Abstract
Intercalated (ITC) amygdala neurons are thought to play a critical role in the extinction of conditioned fear. However, several factors hinder progress in studying ITC contributions to extinction. First, although extinction is usually studied in rats and mice, most ITC investigations were performed in guinea pigs or cats. Thus it is unclear whether their connectivity is similar across species. Second, we lack criteria to identify ITC cells on the basis of their discharge pattern. As a result, key predictions of ITC extinction models remain untested. Among these, ITC cells were predicted to be strongly excited by infralimbic inputs, explaining why infralimbic inhibition interferes with extinction. To study the connectivity of ITC cells, we labeled them with neurobiotin during patch recordings in slices of the rat amygdala. This revealed that medially located ITC cells project topographically to the central nucleus and to other ITC clusters located more ventrally. To study the infralimbic responsiveness of ITC cells, we performed juxtacellular recording and labeling of amygdala cells with neurobiotin in anesthetized rats. All ITC cells were orthodromically responsive to infralimbic stimuli, and their responses usually consisted of high-frequency (∼350 Hz) trains of four to six spikes, a response pattern never seen in neighboring amygdala nuclei. Overall, our results suggest that the connectivity of ITC cells is conserved across species and that ITC cells are strongly responsive to infralimbic stimuli, as predicted by extinction models. The unique response pattern of ITC cells to infralimbic stimuli can now be used to identify them in fear conditioning experiments.
Keywords: extinction, fear
systematic desensitization, the approach clinicians often depend on to treat anxiety disorders, has much in common with the procedure used to extinguish conditioned fear in the laboratory. In both cases, the feared object or situation (conditioned stimulus, CS) is presented repeatedly in the absence of danger (or unconditioned stimulus). These similarities, coupled to the realization that human anxiety disorders are associated with an extinction deficit (Blechert et al. 2007; Milad et al. 2008), have led to an explosion of interest in the mechanisms underlying extinction (Myers and Davis 2007; Quirk and Mueller 2008).
Although extinction likely engages multiple parallel mechanisms, accumulating data suggest that intercalated (ITC) amygdala neurons, a population of GABAergic cells (McDonald and Augustine 1993; Nitecka and Ben-Ari 1987; Paré and Smith 1993a), play a critical role in extinction. Indeed, selective lesions (Likhtik et al. 2008) and pharmacological inhibition of basolateral (BL) inputs to ITC cells (Jungling et al. 2008) interfere with extinction. Moreover, an ex vivo study revealed that extinction training causes a potentiation of BL inputs to ITC cells, an effect dependent on infralimbic (IL) activity for its induction and on postsynaptic mechanisms for its expression (Amano et al. 2010). Thus these results suggest that following extinction, CS-related BL inputs trigger, via ITC cells, more feedforward inhibition in the medial sector of the central (Ce) nucleus (CeM), leading to a reduction in conditioned fear.
Currently, several factors hinder progress in understanding how ITC cells regulate fear expression. First, although experimental studies on extinction are typically performed in rats and mice, the majority of studies on ITC cells have been performed in guinea pigs and cats (Collins and Paré 1999; Paré and Smith 1993a, 1993b; Royer and Paré 2002, 2003; Royer et al. 1999, 2000a, 2002b), with few exceptions (Geracitano et al. 2007; Marowsky et al. 2005). As a result, it is currently unclear whether critical aspects of ITC connectivity also characterize commonly used rodent species. For instance, whereas reports in cats, guinea pigs, and mice (reviewed in Pape and Pare 2010) suggested that ITC cells contribute a strong projection to Ce, the only available study in rats questioned the existence of this projection (Shammah-Lagnado et al. 1999).
Second, we lack criteria to identify ITC cells on the basis of their discharge pattern and have little data about their activity in vivo (Collins and Paré 1999). As a result, it has been impossible to test key predictions of ITC extinction models (Paré et al. 2004). Among these, it was predicted that ITC cells are strongly excited by IL inputs, explaining why IL inhibition interferes with the acquisition of extinction (Sierra-Mercado et al. 2006) and prevents the potentiation of BL inputs to ITC cells (Amano et al. 2010).
Thus this study was undertaken to address these critical gaps in our knowledge focusing on 1) the connections formed by ITC cells in the amygdala, 2) the identification of ITC cells on the basis of their extracellularly recorded activity, and 3) characterizing the impact of IL inputs on rat ITC neurons.
METHODS
Experiments were performed in Sprague-Dawley rats, in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and with the approval of the Institutional Animal Care and Use Committee of Rutgers University (Newark, NJ).
The present report relied on two different approaches. To study the connections of ITC cells with each other and other amygdala nuclei, we prepared coronal slices of the amygdala and labeled ITC cells with neurobiotin during whole cell patch recordings in brain slices kept in vitro. To study the firing pattern and IL responsiveness of ITC cells, we performed juxtacellular recording of ITC cells in urethane-anesthetized rats. We describe these two approaches in turn below.
Whole Cell Patch Recording of ITC Cells In Vitro
Slice preparation.
These experiments were performed using coronal brain slices obtained from 30 Sprague-Dawley rats (100–150 g). The rats were anesthetized with ketamine, pentobarbital, and xylazine (80, 60, and 12 mg/kg ip, respectively). After abolition of all reflexes, they were perfused through the heart with a cold (4°C) modified artificial cerebrospinal fluid (aCSF) that contained (in mM) 126 choline chloride, 2.5 KCl, 1.25 NaH2PO4, 1 MgCl2, 2 CaCl2, 26 NaHCO3, and 10 glucose. The brains were then extracted and cut in 400-μm-thick slices with a vibrating microtome while submerged in the same solution as for the transcardial perfusion. After cutting, slices were transferred to an incubating chamber where they were allowed to recover for at least 1 h at 20°C in a control aCSF with the same composition as that used above with the exception that NaCl was substituted for choline chloride (pH 7.3, 300 mOsm). The slices were then transferred one at a time to a recording chamber perfused with the same solution (7 ml/min). Before the recordings began, the temperature of the chamber was gradually increased to 32°C.
Electrophysiology.
Under visual guidance with differential interference contrast and infrared videomicroscopy, we obtained whole cell patch recordings of ITC neurons using pipettes (7–10 MΩ) pulled from borosilicate glass capillaries and filled with a solution containing (in mM) 130 K-gluconate, 10 N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 10 KCl, 2 MgCl2, 2 ATP-Mg, and 0.2 GTP-tris(hydroxymethyl)aminomethane (pH 7.2, 280 mosM) plus 0.5% neurobiotin. The liquid junction potential was 10 mV with this solution, and the membrane potential was corrected accordingly. Current-clamp recordings were obtained with an Axoclamp 2B amplifier and digitized at 10 kHz with a Digidata 1200 interface (Axon Instruments, Foster City, CA).
To characterize the electroresponsive properties of recorded cells, we applied graded series of depolarizing and hyperpolarizing current pulses (20 pA, 500 ms in duration) from rest and other prepulse potentials. The input resistance of the cells was estimated in the linear portion of current-voltage plots.
No special current injection protocol had to be used to label ITC cells with neurobiotin. It diffused into the cells as we studied their electroresponsive properties. At the conclusion of the recordings, the slices were removed from the chamber and fixed for 1–3 days in 0.1 M phosphate-buffered saline (PBS; pH 7.4) containing 4% paraformaldehyde. Slices were then embedded in 3% agar and sectioned on a vibrating microtome at a thickness of 100 μm. Neurobiotin-filled cells were visualized by incubating the sections in the avidin-biotin-horseradish peroxidase solution (ABC Elite kit; Vector Laboratories, Burlingame, CA) and processed to reveal the horseradish peroxidase staining (Horikawa and Armstrong 1988).
Juxtacellular Recording of ITC Cells In Vivo
Surgery.
