Abstract
Imaging, lesion, and transcranial magnetic stimulation (TMS) studies have implicated a number of regions of the brain in searching for a target defined by a combination of attributes. The necessity of both frontal eye fields (FEF) and posterior parietal cortex (PPC) in task performance has been shown by the application of TMS over these regions. The effects of stimulation over these two areas have, thus far, proved to be remarkably similar and the only dissociation reported being in the timing of their involvement. We tested the hypotheses that 1) FEF contributes to performance in terms of visual target detection (possibly by modulation of activity in extrastriate areas with respect to the target), and 2) PPC is involved in translation of visual information for action. We used a task where the presence (and location) of the target was indicated by an eye movement. Task disruption was seen with FEF TMS (with reduced accuracy on the task) but not with PPC stimulation. When a search task requiring a manual response was presented, disruption with PPC TMS was seen. These results show dissociation of FEF and PPC contributions to visual search performance and that PPC involvement seems to be dependent on the response required by the task, whereas this is not the case for FEF. This supports the idea of FEF involvement in visual processes in a manner that might not depend on the required response, whereas PPC seems to be involved when a manual motor response to a stimulus is required.
Keywords: conjunction search, voluntary saccades
the apparently straightforward task of detection of the presence or absence of a target among distractors is associated with a number of regions in the brain in circumstances where the target is defined by a combination of features, such as shape and color, that can be shared with distractors. Involvement of the posterior parietal cortex (PPC) has been implicated by imaging studies (Corbetta et al. 1995; Donner et al. 2002) and the necessity of the area shown by both patient studies (Friedman-Hill et al. 1995) and the application of transcranial magnetic stimulation (TMS; Ashbridge et al. 1997; Kalla et al. 2008; Walsh et al. 1998). Similar involvement of frontal eye fields (FEF) has been illustrated (Donner et al. 2002; Muggleton et al. 2003; O'Shea et al. 2004). The pattern of disruption seen as a consequence of TMS application for these areas has been, to date, broadly similar. Elevated response times are found when large search arrays (which allow eye movements) are presented (Ashbridge et al. 1997; Walsh et al. 1998) and reduced sensitivity is seen when small, short-duration displays (without eye movements) are used (Kalla et al. 2008; Muggleton et al. 2003; O'Shea et al. 2004). The most notable differences have been seen in the timing of the involvement of the two areas. O'Shea et al. (2004) showed that the time window of disruption of performance with FEF TMS was early (40–80 ms) following search array presentation, broadly in line with the timing of target selection in this region seen in electrophysiology studies (Nowak et al. 1997; Sato and Schall 2003; Schmolesky et al. 1998). This is earlier than the disruption previously seen with PPC stimulation for large search arrays (100–160 ms; Ashbridge et al. 1997) and a recent comparison of the involvement of the two areas using the same task and subjects obtained a similar temporal dissociation (0–40 ms for FEF, 120–160 ms for PPC; Kalla et al. 2008).
Other than this temporal dissociation, differences in the relative contribution of these two areas to search performance remain to be clarified. Both have been suggested to be part of a distributed network involved in visual processing (Corbetta and Shulman 1998). FEF contains neurons that respond to behaviorally relevant stimuli rather than to visual attributes (Goldberg and Segraves 1989; Mohler et al. 1973) and has been described in terms of a saliency map for saccades (Schall and Bichot 1998). Target discrimination in FEF occurs independently of saccade programming (Juan et al. 2004, 2008), and it has been suggested that FEF plays a role in modulating extrastriate cortex activity with respect to the target (Moore et al. 2003; Taylor et al. 2007). If this were the case, then altered performance following FEF TMS might be a consequence of disruption of FEF modulation of extrastriate cortex with respect to the search target (Grosbras and Paus 2003; Ruff et al. 2006; Silvanto et al. 2006). Similarly, PPC has also been shown to respond to behaviorally relevant stimuli, frequently being argued to play a role in the binding of features to a single-object representation (Friedman-Hill et al. 1995; Treisman 1996). PPC has also been proposed to have a role in spatial localization of the target, consistent with a role in coordinate transformation and spatial updating across saccades (van Donkelaar and Müri 2002), as well as having saccade-related functions (e.g., Elkington et al. 1992; Müri et al. 2000; Priori et al. 1993). Deficits following TMS might represent spatial uncertainty in localizing the target if it is present (Ellison et al. 2003). The later role of the PPC may also be consistent with modulatory effects on visual cortex activity. However, the later timing and the fact that PPC, unlike FEF, is important in trials in a visual search task when no target is presented (target-absent trials; Ashbridge et al. 1997) may be more consistent with the visuomotor transformation required for manual responses (Ellison et al. 2003).
