets, including a late (011) and a long-interval (101) doublet.
We additionally tested whether this intriguing variability of spike patterns was attributable to an eventually inaccurate spike sorting. First, we observed visually that spikes elicited in the predefined bins had the same waveform (Fig. 2A). We found that consecutive spikes were very similar (cf. also Supplemental Fig. S2); minor differences in amplitude were probably due to the nonstationarity of spike waveforms generated within a burst and/or a change of spike waveform during the recording (Lewicki 1998). Specifically, we verified that the spikes elicited within different spike patterns did not reflect responses of different cells erroneously combined in a single-cell spike train (cf. isolation score, materials and methods). Furthermore, we analyzed whether gaps in responses reflected spikes missed in discrimination. We calculated the pattern-specific PSTH and plotted it together with raw extracellular recordings from which the spikes were discriminated (for 2 example patterns, 110 and 011, see Fig. 2E and insets). Evidently, both PSTHs and raw spike traces show a lull of activity in bins where no spikes were present (0s in the corresponding digits of the binary string), confirming the accuracy of spike sorting and spike pattern classification procedures. Interestingly, the timing of the peaks of the pattern-specific PSTH is slightly different from the total PSTH (gray shading in Fig. 2E), indicating that apart from the spike count, the first spike latency and ISIs may also vary across patterns.
To study the relation of the identified spike patterns to the hf-EEG wavelets, the hf-EEG trials coincident with each of the patterns were averaged separately. The resulting waveforms differed in some of the oscillation periods (Fig. 2F). These differences cannot be explained by chance fluctuations originating from measurement noise and ongoing brain activity, as the RMS amplitude of the waveforms was significantly different (P < 0.05, bootstrap test). Other identified spike patterns yielded further instances of significant hf-EEG RMS amplitude differences. The differences were not necessarily localized to the time window concomitant with a missed/extra spike, but rather often extended over multiple PSTH peaks (Fig. 2G).
In the 17 cells selected for analysis we identified a total of 85 spike patterns (median 4 patterns per cell, range 3–8). All patterns identified in a single cell were compared pairwise, providing 188 independent comparisons (cf. Supplemental Table S1). In 15 of 17 cells, at least one pair of spike patterns was accompanied by significantly different hf-EEG RMS amplitudes. Significant differences were found in 48% of all evaluated comparisons (91 of 188 pairs, corrected for multiple comparisons: FDR, q < 0.05; Fig. 3B). Spike pattern-related differences accounted for an average of 17 ± 10% of the total variance in single-trial hf-EEG RMS amplitudes (mean ± SD, range 3–33%). This fraction is remarkably high given that the remaining variance includes both neuronal background activity and the band-limited amplifier noise known to permeate EEG recordings.
Next, we sought to demarcate possible sources of the correlated trial-to-trial variability between single-cell spike patterns and macroscopic EEG responses. Two possibilities are fluctuations entering the somatosensory pathways either at early stages (periphery, brain stem) or upstream within thalamocortical circuitry. Accordingly, in most sessions (15 of 17 cells) we additionally recorded evoked responses at the brain stem and obtained a recruitment curve relating the RMS amplitude of the brain stem evoked potential to that of the cortical hf-EEG (see materials and methods for details). Pattern-specific averages of brain stem recordings were then compiled (Fig. 3C, top insets) and their RMS amplitude determined. These amplitudes were used to read off from the calibration curve the difference in hf-EEG RMS amplitude that would be expected, given the difference in brain stem evoked potential amplitude (Fig. 3C, main panel). This was compared with the actual difference in hf-EEG RMS amplitude seen in the pattern-specific averages (Fig. 3C, right insets). Although part of the cortical hf-EEG variance could be explained by fluctuations of the subcortical response RMS amplitude, in 79/154 (51%) of single-cell spike pattern pairs with brain stem recordings available there were residual differences exceeding those predicted from the brain stem evoked responses. These differences accounted for, on average, 14 ± 9% of total variance in hf-EEG RMS (mean ± SD, range: 0–30%).