A total of 150 Sprague-Dawley rats (250–350 g) were anesthetized with urethane (1.8 g/kg) and administered atropine (0.05 mg/kg im) to reduce salivation. Throughout the experiment, body temperature was kept at 37°C with a heating pad. After placing the rats in a stereotaxic frame and shaving the scalp, we made numerous, evenly spaced, small injections of the analgesic bupivacaine (0.125% solution, subcutaneously) around the sites to be incised. Ten minutes later, the scalp was incised above the amygdala, IL cortex, and upper brain stem, and small openings were drilled into the skull. After the dura mater was opened, pairs of stimulating electrodes were stereotaxically inserted in the IL cortex and just dorsal to the substantia nigra, where Ce axons en route to the brain stem form a compact bundle (Hopkins and Holstege 1978), all in the same hemisphere. These stimulating electrodes were used to antidromically invade BL cells projecting to the IL cortex and Ce cells projecting to the brain stem, respectively (Hopkins and Holstege 1978; Krettek and Price 1977; McDonald 1991). In a subset of experiments, IL stimulating electrodes were inserted ipsi- and contralaterally to the recording site in the amygdala. It should be noted that because central lateral (CeL) neurons send strong projections to the parabrachial nucleus (Petrovich and Swanson 1997) and their axons initially course through the same region as CeM axons en route to their brain stem targets (Hopkins and Holstege 1978), the brain stem stimuli we delivered, just dorsal to the substantia nigra, could elicit antidromic responses in both CeL and CeM cells.
Recording, stimulation, and labeling with neurobiotin.
Juxtacellular ITC recordings were obtained with pipettes (0.5-μm tip, 20–30 MΩ) pulled from borosilicate glass capillaries and filled with a solution containing 1 M NaCl plus 1.5% neurobiotin. The pipettes were aimed to Ce with a slight (18°) mediolateral angle and gradually lowered with a micromanipulator. Every 25 μm, electrical stimuli were delivered at a low frequency (0.3 Hz) to the brain stem and IL cortex, alternating between the two sites. Although we used a range of stimulation intensities (0.2–1.5 mA, 100 μs), in the results we report the response patterns obtained with the same stimulation intensity at the two stimulated sites (1 mA). Each time a responsive cell was encountered, we recorded its spontaneous activity for ≥3 min and the responsiveness to IL and brain stem stimuli. The term “spike burst” refers to a cluster of spikes with relatively stereotyped features (comprising 2 or more spikes occurring at a frequency ≥150 Hz).
Extracellular data were amplified 1,000 times with Multiclamp700B (Molecular Devices, Sunnyvale, CA) using a bandwidth of 0.1–6 kHz. The data were sampled at 20 kHz, stored on a hard drive, and analyzed off-line with custom-made programs written in MATLAB (The MathWorks, Natick, MA) and Igor (Wavemetrics, Lake Oswego, OR). Analyses of spike shapes were performed in MATLAB.
Because Ce and BL neurons have differential projections to the brain stem and IL cortex (Hopkins and Holstege 1978; Krettek and Price 1977; McDonald 1991), at some point during a proportion of electrode tracks we observed an abrupt transition in the stimulating sites effective in backfiring recorded neurons (from brain stem to IL stimuli). The region located between the last Ce cell antidromically responsive to brain stem stimuli and the first BL cell backfired from the IL cortex therefore corresponded to the Ce-BL border where ITC cells are known to be located. Thus we then moved the electrode back to the position of the last antidromically responsive Ce cell and, without withdrawing the pipette from the brain, moved the pipette 20–40 μm laterally or medially. After a delay of 20 min, the pipette was moved ventrally again, and the first juxtacellularly recorded neuron that was spontaneously active and/or orthodromically responsive to stimulation of any of our stimulation sites (IL, brain stem) was labeled with neurobiotin. This was achieved by applying positive current pulses (100 ms) at 5 Hz for 1–5 min via the recording pipette. The current amplitude was adjusted to the minimum required to make the cell discharge at each current injection cycle. During this period, if the cell was responsive to IL stimulation before current injection, electrical stimuli were delivered at the same site every 30 s to make sure the firing properties of the cell remained constant. We attempted to label only one cell per experiment.
After labeling, the rats were given an overdose of pentobarbital (80 mg/kg) and perfused with 150 ml of 0.9% saline followed by 150 ml of a fixative containing 4% paraformaldehyde and 0.5% glutaraldehyde in 0.1 M phosphate buffer (PB; pH 7.4). The brain was then removed from the skull and sectioned at 100 μm on a vibrating microtome.
Neurobiotin Revelation
Slices from in vitro recordings were embedded in 3% agar and resectioned at 100 μm. After this, the same approach was used to reveal neurobiotin-labeled neurons recorded in vitro and in vivo. Sections were washed several times in PB (0.1 M, pH 7.4) and then transferred to a 0.5% H2O2 solution in PB for 15 min. After numerous washes in PB, sections were incubated for 12 h at 20°C in a solution containing 0.5% Triton and 1% solutions A and B of an ABC kit (Vector Laboratories) in PB. The next day, they were washed in PB (5 × 10 min). Neurobiotin was visualized by incubating the sections in a 0.1 M PB solution that contained 0.05% diaminobenzidine tetrahydrochloride (Sigma), 2.5 mM nickel ammonium sulfate (Fisher), and 0.003% H2O2 for 5–10 min. The sections were then washed in PB (5 × 10 min), mounted on gelatin-coated slides, and air-dried. The sections were then counterstained with cresyl violet and coverslipped with Permount for later reconstruction.
All visible processes of the labeled neurons were observed in a microscope with a ×40 objective and then photographed. Typically, their processes extended over several sections. To align the sections, we layered the photographs in Photoshop (Adobe Systems, San Jose, CA) and used blood vessels or other obvious landmarks present in the various sections to align them. The layers were then collapsed and the entire neuron drawn. Note that for a neuron to be considered as projecting to a particular nucleus, it had to meet two conditions. First, it had to contribute an axon to this nucleus. Second, the axonal segment seen in this nucleus had to bear axonal varicosities.
Processing for μ-Opioid Receptor Immunohistochemistry
The transcardial perfusion was performed as described above for the in vivo experiments with the exception that the fixative also contained 0.2% picric acid but no glutaraldehyde. After extraction of the brain, overnight postfixation, and cutting (all as described for the in vivo experiments), half the sections (100 μm) were stained with cresyl violet and the other half processed to reveal μ-opioid receptor (μOR) immunoreactivity. The μOR antibody was obtained from DiaSorin (Stillwater, MN). This antibody was previously characterized for specificity and cross-reactivity (Kalyuzhny et al. 1996; Wilson et al. 2002). For instance, absorption of the μOR antibody with its antigen (35 mg/ml) abolished all immunostaining. Furthermore, in our experiments, omission of the antibody from the following protocol abolished all differentiated staining. The sections were washed in PBS (4 × 5 min) and then incubated in 0.5% H2O2 in PBS for 15 min, washed in PBS (4 × 5 min), preincubated in a blocking solution (10% normal goat serum, 1% BSA, and 0.3% Triton X-100), and incubated overnight in the primary antibody solution containing a μOR antibody (1:4,000), 1% normal goat serum, 1% BSA, and 0.3% Triton X-100 in PBS. Sections were then incubated in the secondary antibody solution (Jackson, West Grove, PA; 1:200), followed by the avidin-biotin-complex (Vector Laboratories). After several washes in PBS, the immunoreactivity was then revealed by incubating the sections in a 0.1 M PB solution that contained 0.05% diaminobenzidine tetrahydrochloride (Sigma), 2.5 mM nickel ammonium sulfate (Fisher), and 0.003% H2O2 for 5–10 min. The sections were then washed in PB (5 × 10 min), mounted on gelatin-coated slides, and air-dried. We then compared the position of ITC cell clusters on adjacent sections processed to reveal μOR or stained with cresyl violet.