Work to date using TMS and visual search tasks has typically required the indication of the presence or absence of a target in a search array, and target localization is not usually explicitly required. Additionally, few studies have directly compared the effects of FEF and PPC TMS within the same study using the same task and response requirements. We used a visual search task where the presence or absence of the target was indicated by generating or withholding an eye movement to the target location to test the hypotheses that 1) FEF contributes to performance in terms of visual target detection, and 2) PPC is involved in translation of visual information for action. Thus we predicted differing patterns of disruption for the two areas with FEF TMS expected to disrupt task accuracy in terms of identifying the presence or absence of the target and PPC TMS to result in spatial uncertainty, reflected in variability of the endpoints of generated saccades.
MATERIALS AND METHODS
Subjects.
Eight subjects (5 males, 3 females; mean age 23 yr; all right handed) took part in the study. All were normal, healthy volunteers and gave informed consent before participating. The University College London Research Ethics Committee approved the study.
Task.
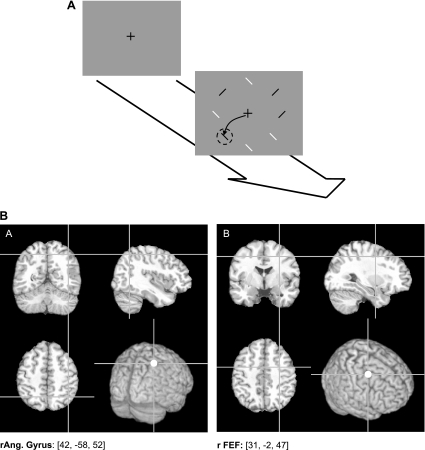
The task was programmed in Microsoft Visual C++ 6.0 and presented using an International Business Machines (IBM)-compatible personal computer (PC) on a 17-in. cathode ray tube (CRT) monitor with a refresh rate of 100 Hz. Eye position was monitored using a Sensomotoric Instruments (SMI) EyeLink I system, modified for use with TMS, with a sampling rate of 250 Hz. The time course of a typical trial is illustrated in Fig. 1. The EyeLink system was calibrated at the start of each block, and each trial began with a correction of any eye drift. This was followed by the presentation of a fixation cross for a random duration between 300 and 700 ms, after which the trial array was presented. This consisted of a circular array of 8 stimuli, presented equally spaced and equidistant from the fixation cross at 10° of visual angle. Distractors were red or blue diagonals with each color associated with a different orientation (e.g., red / and blue \). A target, present in 50% of the trials, was either a red diagonal in the same orientation as the blue diagonals or vice versa. Each subject was assigned a target that remained the same for all blocks for that individual. Half of the subjects had a red target, and half a blue target. When the array was presented, subjects were required to either make a saccade to the target (if there was 1 present) or remain looking at the fixation cross (if no target was present). The array remained on the screen for 1 s. This duration was selected to allow sufficient time for performance of the task, meaning any effects on saccade latency due to TMS would not affect accuracy while being a reasonable time to maintain fixation. Each block consisted of 40 trials. Subjects were tested on 2 occasions, 1 for each stimulation site (see below) with 2 blocks for the relevant site and 2 no-TMS blocks on each occasion. The order of blocks was randomized for each subject.
