Abstract
Successful navigation requires a constantly updated neural representation of directional heading, which is conveyed by head direction (HD) cells. The HD signal is predominantly controlled by visual landmarks, but when familiar landmarks are unavailable, self-motion cues are able to control the HD signal via path integration. Previous studies of the relationship between HD cell activity and path integration have been limited to two or more arenas located in the same room, a drawback for interpretation because the same visual cues may have been perceptible across arenas. To address this issue, we tested the relationship between HD cell activity and path integration by recording HD cells while rats navigated within a 14-unit T-maze and in a multiroom maze that consisted of unique arenas that were located in different rooms but connected by a passageway. In the 14-unit T-maze, the HD signal remained relatively stable between the start and goal boxes, with the preferred firing directions usually shifting <45° during maze traversal. In the multiroom maze in light, the preferred firing directions also remained relatively constant between rooms, but with greater variability than in the 14-unit maze. In darkness, HD cell preferred firing directions showed marginally more variability between rooms than in the lighted condition. Overall, the results indicate that self-motion cues are capable of maintaining the HD cell signal in the absence of familiar visual cues, although there are limits to its accuracy. In addition, visual information, even when unfamiliar, can increase the precision of directional perception.
Keywords: vestibular, path integration, anterior thalamus, spatial orientation, dead reckoning
accurate navigation from a start location to a goal depends on a constantly updated perception of one's location and directional heading, as well as the appropriate trajectory to reach the goal. In general, two forms of spatial information are available for orientation and navigation: path integration and landmark navigation. Path integration refers to the process whereby animals continuously update their orientation and location as they move through the environment by monitoring various internal (idiothetic) cues, such as vestibular, proprioceptive, and motor efference information. In contrast, landmark navigation uses allothetic cues external to the body, such as visual, auditory, or tactile objects, in an episodic manner to maintain orientation relative to a reference point. Impressive feats of navigation are often attributed to highly accurate systems of path integration, and these feats have been noted across different species (Bingman and Cheng 2005). Although rats are known to path integrate accurately over short distances (Etienne and Jeffery 2004; Whishaw and Tomie 1997) their accuracy in complex environments or over distances longer than a typical laboratory arena is not well known.
In rats, directional heading is represented in the brain by head direction (HD) cells (Taube et al. 1990a, 1990b). Each HD cell fires maximally when the rat's head is pointed in one direction in the yaw plane, and the population of HD cells collectively represents all directions. Current evidence suggests that the HD signal is generated in the reciprocal connections between the dorsal tegmental nuclei and the lateral mammillary nuclei using primarily vestibular and motor information, but the HD signal is updated by visual information (Bassett et al. 2007; Sharp et al. 2001; Stackman and Taube 1998; Taube 2007). When both types of information are available, landmark information usually dominates over idiothetic cues (Blair and Sharp 1996; Goodridge and Taube 1995; Taube and Burton 1995; Zugaro et al. 2,000, 2003), although the reverse can be observed when landmark cues become unstable (Knierim et al. 1995) or when landmarks are manipulated in a way that results in large disparities between current and previously familiar conditions (Knierim et al. 1998). HD cell dependence on path integration processes has been demonstrated in experiments where the HD signal remains stable during locomotion from a familiar to a novel environment, where familiar landmark cues are unavailable for use as a reference point (Dudchenko and Zinyuk 2005; Stackman et al. 2003; Taube and Burton 1995). However, all of these experiments were performed within the same recording room, which leaves open the possibility that animals may have used some unidentified room cue to keep track of their orientation. In addition, none of these experiments required path integration over long distances or required the animal to make a large number of turns.
To further test the relationship between HD cell activity and angular path integration, we evaluated HD cell responses in the anterodorsal thalamus (ADN) (Taube 1995) in two different tasks, both of which were more spatially challenging and required a greater number of turns and a greater amount of time to complete than in previous experiments. The first experiment involved recording HD cells while the rats walked from a start box to a goal box via a 14-unit T-maze. Because the rats had no previous experience in the maze and no reward was available during their journey, this experiment allowed us to measure HD signal stability while rats acquired spatial information before their association with a reward. The second experiment monitored HD cells as rats traversed a long route between two separate rooms under one of three conditions: 1) in light, 2) in darkness, and 3) passive transport. The dark condition allowed us to test whether the rats were using a series of visual “snapshots” during their journey under lit conditions to update their orientation, rather than solely using idiothetic cues for path integration. We presently report that although the HD signal was generally maintained by path integration mechanisms, it was improved somewhat by the presence of visual cues.
MATERIALS AND METHODS
Subjects
The present study included adult female Long-Evans hooded rats (age 4–9 mo, 250–360 g, n = 36; Harlan Laboratories, Boston, MA). All rats were group housed preoperatively and individually housed postoperatively within the same colony room on a 12:12-h light-dark cycle. All rats received food and water ad libitum. The estrous cycle at the time of recording was not evaluated; all group members had equal probability of being used for experimental procedures at any point in the cycle. All procedures involving rats were approved by the Dartmouth Institutional Animal Care and Use Committee in compliance with standards set forth by the U.S. Public Health Service's “Policy on Humane Care and Use of Laboratory Animals” and the Guide for the Care and Use of Laboratory Animals.
Electrodes
Electrodes were constructed as described previously (Kubie 1984). Briefly, each microdrive was constructed from ten 25-μm Teflon-insulated nichrome wires (California Fine Wire, Grover Beach, CA) encased by a 26-gauge stainless steel cannula. Each electrode in the array had a tip impedance of ∼2 MΩ. Dental acrylic was used to encase the cannula, wires, and connector and to hold the heads of three custom drive screws. Tips of the drive screws were threaded into custom-built plastic cuffs, which were later cemented to the skull as described below. These cuffs provided a fixed base into which the screws were incrementally advanced to move the electrodes through the brain.
Presurgical Training
Presurgical training was performed in a gray wooden cylinder (diameter = 76 cm, height = 50 cm) containing a white cue card that covered ∼100° of the wall surface. The position of the cue card remained constant throughout presurgical training and HD cell screening procedures. The cylinder floor was covered by gray photographic backdrop paper. A black curtain extending from the ceiling to the floor surrounded the arena (diameter = 2.44 m) to discourage animals from using visual cues other than the white cue card. An overhead speaker controlled by a white noise generator was used to prevent the use of auditory cues. For 1 wk before surgical procedures, rats were trained to forage for 20-mg sucrose pellets (Bio-Serv, Frenchtown, NJ) that dropped onto the cylinder floor at pseudorandom intervals (mean interval = 30 s). All rats demonstrated proficiency in obtaining sucrose pellets before inclusion in the study.
Surgery
Rats were anesthetized with a ketamine-xylazine cocktail (90 and 10 mg/kg, respectively) and positioned in a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA) such that bregma and lambda were in the same horizontal plane. The scalp was retracted, and a hole was drilled through the skull above ADN. Additional holes were drilled in the frontal, parietal, and occipital bones to provide anchor points for miniature screws (Small Parts, Miramar, FL). Each electrode bundle was sterilized and coated, except for the tips, with polyethylene glycol (Carbowax) before being positioned dorsal to ADN (1.5 mm posterior, 1.3 mm lateral, 3.7 mm ventral to bregma). With the electrode bundle in position, the drive screw/cuff assemblies were fastened to the skull and jeweler's screws with Grip cement (Dentsply International, Milford, DE). The scalp was sutured around the electrode drive, and the animal was allowed to recover 1 wk before screening and recording procedures commenced. Buprenorphine (0.015 mg/kg) was administered as a postoperative analgesic.
HD Cell Screening and Signal Processing
Animals were placed into the cylinder in room 1, and each electrode was assessed for cellular activity while they foraged for food pellets. During screening, the electrical signal from each electrode was passed through a 10-channel headstage containing a unity gain operational amplifier. A flexible cable connected the headstage to an overhead commutator, which then connected to a patch panel. From the patch panel, electrical signals were amplified (P5 series; Grass, West Warwick, RI) and band-pass filtered (300–10,000 Hz) before auditory and visual display on a loudspeaker and oscilloscope (model 2214; Tektronix, Beaverton, OR), respectively. A dual time and amplitude window discriminator (model DDIS-1; BAK Electronics, Mount Airy, MD) was used to isolate single-unit spikes from background noise and to generate a square-wave pulse upon spike detection. An overhead video camera (SONY XC-711; Tokyo, Japan) was used to monitor the animal's HD at 60 Hz by detecting the position of one red and one green light-emitting diode (LED) attached to the animal's headstage, separated by 11 cm. Signals generated by the window discriminator at the occurrence of single-unit spikes and the x-y coordinates of the concurrent LED positions were acquired by a computer (Macintosh G4) running LabVIEW software (version 5.0; National Instruments, Austin, TX). Data were analyzed with LabVIEW.