To understand which features of the spike patterns are the best predictors for the differences in hf-EEG RMS amplitude, we calculated the mean hf-EEG RMS specific for each pattern and averaged the obtained amplitudes across cells. In agreement with the previous results based on within-cell comparisons, the ensemble analysis revealed covariations between hf-EEG RMS amplitude and single-cell responses (Fig. 3D). Nevertheless, the differences were not equally pronounced for all spike patterns: the largest hf-EEG RMS differences were obtained between patterns that differed in number of spikes (Fig. 3D, compare singlets, doublets, and triplets), but there was also a tendency for longer first spike latency to be correlated with lower hf-EEG RMS (for example, 0110 and 1100). Thus both the number of spikes and spike response latency can factor in the prediction of hf-EEG RMS amplitudes.
Building on these findings, we investigated quantitatively whether the temporal variations in the hf-EEG amplitude carry additional information about spike patterns. To this end, we looked for spike pattern-specific differences between RMS values calculated within single hf-EEG peaks (Fig. 4A, bottom left) and repeated the significance analysis presented above after centering the hf-EEG RMS window on a single peak of the wavelet at a time. The fraction of cells in which significant RMS differences were found was nonuniformly distributed over the hf-EEG peaks (Fig. 4A, top right). The shape of the distribution was dependent on the exact pair of compared spike patterns, indicating that the individual peak amplitude may provide additional information about the spike pattern underlying a particular hf-EEG response. However, differences in hf-EEG were seen not just at the peaks corresponding to digits that differed between spike patterns (filled bars, Fig. 4A) but also in hf-EEG components before and after the time when spike patterns differed (open bars, Fig. 4A).
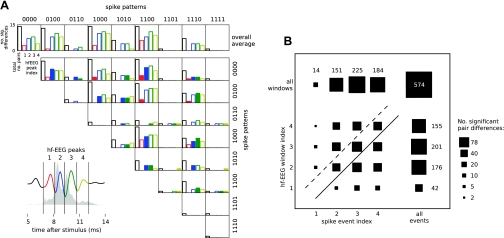
Fig. 4.
The amplitudes of individual hf-EEG peaks covary with spike patterns of a single cell but do not show a regular one-to-one relation to individual spike events. A: 4 individual peaks of hf-EEG were identified visually after averaging across all trials and all recorded cells (inset at bottom left; each peak is shown in a different color). The peaks were segmented into nonoverlapping windows (marked by vertical lines) whose positions are superimposed on the ensemble PSTH (gray-shaded area, average across all cells) to emphasize their relation to spike events used for spike pattern classification (cf. Fig. 2B). The RMS amplitude within each of those hf-EEG windows was corrected for input variations (based on recruitment curves calculated for each window) and compared between all possible pairs of concomitant spike patterns. Each row and column of the mosaic plot (top right) corresponds either to a single pattern from the pair as identified by its respective label (top and right of the plot; for cells with <4 spike events 0s were appended to the spike pattern string) or to the overall average across all patterns (top row). Bars within each frame show the total number of comparisons (black bars) and the number of those comparisons that yielded significant (P < 0.05) RMS differences in each of the hf-EEG windows (color-coded bars). In many instances the differences in hf-EEG peaks paralleled differences in spike occurrence in the same latency window (filled bars; compare also the digits of spike pattern labels), but significant RMS differences were also detected in peaks that did not coincide with spike pattern differences (open bars). B: contingency table summarizing the correspondence between significant differences in hf-EEG peaks and spike occurrence/omissions. The area of each square reflects the number of comparisons where differences in a specific hf-EEG window (rows) and spike event (columns) coincided; top row and rightmost column show marginals after summing all rows and columns, respectively. The data points can be divided into 3 main classes: hf-EEG differences following spike pattern differences (above dashed line), hf-EEG differences occurring simultaneously with spike pattern differences (between dashed and solid lines), and hf-EEG differences preceding spike pattern differences (below solid line).