Fig. 1.
A: time line of a typical trial (black and white are used for target and distractors for illustrative purposes). Trials were initiated by subjects by means of the keyboard spacebar. Following a random-duration fixation (300–700 ms), the array was presented. This remained on screen until either a saccade was made or 1 s had passed. An example correct response is shown by the arrow, and the dashed circle highlights the target in the display (neither shown during the task). B: stimulation sites. “A”: posterior parietal cortex (PPC) (coordinates equivalent to 42, −58, 52). “B”: frontal eye fields (FEF) (coordinates 31, −2, 47). These were localized in each subject by transforming the coordinates for each subject individually for their structural MRI scans using FSL [Oxford Centre for Functional MRI of the Brain (FMRIB Centre), University of Oxford, Oxford, United Kingdom] and then identifying the overlying scalp location using the Brainsight system (Rogue Research). r, Right; Ang., angular gyrus.
TMS.
TMS at 10 Hz for 500 ms starting concurrent with the onset of the search array was delivered by means of a Magstim 200 Super Rapid Stimulator. A 70-mm coil was used for PPC stimulation, whereas a 50-mm coil was used for FEF as use of the larger coil over this area can result in twitches and blinks. A fixed stimulation level of 60% of maximum stimulator output was used because it has proven successful and replicable in many studies and over a wide range of tasks (Ashbridge et al. 1997; Ellison and Cowey 2007; Hung et al. 2005; Juan et al. 2008; Muggleton et al. 2003; Rushworth et al. 2002) and because motor cortex excitability does not provide a good guide to TMS thresholds in other cortical areas (Stewart et al. 2001).
Site localization.
Sites for TMS stimulation were localized using the Brainsight system (Rogue Research). This was used to coregister each subject with their structural MRI scans on which the right FEF and right PPC had been marked. Only sites in the right hemisphere were used as stimulation of these produces effects in visual search tasks, irrespective of target location, whereas left-hemisphere stimulation typically produces less consistent effects (Ashbridge et al. 1997; Muggleton et al. 2003). As we were interested in differences in the type of effect seen, rather than any specific investigation of laterality, we chose to stimulate only the right hemisphere in the present experiment. The site marks were obtained by means of transformation of the FEF coordinates from Paus et al. (1997) and the coordinates for the PPC location associated with disruption of visual search performance (e.g., Göbel et al. 2001) using the FSL software package [Oxford Centre for Functional MRI of the Brain (FMRIB Centre), University of Oxford, Oxford, United Kingdom]. Briefly, this procedure consisted of normalizing subjects' individual scans against the MRI template image. This produced a file containing a mathematical description of the normalization that was then used to convert the stimulation site coordinates from standard space to that of the individual being tested. This method has proved successful and reliable in identifying both FEF (e.g., Juan et al. 2008; Nuding et al. 2009) and PPC (e.g., Kalla et al. 2008; Muggleton et al. 2008) in previous studies. Stimulation sites are illustrated in Fig. 1B.
Data collection and analysis.
Eye position information on each trial was sampled at 250 Hz by the EyeLink system throughout each trial. Saccades and blinks were also automatically detected and recorded, and trials with blinks were excluded from further analysis. Saccades were automatically identified according to acceleration and amplitude criteria (minimum speed 30°/s, minimum acceleration 8,000°·s−2). The information used in analysis was presence (or absence) of a saccade on any given trial and saccade endpoint (with respect to the target). Performance (sensitivity) was scored in terms of d-prime with hits (saccades made) and misses (saccades withheld) for target-present trials and false alarms (saccades made) and correct rejections (saccades withheld) for target-absent trials. Bias (the signal detection theory value, c) was also calculated from these measures and analyzed. Additionally, for trials where saccades were made, the accuracy of the endpoint was analyzed. Accurate saccades were scored as those made toward the target such that the endpoint was closer to the target element than any other element in the display and of minimum amplitude of 1° (a level chosen as the minimum from which it was assumed a direction had been reasonably selected for the saccade). All other saccades were scored as inaccurate.