Behavioral Apparatus
Fourteen-unit T-maze.
The 14-unit T-maze was similar to a previously reported design (Elliot 1928; Tolman and Honzik 1930) but was modified to allow video surveillance of rats' behavior at all points within the maze (Fig. 1). The maze consisted of square start and goal boxes (61 × 61 cm) connected by a walled (height = 28 cm) pathway containing 14 choice points where the rat could turn left or right. The maze was constructed of gray-painted wood to minimize the use of intramaze cues. Cards with vertical black and white stripes were affixed to the walls of the goal box to differentiate the goal box from the start box, which had uniformly gray-painted walls. Both the start and goal boxes were gated so that the rats could be confined to them for set periods of time. For BRIDGE trials (see below), a wooden bridge was used to connect the start and goal boxes, giving the rat a direct pathway to the goal box. This bridge consisted of a 1.1-m-long, 7.6-cm-wide ramp leading out over the start box wall that connected to a flat, open pathway, which then connected to a second ramp that led down into the goal box. The surfaces of the bridge and ramps were covered with wide metal screen to provide the rats with traction as they traversed this route. The entire apparatus was located on the floor of the recording room and was surrounded by a black curtain that extended from the floor to the ceiling.
Fig. 1.
The 14-unit T-maze. A: photo of the 14-unit T-maze with a rat confined to the goal box. Note: the vertically oriented stripes in the goal box that distinguish it from the start box were added after the photo was taken. B: overhead schematic of the 14-unit T-maze. Solid lines represent walls; arrows indicate path between start and goal boxes; dashed line indicates the start point for the MAZE END condition.
Multiroom maze.
The multiroom maze consisted of two different enclosures that were situated in two different rooms and a long alleyway that connected the two enclosures (Fig. 2). The enclosure in room 1 was a 1.28-m2 arena (height = 40 cm) constructed of gray-painted plywood that contained a single doorway in the middle of one side that led to an extended passageway (Fig. 2A). The apparatus in room 2 was a large circular arena used in previous experiments that involved the food-carrying task (Fig. 2C) (Clark and Taube 2009; Frohardt et al. 2006). It was constructed of gray-painted plywood (diameter = 180 cm, height = 38 cm) and contained eight equally spaced doorways around the perimeter of the cylinder. Seven of the eight doorways were blocked with a piece of wood and contained a black curtain in front of the doorway. These curtains provided a striped appearance that helped to distinguish the circular arena from the square enclosure in room 1. The one doorway that did not contain a curtain led to the passageway. Rooms 1 and 2 were adjacent to each other, but to go from one room to the other required passing through two different rooms and a connecting hallway (Fig. 2E). A passageway (width = 26.7 cm, height = 18.4 cm, length = 11.45 m) connected the two enclosures (Fig. 2B). This passageway contained three 90° turns and one 45° turn. Recording systems located in the two rooms were coupled to provide continuous monitoring of cell activity in both rooms, as well as during the traversal between rooms. Video monitoring of the animal's location and orientation, however, was only conducted while the animal was in one of the two enclosures.
Fig. 2.
The multiroom apparatus. A square arena (A) was located in room 1 with an 11.4-m passageway (B) connecting it to a novel cylinder containing 8 uniformly spaced black curtains (C) in room 2. For the dark condition, a roof (D) was positioned on top of the passageway to prevent visual continuity between rooms. A small slit ran down the middle of the roof to allow the recording cable to pass through. E: overhead schematic of the multiroom apparatus drawn to approximate scale.
Although the animal never experienced the square enclosure in room 1 until the day of testing, this square enclosure was considered the familiar environment because it was located in the room where the animal was trained and screened for several weeks before testing. The enclosure in room 2 was considered a novel, unfamiliar environment because the rat had never been in this room before, nor in the large circular arena. The two enclosures were easily distinguishable from one another based on their geometric shape, the presence of the seven curtained doorways in the circular arena, and other visual cues along the room walls.
Recording Procedures and Experiment-Specific Analyses
Fourteen-unit T-maze.
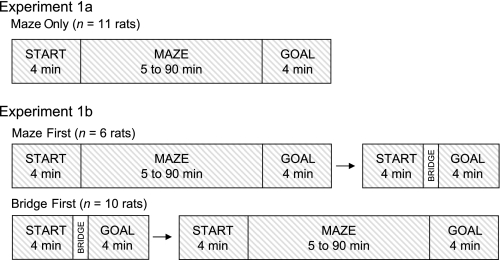
Two procedures were used for this task and are referred to as experiments 1a and 1b. A summary of the design for both experiments is shown in Fig. 3. Eleven rats were used for experiment 1a; five of these rats were only used for experiment 1a, and six rats were subsequently used for experiment 1b. Rats that were used for experiment 1b are referred to as MAZE FIRST. Each trial began with a START session (4 min) with the rat confined to the start box. This session established the preferred direction of the HD cell. Next, the start box door was opened to allow rats to exit the start box, at which point the MAZE session began. HD cell activity was recorded during the MAZE session (unlimited duration), during which rats were free to move from the start box to the goal box via the 14-unit T-maze pathway. The final GOAL session (4 min) began when the rats entered the goal box from the maze pathway and the door to the goal box was closed.
Fig. 3.
Recording procedures for experiment 1. For experiment 1a, head direction (HD) cell activity was recorded while the rat was confined to the start box, during maze traversal, and while the rat was confined to the goal box. In experiment 1b, the same procedure was followed, with the addition of a single bridge trial after 3 maze trials for the MAZE FIRST group. For the BRIDGE FIRST group, 3 bridge trials occurred before a single maze trial. The amount of time spent in the maze varied across rats, and the time ranges are shown for each experiment.
Experiment 1b consisted of two groups of animals and involved four journeys from the start box, across the bridge, and to the goal box for both groups. The MAZE FIRST group consisted of the six rats that had previously navigated the maze between the start box and goal box for three journeys and then used the bridge between the start and goal boxes for the fourth journey. The BRIDGE FIRST group consisted of 10 rats that used the bridge for the first three journeys, after which they traversed the maze from the start to the goal box for one journey only. Recording details were identical to those used in experiment 1a, with 4 min each in the start and goal boxes and unlimited time for navigation. At the end of each 4-min GOAL session, rats were removed from the goal box and returned directly to the start box. At this point, the next journey began with the 4-min START session.
Multiroom maze.
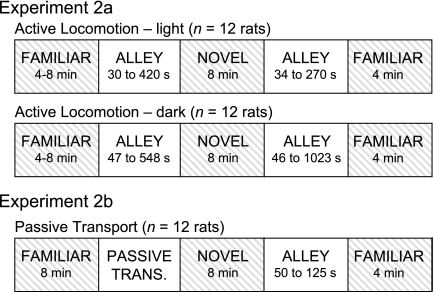
Three different groups of rats were used in this task (n = 12 rats per group). For each group, HD cell activity was recorded in both arenas. Group 1 involved active locomotion between the two rooms under light conditions; group 2 involved active locomotion between the two rooms under dark conditions; group 3 involved disorientation and passive transport in an opaque box between the two rooms (Fig. 4). Each trial began with a 4- or 8-min recording session while the rat was confined to the square arena in room 1. For the lighted trials, the rat was then permitted to walk from the square arena in room 1, through the passageway, to the circular arena in room 2. On entry to the circular arena, the door was closed to confine the rat to the arena, and an 8-min recording session commenced. At the end of the recording session, the arena's door was opened to permit the rat to return, through the passageway, to the square arena in room 1. A 4-min recording session was conducted upon reentry to the familiar square. Time spent in the passageway during the outbound and return trips was noted. For group 1, no effort was made to discourage the animal from using visual cues during navigation between the rooms. In contrast, during dark trials for group 2, a wooden roof was positioned over the passageway (Fig. 2D) and the room lights and tracking LEDs were extinguished to prevent the use of visual cues during navigation between rooms. There was a 3- to 4-mm slit in the top of the roof to allow the recording cable to pass through the passageway unhindered. Although this slit allowed the animal to view a narrow angle of the room outside the passageway, because the rooms were darkened and the LEDs turned off, it was unlikely the rat could discern any features in the room through the slits. Rooms were lighted during the first session in the familiar square arena, after entry to the novel cylindrical arena, and after reentry to the familiar square arena. All other procedures were identical between the light and dark trials. For passive transport trials (group 3), a trial began with a 4- or 8-min recording session while the rat was confined to the square arena in room 1. At the conclusion of this session, the rat was picked up and placed in an opaque box. The box was slowly rotated in both directions while the experimenter walked to the circular arena in room 2, which took about 15 s. The box containing the rat was gently placed into the circular arena, and the rat was lifted out of the box and laid on the arena floor. A block of wood covered the doorway to the passageway and confined the rat to the arena. An 8-min recording session was conducted. At the end of this session, the block of wood was removed and the rat was allowed to walk through the passageway back to the familiar square arena in room 1. On reentry to the familiar arena, the entryway was closed and a 4-min recording session commenced. Time spent in the passageway during the return trip was noted. For all analyses, means ± SE are reported.