Population data on the timing relation between spike pattern and hf-EEG differences are presented in Fig. 4B as a contingency table. The area of each square illustrates the number of instances when an hf-EEG peak differed (rows), given a difference in a particular digit of the spike pattern (columns). If the hf-EEG wavelet altered only at the times when the spike pattern was different, the diagonal elements of this contingency table (between solid and dashed lines) would dominate. If hf-EEG peaks were altered only after the time when spike patterns differed (possibly indicating that spike pattern changes cause hf-EEG changes), elements above the dashed line would dominate. Finally, if hf-EEG fluctuations preceded spike pattern differences, elements below the solid line would dominate. Significant effects were seen in all three sections of the contingency table. There is thus no simple relationship between the timing of trial-to-trial variations in spike patterns and hf-EEG.
DISCUSSION
Scalp recordings of electric potentials and magnetic fields are the only noninvasive methods that allow investigation of human brain activity with high temporal resolution. Here we show that high-frequency components of evoked macroscopic EEG potentials provide a measure of varying cortical spike responses to peripheral nerve stimulation: 1) peaks of the hf-EEG waveform represent discrete windows of opportunity for single-cell spike generation; 2) the amplitude of hf-EEG covaries with spike patterns originating from differential emissions/omissions of spikes in those windows; and 3) a significant part of this correlated variability arises in the thalamocortical loop and not solely in lower levels of the somatosensory pathway. These findings establish a useful link between neuronal spike firing patterns and hf-EEG oscillations, thereby narrowing the gap between microscopic and macroscopic descriptions of neural activity.
As presented above, poststimulus time histograms of individual bursting cells in somatosensory cortex consisted of multiple peaks that aligned with later peaks of the macroscopic oscillatory hf-EEG wavelets. This is in agreement with previous findings showing a close synchrony between macroscopic EEG or magnetoencephalography (MEG) signals and extracellular cortical activity averaged across multiple cells (Baker et al. 2003; Ikeda et al. 2002, 2005). Notably, some peaks of the hf-EEG (e.g., the early peak visible in Fig. 1) were not accompanied by action potentials of single cortical neurons; these components point to additional sources of hf-EEG, such as presynaptic spike activity in thalamocortical axon terminals and other cell types distributed over different layers of cortex (Ikeda et al. 2005).
Building on and extending these findings, the present study shows that the peaks of single-cell PSTHs represent discrete windows of response variability: In each of the windows a cell did or did not fire a single spike, giving rise to a limited set of spike burst patterns. Critically, these spiking windows have been found well aligned across the responding cells, and even cells that fire only a single action potential tend to lock to one of them (Baker et al. 2003). Such intercell coherency enables extracellular action potentials of a large group of neurons to sum constructively (Peterson et al. 1995) and could underlie also the coincidence between peaks of population PSTH and average hf-EEG responses discussed above. Although our detailed analysis of spike waveforms suggests that the identified spike patterns reflect activity of single units, the extracellular recording technique of the present study does not allow us to exclude completely the possibility that a small group of close-by cells contributes to the observed spike pattern variability. Nevertheless, similar spike patterns were detected in somatosensory cortex with intracellular recordings, which, in addition, have shown that the discrete firing windows might be related to deflections of membrane potential due to oscillatory dendritic inputs (Jones et al. 2000; Swadlow et al. 1998). However, intrinsic currents producing bursting are also likely to contribute to the generation of spike patterns (Izhikevich 2010; Krahe and Gabbiani 2004; Lisman 1997; Webster et al. 1997).
The key finding of the present study is that the amplitude of epidural hf-EEG is sensitive to the spike pattern variability observed in putative single cells. Interestingly, the differences between hf-EEG waveforms corresponding to specific spike patterns were present not only in the peak coincident with the time window where the spike patterns differed but appeared also in hf-EEG peaks preceding and following it. Thus the single-cell spike pattern signature is not directly reflected in the waveform of the surface hf-EEG potential but closer related to its overall amplitude. Naturally, the macroscopic hf-EEG amplitude reflects activity generated in a larger pool of neurons, which, however, might produce different spike patterns in response to a single stimulation; nonetheless, mutual interactions between these neurons could make the patterns appear in fixed combinations that repeat across trials. Similar mechanisms have been proposed to underlie differential waveforms of slow wave/ripple complexes in hippocampus (Reichinnek et al. 2010). Further simultaneous recordings from multiple single units should further clarify the specific mechanisms behind the covariation of spike patterns and hf-EEG.