Histological Identification of ITC Cell Clusters
We considered that a neurobiotin-labeled neuron was an ITC cell when counterstaining with cresyl violet revealed that it was located in a densely packed cluster of darkly stained neurons at the BL-Ce border. This approach was selected on the basis of earlier work and additional tests, as described below. Previously, it was reported that in amygdala sections processed to reveal GABA immunoreactivity or counterstained with cresyl violet, ITC cells are conspicuous because they occur as densely packed and darkly stained clusters of small neurons (Paré and Smith 1993a, 1993b, 1994). Moreover, in adjacent sections of the rat amygdala, a close correspondence was found between the positions of the ITC cell clusters labeled with these two methods (see Fig. 1 in Pape and Pare 2010).
Fig. 1.
Identification of intercalated (ITC) cell clusters. Two adjacent coronal sections of the rat amygdala (100-μm thickness) are shown. A: counterstaining with cresyl violet. B: distribution of immunoreactivity for μ-opioid receptors (μORs). Note that darkly stained clusters of cells on the section counterstained with cresyl violet (arrows in A) correspond to patches of dense immunolabeling for μORs (arrows in B). BL, basolateral; CeL, central lateral nucleus; CeM, central medial nucleus; OT, optic tract; ITCd, dorsal ITC cluster; ITCv, ventral ITC cluster; ITCm, large (main) ITC cluster.
Since earlier work had revealed that ITC cell clusters exhibit high levels of μOR, (Jacobsen et al. 2006; Wilson et al. 2002), we further tested whether the ITC cell clusters identified with cresyl violet corresponded to patches of dense μ-OR immunoreactivity. As shown in Fig. 1, a close correspondence was found between the position of ITC cell clusters (arrows) in adjacent sections stained with cresyl violet (Fig. 1A) or processed to reveal μOR immunoreactivity (Fig. 1B). However, pilot tests revealed that the intensity of the μOR immunoreaction was so high that it interfered with visualization of neurobiotin-labeled neurons. To prevent the μOR staining from interfering with the visualization of the neurobiotin-labeled cells, the revelation time had to be reduced. However, we found that at a lower intensity, the μOR staining became no more useful than the cresyl violet to identify ITC cell clusters yet required much more processing. Given that counterstaining with cresyl violet yielded highly reproducible results, we opted for this approach to identify ITC cell clusters for both in vitro and in vivo experiments.
RESULTS
Nomenclature Used to Describe ITC Cell Clusters at the BL-Ce Border
As previously reported (Millhouse 1986), ITC cell clusters are found along the external as well as in and around the fiber bundle located between the BL and Ce. The present report focuses on the latter groups of ITC cells. In sections counterstained with cresyl violet (Fig. 1A) or processed to reveal μOR immunoreactivity (Fig. 1B), several ITC cell clusters can be seen at the BL-Ce border. Dorsally, there is a rather wide cluster close to the dorsolateral edge of the CeL. We refer to this dorsal cluster as ITCd (Fig. 1). More ventrally, one can usually see one or more thinner and elongated ITC clusters immediately lateral or ventrolateral to CeM. We have designated these more ventral clusters as ITCv (Fig. 1). Finally, ventral to CeM, especially at rostral levels of the amygdala, one can see a large ITC cluster. Its size varies depending on the rostrocaudal level, but it can be as wide as 1 mm. We refer to this (main) cluster as ITCm (Fig. 1). In addition to its larger size, ITCm can be distinguished from ITCv because the latter is always found in the fiber bundle separating BL from more medially located amygdala nuclei, whereas ITCm is not.
In Vitro Experiments: Connectivity of ITC Neurons
During patch recordings in coronal slices of the amygdala, a total of 30 ITC cells were labeled with neurobiotin and recovered: 19 were ITCd cells, and 11 were ITCv neurons. The physiological properties of these ITC cells matched the descriptions found in previous reports (Geracitano et al. 2007; Marowsky et al. 2005; Royer et al. 1999, 2000a, 2000b, 2002, 2003), including a very negative membrane potential (−86.7 ± 1.2 mV), a high input resistance (552.0 ± 35.2 MΩ), action potentials of intermediate duration (1.1 ± 0.05 ms at half-amplitude), and limited spike frequency adaptation during prolonged depolarizing current pulses (34.2 ± 3.9% increase in interspike interval duration from the first to the last interval in current pulses eliciting 6–10 spikes).
Figure 2 shows representative examples of morphologically identified ITCd (A) and ITCv neurons (B and C) at low (A1 and B1) or high magnifications (A2–A4 and B2–B4). Irrespective of their position, ITC cells displayed a moderate to high density of dendritic spines (Fig. 2, A4 and B4) and contributed varicose axons (Fig. 2, A3 and B3) that ramified in various ways (see below). The appearance of their dendritic trees matched the shape of the clusters where their somata were located. Indeed, ITC cells located in the larger dorsal clusters tended to have radial and multipolar dendritic trees (Fig. 2A2), whereas those located in the thinner ventral clusters cells typically had flattened and bipolar dendritic arborizations (Fig. 2B2).
Fig. 2.
Morphological properties of ITC neurons. Whole cell patch recordings of ITC cells were obtained in brain slices kept in vitro. ITC cells were labeled with neurobiotin present in the pipette solution. A–C: 3 different ITC cells. A1 and B1 are low-power photomicrographs showing the position of the cells on coronal sections counterstained with cresyl violet. A2–A4, B2–B4, and C are high-power photomicrographs of the same ITC cells. The dendritic trees of ITC cells ranged from stellate (A2) to flattened (B2). They had varicose axons (A3 and B3) that contributed 2 or more collaterals. ITC cells displayed a moderate to high density of dendritic spines (A4 and B4). LA, lateral nucleus; EC, external capsule.
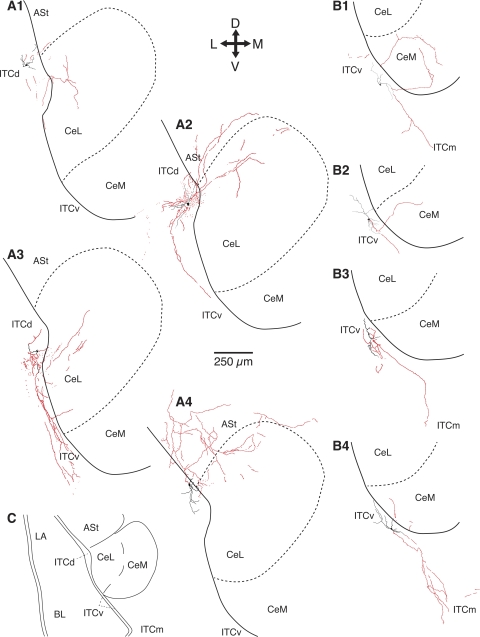
Although the electroresponsive properties of ITCd and ITCv cells were statistically indistinguishable (t-tests, P > 0.05), marked differences in their projection patterns were observed. See Fig. 3 for representative examples of ITCd (Fig. 3, A1–A4) or ITCv neurons (Fig. 3, B1–B4). As a group, ITCd neurons projected to three sites: CeL (Fig. 3, A1–A4), the amygdalostriatal transition area (ASt; Fig. 3, A2 and A4), and other ITC cell clusters located more ventrally (Fig. 3, A2 and A3). There were no significant differences in the incidence of projections to the three sites (Fisher's exact probability test, P = 0.5; CeL, 58% or 11 of 19; ASt, 53% or 10 of 19; ITCv, 37% or 7 of 19), and many ITCd cells (58% or 11 of 19) projected to more than one of these three sites. However, the latter percentage is probably an underestimate, because several axonal branches were seen to exit the plane of the slice close to the parent somata.
Fig. 3.
Axonal projection patterns of ITC cells located in the cluster adjacent to CeL (ITCd; A) or to CeM (ITCv; B). All the depicted cells were recorded in vitro. The scheme in C shows a general view of the amygdala nuclei depicted in A and B. The orientation of the sections is indicated by the cross at top, where L, M, D, and V stand for lateral, medial, dorsal, and ventral, respectively. Red, axons; black, dendritic segments. ITCd cells contributed axon collaterals to one or more of the following sites: CeL (A1–A4), the amygdalostriatal transition area (ASt; A2 and A4), or ITCv cells (A2 and A3). ITCv cells contributed axon collaterals to CeM (B1, B2, and B4) and/or to ITCm (B1, B3, and B4).