Fig. 4.
Recording procedures for experiments 2a and 2b. For both experiments, HD cell activity was recorded while rats were in the familiar and novel arenas. Shaded areas indicate sessions during which HD cell activity was not recorded. The amount of time spent in the alleyway varied across rats, and the time ranges are shown for each experiment.
General Data Analysis
For all recording sessions, HD was determined by calculating the angle between the x-y coordinates of the LEDs within a 256 × 256-pixel field. HDs were sorted into 60 6° bins along with the spikes. The average firing rate as a function of HD was calculated by dividing the total number of spikes by the amount of time in each bin. Each cell's preferred firing direction, henceforth referred to simply as “preferred direction,” was defined as the HD bin at which the cell showed the highest mean firing rate.
HD cell shift analysis.
To determine the amount of angular shift exhibited by a HD cell between the two enclosures, we cross-correlated the tuning curves obtained from both enclosures, as previously described (Taube et al. 1990b). Briefly, the firing rate vs. HD function for the square enclosure in room 1 was shifted in 6° steps and compared with the function from the circular arena in room 2. The amount of shift that produced the maximal correlation was considered to be the amount the preferred direction shifted between the two enclosures. For recording sessions during which multiple HD cells were recorded, the average shift of the preferred directions for simultaneously recorded cells was used for statistical calculations. This averaging method was used because previous studies (as well as the current study) indicated that when multiple HD cells are recorded simultaneously, the preferred direction for all HD cells rotate in register (Taube et al. 1990b), and we did not want to bias our analyses to sessions in which multiple cells were recorded. Statistical evaluations of angular shifts were performed in two ways. First, we measured the amount the preferred direction in room 2 deviated from the preferred direction in room 1. This measurement is simply the absolute value of the difference between the preferred directions in both environments, with a value of 0 representing no difference. We refer to this shift as the “mean absolute shift.” Group comparisons between the absolute shift values from two conditions that involved the same HD cells were performed with a paired t-test, whereas absolute shift values from two conditions that involved different cells were compared with an unpaired t-test (SPSS; IBM, Somers, NY). Group comparisons for the mean absolute shift values are presented in Table 1. Second, we used circular statistics (Oriana; Kovach Computing Services, Anglesey, UK) to compute the mean vector, r, as the mean of the directional shifts, and we refer to this value as the “mean shift.” The mean shift is presented along with the angular standard error of the mean. The vector r is associated with a length and an angle. The length, which varies between 0 and 1, represents how much the data points vary around the mean, with greater values associated with more concentration around the mean and smaller values associated with less concentration. The angle of r represents the mean angle across all values. A Rayleigh test was used to determine whether the distribution of preferred directions was randomly distributed or tended to cluster toward a particular angle (Oriana). The statistic for the Rayleigh test, the mean vector r, is presented along with the P value. To determine whether the distribution of preferred direction shifts was clustered around a predicted angle, we calculated 95% confidence intervals from the distribution of preferred direction shifts (Oriana). If the predicted angle fell within the bounds of the confidence interval, we inferred that the preferred direction shift was not significantly different from the predicted angle. The Watson-Williams F-test was used for group comparisons (Oriana). The test statistic, F, is presented with the P value. An F-test of the concentration parameter obtained from the directional shift scores was used to determine whether the distributions of preferred direction shifts differed in their concentration around their respective mean values (Batschelet 1981). A difference in concentration parameter is expected if one distribution of preferred direction shifts shows greater variability than another. The test statistic, F, and P value are presented.
Table 1.
Mean angular shift and mean absolute shift in PFD between arenas during the first recording session for each condition
| Condition | n | Mean PFD Shift, ° | P Value | Mean Absolute PFD Shift, ° | P Value |
|---|---|---|---|---|---|
| Experiment 1: 14-Unit T-Maze | |||||
| Start → Goal via maze | |||||
| Start vs. Goal | 11 | −3.52 ± 3.37 | < 0.0001 | 8.45 ± 1.85 | |
| Start vs. Maze end | 11 | −21.77 ± 3.44 | < 0.0001 | 17.70 ± 3.15 | 0.052* |
| Start → Goal via bridge | |||||
| Start vs. Goal (MAZE FIRST) | 6 | 0.95 ± 3.44 | < 0.0001 | 9.00 ± 2.74 | 0.775* |
| Start vs. Goal (BRIDGE FIRST) | 10 | 4.79 ± 3.71 | < 0.001 | 8.40 ± 2.40 | 0.986* |
| Experiment 2: Multi-Room Maze | |||||
| Active locomotion | |||||
| Familiar → Novel (light) | 12 | 17.46 ± 9.92 | < 0.0001 | 29.00 ± 5.71 | |
| Familiar → Familiar (light) | 12 | 1.99 ± 2.19 | < 0.0001 | 5.00 ± 1.45 | 0.001† |
| Familiar → Novel (dark) | 12 | 8.22 ± 15.00 | < 0.005 | 41.50 ± 8.40 | 0.232† |
| Familiar → Familiar (dark) | 12 | −0.75 ± 1.52 | < 0.0001 | 3.75 ± 0.84 | 0.001‡ |
| Passive Transport | |||||
| Familiar → Novel (passive) | 12 | 132.07 ± 60.99 | 0.42 | 104.50 ± 13.55 | <0.001† |
| Familiar → Familiar (passive) | 12 | −1.46 ± 2.72 | < 0.0001 | 5.00 ± 2.03 | <0.001§ |
Values are means ± SE (n = no. of sessions) for mean angular shift and mean absolute shift in preferred firing direction (PFD) for each maze condition. P values for mean angular shift in PFD were determined using the Rayleigh test of uniformity; P values for mean absolute shift in PFD were determined using the t-test.
Significant difference compared with Start → goal condition (14-unit T-maze).
Significant difference compared with Familiar → novel (light) condition.
Significant difference compared with Familiar → novel (dark) condition.
Significant difference compared with Familiar → novel (passive) condition.
Histology
After all recording was complete, rats were anesthetized with pentobarbital sodium (150 mg/kg), and one or more electrode tip locations were marked with an iron deposit by passing a constant anodal current (15 μA, 20 s) through the electrode wire(s). Rats were euthanized by transcardial perfusion with normal saline followed by 10% formalin. Brains were then postfixed in 10% formalin containing 2% potassium ferrocyanide for 24–48 h to produce a Prussian blue reaction at the electrode tip locations. Brains were then removed from formalin and placed in 20% sucrose for cryoprotection before being sectioned at 30 μm on a cryostat. Brain sections containing electrode tracks and ADN were mounted on gelatin-coated microscope slides. Brain tissue was then stained with thionin and examined under a light microscope. Recording tracks through the ADN were verified under microscopic analysis. Electrode position in ADN was verified for all rats.
RESULTS
Experiment 1a: 14-Unit T-Maze
The trip through the 14-unit maze to the goal box did not disrupt HD cell signal stability, with very little change in the preferred direction between the start and goal boxes (Fig. 5, A and B); mean absolute shift was 8.45 ± 1.85° [mean shift: −3.52 ± 3.37°, range: −24 to 9°; mean Rayleigh r(11) = 0.99, P < 0.0001] (Table 1). The 95% confidence interval around this mean (−10.13 to 3.09°) indicates that this distribution of shifts included the value of 0° (Fig. 6A, left). No differences in preferred direction shift were seen across the three trips through the maze (data not shown); data are presented from each rat's first trip through the maze.
Fig. 5.