An important conclusion from the present results is the contribution of variable spiking activity to EEG signals, which are usually considered a coarse measure of neuronal activity. Simulation studies have shown that the surface potential generated by sodium spikes can be strong enough to be detected with EEG electrodes or MEG sensors provided that the neuronal synchrony is high enough to overcome the cancellation of their fields due to temporal spike jitter (Murakami et al. 2003; Murakami and Okada 2006; Nunez and Srinivasan 2005; Ray et al. 2008a). Indeed, periods of increased multiunit activity have been previously linked to the high-gamma band (30–100 Hz) of intracortically recorded local field potentials (Belitski et al. 2008; Berens et al. 2008; Eckhorn et al. 1988, 1993; Engel et al. 2001; Frien and Eckhorn 2000; Fries et al. 2001; Gray and Singer 1989; Katzner et al. 2009; König et al. 1995; Ray et al. 2008b; Siegel and König 2003). Recently, it has been shown that variations in the high-gamma power of surface EEG signals are correlated with multiunit activity (Whittingstall and Logothetis 2009). Extending these results from the gamma band, the present findings provide complementary evidence that EEG components above 400 Hz also offer an additional, direct measure of neuronal spike response variability that is characterized by an excellent temporal resolution.
A part of the observed single-neuron and hf-EEG variability arose at early stages of the somatosensory system because of variable stimulus efficacy or subcortical processing. However, even after accounting for response amplitude fluctuations at the first relay station of the somatosensory pathway (brain stem), a significant covariation was still found between hf-EEG and single-cell spike patterns. This indicates further shared variability upstream to the brain stem, i.e., in thalamus and/or cortex. Notably, the first EEG wavelet peak was found to yield a lower number of significant pair differences than any later peak (hf-EEG window index 1 in Fig. 4B), suggesting that the thalamic input contributes less variability than later response components generated intracortically. Since the thalamocortical system is involved in the regulation of arousal and attention (Portas et al. 1998), the unexplained variability could reflect transitions between different brain states, such as phases of sleep, or variations in the levels of vigilance, attention, and expectation (Chapman et al. 1988; Fontanini and Katz 2008; Fox et al. 2006; Kenet et al. 2003; Li et al. 1999; Rosanova and Timofeev 2005). In the present study subjects stayed awake during the recordings, so that the remaining variability might reflect different attentive states or learning. Since the latter usually occurs at a slower time scale, attentional fluctuations may be considered as a prominent source of variability. In support of this, noninvasive experiments in healthy human subjects (Gobbelé et al. 2000; Halboni et al. 2000; Klostermann et al. 2001) showed that the amplitude of scalp hf-EEG is sensitive to a variety of neural conditions preceding the stimulus, such as sleep phases and fluctuating vigilance or attention.
The present findings provide an interesting perspective for human noninvasive neurophysiology because high-frequency response components can also be reliably recorded from the human scalp in a standard paradigm for acquisition of somatosensory evoked magnetic fields or electric potentials (Curio et al. 1994; Hashimoto et al. 1996). However, the signal attenuation due to skull resistivity substantially diminishes the SNR of scalp EEG. While the amplitude ratio between low- and high-frequency EEG responses is similar in scalp and epidural hf-EEG, the SNR of noninvasive measurements is at least one order of magnitude smaller than in the present invasive study (Waterstraat, Telenczuk, Burgoff, Scheer, Curio, manuscript in preparation). Nevertheless, recent advances in EEG amplifier technology (Scheer et al. 2006), denoising algorithms (Celka et al. 2008), and multimodal recording paradigms (Ritter et al. 2008) may help to alleviate this problem. Consequently, recordings of high-frequency activity might eventually provide a noninvasive window on fluctuating cortical spike activity in humans.