RESULTS
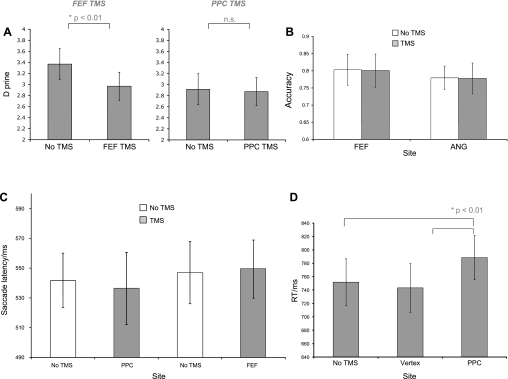
Performance, in terms of sensitivity (d-prime), is illustrated in Fig. 2A and was analyzed using repeated-measures ANOVA with factors of site (FEF or PPC) and stimulation (“yes” or “no”). This showed a significant effect of stimulation [F(1,7) = 13.686, P < 0.01] and a significant site × stimulation interaction [F(1,7) = 15.857, P < 0.01]. Post hoc t-test showed that this was due to reduced sensitivity following FEF TMS relative to the FEF control condition (t = 4.775, P < 0.005), whereas there was no difference for the PPC TMS condition vs. control (t = 0.653, P > 0.5). Bias scores (c) were also analyzed in a similar manner, with the same factors, but no significant effects were seen (all F < 2, P > 0.2).
Fig. 2.
A: d-prime scores for each stimulation condition. Error bars indicate the standard error of the mean. A significant reduction in d-prime was seen following transcranial magnetic stimulation (TMS) delivered over FEF. B: accuracy of saccades directions for stimulation condition. Error bars indicate the standard error of the mean. No significant effects of TMS were seen. C: saccade latencies for each stimulation condition. Error bars indicate the standard error of the mean. No significant effects of TMS were seen. D: effects of TMS on response latency for the conjunction search task requiring manual responses to indicate the presence or absence of the target. Error bars indicate the standard error of the mean. A significant increase in response times (RT) was seen with TMS delivered over PPC. ns, Not significant.
First, saccade accuracy, i.e., regardless of whether the direction of saccades made were toward the location of the target element, was analyzed in a similar manner, again employing an ANOVA with factors of site and stimulation. The data are illustrated in Fig. 2B. No significant effects of site [F(1,7) = 0.693, P > 0.4] or TMS [F(1,7) = 0.005, P > 0.9] were seen.
Thus task performance, in terms of ability to judge correctly regardless of whether a target was present, was reduced when TMS was delivered over FEF but not when delivered over PPC, and neither stimulation site produced any effect in terms of saccade endpoint.
We also analyzed the latencies of saccades with an ANOVA with factors of site and stimulation (as above). These data are illustrated in Fig. 2C. No significant effects were seen (all F < 1.7, P > 0.2).
We further investigated any laterality effects of the FEF TMS effects on target-present trials in terms of target detection accuracy, whether the saccade made on a present trial was in the appropriate direction, saccade latency, and mean deviation of the saccade endpoint (all saccades) from the target. The data were analyzed for the left and right hemisphere targets (the targets located above and below fixation were not included). The data from FEF TMS trials was compared with that from trials with no stimulation using paired t-tests. No significant effects were seen for any of the measures (all P > 0.19; accuracy: left targets, FEF: 94.7%, no TMS: 94.8%; right targets, FEF: 95.3%, no TMS: 96.4%; saccade direction accuracy: left targets, FEF: 77.5%, no TMS: 81.2%; right targets, FEF: 84.0%, no TMS: 83.9%; saccade latency: left targets, FEF: 605.6 ms, no TMS: 600.9 ms; right targets, FEF: 575.3 ms, no TMS: 589.8 ms; saccade endpoint error: left targets, FEF: 1.49°, no TMS: 1.41°; right targets, FEF: 1.48°, no TMS: 1.30°).