Directional tuning curves for 3 representative HD cells recorded during performance of the 14-unit maze. A and B: during traversal of the maze, the preferred direction of HD cells showed a slight shift between the start box and maze end. The amount of time in which the animal's head was pointed within each 6° directional bin during the MAZE END condition (directional sampling) is indicated by the dashed line. In A, the cell's preferred firing direction shifted 30° clockwise (CW) between the start box and maze end. On entry to the goal box, however, the preferred direction shifted 42° counterclockwise (CCW) and was 12° CCW relative to the start box. In B, the cell's preferred firing direction shifted 12° CW between the start box and maze end. On entry to the goal box, the preferred firing direction shifted 24° CCW to become 6° CCW relative to the start box. C: during traversal of the linear bridge that connected the start and goal boxes, the preferred direction shifted −6°.
Fig. 6.
Distribution of shifts in the preferred firing direction of cells recorded during the 14-unit T-maze. For all plots, 0° represents each cell's preferred direction during the first recording session in room 1. A, left: after locomotion from the start box to the goal box via the maze, the mean shift in the preferred directions did not differ from 0°. Right, during the second half of the maze (before entry to the goal box), the preferred directions showed a significant shift, relative to the start box, as well as to the START vs. GOAL comparison at left. B: rats that used the bridge to navigate from the start box to the goal box showed little shift in the cells' preferred directions after traversing the bridge to the goal box. Shaded lines represent 95% confidence intervals. Points that fell between 6° bins are due to sessions that contained multiple cell recordings, and the point represents the average shifts of the preferred firing directions.
Interestingly, although the preferred direction did not show an overall shift between the start and goal boxes, many cells' preferred directions appeared to shift a little by the time the rat reached the end of the maze, only to become realigned with the preferred direction of the start box when the rat entered the goal box (e.g., Fig. 5A). Unfortunately, the nature of the maze design prevented adequate directional sampling of each cell's preferred direction at all points along the maze. To maximize directional sampling, we determined each HD cell's preferred direction during its trip through the second half of the maze, which began when the rat reached a point in the maze that was halfway between the start and goal boxes and ended just before entry into the goal box (Fig. 1B). This condition is referred to as MAZE END. Between the START and MAZE END conditions, the mean absolute shift was 17.70 ± 3.15° [mean shift: −18.29 ± 3.48°, range: −33 to 0°; Rayleigh r(11) = 0.985, P < 0.0001]. The 95% confidence interval around this mean (−25.11 to −11.48°) showed that this distribution of shifts did not include the predicted value of 0° (Fig. 6A, right). Consequently, the preferred firing direction shift between START and GOAL differed from the shift between the START and MAZE END [F(1, 20) = 11.37, P = 0.003]. Importantly, the amount of time required to walk through the 14-unit maze was not correlated with the amount of shift between the start and goal boxes [Pearson r(11) = 0.366, P = 0.268] (Fig. 7A) or between the START and MAZE END [Pearson r(11) = −0.369, P = 0.264] (Fig. 7B). Thus, although the preferred direction at the maze end remained relatively similar to that in the start box, there was a small, but statistically significant, shift as rats walked from the start box through the maze, until just before they entered the goal box. However, the cells' preferred directions became realigned with the start box on the animal's entry into the goal box. It is important to note that although all directions were sampled in the MAZE END, the preferred direction was measured from limited directional sampling, which could have led to variability in the cell's preferred direction, particularly if directional sampling varied across bins that were within the cell's directional firing range. Because of this potential for increased variability, it is possible that the observed shifts reflect sampling error instead of actual shifts. However, we feel this possibility is unlikely because 1) the tuning curves obtained during the MAZE END condition had a similar appearance to those that were recorded in the START and GOAL conditions (Fig. 5, A and B), 2) preferred firing directions measured from subsampled data from entire recording sessions did not significantly shift away from the cell's preferred firing direction in the original session (data not shown), and 3) the preferred directions of two simultaneously recorded cells shifted by the same amount (in register) between the START and MAZE END, despite greater directional sampling within the directional firing range of one cell versus the other cell (data not shown).
Fig. 7.
Absolute angular shift of the preferred firing direction as a function of time in maze. A: during traversal of the maze, time was not significantly correlated with the amount of shift in the preferred firing direction between start and goal boxes (Pearson r = 0.366). B: between the start box and maze end, time in the maze was not correlated with the amount of shift in the preferred firing direction (Pearson r = −0.369). PFD, preferred firing direction.
Experiment 1b: MAZE vs. BRIDGE in the 14-Unit T-Maze
One explanation for the drift of the preferred direction during the maze traversal in experiment 1a is that the maintenance of a stable preferred direction is influenced by route complexity, with short linear routes producing less drift than longer, more complex routes, which involve more turns and increased path time. The drift in preferred direction may be caused by an imprecise path integrator, which has been observed in some behavioral tasks (Etienne et al. 2004; Séguinot et al. 1993). To test this possibility, we evaluated the amount of shift in the preferred direction between the start and goal boxes after rats walked across a short, linear bridge that connected the two arenas. This route was direct and did not involve any turns between the same start and goal boxes that were used in experiment 1a.
The BRIDGE condition was conducted for two groups of rats, with one group experiencing the bridge condition first and then the maze, whereas the order was switched for the second group of rats. For rats that participated in the MAZE FIRST condition (n = 6), HD cells showed little shift in their preferred direction between the start and goal boxes after rats traversed the bridge, with a mean absolute shift of 10.00 ± 6.51° [mean shift: −5.63 ± 9.01°, range: −42 to 6°; Rayleigh r(6) = 0.959, P < 0.001] (Figs. 5B and 6B; Table 1). For rats that participated in the BRIDGE FIRST condition (n = 10), HD cells also showed little shift in their preferred directions between the start and goal boxes after rats traversed the bridge, with a mean absolute shift of 8.40 ± 2.40° [mean shift: 4.79 ± 3.71°, range: −12 to 24°; Rayleigh r(10) = 0.985, P < 0.0001] (Fig. 6B). An F-test showed that there was no difference in the preferred direction shifts between the two groups, based on the mean vector values [F(1, 14) = 2.162, P = 0.164], and data from both groups were combined for subsequent comparisons. Overall, the mean shift between the start and goal boxes for the BRIDGE condition did not differ from 0°, with a mean absolute shift of 9.00 ± 2.74° [mean shift: 0.95 ± 3.44°, range: −42 to 24°; Rayleigh r(16) = 0.972, P < 0.0001]. The 95% confidence interval around the mean (−5.80 to 7.70°) included the 0° value, indicating no shift in the preferred direction. Furthermore, the distribution of preferred direction shifts between the START and GOAL boxes after rats traversed the bridge did not differ from the shifts between the START and GOAL boxes after rats traversed the maze [F(1, 25) = 0.81, P = 0.377] but did differ from the shifts that occurred between the START and MAZE END conditions [F(1, 25) = 14.71, P < 0.001]. Because the rats generally ran straight forward on the bridge without turning and had a relatively short distance to travel, there was not sufficient directional sampling for all directions to evaluate HD cell activity effectively while the rat traversed the bridge.
After the third trip across the bridge, the BRIDGE FIRST group walked through the maze for a single trip. On entry to the goal box, the preferred direction did not shift relative to the start box; mean absolute shift was 15.60 ± 3.82° [mean shift: 8.38 ± 6.52°, range: −18 to 36°; Rayleigh r(10) = 0.954, P < 0.0001]. However, similar to the MAZE FIRST rats, the preferred firing direction shifted slightly relative to the MAZE END; mean absolute shift was 15.60 ± 3.82° [mean shift: −25.77 ± 7.31°, range: −60 to 0°; Rayleigh r(10) = 0.943, P < 0.0001]. The shift between the START and GOAL was not different from 0°, as indicated by the 95% confidence interval around the mean (−4.40 to 21.15°). However, the 95% confidence interval for the mean shift between the START and MAZE END (−40.11 to −11.43) did not include 0°. Thus the HD signal showed similar shift characteristics for the BRIDGE FIRST and MAZE FIRST rats in the 14-unit T-maze.
Overall, HD signal stability between the start and goal boxes was similar for trips that involved traversing across a short, linear route and trips that involved a longer, complex route that included 14 turns and took longer to complete. Importantly, however, the HD signal did not remain stable while the rat traversed the maze, suggesting that a strategy other than path integration was used to regain HD signal stability when the rat entered the goal box. One possibility is that the rats used the geometric similarities between the start and goal boxes to maintain orientation. Alternatively, it is possible that some rats were using unintentional, uncontrolled distal room cue(s) to maintain their orientation between the start and goal boxes. To explore this latter possibility, experiment 2 was explicitly designed to monitor HD cell responses in a navigational task across different recording rooms.