GRANTS
This study has been supported by the Deutsche Forschungsgemeinschaft (SFB 618), the Federal Ministry for Education and Research (Bernstein Focus Neurotechnology Berlin), and The Wellcome Trust.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
Footnotes
Supplemental Material for this article is available online at the Journal website.
REFERENCES
- Baker et al., 2001. Baker SN, Spinks R, Jackson A, Lemon RN. Synchronization in monkey motor cortex during a precision grip task. I. Task-dependent modulation in single-unit synchrony. J Neurophysiol 85: 869–885, 2001 [DOI] [PubMed] [Google Scholar]
- Baker et al., 2003. Baker S, Curio G, Lemon R. EEG oscillations at 600 Hz are macroscopic markers for cortical spike bursts. J Physiol 550: 529–534, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth, 2003. Barth D. Submillisecond synchronization of fast electrical oscillations in neocortex. J Neurosci 23: 2502–2510, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belitski et al., 2008. Belitski A, Gretton A, Magri C, Murayama Y, Montemurro MA, Logothetis NK, Panzeri S. Low-frequency local field potentials and spikes in primary visual cortex convey independent visual information. J Neurosci 28: 5696–5709, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini and Hochberg, 1995. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B 57: 289–300, 1995 [Google Scholar]
- Berens et al., 2008. Berens P, Keliris GA, Ecker AS, Logothetis NK, Tolias AS. Comparing the feature selectivity of the gamma-band of the local field potential and the underlying spiking activity in primate visual cortex. Front Syst Neurosci 2: 2, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger, 1929. Berger H. Über das Elektrenkephalogramm des Menschen. Arch Psychiatr Nervenkr 87: 527, 1929 [Google Scholar]
- Bragin et al., 1999. Bragin A, Engel J, Wilson CL, Fried I, Buzsáki G. High-frequency oscillations in human brain. Hippocampus 9: 137–142, 1999 [DOI] [PubMed] [Google Scholar]
- Buzsaki and Draguhn, 2004. Buzsaki G, Draguhn A. Neuronal oscillations in cortical networks. Science 304: 1926–1929, 2004 [DOI] [PubMed] [Google Scholar]
- Celka et al., 2008. Celka P, Le K, Cutmore T. Noise reduction in rhythmic and multitrial biosignals with applications to event-related potentials. IEEE Trans Biomed Eng 55: 1809–1821, 2008 [DOI] [PubMed] [Google Scholar]
- Chapman et al., 1988. Chapman CE, Jiang W, Lamarre Y. Modulation of lemniscal input during conditioned arm movements in the monkey. Exp Brain Res 72: 316–334, 1988 [DOI] [PubMed] [Google Scholar]
- Cracco and Cracco, 1976. Cracco RQ, Cracco JB. Somatosensory evoked potential in man: far field potentials. Electroencephalogr Clin Neurophysiol 41: 460–466, 1976 [DOI] [PubMed] [Google Scholar]
- Curio et al., 1994. Curio G, Mackert B, Burghoff M, Koetitz R, Abraham-Fuchs K, Haerer W. Localization of evoked neuromagnetic 600 Hz activity in the cerebral somatosensory system. Electroencephalogr Clin Neurophysiol 91: 483–487, 1994 [DOI] [PubMed] [Google Scholar]
- Eckhorn et al., 1988. Eckhorn R, Bauer R, Jordan W, Brosch M, Kruse W, Munk M, Reitboeck HJ. Coherent oscillations: a mechanism of feature linking in the visual cortex? Multiple electrode and correlation analyses in the cat. Biol Cybern 60: 121–130, 1988 [DOI] [PubMed] [Google Scholar]
- Eckhorn et al., 1993. Eckhorn R, Frien A, Bauer R, Woelbern T, Kehr H. High frequency (60–90 Hz) oscillations in primary visual cortex of awake monkey. Neuroreport 4: 243–246, 1993 [DOI] [PubMed] [Google Scholar]
- Efron and Tibshirani, 1994. Efron B, Tibshirani R. An Introduction to the Bootstrap. Boca Raton, FL: Chapman & Hall/CRC, 1994 [Google Scholar]