PPC TMS control task.
In light of the results from the main experiment, we conducted a control task to ensure that our stimulation of PPC had been effective and that the absence of an effect of TMS for this condition on the task was not due to, for example, missing the target area. All but 1 of the same subjects was tested on a conjunction search task where the presence or absence of the target was indicated by a manual response rather than a saccade. Search arrays consisted of the same targets and distractors as in the saccade task, again with the target present on 50% of trials, but they were randomly distributed within a square (not shown to the subjects) of approximately 14 × 14° of visual angle on the PC monitor. Additionally, arrays consisted of 10 elements rather than 8. Arrays were presented until either a present or absent key-press response (on a PC keyboard) was made, and subjects were instructed to perform as fast as possible while minimizing errors. Stimulation was delivered over PPC, localized in the same manner as for the saccade task, or vertex. Additionally, trials with no stimulation were presented. TMS was delivered at 10 Hz for 500 ms at 60% of the machine maximum output, and 80 trials per stimulation condition were presented in 2 blocks of 40 trials. Median response times from correct trials are shown in Fig. 2 and were analyzed using repeated-measures ANOVA with factors of site (PPC, vertex, or none) and of trial type (present or absent). This showed a significant effect of site [F(1,6) = 13.093, P = 0.001] and of type [F(1,6) = 9.998, P = 0.02]. The type effect was due to slower responses for absent than present trials, whereas the site effect was due to slower responses for PPC stimulation. This was due to slower responses when TMS was delivered over PPC compared with both vertex and no-TMS conditions (P < 0.01).
DISCUSSION
In humans, the involvement and necessity of both PPC and FEF in visual search for a target defined by a conjunction of attributes has been illustrated previously (Ashbridge et al. 1997; Donner et al. 2002; Muggleton et al. 2003). Dissociation of the contribution of these two areas has so far been limited to the timing of their involvement using TMS (Ashbridge et al. 1997; Kalla et al. 2008; O'Shea et al. 2004), with FEF involvement preceding that of PPC. Using a visual search task where the required response was a saccade to the target (if it was present), we investigated whether FEF and PPC would result in dissociable patterns of disruption.
Task disruption was seen for FEF TMS, which reduced sensitivity on the task (measured with d-prime), but no effects were seen for PPC stimulation. These results show that it is possible to dissociate FEF and PPC contributions to visual search performance, although the absence of an effect of PPC stimulation differed from expectations. This was unlikely to have been a consequence of failure to stimulate PPC effectively. Numerous previous studies have shown visual search effects following PPC stimulation (Ashbridge et al. 1997; Ellison et al. 2003; Walsh et al. 1998), including those localizing the site in the same manner (Kalla et al. 2008). Furthermore, we successfully found disruption on a search task requiring manual indication of the target presence in the same subjects (with the exception of 1 who was not available).
The saccade search task used here offers the first instance of a visual search task in which disruption is not seen for both FEF and PPC TMS. One possibility for the difference in the effects of stimulating these two areas could be related to effects on the required motor response. It could be that the FEF disruption was a consequence of effects on saccade latencies given that TMS over this area is known to delay voluntary saccades, whereas the angular gyrus in PPC has been implicated in reflexive saccades (Mort et al. 2003). Such a delay could result in some saccades not being generated before trials were terminated, which, in turn, would produce a reduced accuracy score. However, the data do not support such a basis for the effect. First, mean saccade latencies were considerably lower than the cutoff time, meaning it would be unlikely that a delay in saccades would push any responses so late as to render them misses rather than hits. Second, and more importantly, there was no indication that there was a significant effect of FEF TMS on saccade latencies, possibly due to the emphasis on accurate performance or the fact that saccade delays caused by TMS are sensitive to the exact timing of the TMS.