Experiment 2: Multiroom Maze
This experiment used an alley with long linear trajectories and relatively few turns to connect two distinct arenas that were located in different rooms (Fig. 2). Each rat performed the multiroom maze task only once, because the circular arena in room 2 could only be defined as novel for one occasion. Three conditions were used for the multiroom task: 1) active locomotion in light, 2) active locomotion in darkness, and 3) passive transport, where the rat was placed in an opaque box, disoriented by the researcher, and then transported between the two rooms.
Active Locomotion in Light
For rats that actively locomoted between rooms under lighted conditions, the preferred directions showed a random distribution along the 360° continuum while the rats were in the square arena of room 1 [Rayleigh r(14) = 0.147, P > 0.744]. After the 8-min recording session in the square arena, the door to the passageway was opened and the rats usually entered the passageway within 2 min. The duration of the animals' trips through the passageway to the circular arena in room 2 varied considerably (mean: 119.58 ± 36.43 s, range: 30 to 420 s). The behavior of the animals during the passageway trips also varied, with some rats moving in a continuous manner through the passageway, whereas other rats often paused several times. Occasionally, rats attempted to return to the familiar arena in room 1, but they were prevented from doing so by a cardboard barrier that was manually moved along the passageway approximately 0.5 m behind the rat. Although we were not able to track the animal's location or directional heading by video in the passageway (because the animal was out of view of the video cameras), we were able to listen to cell firing as the animal moved through the extended passageway. In general, directional firing remained present in the passageway, with each cell's preferred direction approximating the orientation it had in the square chamber in room 1.
On entry to the novel circular arena, the preferred direction of most HD cells appeared to show a slight counterclockwise (CCW) shift relative to the orientation they exhibited in the square arena (Fig. 8A), with a mean absolute shift of 29.00 ± 5.71° [mean shift: 17.46 ± 9.92°, range: −72 to 54°; Rayleigh r(12) = 0.871, P < 0.0001]. However, the 95% confidence interval around this mean (−2.00 to 36.91°) included the value of 0°, indicating no shift. Figure 9A, left, plots the distribution of these shifts across all cells. Although these directional shifts appeared to be biased, the absence of a significant shift in the preferred direction suggests that path integration processes were able to maintain directional heading perception relatively well while the animal walked between rooms/arenas. Overall, the peak firing rate was 18.8% lower in the novel arena compared with the familiar arena. This reduction of peak firing rate indicates that HD cells may have provided a slightly different representation of perceived directional heading in the novel arena than in the familiar arena.
Fig. 8.
Directional tuning curves for 3 representative HD cells recorded during performance of the multiroom maze. A: during active locomotion in light, this cell's preferred direction remained relatively stable between the familiar and novel arenas, with a shift of 6°. On return to the familiar arena, the preferred direction also remained constant, with a shift of 6° relative to that of the first session in the familiar arena. B: during active locomotion in darkness, the preferred direction showed slightly less stability, with a shift of 30°. On return to the familiar arena, the preferred direction returned to its original alignment, with a shift of 0°. C: during passive transport, the preferred direction shifted −78°, only to become precisely realigned on return to the familiar arena, with a shift of 0°.
Fig. 9.
Distribution of shifts in the preferred firing direction across cells recorded during the multiroom task. For all plots, 0° represents each cell's preferred direction during the first recording session in room 1. Dashed circle indicates 0.05 significance criterion. A: during active locomotion from room 1 to room 2 (Outbound) in light, the distribution of shifts of the preferred direction was small and did not differ from 0°. After the return journey from room 2 to room 1 (Return), the distribution of shifts of the preferred direction did not differ from 0°. B: during active locomotion in darkness, the distribution of shifts of the preferred direction was again centered around 0°, but there was a larger range of shift values. After return to room 1, preferred firing directions returned to their original orientation. C: when rats were disoriented and passively transported between rooms, the preferred firing directions shifted to random values. After the return journey, however, the preferred firing directions returned to their previously established orientations. Points that fell between 6° bins are due to sessions that contained multiple cell recordings, and the point represents the average shifts of the preferred firing directions.
After the circular arena session in room 2, animals returned via the extended passageway back to the square arena in room 1. The duration of the return trips ranged from 30 to 420 s. In general, on reentry to the familiar arena, the preferred directions became realigned to their original orientations exhibited during the initial session in the square arena. The mean absolute shift in the preferred directions between the first and second sessions in the square arena was 5.00 ± 1.45° [mean shift: −1.99 ± 2.19°, range: −12 to 6°; Rayleigh r(12) = 0.993, P < 0.0001]. The 95% confidence interval around the mean (−6.29 to 2.30°) included the predicted value of 0°, indicating no shift (Fig. 9A, right). The return of the preferred direction to its original alignment in the familiar arena suggests that the initial recording session in the square arena of 8 min provided sufficient time to enable a stable representation within that arena, despite the fact that the path integrator was less than perfect during the long excursion between rooms.
Although the mean shift of the preferred direction during active locomotion did not differ from 0°, it is important to note that most cells showed greater angular shift values when navigating from the familiar to the novel arena than between the two sessions in the square arena (mean absolute shifts: 5.0 vs. 29.0°; t-test; P = 0.001; Table 1). Furthermore, an F-test on the concentration parameter found a significant difference between the angular variances in the two conditions [F(11, 11) = 19.34, P < 0.0001]. This difference may arise from an imperfect path integration process and may have resulted from the amount of time required to complete the journey. If true, then the amount of time required to complete the journey would be expected to correlate positively with the amount of directional shift observed between arenas. This prediction, however, was not supported by the data, because the correlation between elapsed trip time and shift in the preferred direction was not significant [mean r(12) = −0.405, P = 0.198] (Fig. 10A). Thus trip duration did not play a large role in contributing to the amount of directional shift that occurred between rooms. Another possibility is that the number of head turns and total distance traveled during the journey are significant predictors of HD signal shift. Unfortunately, the absence of video tracking in the passageway prevented quantitative assessment of these measures.
Fig. 10.
Absolute angular shift of preferred firing direction as a function of time in alley. A: during active locomotion in light, time was not significantly correlated with the amount of shift in the preferred firing direction between arenas (r = −0.405). B: during active locomotion in darkness, time in the alley was not significantly correlated with the amount of shift in the cell's preferred firing direction (r = −0.129).
Active Locomotion in the Dark
An alternative explanation for the relatively stable preferred directions between rooms is that the rats used visual cues along the path to the novel room to enable them to keep track of their orientation. According to this view, the rats could have used optic flow cues and/or a series of visual snapshots to associate different views during their trip to maintain their directional heading (Heft 1996; Istomin and Dwyer 2009). Certainly, the finding that novel landmarks can quickly influence the HD signal is consistent with this possibility (Goodridge et al. 1998). To address this possibility, we tested a new group of rats in the same task in the dark by placing a roof over the alleyway and turning off all room lights. The LEDs on the rats' heads were also turned off while they were in the passageway. On entry to the novel arena, the preferred direction appeared to shift somewhat relative to the preferred direction that occurred in the familiar arena (Fig. 8B). Inspection of the circular histograms in Fig. 9, A and B, left, shows that the two distributions of shifts are noticeably different, with a slightly larger spread away from zero in the dark sessions than in the lighted sessions. However, an F-test of the concentration parameter, which was used to determine whether the angular variances were different between groups, found a nonsignificant result [F(11, 11) = 2.485, P = 0.07]. It is noteworthy, however, that the nonsignificant result is largely driven by the one outlier point of 288° in the light condition. With this point excluded from the analysis, the F-test on the concentration parameter is now significant [F(10, 11) = 6.773, P < 0.01]. Similarly, the mean absolute shift in the dark sessions was 41.50 ± 8.40°, which is somewhat larger than the mean absolute shift in the light sessions when all data points are included (29.00 ± 5.71°) and when the outlier is removed (25.09 ± 4.56°) (Table 1). A t-test, however, revealed that the mean absolute shifts in darkness were not significantly different from those in light, whether all data points were included for the light sessions or the outlier was removed [t(22) = −1.23, P = 0.232 and t(21) = −1.672, P = 0.109, respectively]. Comparison of HD cell responses between each room's arena indicated that the preferred directional shifts of most HD cells in darkness were similar to those found in light, with a mean shift of 8.22 ± 15.00° [range: −69 to 99°; Rayleigh r(12) = 0.679, P = 0.002] (Fig. 9B, left). The 95% confidence interval around this mean (−21.18 to 37.62°) included the value of 0°, indicating no shift in the preferred firing direction. Furthermore, an F-test on the mean vectors (r) between the light and dark conditions found no significant difference [F(1, 22) = 0.277, P = 0.604]. Together, these results indicate that there was a trend for greater variability in shifts of the preferred direction in dark sessions, but statistical analyses revealed that the directional shifts observed in dark sessions were no different than the directional shifts in light sessions.