- Engel et al., 2001. Engel AK, Fries P, Singer W. Dynamic predictions: oscillations and synchrony in top-down processing. Nat Rev Neurosci 2: 704–716, 2001 [DOI] [PubMed] [Google Scholar]
- Faisal et al., 2008. Faisal AA, Selen LPJ, Wolpert DM. Noise in the nervous system. Nat Rev Neurosci 9: 292–303, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontanini and Katz, 2008. Fontanini A, Katz DB. Behavioral states, network states, and sensory response variability. J Neurophysiol 100: 1160–1168, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox et al., 2006. Fox MD, Snyder AZ, Zacks JM, Raichle ME. Coherent spontaneous activity accounts for trial-to-trial variability in human evoked brain responses. Nat Neurosci 9: 23–25, 2006 [DOI] [PubMed] [Google Scholar]
- Frien and Eckhorn, 2000. Frien A, Eckhorn R. Functional coupling shows stronger stimulus dependency for fast oscillations than for low-frequency components in striate cortex of awake monkey. Eur J Neurosci 12: 1466–1478, 2000 [DOI] [PubMed] [Google Scholar]
- Fries et al., 2001. Fries P, Reynolds JH, Rorie AE, Desimone R. Modulation of oscillatory neuronal synchronization by selective visual attention. Science 291: 1560–1563, 2001 [DOI] [PubMed] [Google Scholar]
- Funke and Kerscher, 2000. Funke K, Kerscher N. High-frequency (300–800 Hz) components in cat geniculate (dLGN) early visual responses. J Physiol (Paris) 94: 411–425, 2000 [DOI] [PubMed] [Google Scholar]
- Gobbelé et al., 2000. Gobbelé R, Waberski TD, Kuelkens S, Sturm W, Curio G, Buchner H. Thalamic and cortical high-frequency (600 Hz) somatosensory-evoked potential (SEP) components are modulated by slight arousal changes in awake subjects. Exp Brain Res 133: 506–513, 2000 [DOI] [PubMed] [Google Scholar]
- Gray and Singer, 1989. Gray CM, Singer W. Stimulus-specific neuronal oscillations in orientation columns of cat visual cortex. Proc Natl Acad Sci USA 86: 1698–1702, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halboni et al., 2000. Halboni P, Kaminski R, Gobbelé R, Züchner S, Waberski TD, Herrmann CS, Töpper R, Buchner H. Sleep stage dependant changes of the high-frequency part of the somatosensory evoked potentials at the thalamus and cortex. Clin Neurophysiol 111: 2277–2284, 2000 [DOI] [PubMed] [Google Scholar]
- Hanajima et al., 2004. Hanajima R, Chen R, Ashby P, Lozano AM, Hutchison WD, Davis KD, Dostrovsky JO. Very fast oscillations evoked by median nerve stimulation in the human thalamus and subthalamic nucleus. J Neurophysiol 92: 3171–3182, 2004 [DOI] [PubMed] [Google Scholar]
- Hashimoto et al., 1996. Hashimoto I, Mashiko T, Imada T. Somatic evoked high-frequency magnetic oscillations reflect activity of inhibitory interneurons in the human somatosensory cortex. Electroencephalogr Clin Neurophysiol 100: 189–203, 1996 [DOI] [PubMed] [Google Scholar]
- Hazan et al., 2006. Hazan L, Zugaro M, Buzsáki G. Klusters, NeuroScope, NDManager: a free software suite for neurophysiological data processing and visualization. J Neurosci Methods 155: 207–216, 2006 [DOI] [PubMed] [Google Scholar]
- Ikeda et al., 2002. Ikeda H, Leyba L, Bartolo A, Wang Y, Okada YC. Synchronized spikes of thalamocortical axonal terminals and cortical neurons are detectable outside the pig brain with MEG. J Neurophysiol 87: 626–630, 2002 [DOI] [PubMed] [Google Scholar]
- Ikeda et al., 2005. Ikeda H, Wang Y, Okada YC. Origins of the somatic N20 and high-frequency oscillations evoked by trigeminal stimulation in the piglets. Clin Neurophysiol 116: 827–841, 2005 [DOI] [PubMed] [Google Scholar]