PPC TMS disrupted neither target detection performance nor saccade measures on the task, whereas a control task confirmed that PPC TMS did disrupt search performance when the response was a manual one. This suggests that PPC involvement in visual search is dependent on the nature of the response required, whereas this is not the case for FEF. Furthermore, it is known that this is not just a motor effect alone, as stimulation of PPC during search for a target defined by a single attribute, such as color, does not result in disrupted performance. Recent findings with microstimulation (Juan et al. 2004) and TMS (Juan et al. 2008) support dissociation of visual and motor processes in human FEF. Such a dissociation has not been reported for PPC, which, although not meaning that no such dissociation exists, may be consistent with this area performing functions related to the interpretation of extrastriate signals and visuomotor transformation (Ellison et al. 2003). If this is the case, then a single time point of disruption, possibly relative to the response time rather than visual onset, might be expected. Data obtained here suggest that the function performed by this area is necessary when a manual response is required during conjunction visual search but not when the motor response is an eye movement. This means that, rather than being involved generally in the translation of visual information for action in a response-independent manner as we had initially hypothesized, PPC seems to be involved in such a transformation when the response is manual but not when it is a saccade. This is consistent with FEF involvement in the detection of a conjunction search target involving visual processes that may be linked to a saccade response or a manual response, whereas PPC may be only involved when a manual response has to be generated. The findings for PPC stimulation therefore differ from our original hypothesis of a target localization function for this area, being instead indicative of a response-dependent visuomotor transformation.
In summary, FEF is involved in visual search for a target defined by a combination of features when the presence of a target has to be indicated by an eye movement, but PPC is not. When taken alongside previous findings that both of these areas are involved in search conjunction search performance when a manual response is required, these results support a visual processing role for FEF, which is therefore necessary irrespective of the response required. In contrast, PPC involvement appears to be response-dependent, possibly reflecting a role in interpretation of signals for action when that action is a manual response.
GRANTS
N. G. Muggleton, R. Kalla, and V. Walsh were supported by the United Kingdom Medical Research Council. C.-H. Juan was supported by the National Science Council of Taiwan (96-2413-H-008-001-MY3, 97-2511-S-008-005-MY3).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- Ashbridge et al., 1997. Ashbridge E, Walsh V, Cowey A. Temporal aspects of visual search studied by transcranial magnetic stimulation. Neuropsychologia 35: 1121–1131, 1997 [DOI] [PubMed] [Google Scholar]
- Corbetta and Shulman, 1998. Corbetta M, Shulman GL. Human cortical mechanisms of visual attention during orienting and search. Philos Trans R Soc Lond B Biol Sci 353: 1353–1362, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta et al., 1995. Corbetta M, Shulman GL, Miezin FM, Petersen SE. Superior parietal cortex activation during spatial attention shifts and visual feature conjunction. Science 270: 802–805, 1995 [DOI] [PubMed] [Google Scholar]