In contrast with ITCd cells, no ITCv neurons projected to ASt or CeL (Fig. 3, B1–B4). Instead, they projected to CeM (45% or 5 of 11; Fig. 3, B1, B2, and B4). In addition, 73% of ITCv cells (or 8 of 11) contributed one or more axonal branches toward ITCm neurons (Fig. 3, B1, B3, and B4). In this case, too, the above data probably underestimate the extent of the axonal arborization of ITCv cells, because many axonal branches were seen to exit the plane of the slice close to the parent somata.
In summary, the general principles emerging from these results are that ITC cells located at the BL-Ce border project to Ce and to other ITC cells located more ventrally. The main difference between ITCd and ITCv cells resides in the particular sector of Ce they target: CeL in the case of ITCd cells and CeM in the case of ITCv neurons.
Synaptic Responsiveness and Spontaneous Activity of ITC Cells In Vivo
Since the goal of the in vivo experiments was to determine whether ITC neurons could be distinguished from other types of amygdala neurons on the basis of their extracellularly recorded activity, we first describe the behavior of cells in the amygdala nuclei adjacent to ITC cells: BL and Ce. We then compared the activity of these cells to that of ITC neurons. Figure 4 summarizes the responsiveness of BL, Ce, and ITC neurons to brain stem and IL stimuli. Representative examples of unit responses to the same stimuli are provided in Figs. 5 and 7. After completing histological reconstructions of 29 electrode tracks (Fig. 4A), we determined that a total of 71 and 158 spontaneously active and/or anti- or orthodromically responsive neurons were recorded in BL and Ce, respectively.
Fig. 4.
Contrasting responsiveness of BL, central nucleus (Ce), and ITC cells to electrical stimuli delivered in the infralimbic (IL) cortex and brain stem (BS). Single-unit recordings of amygdala neurons were obtained with high-impedance micropipettes in urethane-anesthetized rats. A: scheme showing 29 electrode tracks with dots indicating the position of recorded cells. In all 29 cases, an amygdala neuron was juxtacellularly labeled with neurobiotin and its position used to infer the location of all recorded cells. In 3 of these tracks (arrow and open circles), the labeled cells were located in the LA. In the remaining tracks, we attempted to label cells near the Ce-BL border. The 19 cases where the recovered cells were within ≤0.2 mm of the Ce-BL border are depicted in Fig. 6B. B–D: proportion of responsive BL, Ce, and ITC cells to electrical stimuli delivered in IL (B and C) or BS (D and E). Antidromic responses are shown in B and D; orthodromic responses are shown in C and E. AST, amygdalostriatal transition; BLA, basolateral amygdala; STR, striatum.
Fig. 5.
Examples of unit responses elicited by electrical stimuli delivered in IL (A–C) or BS (D and E). A and B show data for 2 different BL neurons, D shows a Ce cell, and C and E show LA neurons. In a high proportion of BL neurons (but never in Ce cells), IL stimulation elicited antidromic response characterized by fixed latency (A, top) and collision with spontaneous action potentials (A, bottom). In a few BL cells, IL stimulation evoked orthodromic responses consisting of single spikes (B) or, very rarely, spike bursts (C). The latter response pattern was only seen in 3 LA cells (their position is marked by an arrow in Fig. 4A). In a high proportion of Ce neurons (but never in BL cells), BS stimuli elicited antidromic responses characterized by a fixed latency (D, top) and collision with spontaneous action potentials (D, bottom). BS stimuli elicited orthodromic responses in a few rare LA cells (E).
Fig. 7.
ITC cells can be identified during extracellular recordings on the basis of their unusual IL responsiveness. By using the contrasting pattern of antidromic responsiveness of Ce and BL neurons to BS and IL stimuli, the location of the Ce-BL border was identified. Juxtacellular recordings of border cells were then obtained. After their responsiveness to IL and BS stimuli was examined, the cells were labeled with neurobiotin. Bar graphs in A compare the proportion of morphologically and/or histologically identified ITC, Ce, and BL neurons responding to IL stimuli with high-frequency spike bursts (A1), the number of spikes in these bursts (A2), the first interspike interval (ISI) duration in these bursts (A3), and their latency (A4). B–D show examples of morphologically-identified ITC cells (B1, C1, and D1) and their responses to IL stimuli (B2, C2, and D2). Higher power micrographs of the cells shown in B–D are provided in E1, E2, and E4, respectively. A drawing of the cell in D1 and E4 is shown in E3 (red, axon; black, soma and dendrites).
BL neurons.
It should be noted that a minority of the cells described in this section were recorded in the lateral nucleus (LA; n = 17). For simplicity, we pooled these LA neurons with BL cells, because their spontaneous activity and responsiveness did not differ appreciably from that seen in BL cells. Consistent with earlier studies (Jacobs and McGinty 1971; Likhtik et al. 2006; Paré and Gaudreau 1996; Rosenkranz and Grace 1999), BL cells generally exhibited low spontaneous discharge rates with as many as 69% (or 49 of 71) of them firing ≤0.1 Hz. Among the BL cells with significant background activity (31% or 22 of 71), some neurons were firing at rates as high as 7 Hz.
To determine whether this variability was related to the identity of the cells, we compared the firing rates of BL neurons that could be physiologically identified as projection cells by antidromic invasion from IL with those of the unidentified units, a proportion of which likely corresponds to interneurons. Indeed, a minority of BL cells are local-circuit neurons (reviewed in McDonald 1992), many of which are intrinsically more excitable than projection cells (reviewed in Pape and Pare 2010; Sah et al. 2003). In keeping with this, firing rates were significantly higher in the subgroup of unidentified cells (2.86 ± 0.59 Hz, n = 14) than in projection neurons (0.23 ± 0.07 Hz, n = 57; t-test, P = 0.0011).
Consistent with previous reports (Likhtik et al. 2005), IL stimuli elicited antidromic responses in a high proportion of BL cells (80% or 57 of 71; latency of 14.9 ± 0.7 ms; Figs. 4B and 5A). A much lower proportion of BL cells was orthodromically activated by IL stimuli (10% or 7 of 71; Fig. 4C). In most of these orthodromically responsive BL cells (4 of 7), IL stimuli elicited single spikes (Fig. 5B1). See Fig. 5C for an exception to this general rule. Note that when we stimulated IL, it is likely that current diffused from the stimulation site and activated neuronal processes in neighboring cortical fields (such as the prelimbic area) or coursing through or close to IL en route to other cortical regions. This effect could have artificially increased the proportion of antidromically responsive BL cells.
No BL neurons were antidromically responsive to brain stem stimuli (Fig. 4D). However, a few BL cells were orthodromically activated by brain stem stimuli (7% of cells or 5 of 71; latency of 5.8 ± 0.5 ms; Figs. 4E and 5E).
Ce neurons.
As in BL neurons, the spontaneous firing rates of Ce neurons were extremely low, with the majority of cells (77% or 122 of 158) firing ≤0.1 Hz. In response to brain stem stimuli, a high proportion of Ce cells generated antidromic spikes (67% or 106 of 158; latency of 19.2 ± 1.2 ms; Figs. 4D and 5D) and a minority, orthodromic responses (4% or 7 of 158; latency of 9.9 ± 0.7 ms; Fig. 4E). As in BL neurons, Ce projection neurons (identified as such by antidromic invasion from brain stem) fired at significantly lower rates than the unidentified cells (projection cells: 0.07 ± 0.04 Hz, n = 106; unidentified: 2.10 ± 0.65 Hz, n = 52; t-test, P = 0.007). In contrast to BL neurons, however, no Ce neurons were backfired from IL (Fig. 4B), although a few unidentified cells (15% or 24 of 158; Fig. 4C) displayed long-latency (18.4 ± 1.2 ms) orthodromic responses that always consisted of single spikes. Finally, IL stimulation produced a dramatic reduction in the firing rate of all the spontaneously active Ce projection cells we encountered, as previously reported (Quirk et al. 2003).
ITC cells.