- Izhikevich, 2010. Izhikevich EM. Dynamical Systems in Neuroscience: The Geometry of Excitability and Bursting. Cambridge, MA: MIT Press, 2010 [Google Scholar]
- Jirsch et al., 2006. Jirsch JD, Urrestarazu E, LeVan P, Olivier A, Dubeau F, Gotman J. High-frequency oscillations during human focal seizures. Brain 129: 1593–1608, 2006 [DOI] [PubMed] [Google Scholar]
- Jones and Barth, 1999. Jones MS, Barth DS. Spatiotemporal organization of fast (>200 Hz) electrical oscillations in rat vibrissa/barrel cortex. J Neurophysiol 82: 1599–1609, 1999 [DOI] [PubMed] [Google Scholar]
- Jones et al., 2000. Jones MS, MacDonald KD, Choi B, Dudek FE, Barth DS. Intracellular correlates of fast (>200 Hz) electrical oscillations in rat somatosensory cortex. J Neurophysiol 84: 1505–1518, 2000 [DOI] [PubMed] [Google Scholar]
- Joshua et al., 2007. Joshua M, Elias S, Levine O, Bergman H. Quantifying the isolation quality of extracellularly recorded action potentials. J Neurosci Methods 163: 267–282, 2007 [DOI] [PubMed] [Google Scholar]
- Katzner et al., 2009. Katzner S, Nauhaus I, Benucci A, Bonin V, Ringach DL, Carandini M. Local origin of field potentials in visual cortex. Neuron 61: 35–41, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenet et al., 2003. Kenet T, Bibitchkov D, Tsodyks M, Grinvald A, Arieli A. Spontaneously emerging cortical representations of visual attributes. Nature 425: 954–956, 2003 [DOI] [PubMed] [Google Scholar]
- Klostermann et al., 2001. Klostermann F, Nolte G, Curio G. Independent short-term variability of spike-like (600 Hz) and postsynaptic (N20) cerebral SEP components. Neuroreport 12: 349–352, 2001 [DOI] [PubMed] [Google Scholar]
- König et al., 1995. König P, Engel AK, Singer W. Relation between oscillatory activity and long-range synchronization in cat visual cortex. Proc Natl Acad Sci USA 92: 290–294, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krahe and Gabbiani, 2004. Krahe R, Gabbiani F. Burst firing in sensory systems. Nat Rev Neurosci 5: 13–23, 2004 [DOI] [PubMed] [Google Scholar]
- Lewicki, 1998. Lewicki MS. A review of methods for spike sorting: the detection and classification of neural action potentials. Network 9: 53, 1998 [PubMed] [Google Scholar]
- Li et al., 1999. Li B, Funke K, Wörgötter F, Eysel UT. Correlated variations in EEG pattern and visual responsiveness of cat lateral geniculate relay cells. J Physiol 514: 857–874, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman, 1997. Lisman JE. Bursts as a unit of neural information: making unreliable synapses reliable. Trends Neurosci 20: 38–43, 1997 [DOI] [PubMed] [Google Scholar]
- Mountcastle et al., 1991. Mountcastle VB, Reitboeck HJ, Poggio GF, Steinmetz MA. Adaptation of the Reitboeck method of multiple microelectrode recording to the neocortex of the waking monkey. J Neurosci Methods 36: 77–84, 1991 [DOI] [PubMed] [Google Scholar]
- Murakami et al., 2003. Murakami S, Hirose A, Okada YC. Contribution of ionic currents to magnetoencephalography (MEG) and electroencephalography (EEG) signals generated by guinea-pig CA3 slices. J Physiol 553: 975–985, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami and Okada, 2006. Murakami S, Okada Y. Contributions of principal neocortical neurons to magnetoencephalography and electroencephalography signals. J Physiol 575: 925–936, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez and Srinivasan, 2005. Nunez P, Srinivasan R. Electric Fields of the Brain: The Neurophysics of EEG. New York: Oxford Univ. Press, 2005 [Google Scholar]
- Peterson et al., 1995. Peterson NN, Schroeder CE, Arezzo JC. Neural generators of early cortical somatosensory evoked potentials in the awake monkey. Electroencephalogr Clin Neurophysiol 96: 248–260, 1995 [DOI] [PubMed] [Google Scholar]