- Donner et al., 2002. Donner TH, Kettermann A, Diesch E, Ostendorf F, Villringer A, Brandt SA. Visual feature and conjunction searches of equal difficulty engage only partially overlapping frontoparietal networks. Neuroimage 15: 16–25, 2002 [DOI] [PubMed] [Google Scholar]
- Elkington et al., 1992. Elkington PT, Kerr G, Stein JS. The effect of electromagnetic stimulation of the posterior parietal cortex on eye movements. Eye 6: 510–514, 1992 [DOI] [PubMed] [Google Scholar]
- Ellison et al., 2003. Ellison A, Battelli L, Cowey A, Walsh V. The effect of expectation on facilitation of colour/form conjunction tasks by TMS over area V5. Neuropsychologia 41: 1794–1801, 2003 [DOI] [PubMed] [Google Scholar]
- Ellison and Cowey, 2007. Ellison A, Cowey A. Time course of the involvement of the ventral and dorsal visual processing streams in a visuospatial task. Neuropsychologia 45: 3335–3339, 2007 [DOI] [PubMed] [Google Scholar]
- Friedman-Hill et al., 1995. Friedman-Hill SR, Robertson LC, Treisman A. Parietal contributions to visual feature binding: evidence from a patient with bilateral lesions. Science 269: 853–855, 1995 [DOI] [PubMed] [Google Scholar]
- Goldberg and Segraves, 1989. Goldberg ME, Segraves MA. The visual and frontal cortices. Rev Oculomot Res 3: 283–313, 1989 [PubMed] [Google Scholar]
- Göbel et al., 2001. Göbel S, Walsh V, Rushworth MF. The mental number line and the human angular gyrus. Neuroimage 14: 1278–1289, 2001 [DOI] [PubMed] [Google Scholar]
- Grosbras and Paus, 2003. Grosbras MH, Paus T. Transcranial magnetic stimulation of the human frontal eye field facilitates visual awareness. Eur J Neurosci 18: 3121–3126, 2003 [DOI] [PubMed] [Google Scholar]
- Hung et al., 2005. Hung J, Driver J, Walsh V. Visual selection and posterior parietal cortex: effects of repetitive transcranial magnetic stimulation on partial report analyzed by Bundesen's theory of visual attention. J Neurosci 25: 9602–9612, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juan et al., 2008. Juan CH, Muggleton NG, Tzeng OJ, Hung DL, Cowey A, Walsh V. Segregation of visual selection and saccades in human frontal eye fields. Cereb Cortex 18: 2410–2415, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juan et al., 2004. Juan CH, Shorter-Jacobi SM, Schall JD. Dissociation of spatial attention and saccade preparation. Proc Natl Acad Sci USA 101: 15541–15544, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalla et al., 2008. Kalla R, Muggleton NG, Juan CH, Cowey A, Walsh V. The timing of the involvement of the frontal eye fields and posterior parietal cortex in visual search. Neuroreport 19: 1067–1071, 2008 [DOI] [PubMed] [Google Scholar]
- Mohler et al., 1973. Mohler CW, Goldberg ME, Wurtz RH. Visual receptive fields of frontal eye field neurons. Brain Res 61: 385–389, 1973 [DOI] [PubMed] [Google Scholar]
- Moore et al., 2003. Moore T, Armstrong KM, Fallah M. Visuomotor origins of covert spatial attention. Neuron 40: 671–683, 2003 [DOI] [PubMed] [Google Scholar]
- Mort et al., 2003. Mort DJ, Perry RJ, Mannan SK, Hodgson TL, Anderson E, Quest R, McRobbie D, McBride A, Husain M, Kennard C. Differential cortical activation during voluntary and reflexive saccades in man. Neuroimage 18: 231–246, 2003 [DOI] [PubMed] [Google Scholar]
- Muggleton et al., 2008. Muggleton NG, Cowey A, Walsh V. The role of the angular gyrus in visual conjunction search investigated using signal detection analysis and transcranial magnetic stimulation. Neuropsychologia 46: 2198–2202, 2008 [DOI] [PubMed] [Google Scholar]
- Muggleton et al., 2003. Muggleton NG, Juan CH, Cowey A, Walsh V. Human frontal eye fields and visual search. J Neurophysiol 89: 3340–3343, 2003 [DOI] [PubMed] [Google Scholar]