Because of the small size of ITC cell clusters, post hoc histological reconstructions of electrode tracks marked with electrolytic lesions do not provide sufficient resolution to determine whether a given recorded cell is indeed an ITC cell. To circumvent this difficulty, we used the following approach. Micropipettes containing 1.5% neurobiotin were aimed to Ce and gradually lowered, applying brain stem and IL stimuli at regular intervals along the electrode track. Because Ce and BL neurons have differential projections to the brain stem and IL, at some point during most tracks we observed an abrupt transition in the stimulation sites effective in backfiring recorded neurons (from brain stem to IL stimuli; Fig. 6A). The region located between the last Ce cell antidromically responsive to brain stem stimuli and the first BL cell backfired from IL therefore corresponded to the Ce-BL border, where ITC cells are known to be located. Thus the electrode was then retracted to the position of the last antidromically responsive Ce cell and moved 20–40 μm laterally or medially while still in the brain. After a 20-min delay, the pipette was moved ventrally again and the first juxtacellularly recorded neuron that was spontaneously active and/or orthodromically responsive was labeled with neurobiotin. In other cases, the pattern of antidromic responsiveness only allowed estimation of the ventral border of Ce or dorsal border of BL. In such cases, the pipette was again retracted to the estimated position of the BL-Ce border, and we then used the same approach as described above.
Fig. 6.
The position of the BL-Ce border can be identified on the basis of the contrasting pattern of antidromic responsiveness of Ce and BLA neurons to BS and IL stimuli, respectively. A: number of cells backfired from the BS (solid bars) or IL (open bars) plotted as a function of depth relative to the BLA-Ce border in 29 microelectrode tracks. B: position of 19 neurons juxtacellularly labeled with neurobiotin in, or at proximity of (≤0.2 mm), the BLA-Ce border as identified during the experiments using physiological criteria. Filled circles represent positively identified ITC cells (n = 12), whereas open squares represent Ce, ASt, or BLA neurons.
The position of the Ce-BL border could be estimated based on the pattern of antidromic responsiveness of Ce and/or BL neurons in 47 of 150 rats. In 26 of these 47 rats, a neurobiotin-labeled cell was successfully recovered. Of these, 19 were located immediately adjacent to (n = 7, Fig. 6B, open squares) or within ITC cell clusters (n = 12, Fig. 6B, filled circles). Except for notable variations in spontaneous firing rates (0.36 ± 0.17 Hz; range 0–1.8 Hz), ITC cells exhibited remarkably stereotyped features, some of which unambiguously distinguished them from BL and Ce neurons. Indeed, none of these ITC cells could be backfired from IL (Fig. 4B) or brain stem (Fig. 4D), and all were orthodromically responsive to IL stimuli (Fig. 4C). In all ITC cells in which we tested the impact of ipsi- and contralateral IL stimuli (n = 2), orthodromic discharges could be evoked from both hemispheres.
Figure 7A contrasts the properties of IL-evoked orthodromic responses in morphologically identified ITC cells with those of BL and Ce neurons. In most ITC cells (75% or 9 of 12), IL shocks evoked high-frequency orthodromic spike bursts. The proportion of cells generating two or more orthodromic spikes in response to IL stimulation was significantly higher in ITC cells than in BL (4%) or Ce (0%) neurons (Fisher's exact probability test, P < 0.0001; Fig. 7A1). These response patterns were obtained with IL stimuli of 1 mA (0.1 ms). However, we routinely tested higher stimulation intensities (up to 1.5 mA) and could never make BL or Ce neurons change their response pattern from single spikes to spike bursts. Moreover, for those cells generating more than one spike in response to IL stimuli, the number of spikes per bursts was significantly higher in ITC cells (t-test, P = 0.03; Fig. 7A2). Finally, the peak instantaneous firing frequency during IL-evoked spikes bursts was significantly higher in ITC cells (t-test, P = 0.00013; Fig. 7A3). However, there were no significant differences between the latency of IL-evoked responses between ITC and BL neurons (Fig. 7A4).
Figure 7, B–D, depicts three examples of morphologically identified ITC cells (B1, C1, and D1), their responses to IL stimulation (B2, C2, and D2), and their morphological properties (E). As seen in the in vitro experiments, the shape of their dendritic trees generally matched the shape of the clusters where their somata were located, with ITC cells located in ITCd clusters having radial and multipolar dendritic trees (Fig. 7B1), whereas those located in ITCv clusters had flattened and usually bipolar dendritic arborizations (Fig. 7C1). These cells also had spiny dendrites (insets in Fig. 7, E1 and E2). Their axonal projections were also consistent with those seen in the in vitro experiments (Fig. 7, E3 and E4). The incidence of IL-evoked bursting was similar in ITCd (Fig. 7B2) and ITCv cells (Fig. 7, C2 and D2). The latency of IL-evoked responses typically ranged between 10 and 14 ms (Fig. 7, B2 and D2). The case illustrated in Fig. 7C2 was exceptional in this respect.
Can ITC Neurons Be Distinguished From BL and CE Cells Using Spike Shapes, Firing Patterns, or Rates?
The above findings suggest that a majority of ITC cells can be unambiguously distinguished from BL and Ce neurons on the basis of their responsiveness to IL stimuli. However, routinely testing the infralimbic responsiveness of amygdala cells in behaving animals constitutes a significant technical complication. Thus it would be useful if ITC cells could be distinguished from other types of amygdala neurons using other, more easily accessible measures such as spike shape or firing patterns. To test this possibility, we therefore compared ITC with BL and Ce neurons along all available extracellularly recorded parameters.
Unfortunately, as shown in Fig. 8, ITC cells proved indistinguishable from other amygdala neurons with the use of these more accessible properties. For instance, whether we considered the overall duration (Fig. 8A) or rising slope of action potentials (Fig. 8B), distributions in the three nuclei overlapped extensively. Similarly, firing rate (Fig. 8C) and interspike interval distributions could not be used to distinguish BL, Ce, and ITC cells. The latter variable proved especially indiscriminating, because a vast majority of cells at the three sites exhibited extremely sparse spike trains (Fig. 8D) that consisted of isolated single spikes (generally <2% of interspike intervals below 100 ms).
Fig. 8.
Overlapping distributions of action potential properties and firing rates in ITC, BL, and Ce neurons. Graphs are frequency distributions for 3 different variables: spike duration (A), rising slope of action potentials (B; inferred from interval between 25 and 75% of peak amplitude; absolute values, normalized for amplitude), and firing rates (C). Data were obtained in ITC (A1, B1, and C1; n = 12), BL (A2, B2, and C2; n = 71), and Ce neurons (A3, B3, and C3; n = 158). In A–C, identified projection cells of BL and Ce are represented by open bars, whereas filled bars indicate cells that could not be backfired. The 4 arrows in A2, B2, and C2 indicate 4 morphologically identified aspiny neurons. These presumed local-circuit cells had shorter spikes with faster rise times and relatively high firing rates compared with the rest of the population. Inset in B1 shows how the spike rising slope was measured. Various methods were used to estimate action potential durations (as in Likhtik et al. 2006), but all failed to reveal differences between cells in the 3 nuclei. D: spontaneous activity of a representative ITC cell (depicted in Fig. 7, E3 and E4). ITC firing rates were very low. The firing rate of this cell was around 0.3 Hz.
Last, we also tried to distinguish ITC, Ce, and BL cells by taking into account the entire spike shape using principal component analysis. Whether we considered all Ce and BL cells or restricted our attention to identified projection cells at these two sites, ITC cells could not be discriminated from Ce and BL neurons with the use of this method, either.