On return to the familiar square arena in room 1, the lights were turned on for the second familiar recording session. During this session, the preferred directions became realigned with their orientation during the initial recording session; mean absolute shift between the first and second sessions in the square arena was 3.75 ± 0.84° [mean shift: −0.75 ± 1.52°, range: −6 to 6°; Rayleigh r(12)= 0.997, P < 0.0001] (Table 1). The 95% confidence interval around the mean (−3.73 to 2.23°) included the value of 0°, indicating no shift in the preferred firing direction (Fig. 9B, right). Again, the shifts in the return session appear much smaller than the shifts in the multiroom session that required path integration between the two rooms (compare Fig. 9B, left and right). An F-test on the concentration parameter found a significant difference between the angular variances across these two conditions [F(11, 11) = 99.70, P < 0.001]. In addition, the distribution of shifts in the return phase of the dark condition did not differ from that of the return phase in the light condition [F(1, 22) = 0.261, P = 0.615]. As with locomotion in light, the amount of time spent in the novel alleyway was not significantly correlated with the amount of shift in the cell's preferred direction [r(12) = −0.129, P = 0.696] (Fig. 10B). Unlike locomotion in light, the peak firing rate did not differ between the familiar and novel arenas in the dark; on average, each cell's peak firing rate in the novel arena was 98.0% of the peak rate in the familiar arena.
In summary, as with the light condition, the preferred directions were relatively stable as the rat moved from one room to the other, but there was drift/shift in the preferred direction, and this shift was significantly larger than the directional shifts between the initial and return sessions in the room 1 square arena. These results appear to place a limitation on the accuracy of the path integration system.
Passive Transport
With a new group of animals (n = 12), HD cells were first recorded in the square enclosure of room 1, and then rats were placed in an opaque box and brought to room 2 while undergoing disorientation treatment. After passive transport, the cells' preferred directions shifted by a relatively random amount with the mean absolute shift = 104.50 ± 13.55° [mean angular shift: 132.07 ± 60.99°, range: 42 to 318°; Rayleigh r(12) = 0.272, P = 0.42] (Figs. 8C and 9C, left; Table 1). A reliable confidence interval could not be calculated from these data because the distribution was random (low concentration). An F-test on the concentration parameter revealed that the angular shifts in the passive condition were significantly different from those that occurred during active locomotion in light [F(12, 12) = 5.64, P < 0.001] but not in darkness [F(12, 12) = 2.488, P > 0.05]. Furthermore, the mean shift in the passive transport condition differed from that of the active locomotion in the light condition [F(1, 22) = 11.26, P = 0.003] and from the active locomotion in the dark condition [F(1, 22) = 10.66, P = 0.004]. The peak firing rate did not differ between the familiar and novel arenas, with each cell's peak firing rate in the novel arena being, on average, 100.1% of the peak rate in the familiar arena.
The random shift between the two arenas in the passive transport condition provided an excellent opportunity to assess the extent to which idiothetic cues could override, or be overridden by, familiar visual landmark information following active return to the familiar arena. After active locomotion back to the square arena in room 1, which ranged from 50 to 180 s, the preferred directions became realigned with the values they exhibited during the initial session in the square arena; the mean absolute shift between the two arenas was 5.00 ± 2.03° [mean angular shift; −1.46 ± 2.72°, range: −24 to 6°; Rayleigh r(12) = 0.99, P < 0.0001] (Fig. 9C, right). These shift values are comparable to the shift values in the return session for the light and dark conditions and are not significantly different (both Ps > 0.05). The accurate return of the preferred directions to the previously established alignments suggests that a single episode in the square arena was sufficient to establish cues within it as stable landmarks that persisted across sessions.
DISCUSSION
Our experiments provide evidence that the HD signal can be maintained relatively well by path integration mechanisms across complex routes involving many turns and some length of time, but that greater stability occurs when rats are able to use a combination of path integration and visual strategies. Furthermore, our results show that although the HD cell preferred directions were relatively stable during path integration, the variability of shifts across path integration sessions was greater than the variability observed across bridge sessions (experiment 1) or sessions in the same environment (experiment 2). This finding appears to place constraints on the accuracy of the path integration system and highlights the importance of landmarks for correcting errors over time.
As rats walked from a start box to a goal box via a 14-unit T-maze or via a short bridge, the HD signal remained relatively stable. One drawback of the 14-unit T-maze is that it was entirely contained within a single recording room. To test whether the HD cell signal was controlled by extramaze cue(s) located within the recording room, we used the multiroom maze to eliminate this problem. Results show that the HD cell signal remained relatively stable between rooms when rats walked from a familiar square arena in one room to a novel cylinder in a different room in lighted conditions. When this same task was performed in darkness, the HD cell's preferred direction showed a little more error than in light, but still remained relatively consistent between arenas. Importantly, however, there was a trend for greater variation of shifts observed in the dark condition, suggesting that vision played some role in maintaining a stable preferred direction between the two environments. When rats were disorientated and passively transported to the novel arena, HD cells showed a random shift in the cell's preferred direction between rooms 1 and 2. Under all conditions, HD cell preferred directions returned to their original alignment following active locomotion back to the familiar arena. Together, these findings suggest that a single, brief episode in an arena is sufficient to establish a familiar reference frame that persists across trials and that idiothetic cues, together with visual information along the route, are used to maintain a stable preferred direction when locomoting into an unfamiliar environment.
HD Signal Maintenance by Path Integration
Previous studies have demonstrated that HD cell preferred firing directions can be maintained by path integration information during performance of various spatial tasks (Blair and Sharp 1996; Dudchenko and Zinyuk 2005; Taube and Burton 1995). However, all of these tasks were performed within the same recording room; the HD cell stability that was attributed to path integration mechanisms could have been maintained by subtle landmark cues located within the recording environment or by a combination of landmark cues and path integration. Moreover, in each of these experiments, the distance the animal traveled between environments was very short (<2 m). These limitations were ruled out in the present multiroom experiments, because animals locomoted to a completely novel environment over a distance >10 m that was completely out of sight of the familiar environment. To our knowledge, these recordings are the first to demonstrate accurate updating of directional information in HD cells across different environments that involved animals moving between rooms.
Across cells, the preferred direction remained relatively stable while rats walked from the familiar square arena to the novel cylindrical arena in the multiroom experiment. Because no familiar landmarks were available during the journey and after arrival at the novel arena, the HD signal was presumably maintained by a path integration mechanism. For many individual cells, however, the preferred direction shifted a larger amount between rooms than between the initial and final sessions in the square arena. This result was also true for the 14-unit T-maze, where there was a larger shift in the preferred direction between the START and MAZE END conditions than between the START and GOAL conditions. These results indicate that path integration mechanisms were far from perfect while locomoting between environments. Path integration is thought to be a mechanism that can maintain directional orientation during long-range navigation, although this mechanism is susceptible to error accumulation (Benhamou et al. 1990). This error may result from a number of factors, including the number and direction of turns taken during the journey. This factor may explain the predominantly clockwise (CW) shifts observed in the preferred firing direction when rats made two overall CW 90° turns during their journey between familiar and novel arenas in previous studies (Stackman et al. 2003; Taube and Burton 1995). If the numbers of CW and CCW turns predict the direction of shift in the preferred firing direction, then we would expect the preferred firing direction to show a CW shift between the START and MAZE END conditions of the 14-unit T-maze experiment, given the greater number of CW than CCW turns (8 CW and 6 CCW). Our data support this suggestion, with most cells showing a CW shift, either at some point during the maze or accumulating gradually during maze traversal. If this relation between turn direction and shift of preferred firing direction also occurs in the multiroom experiment, the cells should have shown no shift, given the equal number of CW and CCW turns (although the last CCW turn is 45°). However, the preferred firing direction shifts were predominantly CCW and therefore do not support a consistent relation between turn direction and shift of the preferred firing direction. An alternative influence on path integration error is the first turn taken by the animal during the journey; the first turn in the 14-unit maze was CW, which corresponds to the shift of preferred firing direction between the START and MAZE END, and the first turn in the alley of the multiroom maze was CCW, which corresponds to the shift of preferred firing direction between rooms.