- Müri et al., 2000. Müri RM, Gaymard B, Rivaud S, Vermersch A, Hess CW, Pierrot-Deseilligny C. Hemispheric asymmetry in cortical control of memory-guided saccades. A transcranial magnetic stimulation study. Neuropsychologia 38: 1105–1111, 2000 [DOI] [PubMed] [Google Scholar]
- Nowak et al., 1997. Nowak LG, James AC, Bullier J. Corticocortical connections between visual areas 17 and 18a of the rat studied in vitro: spatial and temporal organisation of functional synaptic responses. Exp Brain Res 117: 219–241, 1997 [DOI] [PubMed] [Google Scholar]
- Nuding et al., 2009. Nuding U, Kalla R, Muggleton NG, Büttner U, Walsh V, Glasauer S. TMS evidence for smooth pursuit gain control by the frontal eye fields. Cereb Cortex 19: 1144–1150, 2009 [DOI] [PubMed] [Google Scholar]
- O'Shea et al., 2004. O'Shea J, Muggleton NG, Cowey A, Walsh V. Timing of target discrimination in human frontal eye fields. J Cogn Neurosci 16: 1060–1067, 2004 [DOI] [PubMed] [Google Scholar]
- Paus et al., 1997. Paus T, Jech R, Thompson CJ, Comeau R, Peters T, Evans AC. Transcranial magnetic stimulation during positron emission tomography: a new method for studying connectivity of the human cerebral cortex. J Neurosci 17: 3178–3184, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priori et al., 1993. Priori A, Bertolasi L, Rothwell JC, Day BL, Marsden CD. Some saccadic eye movements can be delayed by transcranial magnetic stimulation of the cerebral cortex in man. Brain 116: 355–367, 1993 [DOI] [PubMed] [Google Scholar]
- Ruff et al., 2006. Ruff CC, Blankenburg F, Bjoertomt O, Bestmann S, Freeman E, Haynes JD, Rees G, Josephs O, Deichmann R, Driver J. Concurrent TMS-fMRI and psychophysics reveal frontal influences on human retinotopic visual cortex. Curr Biol 16: 1479–1488, 2006 [DOI] [PubMed] [Google Scholar]
- Rushworth et al., 2002. Rushworth MF, Hadland KA, Paus T, Sipila PK. Role of the human medial frontal cortex in task switching: a combined fMRI and TMS study. J Neurophysiol 87: 2577–2592, 2002 [DOI] [PubMed] [Google Scholar]
- Sato and Schall, 2003. Sato TR, Schall JD. Effects of stimulus-response compatibility on neural selection in frontal eye field. Neuron 38: 637–648, 2003 [DOI] [PubMed] [Google Scholar]
- Schall and Bichot, 1998. Schall JD, Bichot NP. Neural correlates of visual and motor decision processes. Curr Opin Neurobiol 8: 211–217, 1998 [DOI] [PubMed] [Google Scholar]
- Schmolesky et al., 1998. Schmolesky MT, Wang Y, Hanes DP, Thompson KG, Leutgeb S, Schall JD, Leventhal AG. Signal timing across the macaque visual system. J Neurophysiol 79: 3272–3278, 1998 [DOI] [PubMed] [Google Scholar]
- Silvanto et al., 2006. Silvanto J, Lavie N, Walsh V. Stimulation of the human frontal eye fields modulates sensitivity of extrastriate visual cortex. J Neurophysiol 96: 941–945, 2006 [DOI] [PubMed] [Google Scholar]
- Stewart et al., 2001. Stewart LM, Walsh V, Rothwell JC. Motor and phosphene thresholds: a transcranial magnetic stimulation correlation study. Neuropsychologia 39: 415–419, 2001 [DOI] [PubMed] [Google Scholar]
- Taylor et al., 2007. Taylor PC, Nobre AC, Rushworth MF. FEF TMS affects visual cortical activity. Cereb Cortex 17: 391–399, 2007 [DOI] [PubMed] [Google Scholar]
- Treisman, 1996. Treisman A. The binding problem. Curr Opin Neurobiol 6: 171–178, 1996 [DOI] [PubMed] [Google Scholar]
- van Donkelaar and Müri, 2002. van Donkelaar P, Müri R. Craniotopic updating of visual space across saccades in the human posterior parietal cortex. Proc Biol Sci 269: 735–739, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh et al., 1998. Walsh V, Ashbridge E, Cowey A. Cortical plasticity in perceptual learning demonstrated by transcranial magnetic stimulation. Neuropsychologia 36: 363–367, 1998 [DOI] [PubMed] [Google Scholar]