DISCUSSION
Based on tracing and physiological studies (Paré and Smith 1993b; Royer et al. 1999, 2000b), it was proposed that ITC cells are in an ideal position to gate the transfer of BL inputs about the CS to fear output CeM neurons (Royer and Pare 2002). It was further proposed that this gating constituted a likely mechanism for the extinction of conditioned fear (Royer and Pare 2002). Although subsequent lesion and pharmacobehavioral studies in rats (Likhtik et al. 2008) and mice (Jungling et al. 2008) supported this extinction model, it has since proven difficult to further our understanding of ITC contributions to extinction because we lacked criteria to identify them in extracellular recordings. As a result, it has been impossible to test key predictions of ITC extinction models. For instance, it was predicted that ITC cells are strongly excited by IL inputs (Paré et al. 2004), explaining why IL inhibition interferes with the acquisition of extinction (Sierra-Mercado et al. 2006) and prevents the potentiation of BL inputs to ITC cells during extinction (Amano et al. 2010). Another difficulty stemmed from the fact that the ITC model of extinction is based on connectivity data obtained in guinea pigs and cats, whereas behavioral studies of extinction are typically conducted in rats and mice. The present study aimed to address these gaps in our knowledge.
Projections of ITC Cells in Different Species
To compare the pattern of findings obtained in guinea pigs and cats to the results obtained presently in rats, one must take into account the differing orientation of the amygdala in these species. In the rat amygdala, Ce is medial to BL, whereas in cats and guinea pigs, Ce is dorsal to BL. Thus the dorsoventral axis of the rat amygdala corresponds to the lateromedial axis of the cat and guinea pigs amygdala. As a result, laterally vs. medially located ITC cell clusters in cats and guinea gigs respectively correspond to ITCd and ITCv clusters in rats.
In previous studies in cats and guinea pigs, it was reported that ITC cells project to Ce (Paré and Smith 1993b; Royer et al. 1999) and to other ITC cells (Royer et al. 2000b). After the labeling of single ITC cells with neurobiotin in slices of the guinea pig amygdala (Royer et al. 1999, 2000b), it was observed that ITC projections to Ce are topographically organized such that laterally located ITC cells (ITCd of rats) project to CeL and ASt, whereas medially located ITC cells (ITCv of rats) project to CeM. In addition, ITC projections to other ITC cells were directionally polarized in that they only targeted more medially located ITC cells (more ventrally located ITC cells in rats).
Thus, once the differing orientation of the amygdala in the two species is taken into account, the results obtained in guinea pigs and rats seem identical, since we have found that ITCd cells projected to CeL but not CeM, whereas the opposite was seen in ITCv neurons. Therefore, it appears that the connectivity of ITC cells is well preserved across species. In keeping with this, the results obtained in mice (Geracitano et al. 2007; Busti et al. 2011) are consistent with our observations in rats.
Distinctive Responsiveness of ITC Cells to IL Inputs
Because several lines of evidence have implicated ITC cells and IL in extinction, it was natural to assume that the two sets of findings were causally related. In support of this, tracing studies revealed that IL sends a dense projection to ITC cells (Cassell and Wright 1986; McDonald et al. 1996) and that IL disinhibition with picrotoxin increases the number of Fos-immunoreactive ITC neurons (Berretta et al. 2005). In behavioral studies, it was observed that CS presentation in the extinction training context induces Fos expression in IL and ITC neurons (Knapska and Maren 2009) and that manipulations that facilitate extinction in extinction-deficient mice have the same effect (Whittle et al. 2010). Furthermore, electrical stimulation of IL facilitates extinction (Milad et al. 2002) and causes an inhibition of CeM responses to BL inputs (Quirk et al. 2003) even though IL has no direct projections to CeM (Cassell and Wright 1986; McDonald et al. 1996). This prompted the suggestion that IL stimulation caused a strong activation of ITC cells, thereby resulting in the feedforward inhibition of CeM cells.
The results of the present study support this suggestion. IL stimuli elicited orthodromic spikes in all tested ITC cells. In 75% of them, we observed that IL stimuli evoked high-frequency (up to 350 Hz) trains of 4–6 action potentials. This pattern of responsiveness was never seen in Ce or BL neurons. In LA neurons, we did see a few cells generating more than one spike in response to IL stimuli. However, these spike bursts comprised significantly fewer spikes, were characterized by significantly lower intraburst frequencies, and were only seen in cells that were far from the Ce-BL border, in the lateral third of LA. As a result, conventional methods for histological reconstructions of electrode tracks would allow investigators to determine that these cells were not ITC neurons.
However, because the present experiments were conducted under urethane anesthesia, it is possible that the IL responsiveness and firing pattern of ITC cells is different in awake behaving animals. Although this possibility cannot be excluded at the present time, since anesthetics generally depress neuronal excitability, we would expect ITC cells to display even stronger IL responses and higher spontaneous firing rates in behaving animals. Consistent with this notion, a previous study in unanesthetized cats (Collins and Paré 1999) reported that ITC cells had much higher spontaneous firing rates than observed in the present study.
Therefore, we propose that the distinctive responses of ITC cells to IL inputs constitute a reliable criterion to identify them in behaving animals. As a result, it will now be possible to test whether the behavior of ITC cells can explain extinction, renewal, and reinstatement.
Multiple Superimposed Layers of Inhibition Between the Input and Output Stations of the Amygdala
Although the above-described studies focused on the role of ITC cells in extinction, our findings have significant implications for the mechanisms supporting the acquisition and expression of conditioned fear. Indeed, by virtue of the fact that ITCd and ITCv cells are respectively adjacent to LA and BL, one can expect that LA outputs constitute a major determinant of ITCd activity, whereas BL outputs should prevalently affect ITCv neurons. In fact, such a topographic arrangement was seen in guinea pigs (Royer et al. 1999). Thus, taking into account the differing orientation of the amygdala in rats in guinea pigs, this would mean that LA neurons target ITCd cells that in turn inhibit CeL neurons, whereas BL neurons drive ITCv cells, resulting in the inhibition of CeM neurons (Fig. 9). Superimposed on these topographic relations between the BL amygdala, ITC cell clusters, and Ce are two additional layers of inhibition. First, there are inhibitory projections from ITCd to ITCv cells, as well as from CeL to CeM (Fig. 9; Lopez De Armentia and Sah 2004; Petrovich and Swanson 1997). Since the ultimate determinant of fear expression is CeM activity (however, see Koo et al. 2004), it is important to consider how these multiple superimposed layers of inhibition affect CeM outputs. We first consider CeL to CeM interactions and then ITC activity below.
Fig. 9.
Multiple superimposed layers of inhibition between the input and output stations of the amygdala. Scheme summarizes the major direct excitatory (solid) and indirect inhibitory (shaded) pathways linking the basolateral amygdala to the central nucleus. LA neurons target ITCd cells (arrow 1) that in turn inhibit CeL neurons (arrow 2), whereas BL neurons drive ITCv cells (arrow 3), resulting in the inhibition of CeM neurons (arrow 4). In addition, there are inhibitory projections from ITCd to ITCv cells (arrow 5), as well as from CeL to CeM (arrow 6). Note that the recruitment of ITCd cells by LA neurons during the conditioned stimulus should produce a parallel inhibition of ITCv cells and CeL neurons, resulting in the disinhibition of fear output neurons in CeM. We hypothesize that fear conditioning produces a potentiation of LA inputs to ITCd cells, whereas extinction training causes a potentiation of BL inputs to ITCv neurons. The latter view is in apparent contradiction with the fact that IL projects to both ITCd and ITCv clusters and that ITCd cells inhibit ITCv neurons. This raises the following question: can IL inputs to ITCv cells overcome the inter-ITC inhibition to inhibit CeM cells? According to a recent modeling study (Li et al. 2011), IL inputs are so strong that they can overcome the inter-ITC inhibition to cause a marked increase in the firing rate of ITCv cells, leading to a persistent decrease in CeM output. Last, it should be mentioned that to improve readability, the scheme does not include 2 well-established facts about this circuit, namely, the existence of BL projections to CeL (Krettek and Price 1978) and the presence of two CeL cell types with opposite responses to conditioned stimuli but unknown connectivity with ITC cells. The latter are discussed in the text.