- Portas et al., 1998. Portas CM, Rees G, Howseman AM, Josephs O, Turner R, Frith CD. A specific role for the thalamus in mediating the interaction of attention and arousal in humans. J Neurosci 18: 8979–8989, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray et al., 2008a. Ray S, Crone NE, Niebur E, Franaszczuk PJ, Hsiao SS. Neural correlates of high-gamma oscillations (60–200 Hz) in macaque local field potentials and their potential implications in electrocorticography. J Neurosci 28: 11526–11536, 2008a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray et al., 2008b. Ray S, Hsiao SS, Crone NE, Franaszczuk PJ, Niebur E. Effect of stimulus intensity on the spike-local field potential relationship in the secondary somatosensory cortex. J Neurosci 28: 7334–7343, 2008b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichinnek et al., 2010. Reichinnek S, Künsting T, Draguhn A, Both M. Field potential signature of distinct multicellular activity patterns in the mouse hippocampus. J Neurosci 30: 15441–15449, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter et al., 2008. Ritter P, Freyer F, Curio G, Villringer A. High-frequency (600 Hz) population spikes in human EEG delineate thalamic and cortical fMRI activation sites. Neuroimage 42: 483–490, 2008 [DOI] [PubMed] [Google Scholar]
- Rosanova and Timofeev, 2005. Rosanova M, Timofeev I. Neuronal mechanisms mediating the variability of somatosensory evoked potentials during sleep oscillations in cats. J Physiol 562: 569–582, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer et al., 2006. Scheer HJ, Sander T, Trahms L. The influence of amplifier, interface and biological noise on signal quality in high-resolution EEG recordings. Physiol Meas 27: 109–117, 2006 [DOI] [PubMed] [Google Scholar]
- Shimazu et al., 2000. Shimazu H, Kaji R, Tsujimoto T, Kohara N, Ikeda A, Kimura J, Shibasaki H. High-frequency SEP components generated in the somatosensory cortex of the monkey. Neuroreport 11: 2821–2826, 2000 [DOI] [PubMed] [Google Scholar]
- Siegel and König, 2003. Siegel M, König P. A functional gamma-band defined by stimulus-dependent synchronization in area 18 of awake behaving cats. J Neurosci 23: 4251–4260, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speckmann and Elger, 2004. Speckmann E, Elger CE. Introduction to the neurophysiological basis of the EEG and DC potentials. In: Electroencephalography: Basic Principles, Clinical Applications, and Related Fields, edited by Niedermeyer E, Lopes Da Silva F. Baltimore, MD: Lippincott Williams & Wilkins, p. 17–31, 2004 [Google Scholar]
- Stern et al., 1992. Stern P, Edwards F, Sakmann B. Fast and slow components of unitary EPSCs on stellate cells elicited by focal stimulation in slices of rat visual cortex. J Physiol 449: 247–278, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swadlow et al., 1998. Swadlow HA, Beloozerova IN, Sirota MG. Sharp, local synchrony among putative feed-forward inhibitory interneurons of rabbit somatosensory cortex. J Neurophysiol 79: 567–582, 1998 [DOI] [PubMed] [Google Scholar]
- Webster et al., 1997. Webster HH, Salimi I, Myasnikov AA, Dykes RW. The effects of peripheral deafferentation on spontaneously bursting neurons in the somatosensory cortex of waking cats. Brain Res 750: 109–121, 1997 [DOI] [PubMed] [Google Scholar]
- Whittingstall and Logothetis, 2009. Whittingstall K, Logothetis NK. Frequency-band coupling in surface EEG reflects spiking activity in monkey visual cortex. Neuron 64: 281–289, 2009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.