Two other possible influences on the stability of the preferred firing direction are time and path length. Time was not significantly correlated with the shift in preferred direction between arenas for either maze. The actual path length of the animal could not be quantified during traversal of the multiroom maze, but the present results indicate that path length alone did not predict stability of the preferred firing direction, because the preferred firing direction shifts exhibited during active locomotion between two rooms did not differ greatly from those that occurred during active locomotion across a short distance reported in previous studies (Stackman et al. 2003; Taube and Burton 1995). Indeed, excluding outliers, an F-test on the concentration parameter found no significant differences in the variability of the directional shifts between our results and these two studies [Stackman et al.: F(10, 20) = 1.25, Taube and Burton: F(10, 19) = 1.54; both P > 0.05]. Thus, although the degree to which turn direction, path length, and time contributed to the small shifts in preferred firing direction could not be assessed in the present experiment, we interpret our results as evidence that directional shifts during long-range navigation do not result from a single factor, but rather are caused by several factors, each of which may be more or less influential at various points or times within an environment.
Despite our attempts to disambiguate the start and goal boxes of the 14-unit T-maze by applying vertical stripes to the walls of the goal box, on entry to the goal box, the preferred direction of many HD cells appeared to become realigned with the orientation exhibited in the start box. One explanation for this realignment is that it may have resulted from the geometric similarities between the start and goal boxes. Indeed, geometric information is known to influence choices in behavioral tasks (Cheng 1986), and the HD signal can be influenced by the geometric contours of the recording arena (Clark et al. 2010; Dudchenko and Zinyuk 2005). In the multiroom maze, HD cell preferred firing directions remained relatively stable during active locomotion in light and in darkness, suggesting that a path integration mechanism is capable of maintaining HD perception. Although not significant, there was a strong trend for the HD signal to show increased variability while the rat navigated in darkness, relative to that in light. Indeed, if the one outlier was removed from the lighted condition, then the variability in shift values in darkness was significantly greater than the variability in light. This ability of vision to increase HD signal stability suggests that animals can quickly update their spatial orientation using unfamiliar landmarks. This result is consistent with an earlier report showing that HD cells showed slightly less shifts in their preferred directions in light than in darkness (Stackman et al. 2003). The present results extend this earlier observation by demonstrating that visual information can also improve HD signal stability across long distances, even when the rat has never seen the novel room. One possibility is that optic flow enhanced the rats' ability to perceive changes in directional heading during the trip between recording rooms. This suggestion is warranted by several previous studies suggesting that humans are able to judge heading direction relatively precisely using only visual information (Ellmore and McNaughton 2004; Kearns et al. 2002; Telford and Howard 1996; Warren and Kurtz 1992), although systematic directional errors exist in some conditions (Peruch et al. 1997). Whether this improved directional perception results from optic flow or the linking of a sequence of visual snapshots is not presently known.
Disruption of HD signal stability by passive transport between rooms is consistent with a previous study (Stackman et al. 2003), where disorientation and passive transport caused the HD signal to become randomly reoriented on entry to the novel arena. Importantly, in the present study the HD signal returned to its previously established orientation on return to the familiar arena, suggesting that path integration processes failed to provide dominant control of HD perception when familiar spatial cues were available (Zhang 1996). In addition, this dominance of allothetic cues occurred despite the fact that the familiar cues were only experienced during a single, unrewarded trial. This visual cue dominance over the HD signal after a long excursion appears to be similar to the visual cue dominance observed in behavioral path integration studies (Etienne et al. 1996, 2000, 2004). The present results, along with those of behavioral studies, suggest that this visual cue dominance can occur even when the view of visual landmarks is brief.
Comparison With Other Spatial Signals
Finally, it is important to contrast the results of the present study with those that have tested entorhinal grid cell and hippocampal place cell responses in complex environments. In a recent experiment by Derdikman et al. (2009), grid cells and CA1/CA3 place cells were recorded while rats navigated to a goal location via a multicompartment alley involving repeating hairpin turns. Grid cells, which typically fire in a hexagonal grid pattern in open environments (Hafting et al. 2005), fragmented into distinct grid maps in each compartment of the maze. In addition, CA1 and CA3 place cell activity disambiguated maze arms with different running direction, a finding that has been replicated in recent reports (Royer et al. 2010; Singer et al. 2010). The fact that grid and CA1/CA3 place cell activity became fragmented in the hairpin maze but ADN HD cell activity remained relatively stable and did not “remap” in the 14-choice T-maze and multiroom tasks suggests that HD cell activity and grid/place cell activity may be decoupled during navigation in compartmental environments. Furthermore, HD cell tuning in the entorhinal cortex may be strongly influenced by afferent input from the ADN HD cell signal, either directly (Shibata 1993) or indirectly via HD cells in the postsubiculum (Taube 2007; van Groen and Wyss 1990).
Conclusions
The HD signal remained relatively stable over long distances across different environments in the absence of visual cues, although there was frequently some error within the path integration system. In addition, access to visual information complemented path integration to improve the spatial updating process, even though this visual information was novel. Although the current study focused on the shifts in the preferred firing direction that occurred between the start and end boxes, small changes in the preferred direction often occurred during route traversal, illustrating the dynamic nature of the HD system during path integration along complex routes. It is therefore important for future studies to monitor these dynamic changes to understand when and how they occur during navigation.
GRANTS
This research was supported by National Institutes of Health Grants NS053907, DC009318 (J. S. Taube), DC008481 (R. M. Yoder), DC008773 (J. E. Brown), and the Natural Sciences and Engineering Council of Canada Postgraduate Scholarships Doctoral award (B. J. Clark).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Jennifer Rilling, Christopher Keene, Mark Harris, and Jeremy Chan for assistance with experimental procedures.
Present address of R. M. Yoder: Department of Psychology, Indiana University-Purdue University Fort Wayne, Fort Wayne, IN 46805.