Recently, it was proposed that release of CeM neurons from inhibitory inputs arising in CeL plays a major role in the expression of conditioned fear (Ehrlich et al. 2009). First, pretraining inhibition of Ce or CeL only with muscimol (Ciocchi et al. 2010; Wilensky et al. 2006), as well as selective silencing of a subset of CeL neurons expressing PKC-δ (Haubensak et al. 2010), prevented the acquisition of conditioned fear, suggesting that conditioning leads to synaptic plasticity in CeL. Moreover, 1 day after fear conditioning in mice, CS presentations were found to inhibit PKC-δ-expressing CeL neurons (CeL− cells) (Ciocchi et al. 2010; Haubensak et al. 2010), whereas a different subset of PKC-δ-negative CeL cells showed a positive response to the CS (CeL+ cells). The presence of reciprocal inhibitory connections between CeL+ and CeL− neurons led to the proposal that when the CS is presented, the excitation of CeL+ cells causes the inhibition of CeL− neurons, resulting in the disinhibition of CeM fear output neurons (Ciocchi et al. 2010; Haubensak et al. 2010). At odds with this model, however, the incidence of CeL+ and CeL− neurons was similar, and both were found to project to CeM (Ciocchi et al. 2010; Haubensak et al. 2010).
In a subsequent study in rats (Duvarci et al. 2011), the CS responsiveness of CeL neurons was compared during habituation, at the end of training, and during a recall test the next day. During habituation and at the end of training, around 10% of CeL cells showed positive or negative responses to the CS. Thus low and high fear states were associated with a similar profile of CS responsiveness in CeL. During the recall test the next day, the incidence of CeL− cells nearly tripled, with no change in that of CeL+ neurons. If CeL+ cells are responsible for the inhibition of CeL− neurons, how can the incidence of CeL− cells increase from training to recall when that of CeL+ neurons does not change? One possibility is that CeL+ to CeL− synapses are potentiated as a result of fear conditioning. Another, and in our opinion, more parsimonious, possibility is that a different inhibitory input, extrinsic to CeL, is involved. For instance, because LA projects to ITCd but not ITCv neurons, CS presentations might cause the glutamatergic activation of ITCd cells, leading to the inhibition of CeL− and ITCv neurons, with the final result of disinhibiting CeM neurons. Now that ITC cells can be identified on the basis of their responsiveness to IL inputs, it is possible to test this idea.
GRANTS
This work was supported by National Institute of Mental Health Grant R01 MH-083710.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Dr. Shuzo Sakata, Sevil Duvarci, and Cagri Temucin Unal for help and advice.
REFERENCES
- Amano et al., 2010. Amano T, Unal CT, Paré D. Synaptic correlates of fear extinction in the amygdala. Nat Neurosci 13: 489–494, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berretta et al., 2005. Berretta S, Pantazopoulos H, Caldera M, Pantazopoulos P, Paré D. Infralimbic cortex activation increases c-Fos expression in intercalated neurons of the amygdala. Neuroscience 132: 943–953, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blechert et al., 2007. Blechert J, Michael T, Vriends N, Margraf J, Wilhelm FH. Fear conditioning in posttraumatic stress disorder: evidence for delayed extinction of autonomic, experiential, and behavioural responses. Behav Res Ther 45: 2019–2033, 2007 [DOI] [PubMed] [Google Scholar]
- Busti et al., 2011. Busti D, Geracitano R, Whittle N, Dalezios Y, Manko M, Kaufmann W, Sätzler K, Singewald N, Capogna M, Ferraguti F. Different fear states engage distinct networks within the intercalated cell clusters of the amygdala. J Neurosci 31: 5131–5144, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassell and Wright, 1986. Cassell MD, Wright DJ. Topography of projections from the medial prefrontal cortex to the amygdala in the rat. Brain Res Bull 17: 321–333, 1986 [DOI] [PubMed] [Google Scholar]
- Ciocchi et al., 2010. Ciocchi S, Herry C, Grenier F, Wolff SB, Letzkus JJ, Vlachos I, Ehrlich I, Sprengel R, Deisseroth K, Stadler MB, Müller C, Lüthi A. Encoding of conditioned fear in central amygdala circuits. Nature 468: 277–282, 2010 [DOI] [PubMed] [Google Scholar]
- Collins and Paré, 1999. Collins DR, Paré D. Spontaneous and evoked activity of intercalated amygdala neurons. Eur J Neurosci 11: 3441–3448, 1999 [DOI] [PubMed] [Google Scholar]
- Duvarci et al., 2011. Duvarci S, Popa D, Paré D. Central amygdala activity during fear conditioning. J Neurosci 31: 289–294, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich et al., 2009. Ehrlich I, Humeau Y, Grenier F, Ciocchi S, Herry C, Luthi A. Amygdala inhibitory circuits and the control of fear memory. Neuron 62: 757–771, 2009 [DOI] [PubMed] [Google Scholar]
- Geracitano et al., 2007. Geracitano R, Kaufmann WA, Szabo G, Ferraguti F, Capogna M. Synaptic heterogeneity between mouse paracapsular intercalated neurons of the amygdala. J Physiol 585: 117–134, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haubensak et al., 2010. Haubensak W, Kunwar PS, Cai H, Ciocchi S, Wall NR, Ponnusamy R, Biag J, Dong HW, Deisseroth K, Callaway EM, Fanselow MS, Lüthi A, Anderson DJ. Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature 468: 270–276, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins and Holstege, 1978. Hopkins DA, Holstege G. Amygdaloid projections to the mesencephalon, pons and medulla oblongata in the cat. Exp Brain Res 32: 529–547, 1978 [DOI] [PubMed] [Google Scholar]
- Horikawa and Armstrong, 1988. Horikawa K, Armstrong WE. A versatile means of intracellular labeling: injection of biocytin and its detection with avidin conjugates. J Neurosci Methods 25: 1–11, 1988 [DOI] [PubMed] [Google Scholar]
- Jacobs and McGinty, 1971. Jacobs BL, McGinty DJ. Amygdala unit activity during sleep and waking. Exp Neurol 33: 1–15, 1971 [DOI] [PubMed] [Google Scholar]
- Jacobsen et al., 2006. Jacobsen KX, Hoistad M, Staines WA, Fuxe K. The distribution of dopamine D1 receptor and mu-opioid receptor 1 receptor immunoreactivities in the amygdala and interstitial nucleus of the posterior limb of the anterior commissure: relationships to tyrosine hydroxylase and opioid peptide terminal systems. Neuroscience 141: 2007–2018, 2006 [DOI] [PubMed] [Google Scholar]
- Jungling et al., 2008. Jungling K, Seidenbecher T, Sosulina L, Lesting J, Sangha S, Clark SD, Okamura N, Duangdao DM, Xu YL, Reinscheid RK, Pape HC. Neuropeptide S-mediated control of fear expression and extinction: role of intercalated GABAergic neurons in the amygdala. Neuron 59: 298–310, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyuzhny et al., 1996. Kalyuzhny AE, Arvidsson U, Wu W, Wessendorf MW. mu-Opioid and delta-opioid receptors are expressed in brainstem antinociceptive circuits: studies using immunocytochemistry and retrograde tract-tracing. J Neurosci 16: 6490–6503, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapska and Maren, 2009. Knapska E, Maren S. Reciprocal patterns of c-Fos expression in the medial prefrontal cortex and amygdala after extinction and renewal of conditioned fear. Learn Mem 16: 486–493, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo et al., 2004. Koo JW, Han JS, Kim JJ. Selective neurotoxic lesions of basolateral and central nuclei of the amygdala produce differential effects on fear conditioning. J Neurosci 24: 7654–7662, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krettek and Price, 1977. Krettek JE, Price JL. Projections from the amygdaloid complex to the cerebral cortex and thalamus in the rat and cat. J Comp Neurol 172: 687–722, 1977 [DOI] [PubMed] [Google Scholar]
- Krettek and Price, 1978. Krettek JE, Price JL. A description of the amygdaloid complex in the rat and cat with observations on intra-amygdaloid axonal connections. J Comp Neurol 178: 255–280, 1978 [DOI] [PubMed] [Google Scholar]
- Li et al., 2011. Li G, Amano T, Pare D, Nair SS. Impact of infralimbic inputs on intercalated amygdala neurons: a biophysical modeling study. Learn Mem 18: 226–240, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likhtik et al., 2005. Likhtik E, Pelletier JG, Paz R, Paré D. Prefrontal control of the amygdala. J Neurosci 25: 7429–7437, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likhtik et al., 2006. Likhtik E, Pelletier JG, Popescu AT, Paré D. Identification of basolateral amygdala projection cells and interneurons using extracellular recordings. J Neurophysiol 96: 3257–3265, 2006 [DOI] [PubMed] [Google Scholar]
- Likhtik et al., 2008. Likhtik E, Popa D, Apergis-Schoute J, Fidacaro GA, Paré D. Amygdala intercalated neurons are required for expression of fear extinction. Nature 454: 642–645, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez De Armentia and Sah, 2004. Lopez De Armentia M, Sah P. Firing properties and connectivity of neurons in the rat lateral central nucleus of the amygdala. J Neurophysiol 92: 1285–1294, 2004 [DOI] [PubMed] [Google Scholar]
- Marowsky et al., 2005. Marowsky A, Yanagawa Y, Obata K, Vogt KE. A specialized subclass of interneurons mediates dopaminergic facilitation of amygdala function. Neuron 48: 1025–1037, 2005 [DOI] [PubMed] [Google Scholar]
- McDonald, 1991. McDonald AJ. Organization of amygdaloid projections to the prefrontal cortex and associated striatum in the rat. Neuroscience 44: 1–14, 1991 [DOI] [PubMed] [Google Scholar]
- McDonald, 1992. McDonald AJ. Cell types and intrinsic connections of the amygdala. In: The Amygdala: Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction, edited by Aggleton JP. New York: Wiley-Liss, 1992, p. 67–96 [Google Scholar]
- McDonald and Augustine, 1993. McDonald AJ, Augustine JR. Localization of GABA-like immunoreactivity in the monkey amygdala. Neuroscience 52: 281–294, 1993 [DOI] [PubMed] [Google Scholar]
- McDonald et al., 1996. McDonald AJ, Mascagni F, Guo L. Projections of the medial and lateral prefrontal cortices to the amygdala: a Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience 71: 55–75, 1996 [DOI] [PubMed] [Google Scholar]
- Milad et al., 2008. Milad MR, Orr SP, Lasko NB, Chang Y, Rauch SL, Pitman RK. Presence and acquired origin of reduced recall for fear extinction in PTSD: results of a twin study. J Psychiatr Res 42: 515–520, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad and Quirk, 2002. Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature 420: 70–74, 2002 [DOI] [PubMed] [Google Scholar]
- Millhouse, 1986. Millhouse OE. The intercalated cells of the amygdala. J Comp Neurol 247: 246–271, 1986 [DOI] [PubMed] [Google Scholar]
- Myers and Davis, 2007. Myers KM, Davis M. Mechanisms of fear extinction. Mol Psychiatry 12: 120–150, 2007 [DOI] [PubMed] [Google Scholar]
- Nitecka and Ben-Ari, 1987. Nitecka L, Ben-Ari Y. Distribution of GABA-like immunoreactivity in the rat amygdaloid complex. J Comp Neurol 266: 45–55, 1987 [DOI] [PubMed] [Google Scholar]
- Pape and Pare, 2010. Pape HC, Pare D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol Rev 90: 419–463, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paré and Gaudreau, 1996. Paré D, Gaudreau H. Projection cells and interneurons of the lateral and basolateral amygdala: distinct firing patterns and differential relation to theta and delta rhythms in conscious cats. J Neurosci 16: 3334–3350, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paré et al., 2004. Paré D, Quirk GJ, LeDoux JE. New vistas on amygdala networks in conditioned fear. J Neurophysiol 92: 1–9, 2004 [DOI] [PubMed] [Google Scholar]
- Paré and Smith, 1993a. Paré D, Smith Y. Distribution of GABA immunoreactivity in the amygdaloid complex of the cat. Neuroscience 57: 1061–1076, 1993a [DOI] [PubMed] [Google Scholar]
- Paré and Smith, 1993b. Paré D, Smith Y. The intercalated cell masses project to the central and medial nuclei of the amygdala in cats. Neuroscience 57: 1077–1090, 1993b [DOI] [PubMed] [Google Scholar]
- Paré and Smith, 1994. Paré D, Smith Y. GABAergic projection from the intercalated cell masses of the amygdala to the basal forebrain in cats. J Comp Neurol 344: 33–49, 1994 [DOI] [PubMed] [Google Scholar]
- Petrovich and Swanson, 1997. Petrovich GD, Swanson LW. Projections from the lateral part of the central amygdalar nucleus to the postulated fear conditioning circuit. Brain Res 763: 247–254, 1997 [DOI] [PubMed] [Google Scholar]
- Quirk et al., 2003. Quirk GJ, Likhtik E, Pelletier JG, Paré D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci 23: 8800–8807, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk and Mueller, 2008. Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology 33: 56–72, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz and Grace, 1999. Rosenkranz JA, Grace AA. Modulation of basolateral amygdala neuronal firing and afferent drive by dopamine receptor activation in vivo. J Neurosci 19: 11027–11039, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer et al., 1999. Royer S, Martina M, Paré D. An inhibitory interface gates impulse traffic between the input and output stations of the amygdala. J Neurosci 19: 10575–10583, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer et al., 2000a. Royer S, Martina M, Pare D. Bistable behavior of inhibitory neurons controlling impulse traffic through the amygdala: role of a slowly deinactivating K+ current. J Neurosci 20: 9034–9039, 2000a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer et al., 2000b. Royer S, Martina M, Paré D. Polarized synaptic interactions between intercalated neurons of the amygdala. J Neurophysiol 83: 3509–3518, 2000b [DOI] [PubMed] [Google Scholar]
- Royer and Paré, 2002. Royer S, Paré D. Bidirectional synaptic plasticity in intercalated amygdala neurons and the extinction of conditioned fear responses. Neuroscience 115: 455–462, 2002 [DOI] [PubMed] [Google Scholar]
- Royer and Paré, 2003. Royer S, Paré D. Conservation of total synaptic weight through balanced synaptic depression and potentiation. Nature 422: 518–522, 2003 [DOI] [PubMed] [Google Scholar]
- Sah et al., 2003. Sah P, Faber ESL, De Armentia ML, Power J. The amygdaloid complex: anatomy and physiology. Physiol Rev 83: 803–834, 2003 [DOI] [PubMed] [Google Scholar]
- Shammah-Lagnado et al., 1999. Shammah-Lagnado SJ, Alheid GF, Heimer L. Afferent connections of the interstitial nucleus of the posterior limb of the anterior commissure and adjacent amygdalostriatal transition area in the rat. Neuroscience 94: 1097–1123, 1999 [DOI] [PubMed] [Google Scholar]
- Sierra-Mercado et al., 2006. Sierra-Mercado D, Jr, Corcoran KA, Lebrón-Milad K, Quirk GJ. Inactivation of the ventromedial prefrontal cortex reduces expression of conditioned fear and impairs subsequent recall of extinction. Eur J Neurosci 24: 1751–1758, 2006 [DOI] [PubMed] [Google Scholar]
- Whittle et al., 2010. Whittle N, Hauschild M, Lubec G, Holmes A, Singewald N. Rescue of impaired fear extinction and normalization of cortico-amygdala circuit dysfunction in a genetic mouse model by dietary zinc restriction. J Neurosci 30: 13586–13596, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilensky et al., 2006. Wilensky AE, Schafe GE, Kristensen MP, LeDoux JE. Rethinking the fear circuit: the central nucleus of the amygdala is required for the acquisition, consolidation, and expression of Pavlovian fear conditioning. J Neurosci 26: 12387–12396, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson et al., 2002. Wilson MA, Mascagni F, McDonald AJ. Sex differences in delta opioid receptor immunoreactivity in rat medial amygdala. Neurosci Lett 328: 160–164, 2002 [DOI] [PubMed] [Google Scholar]