REFERENCES
- Bassett et al., 2007. Bassett JP, Tullman ML, Taube JS. Lesions of the tegmentomammillary circuit in the head direction cell system disrupt the head direction signal in the anterior thalamus. J Neurosci 27: 7564–7577, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batschelet, 1981. Batschelet E. Circular Statistics in Biology. New York: Academic, 1981 [Google Scholar]
- Benhamou et al., 1990. Benhamou S, Sauvé JP, Bovet P. Spatial memory in large-scale environments: efficiency and limitations of the egocentric coding process. J Theor Biol 145: 1–12, 1990 [Google Scholar]
- Bingman and Cheng, 2005. Bingman VP, Cheng K. Mechanisms of animal global navigation: comparative perspectives and enduring challenges. Ethol Ecol Evol 17: 295–318, 2005 [Google Scholar]
- Blair and Sharp, 1996. Blair HT, Sharp PE. Visual and vestibular influences on head-direction cells in the anterior thalamus of the rat. Behav Neurosci 110: 643–660, 1996 [DOI] [PubMed] [Google Scholar]
- Cheng, 1986. Cheng K. A purely geometric module in the rat's spatial representation. Cognition 23: 149–178, 1986 [DOI] [PubMed] [Google Scholar]
- Clark et al. Clark BJ, Harris MJ, Taube JS. Control of anterodorsal thalamic head direction cells by environmental boundaries: comparison with conflicting distal Landmarks. Hippocampus. In press [DOI] [PubMed] [Google Scholar]
- Clark and Taube, 2009. Clark BJ, Taube JS. Deficits in landmark navigation and path integration after lesions of the interpeduncular nucleus. Behav Neurosci 123: 490–503, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derdikman et al., 2009. Derdikman D, Whitlock JR, Tsao A, Fyhn M, Hafting T, Moser MB. Fragmentation of grid cell maps in a multicompartment environment. Nat Neurosci 12: 1325–1332, 2009 [DOI] [PubMed] [Google Scholar]
- Dudchenko and Zinyuk, 2005. Dudchenko PA, Zinyuk LE. The formation of cognitive maps of adjacent environments: evidence from the head direction cell system. Behav Neurosci 119: 1511–1523, 2005 [DOI] [PubMed] [Google Scholar]
- Elliot, 1928. Elliot MH. The effect of change of reward on the maze performance of rats. Publ Psychol 4: 19–30, 1928 [Google Scholar]
- Ellmore and McNaughton, 2004. Ellmore TM, McNaughton BL. Human path integration by optic flow. Spat Cogn Comput 4: 255–272, 2004 [Google Scholar]
- Etienne et al., 2000. Etienne AS, Boulens V, Maurer R, Rowe T, Siegrist C. A brief view of known landmarks reorientates path integration in hamsters. Naturwissenschaften 87: 494–498, 2000 [DOI] [PubMed] [Google Scholar]
- Etienne and Jeffery, 2004. Etienne AS, Jeffery KJ. Path integration in mammals. Hippocampus 14: 180–192, 2004 [DOI] [PubMed] [Google Scholar]
- Etienne et al., 2004. Etienne AS, Maurer R, Boulens V, Levy A, Rowe T. Resetting the path integrator: a basic condition for route-based navigation. J Exp Biol 207: 1491–1508, 2004 [DOI] [PubMed] [Google Scholar]
- Etienne et al., 1996. Etienne AS, Maurer R, Seguinot V. Path integration in mammals and its interaction with visual landmarks. J Exp Biol 199: 201–209, 1996 [DOI] [PubMed] [Google Scholar]
- Frohardt et al., 2006. Frohardt RJ, Bassett JP, Taube JS. Path integration and lesions within the head direction cell circuit: comparison between the roles of the anterodorsal thalamus and dorsal tegmental nucleus. Behav Neurosci 120: 135–149, 2006 [DOI] [PubMed] [Google Scholar]
- Goodridge et al., 1998. Goodridge JP, Dudchenko PA, Worboys KA, Golob EJ, Taube JS. Cue control and head direction cells. Behav Neurosci 112: 749–761, 1998 [DOI] [PubMed] [Google Scholar]
- Goodridge and Taube, 1995. Goodridge JP, Taube JS. Preferential use of the landmark navigational system by head direction cells in rats. Behav Neurosci 109: 49–61, 1995 [DOI] [PubMed] [Google Scholar]
- Hafting et al., 2005. Hafting T, Fyhn M, Molden S, Moser MB, Moser EI. Microstructure of a spatial map in the entorhinal cortex. Nature 436: 801–806, 2005 [DOI] [PubMed] [Google Scholar]
- Heft, 1996. Heft H. The ecological approach to wayfinding: A Gibsonian perspective. In: The Construction of Cognitive Maps, edited by Portugali J. Dordrecht, The Netherlands: Kluwer Academic, 1996, p. 105–801–132 [Google Scholar]
- Istomin and Dwyer, 2009. Istomin KV, Dwyer MJ. Finding the way: a critical discussion of anthropological theories of human spatial orientation with reference to reindeer herders of northeastern Europe and western Siberia. Curr Anthropol 50: 29–49, 2009 [DOI] [PubMed] [Google Scholar]
- Kearns et al., 2002. Kearns MJ, Warren WH, Duchon AP, Tarr MJ. Path integration from optic flow and body senses in a homing task. Perception 31: 349–374, 2002 [DOI] [PubMed] [Google Scholar]
- Knierim et al., 1998. Knierim JJ, Kudrimoti HS, McNaughton BL. Interactions between idiothetic cues and external landmarks in the control of place cells and head direction cells. J Neurophysiol 80: 425–446, 1998 [DOI] [PubMed] [Google Scholar]
- Knierim et al., 1995. Knierim JJ, Kudrimoti HS, McNaughton BL. Place cells, head direction cells, and the learning of landmark stability. J Neurosci 15: 1648–1659, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubie, 1984. Kubie JL. A driveable bundle of microwires for collecting single-unit data from freely-moving rats. Physiol Behav 32: 115–118, 1984 [DOI] [PubMed] [Google Scholar]
- Peruch et al., 1997. Peruch P, May M, Wartenberg F. Homing in virtual environments: effects of field of view and path layout. Perception 26: 301–311, 1997 [DOI] [PubMed] [Google Scholar]
- Royer et al., 2010. Royer S, Sirota A, Patel J, Buzsaki G. Distinct representations and theta dynamics in dorsal and ventral hippocampus. J Neurosci 30: 1777–1787, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Séguinot et al., 1993. Séguinot V, Maurer R, Etienne AS. Dead reckoning in a small mammal: the evaluation of distance. J Comp Physiol A 173: 103–113, 1993 [DOI] [PubMed] [Google Scholar]
- Sharp et al., 2001. Sharp PE, Blair HT, Cho J. The anatomical and computational basis of the rat head-direction cell signal. Trends Neurosci 24: 289–294, 2001 [DOI] [PubMed] [Google Scholar]
- Shibata, 1993. Shibata H. Direct projections from the anterior thalamic nuclei to the retrohippocampal region in the rat. J Comp Neurol 337: 431–445, 1993 [DOI] [PubMed] [Google Scholar]
- Singer et al., 2010. Singer AC, Karlsson MP, Nathe AR, Carr MF, Frank LM. Experience-dependent development of coordinated hippocampal spatial activity representing the similarity of related locations. J Neurosci 30: 11586–11604, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stackman et al., 2003. Stackman RW, Golob EJ, Bassett JP, Taube JS. Passive transport disrupts directional path integration by rat head direction cells. J Neurophysiol 90: 2862–2874, 2003 [DOI] [PubMed] [Google Scholar]
- Stackman and Taube, 1998. Stackman RW, Taube JS. Firing properties of rat lateral mammillary single units: head direction, head pitch, and angular head velocity. J Neurosci 18: 9020–9037, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube, 1995. Taube JS. Head direction cells recorded in the anterior thalamic nuclei of freely moving rats. J Neurosci 15: 70–86, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube, 2007. Taube JS. The head direction signal: origins and sensory-motor integration. Ann Rev Neurosci 30: 181–207, 2007 [DOI] [PubMed] [Google Scholar]
- Taube and Burton, 1995. Taube JS, Burton HL. Head direction cell activity monitored in a novel environment and during a cue conflict situation. J Neurophysiol 74: 1953–1971, 1995 [DOI] [PubMed] [Google Scholar]
- Taube et al., 1990a. Taube JS, Muller RU, Ranck JB., Jr Head-direction cells recorded from the postsubiculum in freely moving rats. I. Description and quantitative analysis. J Neurosci 10: 420–435, 1990a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube et al., 1990b. Taube JS, Muller RU, Ranck JB., Jr Head-direction cells recorded from the postsubiculum in freely moving rats. II. Effects of environmental manipulations. J Neurosci 10: 436–447, 1990b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telford and Howard, 1996. Telford L, Howard IP. Role of optical flow field asymmetry in the perception of heading during linear motion. Percept Psychophys 58: 283–288, 1996 [DOI] [PubMed] [Google Scholar]
- Tolman and Honzik, 1930. Tolman EC, Honzik CH. Introduction and removal of reward, and maze performance in rats. Publ Psychol 4: 257–275, 1930 [Google Scholar]
- van Groen and Wyss, 1990. van Groen T, Wyss JM. The postsubicular cortex in the rat: characterization of the fourth region of the subicular cortex and its connections. Brain Res 529: 165–177, 1990 [DOI] [PubMed] [Google Scholar]
- Warren and Kurtz, 1992. Warren WH, Kurtz KJ. The role of central and peripheral vision in perceiving the direction of self-motion. Percept Psychophys 51: 443–454, 1992 [DOI] [PubMed] [Google Scholar]
- Whishaw and Tomie, 1997. Whishaw IQ, Tomie JA. Piloting and dead reckoning dissociated by fimbria-fornix lesions in a rat food carrying task. Behav Brain Res 89: 87–97, 1997 [DOI] [PubMed] [Google Scholar]
- Zhang, 1996. Zhang K. Representation of spatial orientation by the intrinsic dynamics of the head-direction cell ensemble: a theory. J Neurosci 16: 2112–2126, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zugaro et al., 2000. Zugaro MB, Tabuchi E, Wiener SI. Influence of conflicting visual, inertial and substratal cues on head direction cell activity. Exp Brain Res 133: 198–208, 2000 [DOI] [PubMed] [Google Scholar]
- Zugaro et al., 2003. Zugaro MB, Arleo A, Berthoz A, Wiener SI. Rapid spatial reorientation and head direction cells. J Neurosci 23: 3478–3482, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]